Abstract
Purpose
The purpose of this study was to evaluate clinical reports of response-loss in patients with neovascular eye diseases, such as neovascular age-related macular degeneration (AMD) and diabetic macular edema (DME), after repeated anti-vascular endothelial growth factor (VEGF) therapy. To assess experimental evidence of associations of other angiogenic growth factors and endothelial glycolytic pathways with the diseases and to propose the underlying mechanisms.
Methods
Review of published clinical studies and experimental investigations.
Results
Intravitreal injection of anti-VEGF biologic drugs (e.g. bevacizumab, ranibizumab, and aflibercept) is the front-line treatment for neovascular AMD and DME, and acts by halting the progression of excess blood vessel growth and leakage. Despite favorable clinical results, exudation returns in a number of patients after repeated administrations over time. Patients suffering from disease recurrence may have developed an acquired resistance to anti-VEGF therapy. We have analyzed clinical and preclinical findings on changes to angiogenic signaling pathways following VEGF-targeted treatment and hypothesize that switching to alternative pathways could potentially bypass VEGF blockade, accounting for development of resistance to anti-VEGF therapy. We have also discussed potential reprogramming of ocular endothelial glycolysis in response to VEGF antagonism and proposed that metabolic adaptations could impair blood-retinal barrier function, counteracting the clinical efficacy of VEGF-targeted therapies and contributing to a decline of response to them.
Conclusions
Future studies of the mechanisms proposed in this review may shed some light on how these adaptations result in the development of acquired resistance to anti-VEGF therapy, which should help discover new therapeutic strategies for overcoming anti-VEGF resistance and improving clinical efficacy.
Keywords: angiogenesis, anti-vascular endothelial growth factor (VEGF), age-related macular degeneration (AMD), proliferative diabetic retinopathy (PDR), diabetic macular edema (DME)
Age-related macular degeneration (AMD) is a leading cause of blindness within the elderly population and diabetic retinopathy (DR) is a primary cause of blindness in the working-age population.1,2 The growth of elderly, obese, and diabetic populations has resulted in increased prevalence of AMD and DR, both of which are predicted to continue to increase, representing significant challenges for global public health.3 AMD is recognized as a multifactorial disease caused by combined multiple factors of aging, genetic variants, and environment, as well as lifestyle (including ethnicity, dietary habits, and cigarette smoking), whereas diabetes-related hyperglycemia is the key promoter for the development and progression of DR. Despite the differences in their pathophysiological manifestations, both disease states are primarily driven by the aberrant expression of vascular endothelial growth factor (VEGF) upregulated mainly through hypoxia-inducible factor-1 in response to retinal ischemic hypoxia, activating angiogenesis, and increasing vascular permeability.4,5 In the case of AMD, drusen deposits develop between the retinal pigment epithelium (RPE) and the choroid, causing damage to RPE cells through ischemia and hypoxia. This progresses to the angiogenic late-stage of the disease, termed neovascular (or wet) AMD, when hypoxia triggers overexpression and release of VEGF causing aberrant neovascularization of the choroidal blood vessels and increased vascular permeability.6,7 Untreated neovascular AMD can lead to loss of central visual acuity and can rapidly progress over weeks or months.8
DR is the most severe ocular complication of diabetes mellitus. Diabetic macular edema (DME) is the most common cause of visual impairment in patients with DR and primarily manifests when hyperpermeable retinal blood vessels leak into the macula area.9,10 Diabetes and the resulting hyperglycemia progressively affect retinal endothelial cell (EC) metabolism to induce detrimental oxidative stress and cause EC dysfunction with diminished inner retinal microcirculation, resulting in retinal ischemic hypoxia (see Dysregulation of EC Glucose Metabolism in DR). The hyperglycemia-induced ischemia present in DR is considered a major contributing factor to the upregulation of VEGF, which in turn plays a central role in the development of DME and proliferative diabetic retinopathy (PDR). VEGF elicits aberrant angiogenesis of the retinal vasculature (the hallmark of PDR) and disrupts the blood-retinal barrier (the hallmark of DME) to cause vascular leakage, resulting in loss of vision. Thus, VEGF-targeted therapies are a primary treatment option for such neovascular eye diseases (NVEDs). Anti-VEGF biological therapies, such as ranibizumab and bevacizumab (discussed below), directly target ocular VEGF-A in patients with neovascular AMD and DME.
Alongside VEGF-A, other members of the VEGF family include VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF). VEGF-A (referred to hereafter as VEGF), plays a pivotal role in angiogenesis. Multiple VEGF isoforms, including VEGF121, VEGF145, VEGF165, VEGF189, and VEGF206, result from alternative splicing of mRNA from a single VEGF gene. VEGF165 is the most widely expressed VEGF isoform in tissues, with a crucial role in pathological angiogenesis.11 Despite the wide-scale use of anti-VEGF biologics, long-term clinical data suggests that VEGF-targeted therapy can be limited by the return of visual decline after repeated administrations over time, indicating a loss of efficacy following an initial response.12,13 Patients suffering from disease recurrence may have acquired resistance to anti-VEGF therapy, the mechanisms of which are poorly understood. In addition, up to 30% of sufferers of NVEDs do not respond at all to anti-VEGF therapy.10,14
This review aims to provide an overview of clinical observations and experimental evidence on the role of alternative angiogenic pathways which could account for development of resistance to VEGF-targeted treatment, with particular focus on VEGF-independent pathways mediated through neuropilin-1 (NRP-1). The review also will discuss potential perturbation of endothelial glucose metabolism after anti-VEGF therapy and propose how these adaptation mechanisms potentially bypass VEGF blockade and promote resistance to the VEGF inhibitors responsible for recurrence of NVEDs.
Anti-VEGF Therapy in NVEDs
Numerous clinical trials have been conducted to determine the clinical effectiveness of anti-VEGF therapies in neovascular AMD and DME. In particular, landmark studies over recent years have demonstrated improvements in visual outcomes after treatment (Table 1).
Table 1.
Summary of Landmark Clinical Trials Which Pertained to Approval of Anti-VEGF Biologic Therapies
| Author(s) | Year | Trial Name | Treatment | Against | Test Period | Major Findings |
|---|---|---|---|---|---|---|
| Rosenfeld et al.15 | 2006 | MARINA | Ranibizumab (0.3 mg or 0.5 mg) | Sham | 24 mo | Ranibizumab increased VA with low rates of ocular adverse events in patients with AMD compared to sham injections |
| Brown et al.16 | 2006 | ANCHOR | Ranibizumab (0.3 mg or 0.5 mg) | Verteporfin | 24 mo | Ranibizumab increased VA with low rates of ocular adverse events in patients with AMD compared to verteporfin |
| Nguyen et al.25 | 2010 | READ-2 | Ranibizumab (0.5 mg) | Laser PCG | 24 mo | Ranibizumab alone improved BCVA to a greater extent than laser alone or combination therapy in DME |
| Martin et al.19 | 2012 | CATT | Bevacizumab (1.25 mg) | Ranibizumab (0.5 mg) | 24 mo | Bevacizumab and ranibizumab elicited similar effects on visual acuity in patients with AMD over 2 y |
| Heier et al.21 | 2012 | VIEW 1 and VIEW 2 | Aflibercept (0.5 mg) | Ranibizumab (0.5 mg) | 12 mo | Intravitreal aflibercept elicited similar efficacy and safety outcomes as ranibizumab in patients with AMD |
| Brown et al.26 | 2013 | RISE and RIDE | Ranibizumab (0.3 mg) | Ranibizumab (0.5 mg) | 36 mo | 0.3 mg and 0.5 mg doses of ranibizumab in DME had similar efficacies and improve VA over a 36-mo period |
| Korobelnik et al.22 | 2014 | VIVID and VISTA | Aflibercept | Laser PCG | 12 mo | Intravitreal aflibercept demonstrated superior visual outcomes compared to laser therapy in DME |
| Dugel et al.24 | 2020 | HAWK and HARRIER | Brolucizumab (3 mg or 6 mg) | Aflibercept (2 mg) | 48 wk | Brolucizumab showed similar efficacy to aflibercept in patients with AMD in a 12-mo period |
| Heier et al.27 | 2022 | TENAYA and LUCERNE | Faricimab (6 mg) | Aflibercept (2 mg) | 48 wk | Faricimab every 16 weeks was non-inferior to aflibercept every 8 weeks in patients with AMD |
| Wykoff et al.28 | 2022 | YOSEMITE and RHINE | Faricimab (6 mg) | Aflibercept (2 mg) | 12 mo | Faricimab every 8 weeks or up to every 16 weeks was non-inferior to aflibercept every 8 weeks in patients with DME |
VA, visual acuity; PCG, photocoagulation; BCVA, best corrected visual acuity.
VEGF-targeted biological medicines include monoclonal antibodies against VEGF and decoy receptors comprising modified VEGF receptor extracellular domains. Ranibizumab (sold as Lucentis, developed by Genentech/Novartis) is a recombinant humanized monoclonal antibody Fab fragment targeted against VEGF created from the same parental mouse antibody as bevacizumab (discussed below). Approved in 2006 for the treatment of neovascular AMD and in 2012 for the treatment of DME (see Table 1),15,16 ranibizumab inhibits angiogenesis through binding with high affinity to all VEGF isoforms to prevent activation of VEGF receptor-1 (VEGFR1) and VEGF receptor-2 (VEGFR2), located on the surface of endothelial cells.17 Bevacizumab (Avastin, developed by Genentech/Roche) is a recombinant humanized full-length monoclonal antibody which, similarly to ranibizumab, bind all isoforms of VEGF with high affinity, preventing VEGF receptor binding. Approved by the US Food and Drug Administration (FDA) in 2004 for the first-line treatment of metastatic colorectal cancer, current therapeutic indications include a wide range of cancer types.18 Despite the absence of formal approval for the use of bevacizumab in ocular diseases, it is still widely used as an off-label treatment for neovascular AMD due to its cost-effectiveness in comparison with ranibizumab.19,20
Aflibercept (Eyelea/VEGF-TRAP, developed by Regeneron) is a recombinant fusion protein, which combines the extracellular immunoglobulin-like (Ig) domain 2 of VEGFR1 and the extracellular Ig domain 3 of VEGFR2 fused to the Fc portion of human IgG1. It acts as a soluble decoy VEGF receptor and was approved in 2011 for the treatment of AMD and in 2014 for the treatment of DME.21,22 Similar to bevacizumab and ranibizumab, aflibercept binds to multiple VEGF isoforms but with comparatively higher affinity. Having the second Ig domain of human VEGFR1, aflibercept also binds the VEGFR1 ligands VEGF-B and PlGF.23 Brolucizumab (Beovu, developed by Novartis) is recently developed anti-VEGF biologic treatment, which was approved for wet AMD treatment in 2019 after showing promising results in the HAWK and HARRIER clinical trials.24 It is a humanized single-chain antibody fragment, which neutralizes all isoforms of VEGF. As the emerging Angiopoietin-2/Tie-2 pathway has been identified to play an important and complementary role alongside VEGF in NVEDs, faricimab (Vabysmo, developed by Roche), the first bispecific monoclonal antibody to target both VEGF and angiopoietin-2, has very recently demonstrated vision benefits at an extended treatment interval (every 16 weeks) comparable with VEGF pathway inhibition alone with aflibercept given at 8-week intervals for neovascular AMD and DME, thereby reducing treatment burden in patients.27,28 A summary of ocular anti-VEGF treatments is presented in Table 2.
Table 2.
Summary of Anti-VEGF Biologic Therapies
| INN | Trade Name | Molecular Weight | KD for VEGF165 | Indication |
|---|---|---|---|---|
| Bevacizumab | Avastin | 149 kDa | 58 pM | Choroidal neovascularization (in AMD and other - off-label); diabetic macular edema (off-label); central retinal vein occlusion (off-label) |
| Ranibizumab | Lucentis | 48 kDa | 46 pM | Choroidal neovascularization (in AMD and other); diabetic macular edema; macular edema secondary to retinal vein occlusion |
| Aflibercept | Eyelea/VEGF-TRAP | 115 kDa | 0.49 pM | Neovascular AMD; diabetic macular edema; macular edema secondary to retinal vein occlusion; myopic choroidal neovascularization |
| Brolucizumab | Beovu | 26 kDa | 28.4 pM | Neovascular AMD |
| Faricimab | Vabysmo | 150 kDa | 3.5 nM (22 nM for Ang-2) | Neovascular AMD; diabetic macular edema |
INN, international non-proprietary name; Ang-2, angiopoietin-2.
Combined clinical trial data has suggested that around 30% of patients with DME are nonresponsive to intravitreal anti-VEGF treatment.10,14 Similarly, in patients with AMD, the CATT study revealed that even after 2 years of treatment, 67.4% of patients treated with bevacizumab and 51.5% of patients treated with ranibizumab showed persistent retinal fluid accumulation.19 Although there is currently no consensus on the categorization of response status to anti-VEGF therapy, patients manifesting persistent or increased retinal exudation and no improvement in visual acuity despite four or six monthly consecutive injections have been described as nonresponsive patients or nonresponders,5,10 suggesting that other pathological mechanisms are largely involved in the multifactorial disease. Collectively, these findings draw attention to the presence of significant innate resistance to anti-VEGF therapy, highlighting the need for further research elucidating the underlying mechanisms of disease in nonresponsive patients.
Furthermore, these studies show that intravitreal administration of bevacizumab to patients with neovascular AMD results in a progressive decrease in therapeutic and biological responses to treatment over time, a phenomenon that is not counteracted by increased treatment dosage.29–31 Similarly, 17% to 56% of patients with PDR saw recurrence of vitreous hemorrhage after initial intravitreal bevacizumab treatment32,33; however, the repeated treatment effects of bevacizumab in patients with PDR remains unclear. A number of clinical studies have also described the recurrence of DME after intravitreal bevacizumab injection.34,35 The response-loss (known as tachyphylaxis) refers to the phenomenon whereby some patients who had a good initial response with the resolution of exudation after injections of anti-VEGF drug, then developed new fluid after repeated administration over time and became resistant to further treatment.5,13
Retrospective studies of intravitreal ranibizumab treatment in patients with neovascular AMD showed recurrence in 66% to 76% of patients after 12 months of repeated treatment and in 74.8% of patients after 24 months of treatment.36,37 Gasperini et al.38 reported that patients with AMD with choroidal neovascularization following repeated administration of either ranibizumab or bevacizumab over time developed a diminished therapeutic response; both ranibizumab and bevacizumab showed attenuation of efficacy after an average of five and seven injections, respectively. Interestingly, however, switching from one treatment to the other after resistance occurrence largely elicited restoration of therapeutic effect in the majority of eyes. Eleven percent to 31% of patients with PDR had pathophysiological recurrence after initial intravitreal ranibizumab treatment.39,40 However, the long-term effects of repeated ranibizumab treatments in PDR recurrence requires further investigation. Recent studies have also reported disease recurrence in 9% to 55% of patients with neovascular AMD treated with repeated intravitreal aflibercept injections,13,41 demonstrating an acquired resistance to aflibercept-based anti-VEGF therapy. It was noted by Hara et al.13 that occult with no classic type and polypoidal choroidal vasculopathy (lesions beneath the RPE and no intraretinal edema) were the only AMD subtypes that developed the resistance (tachyphylaxis) to aflibercept. The reason for this is unclear, and the intravitreal aflibercept achieved the initial resolution in these two subtypes, suggesting the access of aflibercept into lesions of choroidal neovascularization beneath the RPE. They also found similar percentages of the RPE detachment, a common manifestation of AMD, in eyes without and with tachyphylaxis (30% vs. 32%),13 whereas the lack of intraretinal edema was only observed in eyes with tachyphylaxis. Perhaps, the absence of intraretinal edema indicates less disruption of the RPE-mediated outer blood-retinal barrier, which could limit penetration of aflibercept to lesions beneath the RPE, contributing to the loss of response and the development of resistance.
Overall, although many patients with NVEDs benefit from anti-VEGF treatment, a significant proportion see no effect of treatment, and most responding patients experience recurrence of disease over time. Although studies have endeavored to characterize patients who either lack an anti-VEGF response or experience decline in response over time,5 the mechanisms behind both the failure to respond and decreased responsiveness remains unclear. Whereas there is a potential genetic component to innate anti-VEGF resistance,42,43 the mechanisms of acquired resistance are largely unknown.
Despite this, some pathways have been hypothesized to participate in disease recurrence after anti-VEGF treatment. Neuropilin-1 (NRP-1), a transmembrane glycoprotein implicated in neuronal development, angiogenesis, and immune regulation, is a co-receptor for VEGF and several other cytokines. A number of NRP-1 ligands have been highlighted as potential candidates through which recurrence of NVEDs may occur after anti-VEGF treatment (see Role of NRP-1 and NRP-1-binding Cytokines in NVEDs). Further to this, alterations in the glycolytic pathway also has the potential to elicit some of the pathological recurrences of NVEDs after anti-VEGF treatment (see Endothelial Glycolysis and its Potential Role in NVED Pathology and Recurrence).
Role of NRP-1 and NRP-1-Binding Cytokines in NVEDs
NRP-1
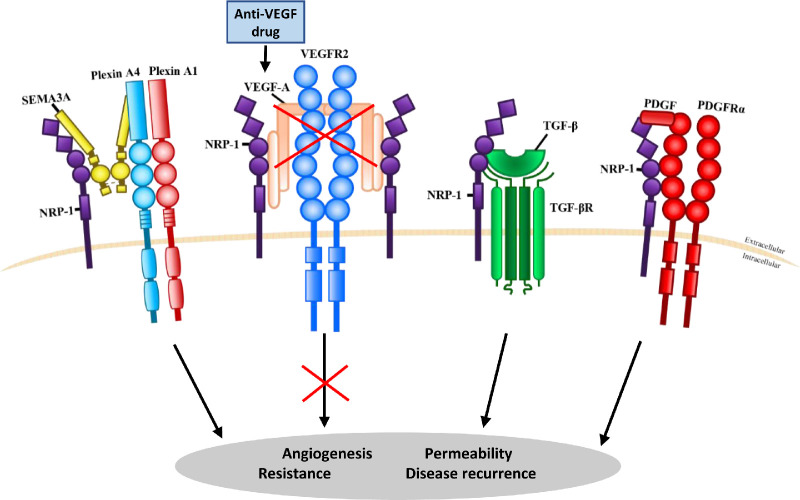
NRP-1 is a receptor for class 3 semaphorins, such as semaphorin 3A (SEMA3A), as well as a co-receptor for a variety of ligands including VEGF, PlGF, transforming growth factor-βs (TGF-βs), and platelet-derived growth factor (PDGF),44,45 as illustrated in Figure 1. By forming a co-receptor complex with VEGFR2, NRP-1 facilitates optimal VEGF signaling via VEGFR2, alongside optimal downstream biological function.46,47 NRP-1 signaling has been widely investigated for its role in angiogenesis and vascular permeability pathways.48,49
Figure 1.
Neuropilin-1 (NRP-1) binding to multiple ligands and their potential involvement in anti-VEGF resistance. NRP-1 can function as a receptor for semaphorin 3A (SEMA3A) and a co-receptor for a variety of ligands, including vascular endothelial growth factor A (VEGF-A) through vascular endothelial growth factor receptor 2 (VEGFR2); transforming growth factor-βs (TGF-βs) through transforming growth factor-β receptors (TGF-βR); and platelet-derived growth factor (PDGF) through the platelet-derived growth factor receptor a (PDGFRα). Following anti-VEGF therapy, NRP-1-mediated VEGF-independent pathways may be switched on to bypass VEGF blockade for pathological angiogenesis and vascular permeability, accounting for disease recurrence due to the development of acquired resistance.
Studies have associated endothelial NRP-1 with tip cell function during angiogenesis, alongside mediation of tumor angiogenesis, vascular permeability, and vascular remodeling mechanisms.50–52 A role of NRP-1 in NVEDs has recently emerged. Fernández-Robredo et al.53 observed that endothelial specific NRP-1 knock-out mice had reduced choroidal and retinal neovascularization in models of laser-induced choroidal neovascularization and oxygen-induced retinopathy compared to wild-type mice, indicating a potential role of NRP-1 in the pathological progression of NVEDs, such as AMD and DR. By using the vaso-permeability Miles assay, Roth et al.52 showed that stimulation of NRP-1 with VEGF or a CendR peptide increased vascular leakage in vivo in both VEGFR2-dependent and independent manners, suggesting a role of NRP-1 signaling in vascular permeability and EC barrier function.
Although these findings indicate a relationship between NRP-1 and pathological ocular neovascularization, there remains a lack of clinical evidence of its role in the development of acquired resistance to anti-VEGF therapy. In vivo studies, such as that of Roth et al.,52 raise the possibility of VEGF-independent pathological activity of endothelial NRP-1. Therefore, further study into the mechanisms behind this is required, investigating the various NRP-1 ligands and the pathways through which they may elicit acquired anti-VEGF resistance (see Fig. 1).
Semaphorin 3A
Semaphorin 3A (SEMA3A) is a class 3 semaphorin which primarily cues axonal guidance through interaction with NRP-1 and members of the plexin family.54,55 Through this pathway, SEMA3A has also been associated with ocular pathophysiology, with Kwon et al.56 identifying SEMA3A as a potential biomarker for DR.
To assess ocular SEMA3A/NRP-1 signaling, Cerani et al.44 evaluated the presence of SEMA3A in the vitreous of patients with DME and found elevated quantities of SEMA3A in patients with DME compared to control patients. Further evaluation of the role of ocular SEMA3A in vivo showed that increased SEMA3A levels contributed to increased ocular vascular leakage resulting from compromised blood-retinal barrier function. Interestingly, these effects were prevented by either using a recombinant mouse soluble NRP-1 in a mouse model of streptozotocin-induced diabetes or knockout of vascular NRP-1 in mice, demonstrating the induction of retinal vascular permeability through NRP-1/SEMA3A interactions. Furthermore, higher levels of SEMA3A were observed in the vitreous of patients suffering from late-stage PDR, and in a murine model of oxygen-induced retinopathy SEMA3A recruited NRP-1-positive mononuclear phagocytes to sites of pathological neovascularization in the retina, which was essential for disease progression.57
In addition, Guo et al.58 investigated the aqueous level of SEMA3A in patients with retinal vein occlusion with macular edema, which often occurs following DR, and observed increased levels of SEMA3A in the aqueous humor of patients. This positively correlated with central retinal thickness as well as negatively correlated with the ganglion cell-inner plexiform layer, suggesting a pathological role of ocular SEMA3A in macular edema and ganglion cell degeneration. Andriessen et al.59 also recently reported elevated SEMA3A and VEGF-A in the vitreous of patients with AMD at times of active choroidal neovascularization and provided evidence that NRP1-expressing myeloid cells promote and maintain choroidal neovascularization in a mouse model.
Placental Growth Factor
The PlGF, originally isolated from human placenta, is a member of the VEGF family,60 binding VEGFR1 and NRP1 but not VEGFR2. Despite this, PlGF is capable of enhancing VEGF activation of VEGFR2 through synergistic crosstalk.61 This occurs through PlGF displacement of VEGF from VEGFR1, increasing availability of VEGF to VEGFR2, enhancing VEGF-induced angiogenic activity.62 In addition to forming homodimers, PlGF and VEGF can also form heterodimers, which exhibit only weak biological activity.63 Unlike VEGF, PlGF is dispensable for developmental angiogenesis, but its expression is associated with pathological angiogenesis and inflammation.61
Although the role of PlGF in tumor growth and metastasis has been extensively studied,64 several studies suggest that PlGF could also be implicated in retinal vascular disease.65,66 Clinical studies revealed the elevation of aqueous and vitreous levels of PlGF in patients with PDR, with neovascular glaucoma due to DR or DME compared to non-diabetic patients or non-DR control patients, suggesting that increased PlGF levels were correlated with progressive ischemic retinopathies.67–70 Furthermore, in an in vivo model of PDR, PlGF overexpression in rat ocular media was associated with abnormal vascular properties, including microaneurysm formation, junction ruptures, and aberrant vascular sprouting.71 Using an electrical cell-impedance sensing system to measure trans-endothelial electrical resistance, Huang et al.72 showed that PlGF negatively regulated retinal endothelial cell barrier function in an in vitro model of the blood-retinal barrier.
Furthermore, Zehetner et al.73 explored the effects of intravitreal administration of ranibizumab, bevacizumab, or aflibercept on systemic PlGF levels in patients with neovascular AMD and reported a significant upregulation of plasma PlGF levels after treatment with aflibercept but not with bevacizumab or ranibizumab, suggesting a counter-regulatory response to aflibercept injection. However, little is known about any changes in ocular PlGF levels after anti-VEGF therapy, and this requires further investigation.
Transforming Growth Factor-β
The TGF-β subfamily of proteins, consisting of TGF-β1 to β3, regulates cellular growth, development, and homeostasis in a cell type and condition specific manner.74 As a co-receptor for TGF-β, NRP-1 is capable of modulating TGF-β-dependent EC development to control sprouting angiogenesis and immune responses through TGF-β receptors.75,76 To further investigate this mechanism, Aspalter et al.77 examined the effect of reduced NRP-1 expression on TGF-β signaling, finding that NRP-1 downregulation increases phosphorylation of vascular SMAD2/3 and drives endothelial stalk cell behavior. These findings may suggest that in the presence of NRP-1, TGF-β/SMAD2/3 signaling-inhibited endothelial tip cell capacity for sprouting angiogenesis may be attenuated. Conversely, inhibition of TGF-β has been associated with decreased phosphorylated SMAD2/3 and impaired vascular development and function in ocular vasculature, suggesting an important role of the TGF-β/SMAD2/3 signaling in ocular neovascularization.78,79
Tosi et al.80 investigated TGF-β1 levels in the aqueous of patients with neovascular AMD before and after repeated doses of ranibizumab. The study found that baseline TGF-β1, prior to ranibizumab injection, was higher in patients with neovascular AMD than control patients and aqueous TGF-β1 levels were persistently elevated with a tendency for further increase after treatment, presenting an upregulating effect of VEGF blockade on ocular TGF-β1. Although TGF-β1 is largely associated with induction of angiogenic mechanisms, other studies are consistent with a therapeutic effect of TGF-β1 secreted from mesenchymal stem cells, through suppression of retinal neovascularization in vivo.81
The vascular effects of TGF-β1 in the presence of NRP-1, or the pathogenic or homeostatic effects of NRP-1/TGF-β interaction on ocular angiogenesis remain poorly understood. Additionally, the ability of TGF-β to both positively and negatively regulate angiogenic pathways in a context-dependent manner warrants much further investigation into the function of upregulated TGF-β1 in NVEDs.
Other Growth Factors
The PDGF family and their receptors play a role during vascular development mainly through their essential functions in pericyte and vascular smooth muscle cell recruitment to developing vessels.82 Several studies indicate a role of NRP-1 activation in the modification of PDGF signalling.82–86 In vivo studies on corneal neovascular rabbits and subretinal neovascular mice have associated combined anti-VEGF and anti-PDGF treatment with reduced AMD pathophysiology, such as reduced choroidal neovascularization, retinal detachment, and subretinal neovascularisation.87–89 This stimulated interest in clinical investigation into dual anti-PDGF and anti-VEGF therapy for AMD, although lack of improvement to visual acuity compared to anti-VEGF alone has been reported recently.90,91 Furthermore, Muhl et al.86 found that in vitro PDGF-D treatment was associated with translocation of NRP-1 to EC junctions and that ex vivo PDGF-D/NRP-1 binding retains pericyte coverage and interaction with ECs during angiogenic sprouting, potentially mediating vascular permeability. Therefore, alternative pathways of PDGF signaling, such as NRP-1 co-activation pathways, may be worth further investigation to examine whether PDGF signaling participates in acquired anti-VEGF resistance.
The hepatocyte growth factor (HGF)/c-MET signaling pathway plays a prominent role in developmental and homeostatic angiogenesis.92,93 There are a number of clinical investigations into the HGF level in association with eye disease progression in PDR and non-PDR, indicating a higher expression level in the disease state.94,95 Further to this, increased aqueous levels of HGF have been observed after anti-VEGF treatment in patients with AMD and DME.96,97 In vivo study of HGF activity in mice with ischemic retinopathy indicated a role of HGF/c-MET activation in retinal neovascularization, with HGF acting as a pro-inflammatory, pro-permeability, and pro-angiogenic factor.98 As a co-receptor for HGF, NRP-1 is capable of enhancing HGF binding and activity.99 Various studies have sought to investigate NRP-1/HGF interactions in cancer.100,101 Although these studies further clarify the role of HGF in angiogenic pathophysiology and the effect of HGF/NRP-1 in disease states, the role of HGF in acquired resistance to anti-VEGF therapy in NVEDs remains unclear.
A recent study found that expression of angiopoietin-like 4 (ANGPTL4), is increased in the eyes of diabetic mice and patients with DME.102 These authors also showed that ANGPTL4 binds to endothelial NRP-1, resulting in activation of RhoA/ROCK signaling and subsequent loss of endothelial cell-cell junction barrier function. Furthermore, a soluble extracellular fragment of NRP-1 (sNRP-1) inhibited ANGPTL4-induced endothelial permeability in diabetic mice, and the permeability-increasing activity of aqueous fluid from patients with DME.102
Endothelial Glycolysis and Its Potential Role in NVED Pathology and Recurrence
Endothelial Glycolysis in Ocular Neovascularization
Increased endothelial glycolysis has recently been recognized as a driving force of angiogenesis alongside the well-established angiogenic growth factor VEGF. Activated ECs rely on glycolysis as opposed to oxidative metabolism for adenosine triphosphate (ATP) production to fuel the migrating tip ECs as well as proliferating stalk ECs during neovascular development.103 The consequence is that less than 1% of glycolytic pyruvate enters the mitochondria to undergo oxidative metabolism for ATP production in physiological conditions.104 In healthy ECs, a common glycolysis pathway is followed in the production of ATP in order to produce the energy required for EC proliferation and migration during angiogenic processes (Fig. 2). During healthy adulthood, quiescent ECs sustain basal levels of glycolysis and other metabolic pathways to serve energy production, biomass synthesis, and redox homeostasis required for their multiple functions, including vascular protection against oxidative stress and maintenance of vascular tone and blood-tissue barrier integrity and stability.105 Healthy quiescent ECs produce up to 85% of their ATP from glycolysis and they are more glycolytic than other healthy cell types.
Figure 2.
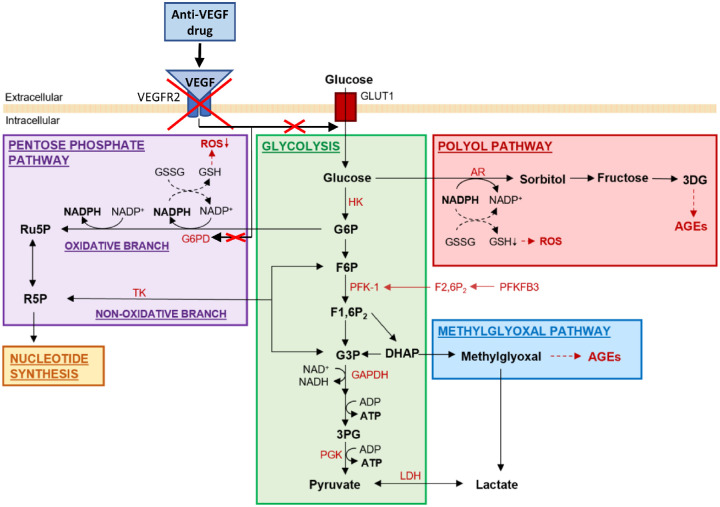
Endothelial cell glycolysis and three glycolytic side pathways potentially influenced by anti-VEGF therapy. The glycolytic main pathway along with the pentose phosphate side pathway physiologically exist in ECs to allow energy production, biomass synthesis and redox homeostasis. The polyol side pathway and the methylglyoxal side pathway become pathologically significant during hyperglycemia. VEGF antagonism may cause EC oxidative stress and cellular damage through abolishment of VEGF-stimulated activity of glucose-6-phosphate dehydrogenase (G6PD) and depletion of reduced nicotinamide adenine dinucleotide phosphate (NADPH) in the pentose phosphate pathway, resulting in decreased reduced glutathione (GSH) and accumulated reactive oxygen species (ROS). Please note for simplicity, not all enzymes, metabolites and glycolysis side pathways are illustrated in this diagram. Abbreviations: 3DG, 3-deoxyglucosone; 3PG, 3-phosphoglycerate; ADP, adenosine diphosphate; AGE, advanced glycation end product; AR, aldose reductase; ATP, adenosine triphosphate; DHAP, dihydroxyacetone phosphate; F1,6P2, fructose 1,6-bisphosphate; F2,6P2, fructose-2,6-bisphosphate; F6P, fructose 6-phosphate; G3P, glyceraldehyde 3-phosphate; G6P, glucose 6-phosphate; GLUT1, glucose transporter 1; GSSG, oxidized glutathione; HK, hexokinase glucokinase; LDH, lactate dehydrogenase; NAD, nicotinamide adenine dinucleotide; NADP, nicotinamide adenine dinucleotide phosphate; PFK-1, 6-phosphofructo-1-kinase; PFKFB3, phosphofructokinase-2/fructose-2,6-bisphosphatase 3; PGK, phosphoglycerate kinase; R5P, ribose 5-phosphate; Ru5P, ribulose 5-phosphate; TK, transketolase.
Although EC metabolism provides the driving force for proliferation during angiogenesis, some studies relate alterations in this mechanism to the progression of pathological angiogenesis in certain disease states.106 Alongside the common glycolysis pathway, glycolysis side pathways exist to allow the generation of macromolecules required for EC proliferation and migration.107 One such pathway, the pentose phosphate pathway (PPP), has an oxidative branch PPP (oxPPP) which produces reduced nicotinamide adenine dinucleotide phosphate (NADPH). This NADPH is utilized in the conversion of oxidized glutathione (GSSG) to reduced glutathione (GSH), a potent reactive oxygen species (ROS) scavenger (see Fig. 2), presenting a major function of the PPP as an antioxidative defense pathway.108
Moreover, the presence and activity of certain growth factors, including some discussed as NRP-1 ligands above, can regulate EC glycolysis. PlGF inhibition has been found to upregulate glucose-6-phosphate dehydrogenase (G6PD), a key regulator of the oxPPP, in human retinal cells in vitro.109 The role of PlGF in downregulation of G6PD has been associated with oxidative cellular damage as well as negative regulation of retinal EC barrier function resulting in vascular hyperpermeability.72 Furthermore, VEGF and TGF-β1 individually have been associated with increased glycolysis in cancer,110,111 and thus could be worth investigating for their effects on retinal EC glycolysis.
Dysregulation of EC Glucose Metabolism in DR
Hyperglycemia is the hallmark of diabetes and perturbs EC metabolism in a number of ways which can cause EC dysfunction, contributing to the pathogenesis of PDR and DME. One such mechanism is the inhibition of the PPP flux through downregulation of G6PD, the rate-limiting enzyme of the pathway that maintains the level of NADPH. This in turn decreases the generation of NADPH, subsequently impairing maintenance of the GSH level, so that ROS is progressively accumulated after GSH depletion (see Fig. 2).104,112 Consequently, hyperglycemia-induced ROS accumulation inhibits GAPDH to stall glycolysis and concomitantly generate upstream glycolytic intermediates, which are subsequently diverted into several pathological glycolytic side pathways including the polyol pathway and the methylglyoxal pathway (see Fig. 2), whereas high levels of glucose continuously overwhelm glycolysis and excess glucose also floods to the polyol pathway.104 These pathways give rise to a further increase in ROS levels and production of advanced glycation end products (AGEs), which can result in cellular inflammation and degradation.104 The degradation of retinal ECs through such mechanisms can give rise to the microaneurysm formation and vascular hyperpermeability present in DR and DME.
Using metabolomic analysis of the vitreous of patients with PDR, Barba et al.113 found lactate to be the most abundant metabolite present compared to non-diabetic patients, evidencing increased glycolytic metabolism in the eyes of patients with PDR. Furthermore, recent studies by Haines et al.114 and Wang et al.115 reported significantly altered metabolites in the vitreous and aqueous humor samples of patients with DR. Of note, increased sn-glycerol-3-phosphate, a product of dihydroxyacetone phosphate (DHAP) reduction, and fructose 6-phosphate (F6P) were observed. As both DHAP and F6P are upstream intermediates in glycolysis (see Fig. 2), it highlights the implications for their diversions from glycolysis to the pathological polyol pathway and the methylglyoxal pathway to generate ROS and AGEs in DR. In addition, Schoors et al.116 determined that in vivo inhibition of phosphofructokinase-2/fructose-2,6-bisphosphatase 3 (PFKFB3), a glycolytic activator which mediates blood vessel formation,107 impairs retinal vessel sprouting and reduces vascular hyperbranching and pathological angiogenesis; this could present another way in which dysregulation of EC glucose metabolism pathways allows the progression of pathological angiogenesis.
Potential Influence of Anti-VEGF Drugs on EC Glucose Metabolism
Whereas clinical investigations have demonstrated the impact of high glucose levels on the progression of DR, accumulating evidence also indicates the importance of glycemic control on the treatment response to VEGF antagonists. Studies by Ozturk et al.117 and Matsuda et al.4 noted that clinical responsiveness to anti-VEGF treatment (ranibizumab and bevacizumab) for NVEDs was influenced by serum glycated hemoglobin A1c (HbA1c) levels, which were negatively correlated with anti-VEGF-mediated improvements in central subfield macular thickness and best-corrected visual acuity. Similar findings from patients with DME were reported by Sharma et al.,118 wherein better treatment outcome of intravitreal bevacizumab was associated with good glycemic control (low HbA1c). These studies also suggest that HbA1c can serve as a predictor for response to anti-VEGF therapy in DME.
Interestingly, it was demonstrated that bevacizumab treatment induced metabolic adaptation in glioblastomas with reduced oxidative metabolism and increased glucose metabolism, alongside upregulated glycolytic enzymes including pyruvate dehydrogenase kinase and lactate dehydrogenase.119 They also observed that bevacizumab led to a depletion in GSH levels, indicating that the treatment caused oxidative stress in the tumors. Retinal ECs are basically glycolytic, and both the oxPPP and non-oxPPP branches are used for production of GSH to provide antioxidative defense and the synthesis of nucleotides to enable cellular growth. The oxPPP flux is regulated by G6PD, which itself is partially regulated by VEGF.103,120 Thus, long-term anti-VEGF treatment in NVEDs may be associated with EC damage leading to macular edema through attenuation of the antioxidative oxPPP. However, whether anti-VEGF therapy reprograms glycolytic metabolism in DR has not been investigated. Given the ability of VEGF to upregulate EC glycolysis121 as well as antioxidative mechanisms,120 it is plausible that long-term anti-VEGF treatment can influence EC metabolism and impair EC function undesirably through the enhancement of hyperglycemia-instigated ROS accumulation and the resulting production of AGEs to worsen oxidative stress and cellular inflammation (see Fig. 2), leading to the disruption of blood-retinal barrier integrity to cause vessel leakage, which counteracts the clinical efficacy of anti-VEGF therapy.
Conclusions and Future Perspectives
Anti-VEGF biologic treatment is a frontline therapy for NVEDs and although many patients benefit from its therapy, a significant proportion either do not respond to treatment, or experience a reduced response after repeated treatments. In this review, we have discussed alternative angiogenic pathways that may be mechanisms underlying acquired resistance to VEGF-targeted therapies. NRP-1 has an emerging role in the development of acquired resistance to anti-VEGF biologic therapy, with a number of its ligands upregulated after anti-VEGF treatment and associated with VEGF-independent angiogenic modulation.73,80,96,97 In spite of these associations, the reason for alteration in expression of these ligands after anti-VEGF ocular therapy remains unclear, and their role in angiogenic modulation post-treatment requires elucidation. Studies into the alterations to ocular EC metabolism such as changes in glycolytic pathways after ocular anti-VEGF treatment are scarce. As glycolysis is a known driver of angiogenic growth and anti-VEGF treatment may mediate altered glucose metabolism, further investigation into alterations or mechanistic switches in these pathways after ocular anti-VEGF therapy is warranted. For the development of an effective therapy for NVEDs, such as wet AMD, PDR, and DME, it is imperative to determine the mechanisms by which anti-VEGF treatment response declines and acquired resistance develops. Closer study of the mechanisms considered in this review may shine a light upon novel targets and allow the development of more rational strategies to treat and prevent progression of neovascular ocular diseases.
Acknowledgments
Funded by a PhD studentship (SCI05P) from Medicines and Healthcare products Regulatory Agency.
Disclosure: D. Sharma, None; I. Zachary, has a paid consultancy providing expert scientific advice for Regeneron, Inc. (C) and Bayer AG (C); H. Jia, None
References
- 1. Virgili G, Parravano M, Menchini F. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev. 2014;(10): CD007419. [DOI] [PubMed] [Google Scholar]
- 2. De Jong EK, Geerlings MJ, den Hollander AI. Age-related macular degeneration. In: Genetics and Genomics of Eye Disease. Salt Lake City, UT: Academic Press; 2020: 155–180. [Google Scholar]
- 3. Bourne RR, Jonas JB, Bron AM, et al.. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: magnitude, temporal trends and projections. Br J Ophthalmol. 2018; 102(5): 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsuda S, Tam T, Singh RP, et al.. The impact of metabolic parameters on clinical response to VEGF inhibitors for diabetic macular edema. J Diabetes Complications. 2014; 28(2): 166–170. [DOI] [PubMed] [Google Scholar]
- 5. Yang S, Zhao J, Sun X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: a comprehensive review . Drug Des Develop Ther. 2016; 10: 1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim LS, Mitchell P, Seddon JM, et al.. Age-related macular degeneration. The Lancet. 2012; 379(9827): 1728–1738. [DOI] [PubMed] [Google Scholar]
- 7. Mitchell P, Liew G, Gopinath B, et al.. Age-related macular degeneration. The Lancet. 2018; 392(10153): 1147–1159. [DOI] [PubMed] [Google Scholar]
- 8. Blumenkranz MS, Leung LS, Martin DF, Rosenfeld PJ, Zarbin MA. Pharmacotherapy of age-related macular degeneration. In Retina Fifth Edition , New York, NY: Elsevier Inc.; 2012: 1213–1255. [Google Scholar]
- 9. Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015; 2(17): 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, et al.. Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res. 2016; 2016: 2156273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019; 176(6): 1248–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rofagha S, Bhisitkul RB, Boyer DS, et al.. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013; 120(11): 2292–2299. [DOI] [PubMed] [Google Scholar]
- 13. Hara C, Wakabayashi T, Fukushima Y, et al.. Tachyphylaxis during treatment of exudative age-related macular degeneration with aflibercept. Graefe's Arch Clin Exp Ophthalmol. 2019; 257(11): 2559–2569. [DOI] [PubMed] [Google Scholar]
- 14. Wells JA, Glassman AR, Ayala AR, et al.. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015; 372(13): 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenfeld PJ, Brown DM, Heier JS, et al.. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355(14): 1419–1431. [DOI] [PubMed] [Google Scholar]
- 16. Brown DM, Kaiser PK, Michels M, et al.. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355(14): 1432–1444. [DOI] [PubMed] [Google Scholar]
- 17. Dhoot DS, Kaiser PK. Ranibizumab for age-related macular degeneration. Expert Opin Biol Ther. 2012; 12(3): 371–381. [DOI] [PubMed] [Google Scholar]
- 18. Garcia J, Hurwitz HI, Sandler AB, et al.. Bevacizumab (Avastin) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treatment Reviews. 2020; 86: 102017. [DOI] [PubMed] [Google Scholar]
- 19. Martin DF, Maguire MG, Fine SL, et al.. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012; 119(7): 1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenfeld PJ, Windsor MA, Feuer WJ, et al.. Estimating Medicare and patient savings from the use of bevacizumab for the treatment of exudative age-related macular degeneration. Am J Ophthalmol. 2018; 191: 135–139. [DOI] [PubMed] [Google Scholar]
- 21. Heier JS, Brown DM, Chong V, et al.. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119(12): 2537–2548. [DOI] [PubMed] [Google Scholar]
- 22. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al.. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014; 121(11): 2247–2254. [DOI] [PubMed] [Google Scholar]
- 23. Papadopoulos N, Martin J, Ruan Q, et al.. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012; 15(2): 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dugel PU, Koh A, Ogura Y, et al.. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020; 127(1): 72–84. [DOI] [PubMed] [Google Scholar]
- 25. Nguyen QD, Shah SM, Khwaja AA, et al.. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010; 117(11): 2146–2151. [DOI] [PubMed] [Google Scholar]
- 26. Brown DM, Nguyen QD, Marcus DM, et al.. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013; 120(10): 2013–2022. [DOI] [PubMed] [Google Scholar]
- 27. Heier JS, Khanani AM, Quezada Ruiz C, et al.. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022; 399: 729–740. [DOI] [PubMed] [Google Scholar]
- 28. Wykoff CC, Abreu F, Adamis AP, et al.. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022; 399: 741–755. [DOI] [PubMed] [Google Scholar]
- 29. Schaal S, Kaplan HJ, Tezel TH. Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration?. Ophthalmology. 2008; 115(12): 2199–2205. [DOI] [PubMed] [Google Scholar]
- 30. Forooghian F, Cukras C, Meyerle CB, et al.. Tachyphylaxis following intravitreal bevacizumab for exudative age-related macular degeneration. Retina. 2009; 29(6): 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hwang RY, Santos D, Oliver SC. Rates of exudative recurrence for eyes with inactivated wet age-related macular degeneration on 12-week interval dosing with bevacizumab therapy. Retina. 2020; 40(4): 679–685. [DOI] [PubMed] [Google Scholar]
- 32. Sinawat S, Rattanapakorn T, Sanguansak T, et al.. Intravitreal bevacizumab for proliferative diabetic retinopathy with new dense vitreous hemorrhage after full panretinal photocoagulation. Eye. 2013; 27(12): 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parikh RN, Traband A, Kolomeyer AM, et al.. Intravitreal bevacizumab for the treatment of vitreous hemorrhage due to proliferative diabetic retinopathy. Am J Ophthalmol. 2017; 176: 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takamura Y, Tomomatsu T, Matsumura T, et al.. The effect of photocoagulation in ischemic areas to prevent recurrence of diabetic macular edema after intravitreal bevacizumab injection. Invest Ophthalmol Vis Sci. 2014; 55(8): 4741–4746. [DOI] [PubMed] [Google Scholar]
- 35. Aksoy S, Yilmaz G, Akkoyun I, et al., Comparison of intravitreal bevacizumab and triamcinolone acetonide therapies for diffuse diabetic macular edema. Int J Ophthalmol. 2015; 8(3): 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuroda Y, Yamashiro K, Miyake M, et al.. Factors associated with recurrence of age-related macular degeneration after anti-vascular endothelial growth factor treatment: a retrospective cohort study. Ophthalmology. 2015; 122(11): 2303–2310. [DOI] [PubMed] [Google Scholar]
- 37. Kim JH, Chang YS, Kim JW, et al.. Recurrence in patients with type 3 neovascularization (retinal angiomatous proliferation) after intravitreal ranibizumab. Retina. 2017; 37(8): 1508–1515. [DOI] [PubMed] [Google Scholar]
- 38. Gasperini JL, Fawzi AA, Khondkaryan A, et al.. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol. 2012; 96(1): 14–20. [DOI] [PubMed] [Google Scholar]
- 39. Chelala E, Nehme J, El Rami H, et al.. Efficacy of intravitreal ranibizumab injections in the treatment of vitreous hemorrhage related to proliferative diabetic retinopathy. Retina. 2018; 38(6): 1127–1133. [DOI] [PubMed] [Google Scholar]
- 40. Chatziralli I, Dimitriou E, Theodossiadis G, et al.. Intravitreal ranibizumab versus vitrectomy for recurrent vitreous haemorrhage after pars plana vitrectomy for proliferative diabetic retinopathy: a prospective study. Int Ophthalmol. 2020; 40(4): 841–847. [DOI] [PubMed] [Google Scholar]
- 41. Dans KC, Freeman SR, Lin T, et al.. Durability of every-8-week aflibercept maintenance therapy in treatment-experienced neovascular age-related macular degeneration. Graefe's Arch Clin Exp Ophthalmol. 2019; 257(4): 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fritsche L, Igl W, Bailey J, et al.. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genetics. 2015; 48(2): 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lorés-Motta L, van Asten F, Muether PS, et al.. A genetic variant in NRP1 is associated with worse response to ranibizumab treatment in neovascular age-related macular degeneration. Pharmacogenet Genomics. 2016; 26(1): 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cerani A, Tetreault N, Menard C, et al.. Neuron-derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1. Cell Metabol. 2013; 18(4): 505–518. [DOI] [PubMed] [Google Scholar]
- 45. Kwiatkowski SC, Guerrero PA, Hirota S, et al.. Neuropilin-1 modulates TGFβ signaling to drive glioblastoma growth and recurrence after anti-angiogenic therapy. PLoS One. 2017; 12(9): e0185065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Soker S, Miao HQ, Nomi M, et al.. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cellular Biochem. 2002; 85(2): 357–368. [DOI] [PubMed] [Google Scholar]
- 47. Jia H, Bagherzadeh A, Hartzoulakis B, et al.. Characterization of a bicyclic peptide neuropilin-1 (NP-1) antagonist (EG3287) reveals importance of vascular endothelial growth factor exon 8 for NP-1 binding and role of NP-1 in KDR signaling. J Biolog Chem. 2006; 281(19): 13493–13502. [DOI] [PubMed] [Google Scholar]
- 48. Zachary I. Neuropilins: role in signalling, angiogenesis and disease. Chem Immunol Allergy.. 2014; 99: 37–70. [DOI] [PubMed] [Google Scholar]
- 49. Raimondi C, Brash JT, Fantin A, et al.. NRP1 function and targeting in neurovascular development and eye disease. Prog Retin Eye Res. 2016; 52: 64–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pan Q, Chanthery Y, Liang W, et al.. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007; 11(1): 53–67 [DOI] [PubMed] [Google Scholar]
- 51. Fantin A, Vieira JM, Plein A, et al.. NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis. Blood, J Am Soc Hematol. 2013; 121(12): 2352–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roth L, Prahst C, Ruckdeschel T, et al.. Neuropilin-1 mediates vascular permeability independently of vascular endothelial growth factor receptor-2 activation. Sci Signal. 2016; 9(425): ra42. [DOI] [PubMed] [Google Scholar]
- 53. Fernández-Robredo P, Selvam S, Powner MB, et al.. Neuropilin 1 involvement in choroidal and retinal neovascularisation. PLoS One. 2017; 12(1): e0169865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sharma A, Verhaagen J, Harvey AR. Receptor complexes for each of the Class 3 Semaphorins. Front Cell Neurosci. 2012; 6: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sabag AD, Smolkin T, Mumblat Y, et al.. The role of the plexin-A2 receptor in Sema3A and Sema3B signal transduction. J Cell Sci. 2014; 127(24): 5240–5252. [DOI] [PubMed] [Google Scholar]
- 56. Kwon SH, Shin JP, Kim IT, et al.. Association of plasma semaphorin 3A with phenotypes of diabetic retinopathy and nephropathy. Invest Ophthalmol Vis Sci. 2016; 57(7): 2983–2989. [DOI] [PubMed] [Google Scholar]
- 57. Dejda A, Mawambo G, Cerani A, et al.. Neuropilin-1 mediates myeloid cell chemoattraction and influences retinal neuroimmune crosstalk. J Clin Invest. 2014; 124(11): 4807–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guo S, Ren J, Li Z, et al.. Aqueous semaphorin 3A level correlates with retinal macular oedema and ganglion cell degeneration in patients with retinal vein occlusion. Acta Ophthalmologica. 2019; 97(3): 273–278. [DOI] [PubMed] [Google Scholar]
- 59. Andriessen EM, Binet F, Fournier F, et al.. Myeloid-resident neuropilin-1 promotes choroidal neovascularization while mitigating inflammation. EMBO Molec Med. 2021; 13(5): e11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maglione D, Guerriero V, Viglietto G, et al.. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci. 1991; 88(20): 9267–9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carmeliet P, Moons L, Luttun A, et al.. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001; 7(5): 575–583. [DOI] [PubMed] [Google Scholar]
- 62. Autiero M, Waltenberger J, Communi D, et al.. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003; 9(7): 936–943. [DOI] [PubMed] [Google Scholar]
- 63. Cao Y, Chen H, Zhou L, et al.. Heterodimers of placenta growth factor/vascular endothelial growth factor: endothelial activity, tumor cell expression, and high affinity binding to Flk-1/KDR. J Biolog Chem. 1996; 271(6): 3154–3162. [DOI] [PubMed] [Google Scholar]
- 64. Dewerchin M, Carmeliet P. Placental growth factor in cancer. Expert Opin Ther Targets. 2014; 18(11): 1339–1354. [DOI] [PubMed] [Google Scholar]
- 65. Nguyen QD, De Falco S, Behar-Cohen F, et al.. Placental growth factor and its potential role in diabetic retinopathy and other ocular neovascular diseases. Acta Ophthalmologica. 2018; 96(1): e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van Bergen T, Etienne I, Cunningham F, et al.. The role of placental growth factor (PlGF) and its receptor system in retinal vascular diseases. Prog Retin Eye Res. 2019; 69: 116–136. [DOI] [PubMed] [Google Scholar]
- 67. Ando R, Noda K, Namba S, et al.. Aqueous humour levels of placental growth factor in diabetic retinopathy. Acta Ophthalmologica. 2014; 92(3): e245–e246. [DOI] [PubMed] [Google Scholar]
- 68. Kovacs K, Marra KV, Yu G, et al.. Angiogenic and inflammatory vitreous biomarkers associated with increasing levels of retinal ischemia. Invest Ophthalmol Vis Sci. 2015; 56(11): 6523–6530. [DOI] [PubMed] [Google Scholar]
- 69. Noma H, Mimura T, Yasuda K, et al.. Aqueous humor levels of soluble vascular endothelial growth factor receptor and inflammatory factors in diabetic macular edema. Ophthalmologica. 2017; 238(1-2): 81–88. [DOI] [PubMed] [Google Scholar]
- 70. Al Kahtani E, Xu Z, Al Rashaed S, et al.. Vitreous levels of placental growth factor correlate with activity of proliferative diabetic retinopathy and are not influenced by bevacizumab treatment. Eye. 2017; 31(4): 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kowalczuk L, Touchard E, Omri S, et al.. Placental growth factor contributes to micro-vascular abnormalization and blood-retinal barrier breakdown in diabetic retinopathy. PLoS One. 2011; 6(3): e17462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang H, Lennikov A, Saddala MS, et al.. Placental growth factor negatively regulates retinal endothelial cell barrier function through suppression of glucose-6-phosphate dehydrogenase and antioxidant defense systems. FASEB J. 2019; 33(12): 13695–13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zehetner C, Bechrakis NE, Stattin M, et al.. Systemic counterregulatory response of placental growth factor levels to intravitreal aflibercept therapy. Invest Ophthalmol Vis Sci. 2015; 56(5): 3279–3286. [DOI] [PubMed] [Google Scholar]
- 74. Kubiczkova L, Sedlarikova L, Hajek R, et al.. TGF-β–an excellent servant but a bad master. J Transl Med. 2012; 10(1): 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hirota S, Clements TP, Tang LK, et al.. Neuropilin 1 balances β8 integrin-activated TGFβ signaling to control sprouting angiogenesis in the brain. Development. 2015; 142(24): 4363–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Powell J, Mota F, Steadman D, et al.. Small molecule neuropilin-1 antagonists combine antiangiogenic and antitumor activity with immune modulation through reduction of transforming growth factor beta (TGFβ) production in regulatory T-cells. J Medicin Chem. 2018; 61(9): 4135–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Aspalter IM, Gordon E, Dubrac A, et al.. Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat Comm. 2015; 6(1): 7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Walshe TE, Saint-Geniez M, Maharaj AS, et al.. TGF-β is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS One. 2009; 4(4): e5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang X, Ma W, Han S, et al.. TGF-β participates choroid neovascularization through Smad2/3-VEGF/TNF-α signaling in mice with Laser-induced wet age-related macular degeneration. Sci Rep. 2017; 7(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tosi GM, Caldi E, Neri G, et al.. HTRA1 and TGF-β1 concentrations in the aqueous humor of patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017; 58(1): 162–167. [DOI] [PubMed] [Google Scholar]
- 81. Kim KS, Park JM, Kong T, et al.. Retinal angiogenesis effects of TGF-β1 and paracrine factors secreted from human placental stem cells in response to a pathological environment. Cell Transplant. 2016; 25(6): 1145–1157. [DOI] [PubMed] [Google Scholar]
- 82. Kofler N, Simons M. The expanding role of neuropilin: regulation of transforming growth factor-β and platelet-derived growth factor signaling in the vasculature. Curr Opin Hematol. 2016; 23(3): 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Develop. 2008; 22(10): 1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pellet-Many C, Frankel P, Evans IM, et al.. Neuropilin-1 mediates PDGF stimulation of vascular smooth muscle cell migration and signalling via p130Cas. Biochem J. 2011; 435: 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pellet-Many C, Metha V, Fields L, et al.. Neuropilins 1 and 2 mediate neointimal hyperplasia and re-endothelialization following arterial injury. Cardiovasc Res. 2015;108:288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Muhl L, Folestad EB, Gladh H, et al.. Neuropilin 1 binds PDGF-D and is a co-receptor in PDGF-D–PDGFRβ signaling. J Cell Sci. 2017; 130(8): 1365–1378. [DOI] [PubMed] [Google Scholar]
- 87. Pérez-Santonja JJ, Campos-Mollo E, Lledó-Riquelme M, et al.. Inhibition of corneal neovascularization by topical bevacizumab (anti-VEGF) and sunitinib (anti-VEGF and anti-PDGF) in an animal model. Am J Ophthalmol. 2010; 150(4): 519–528. [DOI] [PubMed] [Google Scholar]
- 88. Dong A, Seidel C, Snell D, et al.. Antagonism of PDGF-BB suppresses subretinal neovascularization and enhances the effects of blocking VEGF-A. Angiogenesis. 2014; 17(3): 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ding K, Eaton L, Bowley D, et al.. Generation and characterization of ABBV642, a dual variable domain immunoglobulin molecule (DVD-Ig) that potently neutralizes VEGF and PDGF-BB and is designed for the treatment of exudative age-related macular degeneration. mAbs. 2017; 9(2): 269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cohen MN, O'Shaughnessy D, Fisher K, et al.. APEX: a phase II randomised clinical trial evaluating the safety and preliminary efficacy of oral X-82 to treat exudative age-related macular degeneration. Br J Ophthalmol. 2021; 105(5): 716–722. [DOI] [PubMed] [Google Scholar]
- 91. Heier JS, Wykoff CC, Waheed NK, et al.. Intravitreal combined aflibercept+ anti–platelet-derived growth factor receptor β for neovascular age-related macular degeneration: results of the phase 2 CAPELLA trial. Ophthalmology. 2020; 127(2): 211–220. [DOI] [PubMed] [Google Scholar]
- 92. You WK, McDonald DM. The hepatocyte growth factor/c-Met signaling pathway as a therapeutic target to inhibit angiogenesis. BMB Reports. 2008; 41(12): 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ferrara N. The role of the VEGF signaling pathway in tumor angiogenesis. In: Marmé D. (eds). Tumor Angiogenesis: A Key Target for Cancer Therapy. 2019: 211–226. [Google Scholar]
- 94. Yu Y, Zhang J, Zhu R, et al.. The profile of angiogenic factors in vitreous humor of the patients with proliferative diabetic retinopathy. Curr Molec Med. 2017; 17(4): 280–286. [DOI] [PubMed] [Google Scholar]
- 95. Tayyari F, Khuu LA, Sivak JM, et al.. Retinal blood oxygen saturation and aqueous humour biomarkers in early diabetic retinopathy. Acta Ophthalmologica. 2019; 97(5): e673–e679. [DOI] [PubMed] [Google Scholar]
- 96. Cabral T, Lima LH, Mello LGM, et al.. Bevacizumab injection in patients with neovascular age-related macular degeneration increases angiogenic biomarkers. Ophthalmology Retina. 2018; 2(1): 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Juncal VR, Mak MY, Bamakrid M, et al., Changes in aqueous cytokine levels following intravitreal aflibercept in treatment-naive patients with diabetic macular edema. J Ocul Pharmacol Ther. 2020; 36(9): 697–702. [DOI] [PubMed] [Google Scholar]
- 98. Lorenc VE, Lima e Silva R, Hackett SF, et al.. Hepatocyte growth factor is upregulated in ischemic retina and contributes to retinal vascular leakage and neovascularization. FASEB BioAdvances. 2020; 2(4): 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Prud'homme GJ, Glinka Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget. 2012; 3(9): 921–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hu B, Guo P, Bar-Joseph I, et al.. Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene. 2007; 26(38): 5577–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Li L, Jiang X, Zhang Q, et al.. Neuropilin-1 is associated with clinicopathology of gastric cancer and contributes to cell proliferation and migration as multifunctional co-receptors. J Exp Clin Cancer Res. 2016; 35(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sodhi A, Ma T, Menon D, et al.. Angiopoietin-like 4 binds neuropilins and cooperates with VEGF to induce diabetic macular edema. J Clin Invest. 2019; 129(11): 4593–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fitzgerald G, Soro-Arnaiz I, De Bock K. The Warburg effect in endothelial cells and its potential as an anti-angiogenic target in cancer. Front Cell Develop Biol. 2018; 6: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Goveia J, Stapor P, Carmeliet P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Molec Med. 2014; 6(9): 1105–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. De Bock K, Georgiadou M, Schoors S, et al.. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013a; 154(3): 651–663. [DOI] [PubMed] [Google Scholar]
- 106. Eelen G, de Zeeuw P, Treps L, et al.. Endothelial cell metabolism. Physiological Rev. 2018; 98(1): 3–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. De Bock K, Georgiadou M, Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013b; 18(5): 634–647. [DOI] [PubMed] [Google Scholar]
- 108. Bierhansl L, Conradi LC, Treps L, et al.. Central role of metabolism in endothelial cell function and vascular disease. Physiology. 2017; 32(2): 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Saddala MS, Lennikov A, Huang H. Placental growth factor regulates the pentose phosphate pathway and antioxidant defense systems in human retinal endothelial cells . J Proteomics. 2020; 217: 103682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shi S, Xu J, Zhang B, et al.. VEGF promotes glycolysis in pancreatic cancer via HIF1α up-regulation. Curr Molec Med. 2016; 16(4): 394–403. [DOI] [PubMed] [Google Scholar]
- 111. Costanza B, Rademaker G, Tiamiou A, et al.. Transforming growth factor beta-induced, an extracellular matrix interacting protein, enhances glycolysis and promotes pancreatic cancer cell migration. Int J Cancer. 2019; 145(6): 1570–1584. [DOI] [PubMed] [Google Scholar]
- 112. Zhang Z, Yang Z, Zhu B, et al.. Increasing glucose 6-phosphate dehydrogenase activity restores redox balance in vascular endothelial cells exposed to high glucose. PLoS One. 2012; 7(11): e49128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Barba I, Garcia-Ramírez M, Hernández C, et al.. Metabolic fingerprints of proliferative diabetic retinopathy: an 1H-NMR–based metabonomic approach using vitreous humor. Invest Ophthalmol Vis Sci. 2010; 51(9): 4416–4421. [DOI] [PubMed] [Google Scholar]
- 114. Haines NR, Manoharan N, Olson JL, et al.. Metabolomics analysis of human vitreous in diabetic retinopathy and rhegmatogenous retinal detachment. J Proteome Res. 2018; 17(7): 2421–2427. [DOI] [PubMed] [Google Scholar]
- 115. Wang H, Fang J, Chen F, Sun Q, Xu X, Lin SH, Liu K. Metabolomic profile of diabetic retinopathy: a GC-TOFMS-based approach using vitreous and aqueous humor. Acta Diabetol. 2020; 57: 41–51. [DOI] [PubMed] [Google Scholar]
- 116. Schoors S, De Bock K, Cantelmo A, et al.. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014; 19(1): 37–48. [DOI] [PubMed] [Google Scholar]
- 117. Ozturk BT, Kerimoglu H, Adam M, et al.. Glucose regulation influences treatment outcome in ranibizumab treatment for diabetic macular edema. J Diabetes Complications. 2011; 25(5): 298–302. [DOI] [PubMed] [Google Scholar]
- 118. Sharma S, Joshi SN, Karki P. HbA1c as a predictor for response of bevacizumab in diabetic macular oedema. BMJ Open Ophthalmol. 2020; 5(1): e000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fack F, Espedal H, Keunen O, et al.. Bevacizumab treatment induces metabolic adaptation toward anaerobic metabolism in glioblastomas. Acta Neuropathologica. 2015; 129(1): 115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pan S, World CJ, Kovacs CJ, et al.. Glucose 6-phosphate dehydrogenase is regulated through c-Src–mediated tyrosine phosphorylation in endothelial cells. Arterioscl Thromb Vasc Biol. 2009; 29(6): 895–901. [DOI] [PubMed] [Google Scholar]
- 121. Faulkner A, Lynam E, Purcell R, et al.. Context-dependent regulation of endothelial cell metabolism: differential effects of the PPARβ/δ agonist GW0742 and VEGF-A. Sci Rep. 2020; 10(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]