Abstract
Introduction
In the COVID-19 pandemic, healthcare workers (HCWs) were at high risk of infection due to their exposure to COVID infections. HCWs were the backbone of our healthcare response to this pandemic; every HCW withdrawn or lost due to infection had a substantial impact on our capacity to deliver care. Primary prevention was a key approach to reduce infection. Vitamin D insufficiency is highly prevalent in Canadians and worldwide. Vitamin D supplementation has been shown to significantly decrease the risk of respiratory infections. Whether this risk reduction would apply to COVID-19 infections remained to be determined. This study aimed to determine the impact of high-dose vitamin D supplementation on incidence of laboratory-confirmed COVID-19 infection rate and severity in HCWs working in high COVID incidence areas.
Methods and analysis
PROTECT was a triple-blind, placebo-controlled, parallel-group multicentre trial of vitamin D supplementation in HCWs. Participants were randomly allocated in a 1:1 ratio in variable block size to intervention (one oral loading dose of 100 000 IU vitamin D3+10 000 IU weekly vitamin D3) or control (identical placebo loading dose+weekly placebo). The primary outcome was the incidence of laboratory-confirmed COVID-19 infection, documented by RT-qPCR on salivary (or nasopharyngeal) specimens obtained for screening or diagnostic purposes, as well as self-obtained salivary specimens and COVID-19 seroconversion at endpoint. Secondary outcomes included disease severity; duration of COVID-19-related symptoms; COVID-19 seroconversion documented at endpoint; duration of work absenteeism; duration of unemployment support; and adverse health events. The trial was terminated prematurely, due to recruitment difficulty.
Ethics and dissemination
This study involves human participants and was approved by the Research Ethics Board (REB) of the Centre hospitalier universitaire (CHU) Sainte-Justine serving as central committee for participating institutions (#MP-21-2021-3044). Participants provided written informed consent to participate in the study before taking part. Results are being disseminated to the medical community via national/international conferences and publications in peer-reviewed journals.
Trial registration number
Keywords: COVID-19, PREVENTIVE MEDICINE, Clinical trials
Strengths and limitations of this study.
This trial was designed as a hybrid study enabling partially or totally remote screening, randomisation, follow-up, as well as outcome documentation by use of home capillary blood and saliva sampling, visits conducted by videoconference, monitoring by electronic reminders and questionnaires, and communication by phone, text messaging or emails.
The trial used a pragmatic subject selection and easily applicable intervention to maximise subsequent implementation in practice and selected a primary outcome, the risk of laboratory-confirmed infection, that would likely change practice.
A single loading dose followed by regular doses have been shown to lead to rapid and sustained increase in serum level of 25-hydroxyvitamin D and ensure adequate group separation, both properties desired in the context of a rapidly expanding epidemic while facilitating adherence in exhausted front-line health workers.
Given the uncertainty in the progression of the infection rate, the use of a Bayesian adaptive design allowed for adaptations (early stopping or prolongation of duration of follow-up) at the interim analysis according to the projection of infection rates.
Although the trial aimed for high-intensity recruitment, the delay in setting up a remote study in the context of the pandemic, combined with high use of vitamin D and successful vaccination program in healthcare workers, resulted in severe recruitment difficulty and early stopping of the trial for futility.
Introduction
The SARS-CoV-2 disease (COVID-19) outbreak has rapidly expanded to a global pandemic. Healthcare workers (HCWs) played a crucial role in the fight against the COVID-19 pandemic. It was therefore a public health priority to develop strategies to decrease the risk and severity of COVID-19 in this vulnerable population. Indeed, this was a rising concern as HCWs were over-represented in terms of infections (3.8% of infected individuals in Wuhan, China,1 10% in Italy and 12% in Spain, 10–20% in USA)2 3 and perhaps severity. Working in long-term care facilities (LTCF) and with aerosol generating medical procedures (eg, hospitals) further increased the risk (OR: 2.3).4 The risk of reporting COVID-19 infection in front-line HCWs, defined as those in direct contact with patients, was 10-fold greater than the general population at the beginning of the pandemic (HR= 11.61).5 Recent research also indicated that HCWs who were black, Asian or other minority ethnic populations, had a higher likelihood of contracting COVID-19.5 Compared with those unexposed to patients with COVID-19, the risk was twofold to fivefold higher in HCWs exposed to suspected (HR=2.39) or confirmed (HR=4.83) COVID-19 cases, even with adequate personal protection equipment (PPE).5 Although infections may have been due to contact with infected patients, community-acquired disease or family acquired disease, cases were rapidly emerging from cross-infection with asymptomatic infected HCWs.
Vitamin D is an immunomodulatory micronutrient, and its levels in the body may vary due to diet and environmental conditions. Vitamin D insufficiency had been associated with increased risk of respiratory infections, and possibly COVID-19,6 asthma exacerbations and acute respiratory distress syndrome, among others.7–9 Optimal pro-immune and anti-inflammatory impacts likely occur at 25-hydroxyvitamin D (25OHD) levels above 75 nmol/L (30 ng/mL).10 11 In a systematic review of 25 randomised controlled trials (RCTs) of 11 321 individuals, daily/weekly vitamin D supplementation decreased by 19% the rate of acute respiratory infections (two-step analysis; OR 0.81, 95% CI 0.72 to 0.91),12 13 with a stronger effect in subjects with baseline 25OHD<25 nmol/L. Whereas subgroup analyses suggested a protective effect, primary in individuals receiving daily or weekly vitamin D supplement, and not in those with bolus,14 other important differences in population (eg, malnutrition),15 16 age (infant),16 chronic disease (eg, asthma, chronic obstructive pulmonary disease)17–21 and type of infection (eg, bacterial)15 16 could have contributed to the apparent lesser effect. Of interest, vitamin D supplementation significantly reduced the rate of severe exacerbations (ie, requiring rescue systemic corticosteroids), a condition associated with airway inflammation, with no impact according to bolus use or not.14 Vitamin D supplementation was also found to be associated with a decreased load of rhinovirus (common cold), consistent with an increased antiviral immune response.22 A systematic review and several studies reported an inverse association between serum vitamin D levels and COVID-19 severity, inpatient mortality, as well as serum levels of C reactive protein (CRP) and lymphocyte percentage.23 24 These findings suggested that vitamin D status was linked with the severity and mortality of COVID-19 infection in the general population, particularly in severe COVID-19 cases. Whether vitamin D could have prevented or lessened infection and/or the inflammatory response associated with COVID-19 remained to be explored.25 At the time of funding (June 2020) and study initiation (February 2021), no other primary prevention trials were published. Since then, one positive and two negative trials testing different vitamin D interventions as primary prevention were published.26–28
The vitamin D receptor is expressed on innate and adaptive immune cells which also synthesise the active metabolite 1,25-hydroxyvitamin D3 (1,25(OH)2D3); thus, vitamin D could strengthen innate and adaptive cellular immunity by increasing local production of antimicrobial peptides, decreasing secretion of pro-inflammatory cytokines, inhibiting dendritic cell activation, suppressing T helper cell type 1 response, and promoting T regulatory cells induction. These cellular effects are crucial for host responses against infection and can reduce the survival and replication of respiratory viruses.13 24 1,25(OH)2D3 is also produced locally in bronchial epithelial cells and downregulates inflammatory cytokines (eg, interleukin-8) and chemokines (eg, leucocyte attracting CXCL10) expression from stimulated cells.29
The protocol of a placebo-controlled parallel-group triple-blind RCT to explore the impact of vitamin D3 supplementation on reducing the risk and severity of laboratory-confirmed COVID-19 infection in HCWs is described here, as per Standard Protocol Items: Recommendation for Intervention Trials guidelines (online supplemental file 1). After funding, but prior to the start of recruitment, the protocol underwent four amendments (eight protocol versions) in view of the rapidly evolving science, multiple challenges faced with conducting a large-scale COVID-19 trial of high-risk HCWs during the pandemic, including difficulty in obtaining large-scale supplies, as well as favourable pilot results of two novel technologies (table 1). These original and final (V.1.8, 18 January 2021) protocol versions are described below. The trial was initiated but stopped prematurely due to recruitment difficulty.
Table 1.
Study amendments and notifications
| Version number | Clinical trial application (CTA) |
Investigational testing authorisation (ITA) | ||||
| Changes | Description | Submitted | Approved | Submitted | Approval | |
| V.0.0 11-05-2020 | ||||||
| V.1.0 23-08-2020 |
|
|
23 August 2020 | 16 September 2020 | N/A | N/A |
| Amendment 1 V.1.1 23-10-2020 |
|
|
23-10-2020 (CTA-A) * |
02 November 2020 | 23 October 2020 (NADAL) | 14 November 2020 |
| Amendment 2 V.1.2 to V.1.4 V.1.5 27-11-2020 |
|
|
27-11-2020 (CTA-A) * |
30 November 2020 | 23 November 2020 (TASSO-SST and NADAL) | 2 December 2020 |
| Amendment 3 V.1.6 and V.1.7 12-12-2020 |
|
|
12 December 2020 (CTA-A) * |
16 December 2020 | 12 December 2020 (TASSO-SST and NADAL) | 23 December 2020 |
| Amendment 4 V.1.8 18-01-2021 |
|
|
18 January 2021 (CTA-N) † |
N/A | 18 January 2020‡ | 01 February 2021 |
Reverse transcription-quantitative polymerase chain reaction
*CTA-A, Clinical Trial Application—Amendment
†CTA-N, Clinical Trial Application—Notification
‡As there is no ‘notification’ category for the Investigational Testing Authorisation (ITA), each amendment or notification to the Clinical Trial Application (CTA) must be submitted as a new amendment for the devices to be reviewed by ITA.
bmjopen-2022-064058supp001.pdf (131.4KB, pdf)
Objectives
The primary research question was whether one oral dose of 100 000 IU vitamin D3 (administered at baseline) plus weekly supplement of 10 000 IU vitamin D3 could decrease the risk of laboratory-confirmed COVID-19 infection, versus placebo, in front-line HCWs in high COVID-19 incidence areas.
Additionally, the study aimed to examine if, compared with placebo, the vitamin D intervention reduced: (1) Illness severity, (2) Symptom duration, (3) Work absenteeism and (4) Unemployment among front-line HCWs in high COVID-19 incidence areas. This study was to also assess various exploratory outcomes.
Hypothesis
We hypothesised that compared with placebo, vitamin D supplementation would decrease the incidence of laboratory-confirmed symptomatic COVID-19 infection by 20% in front-line HCWs working in high COVID-19 incidence area.
Methods and analysis
Study design
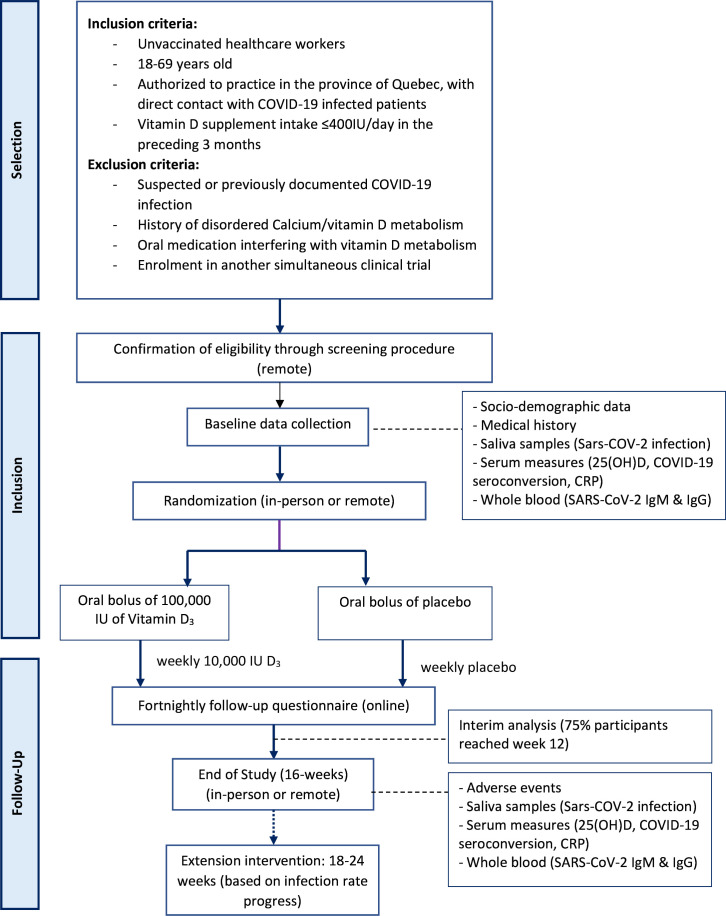
PROTECT was a pragmatic 16-week, triple-blind, placebo-controlled, parallel-group, randomised trial comparing supplemental vitamin D3 and placebo in HCWs with the possibility of extending the study follow-up up to 24 weeks, depending on infection rate progression during an interim analysis (figure 1).
Figure 1.
Study outline. CRP, C reactive protein.
Participants
HCWs (ie, physicians, allied HCWs, orderlies, etc) were eligible if they: (1) Were aged ≥18 years and <70 years old; (2) Were authorised to practice in Quebec; (3) Were working or scheduled to work over the next 16 weeks in a setting at high risk of contact with COVID-19 infected individuals, particularly (but not only) those involved with aerosol-generating medical procedures in hospitals and/or caring for patients in LTCF; (4) Were working in high COVID-19 incidence areas in the greater Montreal area and surroundings; (5) Were covered by the provincial universal public health insurance (Régie de l’assurance-maladie du Québec (RAMQ) for medical services and hospitalisations; (6) Had a personal email or phone (to which to send reminders and questionnaire by email or text messages); (7) Had a fixed address (to which to send the material) in the greater Montreal or surrounding areas.
HCWs were excluded if they met any of the following criteria: vitamin D supplementation (cholecalciferol or calcitriol) intake >400 IU/day (or >12 000 IU/month) in the past 3 months; intention to take >400 IU/day during the study period; suspected or previously documented COVID-19 infection; history of nephrolithiasis, hypercalcaemia, hyperphosphataemia, hyperparathyroidism, granulomatosis disease (eg, tuberculosis, sarcoidosis), renal failure, or active cancer; current intake of medications that may cause hypercalcaemia such as lithium, teriparatide or digoxin; anticipated prolonged absence from work during the study period (ie, pregnancy); anticipated difficult follow-up; enrolment in a concurrent interventional randomised trial; have already received the vaccine against COVID-19. Participation in this trial did not preclude subsequent enrolment in a COVID-19 therapeutic (but not preventive) trial, which would be documented.
Study intervention
Participants in the intervention group received 100 000 IU vitamin D3 (cholecalciferol) at randomisation followed by a weekly dose of 10 000 IU vitamin D3 for 16 weeks. Participants in the control groups received an identical placebo bolus followed by placebo weekly supplement for 16 weeks. Sufficient supply was provided for 24 weeks, in case of prolongation study based on the interim analysis. Participants in both groups were asked to take the study intervention with their most copious meal. Treatment of comorbidities was permitted. Vitamin D intake up to 400 IU per day was allowed.
Randomisation
Randomisation was implemented using a computer-generated random list stratified by one of 11 workplaces (Centre Hospitalier Universitaire (CHU)) or health region (Centre Intégré Universitaire de Santé et Services Sociaux (CIUSSS) or Centre Intégré de Santé et Services Sociaux (CISSS)). HCWs were allocated (1:1) using permuted block randomisation to enhance concealment. Group allocation codes for each stratum was held in a secure location with restricted access by the Central Pharmacy and Data Management.
Patient and public involvement
Participant burden of research measures was ascertained using feedback from patients prior to study participation. Patients were not involved in study design, recruitment of participants or conduct of the study.
Outcomes
Primary outcome
The original primary outcome, incidence of laboratory-confirmed COVID-19 infection, was originally based on (1) Bi-monthly self-obtained mid-turbinate nasopharyngeal (NP) swabs, complemented by (2) NP swabs obtained clinically for screening or diagnostic purposes throughout the study, both analysed by Reverse transcription-quantitative polymerase chain reaction (RT-qPRC) approved by Health Canada. Faced with the unexpected cancellation of our large order of Falcon tubes to collect saliva sample for qPRC, combined with the unacceptable additional delay for a public tender to securing a contract with a private courier service, and in view of the uniform protocol for screening symptomatic or COVID-19-exposed HCWs throughout the Province of Quebec and the reliability of IgG serology, we decided to forgo the twice-monthly saliva sampling for qPRC analysis. The revised definition of the primary outcome became the incidence of laboratory-confirmed COVID-19 infection, documented by RT-qPCR based on salivary (or NP) specimens (1) Obtained for screening or diagnostic purposes throughout the study and (2) Self-obtained salivary specimens obtained at endpoint as well as (3) COVID-19 IgG seroconversion at endpoint (in COVID-unvaccinated individuals: ≥15 UA on the anti-S SARS-CoV-2 IgG Diasorin on Liaison XL platform; in COVID-vaccinated individuals : ≥1.40 index (specimen/calibrator (S/C)) on the anti-N SARS-CoV-2 IgG on ARCHITECT platform from Abbott Core Laboratory Total Solution)
Secondary outcomes
(1) Distribution of disease severity on a five-category ordinal scale (asymptomatic; mild (managed at home); moderate (hospitalisation without supplemental oxygen); severe (oxygen supplementation); critical (mechanical ventilation/death)), (self-reported, RAMQ); (2) Duration of COVID-19 positivity (between first COVID+ to first COVID− test) revised to duration of COVID-19-related symptoms in individuals with laboratory confirmation of COVID infection, (self-reported on diary); (3) COVID-19 IgG seroconversion documented at endpoint (see above); (4) Duration of work absenteeism (self-reported, medical records or human resources databases); (5) Duration of unemployment support (human resource databases); (6) Adverse health events (AHEs) (self-reported). Several exploratory outcomes pertained to the: incidence of postacute and chronic symptoms; long-term (1 year) morbidity and work absence related to COVID-19; change in gene expression of angiotensine converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) in saliva cells; change in inflammatory markers (ie, CRP), immune response postvaccination; other viral infections; and genetic markers (including changes in gene expression).
Study procedures
To facilitate the recruitment of participants, this study was conceived as a hybrid trial enabling partially or totally remote trial participation including screening, randomisation, follow-up and end-of-study visit.
Prescreening
Advertisements were placed in health institutions, newspapers, social media and online, where participants were invited to complete an online prescreening form, read and download the consent forms; and if eligible and interested, to book a virtual screening appointment (via a secured videoconferencing platform) with a research team who would confirm eligibility, explain the study, obtain informed consent, and schedule a virtual or inperson randomisation visit.
Screening
At the virtual screening visit by videoconferencing, research coordinators completed with the individual a more extensive eligibility questionnaire, which included additional questions about: anticipated work exposure over the next 16 weeks to COVID-infected or suspected individuals and to high-risk medical procedures; work place (Centre hospitalier universitaire de Montréal (CHUM) or CHU Sainte-Justine) or health region (CIUSSS or CISSS), serving as randomisation stratum; prior laboratory-confirmed or physician-suspected COVID-19 infection; assessment of the likelihood of prior/current, yet undocumented, COVID-19 infection using the five-item questionnaire developed by Menni et al30 (score >0.50 interpreted as high likelihood of prior infection); and finally, the comfort level with the study design and procedures, including saliva and capillary blood sampling self-collection demonstrated by instructional videos. Eligible and consenting individuals electronically signed an online consent form (with the signed Portable Document Format (PDF) consent form automatically emailed to participants). Then, two additional questionnaires were completed online with the research coordinators namely: (1) The baseline questionnaire collecting information about household, ethnicity, part-time versus full-time work, personal health, skin colour (measured with the Fitzpatrick Scale),31 concomitant medications or supplements, and (2) The nominative Case Report Form (CRF) collecting demographic information essential to opening a medical and pharmaceutical research record (ie, public health insurance number, allergies) and maintaining contact with the research team throughout the study (preferred means to receive electronic reminders/questionnaires and to be notified of positive test results; address to receive study material or for biological sample pick-up; and next-of-kin contact in case of inability to respond to questionnaire due to illness), and to document work absence (employee number).
Finally, at the screening visit, the participant was asked to choose an appointment for a virtual (via a secured videoconferencing platform) or inperson randomisation visit at one of several locations. To help select their preferred visit format, videos of key procedures (such as home blood collection) were shown. An inperson randomisation visit was mandatory only in participants with a significant likelihood of a current or past undocumented COVID-19 infection (Menni Score>0.5) in order to receive the rapid COVID-19 serology test, prior to randomisation.
Preparation and shipment of study drug by research pharmacy
The list of new participants approved by one of the PIs was sent daily by email to the CHUM research team to be open a medical chart and send an electronically signed prescription for the study medication, to the research pharmacy for preparation of study drug.
Prior to randomisation, a list of all consenting and eligible participants was automatically sent every night to the one of the co-principal investigators (FMD or CT) who reviewed screening and baseline questionnaires to approve or refuse study entry and electronically signed their decision. The daily list of new approved participants was sent electronically daily to the CHUM research team. Medical and pharmaceutical records were opened and an electronically signed prescription for the study medication sent to the research pharmacy for preparation of study drug for a given target date.
To enable remote randomisation, the randomisation took place about 1 week prior to the randomisation visit to allow enough time for the preparation and shipment of patient-specific study supplement to the research team and, in turn, the shipment of the study supplement and all materials required for the randomisation visit by the research team to the participant.
Randomisation visit
Seventy-two hours and 24 hours prior to, and at, the randomisation visit, participants were screened by questionnaire for recent travel, symptoms suggestive of SARS-CoV-2 infection, or exposure to COVID-19 infected individuals. Those who responded positively were asked to get tested, notify their institutional health service, and await end of quarantine and/or confirmed negative test to reschedule the randomisation visit.
Randomisation visit (week 0) was performed inperson (60 min) or remotely (90 min), depending on the availability and preference of participants as well as their likelihood of a past COVID-19 infection.
Inperson visits were conducted—by appointment only—in designated rooms with restricted access. The research coordinators wore PPE; all procedures, from participant arrival to departure, were approved by the institutional Infection Control and Safety committee. The inperson visit entailed (1) Ascertainment of the signed consent form, (2) Capillary blood sample collection to perform NADAL COVID-19 IgG/IgM rapid test (Teracero Pharma, Lachine Canada), (3) Venous blood sample collection for baseline serum 25(OH)D and SARS-CoV-2 IgG serology analyses and if genetic consent, DNA; (4) Viewing of the saliva collection video and instruction pamphlet, (5) Collection of the first specimen under supervision, (6) A final verification of the eligibility and exclusion criteria; (7) Randomisation; (8) Oral administration of 100 000 IU vitamin D3 or an identical placebo, and (9) Distribution of the study material including study supplement, saliva sampling kit for end of study, biohazard and sampling bag, and, if a remote visit was anticipated at endpoint, capillary blood collection kits (TASSO-SST device, Tasso Inc., Seattle, USA). Any patient with a positive NADAL COVID-19 IgM/IgG rapid test serology test were excluded prior to randomisation.
The remote randomisation visit, conducted by videoconference, was similar to the inperson randomisation visit with the following additions: (A) Viewing of the capillary blood collection video and instructional pamphlet; (B) Remote capillary sampling under guidance using the TASSO-SST device (Tasso Inc., Seattle, USA); (C) Viewing of the saliva collection video and instructional pamphlet (OG-600 Oragene DNA Collection Kit, DNA Genotek, Ottawa, Canada); (D) Remote DNA salivary sampling under guidance; (E) Preparation of biological samples for shipment with phase change and insulated envelopes under guidance and (F) Organising collection of biological specimens by approved courier service to respective laboratories. Note that a Nadal serology test was not conducted remotely.
Follow-up
Participants received weekly electronic (SMS or email) reminders to take their weekly study supplement (10 000 IU vitamin D or an identical placebo) and to start completing an online daily diary if they tested positive to SARS-CoV-2 or they developed symptoms suggestive of COVID-19 infection.
Every 2 weeks, participants received a link to complete a brief online questionnaire asking to report: their adherence to weekly study supplement intake; health status including recent COVID-19 related exposure, symptoms or testing; AHEs or new comorbidities; change in concomitant medications or supplement intake; work status (active duty, quarantined, holiday, sick) and work setting (emergency department, intensive care unit, etc.); as well as expected/recent COVID-19 vaccination (date and vaccine name) if any; the latter question served to enable timely shipment of materials for additional sampling prior to vaccination, as COVID-19 vaccination was permitted during the study. In participants who planned to get vaccinated during the study, three additional blood, and one additional saliva, samplings, either on-site or remotely, were planned including: saliva (for COVID-19 qPCR analysis) and blood (for SARS-CoV-2 anti-S IgG serology) sampled prior to vaccination, a blood sample (for SARS-CoV-2 anti-S and anti-N IgG serology) collected just prior to the second vaccine dose, and a blood sample (for SARS-CoV-2 anti-S and anti-N IgG serology) collected 1 month after second vaccine dose and endpoint. Regardless of their vaccination status, participants were asked to continue taking the weekly study supplement and complete the bi-monthly questionnaire until the end of the study. If questionnaires were not completed within 2 days of the target date, the research coordinator reached out to the participant to complete the information.
End-of-study visit
An end-of-study visit was conducted either inperson (60 min) or remotely (90 min), depending on the availability and preference of participants and likelihood of a current COVID-19 infection. The inperson end-of-study visit entailed the collection of a (1) Venous blood sample for serum 25(OH)D and SARS-CoV-2 anti-S IgG serological results and in vaccinated participants a SARS-CoV-2 anti-N IgG serology, (2) Capillary blood sample to perform the NADAL COVID-19 IgG/IgM rapid test, (3) A saliva sample for SARS-CoV-2 qPCR analysis as well as guessing of allocation and return of the study supplement bottle to assess adherence and any unused material.
The remote end-of-study visit conducted via videoconference entailed the same procedures as the inperson end-of-study visit with one exception: the self-collection of a capillary (instead of venous) blood using TASSO-SST devices (for the serum 25(OH)D and SARS-CoV-2 anti-S with/without anti-N serology). Individuals were guided into self-performing the pinprick capillary method to perform the NADAL COVID-19 IgG/IgM rapid test and return of biological samples and materials by prepaid approved courier.
Covariates
Several covariates that could act as confounders or interaction variables in the magnitude of effect associated with the intervention were documented, namely: baseline serum 25OHD level; smoking; concomitant supplements or drug(s) that can alter calcium or vitamin D absorption or metabolism such as diuretics and antiepileptics (reported at baseline and every 2 weeks); skin colour (documented at baseline); obesity (documented by height and weight (body mass index (BMI)) at baseline); other comorbidities (ie, diabetes, hypertension, etc) that may affect the severity of COVID-19 infection and receipt of a COVID-19 vaccine (documented by verbal report bi-monthly). All external (governmental and institutional) databases were to be obtained 3 months before, and up to 16 months following, randomisation (as well as 12 months after then study endpoint).
During an event
During COVID-19-related symptoms or documented SARS-CoV infection, participants were instructed to complete a daily symptom diary from date of onset of symptoms or positive test, until 2 days with no symptoms or 14 days if asymptomatic.
Risk management
Clinical and biochemical AHEs were monitored throughout the study and reported for all patients at the end of the study. No specific laboratory safety monitoring was planned given the established safety of the loading dose of 100 000 IU and weekly dose of 10 000 IU.32 33 AHEs were recorded via electronic questionnaires throughout the study. Participants who reported symptoms suggestive of vitamin D intoxication had a venous blood sampling (total and ionised calcium, phosphorus, alkaline phosphatase, albumin, and creatinine). Any abnormal laboratory value was interpreted as ‘clinically significant’ or ‘not clinically significant’ by the site endocrinologist blinded to study allocation. Further investigation or action for individual participants (including interruption, cessation, or unblinding of the study drug via pharmacy or by analysis of serum 25OHD) was determined by the site endocrinologist, if indicated to ensure participant safety. The AHE’s occurrence was reviewed periodically by the Data and Safety Monitoring Board (DSMB). Code breaking was allowed only if deemed essential for participant management. If relevant, summary reports aggregating (or not if requested) both groups were to be provided to the DSMB.
Data management and monitoring
The principal investigator (FMD) and statistical group (SG, RP) oversaw randomisation, data management, progress monitoring and all analyses, including those for the Data Monitoring Safety Board. The DSMB membership included: Lehana Thabane, biostatistican (Chair), Gary Kobinger, infectious disease specialist, Kevin Thorpe, biostatistician, and Edgar Delvin, biochemist and expert in vitamin D. DACIMA was used for online data entry and management.
A combination of remote monitoring activities and inperson routine monitoring visits were conducted by an independent study monitor with the first randomised participants at each site and on a bi-monthly basis, to ensure that each site adhered to the study protocol, Good Clinical Practice guidelines, and data collection completeness.
Sample size calculation
Given uncertainties in infection progression, a Bayesian adaptive design was used where the posterior probability of effectiveness, that is, P(OR<1|data) was the basis of inference and decision making.34 Assuming an expected OR of 0.80 and 1:1 treatment allocation, a total net sample size of 2100 was required to identify a 20% reduction in the risk of COVID-19 in the vitamin D versus control group, with 80% power with the design described above. Considering a dropout rate of 15%, 2414 participants were targeted. An interim analysis was planned when 75% of participants would have reached week 12, at which time the following assessments were to be made: the progression over time in the incidence of infection (slope of the curve of infection) was to be updated and if the probability of effectiveness exceeded 0.95 (p(OR<1)>0.95), the trial would have been terminated for efficacy at the interim point (12 weeks); otherwise, the study would have continued to 16-week follow-up. Simulation results showed that, with the net sample size of 2100 (assuming an expected OR of 0.80 and 1:1 treatment allocation), there was about a 55% chance that the trial would be terminated for efficacy at the interim analysis.35 The overall infection rate was monitored on a monthly basis: note that the study could have been extended to 24 weeks based on the progress of the infection rate, if required.
Statistical analysis
Primary outcome
An intention-to-treat analysis was to be carried out with all randomised participants. For the primary outcome, the posterior distribution of the OR of COVID-19 infection (OR) was the basis of inference in interim and final analyses. The posterior distribution of the OR was to be estimated by drawing samples from the posterior risks under each arm, which could be obtained analytically in a β-binomial model. Flat prior distributions were assumed for the risks (β(1,1)). Posterior 95% credible intervals were to be reported as interval estimates for the OR. Crude analyses as well as analyses adjusted for important covariates (ie, potential confounders, effect modification and baseline group imbalances) were to be conducted. Subgroup analyses would be conducted on baseline 25OHD, age, sex, BMI, occupational risk and COVID-19 vaccination. A stratified analysis on geographical infection rate would be explored; sensitivity analysis censoring to date of COVID-19 vaccination, would be conducted if applicable.
Secondary outcomes
Distribution of disease severity defined as a five-level ordinal outcome would have been examined with a Bayesian analysis using a proportional odds model; the posterior probability of OR would have been obtained by Markov chain Monte Carlo sampling implemented in Stan.34 Duration of symptoms, duration of working day absences and of unemployment would have been examined by a zero-inflated Poisson distribution.
Ethics and dissemination
This study was reviewed and approved by the research ethics board (REB) of the CHU Sainte-Justine, serving as the central REB of all participating institutions (MP-21-2021-3044). A non-objection letter from Health Canada had been obtained to use high-dose vitamin D loading dose as well as the TASSO-SST device for home blood sampling and the NADAL COVID-19 IgM/IgG rapid serology test. Written informed consent for study participation, for biobanking specimens for ancillary studies, and for subsequent publication of results was obtained from all participants, with the knowledge that participation was voluntary and could be withdrawn at any time with no effect on their current/future medical care. As part of the informed consent, enrolees had the option to participate in the HostSeq COVID-19 Canadian biobank conducted under the supervision of CGen, a national Canadian platform for sequencing and genome analysis (online supplemental file 2). In Canada, healthcare is provided to those who suffer harm from trial participation.
bmjopen-2022-064058supp002.pdf (425.6KB, pdf)
All protocol amendments were submitted to Health Canada, investigators and REB; if these changes implied a revision of consent forms, ongoing trial participants were informed of new modifications to provide informed consent. All information obtained during the study were and would continue to be kept confidential as per the law. Data were collected directly by electronic data capture on Dacima Clinical Suite (DACIMA Software, Montreal, Canada). Data safety and confidentiality was upheld at all data collection stages by assigning a unique subject identifier (ID) to each participant, with data and samples kept under lock and key, electronic password protection and access restricted to study personnel. Samples collected during the study were labelled with the unique research code, prior to transfer and storage at the CHU Sainte-Justine biobank, with access restricted to authorised personnel.
This trial used pragmatic patient (irrespective of baseline 25OHD level) and intervention to attempt to maximise subsequent implementation into practice. If the intervention had been shown to be effective in reducing infection and morbidity, this approach would have been readily implementable and could have markedly influenced practice during the COVID-19 pandemic. No participant identifiers were used in the dissemination of this research. Healthcare professionals serving as partners were informed of the study design and pretested all questionnaires.
Results are being disseminated to the medical community via national/international conferences and publications in peer-reviewed journals.
Trial status, challenges and discussion
The study was conducted as per V.1.8 (18 January 2021). The recruitment started on 9 February 2021. On the DSMB recommendation, recruitment was stopped prematurely on 18 March 2021, after 34 participants were enrolled, due to the inability to recruit approximately 200 participants/week required to meet the target sample size of 2415 participants. The DSMB advised that the continuation of the trial, as originally designed, would not be able to answer the research question and recommended that recruitment be stopped for futility. Recruitment difficulties were attributed in part to the high use of vitamin D and high concurrent vaccination rate among our target population, HCWs, the first targeted to be vaccinated from January 2021 onwards. Based on the recommendations of the study’s endocrinologist, a premature end of follow-up after a minimum of 4 weeks from randomisation was deemed sufficient to monitor the safety of the intervention in all participants. The time frame was deemed sufficient to ensure participant safety while learning for the study, that is, transforming the PROTECT Study into a pilot study to document the impact of the study intervention on the rise in vitamin D serum level, participants’ adherence to the study intervention and procedures in the context of a hybrid study, and so on. The last end of visit was conducted on 4 May 2022.
Potential re-directions of the study were discussed. The first option was to change the main outcome for an immunogenicity study in the general adult population. However, after strong consideration of the amount of changes to be made to the protocol and related documents (standards of procedures, case report forms, participant’ instructions and notification, etc), the expected delay in obtaining approval by all regulatory and ethical authorities, the impossible logistic of recruiting participants after the same duration of exposure to the study intervention prior to their vaccination, combined with the government of Quebec announcement that all willing adults would be vaccinated by 24 June 2021, the research team judged that it would unfeasible to perform a scientific solid and feasible trial on immunogenicity if one could not control the timing of immunisation, combined with the expected very short recruitment time frame.
A second option that received very strong consideration was to replicate the PROTECT Trial in children aged 9 years and over. Again, after considering changes to be made to the protocol and related documents, the expected delay for obtaining approval by all regulatory and ethical authorities including school boards, combined with the Pfizer-BioNtech announcement that their vaccine was not only 100% successful for preventing COVID-19 infection in adolescents aged 12–15 years, but that they forecast vaccinating teenagers in time for the September 2021 school entry, the Principle Investigator judged that it was unrealistic to aim for the large recruitment target within such a short time frame.
The protocol was submitted for publication after the last patient end-of-study visit, due to the incredible amount of work done to set up and initiate this large hybrid trial. Of note, the hybrid trial was preceeded by two pilot studies, each to test a new experimental device that enabled partial or totally remote participation. The pandemic also imposed important sanitary protocols and space restrictions for recruiting on-site potentially COVID-19-infected HCWs, as well as several protocol amendments to facilitate and adjust the trial in the context of emerging science, material shortage, and anticipated vaccination campaign; each amendment in turn required significant modification of all related electronic documents and manual of procedures, and resubmission to obtain regulatory approvals. Collectively, this lead to a delay in recruitment initiation which contributed to the premature end-of-follow-up in enrolled participants.
With the gained experience and knowledge, it is crucial that a future trial must begin fast prior to widespread vaccination and in populations where infection rate is high.28 Permitting study entry to individuals with prior infection and prior vaccination (given common re-infections and temporary vaccine protection)36 could have been considered, but it would have significantly reduced the event rate, required prolongation beyond 24 weeks (and additional funding), and compromised study power as was noted in other primary prevention trials.26 27 Restricting eligibility to patients with vitamin D deficiency (<25 nmol/L) would have severely interfered with recruitment ability in population-based or HCWs studies.26–28 Use of calcifediol (25-hydroxyvitamine D or (25OHD)) may have been associated with more potency and rapid rise in serum 25OHD than expected for cholecalciferol37 (vitamin D3) although the choice is debated38 and rapid access to study drug and matching placebo remains a crucial challenge at the onset of a pandemic. Revisiting the intervention dose and frequency of administration in light of the latest literature on SARS-CoV-2 and related virus could be considered, although current evidence suggest that, with similar doses, high-incidence population may be more important than dosing in primary prevention26 28 and high doses are effective in tertiary prevention.35 Of interest, we have demonstrated that the intervention significantly rose 25OHD levels well above 75 nmol/L, that is, in the hypothesised range for optimal pro-immune and anti-inflammatory impact.39 A pragmatic design with fewer outcomes and monitoring via administrative databases appeared theoretically more efficient, but required rapid access to data when interim analyses are planned to monitor event rate; any delay in data access could raise serious challenges and hamper trial decisions. Pursuing a hybrid approach to facilitate enrolment in the context of a pandemic was feasible, although electronic self-screening and outcome monitoring required a lot of programming that have contributed to implementation delays.
The publication of this protocol is meant to share our experience, including conducting a hybrid (virtual and/or inperson) trial and lessons learnt, to serve as template to accelerate protocol writing and its improvement in the context of another epidemic/pandemic, and to serve as reference for the publication of our pilot studies that enabled this trial, and lessons learnt from this experience. As vitamin D supplementation has shown a benefit as tertiary prevention in severe COVID-19 cases, with insufficient data to conclude its impact as primary and secondary prevention, this approach remains worthy of testing.40
Supplementary Material
Acknowledgments
The authors thank, for collaboration, Danny Germain from Quebec Riva Laboratories who agree to provide free of charge Study Preparations (vitamin D and matching placebo), available in bottles of 60 tablets, allowing for study prolongation. The authors also thank Benoit Hebert of Teracero Pharma Inc, for providing free-of-charge the NADAL COVID-19 IgM/IgG Rapid serology test kits, Martin Sauvageau for implementing and coordinating the RT-qPRC analysis of saliva samples at the Montreal Clinical Research Institute, Christian Renaud for coordinating the COVID-19 serology analysis, and Claude Bourassa for coordinating all other blood analyses at the Sainte-Justine University Health Centre. The authors also thank, for collaboration, Raymond Loyer from EFS Solution Santé who adapted their appointment software for the authors’ needs as well as John Padoba, Rabie Razgallah, and Mustapha Gharb who programmed and revised the eCRF to the authors’ needs. The authors thank Anna Smyrnova for coordinating the development of the eCRF and data management. The authors also thank Catherine Lamontague from Orokom Communication Marketing who developed the communication strategy and tools and oversaw the publicity campaign with Marie-Line Bénard-Cyr of the CHU Sainte-Justine who also developed the PROTECT website and Laureanne Marceau of the CHUM. The authors also thank the members of the Data Monitoring Safety Board namely Lehana Thabane (Chair), Gary Kobinger, Kevin Thorpe and Edgar Delvin.
Footnotes
Contributors: FMD designed the study protocol, secured funding, and oversaw the overall conduct of the project. CT contributed to the protocol and amendments, directed the study implementation at the CHUM, and coordinated the prescription of study drug, pharmacy dispensation, as well as salivary sample reception and interpretation. SG conceived the statistical approach and sample size calculation and along with RWP, oversaw randomisation and statistical analysis. CL, JHW and CQ contributed to the study design and amendments. BH wrote the first manuscript draft. LGSM oversaw the safety assessment. DC is responsible for the work absenteeism analysis. All co-authors approved the manuscript. Authorship eligibility on resulting manuscripts will follow standard guidelines.
Funding: This study is funded by a grant awarded through a peer-reviewed process of the COVID-19 May 2020 Rapid Response Funding Opportunity by the Canadian Institute of Health Research, 160 Elgin Street, Ottawa, ON K1A 0W9, Canada (grant number #447317).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. After publication of the primary results, the data sets used and analysed during the current study will be made available by the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The Epidemiological characteristics of an outbreak of 2019 novel Coronavirus diseases (COVID-19) in China. China CDC Wkly 2020;2:113–22. 10.46234/ccdcw2020.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandy A. Estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries. 2020.
- 3.Burrer SL, CDC COVID-19 Response Team, CDC COVID-19 Response Team . CDC COVID-19 response team. characteristics of health care personnel with COVID-19 — United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep 2020;69:477–81. 10.15585/mmwr.mm6915e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ran L, Chen X, Wang Y, et al. Risk factors of Healthcare workers with Coronavirus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis 2020;71:2218–21. 10.1093/cid/ciaa287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 2020;5:e475–83. 10.1016/S2468-2667(20)30164-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. In Review [Preprint] 2020. 10.21203/rs.3.rs-21211/v1 [DOI] [PMC free article] [PubMed]
- 7.Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol 2009;158:20–5. 10.1111/j.1365-2249.2009.04001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herr C, Greulich T, Koczulla RA, et al. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res 2011;12:31. 10.1186/1465-9921-12-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zosky GR, Berry LJ, Elliot JG, et al. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med 2011;183:1336–43. 10.1164/rccm.201010-1596OC [DOI] [PubMed] [Google Scholar]
- 10.Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 2012;76:315–25. 10.1111/j.1365-2265.2011.04261.x [DOI] [PubMed] [Google Scholar]
- 11.Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res 2011;55:96–108. 10.1002/mnfr.201000174 [DOI] [PubMed] [Google Scholar]
- 12.Martineau A, all co-authors of the original study . Vitamin D supplementation to prevent asthma exacerbations - authors' reply. Lancet Respir Med 2018;6:e26–7. 10.1016/S2213-2600(18)30199-1 [DOI] [PubMed] [Google Scholar]
- 13.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583. 10.1136/bmj.i6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martineau AR, Jolliffe DA, Greenberg L, et al. Vitamin D supplementation to prevent acute respiratory infections: Individual participant data meta-analysis. Health Technol Assess 2019;23:1–44. 10.3310/hta23020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manaseki-Holland S, Qader G, Isaq Masher M, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: A randomised controlled trial. Trop Med Int Health 2010;15:1148–55. 10.1111/j.1365-3156.2010.02578.x [DOI] [PubMed] [Google Scholar]
- 16.Manaseki-Holland S, Maroof Z, Bruce J, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: A randomised controlled superiority trial. Lancet 2012;379:1419–27. 10.1016/S0140-6736(11)61650-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen ME, Mailhot G, Alos N, et al. Vitamin D intervention in Preschoolers with viral-induced asthma (DIVA): A pilot randomised controlled trial. Trials 2016;17:353. 10.1186/s13063-016-1483-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro M, King TS, Kunselman SJ, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: The VIDA randomized clinical trial. JAMA 2014;311:2083–91. 10.1001/jama.2014.5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denlinger LC, King TS, Cardet JC, et al. Vitamin D supplementation and the risk of colds in patients with asthma. Am J Respir Crit Care Med 2016;193:634–41. 10.1164/rccm.201506-1169OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martineau AR, MacLaughlin BD, Hooper RL, et al. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3. Thorax 2015;70:451–7. 10.1136/thoraxjnl-2014-206449 [DOI] [PubMed] [Google Scholar]
- 21.Martineau AR, James WY, Hooper RL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (Vidico): A Multicentre, double-blind, randomised controlled trial. Lancet Respir Med 2015;3:120–30. 10.1016/S2213-2600(14)70255-3 [DOI] [PubMed] [Google Scholar]
- 22.Goodall EC, Granados AC, Luinstra K, et al. Vitamin D3 and Gargling for the prevention of upper respiratory tract infections: A randomized controlled trial. BMC Infect Dis 2014;14:273. 10.1186/1471-2334-14-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maghbooli Z, Sahraian MA, Ebrahimi M, et al. Vitamin D sufficiency, a serum 25-Hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS One 2020;15:e0239799. 10.1371/journal.pone.0239799 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Ismailova A, White JH. Vitamin D, infections and immunity. Rev Endocr Metab Disord 2022;23:265–77. 10.1007/s11154-021-09679-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jafarzadeh A, Chauhan P, Saha B, et al. Contribution of monocytes and Macrophages to the local tissue inflammation and cytokine storm in COVID-19: Lessons from SARS and MERS, and potential therapeutic interventions. Life Sci 2020;257:118102. 10.1016/j.lfs.2020.118102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jolliffe DA, Holt H, Greenig M, et al. Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and COVID-19: Phase 3 randomised controlled trial (CORONAVIT). BMJ 2022;378:e071230. 10.1136/bmj-2022-071230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunvoll SH, Nygaard AB, Ellingjord-Dale M, et al. Prevention of COVID-19 and other acute respiratory infections with Cod liver oil supplementation, a low dose vitamin D supplement: Quadruple blinded, randomised placebo controlled trial. BMJ 2022;378:e071245. 10.1136/bmj-2022-071245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villasis-Keever MA, López-Alarcón MG, Miranda-Novales G, et al. Efficacy and safety of vitamin D supplementation to prevent COVID-19 in frontline Healthcare workers. A randomized clinical trial. Arch Med Res 2022;53:423–30. 10.1016/j.arcmed.2022.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeffer PE, Hawrylowicz CM. Vitamin D and lung disease. Thorax 2012;67:1018–20. 10.1136/thoraxjnl-2012-202139 [DOI] [PubMed] [Google Scholar]
- 30.Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med 2020;26:1037–40. 10.1038/s41591-020-0916-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 1988;124:869–71. 10.1001/archderm.124.6.869 [DOI] [PubMed] [Google Scholar]
- 32.Kearns MD, Alvarez JA, Tangpricha V. Large, single-dose, oral vitamin D supplementation in adult populations: A systematic review. Endocr Pract 2014;20:341–51. 10.4158/EP13265.RA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vieth R, Holick MF. Chapter 57B - the IOM—endocrine society controversy on recommended vitamin D targets: In support of the endocrine society position. In: Feldman D, ed. Vitamin D (Fourth Edition). Academic Press, 2018: 1091–107. [Google Scholar]
- 34.Harrell FL C. Statistical design and analysis plan for randomized trial of hydroxychloroquine for treatment of COVID-19: ORCHID. 2020. Available: http://hbiostat.org/proj/covid19/bayesplan.html
- 35.Golchi S. Estimating design operating characteristics in Bayesian adaptive clinical trials. Can J Stat 2022;50:417–36. 10.1002/cjs.11699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eythorsson E, Runolfsdottir HL, Ingvarsson RF, et al. Rate of SARS-Cov-2 Reinfection during an Omicron wave in Iceland. JAMA Netw Open 2022;5:e2225320. 10.1001/jamanetworkopen.2022.25320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pérez-Castrillón JL, Dueñas-Laita A, Brandi ML, et al. Calcifediol is superior to Cholecalciferol in improving vitamin D status in postmenopausal women: A randomized trial. J Bone Miner Res 2021;36:1967–78. 10.1002/jbmr.4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sosa Henríquez M, Gómez de Tejada Romero MJ. Cholecalciferol or Calcifediol in the management of vitamin D deficiency. Nutrients 2020;12:1617. 10.3390/nu12061617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosseini B, Tremblay CL, Longo C, et al. Oral vitamin D supplemental therapy to attain a desired serum 25-Hydroxyvitamin D concentration in essential Healthcare teams. Trials 2022;23:1019. 10.1186/s13063-022-06944-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosseini B, El Abd A, Ducharme FM. Effects of vitamin D supplementation on COVID-19 related outcomes: A systematic review and meta-analysis. Nutrients 2022;14:2134. 10.3390/nu14102134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-064058supp001.pdf (131.4KB, pdf)
bmjopen-2022-064058supp002.pdf (425.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request. After publication of the primary results, the data sets used and analysed during the current study will be made available by the corresponding author on reasonable request.