In comparison to ALK-positive anaplastic large cell lymphoma (ALCL), ALK-negative ALCL has been more difficult to define. It was first characterized by morphological similarity to ALK-positive ALCL, including the presence of pathognomonic ‘hallmark cells’, but lacking expression of the ALK protein. Very early reports suggested a similar prognosis to peripheral T-cell lymphoma – not otherwise specified1 but larger studies indicated that the prognosis, although still unsatisfactory, was better than that of peripheral T-cell lymphoma – not otherwise specified.2 However, outcomes are notably variable across studies, as described by Hapgood and Savage.3 This is in part due to clinical risk factors as captured by the International Prognostic Index score.2,4 Firm recognition of ALK-negative ALCL as a distinct entity came in the World Health Organization’s (WHO) 4th edition update following the description of unique molecular features.5,6
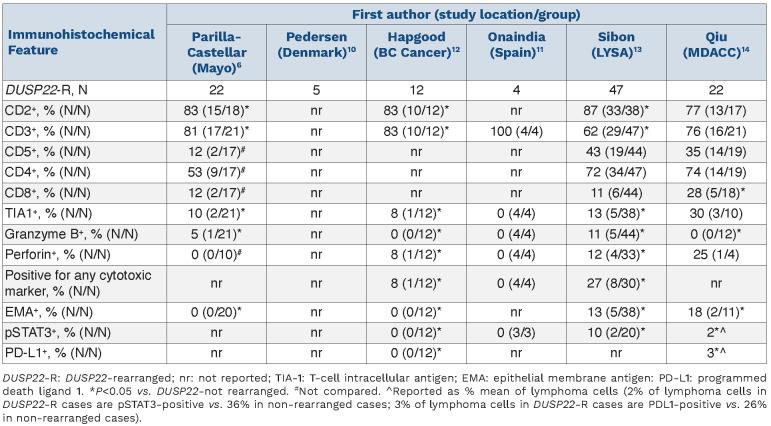
The identification of two recurrent rearrangements represented key milestones in deciphering ALK-negative ALCL. The first rearrangement involves the DUSP22-IRF4 locus on 6p25.3 (DUSP22 rearrangement [DUSP22-R]) and the other involves TP63 on 3p28 (TP63 rearrangement [TP63-R]). The clinical significance of the DUSP22-R was first evaluated in a series of 73 patients with ALKnegative ALCL in which 22 cases were found to harbor the rearrangement, representing 30% of all ALK-negative ALCL. The TP63-R was identified in six cases (8%) and the remainder were deemed to have ‘triple-negative’ ALKnegative ALCL meaning they lacked any rearrangement and are also referred to as DUSP22-NR (non-rearranged)/TP63-NR. Cases with a DUSP22-R had a 5-year overall survival (OS) of 90%, which was similar to that of a comparison group of cases of ALK-positive ALCL. In contrast, those with a TP63-R had a dismal 5-year OS of only 17%. The majority of cases in the series were triple-negative and had an intermediate prognosis (5-year OS of 42%). Pathological evaluation of DUSP22-R tumors revealed sheet-like growth of classic hallmark cells, fewer pleomorphic cells and, as assessed by immunohistochemistry (IHC), reduced expression of epithelial membrane antigen (EMA) and cytotoxic markers (TIA1, granzyme B, perforin) (Table 2). Subsequent studies of these cases described characteristic ‘doughnut’ cells’,7 and a molecular profile characterized by overexpression of immunogenic cancer testis antigen (CTA) genes, a signature of marked DNA hypomethylation and diminished expression of STAT3 and programmed death ligand (PDL), with consequential lack of pSTAT3 and PDL as determined by IHC.8 Exome sequencing identified a recurrent mutation in MSCE116K in almost all cases.9
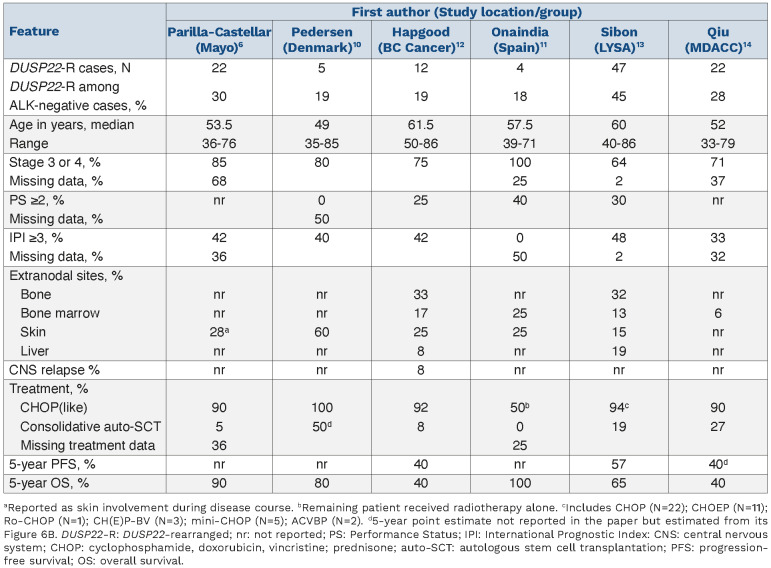
Two subsequent small series of four and five patients each with DUSP22-R ALK-negative ALCL also demonstrated a favorable prognosis10,11 (Table 1). In contrast, in our BC Cancer study of 12 cases, a less favorable prognosis was observed with a 5-year progression-free survival (PFS) and OS of only 40%.12 A range of clinical courses were noted including one patient with central nervous system relapse and another patient managed palliatively had a relapsing/remitting disease course over 4 years, which was reminiscent of cutaneous ALCL. In contrast, IHC features were as expected: EMA-negative, infrequent expression of cytotoxic markers and all cases were pSTAT3- and PDL1-negative (Table 2).
In this issue of Haematologica, two studies have further evaluated the pathological characteristics and prognostic significance of DUSP22-R ALK-negative ALCL.13,14 The study by Sibon and colleagues represents the largest series to date of 47 cases of DUSP22-R ALK-negative ALCL derived from the TENOMIC database, a translational lymphoma research consortium of the LYSA group. In total, 47/104 (45%) cases harbored a DUSP22-R which is a significantly higher proportion than in other studies, and the authors acknowledge a selection bias as cases are submitted to the LYSA TENOMIC database with an aim of compiling those of clinical interest.13 In the subset enrolled on clinical trials, estimates are more in keeping with other studies (23-35%). Regardless, this is an extensively curated database with detailed clinical information which has been lacking in most prior studies (Table 1). Frequent bone involvement was observed, which was also found in the BC Cancer series. Pathologically, cases had the expected morphology and immunophenotype (Table 2) although, curiously, 27% of cases expressed at least one cytotoxic marker (‘cytotoxic profile’), which appears to be higher incidence than in other reports although most other studies only reported on individual marker frequency (Table 2). With a median follow-up of 5 years, the PFS was superior in DUSP22-R cases than in non-rearranged cases (5-year PFS: 48% vs. 25%, respectively; P=0.025); however, the point estimates are far lower than in the original series, and what would be expected for ALK-positive ALCL. Furthermore, OS was not statistically different (5-year OS: 58% vs. 44%, respectively; P=0.20). Confining the analysis to the 39 DUSP22-R cases treated with curative intent, anthracycline chemotherapy (all but 3 cases), and confirmed to have TP63-NR status, demonstrated a more favorable PFS (5-year PFS: 57% vs. 26%, respectively; P=0.001) but not OS (5-year OS: 65% vs. 41%, respectively; P=0.07) although it must be acknowledged that there was limited power to detect a smaller difference. This larger dataset enabled exploration of factors associated with survival. Those cases with a poor performance status (PS) (≥2) and elevated β2-microglobulin had an inferior PFS and OS. Cytotoxic marker expression was also associated with an inferior PFS and the individual factors of granzyme B or perforin expression, but not TIA1, were associated with inferior PFS and OS. However, only PS and DUSP22-NR status were included in the final model because of missing information, and both were associated with PFS, but only PS was also associated with OS. Using these two factors, DUSP22-R patients with a poor PS, a group representing 29% of all cases (11/38, with one patient not included due to missing PS), had a 5-year PFS and OS of only 27% and 29%, respectively, which was indistinguishable from the survival outcomes of triple-negative cases (see Figure 6E, F in the accompanying article).13
Table 1.
Summary of studies to date evaluating the prognosis in DUSP22-rearranged ALK-negative anaplastic large cell lymphoma.
Table 2.
Immunophenotypic features of DUSP22 ALK-negative anaplastic large cell lymphoma cases across studies.
In the second paper, Qiu and colleagues from the MD Anderson Cancer Center evaluated 22 cases of DUSP22-R ALK-negative ALCL, representing 28% of all ALK-negative ALCL cases with pathological features, also consistent with previous reports (Table 2).14 Treatment information was available for 16 patients with DUSP22-R, 13 of whom received anthracycline-based chemotherapy; follow-up information was available for 18 patients and nine (50%) had died. With a median follow-up of 19 months, the projected 5-year OS was only 40%, which was similar to that of DUSP22-NR cases (P=0.275) and inferior to that of ALK-positive ALCL cases (5-year OS 82%; P=0.005). Similarly, 5-year PFS was only 40% (P=0.275 vs. TP63-NR).
The treatment landscape of ALCL has changed over the last decade with the approval of brentuximab vedotin (BV) for the treatment of relapsed/refractory ALCL15 and more recently, approval of CHP (cyclophosphamide, doxorubicin, prednisone)-BV for newly diagnosed systemic ALCL based on superior PFS and OS over CHOP (cyclophosphamide, doxorubicin, vincristine; prednisone), as shown in the ECHELON-2 study in CD30+ peripheral T-cell lymphomas.16 In the LYSA study, survival was notably poor from first relapse/progression, regardless of DUSP22-R status, suggesting that any prognostic relevance may diminish in this high-risk setting (4-year OS 21% [DUSP22-R] vs. 34% [triple-negative]; P=0.62) (Figure 5A in the accompanying article).13 The use of BV in relapsed/refractory ALCL improved outcomes across genetic subgroups but similarly, no outcome difference was noted (Figure 5E, F in the accompanying article).13 Of note, the prognostic impact of DUSP22-R in patients with treatment-naïve ALK-negative ALCL who were treated with CHP-BV remains unknown.
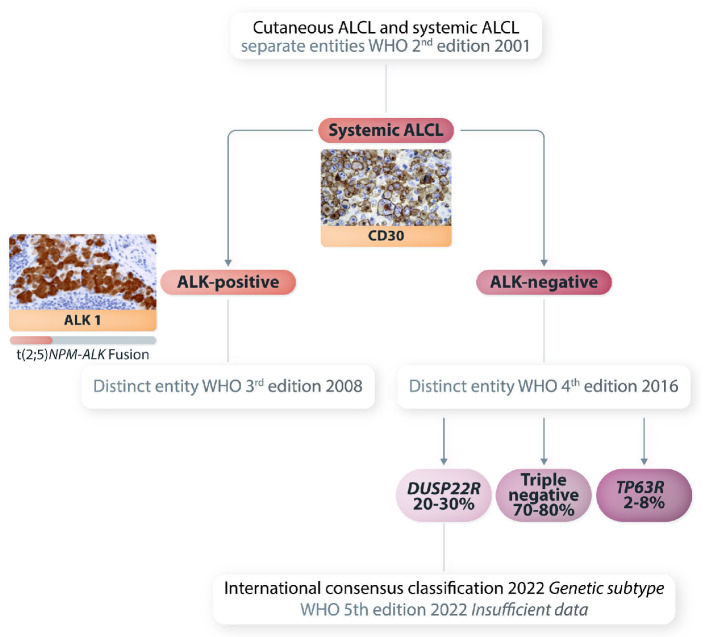
Collectively, unique morphological, immunophenotypic and molecular features support the designation of DUSP22-R ALK-negative ALCL as a distinct entity as proposed by the International Consensus Classification17 although the WHO 5th edition update (WHO-HAEM5) applied only a provisional designation due to uncertainty around prognosis18 (Figure 1). In contrast, cases with a TP63-R are important to recognize given the poor prognosis, but further genetic studies are still required to fully characterize them. Furthermore, although these rearrangements are usually mutually exclusive, rare ‘double-hit’ cases have been reported.19 Considering all studies to date, the prognosis of DUSP22-R ALK-negative ALCL is more variable than that of the typically favorable ALK-positive ALCL, but even that entity can have a poor outcome.2 The LYSA study highlights that clinical information, such as PS, must also be taken into consideration when making management decisions. Of note, as a composite risk score, the International Prognostic Index did not reach statistical significance and, with limited numbers, it was not specifically applied to DUSP22-R cases to judge its utility.
There may also still be unknown pathobiological and genetic factors contributing to outcome in DUSP22-R ALKnegative ALCL. Cases are typically negative for cytotoxic markers but rare cases may be positive, and previous studies have shown an association with inferior outcomes across ALK-negative ALCL.6 DUSP22-R in ALCL was originally shown to occur as a result of a balanced translocation involving the DUSP22 phosphatase gene on 6p25.3 and the FRA7H fragile site on 7q32.3, resulting in downregulation of the DUSP22 gene.20 Subsequent studies assessing DUSP22-R in ALCL have used break-apart fluorescence in situ hybridization and the translocation partner has not been determined. Could alternate translocation partners occur in DUSP22R ALCL and might these account for the more aggressive clinical behavior seen in some cases? Further investigations are required to extend our understanding of the underlying molecular mechanisms that result in these phenotypic and behavioral differences.
Figure 1.
Current classification of systemic anaplastic large cell lymphoma. The most common t(2;5) (ALK-NPM) is shown. Rare variant rearrangements involving the ALK gene on 2p23 and different partner genes on other chromosomes can occur in 15-25% of cases of ALK-positive anaplastic large cell lymphoma. ALCL: anaplastic large cell lymphoma; WHO: World Health Organization; ALK: anaplastic lymphoma kinase.
References
- 1.ten Berge RL, de Bruin PC, Oudejans JJ, Ossenkoppele GJ, van der Valk P, Meijer CJ. ALK-negative anaplastic large-cell lymphoma demonstrates similar poor prognosis to peripheral T-cell lymphoma, unspecified. Histopathology. 2003;43(5):462-469. [DOI] [PubMed] [Google Scholar]
- 2.Savage KJ, Harris NL, Vose JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496-5504. [DOI] [PubMed] [Google Scholar]
- 3.Hapgood G, Savage KJ. The biology and management of systemic anaplastic large cell lymphoma. Blood. 2015;126(1):17-25. [DOI] [PubMed] [Google Scholar]
- 4.Shustov A, Cabrera ME, Civallero M, et al. ALK-negative anaplastic large cell lymphoma: features and outcomes of 235 patients from the International T-Cell Project. Blood Adv. 2021;5(3):640-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iqbal J, Wright G, Wang C, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014;123(19):2915-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parrilla Castellar ER, Jaffe ES, Said JW, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014;124(9):1473-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King RL, Dao LN, McPhail ED, et al. Morphologic features of ALK-negative anaplastic large cell lymphomas with DUSP22 rearrangements. Am J Surg Pathol. 2016;40(1):36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luchtel RA, Dasari S, Oishi N, et al. Molecular profiling reveals immunogenic cues in anaplastic large cell lymphomas with DUSP22 rearrangements. Blood. 2018;132(13):1386-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luchtel RA, Zimmermann MT, Hu G, et al. Recurrent MSC (E116K) mutations in ALK-negative anaplastic large cell lymphoma. Blood. 2019;133(26):2776-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen MB, Hamilton-Dutoit SJ, Bendix K, et al. DUSP22 and TP63 rearrangements predict outcome of ALK-negative anaplastic large cell lymphoma: a Danish cohort study. Blood. 2017;130(4):554-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onaindia A, de Villambrosia SG, Prieto-Torres L, et al. DUSP22-rearranged anaplastic lymphomas are characterized by specific morphological features and a lack of cytotoxic and JAK/STAT surrogate markers. Haematologica. 2019;104(4):e158-e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hapgood G, Ben-Neriah S, Mottok A, et al. Identification of high-risk DUSP22-rearranged ALK-negative anaplastic large cell lymphoma. Br J Haematol. 2019;186(3):e28-e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sibon D, Bisig B, Bonnet C, et al. ALK-negative anaplastic large cell lymphoma with DUSP22 rearrangement has distinctive disease characteristics with better progression-free survival: a LYSA study. Haematologica. 2023;108(6):1590-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu L, Tang G, Li S, et al. DUSP22 rearrangement is associated with distinctive immunophenotype but not outcome in patients with systemic ALK-negative anaplastic large cell lymphoma. Haematologica. 2023;108(6)1604-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190-2196. [DOI] [PubMed] [Google Scholar]
- 16.Horwitz S, O'Connor OA, Pro B, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campo E, Jaffe ES, Cook JR, et al. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood. 2022;140(11):1229-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36(7):1720-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karube K, Feldman AL. "Double-hit" of DUSP22 and TP63 rearrangements in anaplastic large cell lymphoma, ALKnegative. Blood. 2020;135(9):700. [DOI] [PubMed] [Google Scholar]
- 20.Feldman AL, Dogan A, Smith DI, et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively parallel genomic sequencing. Blood. 2011;117(3):915-919. [DOI] [PMC free article] [PubMed] [Google Scholar]