Abstract
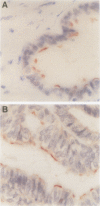
BACKGROUND: The assessment of neoplastic disease in gynaecological histopathology can be complicated by the high incidence of metaplasia seen in tissues of the female genital tract. There is a need to identify specific tissue markers which can be applied in routine histopathological practice. AIM: To examine the clinical potential of a monoclonal antibody, LhS28, which reacts with basal bodies of ciliated epithelial cells. METHODS: A panel of normal and pathological gynaecological tissues was processed and labelled with LhS28. RESULTS: LhS28 immunoreactivity was found in the normal Fallopian tube where it was confined to ciliated rather than secretory epithelial cells. In the remaining specimens, LhS28 was associated exclusively with ciliated cells in tubal metaplasias of the cervix and endometrium and in benign serous lined inclusion cysts. CONCLUSIONS: LhS28 may be a valuable marker for identifying metaplasia of tubal type and may find application in distinguishing tubal metaplasia from low grade cervical glandular intraepithelial neoplasia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Comer M. T., Leese H. J., Southgate J. Induction of a differentiated ciliated cell phenotype in primary cultures of Fallopian tube epithelium. Hum Reprod. 1998 Nov;13(11):3114–3120. doi: 10.1093/humrep/13.11.3114. [DOI] [PubMed] [Google Scholar]
- Comer M. T., Shires M., Goode N. P., Leese H. J., Trejdosiewicz L. K., Southgate J. Expression of an antigen associated with basal bodies of human ciliated epithelial cells. Histochem J. 1999 Jan;31(1):39–43. doi: 10.1023/a:1003470113851. [DOI] [PubMed] [Google Scholar]
- Ducatman B. S., Wang H. H., Jonasson J. G., Hogan C. L., Antonioli D. A. Tubal metaplasia: a cytologic study with comparison to other neoplastic and non-neoplastic conditions of the endocervix. Diagn Cytopathol. 1993;9(1):98–105. doi: 10.1002/dc.2840090121. [DOI] [PubMed] [Google Scholar]
- Fox H. Primary neoplasia of the female peritoneum. Histopathology. 1993 Aug;23(2):103–110. doi: 10.1111/j.1365-2559.1993.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Jonasson J. G., Wang H. H., Antonioli D. A., Ducatman B. S. Tubal metaplasia of the uterine cervix: a prevalence study in patients with gynecologic pathologic findings. Int J Gynecol Pathol. 1992;11(2):89–95. doi: 10.1097/00004347-199204000-00002. [DOI] [PubMed] [Google Scholar]
- Link C. J., Jr, Reed E., Sarosy G., Kohn E. C. Borderline ovarian tumors. Am J Med. 1996 Aug;101(2):217–225. doi: 10.1016/s0002-9343(96)80079-9. [DOI] [PubMed] [Google Scholar]
- Oliva E., Clement P. B., Young R. H. Tubal and tubo-endometrioid metaplasia of the uterine cervix. Unemphasized features that may cause problems in differential diagnosis: a report of 25 cases. Am J Clin Pathol. 1995 May;103(5):618–623. doi: 10.1093/ajcp/103.5.618. [DOI] [PubMed] [Google Scholar]
- Scully R. E. Pathology of ovarian cancer precursors. J Cell Biochem Suppl. 1995;23:208–218. doi: 10.1002/jcb.240590928. [DOI] [PubMed] [Google Scholar]