Abstract
Albizzia julibrissin is empirically used as an antidepressant in clinical practice. Preclinical studies have indicated that its total extracts or bioactive constituents exerted antidepressant-like responses in animal models, providing the molecular basis to reveal its underlying mechanism of action. While attempts have been made to understand the antidepressant effect of A. julibrissin, many fundamental questions regarding its mechanism of action remain to be addressed at the molecular and systems levels. In this review, we conclusively discussed the mechanism of action of A. julibrissin and A. julibrissin formulae by reviewing recent preclinical and clinical studies conducted by using depressive animal models and depressive patients. Several representative bioactive constituents and formulae were highlighted as examples, and their mechanisms of action were discussed. In addition, some representative A. julibrissin formulae that have been shown to be compatible with conventional antidepressants in clinical practice were also reviewed. Furthermore, we discussed the future research directions to reveal the underlying mechanism of A. julibrissin at the molecular and systems levels in depression treatment. The integrated study using both the molecular and systematic approaches is required not only for improving our understanding of its molecular basis and mechanisms of action, but also for providing a way to discover novel agents or approaches for the effective and systematic treatment of depression.
Keywords: Albizia julibrissin Durazz., antidepressants, depression, mechanism of action, clinical application
1. Introduction
Depression is a popular and all age-related mental illness, affecting approximate 10%–15% of the population worldwide (Hasin et al., 2018, WHO, 2017). According to the latest statistics, the lifetime prevalence rate of depression in China is 6.8%, that is, there are nearly 100 million depressive patients, but only 0.5% of the patients are fully treated (Huang et al., 2019, Lu et al., 2021). Depressive patients suffer greatly with poorly function at work, study, and social activities. Severe depression can lead to disability and suicide. The social and economic burdens caused by depression are gradually increasing, and depression has become a global public health problem.
Current medications in depression treatment have many shortcomings, such as slow onset, low efficacy, serious adverse effects, and high costs, all of which limit their use in clinical practice. On the other hand, the use of herbal medicine has been greatly increased as a complementary and alternative medicine in the past decades (WHO, 2019). Herbal medicine is a core part of traditional medicine that has been developed for a long history to maintain health as well as to prevent and treat physical and mental illness. The common reasons for using herbal medicine are that it is more affordable, more personalized health care, and less adverse effects than chemically synthesized drugs (Wachtel-Galor & Benzie, 2011). It should be noted that the use of herbal medicine increased when the synthesized medications are less effective in the treatment of complex diseases, such as depression.
Traditional Chinese medicine (TCM) is an important branch of traditional medicine, and it is broadly used to treat depression in East Asia today. In TCM practice, Chinese herbal medicine is usually used to exert its medicinal effects on depression by using a combination of multiple herbs, so-called formula, in which one herb at least is employed as an antidepressant drug to directly act against the main depressive symptoms, while others serve as modulators to strengthen the antidepressant efficacy or alleviate side effects (Zhang & Cheng, 2019). According to the Chinese Pharmacopoeia, more than 50 single Chinese herbs have empirically been used as antidepressants in clinical practice (Chinese Pharmacopoeia Commission, 2020). During the past two decades, studies have been extensively conducted to uncover the molecular basis and mechanism of action of these herbs in the treatment of depression. These studies have remarkably improved our understanding of Chinese herbal medicine for the effective treatment of depression (Li, Huang, Cheng, & Zhang, 2020).
Albizia julibrissin Durazz., a leguminous deciduous shrub, is one of the most common herbs used for depression treatment. In TCM practice, its dried flowers or bark are generally processed for medicinal purposes. The main ingredients in A. julibrissin include triterpenoids, lignans, flavonoids, saponins, sterols, etc. (Li, Tian, Luo, & Li, 2022). Preclinical studies have shown that these ingredients exhibited a broad array of pharmacological activities ranging from antidepressant and anxiolytic (Li, 2017, Li, Tian, Luo, & Li, 2022, Li, 2017, Wang et al., 2021, Yang & Li, 2019), anti-inflammation (Yang & Li, 2019), anti-oxidation (Sobeh et al., 2017, Shi et al., 2019), and antitumor (Qian et al., 2017, Yu et al., 2016) to enhance immunological function (Sobeh et al., 2017). This narrative review aims to conclusively discuss the mechanism of action as well as the latest progress in preclinical and clinical research of A. julibrissin in the treatment of depression. The literatures that demonstrated A. julibrissin constituents and formulae to produce antidepressant responses were selected for discussion. According to the mechanism of action and structural classification, several representative constituents that have been demonstrated to specifically act on the pathological systems in depression neurobiology are given. In addition, several representative A. julibrissin formulae traditionally used for depression treatment were discussed. Furthermore, we also discussed some A. julibrissin formulae that have been demonstrated to be compatible with conventional antidepressants. Finally, we discussed the future research directions to reveal the mechanism by which A. julibrissin exerts antidepressant-like activity in depression treatment. The comprehensive analysis could improve our understanding of the molecular basis and mechanism of action of A. julibrissin in the treatment of depression, which, in turn, will provide an opportunity to scientifically evaluate its benefits and risks in clinical practice.
2. Mechanisms of antidepression
Tremendous progress in preclinical and clinical studies of depression has revealed many pathophysiological factors across divergent biological systems that are involved in the neurobiology of depression (Han & Yuan, 2021). Remarkably, many pharmacological targets in these biological systems have been employed to uncover the mechanism of action of antidepressants (Duman, Aghajanian, Sanacora, & Krystal, 2016, Gerhard & Duman, 2018). Studies showed that several total extracts or bioactive constituents of A. julibrissin exerted profound effects on the pathophysiological systems through multiple diverse underlying mechanisms of action. Herein, we discussed the mechanism by which A. julibrissin acts on the diverse pathophysiological systems and thereby possesses antidepressant activities (Fig. 1).
Fig. 1.
Proposed mechanisms of action by which A. julibrissin exerted antidepressant responses in animal models. A. julibrissin has been demonstrated to produce multiple antidepressant effects on various divergent pathological systems by modulating monoaminergic neurotransmission, the HPA axis, BDNF signaling cascade, or neuroimmune system.
2.1. Monoamine neurotransmission
Impairment of monoamine neurotransmission is a major cause of depression. Most of the conventional antidepressants, such as fluoxetine and sertraline, selectively inhibit serotonin (5-HT) reuptake transporter, and subsequently enhance 5-HT transmission in the central nerve system (CNS). In addition, monoamine reuptake transporters for norepinephrine (NE) or dopamine (DA), monoamine metabolic enzymes, and postsynaptic monoamine receptors also play important roles in monoamine transmission. Therefore, these proteins are considered to be potential pharmacological targets in depression treatment (Murphy et al., 2021).
Zhang et al. reported that administration of an aqueous extract of Albiziae Flos alleviated chronic stress-induced growth abnormalities by regulating monoamine levels in rat brain (Zhang & Li, 2006). In addition, Kim et al. demonstrated that acute treatment with methylene chloride fraction of A. julibrissin (200 mg/kg, 30 min, p.o.) exerted antidepressant-like effects in mice, which could be specifically reversed by a 5-HT1A receptor antagonist, WAY-100635 (Kim, Kim, Lee, & Jang, 2007). This observation suggested that the A. julibrissin fraction produced antidepressant-like responses by modulating 5-HT transmission (Ji, Kim, Lee, & Jang, 2007). Furthermore, a recent report demonstrated that a 70% ethanol extract of Albiziae Cortex dramatically inhibited serotonin transporter (SERT), the principal target of the conventional antidepressant drugs (Huang et al., 2022). These studies suggested that some bioactive constituents in these A. julibrissin extracts specially act on monoaminergic signaling by modulating monoamine transporters or receptors in CNS, and thereby produce antidepressant-like responses in depressive animal models. However, further studies should be conducted to determine the nature of these constituents as well as the specific interactions with their target proteins.
2.2. Hypothalamic–pituitary–adrenal (HPA) axis
Hyperactivation of the HPA axis induced by stress impairs neuronal survival and neurogenesis, and thereby results in depressive symptoms. Studies have extensively been conducted to reveal pathophysiological factors in the HPA axis, and several pharmacological targets, such as corticotrophin releasing factor, corticotrophin releasing factor 1 receptor, and glucocorticoid receptors, have emerged for developing novel agents that suppress hyperactivation of the HPA axis (Ding et al., 2021, Juruena, Bocharova, Agustini, & Young, 2018, Menke, 2019).
Administration (6 g/kg, 21 d, p.o.) of an aqueous extract of A. julibrissin has been reported to reduce hyperactivation of the HPA axis in depressive rat models induced by chronic unpredictable mild stress (CUMS) (Li, 2017, Li, 2017). Behavioral tests showed that the aqueous extract improved spatial learning and memory in the stressed rats, which was comparable with the action of a conventional antidepressant, fluoxetine. Similarly, another study also indicated that an aqueous extract of A. julibrissin (3.6 g/kg, 28 d, p.o.) exerted an antidepressant-like activity by modulating the HPA axis, characterized by decreasing the release of serum corticotrophin releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and corticosterone (CORT) in chronic restrain-stressed rats (Cui et al., 2019). A lignan glycoside isolated from A. julibrissin (3.6 mg/kg, 7 d, p.o.) has been demonstrated to exhibit an antidepressant-like activity by decreasing plasma concentrations of CRF (corticotropin releasing factor), ACTH, and CORT in the repeated acute restraint-stressed rats (Liu et al., 2017). However, these studies did not address if these extracts or constituents directly act on the pharmacological targets in the HPA axis. Only by understanding the specific drug-target interaction, we are able to uncover their mechanism of action at a molecular level.
It should be notable that several pharmacological targets to suppress stress-induced hyperactivation of the HPA axis have been proposed, however, attempts to develop novel agents directed toward the HPA axis in the treatment of depression have not been successful (Li, Huang, Cheng, & Zhang, 2020). The main reason is that the HPA axis and the neuroinflammatory system bidirectionally regulate through neural, immunological, and humoral intersystem interactions. The neuroendocrine-immune network poses difficulties associated with the development of antidepressant agents directed toward these biological systems for the effective treatment of depression (Li, Huang, & Zhang, 2021). On the other hand, multidrug and multitarget nature of Chinese herbal medicine has a great potential to assist in the development of novel medications for the systematic pharmacotherapy of depression. From this point of view, we expect that A. julibrissin could be an excellent example for the systematic treatment of depression by simultaneously acting multiple pharmacological targets in the diverse pathological systems of depression.
2.3. Neurotrophic signaling cascades
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, plays a key role in maintenance of neural plasticity. Deficiency of BDNF and other neurotrophins or dysfunction of their signaling cascades contributes the pathophysiology of depression (Duman, Deyama, & Fogaca, 2019, Masi and Brovedani, 2011, Voleti & Duman, 2012). The pharmacological targets in BDNF signaling cascade include cAMP response element binding protein (CREB), BDNF, BDNF receptor, TrkB, or its postreceptor signaling cascades such as Ras-Raf-ERK, PI3K-Akt, and PLCγ (Niciu, Ionescu, Mathews, Richards, & Zarate, 2013, Zhang, Yao, & Hashimoto, 2016).
An aqueous extract of A. julibrissin (6 g/kg, 21 d, p.o.) have been demonstrated to produce antidepressant-like effects on cAMP-CREB signaling cascade in the stressed rat models, and subsequently to attenuate behavioral abnormalities (Wang et al., 2015). In addition, total flavonoids isolated from Albiziae Flos (25 mg/kg, 21 d, p.o.) have been shown to increase the expression levels of BDNF and its receptor, TrkB in the hippocampal CA1 and CA3 regions, suggesting that the flavonoids possess an antidepressant-like activity specifically through regulating BDNF signaling in the stressed rat models (Wang et al., 2012, Shi, 2014). It would be interesting to know if these herbal constituents directly act on BDNF signaling cascade and what targets they specifically interact with. Hence, more in-depth studies are required to address these questions, which are important for our understanding of their mechanistic details in order to further refine the use of these herbal antidepressants.
2.4. Inflammatory system
Studies have shown that the immune system bidirectionally communicates with the CNS at several levels through neural and immunological interactions (Jiang et al., 2019, Zunszain, Anacker, Cattaneo, Carvalho, & Pariante, 2011). Dysfunction of immune system has often been observed in many patients with depression and treatment of patients with proinflammatory cytokines can produce depressive symptoms (Chiu, Su, Su, & Chen, 2017, Ng et al., 2018). Therefore, proinflammatory cytokines and their signaling molecules have been suggested as contributing pathophysiological factors for depression (Kim, Na, Myint, & Leonard, 2016, Su et al., 2017, Zhang, Yao, & Hashimoto, 2016). Agents that act on the inflammatory systems, such as proinflammatory cytokines and their receptors, proinflammatory signaling pathways, and inflammasomes, could be effective to treat depressive symptoms (Tonhajzerova et al., 2020, Wohleb, Franklin, Iwata, & Duman, 2016).
Wei reported that an aqueous extract of Albizziae Flos (6 g/kg, 21 d, p.o.) reversed the increased levels of serum proinflammatory cytokines, such as IL-2 and IL-6, and proinflammatory signaling molecules, such as nitric oxide, induced by stress in rats, suggesting that the extract exerted an antidepressant-like activity by down-regulating inflammatory system (Wei, 2016). A earlier report to investigate the effect of an aqueous extract from Albiziae Cortex on immune function in mice showed that administration of the extract (0.5 g/kg, 6 d, p.o.) remarkably increased phagocytic rate of peritoneal macrophages and level of lymphocytic IL-2 in mice, suggesting the aqueous extract from Albiziae Cortex possesses an immune-improving property (Wang et al., 2000). While these studies demonstrated that A. julibrissin acted as a modulator in immune system, either by suppressing proinflammatory cytokines or improving immune function, the specific interaction between the herbal constituents and their acting targets in neuroimmune system as well as the molecular mechanism of action still remain to be addressed. It should be emphasized that agents targeting neuroimmune system alone would not be effective, and an additional medication that directly acts on the HPA axis is also required to achieve a better treatment, because of the intersystem crosstalk between the neuroimmune system and the HPA axis in the neuroendocrine system (Li, Huang, & Zhang, 2021). Hence, it is reasonable to parallelly investigate the effects of herbal bioactive constituents of A. julibrissin on both the pathological systems in depression treatment.
3. Bioactive constituents with antidepressant activities
As mentioned above, total extracts of A. julibrissin exerted antidepressant-like effects in animal models through multiple underlying mechanisms of action. These findings suggest that A. julibrissin must contain certain bioactive constituents that specifically act on these pharmacological targets to correct dysfunction of pathophysiological systems in neurobiology of depression. It is worth noting that depressive and anxious symptoms usually coexist in many patients and share common pathological factors and pharmacological targets for their treatment (Antypa, Vogelzangs, Meesters, Schoevers, & Penninx, 2016, Kaiser, Herzog, Voderholzer, & Brakemeier, 2021). Hence, several A. julibrissin constituents or formulae that have been shown to possess anxiolytic activity in preclinical or clinical studies are also included in our discussion. A. julibrissin is rich in flavonoids, lignan glucosides, and saponins, which have been demonstrated to exert pharmacological effects in animal models. Herein, we list several representative bioactive constituents that show antidepressant or anxiolytic-like effects, based on their chemically structural classification.
3.1. Flavonoids
Guo et al. reported that total flavonoids isolated from Albizziae Flos possessed antidepressant activities by antagonizing the hippocampal apoptosis of CA3 region in CUMS rats (Guo, Xia, Yin, & Shi, 2013, Kim, Kim, Lee, & Jang, 2007). Later, Li et al. demonstrated that the reduction in hippocampal apoptosis results from flavonoids-induced increases in 5-HT and NE levels and BDNF expression as well as a decrease in the expression of Bcl-2 associated X protein in CUMS rats (Li, Wang, & Gao, 2014). In addition, total flavonoids have also been investigated their effects on learning and memory in rat models. These studies indicated that flavonoids significantly alleviated stress-induced learning and memory impairment, probably through regulating monoamine levels in the brain (Ji-Hyun Kim, Kim, Lee, & Jang, 2007, Shi et al., 2013, Shi et al., 2013). Furthermore, anxiolytic effects of total flavonoids have been investigated using anxious animal models. Data showed that total flavonoids possessed an anxiolytic activity, but the molecular mechanism of action has not been fully uncovered (Liu et al., 2015).
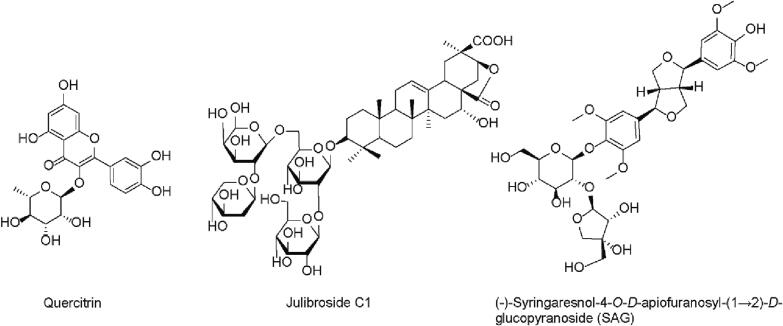
While total flavonoids from A. julibrissin have been shown to exert antidepressant or anxiolytic effects in animal models, quercitrin (Fig. 2) is the only one flavonoid molecule that has been identified to be responsible for anxiolytic activity. In a study conducted by Li et al. (Li et al., 2016), administration of quercitrin (5.0 mg/kg, 7 d, p.o.) produced anxiolytic effects in animal models and its effects on animal behavior were blocked by WAY-100635 (3.0 mg/kg, i.p.), a 5-HT receptor 1A antagonist, but not a γ-aminobutyric acid (GABA) receptor A antagonist, flumazenil (0.5 mg/kg, i.p.). In addition, monoamine levels (5-HT and DA) and their metabolites in the brain were reduced after quercitrin treatment. These data suggest that the anxiolytic-like effects of quercitrin are mediated by 5-HT receptor 1A to enhance monoamine neurotransmission. However, the specific interaction between quercitrin and its molecular targets as well as the specificity for the monoamine signaling pathway still remain to be addressed in the future study.
Fig. 2.
Several representative bioactive compounds shown to exert antidepressant activity, specifically by modulating monoaminergic system.
3.2. Saponins
Julibroside C1 (julibrogenin C 3-O-[β-D-xylopyranosyl1-(1,2)]-β-D-fucopyranosyl-(1,6)-[β-D-glucopyranosyl-(1,2)]-β-D-glucopyranoside) is a saponin-containing compound isolated from the stem bark of A. julibrissin (Fig. 2). Jung et al. performed a study to investigate the anxiolytic effects of julibroside C1 using behavioral and biochemical approaches (Jung et al., 2013). Administration of julibroside C1 (0.5 and 1 mg/kg, 1 h, p.o.) has been shown to increase the time spent in open arms and numbers of entries into the open arms in an elevated plus maze test. In addition, the anxiolytic-like effects of julibroside C1 were blocked by either a 5-HT1A receptor antagonist, WAY-100635, or a GABAA receptor antagonist, bicuculline or flumazenil. Furthermore, an antagonism study was conducted by using quantitative receptor autoradiography in the brain to confirm the involvement of postsynaptic receptor systems in the anxiolytic-like effects of julibroside C1. Data showed that Julibroside C1 significantly decreased [3H]-8-OH-DPAT or [3H]-flunitrazepam binding, with little effect on [3H]-muscimol binding. Taken together, the study suggested that the anxiolytic-like effects of julibroside C1 were mediated by 5-HT1A and GABAA receptor systems (Jung et al., 2013). It is interesting to know the selectivity of julibroside C1 for the two receptor systems as well as the specific drug-receptor interaction for revealing its mechanism of action at the molecular level.
3.3. Lignan glucosides
A study was carried out to evaluate the anxiolytic-like effects of aqueous or several organic solvent extracts of Albiziae Cortex by using behavioral and biochemical approaches (Xiong et al., 2018). A n-butanol extract, which has been demonstrated to be rich in lignan glucosides, was observed to have the strongest anxiolytic-like activity among these extracts. (-)-Syringaresnol-4-O-D-apiofuranosyl-(1 → 2)-D-glucopyranoside (SAG) is one major bioactive constituent of A. julibrissin (Fig. 2). Liu et al. investigated the anxiolytic effects of SAG in acute restraint-stressed rats and subsequently analyzed its potential mechanism of action (Liu et al., 2017). Behavioral tests showed that administration of SAG (3.6 mg/kg, 7 d, p.o.) produced the anxiolytic-like responses in animal models. Moreover, SAG significantly attenuated the acute stress-induced increases in the plasma levels of CRH, ACTH, and CORT, as well as in the levels of neurotransmitters (NE, 5-HT, DA) and their metabolites (5-hydroxyindoleacetic acid, dihydroxyphenylacetic acid, and homovanillic acid) in the cerebral cortex and hippocampus of the rat brain.
Our laboratory has recently isolated and identified two lignan glycosides from A. julibrissin with antidepressant properties, one of which was SAG (Huang et al., 2022), another was a derivative of SAG, (-)-syringaresinol-4,4′-bis-O-β-D-glucopyranoside (SBG). Our results showed that these two lignan glycosides inhibited SERT noncompetitively by decreasing Vmax with little change in Km for its substrate. The two lignan glycosides decreased the accessibility of a cysteine residue placed in the extracellular substrate permeation pathway by inducing a conformational shift toward an outward-closed state of the transport protein. These results indicated that these herbal compounds from A. julibrissin acted on SERT by a novel underlying mechanism of action different from that of conventional antidepressant drugs (Huang et al., 2022). To our knowledge, it is the first example for one bioactive compound isolated from A. julibrissin that specifically targets SERT. However, both SAG and SBG have also been shown to weakly inhibit the transporters for DA and NE. The lack of selectivity for SERT increases our concerns about their addictive side effects caused by elevating synaptic concentrations of dopamine (Poisson, Engel, & Saunders, 2021).
4. A. julibrissin formulae
Depression is a multigenetic and multifactorial syndrome with various underlying pathological mechanisms (Hammen, 2018). Conventional antidepressants with single targets are inadequate for the effective treatment of depression in clinical practice. Hence, agents that are simultaneously directed toward multiple pharmacological targets in the pathophysiology of depression are needed for a better treatment (Li, Huang, & Zhang, 2021). One typical TCM antidepressant formula generally contains multiple herbs, which are thought to act on diverse pathophysiological factors at the same time in the systemic treatment of depression.
There are many empirical A. julibrissin-containing herbal formulae, which have been broadly used for the treatment of depression with comparable efficacy to the conventional antidepressants in clinical practice (Shi et al., 2019, Shi et al., 2019, Shi et al., 2019, Shi et al., 2019; Shi et al., 2019c; Shi et al., 2019d; Shi, Zhang, & Jiang, 2016). As mentioned above, A. julibrissin contains various bioactive constituents that produce multiple antidepressant effects in animal models through diverse underlying mechanisms of action, indicating that A. julibrissin possibly plays a key role in these herbal formulae.
Numerous A. julibrissin formulae have been studied to reveal their mechanisms of action by using both behavioral and biochemical approaches. These preclinical studies demonstrated that an herbal composite formula had a greater efficacy than single herbs, possibly due to their synergistic interactions (Ou et al., 2019). On the other hand, clinical studies are also critical to improve our understanding of the underlying mechanisms of action as well as biological responses in human body. In this section, therefore, we discuss several representative A. julibrissin antidepressant formulae that have been preclinically or clinically studied and others are listed in Table 1, Table 2.
Table 1.
A. julibrissin formulae traditionally used for depression treatment and their efficacy in clinical practice.
| Herbal formulae | Composition | Daily human doses | Types of depression | Evaluation index | Efficacy evaluation | References |
|---|---|---|---|---|---|---|
| Jieyu Granules | Bupleuri Radix, Toosendan Fructus, Citri Reticulatae Pericarpium, Paeoniae Radix Alba, Acori Tatarinowii Rhizoma, Albiziae Cortex, Ziziphi Spinosae Semen, Ambrum | Three times/day | Depression with chronic congestive heart failure | SDS | Relieves symptoms of depression without apparent side effects. | (Wang, Tang, Ji, Hu, & Lin, 2018) |
| Jieyu Granules | Bupleuri Radix, Toosendan Fructus, Citri Reticulatae Pericarpium, Acori Tatarinowii Rhizoma, Albiziae Cortex, Ziziphi Spinosae Semen | One time/day | Depression with permanent atrial fibrillation | HAMD | Alleviates symptoms of depression without adverse effects. The efficacy is better than that of Deanxit. | (Hu, Tang, Wang, & Reng, 2018) |
| Jieyu Granules | Bupleuri Radix, Hyperici Perforati Herba, Albiziae Cortex, Coptidis Rhizoma | Three times/day | Moderate or mild depression |
HAMD SDS SAS |
Relieves symptoms of depression and anxiety without apparent side effects. It is comparable with fluoxetine, but better than Xiaoyao Powder. | (Lin, Hui, Han, Li, & Rong, 2019) |
| Jieyu Shuxin Pills |
Bupleuri Radix, Angelicae Sinensis Radix, Rehmanniae Radix, Aucklandiae Radix, Albizziae Flos, Codonopsis Radix, Glycyrrhizae Radix et Rhizoma | Three times/day | Postpartum depression | EPDS | Alleviates symptoms of postpartum depression without adverse effects. | (Sun & Zhang, 2016) |
| Hehuan Granules | Albizziae Flos, Fossilia, Platycladi Cacumen, Polygalae Radix, Valeriana Officinalis Herba, Citri Exocarpium Rubrum | Two times/day | Depression and insomnia | HAMD | Alleviates depressive symptoms and improves patients’ sleep quality. | (Lei, Zhang, & Chen, 2010) |
| Chaihu Shugan Pills | Bupleuri Radix, Curcumae Radix, Paeoniae Radix Alba, Chuanxiong Rhizoma, Citri Reticulatae Pericarpium, Cyperi Rhizoma, Salviae Miltiorrhizae Radix et Rhizoma, Albiziae Cortex, Polygoni Multiflori Caulis, Coptidis Rhizoma, Scutellariae Radix, Citri Sarcodactylis Fructus, Citri Fructus, Glycyrrhizae Radix et Rhizoma | Two times/day | Post-stroke depression | HAMD | Alleviates symptoms of post-stroke depression, and better than paroxetine in clinical efficacy. |
(Ha, 2020) |
| Yushu Granules | Bupleuri Radix, Gardeniae Fructus, Ziziphi Spinosae Semen, Albiziae Cortex, Fossilia, Ambrum | Three times/day | Depression | HAMD | Comparable with fluoxetine without apparent adverse effects. |
(Mao, Lu, & Song, 2006) |
Note: HAMD, Hamilton depression scale; SDS, Self-rating depression scale; SAS, Self-rating anxiety scale; EPDS, Edinburgh postnatal depression scale; TESS, Treatment emergent symptom scale.
Table 2.
A. julibrissin formulae combined with conventional antidepressants for clinical treatment of depression.
| Herbal formulae | Compositions | Conventional antidepressants |
Types of depression | Evaluation index |
Efficacy evaluation | References |
|---|---|---|---|---|---|---|
| Jieyu Granules | Codonopsis Radix, Albiziae Cortex, Trichosanthis Radix, Curcumae Radix, Chuanxiong Rhizoma, Cyperi Rhizoma, Angelicae Sinensis Radix, Salviae Miltiorrhizae Radix et Rhizoma | Citalopram | Depression in elder people | HAMD | Combinative administration shows a better efficacy than citalopram alone. | (Yuan et al., 2010) |
| Xindanshu Capsules | Albiziae Cortex, Epimedii Folium, Juglandis Semen, Euphorbiae Humifusae Herba, Lilii Bulbus, Bupleuri Radix | Fluoxetine | Depression with lacking in self-confidence | HAMD | Combinative administration shows a better efficacy and less adverse effects than fluoxetine alone. | (Jin & Li, 2009) |
| Qingxin Anshen Granules | Pinelliae Rhizoma, Arisaema Cum Bile, Citri Exocarpium Rubrum, Coptidis Rhizoma, Gardeniae Fructus, Bambusae Caulis in Taenias, Polygalae Radix, Curcumae Radix,Poria, Atractylodis Macrocephalae Rhizoma, Aurantii Fructus, Ziziphi Spinosae Semen, Albiziae Cortex, Fossilia, Glycyrrhizae Radix et Rhizoma | Deanxit | Depression | HAMD TESS |
Combinative administration shows a better efficacy than deanxit alone. | (Jiang & Zhang, 2009) |
| Baihe Anshen Decoction | Bupleuri Radix, Lilii Bulbus, Curcumae Radix, Albizziae Flos, Chuanxiong Rhizoma, Angelicae Sinensis Radix, Paeoniae Radix Alba | Deanxit | Anxiety and depression after coronary heart disease surgery | HAMD | Combinative administration shows a better efficacy than deanxit alone. | (Xu, Ren, & Li, 2020) |
| Baihe Anshen Decoction | Bupleuri Radix, Paeoniae Radix Alba, Chuanxiong Rhizoma, Salviae Miltiorrhizae Radix et Rhizoma, Cyperi Rhizoma, Curcumae Radix, Albiziae Cortex, Ziziphi Spinosae Semen, Scutellariae Radix | Deanxit | Depression with chronic heart failure | HAMD | Combinative administration shows a better efficacy than deanxit alone. | (Wang & Liang, 2016) |
| Baihe Anshen Decoction | Bupleuri Radix, Lilii Bulbus, Curcumae Radix, Albizziae Flos, Chuanxiong Rhizoma, Angelicae Sinensis Radix, Paeoniae Radix Alba, Triticum, Anemarrhenae Rhizoma, Sojae Semen Praeparatum, Gardeniae Fructus, Fossilia, Ostreae Concha, Glycyrrhizae Radix et Rhizoma | Deanxit | Anxiety and depression | SAS SDS |
Combinative administration shows a better efficacy than deanxit. | (Wen & Ji, 2015) |
| Jieyu Hehuan Decoction | Albizziae Flos, Paeoniae Radix Alba, Angelicae Sinensis Radix, Cinnabaris, Poria, Platycladi Semen,Polygalae Radix, Ziziphi Spinosae Semen, Ambrum, Salviae Miltiorrhizae Radix et Rhizoma, Fossilia, Sojae Semen Praeparatum, Nelumbinis Rhizomatis Nodus,Borneolum, Acori Tatarinowii Rhizoma, Ostreae Concha, Margarita, Ziziphi Spinosae Semen, Polygoni Multiflori Caulis, Triticum | Deanxit | Mild depression | HAMD | Combinative administration shows a better efficacy than Deanxit or Jieyu Hehuan decoction alone. | (Li, 2015) |
| Jieyu Hehuan Decoction | Moutan Cortex, Gardeniae Fructus, Angelicae Sinensis Radix, Cinnabaris, Poria, Polygalae Radix, Acori Tatarinowii Rhizoma, Paeoniae Radix Alba, Albizziae Flos, Ambrum, Polygoni Multiflori Caulis, Ziziphi Spinosae Semen, Triticum, Ostreae Concha, Fossilia, Glycyrrhizae Radix et Rhizoma | Paroxetine | Depression | HAMD | Combinative administration shows a better efficacy than paroxetine alone. | (Gong, 2016) |
| Baihe Ningshen Decoction |
Lilii Bulbus, Polygoni Multiflori Caulis, Acori Tatarinowii Rhizoma, Angelicae Sinensis Radix, Glycyrrhizae Radix et Rhizoma,Curcumae Radix, Salviae Miltiorrhizae Radix et Rhizoma, Ziziphi Spinosae Semen, Albizziae Flos, Rosae Rugosae Flos, Scutellariae Radix, Ligustri Lucidi Fructus | Fluoxetine | Post-stroke depression | HAMD | Combinative administration shows better efficacy and fewer side effects than fluoxetine alone. | (Han, Liu, Ji, & Mi, 2010, Li, 2016) |
| Baihe Ningshen Decoction | Lilii Bulbus, Ziziphi Spinosae Semen, Albizziae Flos, Polygoni Multiflori Caulis, Salviae Miltiorrhizae Radix et Rhizoma, Angelicae Sinensis Radix, Glycyrrhizae Radix et Rhizoma | Clomipramine/ Amitriptyline | Depression | HAMD | Combinative administration shows a better efficacy than clomipramine/amitriptyline alone. | (Liu & Zhang, 2002) |
| Yangxin Docoction | Platycladi Cacumen, Schisandrae chinensis Fructus, Bupleuri Radix, Acori Tatarinowii Rhizoma, Glycyrrhizae Radix et Rhizoma, Codonopsis Radix, Curcumae Radix, Salviae Miltiorrhizae Radix et Rhizoma, Ziziphi Spinosae Semen, Polygalae Radix, Aurantii Fructus, Ophiopogonis Radix, Albiziae Cortex, Paeoniae Radix Alba, Citri Fructus | Sertraline | Depression | HAMD | Combinative administration shows better efficacy and fewer side effects than sertraline alone. | (Gao, 2018) |
| Baihe Ganmai Decoction | Lilii Bulbus, Anemarrhenae Rhizoma, Glycyrrhizae Radix et Rhizoma, Triticum, Albiziae Cortex, Ziziphi Spinosae Semen | Agomelatine | First-episode depression | HAMD | Combinative administration shows a better efficacy than agomelatine alone. | (Zhang, Cheng, Feng, Zhao, & Wang, 2021, Zhang et al., 2021, Zhang et al., 2015) |
| Jieyu Pills | Paeoniae Radix Alba, Bupleuri Radix, Angelicae Sinensis Radix, Curcumae Radix, Poria, Lilii Bulbus, Glycyrrhizae Radix et Rhizoma, Albiziae Cortex, Triticum, Ziziphi Spinosae Semen | Duloxetine | Depression with energy deficiency | SDS BDC HAMD |
Better efficacy than Jieyu pills alone. | (Li, Ge, Wang, & Pan, 2020) |
| Duloxetine | Post-stroke depression | HAMD CGI |
Better efficacy than duloxetine alone without increased safety concerns. | (Bai, Guo, Ma, & Wang, 2020, Zhang, Liu, Zhang, Song, & Zhang, 2020) | ||
| Deanxit | Post-stroke depression | HAMD HAMA |
Better efficacy than deanxit alone without increased safety concerns. | (Wu & Yang, 2020) | ||
| Mirtazapine | Depression | HAMD SDSS |
Better efficacy and less adverse effects than mirtazapine alone. | (Ye, Fan, Chen, Li, & Cao, 2020) | ||
| Paroxetine | Postpartum depression | HAMD | Better efficacy and less adverse effects than paroxetine alone. | (Yang & Zhong, 2020) | ||
| Paroxetine | Post-stroke depression | NIHSS HAMD SDS |
Better efficacy than paroxetine alone. | (Tang, Han, & Chen, 2019) | ||
| Escitalopram | Post-stroke depression | HAMD NIHSS |
Better efficacy than escitalopram alone without increased safety concerns. | (Tian, Bai, You, & Yao, 2020) | ||
| Jieyu Shuxin Pills | Bupleuri Radix, Angelicae Sinensis Radix, Rehmanniae Radix, Cyperi Rhizoma, Chuanxiong Rhizoma, Paeoniae Radix Alba, Acori Tatarinowii Rhizoma, Polygalae Radix, Polygoni Multiflori Caulis, Aucklandiae Radix, Albizziae Flos, Codonopsis Radix, Glycyrrhizae Radix et Rhizoma | Citalopram | Postpartum depression | EPDS HAMD SDS |
Better efficacy than citalopram alone without increased safety concerns. | (Fang, Du, & Zheng, 2019, Sun, Li, & Wang, 2016) |
| Shenshuaining Pills | Acori Tatarinowii Rhizoma, Polygoni Multiflori Caulis, Platycladi Cacumen, Ziziphi Spinosae Semen, Albiziae Cortex, Schisandrae chinensis Fructus, Margarita, Cinnabaris, Rehmanniae Radix, Ecliptae Herba, Alpinae Oxyphyllae Fructus | Citalopram | Depression | HAMD TESS SDS |
Better efficacy, faster onset, and fewer adverse effects than citalopram alone. | (Zhang, 2013) |
| Xingnao Jieyu Capsules | Acori Tatarinowii Rhizoma, Polygalae Radix, Curcumae Radix, Bupleuri Radix, Albiziae Cortex, Morindae Officinalis Radix, Salviae Miltiorrhizae Radix et Rhizoma | Deanxit | Post-stroke depression | HAMD SDS |
Better efficacy and faster onset than deanxit alone. | (Yan, Yang, & Yang, 2011) |
| Baicaoxiang Jieyu Anshen Capsules | Prunellae Spica, Paeoniae Radix Alba, Albizziae Flos, Ziziphi Spinosae Semen, Bupleuri Radix, Cyperi Rhizoma, Rehmanniae Radix, Schisandrae chinensis Fructus, Polygoni Multiflori Caulis | Paroxetine | Depression | HAMD PSQI WHOQOL-BREF |
Better efficacy and sleep quality than paroxetine alone. | (Liu, Cui, Li, & Yu, 2017) |
| Yushu Granules | Bupleuri Radix, Gardeniae Fructus, Ziziphi Spinosae Semen, Albiziae Cortex, Fossilia, Ambrum | Fluoxetine | Post-Stroke Depression | HAMD | Better efficacy than fluoxetine alone. | (Kan, 2012) |
| Jiawei Xiaoyao Powder | Bupleuri Radix, Angelicae Sinensis Radix, Paeoniae Radix Alba, Albiziae Cortex, Salviae Miltiorrhizae Radix et Rhizoma, Aurantii Fructus, Atractylodis Macrocephalae Rhizoma, Poria, Chuanxiong Rhizoma, Menthae Haplocalycis Herba, Glycyrrhizae Radix et Rhizoma, Zingiberis Rhizoma | Fluoxetine | Post-Stroke Depression | TESS | Better efficacy and less adverse effects than fluoxetine alone. | (Yan et al., 2015, Yang & Rong, 2009) |
| Anshen Dingzhi Decoction | Bupleuri Radix, Atractylodis Macrocephalae Rhizoma, Poria, Paeoniae Radix Alba, Curcumae Radix, Polygalae Radix, Lilii Bulbus, Acori Tatarinowii Rhizoma, Albiziae Cortex, Glycyrrhizae Radix et Rhizoma | Duloxetine | Depression | HAMD TESS |
Better efficacy, faster onset, and fewer adverse effects than duloxetine alone. | (Gou et al., 2015) |
Note: CGI, clinical global impression; BDC, Burns depression checklist; HAMA, Hamilton anxiety scale; SDSS, social defect screening scale; NIHSS, National Institutes of Health stroke score; EPDS, Edinburgh postpartum depression scale; PSQI, Pittsburgh sleep quality index; WHOQOL-BREF, the world health organization quality of life-BREF.
4.1. Suanzaoren Hehuan Decoction
Suanzaoren Hehuan Decoction is an aqueous extract of an herbal pair comprising Ziziphi Spinosae Semen and A. julibrissin. It has been generally used for the treatment of depression or anxiety in TCM practice. Preclinical studies were conducted to reveal its mechanism of action using behavioral and neurochemical approaches (Li et al., 2019, Shi et al., 2019, Shi et al., 2016, Shi et al., 2018, Shi, Xia, Feng, Zhang, & Jiang, 2016). From these studies, its mechanism of action was proposed to include enhancement of monoaminergic transmission, elevation of neurotrophic factor expression, dehyperactivation of the HPA axis, suppression of inflammation, and attenuation of hippocampal apoptosis. A study was also performed to investigate the synergy and compatibility of the herb pair (Ou et al., 2019). While single herbs showed a potent action in stress-induced depressive animal models, a combination of the herb pair has a greater efficacy. However, this study neither addressed the synergistic interactions between two herbs at the molecular level, nor defined the optimized herb pair ratio that produced the greatest efficacy in the animal models. It would be interesting to know the role of A. julibrissin playing in this herb pair.
Clinical studies indicated that the decoction possessed a comparable efficacy with a conventional antidepressant, venlafaxine, but fewer adverse effects (Shi et al., 2013, Shi et al., 2016). We are encouraged by these clinical studies to explore the mechanism of action of the herb pair as an effective antidepressant in the systematic treatment of depression.
4.2. Jieyu Hehuan Decoction
Jieyu Hehuan Decoction, a combination of 13 herbs, including Albizziae Flos, Curcumae Longae Rhizoma, Aquilariae Lignum Resinatu, Angelicae Sinensis Radix, Paeoniae Radix Alba, Salviae Miltiorrhizae Radix et Rhizoma, Platycladi Cacumen, Gardeniae Fructus, Bupleuri Radix, Menthae Haplocalycis Herba, Poria, Ziziphi Spinaosea Semen, and Citri Exocarpium Rubrum, is an empirical formula for depression treatment (Zhang, 2018). The composition and dosage of Jieyu Hehuan decoction depend on the symptoms of individual patients and can be modified to fit specific individuals more accurately. A preclinical study showed that administration of the decoction (6.8 g/kg, 14 d, p.o.) shortened the immobility time in both the tail suspension and forced swimming tests and increased expression of hippocampal p-GSK3β in mice, suggesting its antidepressant activity is partially mediated by BDNF signaling (Jia et al., 2017). It is extremely difficult to clearly interpret a complicated herbal formula at the molecular level, the system-wide mechanism of action as well as the role of each herb including A. julibrissin, however, should be uncovered in future studies.
Several clinical studies have been performed to investigate the system-wide responses associated with Jieyu Hehuan decoction administration (Chen & Zhang, 2007, Jia et al., 2017, Liang et al., 2018, Tian and Zhang, 2016, Zhang, 2018, Zhang et al., 2009). These studies showed that the decoction produced a better treatment efficacy than conventional antidepressants, such as fluoxetine and paroxetine, with fewer side effects. These clinical results support the idea that the holistic, multidrug, and multitarget nature of one herbal formula is more effective for the treatment of depression.
4.3. Jiawei Wendan Decoction
Jiawei Wendan Decoction, a mixture of eight herbs (Pinellia Rhizoma, Citri Reticulatae Pericarpium, Bambusae Caulis in Taenias, Aurantii Fructus, Albizziae Flos, Acori Tatarinawii Rhizoma, Magnoliae Officinalis Cortex, Poria), has been historically used for the treatment of mood disorders, loss of appetite, and sleep disturbances (Nie and Fang, 2019, Shi et al., 2007, Wu, Tang, Zhang, & Song, 2015). Zhang et al. reported that the decoction exerted both antidepressant and anxiolytic activities in rat models through enhancing 5-HT transmission (Zhang et al., 2021). In addition, studies conducted by Wu et al. (Wu, Tang, Zhang, & Song, 2015) and Wang et al. (Wang et al., 2016) showed that the antidepressant-like effects of Jiawei Wendan decoction were possibly mediated by modulating the levels of hippocampal neuropeptides, neuroendocrine hormone CRH, and Ras protein. Furthermore, studies also indicated that administration of the formula attenuated ultrastructural damage of hypothalamic neurons and gastrointestinal tissues in chronic stress-induced depressive models (Song et al., 2018, Xu, Zhang, Song, & Chen, 2019).
A clinical study demonstrated that Jiawei Wendan decoction had a comparable efficacy with fluoxetine (76.7% vs 80.0%), but fewer adverse effects (2.5% vs 5.5%), assessed by the Hamilton Depression Scale (HAMD) and Treatment Emergent Symptom Scale (TESS), respectively (Shi, Liu, Tang, & Zhang, 2007).
4.4. Jieyu Qingxin Anshen Decoction
Jieyu Qingxin Anshen Decoction is an aqueous extract from an herbal mixture composed of 12 herbs (Bupleuri Radix, Aurantii Fructus, Curcumae Radix, Cyperi Rhizoma, Citri Reticulatae Semen, Citri Reticulatae Pericarpium, Gardeniae Fructus, Sojae Semen Praeparatum, Ziziphi Spinosae Semen, Polysoni Multiflori Caulis, Albiziae Cortex, Margarita), and it has been clinically used for the treatment of depression with “vital energy” deficiency and arrhythmia symptoms (Zhang & Cao, 2013). Clinical studies showed that the herbal decoction is more effective in depression treatment than either conventional antidepressants, such as citalopram and paroxetine or one TCM antidepressant formula, Xiaoyao Powder (Gong, 2020, Guo and Gao, 2019, Sun et al., 2018, Tian, 2017). However, preclinical study to reveal the mechanism by which Jieyu Qingxin Anshen decoction produces antidepressant responses in depressive animal models has not been performed.
5. Clinical studies on combinative use of conventional antidepressants with herbal formulae
Although conventional antidepressants with single targets have been broadly used for the treatment of depression, additional agents that show synergistic activity are needed to act on diverse pathophysiological factors for a better therapeutic efficacy. An herbal formula is usually prescribed to activate blood circulation, eliminate phlegm and dampness, correct digestive and gastrointestinal dysfunction, or improve immune function, all of which are pharmacological targets in systems pharmacology in depression treatment (Wang et al., 2017). In this context, herbal formulae could serve as adjuvants to the conventional medications in the effective treatment of depression. Indeed, clinical studies indicated that treatment with a combination of a conventional antidepressant with an herbal formula showed a better therapeutic efficacy without increased safety concerns (Fu et al., 2021, Lin et al., 2021).
Several clinical attempts using a combination of an A. julibrissin formula with a conventional antidepressant have been conducted to treat patients with depressive symptoms. Conventional antidepressants commonly used with A. julibrissin formulae include fluoxetine, citalopram, paroxetine, flupentixol, clomipramine, etc., all of which exert antidepressant responses through enhancement of monoamine neurotransmission in the brain (Lv et al., 2018, Zhang et al., 2018, Casarotto et al., 2021, MahmoudianDehkordi et al., 2021). Clinical studies demonstrated that A. julibrissin formulae were compatible with these conventional antidepressants, showing a faster onset, shorter course of treatment, lower rate of relapse, and better therapeutic efficacy without increased adverse effects (Table 2). Experimental evidence for the combinative use of conventional antidepressants with herbal formulae have been obtained exclusively from clinical studies. Preclinical study using animal models is required for our understanding of the synergistic mechanism by which the combinative administration exerts a better efficacy.
6. Perspective
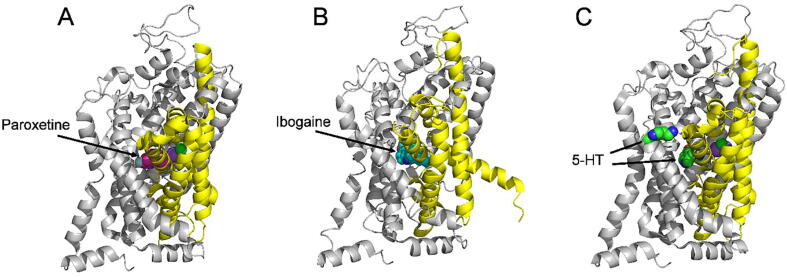
SERT is a well-established molecular target for the antidepressant drugs, such as selective serotonin reuptake inhibitors including fluoxetine and citalopram. The high-resolution structures of SERT in several conformational states have been resolved (Coleman et al., 2019, Coleman, Green, & Gouaux, 2016, Coleman & Gouaux, 2018, Yang and Gouaux, 2021), providing structural insight into antidepressant action on the transporter protein. In these structures, conventional antidepressant molecules occupy the central binding cavity and thus competitively inhibit the conformational conversion required for serotonin transport (Fig. 3). In addition, we previously demonstrated that a natural alkaloid, ibogaine, noncompetitively inhibited SERT by stabilizing an inward-facing conformation of the transporter (Bulling et al., 2012, Jacobs, Zhang, Campbell, & Rudnick, 2007). The cryo-electron microscopy structures of SERT-ibogaine complexes uncovered the ibogaine binding site and mechanism of ibogaine inhibition (Coleman et al., 2019). Furthermore, the recently resolved cryo-electron microscopy structures of SERT revealed an allosteric site formed by an aromatic pocket positioned in the scaffold domain in the extracellular vestibule (Yang & Gouaux, 2021). These structural analyses, thus, shifted our efforts in exploring the molecular mechanism of action toward the herbal compounds that target different conformation or the allosteric site of SERT. Experimental evidence has been recently shown that the lignan glycosides isolated from A. julibrissin, SAG and SBG, directly bind to SERT, presumably to the allosteric site, thus noncompetitively inhibit SERT activity (Huang et al., 2022). Taken together, these works have provided an excellent paradigm to explore the mechanism of action of the herbal compound as an antidepressant agent at the molecular level.
Fig. 3.
High-resolution structures of SERT bound with paroxetine (PDB accession code, 5I6X, paroxetine in the central site, (A) in an outward open conformation, with ibogaine (PDB accession code, 6DZZ, ibogaine in the central site, (B) in an inward open conformation, and with two molecules of 5-HT in the central and allosteric sites (PDB accession code, 7LIA, two molecules of 5-HT in the central and allosteric sites, (C) in an outward open conformation. Cartoon views of the structures are shown from the membrane plane with the extracellular side above and the cytoplasm below the structure. The bundle domain (TMs 1, 2, 6, and 7) and other part of the protein are colored yellow and gray, respectively. The purple and green spheres in the center of the structures represent Na+ and Cl-, respectively. SERT structures in this figure were made by PyMOL.
While several other herbal molecules isolated from A. julibrissin have also been demonstrated to specifically modulate one of the pathological systems in the neurobiology of depression, their targeting proteins have not been fully understood. Identification of bioactive constituents and their specific interactions with pharmacological targets is essential to reveal the molecular mechanism of action by which A. julibrissin exerts antidepressant-like effects. These efforts could also significantly improve our understanding of its benefits and risks at the molecular level. Several advanced identification techniques, such as DNA or RNA microarray (Panossian et al., 2013, Panossian et al., 2014, Panossian, Seo, & Efferth, 2018, Panossian, Seo, Wikman, & Efferth, 2015, Woo, Lim, Myung, Kim, & Lee, 2018) and quantitative proteomics (Wang et al., 2020), have been recently used for identifying potential targeting proteins that are associated with an herb or herbal formula. We expect that these high-throughput technologies can be used for analyzing the acting targets of bioactive constituents isolated from A. julibrissin.
Meanwhile, molecular approaches also play an important role in the identification of specific interactions of herbal molecules with their targeting proteins. The results from high-throughput screening technologies need to be validated by these molecular approaches. Three complementary molecular approaches, including biochemical analysis, genetic interaction, and computational inference, have been extensively used for identifying pharmacological targets (Schenone, Dancik, Wagner, & Clemons, 2013). Biochemical analysis is directly to detect the binding affinity of a bioactive molecule by using a binding assay (Burdine & Kodadek, 2004). Profiling the herbal molecule activity with an available panel of presumed proteins can be used to directly detect its targets. Genetic interaction is used to identify targets by genetic manipulation of putative targets in cells, by changing small molecule sensitivity (Zheng, Chan, & Zhou, 2004). Gene knockout and RNAi can be used to change the action of presumed targets, revealing the dependencies of herbal molecules on biological activity. Computational inference is to compare the responses of small molecules with those of known target molecules by pattern recognition (Daniel et al., 2008). In general, databases can be used to provide useful information about the targets of an herbal molecule by comparing the similarity to compounds with known targets. In practice, a combination of these three methods should be used for defining the pharmacological targets. Using a combinative method of high-throughput screening and molecular approaches will undoubtedly promote to reveal the molecular mechanism of action of A. julibrissin, which, in turn, will improve our understanding of its on-target or off-target effects in the treatment of depression.
As discussed above, A. julibrissin exerts antidepressant-like effects through various underlying mechanisms by acting on multiple pathological factors across divergent biological systems. It is evident that the interaction between a single herbal molecule and its target revealed by the molecular approaches cannot exactly reflect the actions of an herb or an entire herbal formula, which contains numerous bioactive constituents that are proposed to act on multiple systems or targets. Hence, it is necessary to investigate the mechanism of action at the systems level for our understanding of A. julibrissin formulae in the systemic treatment of depression.
It is a challenge to uncover the mechanism of action of an herb or herbal formula at the systems level because we are not clear about all bioactive constituents and their pharmacological profiles. The synergistic interactions (Chatterjee, Verma, Maurya, & Palit, 2011) and compatibility between bioactive constituents or herbs in an herbal formula are fundamental questions to be addressed in systems pharmacology. The synergistic interactions between herb pairs in several herbal formulae have been successfully studied by several laboratories (Adams, Seeram, Hardy, Carpenter, & Heber, 2006, Wang et al., 2012, Yi & Wetzstein, 2011). In addition, a study has been conducted to optimize the compatibility of herb pairs in Kaixin Powder by examining the activation of neurofilament expression in PC12 cells (Lu et al., 2015). Furthermore, Dong et al. have recently used a quantitation-based proteomics approach to identify proteins in response to Kaixin Powder administration (0.6 g/kg, 14 d) across biological systems (Dong et al., 2020). Knowledge obtained from these studies can be employed for the study of A. julibrissin formulae, such as Suanzaoren Hehuan decoction, a simplest herbal formula comprising only two herbs.
In summary, the identification of bioactive constituents and their interactions with pharmacological targets will offer the molecular basis for our understanding of the antidepressant effects of A. julibrissin. Moreover, it is vital to integrate the molecular and systematic approaches into the study of A. julibrissin or formulae for revealing its mechanism of action in its entirety. We expect that the comprehensive study would provide a way to discover novel agents or approaches in the effective and systematic treatment of depression.
Author’s contributions
BH, YW, QT, and YZ wrote the manuscript. YW and CL prepared the figures and tables and assisted in the collection of references. YZ revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was funded by the Innovation Research of the Postgraduates of Guangzhou University (2020GDJC-M28); This research was also funded by the Guangdong Basic and Applied Basic Research Foundation (2019A1515011569 and 2021A1515012067) and National Natural Science Foundation of China (32071233).
References
- Adams L.S., Seeram N.P., Hardy M.L., Carpenter C., Heber D. Analysis of the interactions of botanical extract combinations against the viability of prostate cancer cell lines. Evidence-Based Complementary and Alternative Medicine. 2006;3(1):117–124. doi: 10.1093/ecam/nel001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antypa N., Vogelzangs N., Meesters Y., Schoevers R., Penninx B.W. Chronotype associations with depression and anxiety disorders in a large cohort study. Depression and Anxiety. 2016;33(1):75–83. doi: 10.1002/da.22422. [DOI] [PubMed] [Google Scholar]
- Bai H.J., Guo Y.X., Ma X.Y., Wang X.J. Curative effect of Jieyu Pill combined with Duloxetine on depression and their influences on serum neurotransmitters and related factors of nerve function. Chinese Journal of Pharmacoepidemiology. 2020;29(3):158–162. [Google Scholar]
- Bulling S., Schicker K., Zhang Y.W., Steinkellner T., Stockner T., Gruber C.W.…Sandtner W. The mechanistic basis for noncompetitive ibogaine inhibition of serotonin and dopamine transporters. The Journal of Biological Chemistry. 2012;287(22):18524–18534. doi: 10.1074/jbc.M112.343681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdine L., Kodadek T. Target identification in chemical genetics: The (often) missing link. Chemistry & Biology. 2004;11(5):593–597. doi: 10.1016/j.chembiol.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Casarotto P.C., Girych M., Fred S.M., Kovaleva V., Moliner R., Enkavi G.…Castren E. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184(5):1299–1313. doi: 10.1016/j.cell.2021.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M., Verma P., Maurya R., Palit G. Evaluation of ethanol leaf extract of Ocimum sanctum in experimental models of anxiety and depression. Pharmaceutical Biology. 2011;49(5):477–483. doi: 10.3109/13880209.2010.523832. [DOI] [PubMed] [Google Scholar]
- Chen J.M., Zhang Q. Clinical application of Jieyu Hehuan decoction. Journal of Sichuan of Traditional Chinese Medicine. 2007;25(9):121. [Google Scholar]
- Chiu W.C., Su Y.P., Su K.P., Chen P.C. Recurrence of depressive disorders after interferon-induced depression. Translational Psychiatry. 2017;7(2):e1026. doi: 10.1038/tp.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J.A., Gouaux E. Structural basis for recognition of diverse antidepressants by the human serotonin transporter. Nature Structural & Molecular Biology. 2018;25(2):170–175. doi: 10.1038/s41594-018-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J.A., Green E.M., Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature. 2016;532(7599):334–339. doi: 10.1038/nature17629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J.A., Yang D., Zhao Z., Wen P.C., Yoshioka C., Tajkhorshid E., Gouaux E. Serotonin transporter-ibogaine complexes illuminate mechanisms of inhibition and transport. Nature. 2019;569(7754):141–145. doi: 10.1038/s41586-019-1135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Wang R., Wu J., Wei S., Lin S., Wang Z. Effect of water extract of spina date seed and Albizzia julibrissin flower on the HPA axis and inflammatory cytokines in anxious depression model rats. Journal of Jilin University (Medicine Edition) 2019;45(3):539–545. [Google Scholar]
- Daniel W.Y., Andreas B., Jonathan H., Elizabeth M.W., Gung-Wei C., Charles Y.T.…Yan F. Integrating high-content screening and ligand-target prediction to identify mechanism of action. Nature Chemical Biology. 2008;4(1):59–68. doi: 10.1038/nchembio.2007.53. [DOI] [PubMed] [Google Scholar]
- Ding Y., Wei Z., Yan H., Guo W. Efficacy of treatments targeting hypothalamic-pituitary-adrenal systems for major depressive disorder: A Meta-analysis. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.732157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X.Z., Wang D.X., Zhang T.Y., Liu X., Liu P., Hu Y. Identification of protein targets for the antidepressant effects of Kai-Xin-San in Chinese medicine using isobaric tags for relative and absolute quantitation. Neural Regeneration Research. 2020;15(2):302–310. doi: 10.4103/1673-5374.265555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Aghajanian G.K., Sanacora G., Krystal J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nature Medicine. 2016;22(3):238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Deyama S., Fogaca M.V. Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. European Journal of Neuroscience. 2019;53(1):126–139. doi: 10.1111/ejn.14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X.L., Du J., Zheng Z.L. Effects of Jieyu Shuxin pill combined with citalopram on postpartum depression. Contemporary Medicine. 2019;25(15):38–41. [Google Scholar]
- Fu X., Fu H., Liang L., Kang R. Efficacy of Chaihu Jia Longgumuli decoction in conjunction with paroxetine in the treatment of patients with postpartum depression and its effect on serotonin levels. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2021;30(2):188–191. [Google Scholar]
- Gao M. Clinical efficacy of Yangxin decoction in conjunction with sertraline hydrochloride tablets in the treatment of depression. World Latest Medicine Information. 2018;18(26):160–162. [Google Scholar]
- Gerhard D.M., Duman R.S. Rapid-acting antidepressants: Mechanistic insights and future directions. Current Behavioral Neuroscience Reports. 2018;5(1):36–47. [PMC free article] [PubMed] [Google Scholar]
- Gong H. Therapeutic efficacy of Jieyu Qingxin Anshen decoction in the treatment of depression with liver stagnation and heat disturbance and heart-sorrowing syndrome. Medical Diet and Health. 2020;18(14):34–37. [Google Scholar]
- Gong S. Clinical observation of paroxetine in conjunction with Jieyu Hehuan decoction in treating depression. Shaanxi Journal of Traditional Chinese Medicine. 2016;37(11):1464–1466. [Google Scholar]
- Gou R.H., Dou J.J., Dong H.B., Zou Y.J., Xu D.H., Zhao C.L. Therapeutic efficacy of Anshen Dingzhi decoction in conjunction with duloxetine in the treatment of 56 cases of depression. Hebei Journal of Traditional Chinese Medicine. 2015;37(10):1508–1510. [Google Scholar]
- Guo C.F., Xia M., Yin S.G., Shi X.L. Antidepressant effect by Albizzia julibrissin, flower total flavonoids and its mechanism. Chinese Journal of Experimental Traditional Medical Formulae. 2013;19(13):225–228. [Google Scholar]
- Guo X., Gao W. Jieyu Anshen decoction in conjunction with acupuncture in the treatment of 32 cases of depression with liver-qi stagnation. Traditional Chinese Medicine Research. 2019;32(7):19–20. [Google Scholar]
- Ha M. Clinical observation of the effect of the modified Chaihu Shugan powder in the treatment of post-stroke depression. Cardiovascular Disease Electronic Journal of integrated traditional Chinese and Western Medicine. 2020;8(23):150. [Google Scholar]
- Hammen C. Risk factors for depression: An autobiographical review. Annual Review of Psychology. 2018;14:1–28. doi: 10.1146/annurev-clinpsy-050817-084811. [DOI] [PubMed] [Google Scholar]
- Han Y.Q., Liu D.Y., Ji S.M., Mi J.L. Clinical observation of the treatment of 60 cases of post-stroke depression with Baihe Ningshen decoction in conjunction with low-dose fluoxetine. Shandong Medical Journal. 2010;50(23):69–70. [Google Scholar]
- Han Y.S., Yuan J.X. Advances in neurobiological mechanisms of cognitive dysfunction in depression. Journal of Neuroscience and Mental Health. 2021;21(12):873–878. [Google Scholar]
- Hasin D.S., Sarvet A.L., Meyers J.L., Saha T.D., Ruan W.J., Stohl M., Grant B.F. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346. doi: 10.1001/jamapsychiatry.2017.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.L., Tang K.Q., Wang X., Reng L.Y. Clinical observation of Jieyu granules in treatment of depressive patients with permanent atrial fibrillation. Chinese Journal of Integrative Medicine on Cardio/Cerebrovascular Disease. 2018;16(6):758–760. [Google Scholar]
- Huang B., Liu H., Wu Y., Li C., Tang Q., Zhang Y.W. Two lignan glycosides from Albizia julibrissin Durazz.noncompetitively inhibit serotonin transporter. Pharmaceuticals (Basel, Switzerland) 2022;15(3):344. doi: 10.3390/ph15030344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Wang Y., Wang H., Liu Z., Yu X., Yan J.…Wu Y. Prevalence of mental disorders in China: A cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi: 10.1016/S2215-0366(18)30511-X. [DOI] [PubMed] [Google Scholar]
- Jacobs M.T., Zhang Y.W., Campbell S.D., Rudnick G. Ibogaine, a Noncompetitive inhibitor of serotonin transport, acts by stabilizing the cytoplasm-facing state of the transporter. The Journal of biological Chemistry. 2007;282(40):29441–29447. doi: 10.1074/jbc.M704456200. [DOI] [PubMed] [Google Scholar]
- Jia R., Fang Y., Chen Z., Chen Y., Zhang S., Wang Y. Preliminary screening of antidepressant effects of Jieyu Hehuan decoction, Xuefu Zhuyu decoction and Tianwang Buxin dan. Fujian Journal of Traditional Chinese Medicine. 2017;48(4):31–33. [Google Scholar]
- Jiang L.F., Zhang F.X. Treatment of 40 depressive patients with Qingxin Anshen granules. Journal of New Chinese Medicine. 2009;41(12):76–77. [Google Scholar]
- Jiang Y., Peng T., Gaur U., Silva M., Little P., Chen Z.…Zheng W. Role of corticotropin releasing factor in the neuroimmune mechanisms of depression: Examination of current pharmaceutical and herbal therapies. Frontiers In Cellular Neuroscience. 2019;13:290. doi: 10.3389/fncel.2019.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji-Hyun Kim J.H., Kim S.Y., Lee S.Y., Jang C.G. Antidepressant-like effects of Albizzia julibrissin in mice: Involvement of the 5-HT1A receptor system. Pharmacology Biochemistry and Behavior. 2007;87(1):41–47. doi: 10.1016/j.pbb.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Jin Y.F., Li X.Z. Efficacy of Xindanshu in the treatment of depression with lacking in self-confidence. Journal of Qiqihar Medical College. 2009;30(19):2416. [Google Scholar]
- Jung Y.H., Ha R.R., Kwon S.H., Hong S.I., Lee K.H., Kim S.Y.…Jang C.G. Anxiolytic effects of Julibroside C1 isolated from Albizzia julibrissin in mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;44:184–192. doi: 10.1016/j.pnpbp.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Juruena M.F., Bocharova M., Agustini B., Young A.H. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. Journal of Affective Disorders. 2018;233:45–67. doi: 10.1016/j.jad.2017.09.052. [DOI] [PubMed] [Google Scholar]
- Kaiser T., Herzog P., Voderholzer U., Brakemeier E.L. Unraveling the comorbidity of depression and anxiety in a large inpatient sample: Network analysis to examine bridge symptoms. Depression and Anxiety. 2021;38(3):307–317. doi: 10.1002/da.23136. [DOI] [PubMed] [Google Scholar]
- Kan L. Clinical observation of Yushu granules in conjunction with fluoxetine hydrochloride in the treatment of 54 cases of post-stroke depression. Jilin Journal of Traditional Chinese Medicine. 2012;32(3):266–267. [Google Scholar]
- Kim J.H., Kim S.Y., Lee S.Y., Jang C.G. Antidepressant-like effects of Albizzia julibrissin in mice: Involvement of the 5-HT1A receptor system. Pharmacology Biochemistry and Behavior. 2007;87(1):41–47. doi: 10.1016/j.pbb.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Kim Y.K., Na K.S., Myint A.M., Leonard B.E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2016;64:277–284. doi: 10.1016/j.pnpbp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Lei Y.N., Zhang X.B., Chen S.C. A clinical and medical research on the Albizzia Julibrissin granule to treat depression and improve the sleep quality. Journal of Sichuan of Traditional Chinese Medicine. 2010;28(2):66–68. [Google Scholar]
- Li C., Huang B., Zhang Y.W. Chinese herbal medicine for the treatment of depression: Effects on the neuroendocrine-immune network. Pharmaceuticals (Basel, Switzerland) 2021;14(1):65. doi: 10.3390/ph14010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E.H. Baihe Ningshen Tang combined low-dose fluoxetine treatment depression after stroke randomized controlled study. Journal of Practical Traditional Chinese Medicine. 2016;30(7):55–57. [Google Scholar]
- Li C., Huang J., Cheng Y.C., Zhang Y.W. Traditional Chinese medicine in depression treatment: From molecules to systems. Frontiers in Pharmacology. 2020;11:586. doi: 10.3389/fphar.2020.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Qiao W., Fang D., Chen Q. The effect of Suanzaoren Hehuan formula effective fraction and its main components on depression and insomnia relieving in CUMS model mice. Traditional Chinese Drug Research & Clinical Pharmacology. 2019;30(12):1416–1420. [Google Scholar]
- Li J., Liu Q.T., Chen Y., Liu J., Shi J.L., Liu Y., Guo J.Y. Involvement of 5-HT1A receptors in the anxiolytic-like effects of quercitrin and evidence of the involvement of the monoaminergic system. Evidence-Based Complementary and Alternative Medicine. 2016;2016:6530364. doi: 10.1155/2016/6530364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. The influence of aqueous extract of Hehuanhua on cognitive function and HPA axis in the depression rats. Clinical Journal of Chinese Medicine. 2017;9(4):8–11. [Google Scholar]
- Li R., Tian J.F., Luo X.J., Li R.M. Research progress on chemical components and pharmacological effects of the flowers of Albizia julibrissin Durazz. Tianjin Pharmacy. 2022;34(2):66–71. [Google Scholar]
- Li S.J., Ge X.Y., Wang L.J., Pan Y.H. The efficacy of the duloxetine combined with Jie-Yu pill and psychological intervention to treat with liver-Qi stagnation type of depression. World Journal of Integrated Traditional and Western Medicine. 2020;15(5):794–797. [Google Scholar]
- Li W. Experimental study on improving learning and memory ability of depression rats with Albizia julibrissin. Clinical Journal of Chinese Medicine. 2017;9(20):27–28. [Google Scholar]
- Li W.L., Wang X., Gao Y. Antidepressant effects of Flos Albizziae flavonoids in chronic-stressed rats. Chinese Journal of Public Health. 2014;29(4):515–517. [Google Scholar]
- Li Y.H. Clinical efficacy of Jieyu Hehuan decoction in conjunction with deanxit in the treatment of moderate depression. Shaanxi Journal of Traditional Chinese Medicine. 2015;36(12):1627–1628. [Google Scholar]
- Liang Z., Zhang L., Hu X. Efficacious evaluation of Jieyu Hehuan decoction in the treatment of depression with post-stroke. Guangming Journal of Chinese Medicine. 2018;33(19):2853–2855. [Google Scholar]
- Lin H., Hui B., Han Z.C., Li H., Rong P.H. Clinical observation of Jieyu granules in treating moderate and mild depression. Journal of Guangzhou University of Traditional Chinese Medicine. 2019;36(9):1316–1320. [Google Scholar]
- Lin H., Zhai T., Lin W., Yang F. Wuling capsule combined with escitalopram on the improvement of symptoms in depression patients and the intervention effect of Nrf2 / ARE signaling pathway. Chinese Archives of Traditional Chinese Medicine. 2021;39(10):1–7. [Google Scholar]
- Liu J., Lv Y.W., Shi J.L., Ma X.J., Chen Y., Zheng Z.Q.…Guo J.Y. Anti-anxiety effect of (-)-syringaresnol-4-O-beta-D-apiofuranosyl-(1→2)-beta-D-glucopyranoside from Albizzia julibrissin Durazz (Leguminosae) Molecules. 2017;22(8):1331. doi: 10.3390/molecules22081331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.F., Zhang H.X. Clinical observation of the combinative treatment of depression with traditional Chinese and Western medicines. Hubei Journal of Traditional Chinese Medicine. 2002;24(8):17. [Google Scholar]
- Liu Q.T., Liu J., Guo J.Y., Wu M.X., Lv Y.W., He Y.S., Shi J.L. Anti-anxiety effect of total flavonoids of the flowers of Albizia julibrissin Durazz. Research and Practice on Chinese Medicines. 2015;29(2):33–35. [Google Scholar]
- Liu W., Cui L.J., Li Y.Y., Yu X.Z. Clinical study on Baicaoxiang Jieyu Anshen capsules combined with paroxetine in treatment of depression. Drugs & Clinic. 2017;32(12):2409–2412. [Google Scholar]
- Lu J., Xu X., Huang Y., Li T., Ma C., Xu G.…Zhang N. Prevalence of depressive disorders and treatment in China: A cross-sectional epidemiological study. The lancet Psychiatry. 2021;8(11):981–990. doi: 10.1016/S2215-0366(21)00251-0. [DOI] [PubMed] [Google Scholar]
- Lv T., Li L., Liu P. Clinical observation of efficacy comparison between venlafaxine and clomipramine in the treatment of patients with anxiety and depression. Journal of Medical Theory and Practice. 2018;31(3):364–365. [Google Scholar]
- MahmoudianDehkordi S., Ahmed A.T., Bhattacharyya S., Han X., Baillie R.A., Arnold M.…Kaddurah-Daouk R. Alterations in acylcarnitines, amines, and lipids inform about the mechanism of action of citalopram/escitalopram in major depression. Translational Psychiatry. 2021;11(1):153. doi: 10.1038/s41398-020-01097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L.J., Lu Y., Song W.X. Clinical observation of Yushu granules in the treatment of 70 patient cases with depression. Chinese Journal of Integrative Medicine on Cardio/Cerebrovascular Disease. 2006;4(12):1047–1048. [Google Scholar]
- Masi P., Brovedani P. The hippocampus, neurotrophic factors and depression: Possible implications for the pharmacotherapy of depression. CNS Drugs. 2011;25(11):913–931. doi: 10.2165/11595900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Menke A. Is the HPA axis as target for depression outdated, or is there a new hope? Frontiers in Psychiatry. 2019;10:101. doi: 10.3389/fpsyt.2019.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S.E., Capitão L.P., Giles S.L.C., Cowen P.J., Stringaris A., Harmer C.J. The knowns and unknowns of SSRI treatment in young people with depression and anxiety: Efficacy, predictors, and mechanisms of action. The Lancet Psychiatry. 2021;8(9):824–835. doi: 10.1016/S2215-0366(21)00154-1. [DOI] [PubMed] [Google Scholar]
- Ng A., Tam W.W., Zhang M.W., Ho C.S., Husain S.F., McIntyre R.S., Ho R.C. IL-1beta, IL-6, TNF- alpha and CRP in elderly patients with depression or Alzheimer’s disease: Systematic review and meta-analysis. Scientific Reports. 2018;8(1):12050. doi: 10.1038/s41598-018-30487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu M.J., Ionescu D.F., Mathews D.C., Richards E.M., Zarate C.A., Jr. Second messenger/signal transduction pathways in major mood disorders: Moving from membrane to mechanism of action, part I: Major depressive disorder. CNS Spectrums. 2013;18(5):231–241. doi: 10.1017/S1092852913000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie W., Fang S. Analysis of the prescription, Wendan decoction, and its clinical application. Guide of China Medicine. 2019;17(34):182–183. [Google Scholar]
- Ou X., Li C., Li X., Ding Y., Wang Z. Effects of water extracts of Flos Albiziae and Semen Ziziphi Spinosae based on single herb-mutual reinforcement compatibility on animal model of anxious depression disorder. Journal of Jilin Agricultural University. 2019;41(2):199–206. [Google Scholar]
- Panossian A., Hamm R., Kadioglu O., Wikman G., Efferth T. Synergy and antagonism of active constituents of ADAPT-232 on transcriptional level of metabolic regulation of isolated neuroglial cells. Frontiers in Neuroscience. 2013;7:16. doi: 10.3389/fnins.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panossian A., Hamm R., Wikman G., Efferth T. Mechanism of action of Rhodiola, salidroside, tyrosol and triandrin in isolated neuroglial cells: An interactive pathway analysis of the downstream effects using RNA microarray data. Phytomedicine. 2014;21(11):1325–1348. doi: 10.1016/j.phymed.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Panossian A., Seo E.J., Efferth T. Synergy assessments of plant extracts used in the treatment of stress and aging-related disorders. Synergy. 2018;7:39–48. [Google Scholar]
- Panossian A., Seo E.J., Wikman G., Efferth T. Synergy assessment of fixed combinations of Herba Andrographidis and Radix Eleutherococci extracts by transcriptome-wide microarray profiling. Phytomedicine. 2015;22(11):981–992. doi: 10.1016/j.phymed.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Poisson C.L., Engel L., Saunders B.T. Dopamine circuit mechanisms of addiction-like behaviors. Frontiers in Neural Circuits. 2021;15 doi: 10.3389/fncir.2021.752420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Han Q., Liu D., Tu P., Zeng K., Liang H. Anti-tumor target identification and molecular mechanism study of total saponins from Albizia julibrissin. Journal of Chinese Materia Medica. 2017;42(19):3661–3665. doi: 10.19540/j.cnki.cjcmm.2017.0144. [DOI] [PubMed] [Google Scholar]
- Schenone M., Dancik V., Wagner B.K., Clemons P.A. Target identification and mechanism of action in chemical biology and drug discovery. Nature Chemical Biology. 2013;9(4):232–240. doi: 10.1038/nchembio.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X. Effect of Flos Albizziae total flavonoids on expression of BDNF and TrkB in the hippocampus CA1 of rat depression model. Traditional Chinese Drug Research & Clinical Pharmacology. 2014;15(1):1–4. [Google Scholar]
- Shi X., Cao Z., Du X., Wang Y., Lv J., Wang M., Guo C. Effects of herbal pair of Semen Ziziphi Spinosae and Albizia julibrissin flower on learning and memory ability and cellular signal transduction pathway of BDNF-MEK-ERK-CREB in depression model rats. Chinese Journal of Neuroanatomy. 2019;35(6):617–622. [Google Scholar]
- Shi X., Du X., Liu Q., Xia M., Lv J., Guo C. Effects of herbal pair Spinosae Ziziphi Semen and Albiziae Flos on neuronal apoptosis and GRP78 protein expression in hippocampal CA3 region of depression model rats. Traditional Chinese Drug Research & Clinical Pharmacology. 2019;30(2):162–167. [Google Scholar]
- Shi X., Du X., Xia M., Guo C., Wang M., Liu Q. Effects of herbal pair of Semen Ziziphi Spinosae and Albizia Julibrissin flower on apoptosis and caspase-12 expression in hippocampal CA3 region of depression model rats. Chinese Journal of Neuroanatomy. 2019;35(2):177–181. [Google Scholar]
- Shi X., Guo C., Jiang C., Zhang Y. Effects of herbal pair Flos Albiziae and Semen Ziziphi Spinosae on cognitive function and serum BDNF level in patients with depression. World Science and Technology/Modernization of Traditional Chinese Medicine and Materia Medica. 2013;15(7):1604–1607. [Google Scholar]
- Shi X., Li Z., Cai W., Liu Y., Li S., Ai M.…Qiu L. Chemical constituents from Albiziae Cortex and their ability to ameliorate steatosis and promote proliferation and anti-oxidation in vitro. Molecules (Basel) 2019;24(22):40–41. doi: 10.3390/molecules24224041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Liu T., Tang Q., Zhang L. Observation on clinical effect of regulating spleen-stomach Qi movement on depression. Guangxi Journal of Traditional Chinese Medicine. 2007;30(5):7–9. [Google Scholar]
- Shi X., Zhang Y., Jiang C. Clinical study on the herbal pair Spinosae Ziziphi Semen and Albiziae Flos in depression treatment. Guangxi Journal of Traditional Chinese Medicine. 2016;39(3):21–23. [Google Scholar]
- Shi X., Zhang Z., Yin S., Guo C. The effects of Albizzia julibrissin flower total flavonoids on the learning and memory abilities of the depression model rats. Journal of Pharmacological and Clinical Research of Traditional Chinese Medicine. 2013;29(5):61–63. [Google Scholar]
- Shi X.L., Du X.N., Guo C.F., Meng N.Q., Xia M., Cai T., Feng Q.Y. Effects of different doses of herbal pair of Semen Ziziphi Spinosae and Albizia julibrissin Flower on body weight and ethological indices in a rat model of depression. Guangxi Medical Journal. 2018;40(22):2702–2705. [Google Scholar]
- Shi X.L., Du X.N., Xia M., Men N.Q., Cai T., Guo C.F. Effects of herbal pair of Semen Ziziphi Spinosae and Albizia julibrissin flower on contents of 5-HT and 5- HIAA in hippocampus and serum of rats with sleep deprivation depression. Lishizhen Medical and Materia Medica Research. 2019;30(2):268–270. [Google Scholar]
- Shi X.L., Du Z.C., Xia M., Zhao X.F., Wu C., Cuo C.F. Study on antidepressant effect of Albizzia julibrissin flower total flavonoids hippocampus CA3 of rats with depression hippocampus CA3 of rats with depression. Chinese Journal of Experimental Traditional Medical Formulae. 2013;19(18):198–201. [Google Scholar]
- Shi X.L., Xia M., Feng Q.Y., Zhang Y.Q., Jiang C.L. Effects of herb pair of Semen Ziziphi Spinosae (SZS) and Albizia julibrissin flower (AJF) on the life quality and NEl of patients with depression. Lishizhen Medical and Materia Medica Research. 2016;27(10):2348–2350. [Google Scholar]
- Sobeh M., Hassan S.A., El Raey M.A., Khalil W.A., Hassan M.A.E., Wink M. Polyphenolics from Albizia harveyi exhibit antioxidant activities and counteract oxidative damage and ultra-structural changes of cryopreserved bull semen. Molecules. 2017;22(11):1993. doi: 10.3390/molecules22111993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R., Zhang L., Chen Y., Xu L. Effect of Jiawei Wendan decoction on ultrastructure of hypothalamus neurons in depressive rat models. Acta Chinese Medicine and Pharmacology. 2018;46(6):33–35. [Google Scholar]
- Su W.J., Zhang Y., Chen Y., Gong H., Lian Y.J., Peng W.…Jiang C.L. NLRP3 gene knockout blocks NF-κB and MAPK signaling pathway in CUMS-induced depression mouse model. Brain Research. 2017;322:1–8. doi: 10.1016/j.bbr.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Sun S., Gao Y., Cheng W. Efficacy of Jieyu Qingxin Anshen decoction in treatment of depression with liver Qi stagnation and disturbing heart syndrome. Acta Chinese Medicine. 2018;33(5):870–874. [Google Scholar]
- Sun S.G., Li C.L., Wang J.H. Clinical study of Jieyu Shuxin pills in conjunction with citalopram in the treatment of postpartum depression. Hebei Medical Journal. 2016;38(6):921–922. [Google Scholar]
- Sun S.G., Zhang L.M. Efficacy of Jieyu Shuxin pills in the treatment of postpartum depression. Hebei Medical Journal. 2016;38(9):1399–1400. [Google Scholar]
- Tang X.Q., Han Z.C., Chen J. Study on the clinical efficacy of Jieyu Pill combined with paroxetine in the treatment of post-stroke depression. Tianjin Journal of Traditional Chinese Medicine. 2019;36(11):1061–1064. [Google Scholar]
- The State Commission of Chinese Pharmacopoeia . China Medical Science and Technology Press; Beijing: 2020. Pharmacopoeia of People's Republic of China, part I. [Google Scholar]
- Tian F., Zhang L. Clinical observation of the modified Jieyu Hehuan decoction in the treatment of depression with post-stroke. Journal of Practical Traditional Chinese Medicine. 2016;32(1):12. [Google Scholar]
- Tian J., Bai Y.J., You A.M., Yao J.P. Effect of Jieyu wan in conjunction with escitalopram on neurological junction and serum NF-κB, 5-HT, miR-146 and miR-221-3p in patients with post-stroke depression. Information on Traditional Chinese Medicine. 2020;37(6):96–100. [Google Scholar]
- Tian Y. Clinical significance of Jieyu Qingxin Anshen decoction in the treatment of depression. Guangming Journal of Chinese Medicine. 2017;32(7):1002–1003. [Google Scholar]
- Tonhajzerova I., Sekaninova N., Bona Olexova L., Visnovcova Z. Novel insight into neuroimmune regulatory mechanisms and biomarkers linking major depression and vascular diseases: The dilemma continues. International Journal of Molecular Sciences. 2020;21(7):2317. doi: 10.3390/ijms21072317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voleti B., Duman R.S. The roles of neurotrophic factor and Wnt signaling in depression. Clinical Pharmacology & Therapeutics. 2012;91(2):333–338. doi: 10.1038/clpt.2011.296. [DOI] [PubMed] [Google Scholar]
- Wachtel-Galor S., Benzie I.F.F. In: Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition. Benzie I.F.F., Wachtel-Galor S., editors. CRC Press; Boca Raton, FL: 2011. Herbal medicine: An introduction to its history, usage, regulation, current trends, and research needs; pp. 1–11. [PubMed] [Google Scholar]
- Wang A., Wei X., Chen Y., Li M., Xue D., Liu H. Effects of aqueous extract of Albizzia julibrissin Durazz flower on learning and memory abilities and the expression of CREB of depression model rats. Chinese Journal of Neuroanatomy. 2015;31(6):771–776. [Google Scholar]
- Wang B., Lu S., Zhang C., Zhu L., Li Y., Bai M., Xu E. Quantitative proteomic analysis of the liver reveals antidepressant potential protein targets of Sinisan in a mouse CUMS model of depression. Biomedicine & Pharmacotherapy. 2020;130 doi: 10.1016/j.biopha.2020.110565. [DOI] [PubMed] [Google Scholar]
- Wang B.M., Qiao P., Wang W., Song W., Liu C., Wang X.Y.…Dong X.J. Effect of Albiziae Flos and Polygalae Radix alone and their combination on depression-like Behavior and CREB and NOX2 expression in hippocampus of chronic unpredictable stress-induced rats. Chinese Journal of Experimental Traditional Medical Formulae. 2021;27(17):32–39. [Google Scholar]
- Wang F.Q., Zheng W.D., Ma C.L., Zhang X.Q., Li H.Y., Shi H.Q. The effect of Albiza Cortex on immune functions of mice. Journal of Linyi Medical College. 2000;22(3):201–203. [Google Scholar]
- Wang J.Q., Liang G.L. Treatment of 39 depressive patients with chronic heart failure with Shuxin decoction in conjunction with conventional antidepressants. Traditional Chinese Medicine Research. 2016;29(3):34–37. [Google Scholar]
- Wang M., Xia M., Shi X., Shen X., Fu L., Jiang X. Effects of Jiawei Wendan decoction on Ras protein expression in the hippocampus of depression rat models at different time periods. E-Journal of Translational Medicine. 2016;3(6):1–5. [Google Scholar]
- Wang G.L., Tang K.Q., Ji Z.J., Hu H.L., Lin Y. Jieyu Granule in treatment for patients with chronic congestive heart failure accompanied by depression: A clinical study. Evidence-based Cardiovascular Medicine. 2018;10(8):990–992. [Google Scholar]
- Wang X., Xu X., Tao W., Li Y., Wang Y., Yang L. A systems biology approach to uncovering pharmacological synergy in herbal medicines with applications to cardiovascular disease. Evidence-Based Complementary and Alternative Medicine. 2012;2012 doi: 10.1155/2012/519031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li M., Liang Y., Yang Y., Liu Z., Yao K.…Zhai S. Chinese herbal medicine for the treatment of depression: Applications, efficacies and mechanisms. Current Pharmaceutical Design. 2017;23(34):5180–5190. doi: 10.2174/1381612823666170918120018. [DOI] [PubMed] [Google Scholar]
- Wei X.M. Effects of aqueous extract of Albizzia julibrissin Durazz flower on the level of cytokines in depression model rats. Chinese Journal of Cancer Prevention and Treatment. 2016;14(11):14–17. [Google Scholar]
- Zunszain P.A., Anacker C., Cattaneo A., Carvalho L.A., Pariante C.M. Glucocorticoids, cytokines and brain abnormalities in depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(3):722–729. doi: 10.1016/j.pnpbp.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2017). Depression. https: //www.who.int/news-room/fact-sheets/detail/depression. (Accessed in 2022).
- WHO. (2019). https: //www.who.int/traditional-complementary-integrative-medicine/en/. (Accessed in 2022).
- Wen Y.N., Ji F. Clinical study of the combinative treatment of anxiety and depression with Chinese medicine in conjunction with Western medicine. Journal of Practical Traditional Chinese Medicine. 2015;31(9):829. [Google Scholar]
- Wohleb E.S., Franklin T., Iwata M., Duman R.S. Integrating neuroimmune systems in the neurobiology of depression. Nature Reviews Neuroscience. 2016;17(8):497–511. doi: 10.1038/nrn.2016.69. [DOI] [PubMed] [Google Scholar]
- Woo H.I., Lim S.W., Myung W., Kim D.K., Lee S.Y. Differentially expressed genes related to major depressive disorder and antidepressant response: Genome-wide gene expression analysis. Experimental and Molecular Medicine. 2018;50(8):1–11. doi: 10.1038/s12276-018-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P.R., Tang J., Zhang L., Song R. Research of Jia Wei Wen Dan Tang regulating hypothalamic neuropeptides levels of SS, SP, NPY and CRH in depression model rats. Chinese Journal of Acupuncture and Moxibustion. 2015;4(6):259–262. [Google Scholar]
- Wu Y.H., Yang J. Efficacy of Jieyu pills in conjunction with flupentixol and melitroxine tablets in the treatment of anxiety and depression patients with post-stroke. Medical Journal of Chinese People’s Health. 2020;32(24):97–99. [Google Scholar]
- Xiong Y., Feng B., Liu Y., Cui Y., Wang J., Wang H., Guan J. Effect of Cortex Albiziae on mice's anxiety-like behavior and antianxiety active site screening. World Chinese Medicine. 2018;13(4):790–798. [Google Scholar]
- Xu L., Zhang L.P., Song R.W., Chen Y. Effect of Jiawei Wendan decoction on gastrointestinal motility in depressive rats. Tianjin Journal of Traditional Chinese Medicine. 2019;36(4):387–391. [Google Scholar]
- Xu X.Y., Ren Y.L., Li Z.R. Clinical effect of flupentixol and melitracen combined with Baihe Shuxin decoction on anxiety and depression after coronary heart disease surgery. Clinical Medicine Research and Practice. 2020;5(23):125–126. [Google Scholar]
- Yan L., Xu S.L., Zhu K.Y., Lam K.Y., Xin G., Maiwulanjiang M.…Tsim K.W. Optimizing the compatibility of paired-herb in an ancient Chinese herbal decoction Kai-Xin-San in activating neurofilament expression in cultured PC12 cells. Journal of Ethnopharmacology. 2015;162:155–162. doi: 10.1016/j.jep.2014.12.049. [DOI] [PubMed] [Google Scholar]
- Yan Y.M., Yang W.J., Yang R. Clinical observation of Xingnao Jieyu capsules combined with deanxit for treatment of post-stroke depression patients accompanied by somatoform disorders. Journal of Guangzhou University of Traditional Chinese Medicine. 2011;28(6):586–589. [Google Scholar]
- Yang D., Gouaux E. Illumination of serotonin transporter mechanism and role of the allosteric site. Science Advances. 2021;7(49):eabl3857. doi: 10.1126/sciadv.abl3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Li D.H. Research progress on chemical constituents, pharmacological activities and toxicology of Flos Albizziae. Chinese Journal of Surgery of Integrated Traditional and Western Medicine. 2019;28(6):1061–1064. [Google Scholar]
- Yang L., Rong P.H. Combinative treatment of 30 depressive patient cases with post-stroke and energy deficiency by Jiawei Xiaoyao-San in conjunction with fluoxetine. Shaanxi Journal of Traditional Chinese Medicine. 2009;30(2):150–151. [Google Scholar]
- Yang Y., Zhong X. Clinical observation of Jieyu pills combined with paroxetine in the treatment of postpartum depression. Journal of Guangdong Medical University. 2020;38(3):331–333. [Google Scholar]
- Ye H.S., Fan F.X., Chen X., Li R.J., Cao H. Clinical observation of Jieyu pill combined with mirtazapine in treating refractory depression. World Chinese Medicine. 2020;15(10):1454–1457. [Google Scholar]
- Yi W., Wetzstein H.Y. Anti-tumorigenic activity of five culinary and medicinal herbs grown under greenhouse conditions and their combination effects. Journal of the Science of Food and Agriculture. 2011;91(10):1849–1854. doi: 10.1002/jsfa.4394. [DOI] [PubMed] [Google Scholar]
- Yu Q., Cai K., Tian W. Effects of total saponins from Albiziae Cortex on cellular immunity in H22 tumor-bearing mice. Chinese Journal of Experimental Traditional Medical Formulae. 2016;22(15):143–148. [Google Scholar]
- Yuan J., Li Q.Y., Wang X.H., Liu J., Cheng J., Li X.N., Chen Y.Y. Treatment of 30 patients with depression in elder people by Jieyu granules in conjunction with citalopram. Shaanxi Journal of Traditional Chinese Medicine. 2010;31(6):654–656. [Google Scholar]
- Zhang C.F., Cheng D.J., Feng Y.G., Zhao L.Q., Wang J.B. Effects of morita therapy and Baihe Zhimu Decoction on serum BDNF, DOPAC levels, quality of life, activities of daily living and efficacy in patients with first-episode depression. Chinese Archives of Traditional Chinese Medicine. 2021;39(11):1–6. [Google Scholar]
- Zhang D. Clinical observation on modified Jieyu Hehuan decoction combined with electroencephalogram biofeedback therapy instrument in treating patients with depression. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2018;27(9):108–110. [Google Scholar]
- Zhang F., Li F. Effect of Albizia julibrissin on growth and brain monoamine neurotransmitters in chronic-stressed rats. Zoological Research. 2006;27(6):621–625. [Google Scholar]
- Zhang G., Sun Q., Wang J. Clinical observation of Jieyu Hehuan decoction in the treatment of depression. Qinghai Medical Journal. 2009;39(8):84–85. [Google Scholar]
- Zhang H., Cao X. Analysis of Jieyu Qingxin Anshen decoction prescribed by Cao Xiaolan. Jiangsu Journal of Traditional Chinese Medicine. 2013;45(1):63–64. [Google Scholar]
- Zhang J.C., Yao W., Hashimoto K. Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Current Neuropharmacology. 2016;14(7):721–731. doi: 10.2174/1570159X14666160119094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Y. Study of Citalopram combined with Shenshuaining pill in the treatment of depression, a randomized parallel controlled study. Journal of Practical Traditional Chinese Internal Medicine. 2013;27(1):95–96. [Google Scholar]
- Zhang Q., Wu L., Gao Y., Zang Z., He X., Xia M., Dai J. The study of 5-HT, 5-HT1A content in emotional nervous loop of depression and anxiety rat model and Jiawei Wendan decoction's intervention effect. Chinese Archives of Traditional Chinese Medicine. 2021;39(12):1–12. [Google Scholar]
- Zhang X., Liu Y.Z., Zhang J.X., Song P.R., Zhang J. Clinical study on Jieyu pills combined with duloxetine in treatment of post-stroke depression. Drugs & Clinic. 2020;35(4):717–720. [Google Scholar]
- Zhang Y., Liu L., Liu Y.Z., Shen X.L., Wu T.Y., Zhang T.…Jiang C.L. NLRP3 inflammasome mediates chronic mild stress-induced depression in mice via neuroinflammation. International Journal of Neuropsychopharmacology. 2015;18(8):400–401. doi: 10.1093/ijnp/pyv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhao P., Cai Q. A comparative analysis of sertraline and clomipramine in the treatment of depression in elder people. China Practice Medicine. 2018;13(22):134–135. [Google Scholar]
- Zhang Y.W., Cheng Y.C. Challenge and prospect of traditional Chinese medicine in depression treatment. Frontiers in Neuroscience. 2019;13:190. doi: 10.3389/fnins.2019.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X.S., Chan T.F., Zhou H.H. Genetic and genomic approaches to identify and study the targets of bioactive small molecules. Chemical Biology. 2004;11(5):609–618. doi: 10.1016/j.chembiol.2003.08.011. [DOI] [PubMed] [Google Scholar]