Abstract
Background
Increasing evidence links the gut microbiota (GM) to Alzheimer’s disease (AD) but the mechanisms through which gut bacteria influence the brain are still unclear. This study tests the hypothesis that GM and mediators of the microbiota-gut-brain axis (MGBA) are associated with the amyloid cascade in sporadic AD.
Methods
We included 34 patients with cognitive impairment due to AD (CI-AD), 37 patients with cognitive impairment not due to AD (CI-NAD), and 13 cognitively unimpaired persons (CU). We studied the following systems: (1) fecal GM, with 16S rRNA sequencing; (2) a panel of putative MGBA mediators in the blood including immune and endothelial markers as bacterial products (i.e., lipopolysaccharide, LPS), cell adhesion molecules (CAMs) indicative of endothelial dysfunction (VCAM-1, PECAM-1), vascular changes (P-, E-Selectin), and upregulated after infections (NCAM, ICAM-1), as well as pro- (IL1β, IL6, TNFα, IL18) and anti- (IL10) inflammatory cytokines; (3) the amyloid cascade with amyloid PET, plasma phosphorylated tau (pTau-181, for tau pathology), neurofilament light chain (NfL, for neurodegeneration), and global cognition measured using MMSE and ADAScog. We performed 3-group comparisons of markers in the 3 systems and calculated correlation matrices for the pooled group of CI-AD and CU as well as CI-NAD and CU. Patterns of associations based on Spearman’s rho were used to validate the study hypothesis.
Results
CI-AD were characterized by (1) higher abundance of Clostridia_UCG-014 and decreased abundance of Moryella and Blautia (p < .04); (2) elevated levels of LPS (p < .03), upregulation of CAMs, Il1β, IL6, and TNFα, and downregulation of IL10 (p < .05); (3) increased brain amyloid, plasma pTau-181, and NfL (p < 0.004) compared with the other groups. CI-NAD showed (1) higher abundance of [Eubacterium] coprostanoligenes group and Collinsella and decreased abundance of Lachnospiraceae_ND3007_group, [Ruminococcus]_gnavus_group and Oscillibacter (p < .03); (2) upregulation of PECAM-1 and TNFα (p < .03); (4) increased plasma levels of NfL (p < .02) compared with CU. Different GM genera were associated with immune and endothelial markers in both CI-NAD and CI-AD but these mediators were widely related to amyloid cascade markers only in CI-AD.
Conclusions
Specific bacterial genera are associated with immune and endothelial MGBA mediators, and these are associated with amyloid cascade markers in sporadic AD. The physiological mechanisms linking the GM to the amyloid cascade should be further investigated to elucidate their potential therapeutic implications.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-023-01218-5.
Keywords: Cognitive impairment, Alzheimer’s disease, Gut microbiota, Microbiota-gut-brain axis, Lipopolysaccharide, Endothelial dysfunction
Background
The gut microbiota (GM) represents the most densely populated bacterial community colonizing the human body and covers a wide range of functions critical to several aspects of human health. The GM is a key driver of the innate immune system [1, 2] and is involved in the degradation of macronutrients and production of metabolites [3]. Intestinal bacteria and their products modulate endothelial cell function and, at the same time, intestinal epithelial cells influence immune responses and shape the microbial composition [4] and generate a barrier preventing the passage of antigens and bacteria from the gut into the bloodstream [5].
Alzheimer’s disease (AD) pathology is characterized by the extracellular accumulation of β-amyloid (A), thought to facilitate intracellular cortical deposition and spreading of hyper-phosphorylated tau (T) which in turn drives progressive neurodegeneration (N), ultimately leading to cognitive impairment (CI) [6]. Clinical and preclinical evidence supports GM involvement in promoting AD onset and progression. Individuals with AD dementia and in the AD preclinical state reported alterations in the GM composition compared with healthy controls [7–11], and amyloid deposition was associated with pro-inflammatory bacterial species in the gut and pro-inflammatory cytokines in the blood [12]. Preclinical studies showed that GM is necessary for brain amyloid deposition [13–15] and is associated with neurodegeneration [16–18]. While growing evidence suggests that GM may impact cognitive impairment via signaling molecules of the microbiota-gut-brain axis (MGBA) [19–21], few studies in humans have assessed their relationship with markers of the amyloid cascade (ATN scheme) and CI. Furthermore, to the best of our knowledge, the simultaneous evaluation of a large panel of immune and endothelial mediators and their association with GM in AD patients has not been described previously.
We hypothesized that the fecal microbial alterations of patients with CI due to AD are associated with a specific profile of immune and endothelial MGBA mediators in the blood and that the latter is linked to markers of the amyloid cascade. To evaluate the existence and specificity of such a peripheral signature of sporadic AD, we investigated the association of fecal bacterial genera, MGBA mediators, and amyloid cascade markers in cognitively unimpaired persons (CU), patients with CI not due to AD (CI-NAD) and patients with CI due to AD (CI-AD).
Materials and methods
The present study shares several aspects with two previous papers focused on the evaluation of the role of GM in AD [12, 21]. Differently from those studies, here we applied a whole genome instead of a candidate approach for the GM characterization and we evaluated also tau pathology and neurodegeneration.
Study participants
Participants were community-dwelling persons of 50 to 85 years of age recruited from a large Italian study on amyloid imaging in patients with cognitive complaints, the Incremental Diagnostic Value of [18F]-Florbetapir Amyloid Imaging [INDIA-FBP] study [22]. As previously reported, all participants underwent an extensive neuropsychological battery (Additional file 1) including global cognitive measures (Mini-Mental State Examination (MMSE) and the Alzheimer’s Disease Assessment Scale, cognitive portion (ADAS‐Cog)). The inclusion criteria for participants with normal cognition were at most one neuropsychological test score outside the normal range. CI was defined as (i) presence of cognitive complaints reported by patients or proxy or by the physician; (ii) absence of intracranial metabolic or psychiatric causes of cognitive impairments; (iii) presence of abnormal scores in ≥ 2 cognitive tests; and (iv) history of progression of cognitive symptoms. Amyloid positivity was used to classify participants into amyloid-positive (CI-AD) and amyloid-negative (CI-NAD and CU) and was defined as global [18F]-Florbetapir standardized uptake value ratio versus cerebellum (SUVR) higher than 1.11 [23]. The 85 subjects included in the present study were not under antibiotic nor anti-inflammatory treatment over the past 3 months and accepted to donate stools and blood. Venous blood samples were collected from all participants using 4-ml K3-ethylenediamine tetra-acetic acid (EDTA) vacutainer and centrifuged at 3400 g for 10 min at 4 °C within 2 h of collection to obtain plasma. Plasma samples were then aliquoted and stored at − 80 °C until testing. Stool samples were collected from subjects at their own home in a sterile plastic cup, stored at − 20 °C, and delivered to IRCCS Fatebenefratelli Institute in Brescia within the following 24 h, where they were stored at − 20◦C until their processing.
Fecal bacterial composition
DNA was extracted from 180 to 200 mg of frozen stool using the QIAamp DNA Stool Mini Kit (Qiagen Retsch GmbH, Hannover, Germany) and according to the manufacturer’s instructions. Bead-beating homogenization by TissueLyser II (Qiagen Retsch GmbH, Hannover, Germany) was performed to mechanically disrupt fecal samples before DNA extraction. The samples were homogenized for 10 min at 30 Hz. DNA was quantified using a NanoDrop ND-1000 spectrophotometer, and then stored at + 4 °C until subsequent analyses. The regions V3 and V4 of the bacterial ribosomal RNA 16S gene were amplified and purified according to 16S Metagenomic Sequencing Library Preparation protocol by Illumina (Additional file 2). A paired-end read of 300 cycles per read was performed. The raw 16S data were processed using QIIME2 [24] (64 bit version 2021.4) run on a MacBook Pro with Intel CPU 12 × 2.6 GHz processors and 16 GB of RAM. Sequencing Illumina MiSeq data were already demultiplexed. Forward and reverse primers, reads containing ambiguous bases, or homopolymers greater than eight base pairs in length were removed. Moreover, we set a maximum number of expected errors equal to 2 and reads truncation if the quality score was less than 2. The DADA2 denoising process was applied with the default parameters. Alpha diversity, indicating the richness and abundance of ASVs within each individual, and beta diversity, showing the similarity or difference in microbiota composition between individuals, were calculated using the q2-diversity plugin after rarefaction of the feature table using the sample depth corresponding to the sample with the lowest read count. SILVA reference database (version 138) (https://www.arb-silva.de/), customized following the instructions on the dedicated tutorials and as previously reported (paper 16S), was used to infer the taxonomy of the amplicon sequence variants (ASV) at phylum and genus level. Absolute abundances at the genus and phylum levels were normalized by the total number of reads assigned in each sample. Genera assigned to the Archaea domain or found with relative abundance lower than 10−2, in less than 4 subjects across all samples were removed. A total of 139 ASVs were identified in the whole group after filtering. Alpha and beta diversity were calculated using the q2-diversity plugin and included Bray–Curtis and Jaccard indexes, observed ASVs, phylogenetic diversity, and Shannon and Pielou’s evenness indexes. The feature table was rarefied to the sample depth corresponding to the sample with the lowest read count.
Microbiota-gut-brain axis mediators
A series of markers were selected in order to investigate several possible immune and endothelial mediators of the MGBA: the gut bacteria product LPS; soluble CAMs involved in AD and indicative of endothelial damage (sVCAM-1 and sPECAM-1 [25, 26]), vascular damage (sP- and sL-Selectin [27]) and upregulated in response to infection (sNCAM and sICAM-1 [28, 29]); inflammatory and anti-inflammatory cytokines reported to be altered in AD (IL1β, IL6, TNFα, IL18, IL10 [30]). CAMs were selected because of the key role of endothelial cells in enabling communication between visceral organs and the central nervous system, providing information about physiological conditions in the body, and adapting accordingly to maintain homeostasis [31, 32]. LPS was measured in plasma by ELISA (Pierce LAL Chromogenic Endotoxin Quantitation Kit, Thermo Fisher Scientific). sCAMs were measured using the LEGENDplex™ Human Adhesion Molecule Panel multiplex assay (Cat.#740,945, BioLegend). Data were analyzed with LEGENDplex™ Cloud-based Data Analysis Software (Dec 05, 2019, BioLegend). For cytokines expression level, total RNA was isolated using the PAXgene blood miRNA kit according to the manufacturer’s protocol (PreAnalytiX, Hombrechtikon, CHE), and candidate gene expression analyses were performed using real-time PCR. Each target gene was normalized to the expression of three reference genes, glyceraldehyde 3-phosphate dehydrogenase, beta-actin, and beta-2-microglobulin, using TaqMan Assays on a 384-well Real-Time PCR System (Biorad). The expression levels of each target gene were normalized to the geometric mean of all three reference genes, and the Pfaffl method was used to determine the relative target gene expression of each gene in patients as compared with controls.
Amyloid cascade markers
[18F]-Florbetapir Amyloid PET was performed as previously reported [12, 21]. In addition, other markers altered during the pathological processes of AD were measured. Plasma concentrations of NFL (NF-Light immunoassay Advantage kit; Cat. N° 103,400), GFAP (GFAP Human Discovery Kit; Cat. N° 102,336), and p- tau 181 V2 Advantage Kit (p- tau181; Cat. No. 103714) were measured using the ultrasensitive Simoa SR-X instrument in accordance with the manufacturer’s recommended protocol. General cognition was assessed using MMSE and ADAScog.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (v 8.1.1) (GraphPad Software, San Diego, CA, USA), unless specified otherwise. The comparison among groups of descriptive statistics, GM products, endothelial and inflammatory markers were performed using ANOVA with Bonferroni correction for continuous Gaussian variables (or Kruskall-Wallis test with Dunn correction for non-Gaussian variables) and Chi-square test for categorical data. Two-group comparison (CI-NAD vs CU; CI-AD vs CU) in the abundance of ASVs grouped at phylum and genera levels were determined with Linear discriminant analysis effect size (LEfSe) [33], performed in the Galaxy platform (http://huttenhower.sph.harvard.edu/galaxy/) and visualized using GraphPad Prism. Beta and alpha diversity comparison analyses were performed in QIIME2 by applying the Kruskall-Wallis test and the permutational multivariate analysis of variance (PERMANOVA), respectively. Three-dimensional principal coordinate analysis (PCoA) was used to visualize beta diversity results. Associations of genera with MGBA mediators and of the latter with amyloid cascade markers were assessed with Spearman correlation, the nonparametric version of the Pearson correlation which reduces the influence of extreme values. The effect of age was calculated using the Partial and Semi-Partial (Part) Correlation (“ppcor”) R package (v 1.1) [34]. Significance was set at p < 0.05 (two-tailed) and, for hypothesis validation analyses, associations were selected if their Spearman’s rho value was > 0.4 in order to include only those with at least moderate association.
Results
Demographic and clinical characteristics were as expected for this population (Table 1 and Additional file 3). First, we compared the markers of the physiological systems among the clinical groups to identify those associated with sporadic AD. Then, we assessed the association of genera with MGBA mediators and of the latter with amyloid cascade markers.
Table 1.
Demographic and clinical features of study participants
| CU | CI-NAD | CI-AD | P-value | ||
|---|---|---|---|---|---|
| ANOVA b | CI-NAD vs CI-AD c | ||||
| N | 13 | 38 | 34 | ||
| Age (years) | 69.6 (7.1) | 69.8 (7.4) | 70.8 (6.1) | .796 | > .999 |
| Female | 7 (54%) | 21 (55%) | 16 (47%) | .775 | .487 |
| Education (years) | 9.1 (5.2) | 8.5 (3.9) | 8.7 (4.5) | > .999 | > .999 |
| Body mass indexa | 25.0 (3.4) | 25.3 (3.8) | 24.9 (2.9) | > .999 | > .999 |
| APOEe4 carrier statusd | 2 (18%) | 3 (9%) | 20 (65%) | < .001 | < .001 |
| General cognition | |||||
| Mini-Mental State Examination | 28.3 (1.2) | 24.8 (3.9) | 22.2 (5.1) | < .001 | 0.101 |
| ADAScog | 8.2 (3.3) | 15.3 (9.5) | 17.1 (7.6) | < .001 | .330 |
| Clinical stage | |||||
| Mild cognitive impairment | - | 21 (55%) | 22 (65%) | .415 | |
| Dementia | - | 17 (45%) | 12 (35%) | .415 | |
| Amyloid load (PET SUVr) | .95 (.07) | .92 (.09) | 1.31 (.13) | < .001 | < .001 |
Figure denotes mean (SD) and number (%)
Abbreviations: CU: cognitively unimpaired persons; CI-NAD: patients with cognitive impairment not due to AD; CI-AD: patients with cognitive impairment due to AD; ADAScog: Alzheimer's Disease Assessment Scale, cognitive subscale; PET: Positron emission tomography; SUVr: Standardized uptake value ratio
a Weight/height2 and measured in kg/cm2
b Statistical difference among the 3 groups by ANOVA, Kruskal–Wallis, or chi-squared test
c P values of pairwise comparisons using Bonferroni correction
d Missing data for 8 participants: 2 in CU, 4 in CI-NAD, and 3 in CI-AD
Gut microbial communities
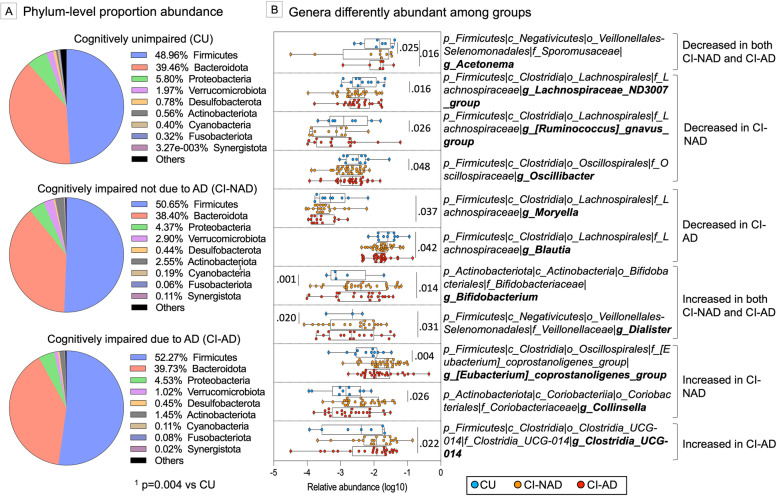
The comparison of beta diversity metrics did not show any GM compositional difference among groups (PERMANOVA, Bray–Curtis, and Jaccard distances, p > 0.133; Additional file 4A-B). Similar results were obtained for alpha diversity where the groups were comparable for all the metrics considered (p > 0.176; Additional file 4C). Concerning phylum taxonomic comparison, a shift in the abundance of Firmicutes (+ 3.3% in CI-NAD and + 6.3% in CI-AD) and Actinobacteria (+ 78% in CI-NAD and + 61% in CI-AD) was observed in CI groups compared to CU (Fig. 1A). However, only the increase of Actinobacteria in CI-NAD was significant (p = 0.004). Genus comparison revealed that both CI groups reported a lower abundance of Acetonema and a higher abundance of Bifidobacterium and Dialister compared to CU (p < 0.031). CI-AD were characterized by decreased abundance of Moryella and Blautia and a higher abundance of Clostridia_UCG-014 and (p < 0.042) while CI-NAD by decreased abundance of Lachnospiraceae_ND3007_group, [Ruminococcus]_gnavus_group and Oscillibacter as well as a higher abundance of [Eubacterium] coprostanoligenes group and Collinsella, and (p < 0.026).
Fig. 1.
Gut microbial communities of study participants grouped at phylum (A) and genus (B) levels. P-values were calculated using the online LEfSe workflow on the Hutlab Galaxy platform. Log10 transformation of genera abundances was used to increase readability. Abbreviations: CU, cognitively unimpaired persons; CI-NAD, patients with cognitive impairment not due to AD; CI-AD, patients with cognitive impairment due to AD
Immune and endothelial microbiota-gut-brain axis mediators
Next, we compared the selected putative modulators of the MGBA among groups and we found a specific association of MGBA mediators and cognitive impairment dependent on amyloid status (Fig. 2). CI-AD were characterized by high levels of LPS (p < 0.034) (Fig. 2A). Increased expression of pro-inflammatory cytokines was found mainly in CI-AD (IL1β, IL6, TNFα, p < 0.038) and to a lesser extent in CI-NAD (TNFα, p < 0.038) (Fig. 2B). Moreover, CI-AD showed decreased expression of the anti-inflammatory cytokine IL10 relative to CI-NAD (p < 0.050). CAMs analysis showed upregulation of sVCAM-1, sPECAM-1 (reflecting endothelial damage), sP-, sL-Selectin (reflecting vascular changes), sNCAM and sICAM-1 (expressed in response to infections) in CI-AD (p < 0.037) and only sP-Selectin (p < 0.030) in CI-NAD compared to CU (Fig. 2C).
Fig. 2.
Blood microbiota-gut-brain axis mediators in the blood of study participants. P-values were calculated by using one-way ANOVA with Bonferroni’s correction for continuous Gaussian variables (or Kruskall-Wallis test with Dunn’s correction for non-Gaussian variables) for the Gram-negative membrane protein LPS (A), the pro (IL1β, IL6, TNFα, IL18) and anti-inflammatory (IL10) cytokines (B) as well as the soluble cell adhesion molecules (C). Data are presented as box-plots with black horizontal lines indicating medians and circles indicating the subject’s values. Abbreviations: CU, cognitively unimpaired persons; CI-NAD, patients with cognitive impairment not due to AD; CI-AD, patients with cognitive impairment due to AD
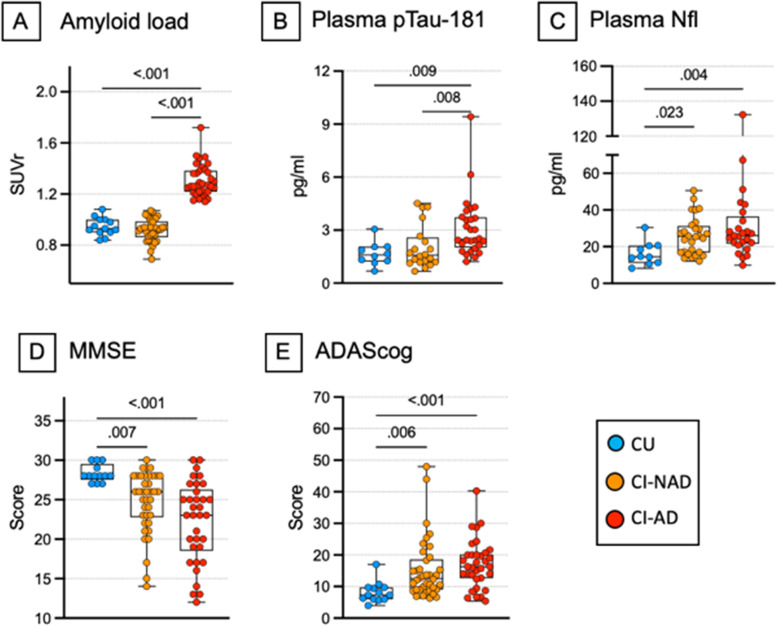
Amyloid cascade markers
In addition to the brain amyloid load measured by PET and used to classify participants into amyloid-positive and amyloid-negative, other markers of the amyloid cascade were measured. Data on plasma pTau-181 showed increased neurofibrillary tangles deposition in CI-AD but not in CI-NAD and CU (p < 0.009; Fig. 3B). Increased levels of plasma NfL and worst cognitive performance was found in CI-AD and CI-NAD when compared to CU (p < 0.023; Fig. 3C–E).
Fig. 3.
Markers of the amyloid cascade of study participants. P-values were calculated by using one-way ANOVA with Bonferroni’s correction for continuous Gaussian variables (or Kruskall-Wallis test with Dunn’s correction for non-Gaussian variables) for the amyloid load (A), plasma NfL (C) and cognitive measures (D–E) but not for pTau-181 (B), where Mann–Whitney test was applied. Data are presented as box-plots with black horizontal lines indicating medians and circles indicating the subject’s values. Abbreviations: CU, cognitively unimpaired persons; CI-NAD, patients with cognitive impairment not due to AD; CI-AD, patients with cognitive impairment due to AD
Association of microbial genera with MGBA markers and of the latter with amyloid cascade markers
In order to test whether AD is associated with distinctive alterations of the GM and MGBA mediator profile, nonparametric association analyses were computed separately in CU and CI-AD or CU and CI-NAD (Fig. 4). Overall, the correlation heatmaps revealed that the genera most abundant in CI groups (i.e., Bifidobacterium, [Eubacterium] coprostanoligenes group and Clostridia_UCG-014) were associated with endothelial damage, endothelial activation in response to infections and increased expression of pro-inflammatory cytokines (|rho|> 0.35, p < 0.044) (Fig. 4A). On the contrary, “protective” genera (i.e., Acetonema, [Ruminococcus]_gnavus_group and Blautia) were associated with endothelial integrity in CI-AD and reduced pro-inflammatory cytokine expression in both groups (|rho|> 0.39, p < 0.039).
Fig. 4.
Association matrices of genera with MGBA mediators (A) and of the latter with amyloid cascade markers (B). Heatmap of the Spearman’s rho coefficient values (pink: positive; green: negative) indicating significant association (age adjusted, p < 0.05) in CU and CI-NAD or CU and CI-AD. The star indicates moderate associations (Spearman’s rho value > 0.4). For MMSE and ADAS-cog, higher values reflected higher cognitive scores. Abbreviations: CU, cognitively unimpaired persons; CI-NAD, patients with cognitive impairment not due to AD; CI-AD, patients with cognitive impairment due to AD
The correlation heatmaps of MGBA and amyloid cascade markers showed different patterns of associations in CI-NAD and CI-AD (Fig. 4). In the analysis including CI-NAD, immune and endothelial markers were mainly associated with cognitive decline (|rho|> 0.36, p < 0.025), only TNFα was associated with neurodegeneration (rho = 0.37 with NfL, p = 0.028) and none with amyloid and tau pathology. In contrast, in the analysis with CI-AD, most of CAMs and cytokines were associated with amyloid and tau pathology and neurodegeneration (|rho|> 0.37, p < 0.025), and only LPS and pro-inflammatory cytokines with CI (|rho|> 0.37, p < 0.019). Of note, high levels of sP-selectin (marker of vascular damage) were associated with increased amyloid and tau pathology as well as neurodegeneration (|rho|> 0.44, p < 0.012). The different relationship of immune and endothelial MGBA mediators with amyloid cascade markers in CI-AD and CI-NAD was even more pronounced when only moderate associations were considered (Spearman’s rho value above 0.4; Fig. 5).
Fig. 5.
Closest associations (Spearman’s rho value > 0.4) between fecal bacterial genera and microbiota-gut-brain axis mediators and between the latter and amyloid cascade markers in CU and CI-NAD (A) and CU and CI-AD (B)
Discussion
This study investigated the association of GM with immune and endothelial MGBA mediators and of the latter with markers related to the amyloid cascade in a cohort of cognitively unimpaired persons, patients with cognitive impairment due to AD, and patients with cognitive impairment not due to AD. We report multiple interactions between circulating molecules involved in the MGBA and amyloid aggregation, tau phosphorylation, and neurodegeneration in CI-AD but not in CI-NAD. Our results suggest that peripheral mediators belonging to the MGBA might represent a peripheral signature of AD. Moreover, they strongly support the key role of peripheral mediators in the pathophysiology of AD although the design of the study cannot provide information on the causal chain.
To the best of our knowledge, 10 reports have previously addressed changes in the whole GM in human AD [7–10, 35–40] and MCI patients [9, 10, 35, 38, 39], with often conflicting results. For example, Blautia was found both decreased (here and [9, 39]) as well as increased [7, 35] in AD patients compared to controls. Similarly, Bifidobacterium was found decreased [7] or increased (here and [35, 40]) depending on the studies. Explanations of these discrepancies can be found in the use of different methods for studying the microbiota profile and to the different criteria for defining Alzheimer’s dementia applied in the studies (Additional file 5). Of the 10 published reports, only 3 reported information on ATN markers [7, 10, 36], and only one used the amyloid positivity finding for the inclusion of patients as in our study [36]. Moreover, sample collection and storage methods, different bioinformatics pipelines, or even the same pipeline on different operating systems have been reported to impact the relative abundance of the dominant bacterial phyla and genera [41, 42]. DNA extraction methods and laboratory locations have been shown to lead to up ten-fold and two-fold differences respectively in the relative abundances of specific bacterial genera, respectively [43].
We confirmed previous human findings demonstrating that blood levels of LPS [44], soluble CAMs (i.e., VCAM-1, PECAM-1, P-Selectin, E-Selectin, NCAM, ICAM-1) [27, 45–49], IL1β, IL6, TNFα [30] are increased in AD patients compared with controls. LPS has been reported to induce amyloid and tau aggregation [50, 51], tau phosphorylation [51], neurodegeneration [52], to reduce synaptic plasticity [53] and to increase microglia density [50] in mouse and rat brains. Our results are consistent with the notion that LPS translocates from the gut to the bloodstream as consequence of increased intestinal permeability [54] and co-localizes with amyloid plaques [55]. The increase in sCAMs reflecting endothelial and vascular damage indicated a general dysfunction of the blood-tissue barriers, including the blood–brain barrier. Furthermore, the upregulation of CAMs involved in the response to infection and controlling leukocyte trafficking is a further indication of widespread vascular inflammation. The latter is thought to promote detrimental processes as it (i) disturbs amyloid-β homeostasis [56] and (ii) facilitates the passage of antigens and bacteria from the gut into the bloodstream [5]. In line with previous findings [57–59], AD patients showed increased pTau-181 and both cognitively impaired groups reported increased NfL and performed worse on the MMSE and ADAScog compared to cognitively normal participants.
Communication between the compartments
The next step was to integrate the data from the 3 systems and to validate our hypothesis by using correlation analysis. According to the hypothesis of the study, we provide evidence revealing that a specific GM community contributes to AD pathology and cognitive impairment by increasing intestinal permeability and via bacteria products and inflammatory mediators. Although a multitude of animal and in-vitro studies established associations between the immune and endothelial MGBA mediators and AD features [18, 60–67], this relationship in human AD patients is still under-studied. Our analysis revealed that, in patients with cognitive impairment due to AD, (i) sPECAM-1, sP-, sE-Selectins, and IL6 were associated with amyloid and tau pathology; (ii) sP-Selectin, sNCAM, and the decrease of IL10 with neurodegeneration; and (iii) LPS and IL1β with cognitive impairment. Conversely, in patients with cognitive impairment not due to AD, no immune and endothelial MGBA mediators were associated with amyloid and tau pathology and neurodegeneration and many of them were associated with cognitive performance (sPECAM-1, sP-Selectin, IL1β, and decreased of IL10), suggesting that the link between GM alterations and cognitive impairment in non-AD patients likely involved different pathways than AD. While the CI-NAD is a clinically and biologically heterogeneous group, we observed significant associations between GM and MGBA vascular mediators. This, together with the higher occurrence of hypertension within this group, suggests that our CI-NAD might be enriched by patients with vascular disease.
Limitation
Although this study is one of the few that includes the amyloid marker for subject inclusion, it has also limitations. First of all, this is a small observational study and should be considered with caution due to participant selection and confounding biases. Replication of the results in an independent, larger cohort is needed, which would allow the evaluation of a broader panel of MGBA markers and the use of more complex statistical models. Although our analysis is limited to selected innate immunity and endothelial mediators, gut-brain communication is much more complex and involves, among others, mediators of cellular immunity and circulating molecules directly produced by the gut microbiota such as gut peptides, neurotransmitters, and metabolites. Of note, despite evidence suggesting that the bacterial metabolites’ short-chain fatty acids (SCFAs) have neuroprotective effects, recent studies showed that SCFAs supplementation could increase AD pathology in several AD animal models, possibly through immune cells activation [18, 61, 68]. This suggests that the involvement of SCFAs in microbiota-gut-brain interactions is much more complex than initially thought and that further studies aimed at establishing their relationship are necessary. Furthermore, here we used non-parametric correlations to study the association of markers belonging to the 3 compartments. This, together with the cross-sectional design, prevents us from drawing causal inferences between GM and MGBA marker alterations and AD pathological changes and symptoms onset. Longitudinal studies investigating also the earlier stages of the disease (i.e., amyloid positive and cognitive intact persons) are required to elucidate whether the AD-related microbiota alterations are upstream or downstream to brain AD pathological changes. Last, some of the studied markers were not available for all study participants (Additional file 6).
Conclusion
This study confirms the presence of a peripheral signature of Alzheimer’s disease featuring microbiota-gut-brain axis markers. The results suggest that the gut microbiota exerts its action on the brain at least in part by modulating endothelial cell function and the levels of circulating inflammatory and microbial products. LPS in the blood and the upregulation of soluble CAMs involved in endothelial damage and vascular changes suggest the presence of a more permeable intestinal barrier in AD. It is therefore plausible that GM products and inflammatory modulators (not only those measured here but also bacteria, fungi, and immune cells) translocate into the bloodstream, reach the brain and trigger the amyloid cascade. The association of LPS and several sCAMs with AD pathology and neurodegeneration provides a new line of evidence for a possible direct link between MGBA and brain pathological changes and offers new biomarkers and treatment targets for AD.
Supplementary Information
Additional file 1. Neuropsychological battery.
Additional file 2. DNA amplification, barcoding and sequencing.
Additional file 3. Risk factors, drugs and nutritional supplements of study participants.
Additional file 4. Beta (A-B) and alpha diversity (C) measures. Beta diversity metrics were computed using normalized data.
Additional file 5. Summary of the criteria used to define AD and methods applied to define the GM profile used in the human studies investigating GM alterations in AD.
Additional file 6. Number of subjects in the Spearman’s rank correlation analyses of Fig. 5.
Acknowledgements
We thank all patients and their families for their participation to the study and all members and collaborators of the INDIA-FBP Working Group.
Abbreviations
- ADAScog
Alzheimer's Disease Assessment Scale–Cognitive Subscale
- CAM
Cell adhesion molecules
- CI-AD
Patients with amyloid-dependent cognitive impairment
- CI-NAD
Patients with amyloid-independent cognitive impairment
- CU
Cognitively unimpaired persons without brain amyloidosis
- LPS
Liposaccharide
- MMSE
Mini-Mental State Examination
- sE-Selectin
Soluble forms of endothelial leukocyte adhesion molecule
- sICAM-1
Soluble forms of intracellular CAM 1
- sNCAM
Soluble forms of neural cell adhesion molecule
- sP-Selectin
Soluble forms of platelet-activation-dependent granule-external protein
- sPECAM-1
Soluble forms of platelet endothelial CAM 1
- sVCAM-1
Soluble forms of vascular CAM 1
Authors’ contributions
MMar analyzed the data and wrote the manuscript. MMar and GBF conceptualized the study and interpreted the data. PM, EM, LC, CF, NL, DL, MMaz, DN, JLB acquired and processed the sample. MA, MS, MP, AC reviewed and edited the manuscript. AC and GBF acquired the funding. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Funding
This study was partially funded by the Alzheimer’s Association Grant (NIRG) and Ricerca Corrente (Italian Ministry of Health). Amyloid PET was collected thanks to an unrestricted grant from Avid Radiopharmaceuticals, Inc which was unrelated to the topic of this study. The Centre de la mémoire is funded by the following private donors under the supervision of the Private Foundation of Geneva University Hospitals: A.P.R.A.—Association Suisse pour la Recherche sur la Maladie d’Alzheimer, Genève; Fondation Segré, Genève; Race Against Dementia Foundation, London, UK; Fondation Child Care, Genève; Fondation Edmond J. Safra, Genève; Fondation Minkoff, Genève; Fondazione Agusta, Lugano; McCall Macbain Foundation, Canada; Nicole et René Keller, Genève; Fondation AETAS, Genève. The Clinical Research Center, University Hospital and Faculty of Medicine, Geneva, provides valuable support for regulatory submissions and data management. The Centre de la mémoire has received unrestricted grants and support for event organizations from ROCHE Pharmaceuticals; OM Pharma; EISAI Pharmaceuticals; and Biogen Pharmaceuticals. Competitive research projects have been funded by H2020, Innovative Medicines Initiative (IMI), IMI2, Swiss National Science Foundation, and VELUX Foundation.
Availability of data and materials
The 16S rRNA sequencing raw data supporting the conclusions of this manuscript are publicly available at https://www.ebi.ac.uk/ena/submit/webin/report/runs/PRJEB55056. The microbiota-gut-brain axis marker dataset analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of “Comitato Etico dell’IRCCS San Giovanni di Dio – Fatebenefratelli” (Brescia, Italy) under registration number 57/2014. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell Host Microbe. 2012;12:496–508. doi: 10.1016/j.chom.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou A, Yuan Y, Yang M, Huang Y, Li X, Li S, et al. Crosstalk between the gut microbiota and epithelial cells under physiological and infectious conditions. Front Cell Infect Microbiol. 2022;12:832672. doi: 10.3389/fcimb.2022.832672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 6.Jack CRJ, Holtzman DM. Biomarker modeling of Alzheimer’s disease. Neuron. 2013;80:1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhuang Z-Q, Shen L-L, Li W-W, Fu X, Zeng F, Gui L, et al. Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis. 2018;63:1337–1346. doi: 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- 9.Liu P, Wu L, Peng G, Han Y, Tang R, Ge J, et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun. 2019;80:633–643. doi: 10.1016/j.bbi.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Verhaar BJH, Hendriksen HMA, de Leeuw FA, Doorduijn AS, van Leeuwenstijn M, Teunissen CE, et al. Gut microbiota composition is related to AD pathology. Front Immunol. 2021;12:794519. doi: 10.3389/fimmu.2021.794519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheng C, Yang K, He B, Du W, Cai Y, Han Y. Combination of gut microbiota and plasma amyloid-β as a potential index for identifying preclinical Alzheimer’s disease: a cross-sectional analysis from the SILCODE study. Alzheimers Res Ther. 2022;14:35. doi: 10.1186/s13195-022-00977-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C, et al. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017;7:2426. doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G, et al. Reduction of abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;8:7–41802. doi: 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Liao J, Xia Y, Liu X, Jones R, Haran J, et al. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut. 2022;71(11):2233–52. [DOI] [PMC free article] [PubMed]
- 16.Erny D, Prinz M. Microbiology: gut microbes augment neurodegeneration. Nature. 2017;544:304–305. doi: 10.1038/nature21910. [DOI] [PubMed] [Google Scholar]
- 17.Chen SG, Stribinskis V, Rane MJ, Demuth DR, Gozal E, Roberts AM, et al. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged fischer 344 rats and caenorhabditis elegans. Sci Rep. 2016;6:34477. doi: 10.1038/srep34477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo D-O, O’Donnell D, Jain N, Ulrich JD, Herz J, Li Y, et al. ApoE isoform- and microbiota-dependent progression of neurodegeneration in a mouse model of tauopathy. Science. 2023;379:eadd1236. doi: 10.1126/science.add1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González Cordero EM, Cuevas-Budhart MA, Pérez Morán D, Trejo Villeda MA, Gomez-Del-Pulgar Ga-Madrid M. Relationship between the gut microbiota and alzheimer’s disease: a systematic review. J Alzheimers Dis. 2022;87:519–528. doi: 10.3233/JAD-215224. [DOI] [PubMed] [Google Scholar]
- 20.Escobar Y-NH, O’Piela D, Wold LE, Mackos AR. Influence of the microbiota-gut-brain axis on cognition in Alzheimer’s disease. J Alzheimers Dis. 2022;87:17–31. doi: 10.3233/JAD-215290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marizzoni M, Cattaneo A, Mirabelli P, Festari C, Lopizzo N, Nicolosi V, et al. Short-chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in Alzheimer’s disease. J Alzheimers Dis. 2020;78:683–697. doi: 10.3233/JAD-200306. [DOI] [PubMed] [Google Scholar]
- 22.Boccardi M, Altomare D, Ferrari C, Festari C, Guerra UP, Paghera B, et al. Assessment of the incremental diagnostic value of florbetapir F 18 imaging in patients with cognitive impairment: the incremental diagnostic value of amyloid PET With [18F]-Florbetapir (INDIA-FBP) study. JAMA Neurol. 2016;73:1417–1424. doi: 10.1001/jamaneurol.2016.3751. [DOI] [PubMed] [Google Scholar]
- 23.Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 24.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet C, Al?Ghalith GA, et al. QIIME 2: reproducible, interactive, scalable, and extensible microbiome data science. Nat Biotechnol. 2019;37(8):852–7. 10.1038/s41587-019-0209-9. [DOI] [PubMed]
- 25.Ilan N, Mohsenin A, Cheung L, Madri JA. PECAM-1 shedding during apoptosis generates a membrane-anchored truncated molecule with unique signaling characteristics. FASEB J. 2001;15:362–372. doi: 10.1096/fj.00-0372com. [DOI] [PubMed] [Google Scholar]
- 26.Haarmann A, Nowak E, Deiß A, van der Pol S, Monoranu C-M, Kooij G, et al. Soluble VCAM-1 impairs human brain endothelial barrier integrity via integrin α-4-transduced outside-in signalling. Acta Neuropathol. 2015;129:639–652. doi: 10.1007/s00401-015-1417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuliani G, Cavalieri M, Galvani M, Passaro A, Munari MR, Bosi C, et al. Markers of endothelial dysfunction in older subjects with late onset Alzheimer’s disease or vascular dementia. J Neurol Sci. 2008;272:164–170. doi: 10.1016/j.jns.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 28.Thoulouze MI, Lafage M, Schachner M, Hartmann U, Cremer H, Lafon M. The neural cell adhesion molecule is a receptor for rabies virus. J Virol. 1998;72:7181–7190. doi: 10.1128/jvi.72.9.7181-7190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner RB, Wecker MT, Pohl G, Witek TJ, McNally E, St George R, et al. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA United States. 1999;281:1797–1804. doi: 10.1001/jama.281.19.1797. [DOI] [PubMed] [Google Scholar]
- 30.Brosseron F, Traschutz A, Widmann CN, Kummer MP, Tacik P, Santarelli F, et al. Characterization and clinical use of inflammatory cerebrospinal fluid protein markers in Alzheimer’s disease. Alzheimers Res Ther. 2018;10:25. doi: 10.1186/s13195-018-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricard N, Bailly S, Guignabert C, Simons M. The quiescent endothelium: signalling pathways regulating organ-specific endothelial normalcy. Nat Rev Cardiol. 2021;18:565–580. doi: 10.1038/s41569-021-00517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciccone V, Genah S, Morbidelli L. Endothelium as a source and target of H(2)S to improve its trophism and function. Antioxidants (Basel) 2021;10:486. doi: 10.3390/antiox10030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S. ppcor: an R package for a fast calculation to semi-partial correlation coefficients. Commun Stat Appl Methods. 2015;22:665–674. doi: 10.5351/CSAM.2015.22.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, He Y, Ma J, Huang P, Du J, Cao L, et al. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement. 2019;15:1357–1366. doi: 10.1016/j.jalz.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Laske C, Müller S, Preische O, Ruschil V, Munk MHJ, Honold I, et al. Signature of Alzheimer’s disease in intestinal microbiome: results from the AlzBiom study. Front Neurosci. 2022;16:792996. doi: 10.3389/fnins.2022.792996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haran JP, Bhattarai SK, Foley SE, Dutta P, Ward DV, Bucci V, et al. Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. MBio. 2019;10:e00632. doi: 10.1128/mBio.00632-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saji N, Murotani K, Hisada T, Tsuduki T, Sugimoto T, Kimura A, et al. The relationship between the gut microbiome and mild cognitive impairment in patients without dementia: a cross-sectional study conducted in Japan. Sci Rep. 2019;9:19227. doi: 10.1038/s41598-019-55851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo M, Peng J, Huang X, Xiao L, Huang F, Zuo Z. Gut microbiome features of Chinese patients newly diagnosed with Alzheimer’s disease or mild cognitive impairment. J Alzheimers Dis. 2021;80:299–310. doi: 10.3233/JAD-201040. [DOI] [PubMed] [Google Scholar]
- 40.Ling Z, Zhu M, Yan X, Cheng Y, Shao L, Liu X, et al. Structural and functional dysbiosis of fecal microbiota in Chinese patients with Alzheimer’s disease. Front Cell Dev Biol. 2020;8:634069. doi: 10.3389/fcell.2020.634069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choo JM, Leong LEX, Rogers GB. Sample storage conditions significantly influence faecal microbiome profiles. Sci Rep. 2015;5:16350. doi: 10.1038/srep16350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marizzoni M, Gurry T, Provasi S, Greub G, Lopizzo N, Ribaldi F, et al. Comparison of bioinformatics pipelines and operating systems for the analyses of 16S rRNA gene amplicon sequences in human fecal samples. Front Microbiol. 2020;11:1262. doi: 10.3389/fmicb.2020.01262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kennedy NA, Walker AW, Berry SH, Duncan SH, Farquarson FM, Louis P, et al. The impact of different DNA extraction kits and laboratories upon the assessment of human gut microbiota composition by 16S rRNA gene sequencing. PLoS ONE. 2014;9:e88982. doi: 10.1371/journal.pone.0088982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang R, Miller RG, Gascon R, Champion S, Katz J, Lancero M, et al. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS) J Neuroimmunol. 2009;206:121–124. doi: 10.1016/j.jneuroim.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rentzos M, Michalopoulou M, Nikolaou C, Cambouri C, Rombos A, Dimitrakopoulos A, et al. Serum levels of soluble intercellular adhesion molecule-1 and soluble endothelial leukocyte adhesion molecule-1 in Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2004;17:225–231. doi: 10.1177/0891988704269822. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen HM, Londos E, Minthon L, Janciauskiene SM. Soluble adhesion molecules and angiotensin-converting enzyme in dementia. Neurobiol Dis. 2007;26:27–35. doi: 10.1016/j.nbd.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Järemo P, Milovanovic M, Buller C, Nilsson S, Winblad B. P-selectin paradox and dementia of the Alzheimer type: circulating P-selectin is increased but platelet-bound P-selectin after agonist provocation is compromised. Scand J Clin Lab Invest. 2013;73:170–174. doi: 10.3109/00365513.2013.764572. [DOI] [PubMed] [Google Scholar]
- 48.Huang C-W, Tsai M-H, Chen N-C, Chen W-H, Lu Y-T, Lui C-C, et al. Clinical significance of circulating vascular cell adhesion molecule-1 to white matter disintegrity in Alzheimer’s dementia. Thromb Haemost. 2015;114:1230–1240. doi: 10.1160/TH14-11-0938. [DOI] [PubMed] [Google Scholar]
- 49.Todaro L, Puricelli L, Gioseffi H, Guadalupe Pallotta M, Lastiri J, Bal de Kier Joffé E, et al. Neural cell adhesion molecule in human serum Increased levels in dementia of the Alzheimer type. Neurobiol Dis. 2004;15:387–393. doi: 10.1016/j.nbd.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Wang L-M, Wu Q, Kirk RA, Horn KP, Ebada Salem AH, Hoffman JM, et al. Lipopolysaccharide endotoxemia induces amyloid-β and p-tau formation in the rat brain. Am J Nucl Med Mol Imaging. 2018;8:86–99. [PMC free article] [PubMed] [Google Scholar]
- 51.Sy M, Kitazawa M, Medeiros R, Whitman L, Cheng D, Lane TE, et al. Inflammation induced by infection potentiates tau pathological features in transgenic mice. Am J Pathol. 2011;178:2811–2822. doi: 10.1016/j.ajpath.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harland M, Torres S, Liu J, Wang X. Neuronal mitochondria modulation of LPS-induced neuroinflammation. J Neurosci Off J Soc Neurosci. 2020;40:1756–1765. doi: 10.1523/JNEUROSCI.2324-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abareshi A, Anaeigoudari A, Norouzi F, Shafei MN, Boskabady MH, Khazaei M, et al. Lipopolysaccharide-induced spatial memory and synaptic plasticity impairment is preventable by captopril. Adv Med. 2016;2016:7676512. doi: 10.1155/2016/7676512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevens BR, Goel R, Seungbum K, Richards EM, Holbert RC, Pepine CJ, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018;67:1555–1557. doi: 10.1136/gutjnl-2017-314759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhan X, Stamova B, Sharp FR. Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in Alzheimer’s disease brain: a review. Front Aging Neurosci. 2018;42. Available from: https://www.frontiersin.org/article/10.3389/fnagi.2018.00042. [DOI] [PMC free article] [PubMed]
- 56.Lyros E, Bakogiannis C, Liu Y, Fassbender K. Molecular links between endothelial dysfunction and neurodegeneration in Alzheimer’s disease. Curr Alzheimer Res. 2014;11:18–26. doi: 10.2174/1567205010666131119235254. [DOI] [PubMed] [Google Scholar]
- 57.Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018;14:989–997. doi: 10.1016/j.jalz.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mattsson N, Andreasson U, Zetterberg H, Blennow K. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74:557–566. doi: 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Podhorna J, Krahnke T, Shear M, Harrison JE. Alzheimer’s disease assessment scale-cognitive subscale variants in mild cognitive impairment and mild Alzheimer’s disease: change over time and the effect of enrichment strategies. Alzheimers Res Ther. 2016;8:8. doi: 10.1186/s13195-016-0170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother. 2018;18:83–90. doi: 10.1080/14737175.2018.1400909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erny D, Dokalis N, Mezö C, Castoldi A, Mossad O, Staszewski O, et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021;33:2260–2276.e7. doi: 10.1016/j.cmet.2021.10.010. [DOI] [PubMed] [Google Scholar]
- 62.Asti A, Gioglio L. Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J Alzheimers Dis. 2014;39:169–179. doi: 10.3233/JAD-131394. [DOI] [PubMed] [Google Scholar]
- 63.Hauss-Wegrzyniak B, Vraniak PD, Wenk GL. LPS-induced neuroinflammatory effects do not recover with time. NeuroReport. 2000;11:1759–1763. doi: 10.1097/00001756-200006050-00032. [DOI] [PubMed] [Google Scholar]
- 64.de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, et al. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 65.Yang F, Wang Z, Wei X, Han H, Meng X, Zhang Y, et al. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab. 2014;34:660–667. doi: 10.1038/jcbfm.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forloni G, Demicheli F, Giorgi S, Bendotti C, Angeretti N. Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells: modulation by interleukin-1. Brain Res Mol Brain Res. 1992;16:128–134. doi: 10.1016/0169-328x(92)90202-m. [DOI] [PubMed] [Google Scholar]
- 67.Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, et al. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colombo AV, Sadler RK, Llovera G, Singh V, Roth S, Heindl S, et al. Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition. Elife. 2021;10:e59826. doi: 10.7554/eLife.59826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Neuropsychological battery.
Additional file 2. DNA amplification, barcoding and sequencing.
Additional file 3. Risk factors, drugs and nutritional supplements of study participants.
Additional file 4. Beta (A-B) and alpha diversity (C) measures. Beta diversity metrics were computed using normalized data.
Additional file 5. Summary of the criteria used to define AD and methods applied to define the GM profile used in the human studies investigating GM alterations in AD.
Additional file 6. Number of subjects in the Spearman’s rank correlation analyses of Fig. 5.
Data Availability Statement
The 16S rRNA sequencing raw data supporting the conclusions of this manuscript are publicly available at https://www.ebi.ac.uk/ena/submit/webin/report/runs/PRJEB55056. The microbiota-gut-brain axis marker dataset analyzed during the current study are available from the corresponding author on reasonable request.