Abstract
Since 2015, the National Institutes of Health has called for its funded preclinical research to include both male and female subjects. However, much of the basic animal research that has studied heart rate and blood pressure in the past has used male rats. Male rats have been preferred for these studies to avoid the possible complicating effects of the female estrous cycle. The aim of the current study was to determine whether blood pressure and heart rates vary as a function of the estrous cycle phase of young normotensive Wistar–Kyoto (WKY) and Spontaneously Hypertensive (SHR) female rats. Blood pressure and heart rate were measured at the same time of day throughout the estrous cycle by using a noninvasive tail cuff sphygmomanometric technique. As expected, 16-wk-old female SHR rats had higher blood pressure and heart rates than did age-matched female WKY rats. However, no significant differences in mean, systolic, or diastolic arterial blood pressure or heart rate were detected across the different stages of the estrous cycle in either strain of female rats. Consistent with previous reports, heart rates were higher and showed less variation in the hypertensive SHR female rats as compared with the normotensive WKY female rats. These results indicate that studies measuring blood pressure and heart rate can include young female SHR and WKY rats with no effect of estrous cycle stage.
Abbreviations: CV, coefficient of variation; BP, blood pressure; DBP, diastolic blood pressure; HR, heart rate; MBP, mean blood pressure; SBP, systolic blood pressure; SHR, spontaneously hypertensive rats; SHRSP, stroke-prone spontaneously hypertensive rats; WKY, Wistar–Kyoto
Introduction
One of the most important human health problems studied in medicine is arterial hypertension. High blood pressure (BP) is a highly prevalent disease that affected around 1.13 billion48 people worldwide in 2015; of these, 24% were adult men and 20% were adult women. Men have higher BP than do premenopausal age-matched women,19 and many studies have found a relationship between sex hormones and BP, as reviewed in reference 37. Estrogen is thought to protect premenopausal women from high BP by causing vasodilation via nitric oxide endothelial production and inhibiting the sympathetic and renin–angiotensin–aldosterone (RAAS) systems.43 In contrast, testosterone is implicated in increasing the incidence of hypertension in men and postmenopausal women;38 elevated BP in postmenopausal women could also be related to a proportional increase of plasma testosterone levels relative to the lower level of plasma estrogens.41,49
Spontaneously Hypertensive Rats (SHR) and the normotensive control strain, Wistar–Kyoto (WKY) rats, have long been used to study the effects of high BP on target organs and to test potential antihypertensive pharmacologic agents.23,34 Recent preclinical research on antihypertensive treatments has been conducted using only male hypertensive SHR rats due to concerns regarding possible confounders associated with the estrous cycle in female SHR rats.7,11,22,35,36,39 However, early research on BP and heart rate (HR) was conducted using female SHR rats without consideration of their estrous cycle3,24,47.
Disregarding sex as a biologic variable can affect the rigor and reproducibility of experimental results and lead to incorrect generalizations. Therefore, NIH has called for the balanced use of female and male animals in the design, analysis and reporting of preclinical studies.5,30 In response to NIH notice NOT-OD-15-102,32 we studied whether the female estrous cycle is relevant to the experimental design and analysis of hypertension in preclinical studies. For this reason, we measured BP and HR throughout the estrous cycle in nonanesthetized 16- to 19-wk-old, hypertensive SHR and control WKY female rats. After age 15 wk, female SHR rats have fully developed and sustained hypertension,4,17 and rats at this age and older are appropriate for preclinical studies. Furthermore, we used a noninvasive tail-cuff and sphygmomanometric measurement method that is appropriate for use in nonanesthetized rats and has been widely used in preclinical studies of hypertensive treatments.
Materials and Methods
Animals.
The present study used 10 SHR and 9 Wistar–Kyoto female rats (Rattus norvegicus; age, 16 wk; weight: SHR, 199 ± 3 g; WKY, 220 ± 4 g). Parental SHR (SHR/NCrl) and WKY (WKY/NCrl) rats were obtained from Charles River (Kingston, NY). The endoparasite-free status of both parents and progeny was checked regularly using fecal flotation testing. SHR and WKY rats were full-sibling inbred and housed in our animal facility, which is accredited by Mexico’s Secretariat of Agriculture and Rural Development (SADER–SENASICA). High-level husbandry was provided: breeding rooms operated under positive pressure and HEPA-filtered air supply and had an anteroom for donning and removing disposable PPE; conventional housing rooms had air showers (bioBUBBLE, Fort Collins, CO) at entry points, and PPE was required for personnel to enter. Rats used in this study were obtained from 4 different litters from different mothers and were identified by ear tags. Rats were transferred to the housing rooms when they were 11 wk old and were kept in groups of 3 or 4 rats in static cages without filter tops (48 × 37.5 × 21 cm). Cages contained autoclaved bedding (7090 Teklad Sani-chips, Envigo, Indianapolis, IN) and sterile cardboard tunnels for environmental enrichment. Cages were cleaned twice weekly, and fresh sterile bedding and cardboard tunnels were added. Housing room conditions were 22 ± 2 °C, 50% to 70% humidity, a 12:12-h light:dark cycle (lights on, 0600 to 1800), and 15 to 20 air changes hourly. Rats had ad libitum access to ozonated water and chow (5001, Lab Diet, St Louis, MO). All procedures conformed to current Mexican law for the care and use of animals in research33 (NOM-062-Z00–1999) and were approved by our IACUC (protocol no. CRC-57- 14), in accordance with the international Guide for the Care and Use of Laboratory Animals.18
Estrous cycle determination.
Estrous cycle stages were determined based on vaginal cytology. Vaginal epithelium was obtained daily from all rats between 0900 and 1100 by using a cotton swab wetted with 0.9% saline. Swab material was transferred to a glass slide, stained with 0.1% crystal violet and evaluated using conventional microscopy with 10× and 40× objective lenses. Estrous stages were identified as follows: proestrus, nucleated cells; estrus, anucleated cornified cells; metestrus, leukocytes, cornified, and nucleated epithelial cells in similar proportions; and diestrus, predominance of leukocytes.27 Estrous cycle determination started when female rats were 13 wk old, and only female rats that had exhibited 2 consecutive 4- to 5-d estrous cycles were used in the study. Rats were handled by the same 2 persons during estrous cycle determination and BP measurements.
BP measurements.
BP measurement began when the rats were 16 wk old. After estrous phase determination, they underwent BP and HR determination that was performed using a noninvasive tail-cuff sphygmomanometric technique (LE 5002, Panlab Harvard Apparatus, Holliston, MA). Rats were habituated to staying in an acrylic rat restrainer for 15 min, with the cuff and transducer placed on their tails, for 4 days before the measurements started. During this habituation process we performed 1 or 2 BP measurements to accustom the rats to the noise of the equipment and the pressure applied by the tail cuff. The room was kept free of environmental noises, other than those produced by the measurement equipment. The same 2 female personnel always handled the rats for habituation and BP measurements. These activities were performed between 1100 and 1300, after body weight and estrous cycle stage had been determined. Rats were prepared for BP and HR measurements by warming them for 10 min using a 38 °C heating pad under their polycarbonate cages, as described previously.22,24,26 Immediately afterward, the rats were introduced into the acrylic rat restrainer that had an adjustable nose piece. Rats in restrainers were placed on a 30 °C heating pad. The pressure cuff and pulse transducer were positioned over the end of the tail, and rats were allowed to stabilize for 5 min before we measured their BP and HR. Acclimation to handling and the vasodilation produced by the elevated body temperature helped the rats to remain calm in the restrainers, allowing reliable measurements. Furthermore, keeping rats’ body temperature above 25 °C increases blood circulation in the tail; therefore, measuring BP and HR at 30 °C facilitated detection of tail artery pulsations.14,26 HR was monitored, and measurements were obtained automatically. Systolic BP (SBP), diastolic BP (DBP), and calculated mean BP (MBP) were presented for visualization in real time. Four readings of SBP and DBP were measured on each day of the estrous cycle, and MBP values were calculated for each reading. One complete estrous cycle was measured each week for 4 consecutive weeks, and the daily mean and standard error data were used to prepare time-course plots.
Statistical analysis.
Four consecutive measures of SBP, DBP, MBP, and HR, which were obtained during 4 estrous cycles from each of the 10 SHR and 9 WKY rats, were averaged for analysis. The effects of estrous stage were analyzed by using the nonparametric Friedman test to compare SBP, DBP, MBP and HR mean values that corresponded to the 4 recorded estrous cycles of both strains of rats. A P value of less than 0.05 was defined as significant. Data from individual rats are displayed by using box plots, which show the average values in percentiles. We also determined the coefficient of variation (CV) by dividing the standard deviation by the mean for all measures from each rat, thus evaluating data variability during the 4 estrous cycles. The Mann–Whitney U test was used to compare WKY and SHR rats, with a P value of less than 0.001 defined as significant. Functions Friedman and Ranksum of the Statistics and Machine Learning Toolbox of MATLAB (The MathWorks, Natick, MA) were used for statistical analysis.
Results
To determine whether the estrous cycle affects BP and HR in young female SHR and WKY rats, we measured SBP, DBP, MBP, and HR during 4 consecutive estrous cycles within the ages of 16 and 19 weeks and generated time-course plots for BP (Figure 1) and HR (Figure 2). As expected, female SHR rats had consistently higher BP and HR than did female WKY rats, indicating that hypertension had developed fully in the SHR rats. Our time course plots show that neither BP nor HR in either SHR or WKY rats showed a periodic pattern related to the stage of the estrous cycle.
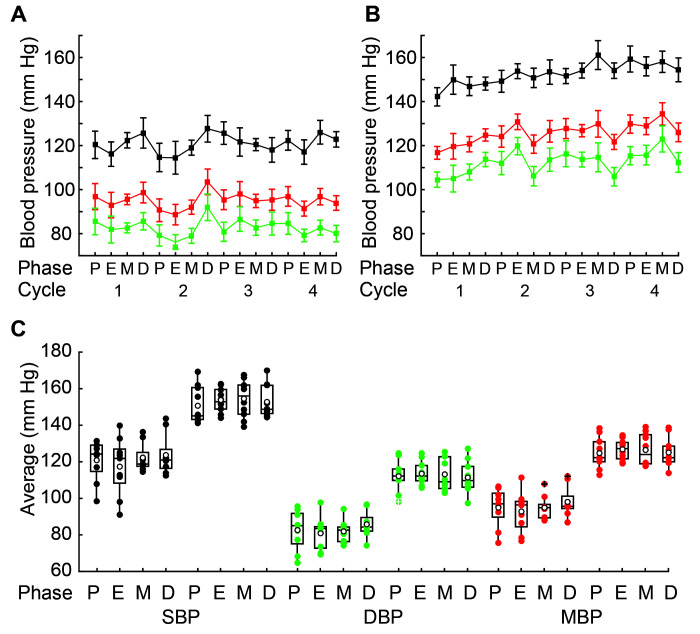
Figure 1.
Blood pressure (BP) of female WKY and SHR rats. Time course of BP values in (A) WKY and (B) SHR female rats across the estrous cycle for 4 consecutive weeks (age, 16 through 19 wk). Lines connect the mean values obtained for all rats during each consecutive estrous phase; vertical lines indicate mean standard error. (C) Box plot representation of average BP values for individual female WKY and SHR rats throughout the estrous cycle. Plots show the distribution of mean values of 4 estrous cycles in percentiles: boxes represent the interquartile range between the 25th and 75th percentiles (second and third quartile), and whiskers indicate the minimum value of the 25th percentile (first quartile) and the 75th percentile and maximum value (fourth quartile). Filled circles indicate the average value for each rat in each estrous stage; open circles show population mean values for each estrous stage; crossed circles depict outliers; and horizontal lines within boxes indicate median values. Black, systolic BP; green, diastolic BP; red, mean BP; P, proestrus; E, estrus; M, metestrus; D, diestrus. Blood pressure values did not differ significantly between any estrous stages of either WKY or SHR rats (P > 0.05 in all cases [Friedman test, Table 1]).
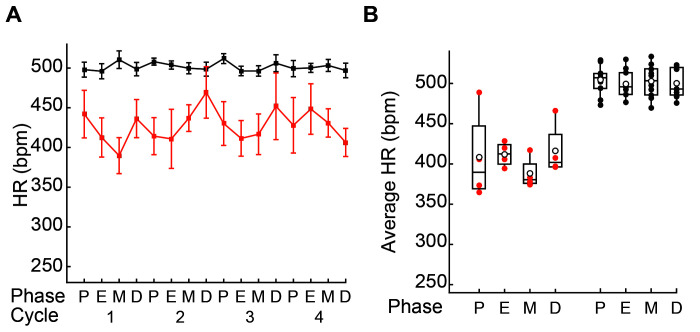
Figure 2.
Heart rate (HR) values in female WKY and SHR rats. (A) Time course of HR values (mean ± SE) in female WKY (orange) and SHR (gray) rats during each phase of 4 consecutive estrous cycles across 4 consecutive weeks (age, 16 through 19 wk). (B) Box plot representation of the average HR values for individual female WKY and SHR rats during each phase of the estrous cycle. Filled circles indicate the average for each rat in each estrous stage; open circles show population mean values for each estrous stage; horizontal lines in the boxes indicate median values; P, proestrus; E, estrus; M, metestrus; D, diestrus. HR values were not significantly different between any estrous stages of either WKY or SHR female rats (P > 0.05 in all cases [Friedman test, Table 1]).
We next averaged the measurements obtained during 4 consecutive estrous cycles during weeks 16 through 19 to obtain a single BP and HR value for each stage of the estrous cycle in 10 SHR and 9 WKY female rats. The individual average BP and HR values obtained for each rat were portrayed in box plots (Figures 1 C and 2 B). The distribution of BP and HR data for the various stages of the estrous cycle had a high degree of overlap in most of the quartiles for both hypertensive SHR and normotensive WKY female rats. Consistent with the data distribution, Friedman tests found no statistical differences in BP or HR among the various stages of the estrous cycle of the SHR and WKY female rats (P > 0.05 in all cases, Table 1). Furthermore, population mean values for BP and HR during each phase of the estrous cycle for SHR and WKY female rats (Figure 1 C and 2 B) showed overlap between the standard errors for these means, indicating that no significant difference existed between the population mean values (data not shown). In other words, neither BP nor HR data differed between various stages of the estrous cycles of normotensive WKY and hypertensive SHR rats.
Table 1.
Results of Friedman tests after multiple comparison of blood pressure (BP) and heart rate values obtained during various stages of the estrous cycle in individual WKY and SHR female rats
| WKY | SHR | |
|---|---|---|
| Systolic BP | F3,35 = 1.52, P = 0.68 | F3,39 = 3.72, P = 0.29 |
| Diastolic BP | F3,35 = 3.53, P = 0.32 | F3,39 = 1.44, P = 0.70 |
| Mean BP | F3,35 = 2.20, P = 0.53 | F3,39 = 0.12, P = 0.99 |
| Heart rate | F3,35 = 3.00, P = 0.39 | F3,39 = 1.08, P = 0.78 |
No statistically significant estrous-dependent differences (P < 0.05) were identified, and no posthoc analysis was applied.
HR data obtained from the normotensive WKY rats had a broader range distribution than the HR data for the hypertensive SHR rats, based on the standard errors for the HR time course data (Figure 2 A). To analyze the variability of values obtained from the 2 strains, we calculated the average CV of the SBP, DBP, MBP, and HR measures (Figures 1 A and B and 2 A) and prepared box plots from the data of each rat (Figure 3). Comparison of the BP average CV distribution of WKY and SHR rats shows strong overlap in most of the quartiles, whereas the HR average CV distribution of the 2 trains did not overlap (i.e., boxes containing half of the data are completely separate). Consistent with the visual inspection of the box plots (Figure 3), we found that BP average CV for SHR and WKY female rats are not significantly different (Table 2). In contrast to BP measurements, HR average CV differed (P < 0.001) between WKY and SHR female rats (Table 2). Overall, our analysis reveals larger variability in the HR values of female WKY rats compared with female SHR rats, whereas female rats of these 2 strains showed similar variability in their BP data.
Figure 3.
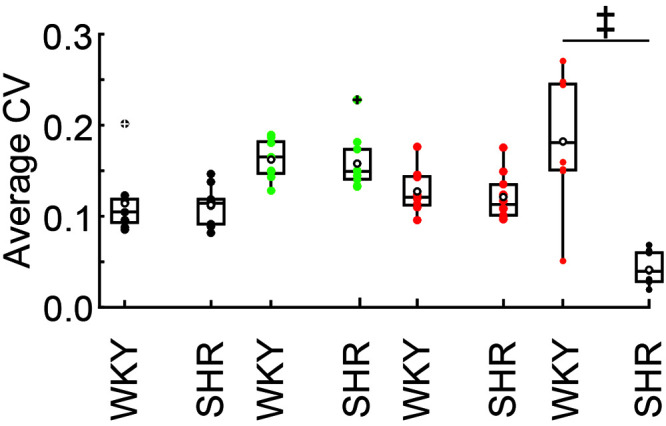
Coefficients of variation (CV) for blood pressure (BP) and heart rate (HR) in female WKY and SHR rats. Filled circles represent average CV values for systolic BP (black), diastolic BP (green), mean BP (red), and HR (orange, WKY; gray, SHR) for individual rats; open circles show population means; and crossed circles depict outliers. The average CV for HR differed significantly (‡, P < 0.001 [Mann–Whitney U test, Table 2]) between WKY and SHR female rats.
Table 2.
Results of Mann–Whitney U tests comparing the average coefficients of variation between WKY and SHR female rats
| Statistics | |
|---|---|
| Systolic blood pressure | U9,10 = 86, P = 0.78 |
| Diastolic blood pressure | U9,10 = 99, P = 0.50 |
| Mean blood pressure | U9,10 = 100, P = 0.45 |
| Heart ratea | U9,10 = 132, P = 0.0002 |
P < 0.001 between values for WKY and SHR female rats
Discussion
The present study used 16- to 19-wk-old nonanesthetized female SHR and WKY rats and a noninvasive tail-cuff sphygmomanometric method to measure HR and BP. The data indicate that daytime SBP, DBP, MBP, and HR do not show a periodic pattern with respect to the stage of the estrous cycle (Figures 1 and 2). We found no statistically significant differences in daytime BP or HR data related to the phase of the estrous cycle in either strain (Figures 1 and 2, Table 1). Consistent with our results, a previous study that measured MBP using carotid artery cannulation of anesthetized rats also found no significant differences throughout the estrous cycle of 16-wk-old WKY and SHR rats.25 Similarly, another study24 found that BP values did not change with regard to the phase of the estrous cycle in nonanesthetized 6- to 15-wk-old SHR when measured using the noninvasive tail-cuff sphygmomanometric method. Furthermore, a study that used telemetry and noninvasive methods12 did not find significant estrous-dependent changes in SBP or DBP in 10- to 34-wk-old normotensive WKY rats. Taken together, these previous studies,12,24,25 and our current results suggest that the changes in physiological hormonal levels that occur during the estrous cycle do not modify daytime BP or HR in SHR or WKY rats. These data indicate that daytime BP and HR data of female normotensive WKY and hypertensive SHR rats can be averaged as a group without consideration of the estrous cycle stage.
Although reported BP values are not different between normotensive WKY male and female rats, BP is higher in male SHR and stroke-prone spontaneously hypertensive rats (SHRSP) as compared with females of those strains.8,13,28 These results suggest a protective role for female gonadal hormones against the development of more severe hypertension in female SHR rats.42,46 Furthermore, previous reports have shown that estrogens generate a rapid vasodilatory effect, presumably attained through the production and release of nitric oxide from the endothelium.2,29 These studies suggest that the acute vasodilatory effect mediated by estrogens could produce variations in BP parameters. In this regard, one study44 reported small variations in MBP and HR based on estrous and circadian phases in normotensive female Wistar–Imamichi rats, as measured by telemetry. The differences were most prominent on the night of proestrus. The author44 concluded that, these changes, although minimal, could be due to the different concentrations of estrogens present at the various stages of the estrous cycle. However, daytime BP measurements throughout the estrous cycle,12,24,25 including our own values for SHR and WKY female rats, do not support that conclusion.44 We and others12,24,25 have found that daytime BP values do not vary significantly across different phases of the estrous cycle, thus undermining the hypothesis of acute estrogen-mediated release of nitric oxide as the main mechanism that regulates BP during the rat estrous cycle.12 However, we cannot rule out the possibility of a significant effect during the evening of proestrus, because we did not measure BP during the circadian dark phase.
HR is reportedly higher and has a lower CV in hypertensive male SHR and SHRSP rats as compared with normotensive male WKY rats.8,20,40,45 In our study, female SHR rats had higher HR (Figure 2 A and 2 B) and a significantly lower CV for HR (Figure 3 and Table 2) than did female WKY rats. Our results for female HR agree with previous studies of HR in normotensive WKY and hypertensive SHR and SHRSP female rats.1,3,8–10,47 However, in contrast to our current results, one study found that daytime HR was considerably higher in WKY females than in SHR females.28 These differences between that study and ours might be related to differences in the age of the rats (6 mo compared with 4 mo in our study), the source of the rats, and the HR measurement method used (telemetry compared with noninvasive tail-cuff sphygmomanometry). The higher HR and lower variation found in hypertensive male and female SHR rats might reflect a deficit in the vagal baroreceptor reflex,6,15,16,21,31 acting in combination with the cardiac hypertrophy that also develops in these rats.1,16
In summary, our study shows that when measured around noon, SBP, DBP, MBP, and HR values are stable throughout the estrous cycle of young female hypertensive SHR and normotensive WKY rats. These results suggest that changes in the physiologic concentrations of estradiol generated during the estrous cycle do not influence BP or HR in either of these strains, as assessed by noninvasive tail-cuff sphygmomanometric BP monitoring during the light phase. Our data lead us to conclude that future research using SHR and WKY strains can average values for BP and HR measured in nonanesthetized female rats during the light phase, regardless of the estrous phase.
Acknowledgments
We thank Héctor A Malagón Rivero, Mario A Arias-García, and Josué Ramírez-Jarquín for their comments on this paper; Carlos Lugo, Alejandro Monroy, and Jesús Moya for providing conscientious care of our animals; Ivett Rosas, Juan Manuel Barbosa, and Gerardo Coello for computer maintenance; Sandra Moncada and Javier Gallegos for library services; and Lourdes Valenzuela, Rolando Hernández-Muñoz, and Humberto Cruzblanca for their invaluable help in critical reading and academic editing of this manuscript.
References
- 1.Adams MA, Bobik A, Korner PI. 1989. Differential development of vascular and cardiac hypertrophy in genetic hypertension. Relation to sympathetic function. Hypertension 14:191–202. 10.1161/01.HYP.14.2.191. [DOI] [PubMed] [Google Scholar]
- 2.Case J, Davison CA. 1999. Estrogen alters relative contributions of nitric oxide and cyclooxygenase products to endothelium-dependent vasodilation. J Pharmacol Exp Ther 291:524–530. [PubMed] [Google Scholar]
- 3.Chandler MP, DiCarlo SE. 1998. Acute exercise and gender alter cardiac autonomic tonus differently in hypertensive and normotensive rats. Am J Physiol 274:R510–R516. 10.1152/ajpregu.1998.274.2.R510. [DOI] [PubMed] [Google Scholar]
- 4.Chen YF, Meng QC. 1991. Sexual dimorphism of blood pressure in spontaneously hypertensive rats is androgen dependent. Life Sci 48:85–96. 10.1016/0024-3205(91)90428-E. [DOI] [PubMed] [Google Scholar]
- 5.Clayton JA, Collins FS. 2014. Policy: NIH to balance sex in cell and animal studies. Nature 509:282–283. 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbett EKA, David ASG, Mary DASG, McWilliam PN, Batten TFC. 2007. Age-related loss of cardiac vagal preganglionic neurons in spontaneously hypertensive rats. Exp Physiol 92:1005–1013. 10.1113/expphysiol.2007.038216. [DOI] [PubMed] [Google Scholar]
- 7.Costa HA, Dias CJM, Martins VA, de Araujo SA, da Silva DP, Mendes VS, de Oliveira MNS, Jr, Mostarda CT, Borges ACR, Ribeiro RM, Filho NS. 2021. Effect of treatment with carvacrol and aerobic training on cardiovascular function in spontaneously hypertensive rats. Exp Physiol 106:891–901. 10.1113/EP089235. [DOI] [PubMed] [Google Scholar]
- 8.Davidson AO, Schork N, Jaques BC, Kelman AW, Sutcliffe RG, Reid JL, Dominiczak AF. 1995. Blood pressure in genetically hypertensive rats. Influence of the Y chromosome. Hypertension 26:452–459. 10.1161/01.HYP.26.3.452. [DOI] [PubMed] [Google Scholar]
- 9.Dias DPM, Oliveira M, Salgado HC, Fazan R, Jr. 2010. Ovariectomy does not affect the cardiac sympathovagal balance of female SHR but estradiol does. Braz J Med Biol Res 43:969–975. 10.1590/S0100-879X2010007500105. [DOI] [PubMed] [Google Scholar]
- 10.Friberg P, Karlsson B, Nordlander M. 1988. Sympathetic and parasympathetic influence on blood pressure and heart rate variability in Wistar–Kyoto and spontaneously hypertensive rats. J Hypertens Suppl 6:S58–S60. 10.1097/00004872-198812040-00014. [DOI] [PubMed] [Google Scholar]
- 11.Gan Z, Huang D, Jiang J, Li Y, Li H, Ke Y. 2018. Captopril alleviates hypertension-induced renal damage, inflammation, and NFκB activation. Braz J Med Biol Res 51:e7338. 10.1590/1414-431x20187338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundt A, Grundt C, Knoth K, Lemmer B. 2010. Rhythmic supplementation of 17β-estradiol according to the physiological estrous cycle: Effect on blood pressure in female ovariectomized rats. Horm Metab Res 42:130–136. 10.1055/s-0029-1241807. [DOI] [PubMed] [Google Scholar]
- 13.Grundt C, Meier K, Lemmer B. 2006. Gender dependency of circadian blood pressure and heart rate profiles in spontaneously hypertensive rats: effects of beta-blockers. Chronobiol Int 23:813–829. 10.1080/07420520600827129. [DOI] [PubMed] [Google Scholar]
- 14.Harvard Apparatus. 2012. [Internet]. LE 5002 noninvasive blood pressure meter V25/05/12. User Manual. Panlab Harvard Apparatus. [Cited 22 June 2021]. Available at: www.harvardapparatus.com/media/manuals/Product%20Manuals/LE5002_NIBP_EN.pdf
- 15.Head GA, Adams MA. 1992. Characterization of the baroreceptor heart rate reflex during development in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 19:587–597. 10.1111/j.1440-1681.1992.tb00509.x. [DOI] [PubMed] [Google Scholar]
- 16.Head GA. 1994. Cardiac baroreflexes and hypertension. Clin Exp Pharmacol Physiol 21:791–802. 10.1111/j.1440-1681.1994.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 17.Hom S, Fleegal MA, Egleton RD, Campos CR, Hawkins BT, Davis TP. 2007. Comparative changes in the blood–brain barrier and cerebral infarction of SHR and WKY rats. Am J Physiol Regul Integr Comp Physiol 292:R1881–R1892. 10.1152/ajpregu.00761.2005. [DOI] [PubMed] [Google Scholar]
- 18.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 19.Joyner MJ, Wallin BG, Charkoudian N. 2016. Sex differences and blood pressure regulation in humans. Exp Physiol 101:349–355. 10.1113/EP085146. [DOI] [PubMed] [Google Scholar]
- 20.Kollarova M, Puzserova A, Balis P, Radosinska D, Tothova L, Bartekova M, Barancik M, Radosinska J. 2020. Age- and phenotype-dependent changes in circulating MMP2 and MMP9 activities in normotensive and hypertensive rats. Int J Mol Sci 21:7286. 10.3390/ijms21197286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau EOC, Lo CY, Yao Y, Tat Mak AF, Jiang L, Huang Y, Yao X. 2016. Aortic baroreceptors display higher mechanosensitivity than carotid baroreceptors. Front Physiol 7:384. 10.3389/fphys.2016.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KH, Bae IY, Park SI, Park JD, Lee HG. 2016. Antihypertensive effect of Korean red ginseng by enrichment of ginsenoside RG3 and arginine–fructose. J Ginseng Res 40:237–244. 10.1016/j.jgr.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leong XF, Ng CY, Jaarin K. 2015. Animal models in cardiovascular research: Hypertension and atherosclerosis. Biomed Res 2015:528757. 10.1155/2015/528757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, Ely D. 2011. Testosterone increases: Sodium reabsorption, blood pressure, and renal pathology in female spontaneously hypertensive rats on a high-sodium diet. Adv Pharmacol Sci 2011:817835. 10.1155/2011/817835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loh SY, Salleh N. 2017. Influence of testosterone on mean arterial pressure: A physiological study in male and female normotensive WKY and hypertensive SHR rats. Physiol Int 104:25–34. 10.1556/2060.104.2017.1.3. [DOI] [PubMed] [Google Scholar]
- 26.López-Fernández-Sobrino R, Margalef M, Torres-Fuentes C, Ávila-Román J, Aragonès G, Muguerza B, Bravo FI. 2021. Enzyme-assisted extraction to obtain phenolic-enriched wine lees with enhanced bioactivity in hypertensive rats. Antioxidants 10:517. 10.3390/antiox10040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcondes FK, Bianchi FJ, Tanno AP. 2002. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz J Biol 62 4a:609–614. 10.1590/S1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- 28.Maris ME, Melchert RB, Joseph J, Kennedy RH. 2005. Gender differences in blood pressure and heart rate in spontaneously hypertensive and Wistar–Kyoto rats. Clin Exp Pharmacol Physiol 32:35–39. 10.1111/j.1440-1681.2005.04156.x. [DOI] [PubMed] [Google Scholar]
- 29.Mendelsohn ME. 2002. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol 90:F3–F6. 10.1016/S0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- 30.Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, McCarthy MM, Sohrabji F, Schiebinger L. 2017. Considering sex as a biological variable in preclinical research. FASEB J 31:29–34. 10.1096/fj.201600781r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy CA, Sloan RP, Myers MM. 1991. Pharmacologic responses and spectral analyses of spontaneous fluctuations in heart rate and blood pressure in SHR rats. J Auton Nerv Syst 36:237–250. 10.1016/0165-1838(91)90047-7. [DOI] [PubMed] [Google Scholar]
- 32.National Institutes of Health. [Internet]. 2015. Consideration of sex as a biological variable in NIH- funded research. [Cited 6 January 2021]. Available at: http://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-102.html. http://orwh.od.nih.gov/sexinscience/overview/pdf/NOT-OD-15-102_Guidance.pdf
- 33.Norma Oficial Mexicana. [Internet]. 1999. Norma Oficial Mexicana NOM-062-ZOO-1999, especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. [Cited 01 February 2021]. Available at: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf [Document in Spanish].
- 34.Okamoto K, Aoki K. 1963. Development of a strain of spontaneously hypertensive rats. Jpn Circ J 27:282–293. 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 35.Peng F, Lin J, Lin L, Tang H. 2012. Transient prehypertensive treatment in spontaneously hypertensive rats: A comparison of losartan and amlodipine regarding long-term blood pressure, cardiac, and renal protection. Int J Mol Med 30:1376–1386. 10.3892/ijmm.2012.1153. [DOI] [PubMed] [Google Scholar]
- 36.Ramírez-Torres G, Ontiveros N, Lopez-Teros V, Ibarra-Diarte JA, Reyes-Moreno C, Cuevas-Rodríguez EO, Cabrera-Chávez F. 2017. Amaranth protein hydrolysates efficiently reduce systolic blood pressure in spontaneously hypertensive rats. Molecules 22:1905. 10.3390/molecules22111905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reckelhoff JF. 2018. Gender differences in hypertension. Curr Opin Nephrol Hypertens 27:176–181. 10.1097/MNH.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 38.Regitz-Zagrosek V. 2012. Sex and gender differences in health: Science and society series on sex and science. EMBO Rep 13:596–603. 10.1038/embor.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribeiro RM, Pinheiro Neto VF, Ribeiro KS, Vieira DA, Abreu IC, Silva Sdo N, Cartágenes Mdo S, Freire SM, Borges AC, Borges MO. 2014. Antihypertensive effect of Syzygium cumini in spontaneously hypertensive rats. Evid Based Complement Alternat Med 2014:605452. 10.1155/2014/605452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricksten SE, Lundin S, Thoren P. 1984. Spontaneous variations in arterial blood pressure, heart rate, and sympathetic nerve activity in conscious normotensive and spontaneously hypertensive rats. Acta Physiol Scand 120:595–600. 10.1111/j.1748-1716.1984.tb07425.x. [DOI] [PubMed] [Google Scholar]
- 41.Secreto G, Sieri S, Agnoli C, Grioni S, Muti P, Zumoff B, Sant M, Meneghini E, Krogh V. 2016. A novel approach to breast cancer prevention: Reducing excessive ovarian androgen production in elderly women. Breast Cancer Res Treat 158:553–561. 10.1007/s10549-016-3901-1. [DOI] [PubMed] [Google Scholar]
- 42.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. 2004. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 62:587–593. 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Tadic M, Cuspidi C, Grassi G, Ivanovic B. 2019. Gender-specific therapeutic approach in arterial hypertension - Challenges ahead. Pharmacol Res 141:181–188. 10.1016/j.phrs.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 44.Takezawa H, Hayashi H, Sano H, Saito H, Ebihara S. 1994. Circadian and estrous cycle–dependent variations in blood pressure and heart rate in female rats. Am J Physiol 267:R1250–R1256. 10.1152/ajpregu.1994.267.5.R1250. [DOI] [PubMed] [Google Scholar]
- 45.Valenti VE, Ferreira C, Meneghini A, Ferreira M, Murad N, Ferreira Filho C, Correa JA, Abreu LC, Colombari E. 2009. Evaluation of baroreflex function in young spontaneously hypertensive rats. Arq Bras Cardiol 92:205–209. 10.1590/s0066-782x2009000300009. [DOI] [PubMed] [Google Scholar]
- 46.Wassmann S, Bäumer AT, Strehlow K, Van Eickels M, Grohé C, Ahlbory K, Rösen R, Böhm M, Nickenig G. 2001. Endothelial dysfunction and oxidative stress during estrogen deficiency in spontaneously hypertensive rats. Circulation 103:435–441. 10.1161/01.CIR.103.3.435. [DOI] [PubMed] [Google Scholar]
- 47.Widdop RE, Verberne AJ, Louis WJ, Jarrott B. 1990. The effect of ketanserin on cardiovascular reflexes in conscious normotensive and spontaneously hypertensive rats. Eur J Pharmacol 186:17–28. 10.1016/0014-2999(90)94056-4. [DOI] [PubMed] [Google Scholar]
- 48.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I. 2018. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 39:3021–3104. 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 49.Yasui T, Matsui S, Tani A, Kunimi K, Yamamoto S, Irahara M. 2012. Androgen in postmenopausal women. J Med Invest 59:12–27. 10.2152/jmi.59.12. [DOI] [PubMed] [Google Scholar]