Abstract
Objective
To evaluate longer term symptoms and health outcomes associated with post-covid-19 condition within a cohort of individuals with a SARS-CoV-2 infection.
Design
Population based, longitudinal cohort.
Setting
General population of canton of Zurich, Switzerland.
Participants
1106 adults with a confirmed SARS-CoV-2 infection who were not vaccinated before infection and 628 adults who did not have an infection.
Main outcome measures
Trajectories of self-reported health status and covid-19 related symptoms between months six, 12, 18, and 24 after infection and excess risk of symptoms at six months after infection compared with individuals who had no infection.
Results
22.9% (95% confidence interval 20.4% to 25.6%) of individuals infected with SARS-CoV-2 did not fully recover by six months. The proportion of individuals who had an infection who reported not having recovered decreased to 18.5% (16.2% to 21.1%) at 12 months and 17.2% (14.0% to 20.8%) at 24 months after infection. When assessing changes in self-reported health status, most participants had continued recovery (68.4% (63.8% to 72.6%)) or had an overall improvement (13.5% (10.6% to 17.2%)) over time. Yet, 5.2% (3.5% to 7.7%) had a worsening in health status and 4.4% (2.9% to 6.7%) had alternating periods of recovery and health impairment. The point prevalence and severity of covid-19 related symptoms also decreased over time, with 18.1% (14.8% to 21.9%) reporting symptoms at 24 months. 8.9% (6.5% to 11.2%) of participants reported symptoms at all four follow-up time points, while in 12.5% (9.8% to 15.9%) symptoms were alternatingly absent and present. Symptom prevalence was higher among individuals who were infected compared with those who were not at six months (adjusted risk difference 17.0% (11.5% to 22.4%)). Excess risk (adjusted risk difference) for individual symptoms among those infected ranged from 2% to 10%, with the highest excess risks observed for altered taste or smell (9.8% (7.7% to 11.8%)), post-exertional malaise (9.4% (6.1% to 12.7%)), fatigue (5.4% (1.2% to 9.5%)), dyspnoea (7.8% (5.2% to 10.4%)), and reduced concentration (8.3% (6.0% to 10.7%)) and memory (5.7% (3.5% to 7.9%)).
Conclusions
Up to 18% of individuals who were not vaccinated before infection had post-covid-19 condition up to two years after infection, with evidence of excess symptom risk compared with controls. Effective interventions are needed to reduce the burden of post-covid-19 condition. Use of multiple outcome measures and consideration of the expected rates of recovery and heterogeneity in symptom trajectories are important in the design and interpretation of clinical trials.
Study registration
Current Controlled Trials ISRCTN14990068
Current Controlled Trials ISRCTN18181860
Introduction
Post-covid-19 condition affects 20-30% of unvaccinated individuals three to six months after SARS-CoV-2 infection.1 2 3 4 5 6 7 8 9 10 11 12 13 To adequately inform patients, healthcare providers, and policy makers, not only is determining prevalence important but also its natural course over time.14 As evidence on the substantial public health burden of post-covid-19 condition accumulates, clinical trials are necessary to establish interventions that accelerate recovery or provide relief for associated symptoms. A solid understanding of trajectories and relevant outcome measures of post-covid-19 condition is required to effectively design and adequately interpret such trials.
Several studies assessed long term outcomes related to post-covid-19 condition and found that 22-75% of individuals had symptoms for more than a year after infection.3 15 16 17 18 19 20 21 22 23 24 25 Most of these studies included specific populations, such as patients in hospital, focused on a specific dimension of post-covid-19 condition, did not have a prospective follow-up, or suffered from relevant attrition rates. Thus, these studies might not reflect the variability of symptoms and recovery over time, and their generalisability across the severity spectrum of acute covid-19 might be limited. Additionally, most studies assessing post-covid-19 condition did not have a comparator group, and their findings were often questioned because many reported symptoms were non-specific and common in the general population without an infection. Limited knowledge and no consensus on core outcomes regarding post-covid-19 condition has led to the use of various outcome measures across observational studies affecting their comparability.26 These issues also impair the interpretation of trials of interventions targeting post-covid-19 condition and their translation into healthcare policy and clinical practice.
In this study, we aimed to address some of the limitations of the existing evidence by comprehensively characterising the course of post-covid-19 condition within a population based, longitudinal cohort of individuals who were infected by SARS-CoV-2. We aimed to describe patterns of recovery and symptom persistence over 24 months and to determine the attributable risk of related symptoms by comparing their prevalence in individuals who were individuals with a general population cohort with no evidence of past infection.
Methods
Study design and participant recruitment
This analysis is based on the Zurich SARS-CoV-2 Cohort (ISRCTN14990068), an ongoing, population based, prospective study of individuals with confirmed SARS-CoV-2 infection. We recruited participants through the Department of Health of the canton of Zurich, which is notified of all diagnosed SARS-CoV-2 cases in the canton through mandatory reporting. We evaluated all individuals for whom information was available for eligibility assessment. Adults (≥18 years) were eligible if they were residing in the canton of Zurich, able to follow study procedures, and had sufficient knowledge of the German language. We invited a daily age stratified (18-39, 40-64, ≥65 years) random sample of eligible individuals to participate.
We enrolled individuals diagnosed with a PCR confirmed SARS-CoV-2 infection between 06 August 2020 and 19 January 2021 on or as soon as possible after diagnosis. All participants were infected with the wildtype (Wuhan-Hu-1) SARS-CoV-2 strain,27 28 enrolled before covid-19 vaccine roll-out, and none had previously been infected.
We obtained written or electronic consent from all participants. The study was prospectively registered and approved by the local ethics committee. This study is reported according to the STROBE statement.29
Uninfected comparator group
To compare health outcomes among individuals who have an infection with those in a comparable population who do not have an infection, we used data from phase 4 of the Corona Immunitas Zurich seroprevalence study (ISRCTN18181860).30 31 For this study, individuals were randomly selected from the general population of the canton of Zurich by use of age stratified sampling (20-64 years, ≥65 years) and invited to participate. For the comparator group, we excluded participants who had a SARS-CoV-2 infection in the past or at the time of enrolment. We used data collected at the time of enrolment, corresponding to the timeframe of the six month assessment of individuals recruited in the Zurich SARS-CoV-2 Cohort (May to August 2021). This method was to minimise any potential influence of the timing of assessments on the outcomes of interest (eg, influence of public health measures or duration of the pandemic on mental health outcomes).
Data sources
We used data collected through electronic questionnaires in the Zurich SARS-CoV-2 Cohort. The baseline questionnaire at enrolment included questions on sociodemographics, self-reported pre-existing comorbidities (ie, hypertension, diabetes mellitus, cardiovascular disease, respiratory disease, chronic renal disease, current or past malignancy, and immune suppression), health status before infection, and details about the acute infection (ie, presence and self-reported severity of symptoms and hospitalisation). Follow-up questionnaires included questions relating to symptoms and physical and mental health; they were completed at two weeks and months 1, 3, 6, 9, 12, 18, and 24 after infection. Response rates up to 12 months ranged between 88% and 96% (supplementary table 1). Participants initially consented to participation for a year and were asked to consent for further biannual assessments after completion of the 12 month assessment.
We further used data collected in the baseline questionnaire of phase 4 of Corona Immunitas. To ensure comparability, the questionnaire was closely aligned with the Zurich SARS-CoV-2 Cohort questionnaires and included identical questions on sociodemographics, comorbidities, symptoms, and physical and mental health. Longer term follow-up data for the Corona Immunitas population was not available.
Outcomes and measurements
In the absence of an established core outcome set for post-covid-19 condition, we determined study outcomes based on commonly reported patient-relevant health measures and symptoms.26 32
Our primary outcome was the overall relative health status of participants at months 6, 12, 18, and 24 after infection, defined using a combination of two self-reported measurements: recovery status, for which participants were asked whether they had recovered compared with their usual health status before infection, and overall health status using the EuroQol visual analogue scale (EQ-VAS), with cut-off values for different levels of health impairment (EQ-VAS scores of >70 for mild; 51-70 for moderate; ≤50 for severe) determined based on population normative values and studies on chronic obstructive pulmonary disease.33 34 35 In a sensitivity analysis, we used alternative EQ-VAS score cut-offs (>60 for mild; 41-60 for moderate; ≤40 for severe).
Secondary outcomes included the point prevalence and severity of 23 symptoms at months 6, 12, 18, and 24 (supplementary table 2). Self-perceived severity was assessed using a five point Likert-scale and recategorised into three levels (minimal or mild, moderate, and severe or very severe). We also asked participants to indicate whether they perceived the symptoms to be related to covid-19 to differentiate post-covid-19 symptoms from those due to other reasons. We report the complete wording of questions in supplementary table 3.
We further assessed the trajectories of the relative health status and symptoms between six months and 24 months. For relative health status, we grouped individuals into five categories: continued recovery (ie, recovered at all follow-up time points), improved (improvement in the health status category or in EQ-VAS by ≥8 points (corresponding to a minimal important difference)), worsened (deterioration of the health status category or in EQ-VAS by ≥8 points), alternating course (alternating periods of recovery and health impairment), and no change (no change in the level of impairment and changes in EQ-VAS of <8 points). For covid-19 related symptoms, we grouped participants into: continued recovery (symptoms absent at all time points), improved (symptom-free by 24 months), worsened (symptoms absent at six months but reported afterwards), alternating course (symptoms alternatingly present and absent), and no change (symptoms present at all time points).
Additionally, we used scale based assessments to assess adverse health outcomes including fatigue (score ≥22 on the fatigue assessment scale (FAS)), dyspnoea (grade ≥1 on modified Medical Research Council dyspnoea scale (mMRC)), depression (score ≥10 on depression, anxiety, and stress scale-21 (DASS-21)), anxiety (score ≥8 on DASS-21), and stress (score ≥15 on DASS-21), and health related quality of life (ie, impaired mobility, self-care, or usual activities, depression or anxiety, pain or discomfort in the EuroQol 5-dimension 5-level (EQ-5D-5L) instrument). Score calculation for these assessments followed official guidance, as described previously.5 36
Statistical analysis
We descriptively evaluated the relative health status and point prevalence and severity of each of the symptoms among individuals infected with SARS-CoV-2 at each follow-up. We visualised changes in health status and covid-19 related symptoms between six and 24 months using alluvial diagrams37 and descriptively compared the characteristics of participants experiencing the different trajectories. Furthermore, we described the proportion of participants with adverse health outcomes on the basis of scale-based assessments before infection and up to 24 months. All proportions are reported with 95% confidence intervals.
Missing data occurred for two reasons: non-response or loss to follow-up and reinfection (supplementary table 4). We excluded all data reported after reinfection (16 participants at 12 months, 290 at 24 months), defined as a new positive SARS-CoV-2 PCR or antigen test more than 60 days after the initial infection,38 from the main analysis to ensure that evaluated outcomes were unrelated to such reinfections. To account for potential selection bias introduced by these missing data, we applied stabilised inverse probability of censoring weights to all estimates. Inverse probability of censoring weighting at each follow-up questionnaire were derived using logistic regression models predicting the probability of non-missingness at that time point. We selected covariables a priori and considered the following to be related to missingness and post-covid-19 condition: age; sex; body mass index; smoking status; education level; monthly income; and presence of diabetes mellitus; cardiovascular, respiratory, or chronic renal disease; current or past malignancy; and immune suppression. To assess the robustness of our findings, we conducted several sensitivity analyses using multivariate imputation by chained equations (supplementary methods), (non-weighted) available case analysis excluding and including data after reinfection, and complete case analysis.
To evaluate the excess risk of symptoms and adverse health outcomes at six months among individuals who had an infection compared with those who had not, we calculated adjusted absolute risk differences39 and odds ratios using generalised linear models. We calculated inverse probability weighted estimates to account for possible confounding.40 We estimated propensity scores to reflect the probability of a participant belonging to the infected or uninfected group given the covariables mentioned previously, in addition to hypertension. We chose these variables because they are probably associated with being infected with SARS-CoV-241 and developing post-covid-19 condition.42 Based on propensity scores, we calculated inverse probability weights, which we subsequently used for estimating average treatment effects of SARS-CoV-2 infection on symptoms and health outcomes in the whole population (supplementary table 5). Propensity scores of less than 0.1 or more than 0.9 were excluded (n=1 with score >0.9).43 We assessed covariate balance using standardised mean differences (differences <0.1 considered negligible; supplementary figure 1) and found both populations to be similar after applying inverse probability weighting.
We performed all statistical analyses using R (version 4.2.2).
Patient and public involvement
Patients were not directly involved in the design and conduct of the cohort, the development of the research questions and aims, or in the interpretation and writing up of the results. The study was conceived and implemented during the early stages of the pandemic and any patient and public involvement would have been challenging and could have delayed its rapid implementation. However, members of the research team are part of a Long Covid citizen science board,44 through which they are in regular exchange with individuals affected by post-covid-19 condition. This relationship has substantially shaped the research questions addressed by the cohort.
Results
Among 3185 individuals who were eligible and invited to participate in the Zurich SARS-CoV-2 Cohort, 1106 (34.7%) agreed to participate (fig 1). 854 (77%) participants consented to study prolongation and 788 (72%) completed the 24 month assessment. 776 participants completed all questionnaires between months six and 24. Just over half of participants were female (566 (51.2%) of 1106). Of 1106 participants, 951 (86.0%) were symptomatic and 48 (4.3%) were admitted to hospital during acute infection. Compared with individuals who did not agree to take part in the study, participants were younger on average, a lower proportion was admitted to hospital for covid-19 (48 (4.3%) of 1106 v 211 (10.1%) of 2079), and a higher proportion was symptomatic (951 (86.0%) of 1106 v 1652 (79.5%) of 2079; supplementary table 6).
Fig 1.
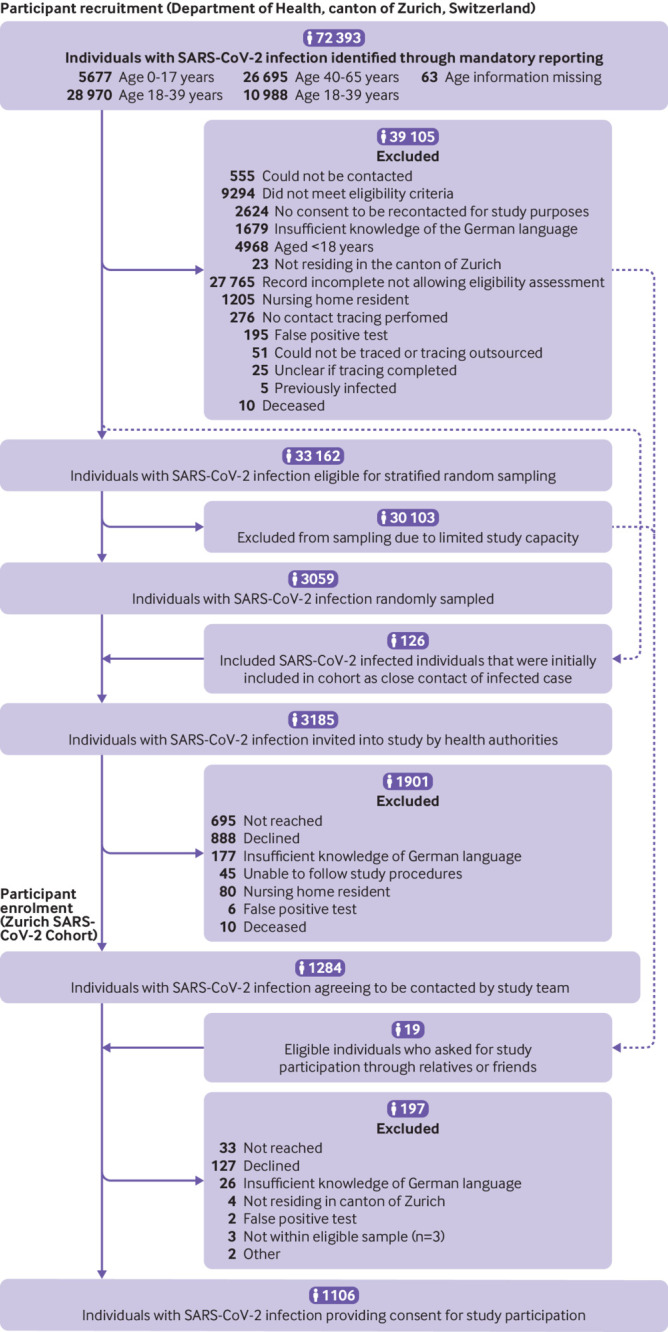
Study enrolment into the Zurich SARS-CoV-2 Cohort study (infected individuals)
Recovery trajectories up to 24 months
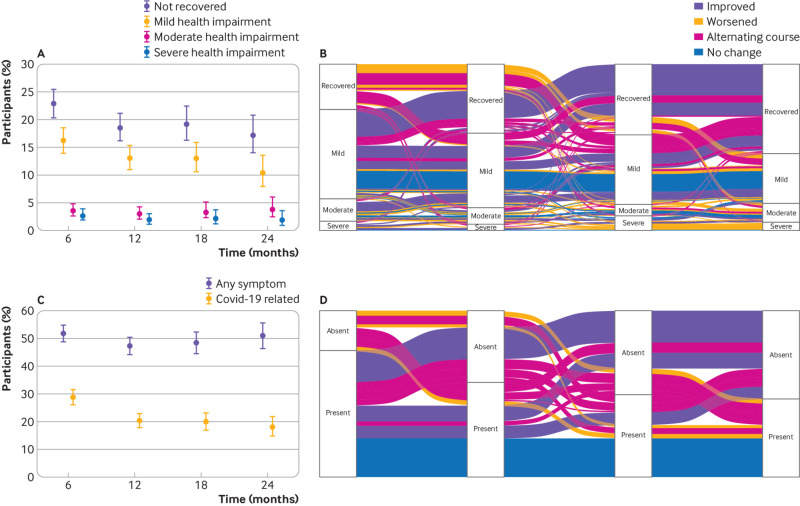
Overall, 55.3% (95% confidence interval 52.3% to 58.3%) reported returning to their normal health status in less than a month after infection, and 17.6% (15.4% to 20.0%) reported recovery within one to three months. By six months, 22.9% (20.4% to 25.6%) of participants reported that they had not yet recovered, with 16.2% (14.1% to 18.6%) having mild, 3.6% (2.6% to 4.9%) having moderate, and 2.7% (1.9% to 3.9%) having severe health impairment (0.4% missing EQ-VAS data) (fig 2A, supplementary table 7). Over time, the proportion of infected individuals reporting non-recovery decreased to 18.5% (16.2% to 21.1%) at 12 months, 19.2% (16.3% to 22.5%) at 18 months, and 17.2% (14.0% to 20.8%) at 24 months. A similar decrease occurred at 24 months in the severity of health impairment with 10.4% (8.0% to 13.5%) having mild, 3.9% (2.5% to 6.0%) having moderate, and 1.9% (1.0% to 3.5%) having severe impairment (1% missing EQ-VAS). Sensitivity analysis using alternative EQ-VAS thresholds resulted in slightly lower proportions with moderate and severe health impairment (supplementary table 8). Analyses restricted to complete cases or including data after reinfection resulted in slightly higher estimates of non-recovery (supplementary figure 2, supplementary table 7).
Fig 2.
(A) Proportions and 95% confidence interval of participants who had not recovered (overall) and with mild, moderate, and severe health impairment at months 6, 12, 18, and 24 after infection. Inverse probability of censoring weighting was applied to all estimates. (B) Alluvial plot showing the transition of participants across the different health states over time (among participants with complete data at all time points and excluding those who reported continued recovery at all follow-up time points). The width of the flow corresponds to the weighted proportion of participants and colours represent the overall trajectory between six and 24 months. (C) Proportions and 95% confidence intervals of participants with any symptom (regardless of whether they were related to covid-19) and symptoms reported by participants to be related to covid-19 at months 6, 12, 18, and 24 after infection. Inverse probability of censoring weighting was applied to all estimates. (D) Alluvial plot showing the transition between presence and absence of self-reported covid-19 related symptoms over time (among participants with complete data at all time points and excluding those who were symptom-free at all follow-up time points). The width of the flow corresponds to the weighted proportion of participants and colours represent the overall symptom trajectory between six months and 24 months
In descriptive trajectory analyses, most participants (68.4% (95% confidence interval 63.8% to 72.6%)) reported continued recovery over time (fig 2B). 13.5% (10.6% to 17.2%) improved or recovered by 24 months, 5.2% (3.5% to 7.7%) worsened, and 4.4% (2.9% to 6.7%) reported stable levels of health impairment. Meanwhile, 8.5% (6.2% to 11.6%) experienced alternating courses of recovery and health impairment (supplementary table 9 for sensitivity analyses of alternative methods to handle missing data). Compared to participants with worsened or unchanged health status, a higher proportion of those who improved were younger than 65 years (55.6% v 40.1%) and a lower proportion had post-exertional malaise (27.3% v 40.6%) at six months (supplementary table 10).
Symptom trajectories up to 24 months
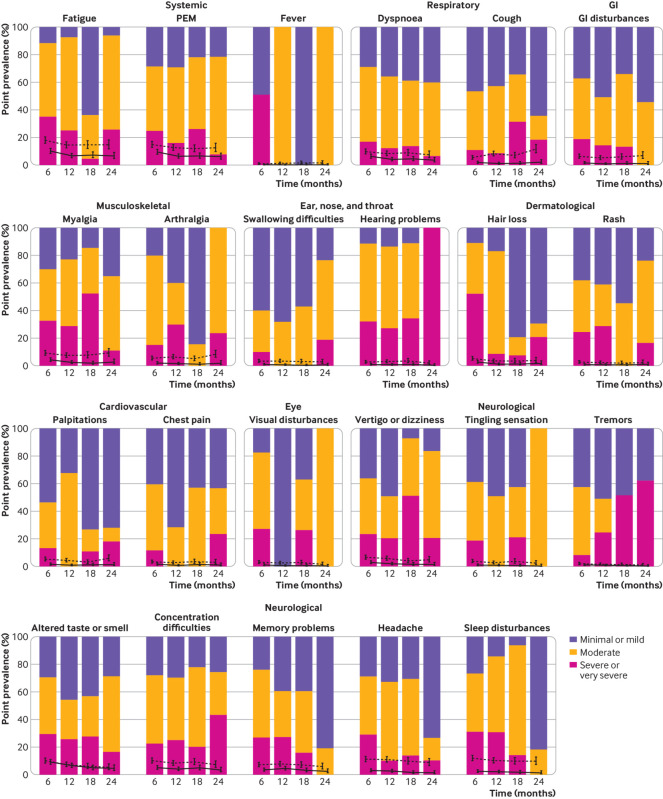
The point prevalence of overall symptoms remained about the same at the follow-up time points (51.7% (48.7% to 54.7%) at six months and 51.0% (46.3% to 55.6%) at 24 months; supplementary table 7). Meanwhile, the prevalence of symptoms considered to be related to covid-19 decreased from 28.9% (26.3% to 31.8%) at six months to 20.3% (17.9% to 23.0%) and 18.1% (14.8% to 21.9%) at 12 and 24 months, respectively (fig 2C, supplementary table 7). Although most (89.2%) participants reporting covid-19 related symptoms also reported non-recovery at 24 months, 5.8% of those with covid-19 related symptoms stated that they had fully recovered. Fatigue, post-exertional malaise , altered taste or smell, dyspnoea, and reduced concentration or memory were the most prevalent symptoms at all time points (fig 3). Sensitivity analyses showed similar patterns (supplementary table 11). Self-reported symptom severity for most symptoms decreased or remained unchanged (fig 3).
Fig 3.
Point prevalence and severity of 23 prespecified symptoms at months 6, 12, 18, and 24 after SARS-CoV-2 infection, grouped by body organ system. Error bars show the prevalence and 95% confidence interval for each of the symptoms. Inverse probability of censoring weighting was applied to all estimates. Solid lines refer to symptoms reported by participants to be related to covid-19. Dotted lines refer to all reported symptoms regardless of whether they were reported to be related to covid-19. Stacked bar plots show self-reported severity of symptoms reported to be related to covid-19 (as a proportion of all reported symptoms). PEM=Post-exertional malaise; GI=gastrointestinal
In descriptive trajectory analyses, symptoms were continuously present at all time points in 8.9% (6.5% to 11.2%) of participants and 12.5% (9.8% to 15.9%) experienced alternating courses (fig 2D, supplementary table 12 for sensitivity analyses). Compared with people who did not have symptoms at all time points or those who improved by 24 months, a higher proportion of participants who worsened or reported symptoms at all time points were 65 years or older (45.7% v 34.1% and 36.0%), had comorbidities (58.8% v 27.5% and 36.0%), and had pre-existing fatigue (FAS; 47.3% v 24.5% and 38.4%), dyspnoea (mMRC scale; 35.2% v 11.9% and 26.6%), and problems on EQ-5D-5L (63.6% v 33.1% and 45.0%). Furthermore, a higher proportion had reported post-exertional malaise at six months (39.1% v 27.5% among those who improved) (supplementary table 13).
Scale-based outcomes up to 24 months
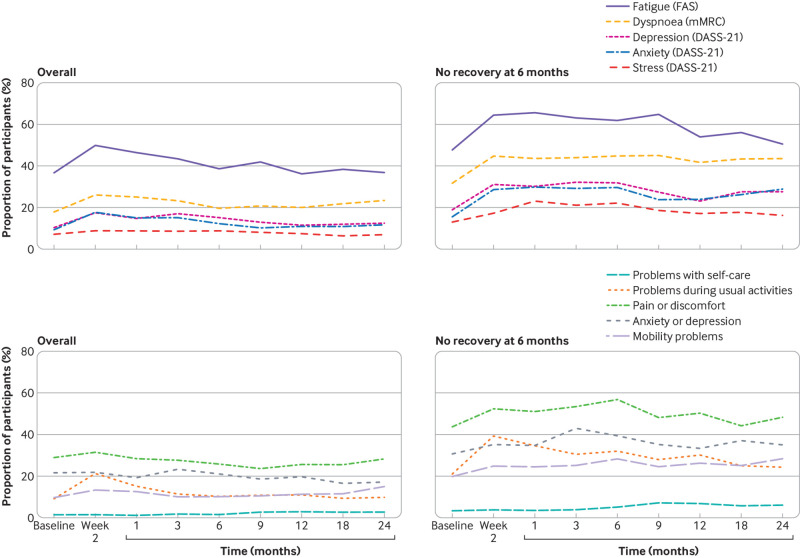
In a descriptive longitudinal assessment of scale-based outcomes among all infected individuals, there was an increase in the proportion of participants reporting adverse health outcomes shortly after infection (fig 4A and fig 4C). By one month, the proportion of participants with adverse outcomes started to decrease, and by 24 months, it was similar to baseline levels before infection: 36.8% (32.4% to 41.5%) with fatigue on the FAS, 23.4% (19.6% to 27.7%) with dyspnoea grade ≥1 on the mMRC scale, 12.5% (9.8% to 15.9%) with depression, 11.7% (9.1% to 15.0%) with anxiety, and 7.0% (5.1% to 9.6%) with stress on DASS-21, and 38.8% (34.4% to 43.4%) with any problem on EQ-5D-5L. Among people who reported that they had not recovered at six months, a higher proportion of participants reported adverse health outcomes at baseline compared with the overall population of infected individuals (fig 4B and fig 4D). Additionally, the proportion of people reporting adverse health outcomes over time more slowly decreased, with all outcomes reported by a considerable proportion of participants at 24 months: 50.5% fatigue (FAS)); 43.5% dyspnoea grade ≥1 (mMRC scale)); 27.6% with depression, 28.9% with anxiety, and 16.2% with stress (DASS-21); and 64.8% with issues with health related quality of life (EQ-5D-5L). Results were consistent across sensitivity analyses (supplementary tables 14-15).
Fig 4.
(A) Proportion of SARS-CoV-2 infected participants with fatigue (measured on FAS), dyspnoea grade ≥1 (on mMRC), depression, anxiety, and stress (on DASS-21) over time. (B) Proportion of participants who have not recovered by six months with fatigue (on FAS), dyspnoea grade ≥1 (on mMRC), depression, anxiety, and stress (on DASS-21) over time. (C) Proportion of SARS-CoV-2 infected participants with problems in the five EQ-5D-5L domains over time. (D) Proportion of participants who have not recovered by six months with problems in the five EQ-5D-5L domains over time. Inverse probability of censoring weighting was applied to all estimates. FAS=fatigue assessment scale; mMRC=modified Medical Research Council dyspnoea scale; DASS-21=21 item depression, anxiety and stress scale
Comparison of outcomes at six months to an uninfected population
We compared symptom point prevalence and adverse health outcomes among Zurich SARS-CoV-2 Cohort participants at six months after infection to uninfected individuals enrolled in Corona Immunitas. Of 2974 individuals invited to Corona Immunitas, 697 (23.4%) participated (fig 5). The age and sex distributions of participants and people who did not agree to take part were similar (supplementary table 16). Of these 697 participants, 69 (9.9%) were excluded from the analysis due to previous infection and 628 (90.1%) were included. Participants enrolled in Corona Immunitas were on average older than those in Zurich SARS-CoV-2 Cohort (65 years v 50 years; table 1).
Fig 5.
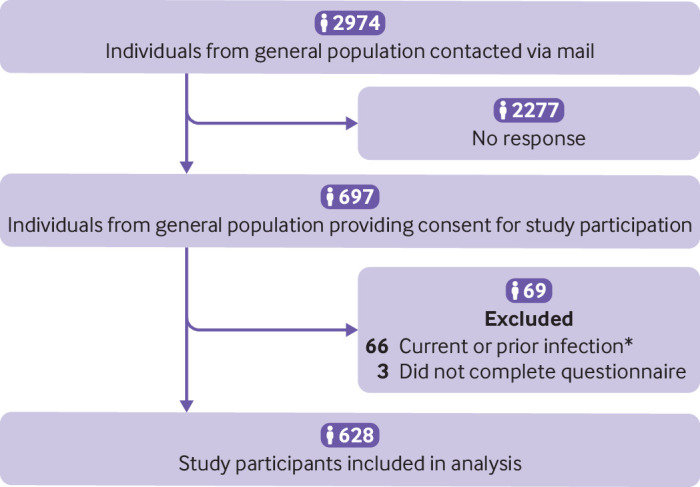
Study enrolment into phase 4 Corona Immunitas Zurich seroprevalence study (uninfected comparator group). *Prior or current infection in the Corona Immunitas study was defined as: self-reported positive SARS-CoV-2 PCR or rapid antigen test, positive anti-spike IgG or IgA antibodies with no history of vaccination, or positive anti-nucleocapsid IgG antibodies on enrolment
Table 1.
Characteristics of individuals with SARS-CoV-2 infection enrolled in the Zurich SARS-CoV-2 Cohort study and individuals with no infection from the Corona Immunitas Zurich seroprevalence study (comparator group)
| SARS-CoV-2 infection* (n=1106) | No SARS-CoV-2 infection† (n=628) | |
|---|---|---|
| Age, years, median (IQR) | 50.0 (35.0-66.0) | 65.0 (45.0-72.0) |
| Age group, years: | ||
| 18-39 | 344 (31.1) | 128 (20.4) |
| 40-64 | 449 (40.6) | 174 (27.7) |
| ≥65 | 313 (28.3) | 326 (51.9) |
| Female sex | 566 (51.2) | 322 (51.3) |
| Initial symptom self-reported severity: | ||
| Asymptomatic | 148 (13.5) | — |
| Mild to moderate | 721 (66.0) | — |
| Severe to very severe | 224 (20.5) | — |
| Missing | 13 | — |
| Initial symptom count, median (IQR): | 5.0 (3.0- 8.0) | — |
| Hospitalisation and ICU stay: | — | |
| Non-hospitalised | 1051 (95.6) | — |
| Hospitalised without ICU stay | 44 (4.0) | — |
| Hospitalised with ICU stay | 4 (0.4) | — |
| Intubation during ICU stay | 1 (25) | — |
| Missing | 7 | — |
| Smoking status: | ||
| Non-smoker | 656 (60.1) | 336 (53.6) |
| Ex-smoker | 288 (26.4) | 208 (33.2) |
| Smoker | 148 (13.6) | 83 (13.2) |
| Missing | 14 | 1 |
| Body mass index, median (IQR) | 24.2 (21.9-26.6) | 24.2 (21.9-26.9) |
| At least one comorbidity: | 324 (29.5) | 225 (35.8) |
| Missing | 9 | 0 |
| Hypertension | 165 (15.1) | 155 (24.7) |
| Diabetes mellitus | 30 (2.8) | 31 (4.9) |
| Cardiovascular disease | 63 (5.9) | 52 (8.3) |
| Respiratory disease | 93 (8.7) | 38 (6.1) |
| Chronic kidney disease | 9 (0.8) | 3 (0.5) |
| Malignancy | 62 (5.8) | 21 (3.3) |
| Immune suppression | 32 (3.0) | 26 (4.2) |
| Monthly household income: | ||
| <6000 CHF | 356 (34.2) | 219 (38.1) |
| 6000-12 000 CHF | 458 (44.0) | 234 (40.8) |
| >12 000 CHF | 227 (21.8) | 121 (21.1) |
| Missing | 65 | 55 |
| Current employment status: | ||
| Employed | 720 (65.9) | 255 (40.7) |
| Student | 53 (4.9) | 25 (4.1) |
| Retired | 274 (25.1) | 322 (51.4) |
| Unemployed or other | 45 (4.1) | 24 (3.8) |
| Missing | 14 | 2 |
| Highest education level reached: | ||
| None or mandatory school | 45 (4.1) | 32 (5.1) |
| Vocational training or specialised baccalaureate | 459 (42.1) | 281 (45.0) |
| Higher technical school or college | 289 (26.5) | 159 (25.5) |
| University | 296 (27.2) | 152 (24.4) |
| Missing | 17 | 4 |
| Swiss nationality | 943 (85.3) | 530 (84.4) |
Values are number of individuals (percentage), unless otherwise specified. IQR=interquartile range; ICU=intensive care unit; CHF=Swiss francs.
Zurich SARS-CoV-2 Cohort.
Corona Immunitas Zurich cohort.
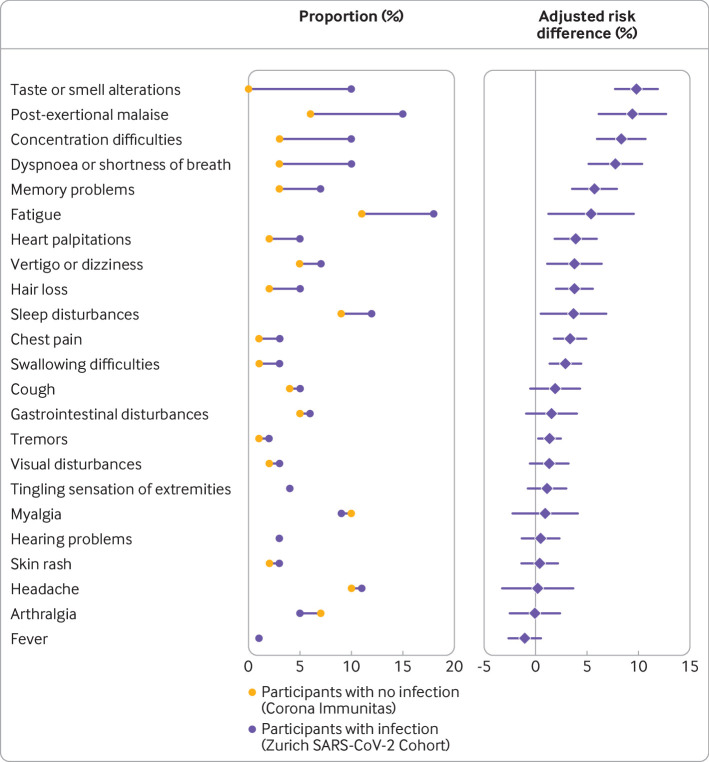
There was strong evidence that the prevalence of any symptom at six months was higher among infected individuals (adjusted absolute risk difference 17.0% (95% confidence interval 11.5% to 22.4%)). Excess risks for individuals infected compared with those who were not were highest for altered taste or smell (9.8% (7.7% to 11.8%)), post-exertional malaise (9.4% (6.1% to 12.7%)), reduced concentration (8.3% (6.0% to 10.7%)) or memory (5.7% (3.5% to 7.9%)), dyspnoea (7.8% (5.2% to 10.4%)), and fatigue (5.4% (1.2% to 9.5%) (fig 6, supplementary table 17 for odds ratios). For adverse health outcomes based on scales (supplementary figure 3, supplementary table 17), there was strong evidence that a higher proportion of infected individuals had symptoms of anxiety (based on DASS-21; 4.1% (0.5% to 7.6%)) at six months compared with individuals who did not have an infection. There was no evidence for a difference in the proportions of participants reporting symptoms of depression or stress or any of the remaining adverse health outcomes (supplementary figure 3, supplementary table 17).
Fig 6.
Observed proportions and excess risk (adjusted absolute risk differences) of each of the symptoms at six months after infection in the infected group (n=1106; Zurich SARS-CoV-2 Cohort) compared with individuals who did not have an infection from the general population (n=628; Corona Immunitas Zurich). Adjusted risk differences were estimated based on inverse probability weighted generalised linear models, adjusted for age, sex, body mass index, smoking status, education level, monthly income, and presence of hypertension, diabetes mellitus, cardiovascular, respiratory, or chronic renal disease, current or past malignancy, and immune suppression
Discussion
In this prospective, population based cohort, we assessed longer term recovery and symptom trajectories up to 24 months after infection among individuals who had infections with wildtype SARS-CoV-2 and who were not vaccinated before infection. Approximately 17% of participants did not return to their normal health status and 18% reported covid-19 related symptoms by 24 months. We found that individuals who had a SARS-CoV-2 infection had different trajectories over time. Although most participants improved over the study period, some had worsening or alternating courses of health impairment and recovery. Although not all adverse outcomes were necessarily attributed to covid-19, our findings imply that a sizable number of people might be affected by post-covid-19 condition and have protracted health issues for many months after infection.
Main findings in context
Other studies on longer term outcomes after SARS-CoV-2 reported a wide range of estimates (22-75% at 12-24 months), probably due to heterogeneity in study populations, designs, definitions, and assessments.1 2 3 4 5 6 7 10 45 Our findings seem to be within the lower bound of what has been previously reported for similar follow-up durations. Although higher estimates in other studies could be related to the inclusion of hospitalised or other selective populations, a contributing factor could be that some assessments did not consider the extent to which the outcomes were attributed to covid-19 and what their impact was on individuals’ daily lives. In our study, we found that half of participants reported at least one symptom up to 24 months after infection. However, symptom prevalence was relevantly lower (18%) when restricting to symptoms that participants felt were related to covid-19. The comparative analysis leveraging data from an uninfected sample from the general population provided further evidence that overall symptom prevalence attributable to SARS-CoV-2 was up to 17%. The estimated excess risks of individual symptoms were relatively consistent with the proportions of symptoms self-reported by participants to be related to covid-19. Yet, for several symptoms, we found insufficient evidence that their prevalence among individuals who had an infection was higher than the comparator individuals. Furthermore, 6% of participants with symptoms at 24 months nevertheless reported full recovery, implying that the symptoms might have had only minimal impact on their daily lives. Overall, findings from this study suggest that, depending on the exact definition used, the reported estimates for post-covid-19 condition based on symptoms alone might be overestimated in studies conducted within similar timeframes.
The evaluation of the course of health outcomes over time showed two main findings. First, several distinct symptom and recovery trajectories might have occurred with some dissimilarities in the characteristics of people who had these different trajectories. Second, the prevalence of people who did not recover and had symptoms decreased mostly between six months and 12 months, followed by a less pronounced decrease after 12 months. This slow recovery has been observed in other studies20 23 46 and could indicate progression into chronic health problems. Nevertheless, the rates of recovery and the overall improvement in the severity of participants’ health impairment over time might also provide some hope for affected individuals. Identification of subgroups at the greatest risks of long term health impairments and acquirement of a deeper understanding of the underlying mechanisms will be necessary for developing interventions targeting post-covid-19 condition.
In this cohort, we used standardised assessment scales in addition to health status and self-reported symptoms, allowing to further contextualise our findings. The use of such measures increases comparability across studies and ensures that additional aspects relevant to patients, such as health-related quality of life and mental health, are also included. The trajectories of scale based outcomes support the observation of an overall improvement in self-reported health and symptoms in individuals who have had an infection. The higher proportion of adverse health outcomes among individuals who had not recovered that was already pre-existing before infection highlights the need to identify individuals who might be at a higher risk of developing post-covid-19 condition and likely to benefit most from prevention and treatment. Our finding of about a 4% excess risk of experiencing symptoms of anxiety among people who had a SARS-CoV-2 infection adds to the existing evidence of a relevant mental health burden of post-covid-19 condition.47 48 However, these scale based assessments have not been validated for post-covid-19 condition. Further investigation of whether other instruments are more sensitive or specific, in addition to the development of scales specific for post-covid-19 condition, are warranted.
Altogether, our findings underscore the complexity in estimating the prevalence and trajectories of post-covid-19 condition and emphasise the importance of assessing multiple outcomes, using standardised scales, and including self-reported measures of recovery to ensure a comprehensive, person centred perspective.
Implications for trial design and outcome selection
Several trials assessing potential interventions for post-covid-19 condition are underway.49 50 Evidence from this study and others can help to effectively design such trials. Our findings provide important information on subgroups of individuals who are at greater risk of long term health issues. These data might help in defining study populations in trials to ensure that individuals who are most likely to benefit from the intervention are included. Additionally, estimated rates of spontaneous recovery could be used to calculate necessary sample sizes to detect an improvement in outcomes. Findings from prospective cohorts can also help to determine the most relevant outcome measures to assess post-covid-19 condition in clinical trials. Due to limited knowledge and emerging evidence, defining outcomes in this context has been challenging and led to substantial heterogeneity across studies. In 2022, a core outcome set for assessing post-covid-19 condition was suggested26 and outcome measures used in this study are consistent with these recommendations. Meanwhile, our findings also emphasise the importance of using a patient reported standardised measure of health status as a complementary outcome to capture the multidimensional impact of post-covid-19 condition. Use of such measures might also help to determine the overall benefit to harm balance of an intervention, especially in the presence of small effect sizes. Finally, evidence on the different symptoms experienced by individuals affected by post-covid-19 condition might help researchers to ensure that these are accurately and consistently captured in both arms of clinical trials at baseline and prospectively. This thoroughness will ensure that potential adverse effects of the interventions are appropriately investigated in the context of the underlying risk due to post-covid-19 condition.
Strengths and limitations
The strengths of our study include the population based approach, the large number of participants, and its prospective design with regular assessments of a broad range of health outcomes. However, several limitations also need to be considered. Firstly, we relied on the relative health status of participants to evaluate persistent health impairments. The relation of symptoms to covid-19 was also determined on the basis of participants’ self-reporting. No clinical validation of self-reported measures was conducted; therefore, we cannot fully exclude information bias through symptoms or health status changes that might have reflected the presence or worsening of other conditions. Although we believe that standardised patient reported measures reflect affected individuals' experiences, future work should consider incorporating objective assessments and examining their correlation with self-reported measures. Secondly, we used a predefined list of symptoms to assess those related to post-covid-19 condition. Although they were informed by what was most commonly reported by participants and in the medical literature, this list might represent only part of the experienced symptoms.51 52 Although we attempted to assess the different symptom patterns over time and observed alternating courses in some individuals, our study was not designed to fully capture the relapsing and remitting nature of symptoms shown by others.20 53 Thirdly, participants from the Zurich SARS-CoV-2 Cohort enrolled when wildtype SARS-CoV-2 was the predominant circulating strain and before vaccination. Further research is needed to assess whether similar courses are noted after vaccination54 55 or infection with other variants.56 57 Fourth, selection might have occurred in the Zurich SARS-CoV-2 Cohort if individuals who were more concerned with their health were more motivated to participate, or if individuals with post-covid-19 condition were more likely to be retained. This bias may have led to an overestimation of the prevalence of post-covid-19 condition. However, we at least partially accounted for differential loss to follow-up by applying inverse probability of censoring weighting. Furthermore, the proportions of individuals who were admitted to hospital and were 65 years or older were lower among individuals participating in our study compared to people who did not participate, which might have led to an underestimation of the prevalence of post-covid-19 condition. Overall, the direction of any potential selection bias is difficult to estimate. Nevertheless, the population based approach, high retention, and similar findings across sensitivity analyses strengthen the credibility of our estimates. Meanwhile, because only a small proportion of the participants were admitted to hospital for covid-19, our findings might not be generalisable to those with most severe acute disease. Similarly, self-selection could have occurred in the uninfected group. However, the age and sex distributions of participants and people who did not participate were similar; although, systematic differences relating to unassessed sociodemographic factors could have occurred. Furthermore, the assessment of post-covid-19 condition was not a primary objective in the Corona Immunitas seroprevalence study. Thus, we consider the potential impact of any selection effects on our estimates to be minimal. Lastly, we pooled data from two separate cohorts to estimate excess risks of outcomes related to post-covid-19 condition. Despite the close alignment of the cohorts and use of inverse probability weighting, residual confounding is possible in relation to differences in further socioeconomic or health related factors between the study and control groups, which we could not fully adjust for in our analysis.
Conclusion
This population based study showed that despite a decrease in the severity of symptoms and health impairment over time, up to 18% of individuals infected with SARS-CoV-2 were affected by post covid-19 condition 24 months after infection. Evidence suggests an excess symptom risk in comparison to individuals who did not have an infection. Persisting health issues create significant challenges for affected individuals and pose an important burden on population health and healthcare services. This underscores the value of infection prevention and emphasises the need for clinical trials to establish effective interventions for post-covid-19 condition. Furthermore, our findings show the importance of using multiple outcome measures and of considering the expected rates of recovery and heterogenous symptom trajectories in the design and interpretation of future trials.
What is already known on this topic
Studies assessing long term outcomes related to post-covid-19 condition report persistence of symptoms in a large proportion of individuals for many months after infection
Many studies included selective populations or conducted cross-sectional assessments, which might not fully reflect the natural history of post-covid-19 condition across the severity spectrum of covid-19
What this study adds
Despite a decrease in the severity of symptoms and health impairment over time, up to 18% of infected individuals are affected by post-covid-19 condition 24 months after infection
Clinical trials are needed to establish effective interventions to reduce the burden of post-covid-19 condition
Patients recovering from covid-19 might experience heterogenous recovery trajectories, which need to be considered in the design and interpretation of such clinical trials
Web extra.
Extra material supplied by authors
Web appendix: Online appendix
Contributors: TB, DM, HEA, JSF, and MAP conceived and planned the Zurich SARS-CoV-2 Cohort study. TB, DM, and MAP coordinated the Zurich SARS-CoV-2 Cohort study. AF, JSF, and MAP conceived and planned the Corona Immunitas study. AF and MAP coordinated the Corona Immunitas study. TB, DM, JSF, and MAP conceived and designed this study. TB and DM prepared the analytic datasets and performed the statistical analysis. TB, DM, JSF, and MAP interpreted the data. TB, DM, AA, AD, HEA, AF, JSF, and MAP provided critical revision for important intellectual content. MAP, and JSF obtained funding. TB drafted the first manuscript. All authors had access to the data in the study and accept responsibility to submit for publication. All authors critically revised the draft manuscript and read and approved the final manuscript. MAP is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study is part of the Corona Immunitas research network, coordinated by the Swiss School of Public Health (SSPH+), and funded by fundraising of SSPH+including funds of the Swiss Federal Office of Public Health and private funders (ethical guidelines for funding stated by SSPH+ were respected), by funds of the cantons of Switzerland (Vaud, Zurich, and Basel) and by institutional funds of the Universities. Additional funding specific to this study was received from the Department of Health of the canton of Zurich, the University of Zurich Foundation, the Swiss Federal Office of Public Health, and the CoVICIS project (grant No 101046041) funded by the European Union Horizon Europe Program. The funding bodies had no influence on the design, conduct, analysis, or interpretation of the study, as well as on the decision to publish, preparation or revisions of the manuscript. TB received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 801076, through the SSPH+ Global PhD Fellowship Programme in Public Health Sciences (GlobalP3HS) of the SSPH+. DM received funding by the University of Zurich Postdoc Grant, grant no. FK-22-053. HEA received a Swiss National Science Foundation (SNSF) Early Postdoc Mobility Fellowship, grant no. 191414.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/disclosure-of-interest (available on request from the corresponding author) and declare: funding for the research project by the Department of Health of the canton of Zurich, the University of Zurich Foundation and the Swiss Federal Office of Public Health; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
The manuscript’s guarantor (MAP) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: The results of this research will be disseminated to the study participants through a study specific newsletter and to the general public through news and social media channels. We will also directly share these results directly with public health policy makers in Switzerland.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study was approved by the responsible ethics committee of the canton of Zurich, Switzerland (Kantonale Ethik-Kommission Zürich; BASEC-Nr. 2020-01739 for the Zurich SARS-CoV-2 Cohort study and BASEC-Nr. 2020-01247 for the Corona Immunitas study). Written or electronic informed consent was obtained from all participants.
Data availability statement
We are open to sharing de-identified individual participant data that underlie the results reported in this article. Requests can be made to the corresponding author at miloalan.puhan@uzh.ch. Data requestors will need to sign a data access agreement.
References
- 1. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ 2021;372:n693. 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with covid-19 four months after hospital discharge. JAMA Netw Open 2021;4:e2036142. 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bull-Otterson L. Post-covid conditions among adult covid-19 survivors aged 18-64 and ≥65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep 2022;71:713-7. 10.15585/mmwr.mm7121e1. [DOI] [Google Scholar]
- 4. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after covid-19 infection. JAMA Netw Open 2021;4:e210830. 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menges D, Ballouz T, Anagnostopoulos A, et al. Burden of post-covid-19 syndrome and implications for healthcare service planning: a population-based cohort study. PLoS One 2021;16:e0254523. 10.1371/journal.pone.0254523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nehme M, Braillard O, Alcoba G, et al. COVICARE TEAM . Covid-19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med 2021;174:723-5. 10.7326/M20-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nittas V, Gao M, West EA, et al. Long COVId through a public health lens: an umbrella review. Public Health Rev 2022;43:1604501. 10.3389/phrs.2022.1604501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morin L, Savale L, Pham T, et al. Writing Committee for the COMEBAC Study Group . Four-month clinical status of a cohort of patients after hospitalization for covid-19. JAMA 2021;325:1525-34. 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV, WHO Clinical Case Definition Working Group on Post-COVID-19 Condition . A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022;22:e102-7. 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. NIHR . Living with covid19–second review. NIHR Evidence. 2021. 10.3310/themedreview_45225 [DOI]
- 11. Whitaker M, Elliott J, Chadeau-Hyam M, et al. Persistent covid-19 symptoms in a community study of 606,434 people in England. Nat Commun 2022;13:1957. 10.1038/s41467-022-29521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM, Lifelines Corona Research Initiative . Persistence of somatic symptoms after covid-19 in the Netherlands: an observational cohort study. Lancet 2022;400:452-61. 10.1016/S0140-6736(22)01214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu Q, Ailshire JA, Crimmins EM. Long covid and symptom trajectory in a representative sample of Americans in the first year of the pandemic. Sci Rep 2022;12:11647. 10.1038/s41598-022-15727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lerner AM, Robinson DA, Yang L, et al. Toward understanding covid-19 recovery: National Institutes of Health Workshop on postacute covid-19. Ann Intern Med 2021;174:999-1003. 10.7326/M21-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heesakkers H, van der Hoeven JG, Corsten S, et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for covid-19. JAMA 2022;327:559-65. 10.1001/jama.2022.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with covid-19: a longitudinal cohort study. Lancet 2021;398:747-58. 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with covid-19: a longitudinal cohort study. Lancet Respir Med 2022;10:863-76. 10.1016/S2213-2600(22)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nehme M, Braillard O, Chappuis F, et al. CoviCare Study Team . One-year persistent symptoms and functional impairment in SARS-CoV-2 positive and negative individuals. J Intern Med 2022;292:103-15. 10.1111/joim.13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sigfrid L, Drake TM, Pauley E, et al. Long covid in adults discharged from UK hospitals after covid-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg 2021;8:100186. 10.1016/j.lanepe.2021.100186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tran V-T, Porcher R, Pane I, Ravaud P. Course of post covid-19 disease symptoms over time in the ComPaRe long covid prospective e-cohort. Nat Commun 2022;13:1812. 10.1038/s41467-022-29513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu T, Wu D, Yan W, et al. Twelve-month systemic consequences of coronavirus disease 2019 (covid-19) in patients discharged from hospital: a prospective cohort study in Wuhan, China. Clin Infect Dis 2022;74:1953-65. 10.1093/cid/ciab703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou F, Tao M, Shang L, et al. Assessment of Sequelae of COVID-19 Nearly 1 Year After Diagnosis. Front Med (Lausanne) 2021;8:717194. 10.3389/fmed.2021.717194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peter RS, Nieters A, Kräusslich H-G, et al. EPILOC Phase 1 Study Group . Post-acute sequelae of covid-19 six to 12 months after infection: population based study. BMJ 2022;379:e071050. 10.1136/bmj-2022-071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hastie CE, Lowe DJ, McAuley A, et al. Outcomes among confirmed cases and a matched comparison group in the long-covid in Scotland study. Nat Commun 2022;13:5663. 10.1038/s41467-022-33415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fumagalli C, Zocchi C, Tassetti L, et al. AOU Careggi COVID-19 Follow-up study Group . Factors associated with persistence of symptoms 1 year after COVID-19: A longitudinal, prospective phone-based interview follow-up cohort study. Eur J Intern Med 2022;97:36-41. 10.1016/j.ejim.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Munblit D, Nicholson T, Akrami A, et al. PC-COS project steering committee . A core outcome set for post-covid-19 condition in adults for use in clinical practice and research: an international Delphi consensus study. Lancet Respir Med 2022;10:715-24. 10.1016/S2213-2600(22)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swiss Federal Office of Public Health. Covid-19 Switzerland: Coronavirus dashboard – Overview. https://www.covid19.admin.ch/en/overview (accessed 12 Mar 2023).
- 28.Swiss Federal Office of Public Health. Covid-19 Switzerland: Coronavirus dashboard – Virus variants. https://www.covid19.admin.ch/en/epidemiologic/virus-variants (accessed 12 Mar 2023).
- 29. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573-7. 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 30. West EA, Anker D, Amati R, et al. Corona Immunitas Research Group . Corona Immunitas: study protocol of a nationwide program of SARS-CoV-2 seroprevalence and seroepidemiologic studies in Switzerland. Int J Public Health 2020;65:1529-48. 10.1007/s00038-020-01494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swiss School of Public Health (SSPH+). Corona Immunitas. https://www.corona-immunitas.ch/en (accessed 12 Mar 2023).
- 32. Tong A, Baumgart A, Evangelidis N, et al. COVID-19-Core Outcomes Set Investigators . Core outcome measures for trials in people with coronavirus disease 2019: respiratory failure, multiorgan failure, shortness of breath, and recovery. Crit Care Med 2021;49:503-16. 10.1097/CCM.0000000000004817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perneger TV, Combescure C, Courvoisier DS. General population reference values for the French version of the EuroQol EQ-5D health utility instrument. Value Health 2010;13:631-5. 10.1111/j.1524-4733.2010.00727.x. [DOI] [PubMed] [Google Scholar]
- 34. Wacker ME, Jörres RA, Karch A, et al. COSYCONET-Consortium . Assessing health-related quality of life in COPD: comparing generic and disease-specific instruments with focus on comorbidities. BMC Pulm Med 2016;16:70. 10.1186/s12890-016-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zanini A, Aiello M, Adamo D, et al. Estimation of minimal clinically important difference in EQ-5D visual analog scale score after pulmonary rehabilitation in subjects with COPD. Respir Care 2015;60:88-95. 10.4187/respcare.03272. [DOI] [PubMed] [Google Scholar]
- 36. Domenghino A, Aschmann HE, Ballouz T, et al. Mental health of individuals infected with SARS-CoV-2 during mandated isolation and compliance with recommendations-A population-based cohort study. PLoS One 2022;17:e0264655. 10.1371/journal.pone.0264655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brunson JC. ggalluvial: layered grammar for alluvial plots. J Open Source Softw 2020;5:2017. 10.21105/joss.02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.European Centre for Disease Prevention and Control. Reinfection with SARS-CoV-2: implementation of a surveillance case definition within the EU/EEA. https://www.ecdc.europa.eu/en/publications-data/reinfection-sars-cov-2-implementation-surveillance-case-definition-within-eueea (accessed 12 Mar 2023).
- 39. Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol 1986;123:174-84. 10.1093/oxfordjournals.aje.a114212. [DOI] [PubMed] [Google Scholar]
- 40. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ 2019;367:l5657. 10.1136/bmj.l5657. [DOI] [PubMed] [Google Scholar]
- 41. de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis 2020;20:1034-42. 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ 2021;374:n1648. 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 43. Crump RK, Hotz VJ, Imbens GW, et al. Dealing with limited overlap in estimation of average treatment effects. Biometrika 2009;96:187-99 10.1093/biomet/asn055. [DOI] [Google Scholar]
- 44. Ziegler S, Raineri A, Nittas V, et al. Long covid citizen scientists: developing a needs-based research agenda by persons affected by long covid. Patient 2022;15:565-76. 10.1007/s40271-022-00579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) condition or long cOVID: a meta-analysis and systematic review. J Infect Dis 2022;226:1593-607. 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nehme M, Braillard O, Chappuis F, Guessous I, CoviCare Study Team . The chronification of post-COVID condition associated with neurocognitive symptoms, functional impairment and increased healthcare utilization. Sci Rep 2022;12:14505. 10.1038/s41598-022-18673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of covid-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 2021;8:416-27. 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xie Y, Xu E, Al-Aly Z. Risks of mental health outcomes in people with covid-19: cohort study. BMJ 2022;376:e068993. 10.1136/bmj-2021-068993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ceban F, Leber A, Jawad MY, et al. Registered clinical trials investigating treatment of long covid: a scoping review and recommendations for research. Infect Dis (Lond) 2022;54:467-77. 10.1080/23744235.2022.2043560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fawzy NA, Shaar BA, Taha R, et al. A systematic review of trials currently investigating therapeutic modalities for post-acute covid-19 syndrome and registered on World Health Organization International Clinical Trials Platform. Clin Microbiol Infect 2023; 10.1016/j.cmi.2023.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hayes LD, Ingram J, Sculthorpe NF. More than 100 persistent symptoms of SARS-CoV-2 (long covid): a scoping review. Front Med (Lausanne) 2021;8:750378. 10.3389/fmed.2021.750378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of covid-19: a systematic review and meta-analysis. Sci Rep 2021;11:16144. 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long covid in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021;38:101019. 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Azzolini E, Levi R, Sarti R, et al. Association between BNT162b2 vaccination and long covid after infections not requiring hospitalization in health care workers. JAMA 2022;328:676-8. 10.1001/jama.2022.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Notarte KI, Catahay JA, Velasco JV, et al. Impact of covid-19 vaccination on the risk of developing long-covid and on existing long-covid symptoms: a systematic review. EClinicalMedicine 2022;53:101624. 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022;399:2263-4. 10.1016/S0140-6736(22)00941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ballouz T, Menges D, Kaufmann M, et al. Post covid-19 condition after wildtype, delta, and omicron SARS-CoV-2 infection and prior vaccination: Pooled analysis of two population-based cohorts. PLoS One 2023;18:e0281429. 10.1371/journal.pone.0281429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Online appendix
Data Availability Statement
We are open to sharing de-identified individual participant data that underlie the results reported in this article. Requests can be made to the corresponding author at miloalan.puhan@uzh.ch. Data requestors will need to sign a data access agreement.