Abstract
Objective:
Body tissue composition plays a crucial role in the multisystemic processes of advanced liver disease and has been shown to be influenced by transjugular intrahepatic portosystemic shunt (TIPS). A differentiated analysis of the various tissue compartments has not been performed until now. The purpose of this study was to evaluate the value of imaging biomarkers derived from automated body composition analysis (BCA) to predict clinical and functional outcome.
Methods:
A retrospective analysis of 56 patients undergoing TIPS procedure between 2013 and 2021 was performed. BCA on the base of pre-interventional CT examination was used to determine quantitative data as well as ratios of bone, muscle and fat masses. Furthermore, a BCA-derived sarcopenia marker was investigated. Regarding potential correlations between BCA imaging biomarkers and the occurrence of hepatic encephalopathy (HE) as well as 1-year survival, an exploratory analysis was conducted.
Results:
No BCA imaging biomarker was associated with the occurrence of HE after TIPS placement. However, there were significant differences in alive and deceased patients regarding the BCA-derived sarcopenia marker (alive: 1.60, deceased: 1.83, p = 0.046), ratios of intra- and intermuscular fat/skeletal volume (alive: 0.53, deceased: 0.31, p = 0.015) and intra- and intermuscular fat/muscle volume (alive: 0.21, deceased: 0.14, p = 0.031).
Conclusion:
A lower amount of intra- and intermuscular adipose tissue might have protective effects regarding liver derived complications and survival.
Advances in knowledge:
Precise characterization of body tissue components with automated BCA might provide prognostic information in patients with advanced liver disease undergoing TIPS procedure.
Introduction
Transjugular intrahepatic portosystemic shunt (TIPS) plays a major role in the therapeutic armamentarium of advanced liver disease. By using a minimal invasive technique, portal hypertension, which contributes to a variety of life-shortening complications, can be treated effectively. 1 However, a higher amount of blood passes the liver metabolism unfiltered and enters the systemic circulation. 2 Consecutively, hepatic metabolization of ammonium is lowered and the risk for hepatic encephalopathy (HE), representing one of the most significant complications of severe liver dysfunction, is increased. 3
Therefore, the identification of prognostic factors for HE and survival is of utmost importance to improve patient selection for this procedure. Here, diet-related factors have gained considerate importance. 4,5 Malnutrition occurs in up to 50% of patients with liver cirrhosis and does not only correlate with typical complications of cirrhosis including ascites, spontaneous bacterial peritonitis (SBP) and gastrointestinal bleeding but also has impact on the prevalence of HE and overall survival. 4–9 However, comprehensive assessment of malnutrition in clinical practice remains challenging and the heterogeneity in the definition of malnutrition in cirrhosis aggravates a systematical, uniform interpretation of study results. 4
Sarcopenia—defined as the loss of skeletal muscle mass and strength—has been proposed as a corollary of the nutritional status. Initial studies hint that TIPS can positively modify body composition and a conversion of sarcopenia to a non-sarcopenia status after TIPS was associated with less clinical complications and improved survival. 10–13
But prior to transferring these observations into clinical practice, a common tool has to be established to unify body tissue measurements. In particular, CT is recommended for the analysis of sarcopenia and body composition. 14,15 However, obtaining these potentially important parameters by manual segmentation of the muscle mass is a cumbersome and time-consuming process that has prevented its introduction into clinical practice. In addition, there is still vivid debate regarding the establishment of cut-off values for the definition of sarcopenia and body tissue data. 16,17
A recently introduced 3D Multires U-Net by Koitka et al, enables the extraction of body tissue data on the basis of deep neural networks and are thereby—unlike the more frequently used analysis just on a L3 slice—able to perform an automated 3D full body composition analysis (BCA) with quantification of multiple BCA imaging markers in a considerably shorter period of time. 18,19
Therefore, the aim of the current study was to elucidate the potential of different BCA imaging biomarkers including a newly developed BCA derived sarcopenia index determined by an evolution of the aforementioned BCA system as prognostic parameters regarding clinical aspects and survival for patients after TIPS placement.
Methods and materials
Study design
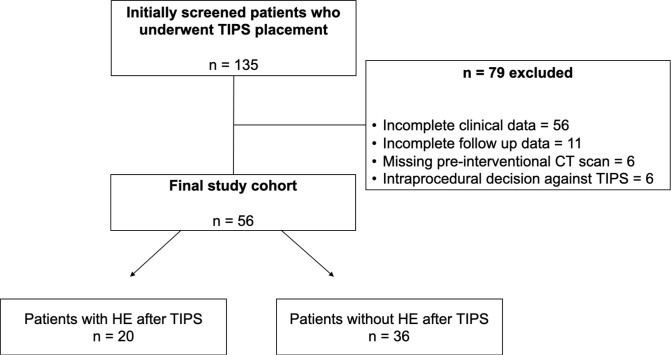
We conducted a retrospective observational study at a tertiary care hospital focusing on the treatment of liver disease. 135 patients who underwent a TIPS procedure between October 2013 and December 2021 were screened for eligibility. Inclusion criteria of the study were: elective and emergency TIPS procedures due to variceal bleeding or refractory ascites regardless of the underlying etiology of liver dysfunction. Exclusion criteria for this study were a patient age of less than 18 years, the lack of imaging data prior to TIPS placement and clinical follow-up data (Figure 1). Epidemiological and clinical data before the TIPS procedure as well as periinterventional reports and clinical parameters in the further course after TIPS were gathered. Outcome parameters were the occurrence of HE and ascites after TIPS as well as survival rates 1 year after TIPS implantation. The study was approved by the institutional review board and the need for informed consent was waived due to the retrospective nature of the study.
Figure 1.
Flow chart of the study cohort. HE, hepatic encephalopathy; TIPS, transjugular intrahepatic portosystemic shunt
TIPS procedure
Indication for TIPS procedure was determined by multidisciplinary evaluation of each case. Vascular access was obtained by jugular vein puncture. A transhepatic puncture device (Gore TIPS Set, Gore, W. L. Gore & Associates, DE) was navigated to one of the hepatic veins (preferably right hepatic vein), followed by visualization of the portal system and measurement of the pre-therapeutical central venous and indirect portal venous pressure (with consecutive calculation of the portosystemic gradient, PSG). Next, the intrahepatic branches of the portal vein were punctured with a TIPS needle and a guidewire (Amplatz Super Stiff, Boston Scientific, Marlborough, MA) was advanced into the portal venous system and exchanged for a 4F vertebralis catheter for portal vein visualization. The established parenchymal portosystemic tract was dilated with a high pressure 6 mm balloon (Mustang Balloon Dilatation Catheter, Boston Scientific, Marlborough, MA). Then, a self-expandable bare metal stent (Epic, Boston Scientific, Marlborough, MA Self-Expanding Nitinol Stent with Delivery System, Epic©) or a partially covered stent (Viatorr TIPS Endoprosthesis, Gore, W. L. Gore & Associates, DE) was deployed and dilated from a diameter of 6 mm up to 10 mm. After each dilatation, central and portal venous pressure were obtained to determine the PSG, respectively. A satisfactory result was individually determined by the operating radiologist, aiming at the established and recommended end point values of a PSG of 8–12 mmHg with certain exceptions, e.g. a very high initial PSG or known history of HE before TIPS. 20,21
Imaging acquisition and tissue body composition assessment
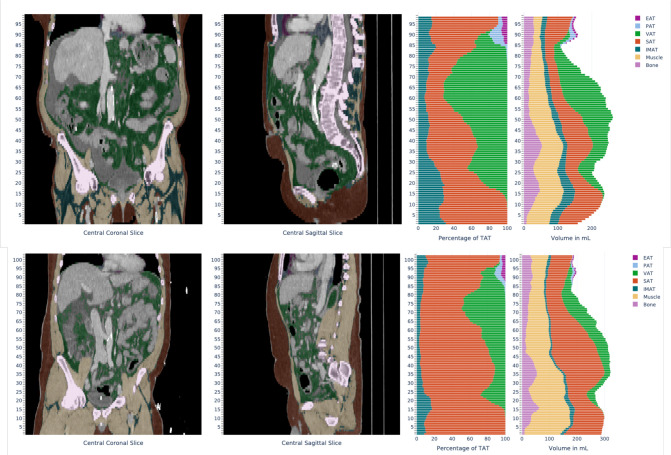
Pre-interventional CT scans were used to obtain the BCA-derived sarcopenia marker as well as various BCA ratios. For the BCA, a previously established system based on 3D Multires U-Netwas used to generate a whole abdominal segmentation and quantification of different tissues such as bone, muscle and fat in ml. 18 The neural networks employed in this system were trained on manually segmented CT scans and were able to segment bone and muscle tissue directly. Total adipose tissue (TAT) was identified using HU-based thresholding from surrounding body region and was further subdivided into subcutaneous (SAT), visceral (VAT) and intra- and intermuscular (IMAT) fat using the predicted semantic body regions (Figure 2). We defined the BCA-derived sarcopenia markeras the relation of muscle volume to bone volume and IMAT using the following formula:
Figure 2.
Exemplary visual impression and report of the tissue quantification system output of two CT-examinations from the BCA network. Coronal and sagittal reformats with the adjacent bar diagram depicting percentage of different adipose tissues in relation to the total adipose tissue and volumes of the different segmented tissues per axial slice. EAT, endocardial adipose tissue; IMAT, Intra- and intermuscular adipose tissue; PAT, pericardial adipose tissue; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue
Sarcopenia marker = muscle volume/(skeletal volume+IMAT)
As absolute volumes are highly dependent on patient size, we calculated BCA ratios of the different tissue components in relation to the skeletal volume and a ratio of IMAT in relation to the total muscle volume as an estimator of myosteatosis:
Myosteatosis = IMAT/muscle volume
Statistical analysis
Continuous variables are summarized as mean (standard deviation) in case of normal distribution, otherwise as median (range) and categorical variables as count (percentage). Continuous data were evaluated for normality of distribution using the Kolmogorov–Smirnov test and by inspection of the plots. The χ2 test for trend and Fisher’s exact test were used for testing association between categorical and the Mann–Whitney U test or t-test for continuous variables.The predictive accuracy of different parameters regarding the overall survival (OS) were assessed by the Kaplan–Meyer analysis with comparison of the different groups with the Log-rank test. Due to the fact that no established cut-off values exist for BCA data, cut-off values were determined using measures of central tendency (mean for sarcopenia (1.66), median for IMAT/skeletal volume (0.420) and IMAT/muscle volume (0.176), respectively). The groups were compared using the Log-rank test. The level of significance was set to 0.05. Due to the explorative nature of this study, no α error correction was applied. All analyses were performed using SPSS (IBM Corp., SPSS Statistics 27.0. Armonk, NY) and GraphPad Prism (GraphPad Software, LLC, Prism 9).
Results
Patient characteristics
The final study cohort consisted of 56 TIPS patients. Most of the patients were male (female: 39.3%, 22/56 vs male 60.7%, 34/56) and the mean age of the study cohort was 58.64 (± 12.31) years. Almost half of the study cohort suffered from alcoholic liver disease (48.2%, 27/56), whereas only a minority of patients developed severe liver dysfunction due to non-alcoholic liver disease (NASH) (12.5%, 7/56). Eight patients suffered from viral liver disease (14.3%, 8/56). The remainder of the study cohort, subsumed as “other”, consisted of patients with autoimmunological diseases such as primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC) or Budd-Chiari-syndrome (25.0%, 13/56). Almost all patients presented with ascites (91.1%, 51/56) and almost one-third of the study cohort suffered from upper gastrointestinal bleeding in the past (30.4%, 17/56) (Table 1).Table 2 illustrates the descriptive data after stratifying the cohort into a group with high and low BCA derived sarcopenia marker.
Table 1.
Baseline characteristics of the study population
| Cohorts | |||
|---|---|---|---|
|
Entire cohort
n = 56 |
HE after TIPS
n = 20 |
No HE after TIPS
n = 36 |
|
| Epidemiological data | |||
| Age, (years)a | 58.64 (12.31) | 57.0 (13.62) | 59.56 (11.63) |
| Gender Male Female |
34 (60.7) 22 (39.3) |
11 (55.0) 9 (45.0) |
23 (63.9) 13 (36.1) |
| Viral liver disease | 8 (14.3) | 4 (20.0) | 4 (11.1) |
| Alcoholic liver disease | 27 (48.2) | 10 (50.0) | 17 (47.2) |
| NASH | 7 (12.5) | 0 (0.0) | 7 (19.5) |
| Other | 14 (25.0) | 6 (30.0) | 8 (22.2) |
| Clinical data | |||
| HE before TIPS | 12 (21.4) | 8 (40.0) | 4 (11.1) |
| Bleeding from esophageal varices | 17 (30.4) | 5 (25.0) | 12 (33.3) |
| Ascites | 51 (91.1) | 18 (90.0) | 33 (91.7) |
| SBP | 9 (16.1) | 3 (15.0) | 6 (16.7) |
| Periinterventional data | |||
| TIPS setting Emergency Elective |
10 (17.9) 46 (82.1) |
6 (30.0) 14 (70.0) |
4 (11.1) 32 (88.9) |
| Complications | 6 (10.7) | 3 (15.0) | 3 (8.3) |
| PSG before TIPSb | 21.55 (7.05) | 20.44 (8.27) | 22.09 (6.45) |
| PSG after TIPSb | 10.33 (3.9) | 10.28 (4.73) | 10.35 (3.51) |
HE = hepatic encephalopathy, NASH = non-alcoholic steatohepatitis, PSG = portosystemic pressure gradient, SBP = spontaneous bacterial peritonitis, TIPS = transjugular intrahepatic portosystemic shunt.
Values represent mean (standard deviation).
Values represent median (range).
Table 2.
Characteristics of the study population in regard of the BCA-derived sarcopenia marker
| Sarcopenia marker high = 28 | Sarcopenia marker low = 28 | |
|---|---|---|
| Epidemiological data | ||
| Age, (years)a | 53.25 (11.69) | 64.68 (10.14) |
| Gender Male Female |
17 (60.7) 11 (39.3) |
17 (60.7) 11 (39.3) |
| Viral liver disease | 6 (21.4) | 2 (7.1) |
| Alcoholic liver disease | 10 (35.7) | 17 (60.7) |
| NASH | 3 (10.7) | 4 (14.3) |
| Other | 9 (32.1) | 5 (17.9) |
| Clinical data | ||
| HE before TIPS | 6 (21.4) | 6 (21.4) |
| Bleeding from esophageal varices | 9 (32.1) | 8 (28.6) |
| Ascites | 26 (92.9) | 25 (89.3) |
| SBP | 7 (25.0) | 2 (7.1) |
| Periinterventional data | ||
| TIPS setting Emergency Elective |
9 (32.1) 19 (67.9) |
1 (3.6) 27 (96.4) |
| Complications | 4 (14.3) | 2 (7.1) |
| PSG before TIPSa | 22.21 (5.53) | 20.92 (8.3) |
| PSG after TIPSa | 9.93 (3.7) | 10.76 (4.15) |
HE = hepatic encephalopathy, NASH = non-alcoholic steatohepatitis, PSG = portosystemic pressure gradient, SBP = spontaneous bacterial peritonitis, TIPS = transjugular intrahepatic portosystemic shunt.
Values represent mean (standard deviation).
Association of BCA imaging markers regarding the presence of HE and ascites after TIPS placement
Approximately, one-third of the patients developed HE after TIPS placement (35.7%, 20/56), whereas the remaining showed no signs of HE (64.3%, 36/56) after the procedure. Comparison of both groups revealed no statistical differences concerning the BCA-derived sarcopenia marker and BCA ratios derived from pre-interventional CT (Table 3). After TIPS placement, recurrent or persistent ascites was observed in 28.6% (16/56). Again, the comparison of patients with and without ascites after TIPS detected no statistical differences for the aforementioned indices (Table 4).
Table 3.
BCA imaging markers and ratios regarding the presence of HE after TIPS placement
| BCA and ratios | HE after TIPS n = 20 |
No HE after TIPS n = 36 |
p-value |
|---|---|---|---|
| BCA-derived sarcopenia markera | 1.70 (0.32) | 1.65 (0.35) | 0.602 |
| Muscle volume/skeletal volumea | 2.48 (0.32) | 2.43 (0.47) | 0.696 |
| Subcutaneous fat/skeletal volumea | 3.53 (2.09) | 2.73 (1.91) | 0.150 |
| Visceral fat/skeletal volumeb | 1.16 (3.57) | 1.17 (5.41) | 0.851 |
| Intra- and intermuscular fat/skeletal volumeb | 0.40 (0.83) | 0.49 (1.28) | 0.837 |
| Total body fat/skeletal volumea | 5.46 (2.89) | 4.67 (2.90) | 0.335 |
| Intra- and intermuscular fat/muscle volumeb | 0.16 (0.29) | 0.19 (0.52) | 0.798 |
BCA = body composition analysis, HE = hepatic encephalopathy, TIPS = transjugular intrahepatic portosystemic shunt.
Values represent mean (standard deviation).
Values represent median (range).
Table 4.
BCA imaging markers regarding the presence of ascites before and after TIPS placement
| BCA and ratios | Ascites after TIPS n = 16 |
No ascites after TIPS n = 40 |
p-value |
|---|---|---|---|
| BCA-derived sarcopenia markera | 1.68 (0.29) | 1.66 (0.36) | 0.821 |
| Muscle volume/skeletal volumea | 2.42 (0.38) | 2.46 (0.44) | 0.740 |
| Subcutaneous fat/skeletal volumea | 2.80 (2.32) | 3.11 (1.87) | 0.607 |
| Visceral fat/skeletal volumeb | 1.28 (2.05) | 1.08 (5.41) | 0.800 |
| Intra- and intermuscular fat/skeletal volumeb | 0.43 (0.72) | 0.41 (1.28) | 0.574 |
| Total body fat/skeletal volumea | 4.49 (2.90) | 5.13 (2.92) | 0.459 |
| Intra- and intermuscular fat/muscle volumeb | 0.16 (0.29) | 0.18 (0.53) | 0.526 |
BCA = body composition analysis, TIPS = transjugular intrahepatic portosystemic shunt.
Values represent mean (standard deviation).
Values represent median (range).
Association of BCA imaging markers regarding the presence of fatty liver disease
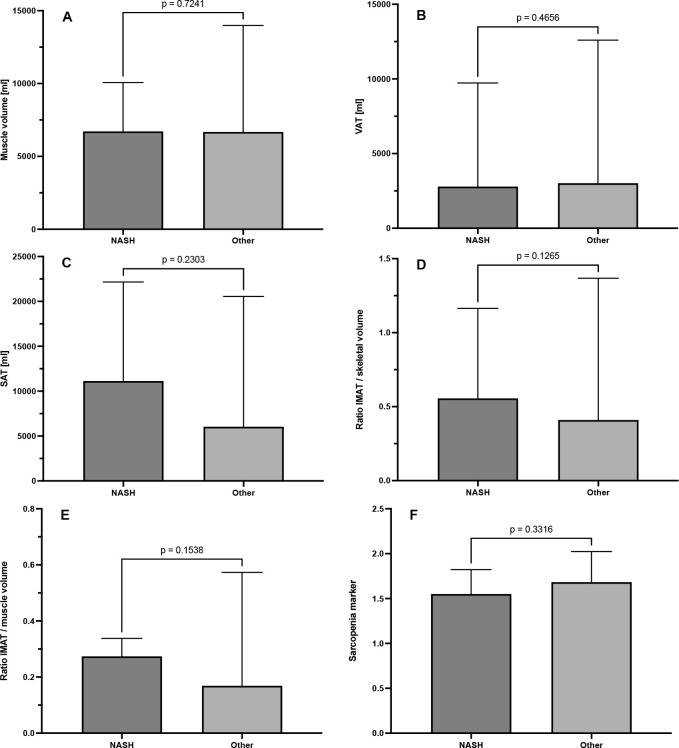
Patients with and without NASH exhibited a similar muscle volume (6890 ml (2168 ml) vs 6611 ml (1912 ml), p = 0.724), VAT (2789 ml (7968 ml) vs 3016 ml (11990 ml), p = 0.466) and also BCA-derived sarcopenia marker (1.55 (0.27) vs 1.68 (0.34), p = 0.33). There was a slight tendency for a higher amount of SAT in patients with NASH, although this was not statistically significant (11,131 ml (20,337 ml) vs 6039 ml (19,857 ml), p = 0.230). We observed similar tendencies when looking at the ratios of IMAT/skeletal volume (0.56 (0.96) vs 0.41 (1.28), p = 0.126) and IMAT/muscle volume as an estimator for myosteatosis (0.27 (0.23) vs 0.17 (0.53), p = 0.154), again without a statistical significance (Figure 3).
Figure 3.
BCA imaging markers in fatty liver disease. BCA, body composition analysis; IMAT, intra- and intermuscular adipose tissue; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue
Association of BCA imaging markers regarding overall survival 6 months and 1 year after TIPS placement
We compared the BCA-derived sarcopenia marker and indices derived from pre-interventional BCA regarding the survival rates 1 year to evaluate possible associations of body composition data with outcome of patients after TIPS placement. Patients deceased within 1 year after TIPS placement exhibited an elevated BCA-derived sarcopenia marker, that indicates a higher amount of muscle tissue (1.83 (0.31) vs 1.60 (0.34), p = 0.046). In addition, deceased patients also showed a lower ratio of IMAT/skeletal volume (0.31 (0.66) vs 0.53 (1.28), p = 0.015) and IMAT/muscle volume(0.14 (0.29) vs 0.21 (0.53), p = 0.031).This underlines the results from theBCA derived sarcopenia marker analysis (Table 5). These parameters exhibited also statistical trends for survival rates after 6 months but failed to show any statistical significance (Supplementary material 1).
Table 5.
BCA imaging markers and ratios regarding the primary outcome 1 year after TIPS implantation
| BCA and ratios | Alive n = 28 |
Death n = 13 |
p-value |
|---|---|---|---|
| BCA-derived sarcopenia markera | 1.60 (0.34) | 1.83 (0.31) | 0.046 |
| Muscle volume/skeletal volumea | 2.43 (0.41) | 2.53 (0.33) | 0.454 |
| Subcutaneous fat/skeletal volumea | 3.36 (1.75) | 2.85 (2.10) | 0.416 |
| Visceral fat/skeletal volumeb | 1.29 (5.41) | 1.03 (1.70) | 0.311 |
| Intra- and intermuscular fat/skeletal volumeb | 0.53 (1.28) | 0.31 (0.66) | 0.015 |
| Total body fat/skeletal volumea | 5.59 (2.77) | 4.44 (2.62) | 0.220 |
| Intra- and intermuscular fat/muscle volumeb | 0.21 (0.53) | 0.14 (0.29) | 0.031 |
BCA = body composition analysis;TIPS, transjugular intrahepatic portosystemic shunt.
Values represent mean (standard deviation).
Values represent median (range).
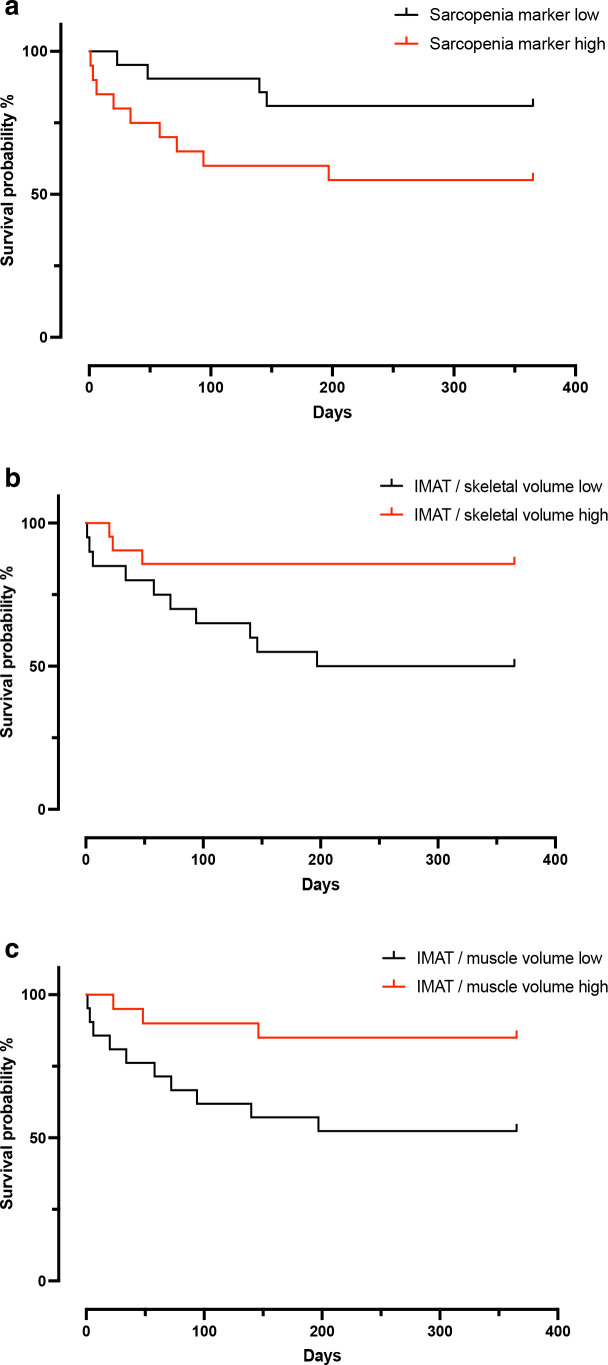
Kaplan-Meier analysis revealed that patients in the high sarcopenia group (mean OS 225 d, 95% CI, 153 d to 297 d) showed a tendency for a higher mortality rate 1 year after TIPS placement than patients in the low sarcopenia group (mean OS 312 d, 95% CI, 264 d to 361 d, log rank test, χ² = 3.53, p = 0.06). In addition, a significant difference of the survival between patients with a high (mean OS 317 d, 95% CI, 266 d to 369 d) and low ratio of IMAT/skeletal volume (mean OS 220 d, 95% CI, 152 d to 288 d, log rank test, χ² = 5.45, p = 0.020) as well as between patients with a high (mean OS 321 d, 95% CI, 273 d to 369 d) and low ratio of IMAT/muscle volume (mean OS 221 d, 95% CI, 152 d to 290 d, log rank test, χ² = 5.08, p = 0.024) became apparent with a higher mortality rate in patients of the lower group, thus lower amount of intra- and intermuscular adipose tissue, respectively (Figure 4).
Figure 4.
Kaplan-Meier analysis on all-cause mortality 1 year after TIPS placement. Division of the study cohort into a high- and low group was conducted using measures of central tendency (mean for sarcopenia and median for IMAT/skeletal volume and IMAT/muscle volume, respectively). IMAT, intra- and intermuscular adipose tissue; TIPS, transjugular intrahepatic portosystemic shunt.
Discussion
In the present study, we aimed to assess the potential value of imaging biomarkers derived from automated BCA to predict clinical and functional outcome in patients with advanced liver disease undergoing TIPS procedure.
We did not find a robust association of the BCA-derived sarcopenia marker regarding the occurrence of HE or ascites in the clinical course of the patients after the procedure. However, we observed higher mortality rates after 1 year in patients with a lowered amount of IMAT in relation to skeletal and muscular volume. Therefore, IMAT could be a potential biomarker in patients with liver disease that should be investigated in further studies.
Although the introduction of TIPS procedure was a major game changer in patients with advanced liver disease, creating an artificial shunt between the portal and caval venous system increases the risk for the development of HE due to various reasons in a complex interplay. 2 Therefore, the identification of reliable predictors is key to improve patient selection for TIPS and consecutively clinical outcome. 2,22,23 Recent studies could demonstrate that TIPS has influence on the body composition and is able to improve nutritional parameters of the involved patients. Consecutively, the nutritional status might provide valuable predictive factors for HE and survival. 10,12,24,25 As comprehensive assessment of the nutritional status remains challenging, sarcopenia has been proposed as a common denominator of the nutritional status. A recent meta-analysis of 1713 patients demonstrated a significantly higher risk of HE among cirrhotic patients with sarcopenia. 26 In TIPS patients, the predictive value of sarcopenia remains mainly unclear: while Farkas et al found inconclusive results regarding the development of HE after TIPS, Benmassaoud et al detected neither an association between HE nor an increased mortality after TIPS implantation. 27,28
Therefore, we wanted to investigate whether the assessment of the whole abdominal muscle mass by automated BCA could improve these results. Consistent with the latter studies, our results did not show any statistical significance of the BCA-derived sarcopenia marker and other body tissue composition data in patients with and without HE after TIPS implantation. However, we even observed a potential association of a higher BCA derived sarcopenia marker—indicating a higher muscle mass—with a reduced 1-year OS rate.
However, automated BCA can be used to analyze different body components as well, most notably body fat. The loss of body fat—a condition called adipopenia—has been identified as an important predictive factor for severity and also survival in patients with liver cirrhosis. 29–31 When decompensation of liver function occurs, different pathophysiological pathways, such as increase in sympathetic nervous system activity, inflammation, recurrent infections and ascites result in a hypermetabolism, which can be observed in up to 35% of patients with advanced liver disease. 32,33 Ebadi et al and Benjamin et al demonstrated that liver cirrhosis patients with a lowered amount of subcutaneous adipose tissue had a higher probability of clinical decompensation but also a higher risk for mortality. 30,31
The results of our study highlight the potential of IMAT as a potential biomarker in TIPS patients. We found that an increase of IMAT in relation to the skeletal and muscle volume was associated with higher survival rates 1 year after the procedure.
A potential reason for our findings of the association of higher amounts of IMAT with improved survival might be the lower number of patients with fatty liver disease such as NAFLD and NASH in our study cohort, as those patients do not show a congruent decline in muscle and fat tissue—which has often been observed in cirrhotic patients—but rather exhibit a discrepancy in muscle and fat metabolism, namely loss of muscle tissue and elevation of adipose tissue. 4 Analysis of body tissue fat might be a more decisive parameter in estimating development and severity of patients with NASH as sarcopenia, not only because established cut-off values for sarcopenia are still lacking in obese patients with liver disease but study results also revealed, that patients with NASH might surprisingly also exhibit a higher amount of muscle tissue. 34 Our results stand in line with the above-mentioned results from Schmitz et al. 34 We also could not detect a decline of muscle volume in patients with NASH and no striking differences regarding the BCA-derived sarcopenia marker in patients with and without NASH. Furthermore, a tendency for higher amounts of subcutaneous adipose tissue, myosteatosis and IMAT in relation to skeletal volume in patients with NASH became evident.
A limitation of our study is its single-center design and the small sample size. Due to the fact, that there are established cut-off values for sarcopenia in patients with severe liver disease, the applicability of our study results into daily practice might be aggravated. Another important fact that should be mentioned is the rather low rate of patients with fatty liver disease in our cohort, whereas this patient cohort already has a huge impact in clinical practice nowadays that should be investigated in more detail. Lastly, we detected an elevated rate of post-TIPS HE in our study cohort with 35.7% vs. 5–35% compared to the current literature. This could be an indicator of a potential selection bias as patients with a favorable outcome might not receive follow-up at a distant tertiary care center but close to home. 2,22
Conclusion
We observed that a lower amount of intra- and intermuscular adipose tissue in relation to total muscle and skeletal volume as well as a lower BCA-derived sarcopenia index were linked to higher mortality rates 1 year after TIPS implantation. Furthermore, pre-procedural BCA imaging markers revealed no associations regarding the development of HE after TIPS placement in our patient cohort. Our study results illustrate the complex interplay of the different body tissue components in patients with advanced liver disease, which can be analyzed for the first time by automated BCA in a fashion that can be readily applied in clinical routine. Therefore, it should stimulate further research to analyze the predictive role of body composition and especially of the adipose tissue component, in patients with advanced liver disease more precisely.
Contributor Information
Georgios Luca Alatzides, Email: georgios.alatzides@uk-essen.de, georgios-alatzides@e-mail.de.
Johannes Haubold, Email: Johannes.Haubold@uk-essen.de.
Hannah Luisa Steinberg, Email: Hannah.Steinberg@uk-essen.de.
Sven Koitka, Email: Sven.Koitka@uk-essen.de.
Johannes Grueneisen, Email: Johannes.Grueneisen@uk-essen.de.
Amos Cornelius Zeller, Email: Amos.Zeller@uk-essen.de.
Hartmut Schmidt, Email: Hartmut.Schmidt@uk-essen.de.
Jens Matthias Theysohn, Email: Jens.Theysohn@uk-essen.de.
Yan Li, Email: Yan.Li@uk-essen.de.
Felix Nensa, Email: Felix.Nensa@uk-essen.de.
Benedikt Michael Schaarschmidt, Email: Benedikt.Schaarschmidt@uk-essen.de.
REFERENCES
- 1. Vizzutti F, Schepis F, Arena U, Fanelli F, Gitto S, Aspite S, et al. Transjugular intrahepatic portosystemic shunt (tips): current indications and strategies to improve the outcomes. Intern Emerg Med 2020; 15: 37–48. doi: 10.1007/s11739-019-02252-8 [DOI] [PubMed] [Google Scholar]
- 2. Li X, Partovi S, Coronado WM, Gadani S, Martin C, Thompson D, et al. Hepatic encephalopathy after tips placement: predictive factors, prevention strategies, and management. Cardiovasc Intervent Radiol 2022; 45: 570–77. doi: 10.1007/s00270-021-03045-3 [DOI] [PubMed] [Google Scholar]
- 3. Patidar KR, Bajaj JS. Covert and overt hepatic encephalopathy: diagnosis and management. Clin Gastroenterol Hepatol 2015; 13: 2048–61. doi: 10.1016/j.cgh.2015.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis 2012; 16: 95–131. doi: 10.1016/j.cld.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huisman EJ, Trip EJ, Siersema PD, van Hoek B, van Erpecum KJ. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol 2011; 23: 982–89. doi: 10.1097/MEG.0b013e32834aa4bb [DOI] [PubMed] [Google Scholar]
- 6. Sam J, Nguyen GC. Protein-Calorie malnutrition as a prognostic indicator of mortality among patients hospitalized with cirrhosis and portal hypertension. Liver Int 2009; 29: 1396–1402. doi: 10.1111/j.1478-3231.2009.02077.x [DOI] [PubMed] [Google Scholar]
- 7. Montano-Loza AJ, Meza-Junco J, Prado CMM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012; 10: 166–73. doi: 10.1016/j.cgh.2011.08.028 [DOI] [PubMed] [Google Scholar]
- 8. Merli M, Giusto M, Lucidi C, Giannelli V, Pentassuglio I, Di Gregorio V, et al. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis 2013; 28: 281–84. doi: 10.1007/s11011-012-9365-z [DOI] [PubMed] [Google Scholar]
- 9. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu European Association for the Study of the Liver , . EASL clinical practice guidelines on nutrition in chronic liver disease. J Hepatol 2019; 70: 172–93. doi: 10.1016/j.jhep.2018.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu J, Ma J, Yang C, Chen M, Shi Q, Zhou C, et al. Sarcopenia in patients with cirrhosis after transjugular intrahepatic portosystemic shunt placement. Radiology 2022; 303: 711–19. doi: 10.1148/radiol.211172 [DOI] [PubMed] [Google Scholar]
- 11. Tsien C, Shah SN, McCullough AJ, Dasarathy S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastroenterol Hepatol 2013; 25: 85–93. doi: 10.1097/MEG.0b013e328359a759 [DOI] [PubMed] [Google Scholar]
- 12. Gioia S, Ridola L, Cristofaro L, Merli M, Faccioli J, Riggio O, et al. The improvement in body composition including subcutaneous and visceral fat reduces ammonia and hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Liver Int 2021; 41: 2965–73. doi: 10.1111/liv.15060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gioia S, Merli M, Nardelli S, Lattanzi B, Pitocchi F, Ridola L, et al. The modification of quantity and quality of muscle mass improves the cognitive impairment after tips. Liver Int 2019; 39: 871–77. doi: 10.1111/liv.14050 [DOI] [PubMed] [Google Scholar]
- 14. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working group on sarcopenia in older people. Age Ageing 2010; 39: 412–23. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kroll L, Mathew A, Baldini G, Hosch R, Koitka S, Kleesiek J, et al. CT-derived body composition analysis could possibly replace DXA and BIA to monitor NET-patients. Sci Rep 2022; 12: : 13419. doi: 10.1038/s41598-022-17611-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One 2017; 12: : e0186990. doi: 10.1371/journal.pone.0186990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Son SW, Song DS, Chang UI, Yang JM. Definition of sarcopenia in chronic liver disease. Life (Basel) 2021; 11(): 349. doi: 10.3390/life11040349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koitka S, Kroll L, Malamutmann E, Oezcelik A, Nensa F. Fully automated body composition analysis in routine CT imaging using 3D semantic segmentation convolutional neural networks. Eur Radiol 2021; 31: 1795–1804. doi: 10.1007/s00330-020-07147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weston AD, Korfiatis P, Kline TL, Philbrick KA, Kostandy P, Sakinis T, et al. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology 2019; 290: 669–79. doi: 10.1148/radiol.2018181432 [DOI] [PubMed] [Google Scholar]
- 20. Boike J.R., et al., North American Practice-Based Recommendations for Transjugular Intrahepatic Portosystemic Shunts in Portal Hypertension, Clinical Gastroenterology and Hepatology. (2021). [DOI] [PMC free article] [PubMed]
- 21. Boyer TD, Haskal ZJ, American Association for the Study of Liver Diseases . The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005; 41: 386–400. doi: 10.1002/hep.20559 [DOI] [PubMed] [Google Scholar]
- 22. Suhocki PV, Lungren MP, Kapoor B, Kim CY. Transjugular intrahepatic portosystemic shunt complications: prevention and management. Semin Intervent Radiol 2015; 32: 123–32. doi: 10.1055/s-0035-1549376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin X, Gao F, Wu X, Cai W, Chen X, Huang Z. Efficacy of albumin-bilirubin score to predict hepatic encephalopathy in patients underwent transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol 2021; 33: 862–71. doi: 10.1097/MEG.0000000000001801 [DOI] [PubMed] [Google Scholar]
- 24. Montomoli J, Holland-Fischer P, Bianchi G, Grønbaek H, Vilstrup H, Marchesini G, et al. Body composition changes after transjugular intrahepatic portosystemic shunt in patients with cirrhosis. World J Gastroenterol 2010; 16: 348–53. doi: 10.3748/wjg.v16.i3.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nardelli S, Lattanzi B, Torrisi S, Greco F, Farcomeni A, Gioia S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol 2017; 15: 934–36. doi: 10.1016/j.cgh.2016.10.028 [DOI] [PubMed] [Google Scholar]
- 26. Wijarnpreecha K, Werlang M, Panjawatanan P, Kroner PT, Cheungpasitporn W, Lukens FJ, et al. Association between sarcopenia and hepatic encephalopathy: a systematic review and meta-analysis. Ann Hepatol 2020; 19: 245–50. doi: 10.1016/j.aohep.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 27. Benmassaoud A, Roccarina D, Arico F, Leandro G, Yu B, Cheng F, et al. Sarcopenia does not worsen survival in patients with cirrhosis undergoing transjugular intrahepatic portosystemic shunt for refractory ascites. Am J Gastroenterol 2020; 115: 1911–14. doi: 10.14309/ajg.0000000000000959 [DOI] [PubMed] [Google Scholar]
- 28. Farkas ZC, Rashid T, Chen YS, Siddiqui TM, Yandrapalli S, Frager S, et al. The correlation between sarcopaenia and post-transjugular intrahepatic portosystemic shunt hepatic encephalopathy: a single-institution review. Arch Med Sci Atheroscler Dis 2019; 4: e89–93. doi: 10.5114/amsad.2019.85380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Luca M, Addario L, Lombardi A, Imparato M, Fontanella L, Addario M, et al. Adipopenia is the rapid screening tool that best predicts mortality in patients with decompensated cirrhosis: results of a prospective study. J Gastrointestin Liver Dis 2021; 30: 94–102. doi: 10.15403/jgld-3071 [DOI] [PubMed] [Google Scholar]
- 30. Ebadi M, Tandon P, Moctezuma-Velazquez C, Ghosh S, Baracos VE, Mazurak VC, et al. Low subcutaneous adiposity associates with higher mortality in female patients with cirrhosis. J Hepatol 2018; 69: 608–16. doi: 10.1016/j.jhep.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 31. Benjamin J, Shasthry V, Kaal CR, Anand L, Bhardwaj A, Pandit V, et al. Characterization of body composition and definition of sarcopenia in patients with alcoholic cirrhosis: a computed tomography based study. Liver Int 2017; 37: 1668–74. doi: 10.1111/liv.13509 [DOI] [PubMed] [Google Scholar]
- 32. Chapman B, Sinclair M, Gow PJ, Testro AG. Malnutrition in cirrhosis: more food for thought. World J Hepatol 2020; 12: 883–96. doi: 10.4254/wjh.v12.i11.883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Müller MJ, Böttcher J, Selberg O, Weselmann S, Böker KH, Schwarze M, et al. Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr 1999; 69: 1194–1201. doi: 10.1093/ajcn/69.6.1194 [DOI] [PubMed] [Google Scholar]
- 34. Schmitz SM-T, Schooren L, Kroh A, Koch A, Stier C, Neumann UP, et al. Association of body composition and sarcopenia with NASH in obese patients. J Clin Med 2021; 10(): 3445. doi: 10.3390/jcm10153445 [DOI] [PMC free article] [PubMed] [Google Scholar]