Abstract
DUSP22 rearrangement (R) has been associated with a favorable outcome in systemic ALK-negative anaplastic large cell lymphoma (ALCL). However, a recent study found that patients with DUSP22-R ALK-negative ALCL have a poorer prognosis than was reported initially. In this study, we compared the clinicopathological features and outcomes of patients with ALKnegative ALCL with DUSP22-R (n=22) versus those without DUSP22-R (DUSP22-NR; n=59). Patients with DUSP22-R ALCL were younger than those with DUSP22-NR neoplasms (P=0.049). DUSP22-R ALK-negative ALCL cases were more often positive for CD15, CD8, and less frequently expressed pSTAT3Tyr705, PD-L1, granzyme B and EMA (all P<0.05). TP63 rearrangement (TP63-R) was detected in three of the 66 (5%) ALK-negative ALCL cases tested and none of these cases carried the DUSP22-R. Overall survival of patients with DUSP22-R ALCL was similar to that of the patients with DUSP22-NR neoplasms regardless of International Prognostic Index score, stage, age, or stem cell transplantation status (all P>0.05), but was significantly shorter than that of the patients with ALK-positive ALCL (median overall survival 53 months vs. undefined, P=0.005). Five-year overall survival rates were 40% for patients with DUSP22-R ALCL versus 82% for patients with ALK-positive ALCL. We conclude that DUSP22-R neoplasms represent a distinctive subset of ALK-negative ALCL. However, in this cohort DUSP22-R was not associated with a better clinical outcome. Therefore, we suggest that current treatment guidelines for this subset of ALK-negative ALCL patients should not be modified at present.
Introduction
Anaplastic large cell lymphoma (ALCL) is a mature T-cell neoplasm characterized by large pleomorphic neoplastic cells with kidney-shaped nuclei (so-called “hallmark” cells) and uniform, strong CD30 expression. Based on the presence or absence of an anaplastic lymphoma kinase gene (ALK) rearrangement and resultant ALK expression, ALCL is further classified into ALK-positive (+) and ALKnegative types.1 Although ALK-negative ALCL is morphologically indistinguishable from ALK+ ALCL, patients with systemic ALK-negative ALCL are usually older and have a more aggressive clinical course and poorer outcome with 5-year overall survival (OS) rates of <50% compared with 80-90% for patients with ALK+ ALCL.2-7
ALK-negative ALCL is a genetically heterogeneous entity, with 13-30% of cases harboring a DUSP22 rearrangement (R) and 2-8% of cases carrying a TP63-R.6,8-11 TP63-R and DUSP22-R are nearly mutually exclusive. DUPS22, also known as c-Jun N-terminal kinase (JNK) pathway-associated phosphatase (JKAP), is a tumor suppressor gene located on chromosome 6p25.3. DUPS22 encodes the dual-specificity phosphatase-22 which plays a role in inhibiting T-cell receptor (TCR) signaling.12,13 DUSP22 knockout enhances T-cell activation and TCR signaling and produces enhanced T-cell-mediated immune responses in a mouse model.12 Restoring expression of DUSP22 in DUSP22-deficient malignant T cells inhibits cellular expansion by stimulating apoptosis and impairs clonogenicity and tumorigenicity.13 DUSP22-R often results from t(6;7)(p25.3;q32.3) and is associated with as much as a 50-fold reduction of DUSP22 expression.14 DUSP22-R ALKnegative ALCL cases appear to represent a distinctive subset of ALK-negative ALCL cases with unique morphology, immunophenotype, and molecular signature.9,11,15-17 TP63 is located at chromosome 3q28. TP63-R results in a p63 fusion protein with structural homology to oncogenic ΔNp63. Among ALK-negative ALCL patients, the subgroup with TP63-R neoplasms has a poor prognosis.6 The prognostic significance of DUSP22-R in ALK-negative ALCL is currently controversial. A Mayo Clinic group initially reported that patients with DUSP22-R ALK-negative ALCL (n=22) have a favorable clinical outcome with a 5-year OS rate of 90%, similar to that of ALK+ ALCL patients.6 Later on, two small studies of five and four patients, respectively, showed a similarly favorable prognosis in patients with DUPS22-R ALCL.9,10 Although the case numbers were limited in these studies, others have wondered if the treatment guidelines for patients with DUSP22-R ALK-negative ALCL should be modified. However, a recent study by Hapgood and colleagues reported a 5-year OS of 40% for their cohort of 12 patients with DUSP22-R ALK-negative ALCL.11
In this study, we used fluorescence in situ hybridization (FISH) to determine the status of DUSP22 and TP63 in cases of ALK-negative ALCL. We further focused on DUSP22-R ALK-negative ALCL cases to characterize their clinicopathological and immunophenotypic features and the patients’ outcomes.
Methods
Case selection
We searched the database of the Department of Hematopathology at the MD Anderson Cancer Center from January 1, 2007 through December 31, 2021 for cases of systemic ALK-negative ALCL. The diagnosis of ALKnegative ALCL was based on criteria specified in the 5th edition of the World Health Organization classification.1 The following cases were considered as primary cutaneous ALK-negative ALCL and were excluded from the study: (i) patients with cutaneous disease alone without extracutaneous involvement; and (ii) patients with concurrent cutaneous disease and involvement of regional lymph nodes but no other extracutaneous involvement.1,18 For comparison of survival, we compared this cohort with a group of patients with ALK+ ALCL seen at our institution during the same time interval. Some data for the group of ALK+ ALCL patients have been published previously.19 Clinical information was obtained by review of medical records. This study was approved by the Institutional Review Board.
Immunophenotypic analysis
Immunohistochemical studies were performed as described previously.20 The antibodies used were specific for: ALK1, BCL2, CD2, CD3, CD4, CD5, CD7, CD8, CD15, CD20, CD30, CD43, CD45, CD56, EMA, granzyme B, Ki-67, MUM1, MYC, PAX5, PD-L1, perforin, phospho-STAT3Tyr705, and TIA1.
The percentages of lymphoma cells positive for CD15, phospho-STAT3Tyr705 and PD-L1 were read by two hematopathologists (JX and SL) with estimation to the closest 5%, and then the average of the two reads was used for the final reading of each case.
Flow cytometry immunophenotypic analysis was performed using either a FACSCanto II or a FACSCalibur cytometer (Becton-Dickinson Biosciences, San Jose, CA, USA) as described previously.21 The panel of antibodies employed included CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD25, CD30, CD45, CD52, CD56, TCR α/b and TCR γ/δ (Becton-Dickinson Biosciences, San Jose, CA, USA).
Fluorescence in situ hybridization
FISH analysis was performed on formalin-fixed, paraffinembedded tissue sections using IRF4/DUSP22 dual-color break-apart probes (3’IRF4/DUSP22 – centromeric, labeled with red; 5’IRF4/DUSP22 – telomeric, labeled with green; CytoTest, Rockville, MD, USA) and TP63 dual-color break-apart probes (Cytocell, Cambridge, UK) according to the manufacturers’ instructions. Two hundred interphase nuclei were analyzed.22 The cutoff value was 10.7% for the IRF4/DUSP22-R and 11.1% for the TP63-R. IRF4/DUSP22-R and TP63-R were assessed in a blinded fashion without knowledge of the pathological diagnosis or results of other FISH analyses.
Statistical analysis
Statistical analyses were performed using Graph-Pad Prism 8 and SPSS 26.0 software (IBM Corporation, Armonk, NY, USA). The Fisher exact test was used to compare clinicopathological features between the DUSP22-R and DUSP22-NR groups in patients with ALK-negative ALCL. OS was calculated from the date of initial diagnosis to the date of death or last follow-up. Progression-free survival was calculated from the date of diagnosis to the date of progression/relapse or, if no progression/relapse, the date of death or last follow-up. Survival was analyzed using the Kaplan-Meier method and was compared using the log rank test. A P value of less than 0.05 was considered statistically significant.
Results
Clinical and fluorescence in situ hybridization findings
The results of FISH analysis showed that a DUSP22-R was present in 22 (28%) cases (DUSP22-R group), and absent in 59 (72%) cases (DUSP22-NR group). The clinical features of these patients are summarized in Table 1. The DUSP22-R group comprised 15 men and seven women with a median age of 52 years (range, 33-79 years) at the time of diagnosis. Six (40%) patients with available data had B symptoms. Lymphadenopathy was identified in 16 of 17 (94%) patients, and nine of 15 (60%) patients had extra-nodal involvement including skin (6 cases; 40%), bone (3 cases; 20%), soft tissue (2 cases; 13%), liver (1 case; 7%), lung (1 case; 7%), and muscle (1 case; 7%). Peripheral blood and bone marrow involvement were seen in one of eight (13%) and one of 18 (6%) patients, respectively. Fifteen patients were fully staged, and 13 (87%) had stage III or IV disease. Five of 15 (33%) patients had an International Prognostic Index (IPI) score of ≥3. One of 14 (7%) patients had leukocytosis and one of 13 (8%) patients had absolute lymphocytosis. Anemia was observed in seven of 14 (50%) patients and thrombocytopenia was present in two of 14 (14%) patients. An elevated serum lactate dehydrogenase level was detected in seven of 14 (50%) patients. The DUSP22-NR group consisted of 38 men and 21 women with a median age of 61 years (range, 21-95 years) at the time of diagnosis. Twenty-seven (63%) patients had B symptoms. Forty-one of 51 (80%) patients had lymphadenopathy and 34 of 45 (76%) patients had extranodal disease. The involved extranodal sites included lung (10 cases; 22%), skin (9 cases; 20%), bone (7 cases; 16%), liver (6 cases; 13%), soft tissue (5 cases; 11%), gingiva/oropharynx/nasphoarynx (3 cases; 7%), spleen (2 cases; 4%), muscle (2 cases; 4%), kidney (1 case; 2%), and stomach (1 case; 2%). Peripheral blood and bone marrow involvement were seen in four of 18 (22%) cases and eight of 46 (17%) patients, respectively. Forty-one patients were fully staged, and 27 (66%) had stage III or IV disease. Eighteen of 36 (50%) patients had an IPI scores of ≥3. Seven of 31 (23%) patients had leukocytosis and no patients (of 30) had absolute lymphocytosis. Anemia was observed in 20 of 31 (65%) patients and thrombocytopenia was present in 4 of 31 (13%) patients. An elevated serum lactate dehydrogenase level was detected in 16 of 26 (62%) patients. Compared to patients in the DUSP22-NR ALK-negative ALCL group, patients with DUSP22-R neoplasms were younger (median age 52 vs. 61 years, P=0.049). There were no other significant differences in clinical features between patients in these two groups (all P>0.05) (Table 1).
Table 1.
Clinical features of patients with ALK-negative anaplastic large cell lymphoma with or without a DUSP22-rearrangement.
TP63 rearrangement by fluorescence in situ hybridization analysis
FISH analysis was performed to evaluate the status of TP63-R in 66 cases (19 DUSP22-R and 47 DUSP22-NR) with material available. Three (~5%) cases were positive for TP63-R and all were in the DUSP22-NR group (Table 1). None (n=19) of the DUSP22-R ALK-negative ALCL cases tested carried a TP63-R. The difference in the frequency of TP63-R between the DUSP22-R and DUSP22-NR groups was not statistically significant (P=0.55).
Morphological and immunophenotypic findings
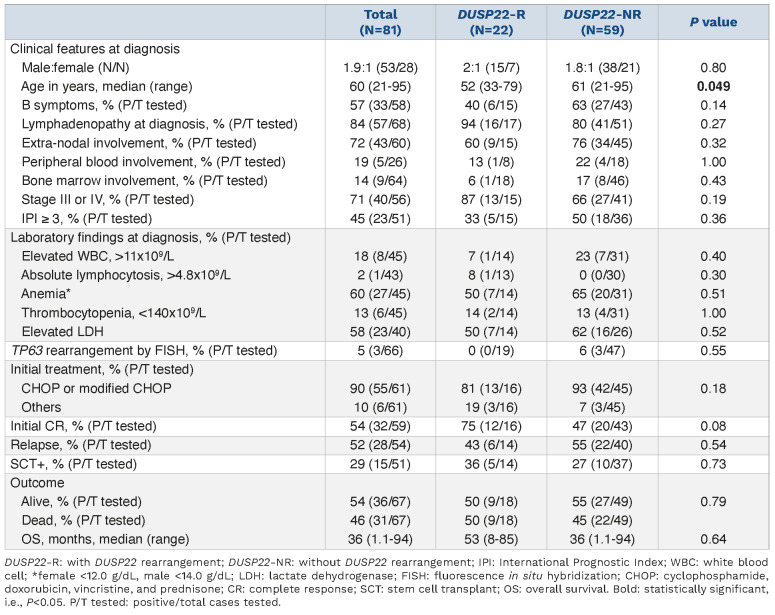
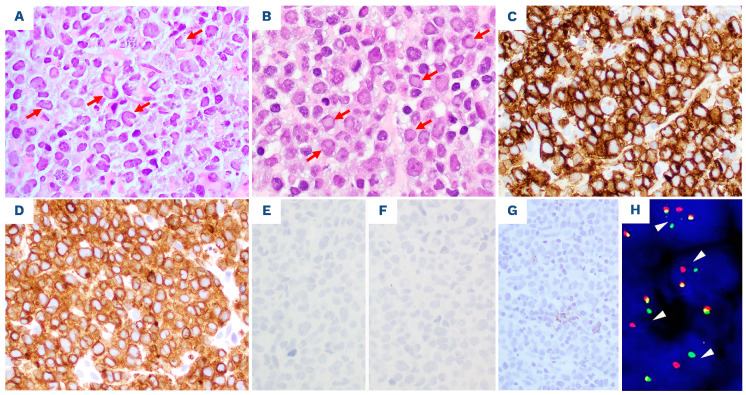
DUSP22-NR ALK-negative ALCL cases in tissue specimens have morphological features of the so-called “common pattern” described in ALK+ ALCL. In comparison, the lymphoma cells in DUSP22-R neoplasms were relatively more monotonous in appearance, smaller, and more often had central nuclear pseudoinclusions (“doughnut” cells). Hallmark cells were present in all cases of DUSP22-R ALCL, but large pleomorphic cells were seen only occasionally. A DUPS22-R ALK-negative ALCL case is shown in Figure 1 (lymph node) and Figure 2 (bone marrow and peripheral blood).
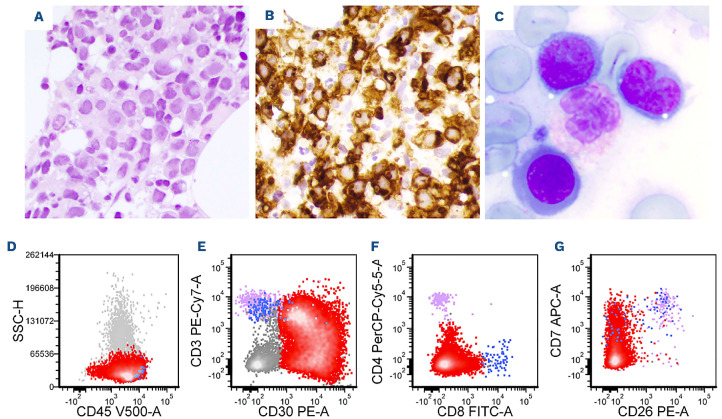
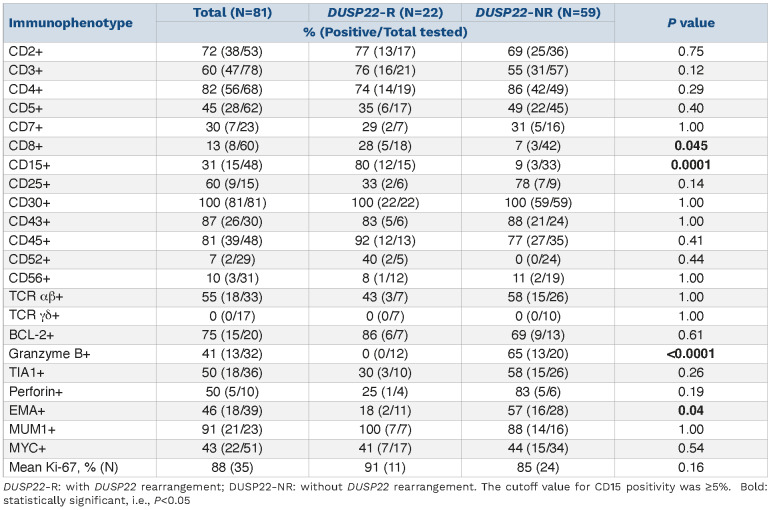
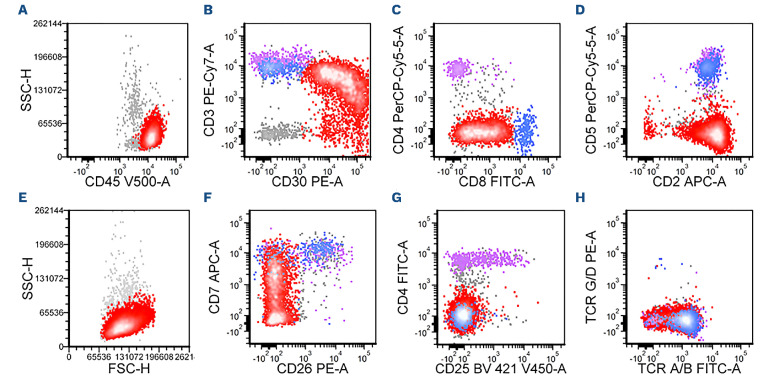
The lymphoma cells of DUSP22-R ALK-negative ALCL cases were positive for CD45 (12/13; 92%), BCL-2 (6/7; 86%), CD43 (5/6; 83%), CD2 (13/17; 77%), CD3 (16/21; 76%) and CD4 (14/19; 74%) (Table 2 and Figure 3). Expression of other T-cell-associated antigens was less frequent: TCR α/b (3/7; 43%), CD52 (2/5; 40%), CD5 (6/17; 35%), CD25 (2/6; 33%), CD7 (2/7; 29%) and CD8 (5/18; 28%). Cytotoxic markers were positive in only a few cases of DUSP22-R ALK-negative ALCL including TIA1 (3/10; 30%) and perforin (1/4; 25%) and granzyme B was consistently negative (n=12). Small subsets of cases were positive for EMA (2/11; 18%) and CD56 (1/12; 8%). All seven cases examined for TCR γ/δ were negative. The proliferation index as assessed by Ki67 was high (~90%).
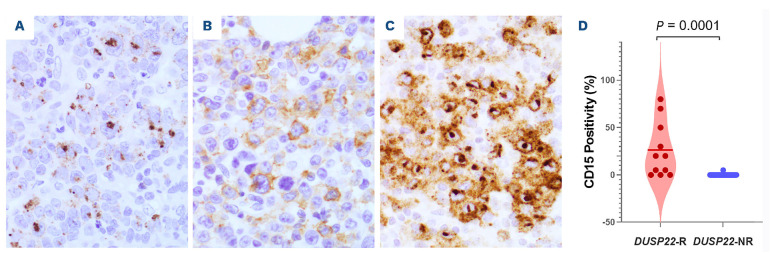
CD15 was positive in 12 of 15 (80%) DUSP22-R cases examined. A mean of 26% lymphoma cells in DUSP22-R cases were positive for CD15. Three types of CD15 staining pattern were observed: a Golgi-like (perinuclear dot) pattern, a membranous/cytoplasmic pattern, and a combination of the Golgi and membranous/cytoplasmic patterns (Figure 4).
The immunophenotype of DUSP22-NR ALK-negative ALCL cases was similar except for the following significant differences that included CD15 being usually negative (9% vs. 80%, P=0.0001), lower frequency of CD8 (7% vs. 28%, P=0.045), and more common expression of granzyme B (65% vs. 0%, P<0.0001) and EMA (57% vs. 18%, P=0.04).
Figure 1.
Lymph node biopsy from a case with DUSP22-rearranged ALK-negative anaplastic large cell lymphoma. (A, B) The nodal architecture is effaced by sheets of monotonous, intermediate sized lymphoma cells. Some lymphoma cells show kidney-shaped nuclei (hallmark cells) (arrows, A) or central nuclear pseudoinclusions (“doughnut” cells) (arrows, B). (C-G) The lymphoma cells are strongly and diffusely positive for CD30 (C) and CD3 (D), and are negative for ALK1 (E), granzyme B (F) and EMA (G). (H) Fluorescence in situ hybridization analysis using IRF4/DUSP22 break-apart probes, ×600. The nuclei showing a DUSP22 rearrangement (with red and green split signals) are indicated by white arrow heads. (A, B) Hematoxylin-eosin stain, x600 (A) and x600 (B). (C-G) Immunohistochemistry, x400.
Figure 2.
The DUSP22-rearranged ALK-negative anaplastic large cell lymphoma shown in Figure 1 also involves bone marrow and peripheral blood. (A) This bone marrow core biopsy specimen shows a hypercellular bone marrow infiltrated by lymphoma cells in an interstitial pattern. The lymphoma cells are morphologically similar to those in the lymph node described in Figure 1. (B) The lymphoma cells in the bone marrow are positive for CD30 by immunohistochemistry. (C) Bone marrow aspirate smear showing lymphoma cells, mostly small to intermediate size, with irregular nuclear contours and basophilic cytoplasm. A lymphoma cell shows kidney-shaped nuclei. (D-G) Flow cytometric immunophenotypic analysis of the peripheral blood shows a large population (75%) of lymphoma cells (red dots) which are positive for CD45 (D), CD30 (E), CD3 (partial/decreased, E), CD8 (dim, F), and CD7 (partial, G), and negative for CD4 and CD26, immunophenotypically similar to the lymphoma cells in the lymph node described in Figure 2. The purple and blue dots represent background benign CD4+ and CD8+ T cells, respectively. (A) Hematoxylin-eosin stain, x500. (B) Immunohistochemistry, x500. (C) Wright-Giemsa stain, x1000.
STAT3 activation and PD-L1 expression
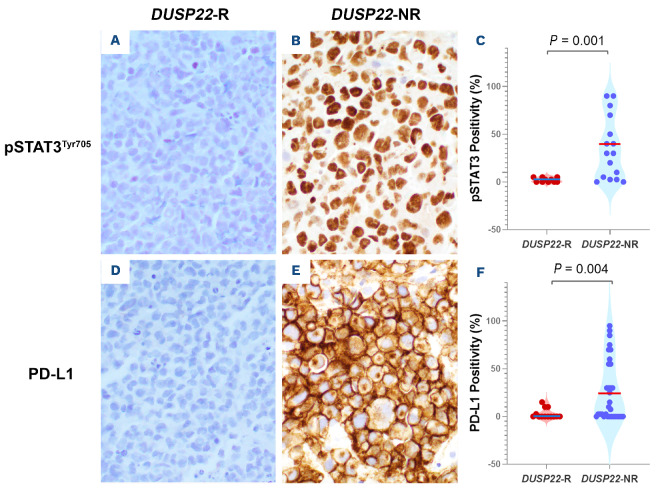
Activation of STAT3 was examined by assessing nuclear expression of phosphorylated STAT3 (pSTAT3Tyr705). A mean of 2% lymphoma cells in DUSP22-R ALK-negative ALCL cases showed nuclear staining for pSTAT3Tyr705, a value significantly lower than the mean of 36% observed in DUSP22-NR tumors (P=0.001) (Figure 5A-C). PD-L1, a downstream molecule regulated by the JAK/STAT3 signaling pathway, was positive in 3% lymphoma cells in the DUSP22-R ALCL group compared to 26% positive cells in DUSP22-NR tumors (P=0.01) (Figure 5D-F).
Treatment and response
Treatment information was available for 16 patients with DUSP22-R ALK-negative ALCL and 45 patients with DUSP22-NR neoplasms. All patients were treated with chemotherapy regimens over the time interval of this study, with or without consolidation with stem cell transplant (SCT). Fifty-five of 61 (90%) patients were treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or modified CHOP: 13 of 16 (81%) patients in the DUSP22-R group and 42 of 45 cases (93%) in the DUSP22-NR group. Of the six patients treated with non-CHOP-based chemotherapy regimens, four received brentuximab vedotin-based therapy, one received ifosfamide, carboplatin and etoposide (ICE), and one received etoposide, methylprednisolone, high-dose cytarabine and cisplatin (ESHAP) in combination with gemcitabine and vinorelbine. After initial induction chemotherapy, 12 of 16 (75%) patients in the DUSP22-R group and 20 of 43 patients (47%) in the DUSP22-NR group achieved complete remission. Patients with DUSP22-R ALCL tended to have a higher initial complete response rate than the patients with DUSP22-NR neoplasms, but this difference did not reach statistical significance (P=0.08). Five of 14 (36%) patients in the DUSP22-R group and ten of 37 (27%) patients in the DUSP22-NR group underwent SCT. There was no significant difference in initial treatment or SCT rates between patients with DUSP22-R or DUSP22-NR ALK-negative ALCL (all P>0.05) (Table 1).
Table 2.
Immunophenotypic features of ALK-negative anaplastic large cell lymphoma with or without a DUSP22-rearrangement
Outcome
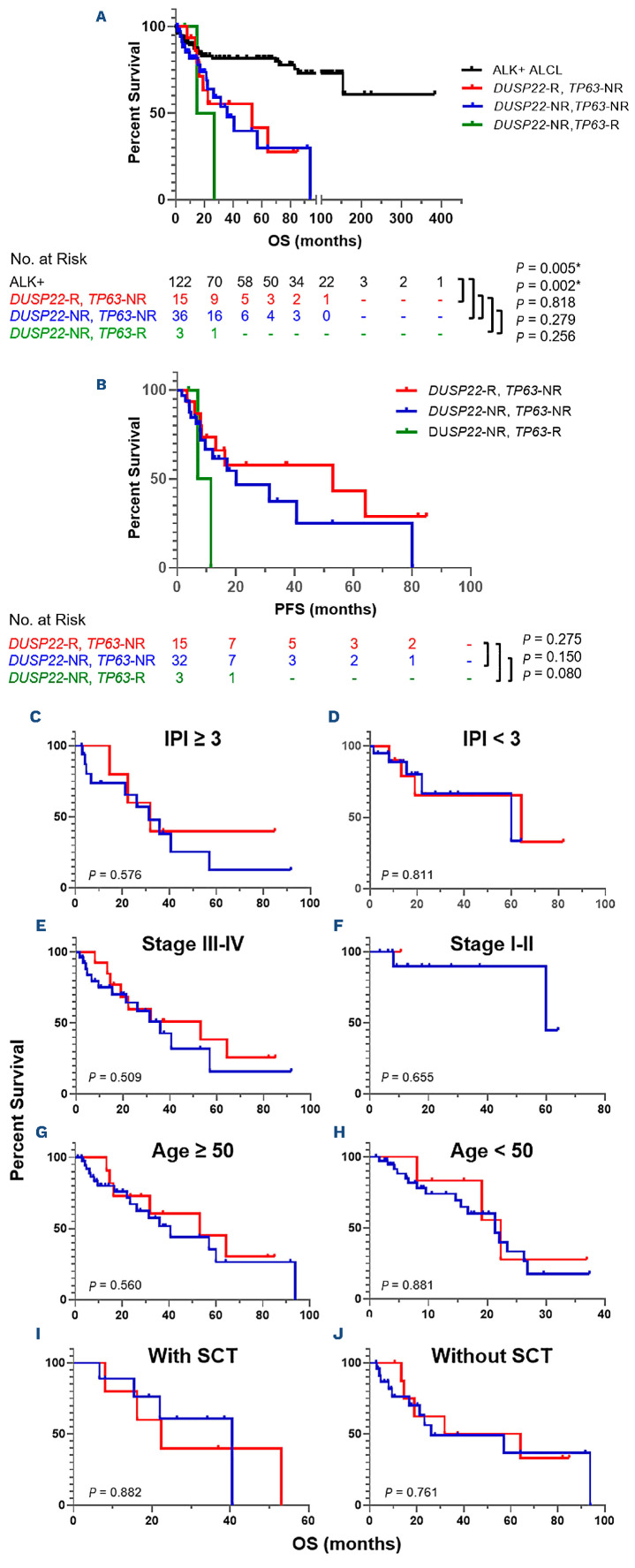
After a median follow-up of 19.2 months (range, 1.1-94 months), 31 of 67 (46%) patients with clinical follow-up data died, including nine of 18 (50%) patients in the DUSP22-R group and 22 of 49 (45%) patients in the DUSP22-NR group. In the DUSP22-NR group, two of three (67%) patients carrying a TP63-R died. Comparing the patients with DUSP22-R, DUSP22-NR/TP63-NR (so called “triple-negative”), and DUSP22-NR/TP63-R ALK-negative ALCL, no significant differences were observed in OS (median 53 vs. 36 vs. 21 months, all P>0.05) or in progression-free survival (median 53 vs. 20 vs. 9 months, all P>0.05) between these groups (Figure 6A, B). Although the patients with TP63-R neoplasms tended to have shorter progression-free survival than those without TP63-R, this difference did not reach statistical significance. After excluding the TP63-R cases, the OS of the DUSP22-R and DUSP22-NR groups was further compared after stratifying patients by IPI score (≥3 or <3), clinical stage (stage III/IV or I/II), age (≥50 or <50 years [Figure 6C]; ≥40 or <40 years, data not shown; ≥60 or <60 years, data not shown), and SCT status, there were still no significant differences in OS between the DUSP22-R and DUSP22-NR groups (all P>0.05) (Figure 6C-J).
We also compared the OS of patients with DUSP22-R ALKnegative ALCL to that of a group of patients with ALK+ ALCL. The OS of patients with DUSP22-R ALK-negative ALCL was significantly poorer than that of patients with ALK+ ALCL (median OS 53 months vs. undefined, P=0.005) (Figure 6A). The 5-year OS rates were 40% for patients with DUSP22-R ALK-negative ALCL as compared to 82% for patients with ALK+ ALCL.
Discussion
The DUSP22-R has been reported most often in cases of systemic ALK-negative ALCL and primary cutaneous ALCL, occasionally in lymphomatoid papulosis and rarely in peripheral T-cell lymphoma, not otherwise specified, but not in ALK+ ALCL.23,24 A DUSP22-R occurs in 13-30% cases of ALK-negative ALCL.6,8-11 Consistent with prior reports, 27% of systemic ALK-negative ALCL cases in the present study harbored a DUSP22-R. We also showed that patients with DUSP22-R ALCL were ~10 years younger than patients with DUSP22-NR neoplasms. Except for age, the present study showed no significant differences in other clinical features between patients with DUSP22-R versus DUSP22-NR ALKnegative ALCL.
Constitutive activation of the JAK/STAT3 signaling pathway is a central pathogenic feature of ALK+ as well as ALKnegative ALCL. DUSP22 has been shown to inhibit STAT3 signaling.25 Since DUSP22 expression was significantly decreased in the presence of a DUSP22-R, it seemed reasonable to expect enhanced STAT3 activation in DUSP22-R ALCL. Surprisingly, total STAT3 protein level and STAT3 activation (measured by pSTAT3Y705) in DUSP22-R ALCL was significantly decreased, at least partially due to significantly reduced expression of STAT3 and other genes in the JAK/STAT3 pathway.16 Lack of STAT3 activation in DUSP22-R ALCL has also been shown by others.9,11 The JAK/STAT3 pathway regulates expression of many downstream molecules including PD-L1.20,26,27 Therefore, one would predict that lack of pSTAT3 in DUSP22-R ALCL would result in absent PD-L1 expression, as shown here and elsewhere.11,16 Expression of other known STAT3 target genes, such as granzyme B and CD25, was also low in DUSP22-R ALCL in this cohort.16 DUSP22-R ALCL cases were nearly always negative or infrequently expressed cytotoxic markers and EMA.6,9,11,15 Based on gene expression profiling, ALK-negative ALCL cases included two distinct subgroups: cytotoxic and non-cytotoxic. The non-cytotoxic subgroup showed high expression of CD30 but not perforin or granzyme B, with half of cases harboring a DUSP22-R.28 In keeping with prior reports, the levels of expression of granzyme B and EMA in our DUSP22-R ALK-negative ALCL cases were significantly lower than those in the DUSP22-NR cases. DUSP22-R results in inhibition of the JAK/STAT3 pathway, but it may also lead to activation of other pathways, as supported by the increased frequency of CD15 observed in the current study. A high CD15 positivity rate in DUSP22-R ALK-negative ALCL cases has not been previously reported. Three CD15 staining patterns were observed in DUSP22-R ALCL cases: a Golgi-like pattern, a membranous/cytoplasmic pattern, and a combination of the Golgilike and membranous/cytoplasmic patterns. CD15 positivity with a Golgi-like staining pattern is unusual but has been reported in a case of DUSP22-R ALK-negative ALCL.29 Another case report of ALK-negative ALCL showed membranous and Golgi-like staining pattern of CD15, but DUSP22-R status was unknown.30
Figure 3.
Flow cytometric immunophenotypic analysis of the lymph node biopsy specimen shown in Figure 1. (A-H) The lymphoma cells (red dots) are positive for CD45 (A), CD30 (B), CD3 (decreased, B), CD8 (partial, C), CD2 (D), CD7 (partial/decreased, F), and T-cell receptor (TCR) α/β (decreased, H), and negative for CD4 (C), CD5 (D), CD26 (F), CD25 (G), and TCR γ/d (H). The lymphoma cells are medium-sized by forward scatter (E). The purple and blue dots represent background benign CD4+ and CD8+ T cells, respectively.
Figure 4.
CD15 expression with three staining paterns in DUSP22-rearranged cases of ALK-negative anaplastic large cell lymphoma. (A) Golgi-like pattern. (B) Membranous/cytoplasmic pattern. (C) Combination of the Golgi-like and membranous/cytoplasmic patterns. (D) The percentage of CD15+ lymphoma cells in DUSP22-rearranged cases of anaplastic large cell lymphoma cases is significantly higher than that in the cases without a DUSP22 rearrangement. (A-C) Immunohistochemistry, x500. DUSP22-R: with DUSP22 rearrangement; DUSP22-NR: without DUSP22 rearrangement.
Although CD15 has received little attention in DUSP22-R ALK-negative ALCL, CD15 expression was observed in ALCL previously. Felgar et al. reported CD15 expression in three of 17 (18%) T- or null cell ALCL with unknown ALK status; these cases were all negative for TIA1, suggesting to the authors that the CD15+ ALCL cases may be different from other ALCL cases.31 Gorczyca et al. reported CD15 positivity in two of 26 (8%) ALK+ ALCL cases and seven of 30 (23%) ALK-negative CD30+ T-cell lymphomas.32 Four CD15+ ALKnegative ALCL cases were submitted to the 2005 Society of Hematopathology/European Association for Hematopathology Workshop: one of three (33%) cases was positive for EMA, and one of two (50%) cases was positive for TIA1.33 Retrospectively, we speculate that at least some of previously reported CD15+ ALK-negative ALCL cases were likely neoplasms with DUSP22-R. The potential explanation for CD15 expression in DUSP22-R ALCL is unknown and needs to be investigated further. CD15 has been a valuable marker for distinguishing classic Hodgkin lymphoma from other CD30+ lymphomas including ALK-negative ALCL. The differential diagnosis between ALK-negative ALCL and classic Hodgkin lymphoma is usually not difficult based on their morphological and immunophenotypic differences. However, a subset of tumors may occasionally show morphological and/or immunophenotypic overlap, posing a diagnostic challenge. For the CD30+ CD15+ cases with overlapping features of ALCL and classic Hodgkin lymphoma, FISH for DUSP22-R may be helpful in reaching the correct diagnosis because a positive result for DUSP22-R points to the diagnosis of ALKnegative ALCL. The unique morphological, immunophenotypic, and molecular/genetic features of DUSP22-R ALCL have been reported consistently, suggesting that DUSP22-R cases are a distinct subset of ALK-negative ALCL.9,11,15-17 However, the prognostic significance of DUSP22-R in ALK-negative ALCL has been controversial. DUSP22-R was initially reported to be associated with a favorable clinical outcome in systemic ALK-negative ALCL patients with a 5-year OS rate of 90%, similar to that of patients with ALK+ ALCL.6 However, in a recent study from Vancouver, the 5-year OS of DUSP22-R ALCL patients was only 40%.11 Unlike earlier studies with small cohorts, the present study of 81 ALK-negative ALCL patients shows that patients with DUSP22-R neoplasms have a relatively poorer outcome, similar to that of patients in the DUSP22-NR group and substantially poorer than that of ALK+ ALCL patients. The discrepancies observed between studies on the outcome of DUSP22-R cases could potentially be attributable to some missed breakpoints. Different FISH probes were used in different studies: some were prepared in-house6,10,11 whereas others were purchased,8,9 as in the current study. It is possible that break-apart FISH probes may not detect all DUSP22-R if the breakpoints are located outside of the probe coverage or if the rearrangement is through insertion. If true, differences in probes used could alter the results of survival analyses. We note that patients with DUSP22-R ALK-negative ALCL in this cohort tended to have a higher initial complete response rate than the patients with DUSP22-NR neoplasms, although this difference did not reach statistical significance. This result might be due to the relatively small size of this cohort and larger-sized studies are needed for further investigation.
Figure 5.
Comparison of STAT3 activation (pSTAT3Tyr705) and PD-L1 expression in ALK-negative anaplastic large cell lymphoma with or without a DUSP22 rearrangement. (A, D) Negative pSTAT3Tyr705 (A) and PD-L1 (D) expression in a DUSP22-rearranged case. (B, E) Diffuse positivity of pSTAT3Tyr705 (B) and PD-L1 (E) in a case without a DUSP22-rearrangement. (C, F) The percentage of pSTAT3Tyr705-positive (C) or PD-L1-positive (F) lymphoma cells in DUSP22-rearranged cases of anaplastic large cell lymphoma is significantly lower than that in cases without a DUSP22 rearrangement. DUSP22-R: with DUSP22 rearrangement; DUSP22-NR: without DUSP22 rearrangement.
Figure 6.
DUSP22 rearrangement has no prognostic significance in patients with ALK-negative anaplastic large cell lymphoma regardless of International Prognostic Index score, stage, age, or transplantation status. (A, B) Comparison of overall survival (A) and progression-free survival (B) among the patients with ALK+ anaplastic large cell lymphoma (ALCL), DUSP22-R (all were TP63-NR) ALK-negative ALCL, DUSP22-NR/TP63-NR (triple-negative) ALK-negative ALCL, and DUSP22-NR/TP63-R ALK-negative ALCL. *P<0.05, when comparing ALK+ ALCL versus DUSP22-R ALK-negative ALCL or comparing ALK+ ALCL versus DUSP22-NR/TP63-NR ALK-negative ALCL. (C, D) Comparison of overall survival in patients with International Prognostic Index score ≥3 (C) and <3 (D). (E, F) Comparison of overall survival in patients with stage III-IV (E) and stage I-II (F) disease. (G, H) Comparison of overall survival in patients aged ≥50 years (G) and <50 years (H). (I, J) Comparison of overall survival in patients with stem cell transplantation (I) and without (J). DUSP22-R: with DUSP22 rearrangement; DUSP22-NR: without DUSP22 rearrangement; TP63-R: with TP63 rearrangement; TP63-NR: without TP63 rearrangement; IPI: International Prognostic Index; OS: overall survival; PFS: progression-free survival; SCT: stem cell transplant. (C-J) TP63-R cases were excluded.
In this cohort, ~5% of patients with ALK-negative ALCL had TP63-R, consistent with the reported frequency (2-8%) of TP63-R cases. All TP63-R cases in this study were negative for DUSP22-R. TP63-R and DUSP22-R are nearly mutually exclusive,6 although rare cases harboring both rearrangements have been reported.34,35 In a past study TP63-R (n=6) was reported to be associated with poorer prognosis in patients with ALK-negative ALCL.6 The present study shows no association between TP63-R and OS, but the patients with TP63-R neoplasms tended to have shorter progression-free survival than those without TP63-R. Given the limited number of TP63-R cases in our cohort, the prognostic significance of TP63-R cannot be determined.
In summary, our data support the idea that DUSP22-R ALK-negative ALCL is a distinctive subset of ALCL. As suggested by others, our data showed that these neoplasms have minimal STAT3 activation and PD-L1 expression, and no/low expression of cytotoxic markers. We report a novel finding in this study that CD15 is commonly expressed in DUSP22-R ALK-negative ALCL cases. Our data also suggest that patients with DUSP22-R ALK-negative ALCL do not have an excellent prognosis as earlier studies suggested, but instead have a prognosis similar to that of patients with DUSP22-NR ALK-negative ALCL. We therefore suggest that further investigation is needed before modifications to the treatment of patients with DUSP22-R ALK-negative ALCL are proposed.
References
- 1.Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36(7):1720-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130. [DOI] [PubMed] [Google Scholar]
- 3.Falini B, Pileri S, Zinzani PL, et al. ALK+ lymphoma: clinicopathological findings and outcome. Blood. 1999;93(8):2697-2706. [PubMed] [Google Scholar]
- 4.Sibon D, Fournier M, Briere J, et al. Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte trials. J Clin Oncol. 2012;30(32):3939-3946. [DOI] [PubMed] [Google Scholar]
- 5.ten Berge RL, de Bruin PC, Oudejans JJ, et al. ALK-negative anaplastic large-cell lymphoma demonstrates similar poor prognosis to peripheral T-cell lymphoma, unspecified. Histopathology. 2003;43(5):462-469. [DOI] [PubMed] [Google Scholar]
- 6.Parrilla Castellar ER, Jaffe ES, Said JW, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014;124(9):1473-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellin F, Landstrom J, Jerkeman M, et al. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014;124(10):1570-1577. [DOI] [PubMed] [Google Scholar]
- 8.Parkhi M, Bal A, Das A, et al. ALK-negative anaplastic large cell lymphoma (ALCL): prognostic implications of molecular subtyping and JAK-STAT pathway. Appl Immunohistochem Mol Morphol. 2021;29(9):648-656. [DOI] [PubMed] [Google Scholar]
- 9.Onaindia A, de Villambrosia SG, Prieto-Torres L, et al. DUSP22-rearranged anaplastic lymphomas are characterized by specific morphological features and a lack of cytotoxic and JAK/STAT surrogate markers. Haematologica. 2019;104(4):e158-e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen MB, Hamilton-Dutoit SJ, Bendix K, et al. DUSP22 and TP63 rearrangements predict outcome of ALK-negative anaplastic large cell lymphoma: a Danish cohort study. Blood. 2017;130(4):554-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hapgood G, Ben-Neriah S, Mottok A, et al. Identification of high-risk DUSP22-rearranged ALK-negative anaplastic large cell lymphoma. Br J Haematol. 2019;186(3):e28-e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JP, Yang CY, Chuang HC, et al. The phosphatase JKAP/DUSP22 inhibits T-cell receptor signalling and autoimmunity by inactivating Lck. Nat Commun. 2014;5:3618. [DOI] [PubMed] [Google Scholar]
- 13.Melard P, Idrissi Y, Andrique L, et al. Molecular alterations and tumor suppressive function of the DUSP22 (Dual Specificity Phosphatase 22) gene in peripheral T-cell lymphoma subtypes. Oncotarget. 2016;7(42):68734-68748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman AL, Dogan A, Smith DI, et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively parallel genomic sequencing. Blood. 2011;117(3):915-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King RL, Dao LN, McPhail ED, et al. Morphologic features of ALKnegative anaplastic large cell lymphomas with DUSP22 rearrangements. Am J Surg Pathol. 2016;40(1):36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luchtel RA, Dasari S, Oishi N, et al. Molecular profiling reveals immunogenic cues in anaplastic large cell lymphomas with DUSP22 rearrangements. Blood. 2018;132(13):1386-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravindran A, Feldman AL, Ketterling RP, et al. Striking association of lymphoid enhancing factor (LEF1) overexpression and DUSP22 rearrangements in anaplastic large cell lymphoma. Am J Surg Pathol. 2021;45(4):550-557. [DOI] [PubMed] [Google Scholar]
- 18.Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95(12):3653-3661. [PubMed] [Google Scholar]
- 19.Khanlari M, Li S, Miranda RN, et al. Small cell/lymphohistiocytic morphology is associated with peripheral blood involvement, CD8 positivity and retained T-cell antigens, but not outcome in adults with ALK+ anaplastic large cell lymphoma. Mod Pathol. 2022;35(3):412-418. [DOI] [PubMed] [Google Scholar]
- 20.Shen J, Li S, Medeiros LJ, et al. PD-L1 expression is associated with ALK positivity and STAT3 activation, but not outcome in patients with systemic anaplastic large cell lymphoma. Mod Pathol. 2020;33(3):324-333. [DOI] [PubMed] [Google Scholar]
- 21.Shen J, Medeiros LJ, Li S, et al. CD8 expression in anaplastic large cell lymphoma correlates with noncommon morphologic variants and T-cell antigen expression suggesting biological differences with CD8-negative anaplastic large cell lymphoma. Hum Pathol. 2020;98:1-9. [DOI] [PubMed] [Google Scholar]
- 22.Lyapichev KA, Tang G, Li S, et al. MYC expression is associated with older age, common morphology, increased MYC copy number, and poorer prognosis in patients with ALK+ anaplastic large cell lymphoma. Hum Pathol. 2021;108:22-31. [DOI] [PubMed] [Google Scholar]
- 23.Feldman AL, Law M, Remstein ED, et al. Recurrent translocations involving the IRF4 oncogene locus in peripheral T-cell lymphomas. Leukemia. 2009;23(3):574-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24(4):596-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekine Y, Tsuji S, Ikeda O, et al. Regulation of STAT3-mediated signaling by LMW-DSP2. Oncogene. 2006;25(42):5801-5806. [DOI] [PubMed] [Google Scholar]
- 26.Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci U S A. 2008;105(52):20852-20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atsaves V, Tsesmetzis N, Chioureas D, et al. PD-L1 is commonly expressed and transcriptionally regulated by STAT3 and MYC in ALK-negative anaplastic large-cell lymphoma. Leukemia. 2017;31(7):1633-1637. [DOI] [PubMed] [Google Scholar]
- 28.Drieux F, Ruminy P, Abdel-Sater A, et al. Defining signatures of peripheral T-cell lymphoma with a targeted 20-marker gene expression profiling assay. Haematologica. 2020;105(6):1582-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman J, Vega F: Indolent ALK-negative anaplastic large-cell lymphoma, DUSP22 rearranged, with an unusual immunophenotype in a human immunodeficiency virus patient. Histopathology. 2017;70(7):1173-1175. [DOI] [PubMed] [Google Scholar]
- 30.Arun I, Roy P, Arora N, et al. PAX-5 positivity in anaplastic lymphoma kinase-negative anaplastic large cell lymphoma: a case report and review of literature. Int J Surg Pathol. 2017;25(4):333-338. [DOI] [PubMed] [Google Scholar]
- 31.Felgar RE, Salhany KE, Macon WR, et al. The expression of TIA-1+ cytolytic-type granules and other cytolytic lymphocyte-associated markers in CD30+ anaplastic large cell lymphomas (ALCL): correlation with morphology, immunophenotype, ultrastructure, and clinical features. Hum Pathol. 1999;30(2):228-236. [DOI] [PubMed] [Google Scholar]
- 32.Gorczyca W, Tsang P, Liu Z, et al. CD30-positive T-cell lymphomas co-expressing CD15: an immunohistochemical analysis. Int J Oncol. 2003;22(2):319-324. [PubMed] [Google Scholar]
- 33.Medeiros LJ, Elenitoba-Johnson KS. Anaplastic large cell lymphoma. Am J Clin Pathol. 2007;127(5):707-722. [DOI] [PubMed] [Google Scholar]
- 34.Karube K, Feldman AL. "Double-hit" of DUSP22 and TP63 rearrangements in anaplastic large cell lymphoma, ALKnegative. Blood. 2020;135(9):700. [DOI] [PubMed] [Google Scholar]
- 35.Klairmont MM, Ward N. Co-occurring rearrangements of DUSP22 and TP63 define a rare genetic subset of ALK-negative anaplastic large cell lymphoma with inferior survival outcomes. Leuk Lymphoma. 2022;63(2):506-508. [DOI] [PubMed] [Google Scholar]