Abstract
In poplars (Populus), bspA encodes a 32-kD bark storage protein that accumulates in the inner bark of plants exposed to either short-day (SD) photoperiods or elevated levels of nitrogen. In this study, poplars transformed with a chimeric gene consisting of the bspA promoter fused to β-glucuronidase (uidA) were used to investigate the transcriptional regulation of the bspA promoter. Photoperiodic activation of the bspA promoter was shown to involve perception by phytochrome and likely involves both a low fluence response and a parallel very low fluence response pathway. Activity of the bspA promoter was also influenced by shoot growth. High levels of bspA expression usually occur in the bark of plants during SD but not long day or SD with a night break. When growth was inhibited under growth permissive photoperiods (SD with night break) levels of bark β-glucuronidase (GUS) activity increased. Stimulating shoot growth in plants treated with SD inhibited SD-induced increases in bark GUS activity. Because changes in photoperiod and growth also alter carbon and nitrogen partitioning, the role of carbon and nitrogen metabolites in modulating the activity of the bspA promoter were investigated by treating excised stems with amino acids or NH4NO3 with or without sucrose. Treatment with either glutamine or NH4NO3 resulted in increased stem GUS activity. The addition of sucrose with either glutamine or NH4NO3 resulted in synergistic induction of GUS, whereas sucrose alone had no effect. Glutamine plus sucrose induction of GUS activity was inhibited by EGTA, okadaic acid, or K-252A. Inhibition by EGTA was partially relieved by the addition of Ca2+. The Ca2+ ionophore, ionomycin, also induced GUS activity in excised shoots. These results indicate that transcriptional activation of bspA is complex. It is likely that SD activation of bspA involves perception by phytochrome coupled to changes in growth. These growth changes may then alter carbon and nitrogen partitioning that somehow signals bspA induction by a yet undefined mechanism that involves carbon and nitrogen metabolites, Ca2+, and protein phosphorylation/dephosphorylation.
Plants have evolved mechanisms to store and recycle nutrients that can be used during vegetative and reproductive growth (Chapin et al., 1990; Staswick, 1994). Nutrient reserve formation is a regulated process involving the re-use of metabolites that might otherwise be lost as litter. Nutrient recycling and storage occurs on a number of time scales including daily, weekly, seasonal, and lifetime storage (Chapin et al., 1990). In temperate deciduous woody perennials, N recycling involves the resorption of N from fall senescing leaves, storage in perennial tissues during over wintering, and subsequent re-use the following spring (Ryan and Bormann, 1982; Boerner, 1984). The majority of N in over wintering temperate deciduous trees is stored as a specialized storage protein termed bark storage protein (BSP) (Sauter et al., 1989; Wetzel et al., 1989). During fall and winter, BSP accumulates in protein storage vacuoles of phloem parenchyma, cortical parenchyma, and xylem ray cells (Sauter et al., 1989; Stepien and Martin, 1992).
In poplars (Populus), a 32-kD BSP accumulates in bark phloem parenchyma (Wetzel et al., 1989) and xylem ray cells (Sauter et al., 1989; Clausen and Apel, 1991) during autumn. Poplar BSP is encoded by a small multigene family and one of the gene family members, bspA, has been cloned and sequenced (Coleman and Chen, 1993). The bsp gene family is clustered and linked to a related gene (WIN4) of a family of wound-inducible genes (Davis et al., 1993). Environmental factors including photoperiod (Coleman et al., 1991; Langheinrich and Tischner, 1991), nitrogen availability (van Cleve and Apel, 1993; Coleman et al., 1994), temperature (van Cleve and Apel, 1993), and wounding (Davis et al., 1993) influence the expression of poplar bsp. Although the influence of environmental factors on bsp expression has been characterized, the physiological and molecular mechanisms by which these factors regulate bsp expression are not well understood.
In temperate woody plants, short-day (SD) photoperiods trigger a number of adaptive responses including nitrogen storage, stem growth cessation, terminal bud formation, bud dormancy, cold acclimation, and leaf senescence (Howe et al., 1996; Coleman, 1997; Olsen et al., 1997; Thomas and Vince-Prue, 1997). In poplar, SD photoperiod induced growth cessation and terminal bud formation involves perception by phytochrome (Howe et al., 1996; Olsen et al., 1997). Because photoperiod influences a wide array of physiological and developmental processes in woody plants, knowledge of photoperiodic regulation of bsp expression requires understanding the relationship between photoperiod, growth cessation, source-sink changes, and how these factors influence bsp expression.
We are using poplars as a model to investigate the regulation of bsp expression to understand how seasonal nitrogen storage is regulated in deciduous trees. In the present paper, poplars transformed with a gene consisting of the bspA promoter fused to β-glucuronidase (GUS) were used to investigate the activity of the bspA promoter in response to environmental and metabolic factors. We previously showed that the bspA promoter can confer SD responsiveness to GUS (Zhu and Coleman, 2001). In this report we show that SD activation of the bspA promoter involves perception by phytochrome. In addition, irrespective of the photoperiod that plants were exposed, the activity of the bspA promoter correlated with shoot growth. We also show that specific nitrogen compounds activate the bspA promoter, and this induction involves secondary messengers including Ca2+ and protein phosphorylation. Together, these results suggest that induction of bspA by SD involves perception by phytochrome that signals downstream events including altered carbon and nitrogen partitioning that probably regulates bspA induction.
RESULTS
SD Activity of the bspA Promoter Involves Photoperception by Phytochrome
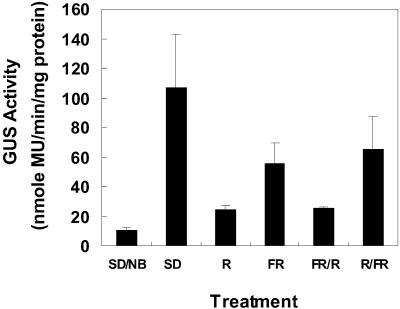
It was shown previously that bsp expression is induced during exposure to SD photoperiods (Coleman et al., 1991) and that the bspA promoter is responsive to photoperiod (Zhu and Coleman, 2000). Photoperiod perception in plants involves the photoreceptor phytochrome, and in SD plants the response is inhibited by a light interruption during the dark cycle (night break) (for review, see Thomas and Vince-Prue, 1997). The photoreceptor phytochrome exists in two interconvertible forms. Pr, the physiologically inactive form, is converted to Pfr the active form by red (R) light, whereas Pfr is converted to Pr by far-red (FR) light (Smith and Whitelam, 1990). This photoreversibility can be used to identify phytochrome-mediated events including gene expression (Quail et al., 1995). To determine if SD-induced bspA expression involves photoperception by phytochrome we examined transcriptional activity of the bspA promoter by assaying GUS activity in poplars transformed with the bspA-promoter::uidA chimeric gene that were exposed to either SD or SD with night breaks (NB). NB treatments consisted of either incandescent, R, FR, FR followed by R (FR/R), or R followed by FR (R/FR). Because results were similar among the five lines, only representative results from line B101.3.3 are presented. As shown in Figure 1, a 10-fold increase in bark GUS activity occurred after 4 weeks of SD treatment compared with plants exposed to SD with a 15-min NB of incandescent light. NB with R or FR followed by R (FR/R) reduced the levels of bark GUS activity by almost 80% compared with SD. R inhibition of SD induction of GUS was reversed when followed by FR (R/FR) but reversal was not to SD levels. The reversal of R inhibition by FR was to the levels of the FR treatment alone. Since FR alone partially inhibited SD induction of GUS complete reversal of R inhibition by FR is unlikely since the FR treatment reduced SD GUS induction. This suggests that a very low fluence response (VLFR) may exist during SD activation of the bspA promoter and complete FR reversibility would not be expected since VLFR are generally not photoreversible (Mancinelli, 1994).
Figure 1.
Inhibition of SD induced GUS activity by NBs. Poplars transformed with the bspA-promoter::uidA gene were grown at 25°C for 4 weeks in either SD photoperiods (SD; 8-h light/16-h dark) or SD supplemented with NBs. NB treatments consisted of either 15 min of incandescent light (SD/NB), 10 min of R (R), 10 min of FR (FR), 10 min of FR immediately followed by 10 min of R (FR/R), or 10 min of R immediately followed by 10 min of FR (R/FR). Each NB treatment was given nightly during the middle of the dark cycle. After 4 weeks, bark from young stems was assayed for GUS activity. Because results were similar among all lines, representative results from line B101.3.3 are shown. Each value is the mean of at least three independent measurements. Bars are sd of the mean.
Altered Growth Influences the Activity of the bspA Promoter
The expression of vegetative storage protein (VSP) genes in herbaceous plants is related to growth and source-sink relations (Staswick, 1994). Poplars exposed to SD photoperiods form terminal buds and stem elongation ceases. Because photoperiod influences growth, it was asked if growth and source-sink relationships have a role in modulating the activity of the bspA promoter by treating transformed poplars in such a way as to suppress or reduce shoot growth under growth permissive photoperiods (SD-NB) or stimulate shoot growth under growth suppressive photoperiods (SD). Because results were similar among the five lines, only representative results from line B6-9-3 are presented.
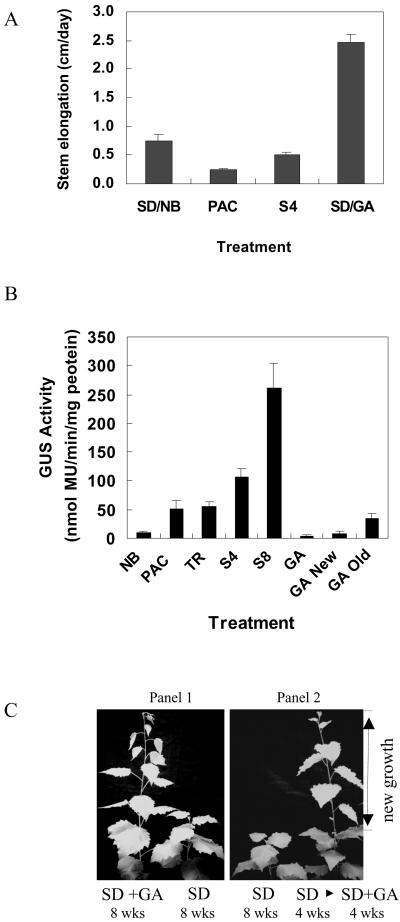
Shoot growth was suppressed or eliminated in SD-NB (15 min NB with incandescent light and growth permissive) conditions by removing the shoot tips or treating plants with paclobutrazol, a gibberellin (GA) biosynthesis inhibitor. Stem growth in plants exposed to SD-NB averaged 0.8 cm per day, whereas SD-treated plants averaged 0.5 cm per day (over a 4-week time interval) (Fig. 2A). Treatment of plants with paclobutrazol under SD-NB conditions reduced shoot elongation to 0.2 cm per day (Fig. 2A), whereas shoot-tip removal eliminated shoot growth (data not shown). Plants treated with either paclobutrazol or shoot tip removal developed a phenotypic appearance similar to that observed in SD conditions (data not shown). Both shoot-tip removal and treatment of intact plants with paclobutrazol resulted in a 5-fold increase in bark GUS activity compared with plants grown in SD-NB (Fig. 2B). The increase of bark GUS activity in plants with shoot-tips removed or sprayed with paclobutrazol was approximately 50% of that observed in plants exposed to 4 weeks of SD. It is interesting that shoot-tip removal and paclobutrazol treatment resulted in similar levels of GUS activity. Since the difference in light duration between SD and SD-NB is only a 15-min interval of incandescent light, it is unlikely that total photosynthesis differs between SD and SD-NB. Therefore, increased GUS activity in the bark is most likely a consequence of reduced growth.
Figure 2.
Influence of shoot tip removal, paclobutrazol, or GA on growth and GUS activity. A, Stem growth (cm/day) of poplars transformed with the bspA-promoter::uidA gene (line B6-9-3). Plants were treated as follows: 4 weeks of SD with a nightly 15-min night interruption with incandescent light (SD/NB); SD plus NB and a weekly spray with 50 μm paclobutrazol for 4 weeks (PAC); 4 weeks of SD (S4); and 8 weeks of SD with a weekly spray of a mixture of GA3, GA4, and GA7 at a combined concentration of 50 μm (SD/GA). B, Gus activity of poplars transformed with the bspA-promoter::uidA gene that were grown at 25°C and treated as follows: 4 weeks of SD with a nightly 15-min night interruption with incandescent light (NB); SD with NB and a weekly spray with 50 μm paclobutrazol for 4 weeks (PAC); SD with NB for 4 weeks and shoot tips removed from the plants (TR); 4 weeks of SD (8-h light/16-h dark; S4); 8 weeks of SD (S8); 8 weeks of SD with a weekly spray of a 50 μm combined solution of GA3, GA4, and GA7 (GA); and 4 weeks of SD without GA treatment followed by an additional 4 weeks of SD with weekly sprays of 50 μm GAs and GUS activity was assayed after the com- bined 8 weeks of SD in stems produced during the GA treatment (GA new) or in stems produced prior to the GA treatment (GA old). Bark from young stems was collected after each treatment and assayed for GUS activity. Because results were similar among lines, representative results from line B6-9-3 are shown. Each value is the mean of at least three independent measurements. Bars are SD of the mean. C, Examples of plants (line B6-9-3) grown in SD and treated with or without GA. Panel 1, Example of plants grown in either SD for 8 weeks and sprayed weekly with a 50 μm mixture of GA3, GA4, and GA7 (SD+GA) or sprayed with water (SD). Panel 2, Example of plants grown either for 8 weeks in SD without GA (SD) treatment or for 8 weeks in SD and sprayed with a 50 μm mixture of GA3, GA4, and GA7 during the final 4 weeks of SD treatment (SD 8594'3f SD + GA).
To further establish a role for growth in modulating the activity of the bspA promoter, transformed poplars were exposed to SD conditions (growth suppressive) and treated with GAs during the entire 8 weeks of SD exposure. A second group of plants were exposed to SD for 4 weeks without GA treatment followed by an additional 4 weeks of SD with GA treatment. Plants that were treated for 8 weeks of SD formed a terminal bud, and stem elongation ceased while the plants treated with GA during the 8 weeks of SD treatment averaged 2.5 cm of new growth per day (Fig. 2, A and C). As shown in Figure 2B, bark GUS activity increased 25-fold in transformed poplars grown for 8 weeks in SD compared with control plants (SD-NB). However, if GAs were applied during the 8 weeks of SD treatment (GA), induction of GUS was inhibited (Fig. 2B). In addition, induction of bark GUS activity was also inhibited in SD exposed plants when they were treated with GAs during the last 4 weeks of the 8 weeks of SD treatment. Inhibition of GUS activity in plants treated with GAs during the final 4 weeks of the 8 weeks of SD treatment occurred in the bark of shoots produced during the final 4 weeks (GA new) as well as shoots formed prior to the GA treatment (GA old) (Fig. 2B).
Nitrogen and Suc Act Synergistically to Modulate bspA Promoter Activity
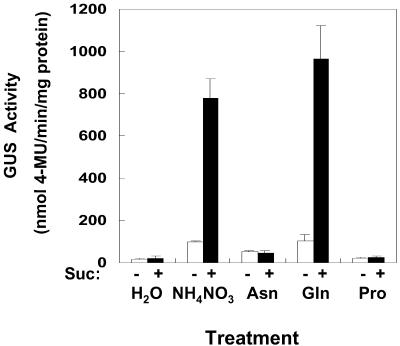
SD photoperiods induce changes in growth that result in alterations of N and C partitioning. Because altered N and C partitioning could influence bspA induction, we determined if different N-containing compounds, Suc, or N-containing compounds in combination with Suc influenced the activity of the bspA promoter under long day (LD) conditions. Preliminary experiments (data not shown) showed that excised poplar stems placed in aqueous solutions readily take up the solutions and continue to elongate for several days. Using this technique, excised stems of poplars transformed with the bspA-promoter::uidA gene were incubated under LDs in either 10 mm NH4NO3, 25 mm Asn, 25 mm Gln, or 25 mm Pro with or without 200 mm Suc. Because results were similar among the five lines, only representative results from line B25-1-2 are presented. As shown in Figure 3, after 4 d of incubation with either NH4NO3 or Gln a 5-fold increase in bark GUS activity was detected compared with incubation in water. Asn also induced GUS activity but was less effective than Gln, whereas Pro had no effect. When Suc was included with NH4NO3 or Gln a large synergistic increase in GUS activity occurred after 4 d (36- and 45-fold increase, respectively), whereas Suc alone was no different than water. Suc in combination with Asn or Pro had no effect on bspA promoter activity and GUS induction (Fig. 3).
Figure 3.
Nitrogen and Suc induction of GUS activity in excised shoots. Shoots with approximately 10 nodes were excised from poplars transformed with the bspA-promoter::uidA gene and incubated for 4 d in either water (H2O) or solutions containing 10 mm ammonium nitrate (NH4NO3), 25 mm Asn, 25 mm Gln, and 25 mm Pro in the presence (+) or absence (−) of 200 mm Suc. All incubations were under an LD photoperiod at 25°C. After 4 d of treatment bark tissue from young shoots was assayed for GUS activity. Because results were similar among lines, representative results from line B25-1-2 are presented. Each value is the mean of at least three independent measurements. Bars are sd of the mean.
Calcium and Protein Phosphorylation Influence bspA Promoter Activity
The previous results are consistent with a model in which photoperiod regulation of bspA involves perception by phytochrome leading to altered source-sink relations and changes in the cellular carbon and nitrogen status that could activate bspA transcription. Since calcium and protein phosphorylation have previously been shown to be involved in the signaling associated with amino acid and sugar inducible gene expression (Tekeda et al., 1994; Karchi et al., 1995; Ohto et al., 1995), we investigated whether Ca2+ or protein phosphorylation are involved in transcriptional activation of bspA by Gln plus Suc. Because results were similar among the five lines, only representative results from line B25-1-4 (Ca2+ study) or B6-9-6 (protein phosphorylation study) are presented.
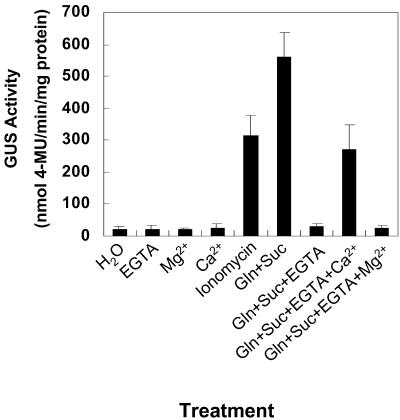
One method to study the role of Ca2+ is to reduce the levels by chelation with EGTA. When excised shoots were incubated in 25 mm Gln plus 200 mm Suc a 26-fold increase in bark GUS activity was detected compared with incubation in water (Fig. 4). If 5 mm EGTA was included with Gln and Suc no increase in bark GUS activity was detected (Fig. 4). The inhibition by EGTA was partially overcome by the addition of 50 mm CaCl2 (13-fold increase in GUS activity relative to water), whereas 50 mm MgCl2 had no effect. Incubating excised shoots in a 100 nm solution of ionomycin, an ionophore that increases Ca2+ flow intracellularly, also increased GUS activity in excised poplar stems almost 15-fold (Fig. 4). GUS activity in stems treated with EGTA, CaCl2, or MgCl2 was no different from water controls.
Figure 4.
Influence of calcium on Gln plus Suc induction of GUS activity in excised shoots. Shoots with approximately 10 nodes were excised from poplars transformed with the bspA-promoter::uidA gene and incubated in either water (H2O) or in solutions of either 5 mm EGTA, 50 mm MgCl2 (Mg2+), 50 mm CaCl2 (Ca2+), 100 nm ionomycin, 25 mm Gln plus 200 mm Suc (Gln+Suc), 25 mm Gln plus 200 mm Suc and 5 mm EGTA (Gln+Suc+EGTA), 25 mm Gln and 200 mm Suc plus 5 mm EGTA and 50 mm CaCl2 (Gln+Suc+EGTA+ Ca2+), or 25 mm Gln and 200 mm Suc plus 5 mm EGTA and 50 mm MgCl2 (Gln+Suc+EGTA+ Mg2+). Bark from young stems was harvested 4 d after incubation and assayed for GUS activity. Because results were similar among lines, representative results from B25-1-4 are shown. Each value is the mean of at least three independent measurements. Bars are sd of the mean.
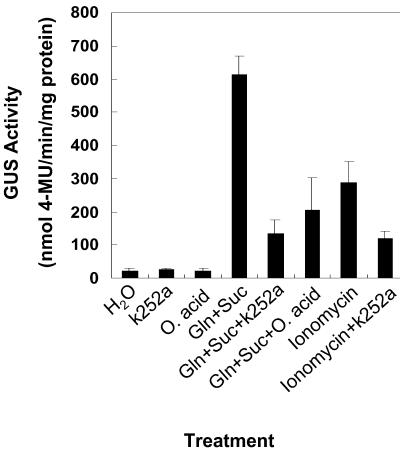
The influence of protein phosphorylation on bspA promoter activity during Gln plus Suc induction was investigated by incubating excised stems of transformed poplar in solutions of the protein kinase inhibitor K-252a (200 nm) or the protein phosphatase inhibitor okadaic acid (100 nm) (Fig. 5). Consistent with the previous experiments, incubation of stems in Gln and Suc resulted in a large increase in bark GUS activity (25-fold increase compared with water). When either K-252a or okadaic acid was included in the Gln plus Suc solution, induction of GUS activity was reduced by approximately 75%.
Figure 5.
Influence of K-252a and okadaic acid on Gln plus Suc induction of GUS activity in excised shoots. Shoots with approximately 10 nodes were excised from poplars transformed with the bspA-promoter::uidA gene and incubated in either water (H2O) or solutions of either 200 nm K-252a (k252a), 100 nm okadaic acid (O. acid), 25 mm Gln plus 200 mm Suc (Gln+Suc), 25 mm Gln plus 200 mm Suc and 200 nm K-252a (Gln+Suc+k252a), 25 mm Gln plus 200 mm Suc and 100 nm okadaic acid (Gln+Suc+O. acid), 100 nm ionomycin, or 100 nm ionomycin and 200 nm K-252a (Ionomycin+k252a). For the ionomycin with K-252a treatment, stems were first pre-incubated for 8 h with 200 nm K-252a before the ionomycin treatment. Bark from young stems was harvested 4 d after incubation and assayed for GUS activity. Because results were similar among lines, representative results from line B6-9-6 are presented. Each value is the mean of at least three independent measurements. Bars are sd of the mean.
The previous experiment showed that treatment in ionomycin without Gln plus Suc induced GUS activity. Because Ca2+ activation could involve the activation of a calcium dependent protein kinase, we conducted another experiment where excised shoots were first incubated with K-252a prior to treatment with ionomycin. As shown in Figure 5, ionomycin induction of GUS activity was reduced by approximately 60% when the poplar shoots were pre-incubated with K-252a prior to the addition of ionomycin.
DISCUSSION
Over-wintering adaptive traits in temperate perennial woody plants involve an integrated physiological response directed at plant survival and nutrient storage including BSP synthesis and storage (Wetzel et al., 1989). For many perennial woody plants, adaptive traits associated with over wintering such as cessation of shoot growth and bud dormancy are photoperiodic responses (Thomas and Vince-Prue, 1997). We previously showed that bsp expression was induced by SD photoperiods (Coleman et al., 1991) and NBs inhibited bsp mRNA accumulation and transcriptional activation of the bspA promoter (Zhu and Coleman, 2001). The results presented here show for the first time that transcriptional activation of the bspA promoter during SD involves photoperception by phytochrome. Inhibition of bspA promoter activity by NBs using either white or R light is consistent with SD responses (for review, see Thomas and Vince-Prue, 1997). To the best of our knowledge, this is the first example of photochrome-mediated regulation of VSP gene expression.
Photoperiodic responses are related to the light and dark periods of a 24-h cycle. In addition to photoperiodic responses, phytochrome mediated responses can also include low fluence response (LFR) and a VLFR, which can be distinguished based on photobiological properties of the response (Mancinelli, 1994). LFR responses are the classical phytochrome mediated responses that are R-FR reversible, whereas VLFR are induced by very low photon fluences and do not show R-FR reversibility (Mancinelli, 1994). In this study, SD activation of the bspA promoter was significantly reduced by NBs with R and showed R-FR reversibility. However R-FR reversibility was not complete and was only reversed to the level of the FR treatment. Given that FR alone reduced GUS induction it would not be expected that the R-FR reversibility would exceed the effect of the FR treatment. The effect of FR probably represents a VLFR since these responses occur at very low fluences of any light and are not reversible (Mancinelli, 1994). These results are consistent with both LFR and VLFR pathways being involved in the photoperiodic activation of the bspA promoter. Although it is possible that photoconversion of phytochrome was incomplete, given the high fluences of R and FR used in the treatments (18 μE m−2 s−1) this seems unlikely. Furthermore, studies were also conducted with a 5-min NB with R and FR, giving identical results (data not shown). Based on the studies using 5-min treatments, the experiments reported here used a 10-min NB to eliminate the possibility of incomplete photoconversion.
Because SD photoperiod influences growth, which alters source-sink relations, experiments were conducted to determine if activation of the bspA promoter was influenced by growth. If growth influences bspA expression, then we predicted that stimulating growth in SD treated plants should inhibit activation of the bspA promoter. Reducing or inhibiting growth under growth permissive conditions (SD-NB), conversely, should induce bspA expression. As reported here, when shoot growth was reduced under permissive growth conditions (SD-NB) either by treatment with a GA inhibitor or removing shoot tips, the levels of bark GUS activity increased. When SD plants where treated with GAs, which stimulated shoot growth, the levels of bark GUS activity were similar to SD-NB levels. The results are consistent with a role for shoot growth in regulating bspA expression.
How changes in shoot growth might signal bspA induction is not known. In perennial woody plants, SD photoperiods alter GA metabolism, which correlates with growth cessation and terminal bud set (Junttila, 1990; Olsen et al., 1995, 1997). It is possible that meristematic changes in GA metabolism during SD-induced growth cessation and terminal bud set alter source-sink relations of the plant. These altered source-sink relations may then signal the activation of the bspA promoter. Since source-sink relations modulated by developmental and environmental signals have been shown to influence VSP gene expression in herbaceous plants (Staswick, 1994; Bunker et al., 1995), it is possible that such factors are also important in poplar. Whether phytohormones and/or other metabolic signals regulate bspA expression is unclear. There currently is no direct evidence that bspA is directly regulated by phytohormones including abscisic acid, GA, cytokinin, and auxin (G.D. Coleman, unpublished data). Therefore, it is likely that metabolic signals linked to source-sink relations modulate the bspA promoter resulting in bspA expression and subsequent N storage.
Gln is a major transport amino acid in poplar (Sagisaka, 1974; Sauter, 1981; Sauter and van Cleve, 1992). During fall leaf senescence, N is largely transported from leaves to stems as Gln (Sauter and van Cleve, 1992). As reported here, the bspA promoter was activated by Gln, and this activation was synergistically enhanced by Suc. The response was specific to Gln since GUS activity did not differ among stems treated with water, Suc, Pro, Pro with Suc, or Asn with Suc. Although NH4NO3 also induced GUS, it is likely that this was through increased levels of Gln since NH4 and NO3 are used in the synthesis of Gln through nitrate reductase/nitrite reductase reduction and the GS-GOGAT pathway (Miflin and Lea, 1980).
Gln also accumulates in the living bark and xylem of poplars (Populus) and willows (Salix; Sagisaka, 1974; Sauter, 1981; Sauter and van Cleve, 1992) during spring bud break and shoot growth. Accumulated Gln is either stored or mobilized to active sinks such as developing leaves and shoot apices (Dickson et al., 1985; Vogelmann et al., 1985). Furthermore, xylem-fed [14C]Gln moved either directly upward in stem xylem or from stem xylem to phloem, followed by accumulation in developing leaves (Dickson et al., 1985; Vogelmann et al., 1985). Stem-localized [14C]Gln was distributed in the metaxylem next to the pith, phloem parenchyma, and xylem ray cells (Dickson et al., 1985; Vogelmann et al., 1985). During shoot growth bsp expression is also detected in young sinks, including the shoot apex (Lawrence et al., 1997; Zhu and Coleman, 2001). This correlates with one of the sites where Gln accumulates during growth, further arguing for a role for Gln in regulating bsp expression. In addition, the stem localization of [14C]Gln (Dickson et al., 1985; Vogelmann et al., 1985) coincides with the locations of bspA promoter activity as revealed by histochemical analysis of GUS activity in N- and SD-treated poplars transformed with the bspA-promoter::uidA chimeric gene (Zhu and Coleman, 2001).
Expression of the VSP genes of soybean is influenced by developmental and environmental factors. Although N availability influences soybean vsp expression, this effect appears to be related to source-sink relations as opposed to a direct N effect since various forms of mineral and organic N had no effect of vsp mRNA abundance in cell cultures of leaf explants (Staswick et al., 1991). In contrast to soybean, both Gln and NH4NO3 appear to influence bspA induction in poplar. Accumulation of the soybean VSP, the potato storage protein, patatin, and the sweet potato storage protein, sporamin, can also be induced by Suc (Wenzler et al., 1989; Hattori et al., 1991; Mason et al., 1992). In poplar, Suc alone had no effect on the activation of the bspA promoter but acted synergistically with either Gln or NH4NO3. Although the regulation of poplar BSP accumulation is certainly influenced by source-sink relations, it appears that induction of bspA is more directly related to N compared with other VSP. In particular, it seems probable that Gln levels may serve as a metabolic signal that regulates bspA induction.
Changes in cytosolic Ca2+ and/or protein phosphorylation interact in regulating the expression of many plant genes (for review, see Bush, 1995). A number of non-photosynthetic genes involved in primary C and N metabolism are affected by changes in phosphorylation state (for review, see Smith and Walker, 1996). It is also known that phytochrome-mediated gene expression requires Ca2+ (Neuhaus et al., 1997) as well as protein phosphorylation (Sheen, 1993). Ca2+ and protein phosphorylation have also been shown to be involved in sugar inducible expression of the vsp encoding sweet potato sporamin and β-amylase (Tekeda et al., 1994; Ohto et al., 1995).
In this study we used the Ca2+ chelator EGTA, the Ca2+ ionophore ionomycin, the protein kinase inhibitor K-252a, and the protein phosphatase inhibitor okadaic acid to show that the signaling cascade involved in activation of the bspA promoter by Gln plus Suc is mediated, at least in part, by Ca2+ and protein phosphorylation. EGTA and Ca2+ were used at millimolar concentrations, whereas ionomycin, K-252a, and okadaic acid were used in nanomolar concentrations. These concentrations are within the ranges that have been previously used for similar types of studies (Tekeda et al., 1994: Karchi et al., 1995; Ohto et al., 1995). At this concentration, okadaic acid inhibits type 1 and 2A protein phosphatases (Sheen, 1993; Karchi et al., 1995), therefore it cannot be concluded if one type or both types of protein phosphatases are involved. The inhibitory effect of EGTA on Gln plus Suc induction of GUS suggests that Ca2+ influx from the extracellular spaces is involved. This is also supported by the stimulatory effects of ionomycin since ionomycin increases cytoplasmic Ca2+ levels by membrane transport (Liu and Hermann, 1978). Although it is possible that inhibition of Gln plus Suc induction of GUS could result from altered uptake or transport of Gln or Suc by EGTA, this seems unlikely since the addition of Ca2+ overcame the inhibition by EGTA.
It is interesting that both K-252a and okadaic acid inhibited Gln plus Suc induction of GUS. Such results would be expected if both protein phosphorylation and dephosphorylation were involved in the signal transduction leading to gene expression. Although it is possible that both K-252a and okadaic acid somehow inhibited the uptake and transport of Gln and/or Suc, this seems unlikely since K-252a was also inhibitory to ionomycin induction of GUS. The inhibitory effect of K-252a on ionomycin induction could mean that Ca2+ and phosphorylation are both involved in regulating Gln plus Suc induction. Whether these factors operate within the same pathway, different pathways, or different pathways that converge cannot be concluded from these experiments. For example it is possible that a Ca2+-dependent protein kinase may be involved in signal transduction. Such a signal transduction scheme is similar to sugar-inducible vsp coding for the sweet potato sporamin and β-amylase (Tekeda et al., 1994; Ohto et al., 1995).
C and N metabolism are tightly linked physiological processes, and C/N partitioning between source and sink tissues is highly regulated in higher plants. Genes modulated by C and/or N metabolites appear to use a “sensing mechanism” to regulate expression (Alderson et al., 1991; Moorhead et al., 1999). The activation of the bspA promoter by Suc and Gln may well be another example of gene regulation modulated by C and N metabolites, a mechanism that widely exists in bacteria, yeast, and higher plants (Koch, 1996). Like bspA the regulation of many photosynthetic and non-photosynthetic genes involves changes in C/N metabolism (Koch, 1996). However, in contrast to bspA, photosynthetic genes such as rbcS and cab are inhibited by sugars, whereas nitrate reductase (Vincentz et al., 1993) and glutamine synthetase (Oliveira and Coruzzi, 1999) are induced by Suc and Gln antagonizes Suc inducibility.
In Arabidopsis and Ricinus communis a PII-like homolog to the Escherichia coli regulatory protein that is involved in regulating Gln synthetase activity, has recently been characterized (Hsieh et al., 1998). Expression of the PII homolog (GLB1) is affected by light and organic nitrogen and Gln appears to repress expression (Hsieh et al., 1998). How Gln and Suc are perceived in poplar leading to the induction bspA is not clear. However, it is intriguing to speculate that a poplar homolog of PII is involved in the perception and signaling of cellular carbon and nitrogen status that ultimately activates the bspA promoter via signal transduction involving Ca2+ and protein phosphorylation/dephosphorylation.
MATERIALS AND METHODS
BspA-promoter::uidA Gene Construction
The bspA-promoter::uidA gene fusion was made by gel purifying a 2.8-kb AccI to AccI DNA fragment that contained the bspA promoter DNA from a 7.0-kb SalI-XbaI genomic subclone (Coleman and Chen, 1993; GenBank accession no. X70064). The purified AccI-AccI fragment was filled in with the Klenow fragment of DNA polymerase and ligated into the SmaI site of the binary vector pGPTV-BAR (Becker et al., 1992), which is located upstream of the uidA coding region. The binary plasmid was transformed into Escherichia coli DH5α and colonies were screened for correct orientation of the bspA promoter and the junction between the bspA promoter and uidA was verified by DNA sequencing. A selected plasmid with the bspA promoter in the correct orientation was transformed into Agrobacterium tumefaciens strain C58/pM90 (Konz and Schell, 1986) and used to transform poplar.
Plant Material and Transformation
Hybrid aspen (Populus tremula × Populus alba; clone N717-B4) were grown and propagated in vitro on half-strength Murashige and Skoog medium (Murashige and Skoog, 1962). Internodal stem sections were transformed with the bspA-promoter::uidA chimeric gene essentially as described by Leple et al. (1992). Independent R0 transformants were selected for resistance to glufonisate ammonium at a concentration of 5 mg L−1 and chimeric gene integration was confirmed by DNA gel-blot analysis using the coding region of GUS as a hybridization probe. Rooted plantlets were maintained in vitro or transferred to soil and grown in a greenhouse. Ten independent bspA-promoter::uidA transgenic poplar lines were regenerated and the induction of stem bark GUS activity was shown to be induced by SD or N treatment in all 10 lines (Zhu and Coleman, 2001). In this study five transformed bspA-promoter::uidA poplar lines (B101.3.3, B6-9-3, B25-1-2, B25-1-4, and B6-9-6) that are representative of the 10 previously characterized lines were used for each experiment. The results between the five lines were similar with only the absolute values differing and no single line showed a different response. Because the treatment effects were similar among individual lines and the important comparison is how treatments affected GUS activity within a line, only the results from a representative line are presented for each experiment. However, results from a different line (as specified in the figure legend) are presented for each experiment.
Experimental Treatments
Photoperiod Treatments
Photoperiod, including NB, treatments were conducted in controlled environment chambers using 2-month-old greenhouse-grown plants. Light was provided by a mixture of fluorescent (F48T12/CW/VHO fluorescent lamps, GTE-Sylvania, Danvers, MA) and incandescent lamps (75 W) at a photosynthetic photon flux (400–700 nm) of 250 μE m−2 s−1. R light was provided by F48T12/236/VHO lamps (GTE-Sylvania) filtered through two layers of Roscolux number 823 red cellophane (Kliegle Bros., New York) and FR light was provide by F48T12/232/VHO lamps (GTE-Sylvania) filtered through a one-eighth-inch-thick F-700 Plexiglas filter (Westlake Plastics, Lenni Mills, PA). The intensity of both R and FR was 18 μE m−2 s−1. SD photoperiod consisted of 8 h of light followed by 16 h of dark. NB treatments were conducted by interrupting the middle of dark period during each night cycle with either incandescent, R, or FR light. The incandescent night interruptions were for 15 min, whereas the R or FR interruptions were for 10 min. The R followed by FR and FR followed by R reversal treatments were carried out by irradiating R- or FR-treated plants immediately with FR or R light, respectively, for 10 min.
Growth Treatments
Plant growth was inhibited in plants growing in SD-NB conditions by physically removing shoot-tips from growing plants or by treating growing plants with paclobutrazol, a GA biosynthesis inhibitor (Jacobsen and Olszewski, 1993). Shoot-tip removal treatments were performed by surgically removing the apical shoot tip (0.5 cm) from transformed poplars that were grown in SD with a 15-min NB of incandescent light (SD-NB). Throughout the shoot-tip removal experiment, plants were examined for any growth from axillary buds, which if detected was surgically removed. Four weeks after shoot-tip removal, bark was collected and assayed for GUS activity. Control plants (shoot-tips intact) continued to grow and did not form a terminal bud under these photoperiod conditions (SD with a 15-min NB with incandescent light). Shoot growth inhibition with paclobutrazol was performed by weekly spraying of intact plants growing in SD-NB (15 min of incandescent light) conditions with a 50-μm foliar spray. Bark GUS activity was assayed after 4 weeks of paclobutrazol treatment. Control plants were sprayed with water. The use of SD with night interruption ensures that differences in GUS activity were related to the experimental treatments and not light duration and/or photosynthetic differences, since a 16-h LD would expose plants to twice as much light as an SD.
Shoot growth was stimulated in plants exposed to SD by treating transformed poplars with an equal molar solution of GA3, GA4, and GA7 at a combined concentration of 50 μm. One group of plants received a weekly foliar spray of GA during the entire 8 weeks of SD treatment. Another group of plants were first exposed to SD for 4 weeks without GA treatment and then sprayed weekly with GA during an additional 4 weeks of SD. At the end of each GA treatment bark GUS activity was assayed. Control plants were sprayed with water.
Stem lengths were measured daily for plants treated with SD-NB, paclobutrazol, SD plus sprayed with GA, and plants exposed to SD for 4 weeks. Because shoot tip removal eliminates growth, stem lengths were not measured for those plants. In addition, growth was not measured for plants exposed to 8 weeks of SD since these plants form a terminal bud and cease growth.
Excised Shoot Treatments
Transformed poplar shoots with approximately 10 nodes were excised from greenhouse (LD photoperiod) grown stock plants and the basal end of the excised shoots were placed in water for 48 h in a growth chamber (25°C, 16-h light/8-h dark). After the 48-h pre-incubation, excised shoots were placed in aqueous solutions of various chemicals and incubated at 25°C with a LD photoperiod (16-h light/8-h dark). For the nitrogen and Suc induction study excised shoots were treated with either 10 mm NH4NO3, 25 mm Asn, 25 mm Gln, or 25 mm Pro with or without 200 mm Suc. For the calcium study excised shoots were treated with 5 mm EGTA, 50 mm MgCl2, 50 mm CaCl2, 100 nm ionomycin, 25 mm Gln plus 200 mm Suc, 25 mm Gln plus 200 mm Suc and 5 mm EGTA, 25 mm Gln plus 200 mm Suc and 50 mm CaCl2, or 25 mm Gln plus 200 mm Suc and 50 mm MgCl2. For the phosphorylation study excised stems were treated with 200 nm K-252a, 100 nm okadaic acid, 100 nm ionomycin, 25 mm Gln plus 200 mm Suc, 25 mm Gln plus 200 mm Suc and 100 nm okadaic acid, 25 mm Gln plus 200 mm Suc and 200 nm K-252a, or 100 nm ionomycin and 200 nm K-252a. For the ionomycin plus K-252a treatment, shoots were first incubated for 8 h with 200 nm K-252a prior to the addition of ionomycin. The concentrations of these chemicals were based on preliminary experiments (data not shown) and are within the range of concentrations used in similar studies (Tekeda et al., 1994; Karchi et al., 1995; Ohto et al., 1995). Bark from young shoots (apical 5 cm) was harvested after 4 d of each treatment and assayed for GUS activity. Poplars transformed with 35s::uidA gene were included as positive controls while poplars transformed with promoterless::uidA gene served as negative controls (data not shown).
Analysis of GUS Activity
Fluorometric assays for GUS activity was performed according to standard procedures (Jefferson, 1987).
Footnotes
This work was supported by the Plant Responses to the Environment Program of the National Research Initiative, U.S. Department of Agriculture (grant no. 98–35100–6108).
LITERATURE CITED
- Alderson A, Sabelli PA, Dickinson JR, Cole D, Richardson M, Kreis M, Shewry PR, Halford NG. Complementation of snf1, a mutation affecting global regulation of carbon metabolism in yeast, by a plant protein kinase cDNA. Proc Natl Acad Sci USA. 1991;88:8602–8605. doi: 10.1073/pnas.88.19.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D, Kemper E, Schell J, Masterson R. New plant binary vectors with selectable markers located proximal to the left T-DNA borders. Plant Mol Biol. 1992;20:1195–1197. doi: 10.1007/BF00028908. [DOI] [PubMed] [Google Scholar]
- Boerner RE. Foliar nutrient dynamics and nutrient use efficiency of four deciduous tree species in relation to site fertility. J Appl Ecol. 1984;21:1029–1040. [Google Scholar]
- Bunker TW, Koetje DS, Stephenson LC, Creelman RA, Mullet JE, Grimes HD. Sink limitation induces the expression of multiple soybean vegetative lipoxygenase mRNAs while the endogenous jasmonic acid level remains low. Plant Cell. 1995;7:1319–1331. doi: 10.1105/tpc.7.8.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Chapin FS, Schulze E-D, Mooney HH. The ecology and economics of storage in plants. Annu Rev Ecol Syst. 1990;21:423–447. [Google Scholar]
- Clausen S, Apel K. Seasonal changes in the concentration of the major storage protein and its mRNA in xylem ray cells of poplar trees. Plant Mol Biol. 1991;17:669–678. doi: 10.1007/BF00037052. [DOI] [PubMed] [Google Scholar]
- Coleman GD. Seasonal vegetative storage proteins of poplar. In: Kloptenstein NB, Chun YW, Kim MS, Ahuja MR, editors. Micropropagation, Genetic Engineering, and Molecular Biology of Populus, Gen Tech Rep RM–GTR–297. Fort Collins, CO: U.S. Department of Agriculture Rocky Mountain Forest and Range Experiment Station; 1997. pp. 124–130. [Google Scholar]
- Coleman GD, Bañados MP, Chen THH. Poplar bark storage protein and a related wound-induced gene are differentially induced by nitrogen. Plant Physiol. 1994;106:211–215. doi: 10.1104/pp.106.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman GD, Chen THH. Sequence of a poplar bark storage protein gene. Plant Physiol. 1993;102:1347–1348. doi: 10.1104/pp.102.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman GD, Chen THH, Ernst SG, Fuchigami LH. Photoperiod control of poplar bark storage protein accumulation. Plant Physiol. 1991;96:686–692. doi: 10.1104/pp.96.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Egelkrout EE, Coleman GD, Chen THH, Haissig BE, Riemenschneider DE, Gordon MP. A family of wound-induced genes in Populus shares common features with genes encoding vegetatie proteins. Plant Mol Biol. 1993;23:135–143. doi: 10.1007/BF00021426. [DOI] [PubMed] [Google Scholar]
- Dickson RM, Vogelmann TC, Larson PR. Glutamine transfer from xylem to phloem and translocation to developing leaves of Populus deltoides. Physiol Plant. 1985;77:412–417. doi: 10.1104/pp.77.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Fukumoto H, Nakagawa S, Nakamura K. Sucrose-induced expression of genes coding for the tuberous root storage protein, sporamin, or sweet potato in leaves and petioles. Plant Cell Physiol. 1991;32:79–86. [Google Scholar]
- Howe GT, Gardner G, Hackett WP, Furnier GR. Phytochrome control of short-day-induced bud set in black cottonwood. Physiol Plant. 1996;97:95–103. [Google Scholar]
- Hsieh MH, Lam HM, van de Loo FJ, Coruzzi G. A PII-like protein in Arabidopsis: putative role in nitrogen sensing. Proc Natl Acad Sci USA. 1998;95:13965–13670. doi: 10.1073/pnas.95.23.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Olszewski NE. Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell. 1993;8:887–896. doi: 10.1105/tpc.5.8.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. [Google Scholar]
- Junttila O. Gibberellins and the regulation of shoot elongation in woody plants. In: Takahashi N, Phinney BO, MacMillan J, editors. Gibberellins. Berlin: Springer-Verlag; 1990. pp. 119–210. [Google Scholar]
- Karchi H, Miron D, Ben-Yaacov S, Galili G. The lysine-dependent stimulation of lysine catabolism in tobacco seed requires calcium and protein phosphorylation. Plant Cell. 1995;7:1963–1970. doi: 10.1105/tpc.7.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Konz C, Schell J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- Langheinrich U, Tischner R. Vegetative storage proteins in poplar: induction and characterization of a 32- and a 36-kilodalton poplypeptide. Plant Physiol. 1991;97:1017–1025. doi: 10.1104/pp.97.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence SD, Greenwood JS, Korhnak TE, Davis JM. A vegetative storage protein homolog is expressed in the growing shoot-apex of hybrid poplar. Planta. 1997;203:237–244. doi: 10.1007/s004250050187. [DOI] [PubMed] [Google Scholar]
- Leple JC, Brasilerio ACM, Michel MF, Delmotte F, Jouanin L. Transgenic poplars: expression of chimeric genes using four different constructs. Plant Cell Rep. 1992;11:137–141. doi: 10.1007/BF00232166. [DOI] [PubMed] [Google Scholar]
- Liu C-M, Hermann TE. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978;253:5892–5894. [PubMed] [Google Scholar]
- Mancinelli AL. The physiology of phytochrome action. In: Kendrick RE, Kronberg GHM, editors. Photoporphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 211–269. [Google Scholar]
- Mason HS, DeWald DB, Creelman RA, Mullet JE. Coregulation of soybean vegetative storage protein gene expression by methyljasmonate and soluble sugars. Plant Physiol. 1992;98:859–867. doi: 10.1104/pp.98.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin BJ, Lea PJ. Ammonia assimilation. In: Miflin BJ, editor. The Biochemistry of Plants. Vol. 5. New York: Academic Press; 1980. pp. 169–202. [Google Scholar]
- Moorhead G, Douglas P, Cotelle V, Harthill J, Morrice N, Meek S, Deiting U, Stitt M, Scarabel M, Aitken A. Phosphorylation-dependent interactions between enzymes of plant metabolism and 14-3-3 proteins. Plant J. 1999;18:1–12. doi: 10.1046/j.1365-313x.1999.00417.x. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant. 1962;15:473–479. [Google Scholar]
- Neuhaus G, Bowler C, Hiratsuka K, Yamagata H, Chua NH. Phytochrome-regulated repression of gene expression requires calcium and cGMP. EMBO J. 1997;16:2554–2564. doi: 10.1093/emboj/16.10.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto M, Hayashi K, Isobe M, Nakamura K. Involvement of Ca2+-signaling in sugar-inducible expression of genes coding for sporamin and β-amylase of sweet potato. Plant J. 1995;7:297–307. [Google Scholar]
- Oliveira IC, Coruzzi GM. Carbon and amino acids reciprocally modulate the expression of glutamine synthetase in Arabidopsis. Plant Physiol. 1999;121:301–310. doi: 10.1104/pp.121.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JE, Jensen E, Junttila O, Moritz T. Photoperiod control of endogenous gibberellins in seedlings of Salix pentandra. Physiol Plant. 1995;93:639–644. [Google Scholar]
- Olsen JE, Junttila O, Nilsen J, Eriksson ME, Martinussen I, Olsson O, Sandberg G, Moritz T. Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimation. Plant J. 1997;12:1339–1350. [Google Scholar]
- Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wanger D. Phytochromes: photosensory perception and signal transduction. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- Ryan DF, Bormann FH. Nutrient resorption in northern hardwood forests. Bioscience. 1982;32:29–32. [Google Scholar]
- Sagisaka S. Effect of low temperature on amino acid metabolism in wintering poplar. Plant Physiol. 1974;53:319–322. doi: 10.1104/pp.53.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter JJ. Seasonal variation of amino acids in the xylem sap of Salix. Z Pflanzenphysiol. 1981;101:399–411. [Google Scholar]
- Sauter JJ, van Cleve B. Seasonal variation of amino acids in the xylem sap of Populus × canadensis and its relation to protein body mobilization. Trees. 1992;7:26–32. [Google Scholar]
- Sauter JJ, van Cleve B, Wellenkamp S. Ultrastructural and biochemical results on the localization and distribution of storage proteins in a poplar tree and in twigs of other tree species. Holzforschung. 1989;43:1–6. [Google Scholar]
- Sheen J. Protein phosphatase activity is required for light-inducible gene expression in maize. EMBO J. 1993;12:3497–3505. doi: 10.1002/j.1460-2075.1993.tb06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Whitelam GC. Phytochrome, a family of photoreceptors with multiple physiological roles. Plant Cell Environ. 1990;13:695–707. [Google Scholar]
- Smith RD, Walker JC. Plant protein phosphatases. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:101–125. doi: 10.1146/annurev.arplant.47.1.101. [DOI] [PubMed] [Google Scholar]
- Staswick PE. Storage proteins of vegetative plant tissues. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:3030–3322. [Google Scholar]
- Staswick PE, Huang J, Rhee Y. Nitrogen and methyl jasmonate induction of soybean vegetative storage protein genes. Plant Physiol. 1991;96:130–136. doi: 10.1104/pp.96.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien V, Martin F. Purification, characterization and localization of the bark storage proteins of poplar. Plant Physiol Biochem. 1992;30:399–407. [Google Scholar]
- Tekeda S, Mano S, Masa-Aki O, Nakamura K. Inhibitors of protein phosphatases 1 and 2A block sugar inducible gene expression in plants. Plant Physiol. 1994;106:567–574. doi: 10.1104/pp.106.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B, Vince-Prue D. Photoperiodism in Plants. London: Academic Press; 1997. [Google Scholar]
- van Cleve B, Apel K. Induction by nitrogen and low temperature of storage protein synthesis in poplar tress exposed to long days. Planta. 1993;189:157–160. [Google Scholar]
- Vincentz M, Moureaux T, Leydecker MT, Vaucheret H, Caboche M. Regulation of nitrate and nitrite reductase expression in Nicotiana plumbaginigolia leaves by nitrogen and carbon metabolites. Plant J. 1993;3:315–324. doi: 10.1111/j.1365-313x.1993.tb00183.x. [DOI] [PubMed] [Google Scholar]
- Vogelmann TC, Dickson RE, Larson PR. Comparative distribution and metabolism of xylem-borne amino compounds and sucrose in shoots of Populus deltoides. Plant Physiol. 1985;77:418–423. doi: 10.1104/pp.77.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzler H, Mignery G, Fisher L, Park W. Sucrose-regulated expression of a chimeric potato tuber gene in leaves of transgenic tobacco plants. Plant Mol Biol. 1989;13:347–354. doi: 10.1007/BF00015546. [DOI] [PubMed] [Google Scholar]
- Wetzel S, Demmers C, Greenwood JS. Seasonally fluctuating bark proteins are a potential form of nitrogen storage in three temperate hardwoods. Planta. 1989;178:275–281. doi: 10.1007/BF00391854. [DOI] [PubMed] [Google Scholar]
- Zhu B, Coleman GD (2001) The poplar bark storage protein gene (bspA) promoter is responsive to photoperiod and nitrogen in transgenic poplar and is active in floral tissues, immature seeds and germinating seeds of transgenic tobacco. Plant Mol Biol (in press) [DOI] [PubMed]