Abstract
Introduction
Pancreatic cancer has one of the worst prognoses of all cancers. Patients with locally advanced pancreatic cancer have a 12.7–20.2 per cent chance of receiving curative surgery after induction systemic chemotherapy. Intratumoral injection therapies have been studied as complementary treatment options for improved local tumour control. The aim of this systematic review was to provide an overview of intratumoral injection therapies, their safety, and oncological outcome in patients with locally advanced pancreatic cancer.
Methods
A literature search was conducted in PubMed, Embase and the Cochrane Library for articles written in English up to 28 November 2022. All study designs involving at least five patients with locally advanced pancreatic cancer who were treated with an intratumoral injection therapy were included. Critical appraisal of the included studies was performed using the Newcastle–Ottawa scale.
Results
After evaluation of the 1680 articles yielded by the systematic search, 52 studies treating 1843 patients were included. Included intratumoral injection treatment modalities comprised iodine-125 (125I) seed brachytherapy (32 studies, 1283 patients), phosphorus-32 (32P) microbrachytherapy (5 studies, 133 patients), palladium-103 (103Pd) seed brachytherapy (2 studies, 26 patients), immunotherapy (9 studies, 330 patients), and chemotherapy (4 studies, 71 patients). Overall survival ranged between 7.0 and 16.0 months for 125I, 5.2 and 15.5 months for 32P, 6.9 and 10.0 months for 103Pd, 5.8 and 13.8 months for immunotherapy, and 9.0 and 16.2 months for chemotherapy. Severe complication (greater than or equal to grade III complications using Clavien–Dindo classification) rates were 6.2 per cent for 125I, 49.2 per cent for 32P, 15 per cent for 103Pd, 57.9 per cent for immunotherapy, and 0 per cent for chemotherapy.
Conclusion
Five intratumoral injection therapies are described and an overview is reported. Some intratumoral injection therapies for patients with locally advanced pancreatic cancer seem safe, although 32P microbrachytherapy and immunotherapy require additional evidence. Currently available data are insufficient to provide firm conclusions regarding the added value to survival. The potential advantage of intratumoral injection therapies complementary to conventional care should be studied in well designed RCTs.
Intratumoral injection therapies have been studied as novel (complementary) treatment options for improved local tumour control of locally advanced pancreatic cancer. A literature search was conducted in PubMed, Embase, and the Cochrane Library, including 52 studies treating 1843 patients with 5 different modalities of intratumoral injection therapies. There was a large variation in safety and survival; however, most intratumoral injection therapies seem safe, although current data are insufficient for firm conclusions regarding the added value to survival.
Introduction
Pancreatic cancer is diagnosed in over 440 000 people worldwide every year and the incidence has increased by 55 per cent over the past 25 years1. The mortality is similar to the incidence due to the poor prognosis of this malignancy. With a 1-year overall survival (OS) of just 20 per cent and 5-year survival of 9 per cent, pancreatic cancer is one of the most aggressive forms of all common cancers2. When untreated, 5-year survival decreases to 3 per cent3. Resection can be performed in just 20 per cent of all patients and is the only potentially curative treatment option. At the time of diagnosis, around 50 per cent of all patients with pancreatic cancer are affected by distant metastases and the remaining 30 per cent have locally advanced pancreatic cancer (LAPC), making resection futile4,5. The most commonly used criteria for LAPC are those from the National Comprehensive Cancer Network (NCCN) guidelines, defining LAPC as greater than 180° arterial encasement or unreconstructible venous involvement without evidence of distant metastases6. Commonly, tumour involvement in the superior mesenteric artery, celiac axis, or common hepatic artery or definite occlusion of the superior mesenteric vein or portal vein make pancreatic cancer unresectable7.
The current therapy of choice for LAPC is induction/palliative chemotherapy with FOLFIRINOX (a combination of 5-fluorouracil, irinotecan, leucovorin, and oxaliplatin) or gemcitabine with nab-paclitaxel, and response evaluation after 8 weeks8. During re-evaluation, if metastases remain absent and no tumour progression is observed, approximately 28.0–31.3 per cent become eligible for exploration surgery, 12.6–20.2 per cent receive surgical resection, and 15.9–18.1 per cent have an R0 (greater than 1 mm) outcome9,10. Of the 79.8–87.3 per cent of patients that do not receive surgical resection, approximately 25.0 per cent have local tumour progression without metastatic disease and may benefit from local therapies9,10. Gemcitabine has been the recommended induction therapy for LAPC for over a decade and is still used in patients with a WHO performance score of 2 and higher8. OS for LAPC is approximately 14.8–24.2 months for FOLFIRINOX chemotherapy11,12 and 9.0–16.0 months for gemcitabine-based chemotherapy9,13; however, most patients also undergo radiotherapy, ablation therapies, second-line chemotherapy, or resection before, during, or after the first-line chemotherapy treatment. For patients with severe co-morbidities these extensive combination treatments are often considered impossible14. Some studies suggest that almost half of elderly patients (greater than 65 years) with no metastatic pancreatic cancer do not undergo chemotherapy or surgery, possibly due to co-morbidities15.
For patients with stable unresectable disease after chemotherapy, local ablation is occasionally applied in clinical trials aiming to control local progression and to prolong survival16,17. Although ablation is considered feasible, it is also associated with substantial morbidity and mortality16. The effectiveness of additional local ablation is disputable because of the paucity of high-level evidence. Overall, small non-comparing case studies, hampered by selection bias, show a wide variation in OS from 5.0 up to 25.6 months16. Although some survival outcomes after ablation seem promising, the clinical demand for a minimally invasive therapy to improve local tumour control is still unmet.
Optimally, a local therapy for pancreatic cancer is minimally invasive, offers accurate treatment delivery with complete tumour coverage, spares healthy surrounding tissue, and has accurate therapy prediction and control. To meet these demands, over the past decades, novel intratumoral injection therapies for pancreatic cancer have been studied worldwide. Advancements in therapy control, advanced image acquisition and processing, personalized treatment planning and immunological pathways have changed the perspective to achieve optimal local tumour control. With less invasive therapies, hospital stay and healthcare costs may decrease18. Safe treatment delivery reduces complication rates and benefits the quality of life. The aim of this systematic review was to provide an overview of intratumoral injection therapies, their safety, and oncological outcome in patients with LAPC.
Methods
This systematic review was conducted and reported conforming to PRISMA guidelines19. The methodology and inclusion/exclusion criteria were defined in advance by a Biomedical Information Specialist and the authors. This study was registered on PROSPERO, the international prospective register of systematic reviews (registration ID: CRD42020212862).
Search strategy
A literature search was conducted in PubMed, Embase, and the Cochrane Library for articles written in English from dates of inception up to 28 November 2022. The literature search was performed using medical domains combined by ‘AND’ between domains and within the domain by ‘OR’. The first domain contained terms regarding pancreatic cancer, the second regarding intratumoral therapy, and the third regarding LAPC. Search terms were restricted to Medical Subject Headings, title, abstract, and keywords. Study selection and organization were performed using EndNote X9.2. The complete search strategy for each library is presented in Appendix S1. After a first scan to remove duplicate publications, the titles and abstracts were scanned for inclusion and exclusion criteria. Publications limited to an abstract were not excluded if the information was adequate, as described below. If multiple studies contained the same patient cohort, only the latest published article was included. If there was uncertainty regarding inclusion, a second author was consulted.
Definitions
LAPC was defined as an irresectable tumour due to vascular involvement without distant metastasis. Patients with vascular involvement resected at diagnosis or at any time during follow-up (for example after induction chemotherapy) were not considered. A more detailed definition of LAPC (for example type and extent of vascular involvement)20 could not be applied due to the time span and heterogeneity of the included studies.
Intratumoral injection therapy was defined as the injection of an active substance in the pancreatic tumour mass with the intention to treat or control the primary pancreatic cancer. Angiographically delivered therapy, infusion therapy, stenting, ablation, or post-resection treatments were not defined as intratumoral injection therapy. Studies performing non-resection surgical procedures, including cholangiojejunostomy, gastrojejunostomy, biliary/gastric bypass, and stent placement, were included if performed complementary or secondary to intratumoral injection therapy.
Study selection
Studies were included if they treated human patients suffering from LAPC with a single intratumoral injection therapy and presented outcomes regarding survival and/or safety. Articles had to be published in a registered journal defined by the SCImago Journal & Country Rank21. Studies were excluded if one or more of the following criteria was met: reviews, non-English articles, animal studies, case studies (or less than five patients in a single treatment population), minority LAPC in a treatment population, and resection immediately after intratumoral injection therapy.
Quality assessment
All studies passing the full-text assessment were critically appraised according to the Newcastle–Ottawa scale (NOS) for assessing the quality of non-randomized studies. The NOS is a validated scoring system with appraisals for case–control and cohort studies. RCTs were assessed using the cohort evaluation. A total of nine points could be appraised per study; four by selection, two by comparability, and the last three by either exposure or outcome of interest for case–control and cohort studies respectively. The complete scoring criteria are presented in Appendix S2. Studies with five stars or more were considered of good quality. Studies with less than five stars were not excluded.
Data extraction
Data on intratumoral injection therapy, dose, approach, cancer stage, metastases, combination therapies, median OS, and complications by the Clavien–Dindo classification22 were extracted when available23. Furthermore, study characteristics, such as design, country, population characteristics, and sample size, were extracted from the included studies. Data extraction and organization were performed using Microsoft® Excel® for Microsoft 365.
Statistical analysis
Most outcomes were descriptive and, due to the heterogeneity of the included studies, no meta-analysis or statistical analysis was performed.
Results
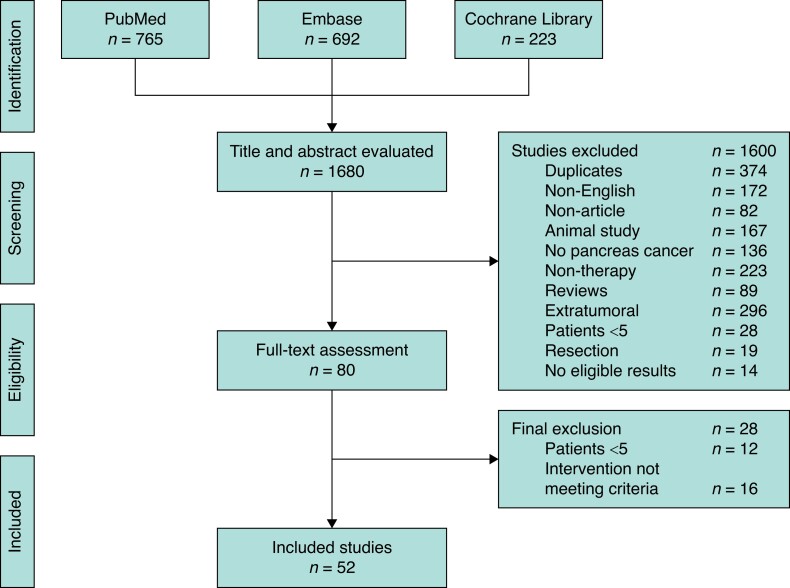
Starting with 1680 articles, after title and abstract screening for duplicates and exclusion criteria, 1600 studies were excluded. Eighty studies entered full-text assessment. Of these, 28 studies were excluded because of small sample size (12) and/or intervention not meeting the inclusion criteria (16). Some 52 clinical studies with 1843 patients were included for quality assessment. The complete results of the quality assessment are reported in Appendix S3. A detailed selection flow chart is shown in Fig. 1.
Fig. 1.
PRISMA flow chart of the study selection
The included studies comprised five different intratumoral injection treatment modalities: iodine-125 (125I) seed brachytherapy (32 studies, 1283 patients), phosphorus-32 (32P) microbrachytherapy (5 studies, 133 patients), palladium-103 (103Pd) seed brachytherapy (2 studies, 26 patients), immunotherapy (9 studies, 330 patients), and chemotherapy (4 studies, 71 patients). Most of the included studies had the following inclusion criteria in common: age greater than or equal to 18 years, adequate performance status (WHO/Eastern Cooperative Oncology Group (ECOG), Karnofsky), and an adequate hepatic, haematological, immune, and/or renal function. Gemcitabine-based chemotherapy was the most frequently used form of induction/palliative chemotherapy. One study combined intratumoral injection therapy with FOLFIRINOX chemotherapy24. All results are presented per modality and an overview of all intratumoral injection therapies is presented in Table 1.
Table 1.
Results of all intratumoral injection therapies for locally advanced pancreatic cancer
| Intratumoral injection therapy | No. of studies | No. of patients | Metastasis | Chemotherapy | Chemoradiotherapy | Radiotherapy | Overall complications, n | Complications ≥grade III | OS range (months) |
|---|---|---|---|---|---|---|---|---|---|
| Iodine-125 | 32 | 1283 | 212 of 1026 (20.6) | 538 (47.5) | 161 (14.2) | 137 (12.1) | 324 in 771 | 37 (6.2) | 7–16 |
| Phosphorus-32 | 5 | 133 | 19 of 133 (14.3) | 53 (39.8) | 65 (48.9) | 0 | 1122 in 89 | 61 (49.2) | 5.2–15.5 |
| Palladium-103 | 2 | 26 | 1 of 26 (4) | 2 (8) | 20 (77) | 0 | 6 in 26 | 4 (15) | 5–7 |
| Immunotherapy | 9 | 330 | 147 of 294 (50.0) | 72 (21.8) | 252 (76.4) | 0 | 316 in 223 | 33 (57.9) | 5.8–13.8 |
| Chemotherapy | 4 | 71 | 15 of 53 (28) | 18 (33) | 36 (67) | 0 | 3 in 41 | 0 (0) | 9–16.2 |
Values are n (%) unless otherwise stated. OS, overall survival.
Iodine-125 seed brachytherapy
An overview of the results of intratumoral injection 125I brachytherapy is presented in Table 2. Of the 32 studies applying 125I brachytherapy in 1283 patients suffering from pancreatic adenocarcinoma, 15 had a retrospective design25–39, 16 had an open-label prospective design40–55, and one compared 125I combined with chemotherapy versus chemotherapy alone in an RCT56. An overview of the characteristics of all applied radioactive isotopes is presented in Appendix S4.
Table 2.
Results of intratumoral iodine-125 seed brachytherapy for locally advanced pancreatic cancer
| Reference | n | Metastasis | Tumour dose (Gy), median* (range) | Combination therapy | Overall complications, n | Complications ≥grade III | Median* OS (months); OS initiation | NOS 1–9 |
|---|---|---|---|---|---|---|---|---|
| Intraoperative | ||||||||
| Dobelbower et al., 198640 | 12 | 2 (17) | 140 (120–210) | CRT 6 (50) | 5 | NR | 15; D | 8 |
| Goertz et al., 199027 | 11 | 2 (18) | 160 | RT 11 (100) | 11 | 1 (9) | 8 | 7 |
| Li et al., 202028 | 50 | 0 (0) | (110–160) | CHT 26 (52) | 4 | NR | 12 | 9 |
| Li et al., 201630 | 137 | NR | NR | CHT 137 (100.0) | 21 | 0 (0.0) | 9.4; T | 9 |
| Montemaggi et al., 199144 | 7 | 0 (0) | 82 (60–100) | RT 4 (57) | NR | 3 (43) | 7; T | 5 |
| Morrow et al., 198434 | 33 | 9 (27) | NR | NR | NR | 8 (24) | 8; T | 4 |
| Peretz et al., 198935 | 98 | 0 (0) | 136 | CHT 27 (28), RT 27 (28) | 9 | NR | 7 | 4 |
| Schuricht et al., 199136 | 42 | 0 (0) | (120–150) | CRT 42 (100) | 24 | NR | 12.8 | 8 |
| Shipley et al., 198046 | 12 | 6 (50) | 160 | RT 12 (100) | 5 | NR | 11 | 6 |
| Syed et al., 198348 | 18 | 1 (6) | (100–150) | RT 18 (100) | NR | 4 (22) | 14 | 7 |
| Wang et al., 201350 | 28 | 0 (0) | 120 | CHT 10 (36), RT 7 (25) | NR | NR | 10.1 | 4 |
| Wang et al., 201151 | 27 | 15 (56) | (110–160) | CRT 6 (22), RT 1 (4) | NR | NR | 8 | 4 |
| Whittington et al., 198438 | 33 | 0 (0) | 120 | CRT 20 (61), RT 13 (39) | 37 | NR | 9; D | 8 |
| Zheng et al., 201754 | 34 | 0 (0) | NR | CHT 8 (24) | 6 | NR | 11; T | 7 |
| Total intraoperative | 542 | 70 of 405 (17.3) | NA | CHT 208 (40.8), CRT 74 (14.5), RT 93 (18.3) | 122 of 429 | 16 of 206 (7.8) | NA | NA |
| Percutaneous | ||||||||
| Chen et al., 202125 | 22 | 4 (18) | 130 | CHT 22 (100) | 22 | NR | 11.7; T | 9 |
| Chi et al., 202126 | 21 | 0 (0) | 130 | CHT 21 (100) | 24 | 0 (0) | 13.2; T | 4 |
| Joyce et al., 199043 | 19 | NR | 160 | RT 12 (63) | 22 | NR | 8.1; T | 8 |
| Liu et al., 201431 | 30 | 0 (0) | NR | NR | 6 | NR | 16 | 8 |
| Lun et al., 201556 | 38 | NR | NR | CHT 38 (100) | NR | NR | 11.8 | 4† |
| Luo et al., 201932 | 35 | NR | NR | CHT 35 (100) | NR | NR | 9.5 | 7 |
| Niu et al., 201645 | 60 | 0 (0) | 115 (110–130) | NR | NR | 0 (0) | 10.4 | 6 |
| Wang et al., 202149 | 28 | NR | NR | NR | 2 | 0 (0) | 11.6 | 5 |
| Wang et al., 201752 | 32 | 25 (78) | 120 | CHT 16 (50) | NR | 0 (0) | 14 | 6 |
| Yang et al., 201653 | 18 | 0 (0) | 167 (164–170) | CHT 18 (100), RT 1 (6) | 10 | 3 (17) | 7.3; T | 5 |
| Zhongmin et al., 201055 | 31 | 12 (39) | 52 | CHT 10 (32) | NR | NR | 10.3; T | 7 |
| Zhou et al., 202139 | 67 | 0 (0) | NR | CHT 6 (9) | 20 | 0 (0) | 11; T | 8 |
| Total percutaneous | 401 | 41 of 281 (14.6) | NA | CHT 166 (58.7), RT 13 (4.6) | 106 of 205 | 3 of 226 (1.3) | NA | NA |
| EUS | ||||||||
| Du et al., 201341 | 100 | 40 (40.0) | 140 (120–210) | CHT 100 (100.0) | 52 | 0 (0.0) | 7; T | 8 |
| Jin et al., 200842 | 22 | 8 (36) | NR | CHT 22 (100) | 13 | NR | 9 | 6 |
| Sun et al., 200647 | 15 | 7 (47) | 140 | RT 1 (7) | 31 | 4 (27) | 10.6; T | 8 |
| Sun et al., 201737 | 42 | 24 (57) | 95 | CHT 42 (100) | NR | 0 (0) | 9; T | 2 |
| Total EUS | 179 | 79 of 179 (44.1) | NA | CHT 164 (91.6), RT 1 (0.6) | 96 of 137 | 4 of 157 (2.5) | NA | NA |
| Other | ||||||||
| Li et al., 202029 | 50 | 22 (44) | NR | CRT 6 (12) | 17 | 0 (0) | 8.8 | 8 |
| Mohiuddin et al., 199433 | 111 | 0 (0.0) | NR | CRT 81 (73.0), RT 30 (27.0) | NR | NR | 11.4 | 3 |
| Total (intraoperative, percutaneous, EUS, and other) | 1283 | 212 of 1026 (20.7) | NA | CHT 538 (47.5), CRT 161 (14.2), RT 137 (12.1) | 324 of 771 | 37 out of 600 (6.2) | NA | NA |
Values are n (%) unless otherwise stated. *If the median was unavailable the mean is presented. †RCT. OS, overall survival; NOS, Newcastle–Ottawa scale; CRT, chemoradiotherapy; NR, not reported; D, diagnosis; RT, radiotherapy; CHT, chemotherapy; T, treatment; NA, not applicable; EUS, endoscopic ultrasonography.
In 27 studies reporting metastases, 212 of 1026 patients (20.7 per cent) had or developed stage IV pancreatic cancer. The included studies utilized 125I seeds with a length of 4.4 to 4.6 mm with a diameter of less than 1 mm42,57. Each patient received between 10 and 150 seeds in one or multiple operations depending on tumour volume, characteristics, and response. The median tumour dose ranged from 52 Gy55 to 167 Gy53, with most of the studies ranging between 100 and 150 Gy. For the application method, 14 studies (542 patients) implanted the seeds intraoperatively in an open approach using X-ray, CT, or ultrasonography guidance27,28,30,34–36,38,40,44,46,48,50,51,54. Twelve studies (401 patients) used percutaneous implantation guided by ultrasonography or CT25,26,31,32,39,43,45,49,52,53,55,56. Since 2006, four studies (179 patients) implemented endoscopic ultrasonography (EUS) to deliver the radioactive seeds to the tumour with a transgastric or transduodenal injection37,41,42,47. In 28 studies with 1132 patients, 538 patients (47.5 per cent), 161 patients (14.2 per cent), and 137 patients (12.1 per cent) received chemotherapy, chemoradiotherapy, or radiotherapy respectively.
Out of 600 patients in 15 studies reporting complications, 37 (6.2 per cent) suffered from greater than or equal to grade III complications (using Clavien–Dindo classification). Three studies reported postprocedural mortality34,38,44. Four deaths were caused by abscesses or anastomotic leakage38, three were caused by a pulmonary embolism38,44, two were caused by duodenal ulcers38, and one cause was not reported34. The most common complications reported were gastrointestinal haemorrhages35,38,46,54, pancreatic fistula34,35,44,54, leucocytopenia47, and different intra-abdominal infections like pancreatitis and cholangitis35,38,42,47,48,54. The median OS ranged from 7.0 months35,41 to 16 months31. In the RCT, no significant difference (P > 0.05) was found in adverse events between 125I combined with chemotherapy versus chemotherapy alone56. However, a statistically significant difference (P < 0.05) was established between the OS of 125I combined with chemotherapy (11.84 months) versus chemotherapy alone (10.40 months)56.
Phosphorus-32 microbrachytherapy
An overview of the results of intratumoral injection 32P microbrachytherapy is presented in Table 3. All five included studies (133 patients) using 32P had a prospective design. One study compared 32P combined with previous 5-fluorouracil and gemcitabine chemotherapy after injection versus chemotherapy alone in an RCT58.
Table 3.
Results of intratumoral phosphorus-32 microbrachytherapy for locally advanced pancreatic cancer
| Reference | n | Metastasis | Type of 32P therapy | Tumour dose (Gy), median* (range) | Combination therapy | Overall complications, n | Complications ≥grade III | Median* survival (months); OS initiation | NOS 1–9 |
|---|---|---|---|---|---|---|---|---|---|
| Percutaneous | |||||||||
| Order et al., 199659 | 47 | 19 (40) | MAA and colloidal chromic 32P | (9000–17 000) | CRT 47 (100) | 27 | 10 (53) | 9.9; T | 8 |
| Rosemurgy et al., 200858 | 18 | 0 (0) | Colloidal chromic 32P | 1255 | CRT 18 (100) | NR | 16 (89) | 5.2 | 8† |
| Westlin et al., 199761 | 17 | 0 (0) | MAA and colloidal chromic 32P | 5000 (1390–19 000) | CHT 2 (12) | NR | 1 (6) | 7.6; T | 6 |
| Total percutaneous | 82 | 19 of 82 (23) | MAA and/or colloidal chromic 32P | NA | CHT 2 (2), CRT 65 (79) | 27 of 47 | 27 of 82 (33) | NA | NA |
| EUS | |||||||||
| Bhutani et al., 201960 | 9 | 0 (0) | Microparticle | 100 | CHT 9 (100) | NR | 24 (NR)‡ | NR | 4 |
| Ross et al., 202124 | 42 | 0 (0) | Microparticle | 100 | CHT 42 (100) | 1095 | 34 (81) | 15.5; Inc | 5 |
| Total EUS | 51 | 0 of 51 (0) | Microparticle | NA | CHT 51 (100) | 1095 of 42 | 34 of 42 (81) | NA | NA |
| Total | 133 | 19 of 133 (14.3) | MAA and/or colloidal chromic 32P or Microparticle | NA | CHT 53 (39.8), CRT 65 (48.9) | 1122 of 89 | 61 of 124 (49.2) | NA | NA |
Values are n (%) unless otherwise stated. *If the median was unavailable the mean is presented. †RCT. ‡n = number of complications. OS, overall survival; NOS, Newcastle–Ottawa scale; MAA, macroaggregated albumin; CRT, chemoradiotherapy; T, treatment; NR, not reported; CHT, chemotherapy; NA, not applicable; EUS, endoscopic ultrasonography; Inc, inclusion.
One study included 19 patients (40 per cent) with stage IV pancreatic cancer59. The remaining studies had no patients with metastases (total 14.3 per cent)24,58,60,61. 32P was only injected percutaneously with CT guidance and achieved a tumour dose between 1255 and 19 000 Gy58,59,61. This dose was a notably higher dose than the dose of 100 Gy achieved by the more recent microparticle brachytherapy utilizing EUS application24,60.
Four studies (124 patients) reported complications. Some 61 patients (49.2 per cent) suffered from greater than or equal to grade III complications. The most frequently reported complications included haematological toxicities24,58–60, gastrointestinal haemorrhage58,61, fatigue24,58, and nausea24,58. The median OS of all five studies ranged from 5.2 months58 to 15.5 months after inclusion24. The RCT by Rosemurgy et al.58 (2008) was abandoned at a preliminary stage after treating 18 of 40 intended patients with 32P, due to a statistically significant higher complication rate (P = 0.03) and lower survival (P = 0.18) in the 32P group. The authors also found the highest complication rate, with 75 greater than or equal to grade III complications in 16 of 18 patients (89 per cent)58, followed closely by Ross et al.24 (2021) with 139 greater than or equal to grade III complications in 34 of 42 patients (81 per cent). In contrast, Ross et al.24 (2021) did find the highest survival of 15.5 months in the intratumoral injection 32P microbrachytherapy treatment group.
Palladium-103 seed brachytherapy
An overview of the results of 103Pd seed brachytherapy is presented in Table 4. In 1996, two prospective studies applied 103Pd seed brachytherapy in 26 patients62,63.
Table 4.
Results of intratumoral palladium-103 seed brachytherapy for locally advanced pancreatic cancer
| Reference | n | Metastasis | Median* tumour dose (Gy) | Combination therapy | Overall complications, n | Complications ≥grade III | Median* survival (months); OS initiation | NOS 1–9 |
|---|---|---|---|---|---|---|---|---|
| Nori et al., 199662 | 15 | 0 (0) | 110 | CRT 15 (100) | NR | 0 (0) | 10; T | 5 |
| Raben et al., 199663 | 11 | 1 (9) | 124.4 | CHT 2 (18), CRT 5 (45) | 6 | 4 (36) | 6.9 | 7 |
| Total | 26 | 1 of 26 (4) | NA | CHT 2 (8), CRT 20 (77) | 6 of 26 | 4 of 26 (15) | NA | NA |
Values are n (%) unless otherwise stated. *If the median was unavailable the mean is presented. OS, overall survival; NOS, Newcastle–Ottawa scale; CRT, chemoradiotherapy; NR, not reported; T, treatment; CHT, chemotherapy; NA, not applicable.
From the included 26 patients, one patient suffered stage IV pancreatic cancer63. On average, all patients were submitted to a dose of 110–124.2 Gy after intraoperative implantation. Two patients also underwent complementary chemotherapy63 and 20 underwent chemoradiotherapy62,63. Four patients suffered greater than or equal to grade III complications, including duodenal perforation, sepsis, cerebral vascular accident, and radiation enteritis63. An OS was found of 6.9 months63 and 10 months62.
Immunotherapy
An overview of the results of intratumoral injection immunotherapy is presented in Table 5. Nine studies applied immunotherapy to 330 patients within a prospective design64–72, of which two were RCTs67,71. The two RCTs compared chemotherapy alone versus chemotherapy with an oncolytic virus67,71. The first RCT used TNFerade adenovirus with 5-fluorouracil chemoradiotherapy67 and the other one an H101 adenovirus for p53 activation with gemcitabine71.
Table 5.
Results of intratumoral immunotherapy for locally advanced pancreatic cancer
| Reference | n | Metastasis | Type of immunotherapy | Imaging | Combination therapy | Overall complications, n | Complications ≥grade III | Median* survival (months); OS initiation | NOS 1–9 |
|---|---|---|---|---|---|---|---|---|---|
| p53 activation pathway | |||||||||
| Gong et al., 201164 | 9 | NR | H101 adenovirus | EUS (100) | CHT 9 (100) | NR | NR | 7 | 7 |
| Xiao et al., 201171 | 19 | NR | H101 adenovirus | EUS (100) | CHT 19 (100) | NR | NR | 9 | 6† |
| Yunwei et al., 201072 | 8 | NR | H101 adenovirus | EUS (100) | CHT 8 (100) | NR | NR | 6 | 3 |
| Hecht et al., 200365 | 21 | 12 (57) | ONYX-015 adenovirus | EUS (100) | CHT 21 (100) | NR | 21 (100) | 7.5; T | 4 |
| Li et al., 201169 | 15 | 8 (53) | p53 adenovirus | Percutaneous ultrasonography (100) | CRT 15 (100) | 64 | 8 (53) | 13.8 | 7 |
| Total p53 activation pathway | 72 | 20 of 36 (56) | NA | EUS (79), Percutaneous ultrasonography (21) | CHT 57 (79), CRT 15 (21) | 64 of 15 | 29 of 36 (81) | NA | NA |
| Tumour necrosis factor-α pathway | |||||||||
| Hecht et al., 201266 | 50 | 0 (0) | TNFerade biologic | EUS (54), percutaneous (46) | CRT 50 (100) | NR | 65 (NR)‡ | 9.9; Inc | 8 |
| Herman et al., 201367 | 187 | 132 (70.6) | TNFerade biologic | EUS (50.8), Percutaneous, ultrasonography/CT (49.2) | CRT 187 (100.0) | 219 | 116 (NR)‡ | 10; R | 8† |
| Total tumour necrosis factor-α pathway | 237 | 132 of 237 (55.7) | NA | EUS (51.5), percutaneous ultrasonography/CT (48.5) | CRT 237 (100.0) | 219 of 187 | NR | NA | NA |
| Other immunotherapy | |||||||||
| Hirooka et al., 201868 | 15 | 0 (0) | Zoledronate-pulsed dendritic cells | EUS | CHT 15 (100) | 33 | 4 (27) | 11.5 | 8 |
| Nishimura et al., 201870 | 6 | 5 (83) | STNM01 oligonucleotide | EUS | None | 0 | 0 (0) | 5.8 | 5 |
| Total | 330 | 147 of 294 (50.0) | NA | NA | CHT 72 (21.8), CRT 252 (76.4) | 316 of 223 | 33 of 57 (57.9) | NA | NA |
Values are n (%) unless otherwise stated. *If the median was unavailable the mean is presented. †RCT. ‡n = number of complications. OS, overall survival; NOS, Newcastle–Ottawa scale; NR, not reported; EUS, endoscopic ultrasonography; CHT, chemotherapy; T, treatment; CRT, chemoradiotherapy; NA, not applicable; Inc, Inclusion; R, randomization.
Six studies reported metastases and half of the patients (147) had metastatic disease65–70. Five studies injected adenoviruses to increase p53 activation64,65,69,71,72. Two studies implanted TNFerade biologic, which enables tumour-specific delivery of TNF-α by radiation-inducible gene transfer66,67. One study injected zoledronate-pulsed dendritic cells combined with intravenous adoptive activated T lymphocytes to induce a CD8+ response68. Another study injected a double-stranded RNA oligonucleotide, called STNM01, to suppress a specific tumour growth factor (CHST15)70. In six studies the injection was guided by EUS64,65,68,70–72, in one study the injection was percutaneous69, and two studies used both methods66,67. From the 330 patients receiving immunotherapy, 72 patients (21.8 per cent) also underwent chemotherapy64,65,68,71,72 and 252 (76.4 per cent) underwent chemoradiotherapy66,67,69.
Four studies reported complication rates; 9 out of 36 patients (81 per cent) suffered greater than or equal to grade III complications after p53 adenovirus therapy65,69, four of 15 patients (27 per cent) suffered greater than or equal to grade III complications after zoledronate-pulsed dendritic cell injection68, and zero of 6 patients (0 per cent) suffered greater than or equal to grade III complications after STNM01 injection70. One study reported two cases of postprocedural mortality. One was caused by progressive disease and one was caused by a splenic artery thrombosis within 30 days post-intervention66. The most frequently presented complications included leucocytopenia65,67–69, severe pain66,67, fever64,65,67–69,71,72, gastrointestinal bleeding66,67, and intra-abdominal infection66,67. The median OS ranged between 5.8 months70 and 13.8 months69. In the first RCT, no significant difference in greater than or equal to grade II complications (P = 0.08) or OS (P = 0.26) was found between the TNFerade adenovirus injection combined with 5-fluorouracil chemoradiotherapy (greater than or equal to grade II complications 75.9 per cent, OS 10 months) and the chemoradiotherapy alone (greater than or equal to grade II complications 65.6 per cent, OS 10 months)67. The RCT applying H101 adenovirus for p53 activation did not report complications; this RCT found a significant difference (P = 0.004) in OS between the H101 adenovirus injection combined with gemcitabine (9 months) versus gemcitabine alone (6 months)71.
Intratumoral chemotherapy
An overview of the results of intratumoral injection chemotherapy is presented in Table 6. Four prospective studies performed intratumoral chemotherapy in 71 patients73–76. One RCT studied a chemotherapy capsule implant (5-fluorouracil) combined with systemic chemotherapy of gemcitabine versus systemic gemcitabine alone74.
Table 6.
Results of intratumoral chemotherapy for locally advanced pancreatic cancer
| Reference | n | Metastasis | Type of intratumoral CHT | Intratumoral CHT dose (mg), median* (range) | Combination therapy | Overall complications, n | Complications ≥grade III | Median* survival (months); OS initiation | NOS 1–9 |
|---|---|---|---|---|---|---|---|---|---|
| Levy et al., 201173 | 36 | 11 (30) | Gemcitabine | 90 (28–280) | CRT 36 (100) | 0 | 0 (0) | 9.3; T | 5 |
| Li et al., 201674 | 18 | NR | 5-Fluorouracil capsule | (800–1500) | CHT 18 (100) | NR | NR | 10.3 | 7† |
| Mohamadnejad et al., 201575 | 12 | 0 (0) | Gemcitabine | 168 (80–200) | CHT (NR), CRT (NR) | NR | 0 (0) | 9 | 8 |
| Yang et al., 201776 | 5 | 4 (80) | Gemcitabine | (400–600) | NR | 3 | 0 (0) | 16.2 | 7 |
| Cisplatin | (11.25–22.5) | ||||||||
| Total | 71 | 15 of 53 (28) | NA | NA | CHT 18 (33), CRT 36 (67) | 3 of 41 | 0 of 53 (0) | NA | NA |
Values are n (%) unless otherwise stated. *If the median was unavailable the mean is presented. †RCT. CHT, chemotherapy; OS, overall survival; NOS, Newcastle–Ottawa scale; CRT, chemoradiotherapy; T, treatment; NR, not reported; NA, not applicable.
Three studies reported patients with metastases (15 of 53 patients (28 per cent))73,75,76. Gemcitabine injection was guided by EUS73,75. One study analysed the intratumoral distribution of percutaneous injection by injecting 1–2 ml of radiopaque agent before injecting gemcitabine and cisplatin with fibrin glue76. Capsules incorporating 5-fluorouracil were implanted intraoperatively followed by fibrin gel to prevent pancreatic fistula74. Two studies, including 54 patients, reported 18 patients (33 per cent) with combined systemic chemotherapy and 36 (67 per cent) with chemoradiotherapy73,74.
Three studies reported no occurrence of greater than or equal to grade III complications73,75,76. OS ranged from 9 months75 to 16.2 months76. The RCT did not report complication rates and no significant difference (P = 0.07) was found in the survival between treatment with implanted 5-fluorouracil capsules combined with systemic gemcitabine (10.3 months) versus systemic gemcitabine alone (8.1 months)74.
Discussion
This systematic review reveals data on five types of intratumoral injection therapy with widely heterogeneous safety and survival outcomes in patients with LAPC.
125I brachytherapy, intratumoral chemotherapy, and 103Pd brachytherapy are associated with low rates of greater than or equal to grade III complications in the current literature review. In contrast, the complication rates of 32P brachytherapy and intratumoral immunotherapy were at least three-fold higher. Within the 32P and immunotherapy intervention groups, less complications seemed to be related to the injection procedure and more to the injected agents24,60,61,64,68,69. A common procedure-related complication was bacterial infection from the gastrointestinal tract into the pancreas, which was easily treated with antibiotics65. For immunotherapy, the method of injection (EUS or percutaneous) did not seem to influence complication rates65,66. An explanation for the high severe complication rate after immunotherapy is the triggered autoimmune response. After immunotherapy, intra-abdominal infection66,67 and fever64,65,67–69,71,72 were often observed. These are clear signs of an autoimmune response77. Current research into upcoming immunotherapies also attempts to identify and control these side effects78. The high complication rate after 32P microbrachytherapy is possibly due to a radiation overdose and therapy diffusion into healthy tissues58. Rosemurgy et al.58 (2008) reported therapy diffusion of 32P into nearby tissues and found a high complication rate (89 per cent), whereas Westlin et al.61 (1997) did not report therapy diffusion and found a much lower complication rate (6 per cent) with an exceptionally higher median tumour dose (1227 versus 11 050 Gy respectively). Ross et al.24 (2021) also found a high complication rate (81 per cent) after a median tumour dose of only 100 Gy (±20 per cent); however, they also claimed that only 8 of 139 (5.8 per cent) severe complications were 32P or procedure related, and reported that almost no therapy diffusion occurred outside the tumour. These heterogeneous results might suggest that, with therapy deposition central within the tumour, possibly with image guidance for improved treatment control and clear safety margins, microbrachytherapy could still prove to be a safe treatment method for LAPC.
The rate of severe complications after 125I brachytherapy, intratumoral chemotherapy, and 103Pd brachytherapy was below the complication rate of the most common ablative treatment for LAPC (radiofrequency ablation (RFA)). Rombouts et al.16 (2015) published a systematic review concerning ablative treatment methods for LAPC. In the RFA group an overall complication rate of 24.2 per cent was found, with a 13.6 per cent RFA-procedure-related complication rate16.
LAPC patients undergoing intratumoral injection therapy are generally also treated with systemic chemotherapy. Systemic chemotherapy is associated with side-effects, such as leucocytopenia and thrombocytopenia24,47,58–60,65,67–69,79. Even after modern chemotherapy regimens, such as FOLFIRINOX, complication rates range from 19.1–23.2 per cent80 up to 50 per cent11. Whether complications are related to intratumoral injection therapy or systemic chemotherapy can be difficult to identify. Overall, 11 cases of short-term postprocedural mortality were reported. Three RCTs compared complication rates of systemic chemotherapy combined with intratumoral injection therapy versus systemic chemotherapy alone. Two found no significant difference (125I and TNFerade)56,67 and one did find a significant difference with disadvantage towards 32P58. An RCT with modern chemotherapy regimens, with and without intratumoral injection therapy, should be the cornerstone to assess safety in this patient population.
The survival outcomes of the intratumoral injection modalities varied considerably between 5.0 and 16.2 months. No single intratumoral injection modality showed consistent high survival outcomes. Regarding survival outcome, Ross et al.24 (2021) showed the most promising results with a median OS of 15.5 months in 42 patients after receiving 32P microbrachytherapy. Considering the absence of a control group and, therefore, the high chance of selection bias, the benefit of 32P is still questionable24.
With regards to ablative treatment, Rombouts et al.16 (2015) found an OS of between 5.0 and 25.6 months in the RFA group. The highest survival of 25.6 months was found when RFA was combined with several different therapies, including intra-arterial plus systemic chemotherapy81. When RFA was applied as monotherapy, the median survival was 14.7 months. Still, evident selection bias was present81. More recent studies applying RFA for LAPC patients found a survival between 5.0 and 9.0 months with and without a combination of chemotherapy82,83. Overall, similar survival results are shown for most intratumoral therapies in this review.
Whether intratumoral injection therapy contributes to the survival of patients with LAPC remains questionable with the currently available literature. Due to the insidious onset and probable microscopic spread at the time of diagnosis, pancreatic cancer is essentially a systemic disease and local therapies may not contribute to survival36. Even if no metastases are found at the time of diagnosis, the disease may have already spread to the pancreatic surroundings. The OS results of the current review substantiate this theory by showing slightly improved survival after chemotherapy compared with no chemotherapy in the 125I group and similar survival between studies with and without metastatic disease69,76. The potential clinical benefit of local tumour therapy in patients with pancreatic cancer is not limited to survival. Local tumour response and local progression-free survival can be of great value for patients, especially when providing pain relief and improving and prolonging the performance score and quality of life.
The included studies have several limitations. Most studies were case series and cohort studies with small sample sizes. Selection bias in several forms hampers the quality of the studies, such as the type of LAPC classification guideline (NCCN or AJCC)17, local diagnostic and treatment protocols, additional diagnostic research, and prior treatment completion. To take selection bias into consideration, the NOS was applied.
To present a clear overview, many results had to be filtered or adjusted to fit certain classifications. Therefore, undetailed data were often excluded from the analysis.
Combination therapies have been categorized by the type of therapy (for example chemotherapy, chemoradiotherapy, or radiotherapy) and not by the technical aspects, start, duration, dose, and iteration of the cycles. Even though all studies, except one, used gemcitabine-based chemotherapy, the current movement towards FOLFIRINOX-based chemotherapy might have a radical impact on oncological outcomes soon. Metastases were present at different rates, locations, and quantities within each study. Additionally, studies were not excluded if metastatic disease was present or occurred in a minority of the included patients. A large variation in survival was seen, which could partly be explained by the moment from which survival was measured (for example the initial diagnosis, inclusion in the study, or the intervention); however, this was not consistently reported. Potential differences in lead time of several months may have had a great impact on OS differences.
Five intratumoral injection therapies are described and an overview is reported. Some intratumoral injection therapies for patients with LAPC seem safe, although 32P microbrachytherapy and immunotherapy require additional evidence. Currently available data on all modalities are insufficient to provide firm conclusions regarding the added value to survival. Clinical benefits of these procedures are potentially not limited to survival, but control of local tumour growth could be of great value for patients, especially when providing pain relief and improving quality of life. The potential advantage of intratumoral injection therapies complementary to conventional care should therefore be studied in well designed RCTs.
Supplementary Material
Contributor Information
Coen Ysbrand Willink, Department of Medical Imaging, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
Sjoerd Franciscus Maria Jenniskens, Department of Medical Imaging, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
Nienke Johanna Maria Klaassen, Department of Medical Imaging, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
Martijn Willem Jan Stommel, Department of Surgery, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
Johannes Frank Wilhelmus Nijsen, Department of Medical Imaging, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
Funding
No funding was made available specific for this research or publication. All authors are under contract at the institute mentioned above. All authors contribute to ongoing intratumoral holmium microbrachytherapy research.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data Availability
Research data can be made available on request.
References
- 1. Lippi G, Mattiuzzi C. The global burden of pancreatic cancer. Arch Med Sci 2020;16:820–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019;10:10–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Cancer Society . Cancer Facts and Figures 2020. cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf (accessed 29 June 2020)
- 4. Keane MG, Bramis K, Pereira SP, Fusai GK. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol 2014;20:2267–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30 [DOI] [PubMed] [Google Scholar]
- 6. Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SWet al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1028–1061 [DOI] [PubMed] [Google Scholar]
- 7. Verslype C, Van Cutsem E, Dicato M, Cascinu S, Cunningham D, Diaz-Rubio Eet al. The management of pancreatic cancer. Current expert opinion and recommendations derived from the 8th World Congress on Gastrointestinal Cancer, Barcelona, 2006. Ann Oncol 2007;18(Suppl 7):vii1–vii10 [DOI] [PubMed] [Google Scholar]
- 8. Dutch Federation of Medical Specialists (Federatie Medisch Specialisten) . Pancreas Carcinoma. richtlijnendatabase.nl/richtlijn/pancreascarcinoom/ (accessed 17 June 2020)
- 9. Gemenetzis G, Groot VP, Blair AB, Laheru DA, Zheng L, Narang AKet al. Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg 2019;270:340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brada LJH, Walma MS, Daamen LA, van Roessel S, van Dam RM, de Hingh IHet al. Predicting overall survival and resection in patients with locally advanced pancreatic cancer treated with FOLFIRINOX: development and internal validation of two nomograms. J Surg Oncol 2021;124:589–597 [DOI] [PubMed] [Google Scholar]
- 11. Rombouts SJ, Mungroop TH, Heilmann MN, van Laarhoven HW, Busch OR, Molenaar IQet al. FOLFIRINOX in locally advanced and metastatic pancreatic cancer: a single centre cohort study. J Cancer 2016;7:1861–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EAet al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016;17:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walma MS, Brada LJ, Patuleia SIS, Blomjous JG, Bollen TL, Bosscha Ket al. Treatment strategies and clinical outcomes in consecutive patients with locally advanced pancreatic cancer: a multicenter prospective cohort. Eur J Surg Oncol 2021;47:699–707 [DOI] [PubMed] [Google Scholar]
- 14. Chen YG, Pan HH, Dai MS, Lin C, Lu CS, Su SLet al. Impact of comorbidity and age on determinants therapeutic strategies in advanced pancreatic head cancer patients with obstructive jaundices. Medicine (Baltimore) 2015;94:e1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parmar AD, Vargas GM, Tamirisa NP, Sheffield KM, Riall TS. Trajectory of care and use of multimodality therapy in older patients with pancreatic adenocarcinoma. Surgery 2014;156:280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rombouts SJ, Vogel JA, van Santvoort HC, van Lienden KP, van Hillegersberg R, Busch ORet al. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br J Surg 2015;102:182–193 [DOI] [PubMed] [Google Scholar]
- 17. He J, Page AJ, Weiss M, Wolfgang CL, Herman JM, Pawlik TM. Management of borderline and locally advanced pancreatic cancer: where do we stand? World J Gastroenterol 2014;20:2255–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jusoh AC, Ammori BJ. Laparoscopic versus open distal pancreatectomy: a systematic review of comparative studies. Surg Endosc 2012;26:904–913 [DOI] [PubMed] [Google Scholar]
- 19. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPet al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Comprehensive Cancer Network (NCCN) . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Pancreatic Adenocarcinoma. Version 1.2022, 24 February 2022
- 21. SCImago . SJR — SCImago Journal & Country Rank. scimagojr.com (accessed 23 March 2020)
- 22. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford Ret al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247 [DOI] [PubMed] [Google Scholar]
- 24. Ross PJ, Wasan HS, Croagh D, Nikfarjam M, Nguyen N, Aghmesheh Met al. Results of a single-arm pilot study of 32P microparticles in unresectable locally advanced pancreatic adenocarcinoma with gemcitabine/nab-paclitaxel or FOLFIRINOX chemotherapy. ESMO Open 2021;7:100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen C, Wang W, Wang Y, Yu Z, Li Y. Locally advanced pancreatic carcinoma with jaundice: the benefit of a sequential treatment with stenting followed by CT-guided 125I seeds implantation. Eur Radiol 2021;31:6500–6510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chi Z, Chen L, Huang J, Jiang N, Zheng Q, Huang Net al. A novel combination of percutaneous stenting with iodine-125 seed implantation and chemotherapy for the treatment of pancreatic head cancer with obstructive jaundice. Brachytherapy 2021;20:218–225 [DOI] [PubMed] [Google Scholar]
- 27. Goertz SR, Ali MM, Parker GA. Local management of pancreatic carcinoma: iodine-125 implantation. Clin Oncol (R Coll Radiol) 1990;2:22–26 [DOI] [PubMed] [Google Scholar]
- 28. Li CG, Zhou ZP, Jia YZ, Tan XL, Song YY. Radioactive 125I seed implantation for locally advanced pancreatic cancer: a retrospective analysis of 50 cases. World J Clin Cases 2020;8:3743–3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li W, Wang X, Wang Z, Zhang T, Cai F, Tang Pet al. The role of seed implantation in patients with unresectable pancreatic carcinoma after relief of obstructive jaundice using ERCP. Brachytherapy 2020;19:97–103 [DOI] [PubMed] [Google Scholar]
- 30. Li YF, Liu ZQ, Zhang YS, Dong LM, Wang CY, Gou SMet al. Implantation of radioactive 125I seeds improves the prognosis of locally advanced pancreatic cancer patients: a retrospective study. J Huazhong Univ Sci Technolog Med Sci 2016;36:205–210 [DOI] [PubMed] [Google Scholar]
- 31. Liu K, Ji B, Zhang W, Liu S, Wang Y, Liu Y. Comparison of iodine-125 seed implantation and pancreaticoduodenectomy in the treatment of pancreatic cancer. Int J Med Sci 2014;11:893–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luo M, Zhang F. CT-guided 125I brachytherapy combined with transarterial infusion for the treatment of unresectable or locally advanced pancreatic carcinoma. Brachytherapy 2019;18(Suppl):S83–SS4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mohiuddin M, Rosato F, Schuricht A, Barbot D, Biermann W, Cantor R. Carcinoma of the pancreas–the Jefferson experience 1975-1988. Eur J Surg Oncol 1994;20:13–20 [PubMed] [Google Scholar]
- 34. Morrow M, Hilaris B, Brennan MF. Comparison of conventional surgical resection, radioactive implantation, and bypass procedures for exocrine carcinoma of the pancreas 1975–1980. Ann Surg 1984;199:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peretz T, Nori D, Hilaris B, Manolatos S, Linares L, Harrison Let al. Treatment of primary unresectable carcinoma of the pancreas with I-125 implantation. Int J Radiat Oncol Biol Phys 1989;17:931–935 [DOI] [PubMed] [Google Scholar]
- 36. Schuricht AL, Barbot DJ, Mohiuddin M, Rosato FE. Adenocarcinoma of the pancreas: a multimodality approach–a single surgeon's experience (1979–1988). J Surg Oncol 1991;48:56–61 [DOI] [PubMed] [Google Scholar]
- 37. Sun X, Lu Z, Wu Y, Min M, Bi Y, Shen Wet al. An endoscopic ultrasonography-guided interstitial brachytherapy based special treatment-planning system for unresectable pancreatic cancer. Oncotarget 2017;8:79099–79110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whittington R, Solin L, Mohiuddin M, Cantor RI, Rosato FE, Biermann WAet al. Multimodality therapy of localized unresectable pancreatic adenocarcinoma. Cancer 1984;54:1991–1998 [DOI] [PubMed] [Google Scholar]
- 39. Zhou S, Zhu C, Chen SL, Li JA, Qu KL, Jing Het al. 125I intracavitary irradiation combined with 125I seeds implantation for treatment of locally advanced pancreatic head cancer: a retrospective analysis of 67 cases. Int J Gen Med 2021;14:2645–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dobelbower RR Jr, Merrick HW 3rd, Ahuja RK, Skeel RT. 125I interstitial implant, precision high-dose external beam therapy, and 5-FU for unresectable adenocarcinoma of pancreas and extrahepatic biliary tree. Cancer 1986;58:2185–2195 [DOI] [PubMed] [Google Scholar]
- 41. Du Y, Jin Z, Meng H, Zou D, Chen J, Liu Yet al. Long-term effect of gemcitabine-combined endoscopic ultrasonography-guided brachytherapy in pancreatic cancer. J Interv Gastroenterol 2013;3:18–24 [Google Scholar]
- 42. Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy 2008;40:314–320 [DOI] [PubMed] [Google Scholar]
- 43. Joyce F, Burcharth F, Holm HH, Stroyer I. Ultrasonically guided percutaneous implantation of iodine-125 seeds in pancreatic carcinoma. Int J Radiat Oncol Biol Phys 1990;19:1049–1052 [DOI] [PubMed] [Google Scholar]
- 44. Montemaggi P, Dobelbower R, Crucitti F, Caracciolo F, Morganti AG, Smaniotto Det al. Interstitial brachytherapy for pancreatic cancer: report of seven cases treated with 125I and a review of the literature. Int J Radiat Oncol Biol Phys 1991;21:451–457 [DOI] [PubMed] [Google Scholar]
- 45. Niu L. Combination of iodine-125 seed implantation with cryosurgery for locally advanced pancreatic carcinoma. Brachytherapy 2016;15(Suppl 1):S141 [Google Scholar]
- 46. Shipley WU, Nardi GL, Cohen AM, Ling CC. Iodine-125 implant and external beam irradiation in patients with localized pancreatic carcinoma: a comparative study to surgical resection. Cancer 1980;45:709–714 [DOI] [PubMed] [Google Scholar]
- 47. Sun S, Xu H, Xin J, Liu J, Guo Q, Li S. Endoscopic ultrasound-guided interstitial brachytherapy of unresectable pancreatic cancer: results of a pilot trial. Endoscopy 2006;38:399–403 [DOI] [PubMed] [Google Scholar]
- 48. Syed AM, Puthawala AA, Neblett DL. Interstitial iodine-125 implant in the management of unresectable pancreatic carcinoma. Cancer 1983;52:808–813 [DOI] [PubMed] [Google Scholar]
- 49. Wang B. Individualized 3D-printing coplanar template-assisted iodine-125 seed implantation for inoperable pancreatic cancer. Brachytherapy 2021;20(Suppl):S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang H, Junjie W, Yuliang J, Jinna L, Suqing T, Weiqiang Ret al. Prognostic factors for intraoperative 125I seeds implantation for treatment of locally-advanced pancreatic carcinoma. Int J Radiat Oncol Biol Phys 2013;87(Suppl):S310 [Google Scholar]
- 51. Wang J, Li J, Tian S, Jiang Y, Ran W, Xiu D. Intraoperative ultrasound-guided 125I implantation in the treatment of unresectable pancreatic carcinoma. Brachytherapy 2011;10(Suppl):S70 [Google Scholar]
- 52. Wang W, Wang Y, Li Y. CT-guided iodine-125 seeds implantation combined with chemotherapy for locally advanced pancreatic carcinoma. Brachytherapy 2017;16(Suppl):S47 [Google Scholar]
- 53. Yang M, Yan Z, Luo J, Liu Q, Zhang W, Ma Jet al. A pilot study of intraluminal brachytherapy using 125I seed strand for locally advanced pancreatic ductal adenocarcinoma with obstructive jaundice. Brachytherapy 2016;15:859–864 [DOI] [PubMed] [Google Scholar]
- 54. Zheng Z, Xu Y, Zhang S, Pu G, Cui C. Surgical bypass and permanent iodine-125 seed implantation vs. surgical bypass for the treatment of pancreatic head cancer. Oncol Lett 2017;14:2838–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhongmin W, Yu L, Fenju L, Kemin C, Gang H. Clinical efficacy of CT-guided iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. Eur Radiol 2010;20:1786–1791 [DOI] [PubMed] [Google Scholar]
- 56. Lun J-J, Zhao J-L, Sun J-Y, Hu X-K, Yin H-Z. CT-guided 125I radioactive seed interstitial implantation combined with chemotherapy for advanced pancreatic carcinoma: analysis of therapeutic efficacy. J Interventional Radiol (China) 2015;24:494–497 [Google Scholar]
- 57. Heintz BH, Wallace RE, Hevezi JM. Comparison of I-125 sources used for permanent interstitial implants. Med Phys 2001;28:671–682 [DOI] [PubMed] [Google Scholar]
- 58. Rosemurgy A, Luzardo G, Cooper J, Bowers C, Zervos E, Bloomston Met al. 32P as an adjunct to standard therapy for locally advanced unresectable pancreatic cancer: a randomized trial. J Gastrointest Surg 2008;12:682–688 [DOI] [PubMed] [Google Scholar]
- 59. Order SE, Siegel JA, Principato R, Zeiger LE, Johnson E, Lang Pet al. Selective tumor irradiation by infusional brachytherapy in nonresectable pancreatic cancer: a phase I study. Int J Radiat Oncol Biol Phys 1996;36:1117–1126 [DOI] [PubMed] [Google Scholar]
- 60. Bhutani MS, Klapman JB, Tuli R, El-Haddad GE, Hoffe S, Wong FCLet al. OncoPaC-1: an open-label, single-arm pilot study of phosphorus-32 microparticles brachytherapy in combination with gemcitabine +/- nab-paclitaxel in unresectable locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2019;105(Suppl):E236–E237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Westlin JE, Andersson-Forsman C, Garske U, Linne T, Aas M, Glimelius Bet al. Objective responses after fractionated infusional brachytherapy of unresectable pancreatic adenocarcinomas. Cancer 1997;80:2743–2748 [DOI] [PubMed] [Google Scholar]
- 62. Nori D, Merimsky O, Osian AD, Heffernan M, Cortes E, Turner JW. Palladium-103: a new radioactive source in the treatment of unresectable carcinoma of the pancreas: a phase I-II study. J Surg Oncol 1996;61:300–305 [DOI] [PubMed] [Google Scholar]
- 63. Raben A, Mychalczak B, Brennan MF, Minsky B, Anderson L, Casper ESet al. Feasibility study of the treatment of primary unresectable carcinoma of the pancreas with 103Pd brachytherapy. Int J Radiat Oncol Biol Phys 1996;35:351–356 [DOI] [PubMed] [Google Scholar]
- 64. Gong T, Zhu Q, Zhang Y, Xu K, Chen X, Wu Jet al. Study on EUS guided oncolytic adenovirus implantation in patients with unresectable pancreatic cancer. Digestion 2011;83:235 [Google Scholar]
- 65. Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RMet al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res 2003;9:555–561 [PubMed] [Google Scholar]
- 66. Hecht JR, Farrell JJ, Senzer N, Nemunaitis J, Rosemurgy A, Chung Tet al. EUS or percutaneously guided intratumoral TNFerade biologic with 5-fluorouracil and radiotherapy for first-line treatment of locally advanced pancreatic cancer: a phase I/II study. Gastrointest Endosc 2012;75:332–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Herman JM, Wild AT, Wang H, Tran PT, Chang KJ, Taylor GEet al. Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J Clin Oncol 2013;31:886–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hirooka Y, Kawashima H, Ohno E, Ishikawa T, Kamigaki T, Goto Set al. Comprehensive immunotherapy combined with intratumoral injection of zoledronate-pulsed dendritic cells, intravenous adoptive activated T lymphocyte and gemcitabine in unresectable locally advanced pancreatic carcinoma: a phase I/II trial. Oncotarget 2018;9:2838–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li JL, Cai Y, Zhang SW, Xiao SW, Li XF, Duan YJet al. Combination of recombinant adenovirus-p53 with radiochemotherapy in unresectable pancreatic carcinoma. Chin J Cancer Res 2011;23:194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nishimura M, Matsukawa M, Fujii Y, Matsuda Y, Arai T, Ochiai Yet al. Effects of EUS-guided intratumoral injection of oligonucleotide STNM01 on tumor growth, histology, and overall survival in patients with unresectable pancreatic cancer. Gastrointest Endosc 2018;87:1126–1131 [DOI] [PubMed] [Google Scholar]
- 71. Xiao B, Jin ZD, Du YQ, Wu RP, Li ZS. Intratumoral injection of E1B gene-deleted adenovirus combined with intravenous gemcitabine in treating unresectable pancreatic carcinoma. J Gastroenterol Hepatol 2011;26:12–13 [Google Scholar]
- 72. Yunwei S, Qi Z, Kai X, Xi C, Lu X, Ji-Hong T. Preliminary studies of EUS guided oncolytic adenovirus implantation combined with chemotherapy in patients of non-operative pancreatic cancer. Digestion 2010;81:166 [Google Scholar]
- 73. Levy MJ, Alberts SR, Chari ST, Farnell MB, Haddock MG, Kendrick MLet al. EUS guided intra-tumoral gemcitabine therapy for locally advanced and metastatic pancreatic cancer. Gastrointest Endosc 2011;73(Suppl):AB144–AB145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li JQ, Yang JC, Liang JX, Wang SL. Pharmacokinetic study and clinical evaluation of a slow-release 5-fluorouracil implant in pancreatic cancer patients. Anticancer Drugs 2016;27:60–65 [DOI] [PubMed] [Google Scholar]
- 75. Mohamadnejad M, Zamani F, Setareh M, Nikfam S, Malekzadeh R. EUS-guided intratumoral gemcitabine injection in locally advanced non-metastatic pancreatic cancer. Gastrointest Endosc 2015;81(Suppl):AB440–AB441 [Google Scholar]
- 76. Yang B, He JP, Yuan ML, Li W, Jiao H, You Xet al. Percutaneous intratumoral injection of gemcitabine plus cisplatin mixed with fibrin glue for advanced pancreatic carcinoma: case report. Medicine (Baltimore) 2017;96:e8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Barber FD. Adverse events of oncologic immunotherapy and their management. Asia Pac J Oncol Nurs 2019;6:212–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. National Cancer Institute . New Drugs, New Side Effects: Complications of Cancer Immunotherapy. cancer.gov/news-events/cancer-currents-blog/2019/cancer-immunotherapy-investigating-side-effects (accessed 11 August 2020)
- 79. Moysan E, Bastiat G, Benoit JP. Gemcitabine versus modified gemcitabine: a review of several promising chemical modifications. Mol Pharm 2013;10:430–444 [DOI] [PubMed] [Google Scholar]
- 80. Rombouts SJ, Walma MS, Vogel JA, van Rijssen LB, Wilmink JW, Mohammad NHet al. Systematic review of resection rates and clinical outcomes after FOLFIRINOX-based treatment in patients with locally advanced pancreatic cancer. Ann Surg Oncol 2016;23:4352–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cantore M, Girelli R, Mambrini A, Frigerio I, Boz G, Salvia Ret al. Combined modality treatment for patients with locally advanced pancreatic adenocarcinoma. Br J Surg 2012;99:1083–1088 [DOI] [PubMed] [Google Scholar]
- 82. Fegrachi S, Walma MS, de Vries JJJ, van Santvoort HC, Besselink MG, von Asmuth EGet al. Safety of radiofrequency ablation in patients with locally advanced, unresectable pancreatic cancer: a phase II study. Eur J Surg Oncol 2019;45:2166–2172 [DOI] [PubMed] [Google Scholar]
- 83. He C, Wang J, Zhang Y, Cai Z, Lin X, Li S. Comparison of combination therapies in the management of locally advanced pancreatic cancer: induction chemotherapy followed by irreversible electroporation vs radiofrequency ablation. Cancer Med 2020;9:4699–4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data can be made available on request.