Abstract
Epithelial mesenchymal transition (EMT) and mesenchymal epithelial transition (MET) are genetic determinants of cellular plasticity. These programs operate in physiological (embryonic development, wound healing) and pathological (organ fibrosis, cancer) conditions. In cancer, EMT and MET interfere with various signalling pathways at different levels. This results in gross alterations in the gene expression programs, which affect most, if not all hallmarks of cancer, such as response to proliferative and death-inducing signals, tumorigenicity, and cell stemness. EMT in cancer cells involves large scale reorganisation of the cytoskeleton, loss of epithelial integrity, and gain of mesenchymal traits, such as mesenchymal type of cell migration. In this regard, EMT/MET plasticity is highly relevant to the Go-or-Grow concept, which postulates the dichotomous relationship between cell motility and proliferation. The Go-or-Grow decisions are critically important in the processes in which EMT/MET plasticity takes the central stage, mobilisation of stem cells during wound healing, cancer relapse, and metastasis. Here we outline the maintenance of quiescence in stem cell and metastatic niches, focusing on the implication of EMT/MET regulatory networks in Go-or-Grow switches. In particular, we discuss the analogy between cells residing in hybrid quasi-mesenchymal states and GAlert, an intermediate phase allowing quiescent stem cells to enter the cell cycle rapidly.
Keywords: Cancer, EMT, MET, Stem cells, Quiescence, Cancer stem cells
Introduction
Epithelial mesenchymal transition (EMT) is a process of conversion of polarised epithelial cells into mesenchymal cells. First discovered in embryonic development, EMT and a reverse process of mesenchymal epithelial transition (MET) were later implicated in human diseases, organ fibrosis, and cancer. EMT and MET are vital in embryogenesis for the early (gastrulation, neural crest delamination) and late (formation of various organs, such as the heart) stages. The classical physiological function of EMT in normal and pathological conditions is the generation of motile cells capable of degrading and crossing basement membranes [1]. A basement membrane is a specialised extracellular matrix built by proteins deposited by epithelial cells and cells of the underlying stroma. It is composed of polymerized laminin, glycoproteins, and collagen fibrils and separates epithelial layers from stromal tissues [2]. After passing through basement membranes, cells undergoing an EMT may penetrate surrounding tissues and migrate to new destinations.
Epithelial cells are linked to the basement membranes via focal adhesions and epithelium-specific adhesion structures, hemidesmosomes. The structural integrity and functionality of epithelial tissues depend on intercellular adhesion complexes, tight junctions, adherens junctions (AJ), and desmosomes, which mediate attachments between adjacent cells [3]. These differentiated, highly specialised structures are essential for maintaining the basolateral polarity of epithelial cells and tissue homeostasis [1].
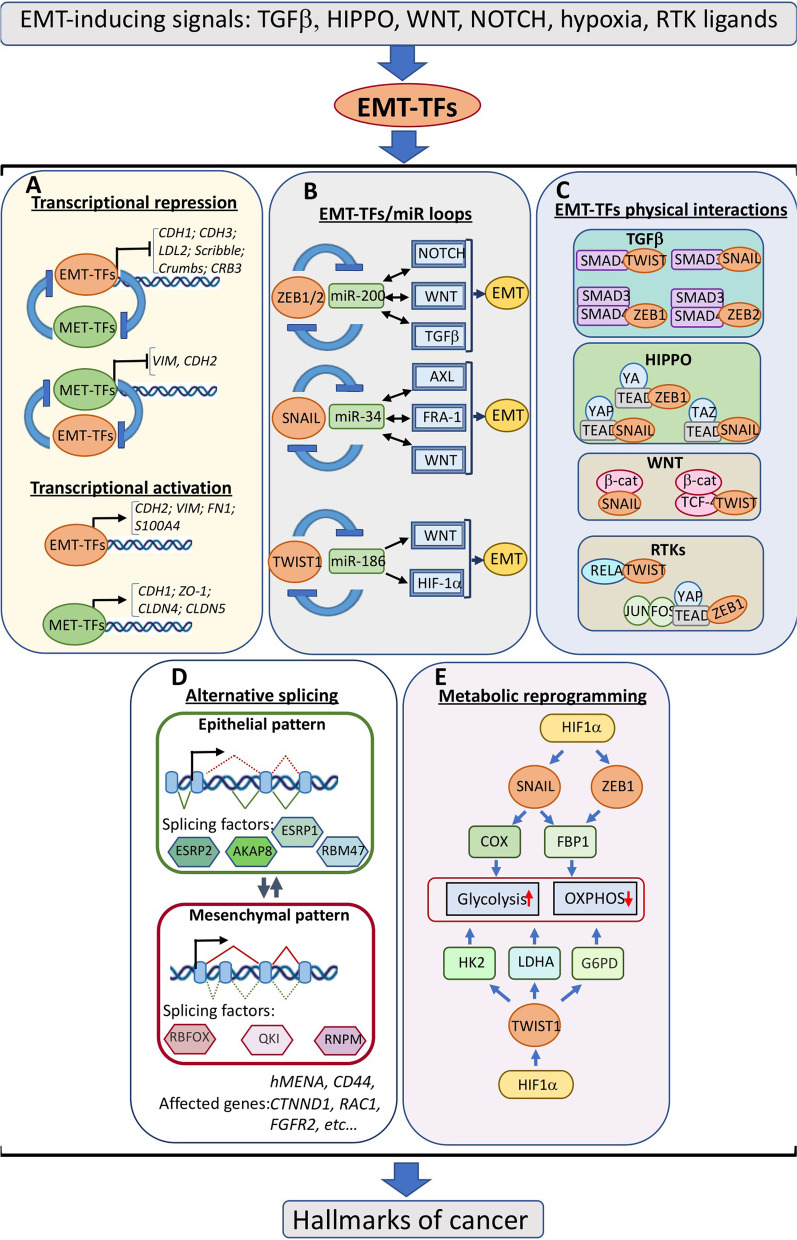
During EMT and MET, cells experience drastic changes in their physiology. In the course of EMT, the alterations involve the disappearance of intercellular adhesions, loss of epithelial polarity, dynamic restructuring of the cytoskeleton, degradation of basement membranes, etc. Several transcription factors collectively termed EMT-TFs govern the coordinated changes in cell physiology manifesting EMTs. Zn-finger transcription factors of SNAIL (SNAIL, SLUG) and ZEB (ZEB1, ZEB2) families and basic helix-loop-helix proteins TWIST1 and TWIST2 are best-studied EMT-TFs in the context of normal and pathological conditions [4–6]. Mechanistically, EMT-TFs repress transcription of a set of genes, whose function is critical for maintaining the epithelial state. Repression of the E-cadherin-encoding CDH1 gene results in the dissolution of AJ; reduction in the levels of the polarity complexes components, Crumbs and Scribble, CRB3 and LDL2 (Lethal Giant Larvae 2) leads to the loss of basolateral polarity [7, 8] (Fig. 1A). EMT-TFs repress transcription by recruiting various co-repressor complexes with HDAC and HMTase activities, Mi2/NuRD/G9A [9, 10], SIN3a/HDAC1/HDAC2 [11], coREST/CtBP1/HDAG2/G9A [12], SWI/SNF [13], and EZH2/HDAC1[2] [14] among others (reviewed in [15]). Concurrently, EMT-TFs directly or indirectly activate transcription of mesenchymal genes, including a critical component of mesenchymal intermediate filaments vimentin, CDH2 (codes for N-cadherin), matrix metalloproteinases specializing in the degradation of the basement membrane, and others. Direct activation requires binding co-activators with HAT activities, CBP, p300, and PCAF [15–18]. The binding of EMT-TFs to the effectors of some signal transduction pathways may result in their conversion from transcriptional repressors to activators (see Sect. 2). The EMT/MET balance is regulated by yet another group of transcription factors, which can be collectively referred to as MET-TFs. This relatively less explored set of factors includes diverse proteins belonging to various families, namely, zinc-finger-containing proteins Ovo-Like Transcriptional Repressor 2 OVOL2 [19] and Krüppel-like factor 4 (KLF4) [20], Grainyhead-like protein GRHL2 [21], and ETS family members E74-like ETS transcription factor 3 and 5 [22, 23] among others. MET-TFs promote epithelization in normal physiological conditions and cancer cells by direct transcriptional repression of mesenchymal markers and by establishing mutual bidirectional inhibitory circuits with EMT-TFs [24–27]. Moreover, some MET-TFs directly activate transcription of the genes encoding proteins specifying epithelial lineages, such as CDH1, ZO-1, CLDN4, and CLDN5 (encode Claudin-4 and -5) [28, 29] (Fig. 1A).
Fig. 1.
EMT pathways are embedded in signalling networks in cancer cells. Various signalling pathways exemplified on the top promote EMT by activating EMT-TFs. A Transcriptional regulation of epithelial-mesenchymal plasticity. In cells undergoing EMT, EMT-TFs repress transcription of epithelial genes encoding components of various epithelial structures, such as polarity complexes and adherens junctions, and activate the expression of mesenchymal genes. MET-inducing transcription factors (MET-TFs) repress transcription of mesenchymal markers, activate epithelial transcription programs, and act in double-negative feedback loops involving EMT-TFs. B EMT-inducing signals are modified by EMT-TFs/microRNA loops. EMT-TFs and microRNAs form interrelated double-negative feedback loops which affect expression levels of certain components of EMT-inducing signalling network. C EMT-TFs physically interact with components of signalling pathways forming complexes that in turn influence target genes. D EMT/MET mutually regulate alternative splicing. EMT-TFs regulate expression of epithelial (e.g., ESRP1/2) or mesenchymal (e.g., QKI) splicing factors, which in turn determine formation of epithelial- or mesenchymal-specific protein isoforms required for the accomplishment of EMT or MET programs. E Expression of metabolic genes of glycolysis and oxidative phosphorylation pathways are controlled by EMT-TFs
EMT and cellular signalling networks
EMT-TFs are induced in response to various signalling cues in embryonic or fibrotic tissues or tumour microenvironment, such as TGFβ, RTK ligands, HIPPO, WNT, NOTCH, or inflammatory cytokines [5, 30]. Importantly, EMT programs often modify EMT-inducing signal transduction pathways by activating positive or negative feedback regulatory loops. The underlying mechanisms vary in their nature. EMT-TFs form double negative self-enforcing loops with several classes of microRNA (Fig. 1B). For instance, ZEB proteins are repressors of microRNA clusters encoding miR-200 family members, which in turn are ZEB repressors [31, 32]. Similar connections exist between miR-34a and SNAIL family members and miR-186 and TWIST1. ZEB1/2-miR-200 module affects expression levels of critical components of TGFβ[33, 34], WNT [35–37], and NOTCH (up-regulation of MAML2/3; [38]) signalling pathways. When the balance in ZEB/miR-200 equilibrium is moved towards EMT-TFs, this may lead to the activation of TGFβ, WNT, or NOTCH pathways and thereby potentiate EMT via positive feedback loops. Likewise, SNAIL- or SLUG-induced EMT programs involve similar feedback regulation: repressing miR-34a subsequently leads to the increased expression of its EMT-potentiating targets, such as TGFβ1, LEF1, AXL, FRA-1, etc. [39–42]. TWIST1-targeted miR-186 is a repressor of the WNT pathway as well as HIF1α, among other targets known to contribute to the EMT programs in various cancer types [43, 44] (Fig. 1B). Long non-coding RNAs (lncRNAs) represent one more class of RNA molecules closely associated with regulating the EMT/MET balance. Various lncRNA species modulate EMT pathways via different mechanisms and at different levels. For example, lncRNA-ATB or lncRNA-OC1 may act as competing endogenous RNAs (ceRNAs) to promote EMT by sponging miR-200 family members or miR-34a respectively [45, 46]. Additionally, lncRNAs may serve as scaffolds for essential interacting components of EMT pathways or participate in the recruitment of chromatin-modifying complexes to the promoters of genes implicated in EMT or MET programs. The interactions of lncRNA with various signalling pathways implicated in epithelial plasticity are covered in recent comprehensive reviews [47, 48].
EMT-TFs also interfere with cellular signalling networks via direct physical interactions with the effectors of TGFβ, RTK, HIPPO, and WNT pathways (Fig. 1C). The resultant complexes recruit chromatin modifiers and ensure cooperative transcriptional control of target genes (reviewed in [49]. In particular, TGFβ-induced EMT programs involve physical and functional interactions between transcription factors SMAD2/3 and ZEB proteins or SNAIL [50, 51] or between TWIST1 and SMAD4 [52]. Binding of HIPPO effectors YAP and TAZ to SNAIL family members [53] or formation of multimeric complexes composed of sequence-specific transcription factors of TEAD, AP-1, NF κB families, and EMT-TFs [54–56] are essential for cooperation between EMT, HIPPO and RTK signalling. Binding the HIPPO effector YAP and AP-1 complexes results in the release of CtBP, and recruitment of HAT CBP and shift of ZEB1 from transcriptional repression to activation [54]. Complexes containing SNAIL and β-catenin were reported in colorectal cancer cells, and this interaction was essential for activating WNT-dependent genes [57]. TWIST1 was co-immunoprecipitated with TCF4/β-catenin complex in dermal papilla cells [58] (Fig. 1C).
The above-cited reports exemplify interactions between EMT and several signal transduction pathways, but they do not cover the impact of EMT on gene regulation in its full complexity. Indeed, EMT-microRNA circuits deeply interfere with fundamental cell signalling mechanisms at several levels. EMT pathways activate mesenchymal splicing programmes [59, 60] and are implicated in metabolic reprogramming [61, 62] (Fig. 1D, E). EMT is responsible for the rewiring of RTK signalling [63]; it alters the cellular epigenetic landscape [64]. Through the analysis of current literature and public databases, a recent study identified 932 genes in total that are associated with EMT/MET across various cancer types [65]. Therefore, it is not surprising that in addition to the classical role of EMT/MET programmes in determining phenotypic cell plasticity, they affect most, if not all, cancer features. These features classified as “hallmarks of cancer” by R Weinberg and D Hanahan include cell cycle regulation, replicative immortality, apoptotic resistance, immune evasion, evading growth suppressors, self-renewal ability, neovascularization and altered energetics (Fig. 1) [66].
The deregulated cell cycle and acquired cell stemness are among cancer characteristics closely associated with EMT/MET-controlled cell plasticity and will be discussed in the following sections.
Go-or-Grow: EMT and cell cycle control in normal tissues
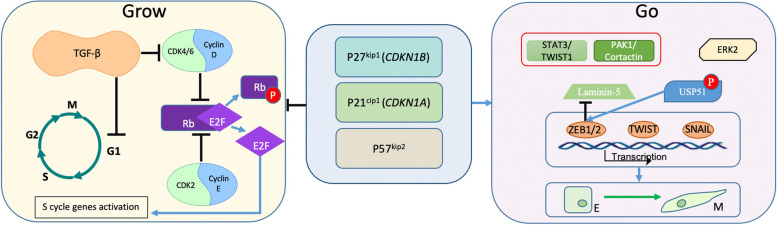
From a general perspective, molecular mechanisms controlling cell proliferation and migration must be connected. Indeed, migrating and mitotic cells share common signalling modules and structural elements (e.g., microfilaments and microtubules). The critical pathway which integrates growth-promoting and growth-suppressing signals into a decision for proliferation or cell cycle exit is Rb-E2F. Cyclin D-CDK4/6 and cyclin E-CDK2 complexes consecutively phosphorylate RB to drive G1 progression and entry into the S phase. Cyclin-dependent kinase inhibitors (CKIs), p27KIP1, p21CIP1, and p57KIP2, are key regulators of CDK activity [67] (Fig. 2). In addition to their canonical function in cell cycle regulation, CKIs are also implicated in cytoskeletal dynamics, cell migration, and invasion [68]. For instance, the central inhibitor of G1-S transition, p27KIP1, facilitates cell motility and invasion by increasing the turnover of invadopodia through PAK1/cortactin signalling [69, 70], affecting actomyosin dynamics [71], and by inducing EMT via STAT3-TWIST1 pathway [72] (Fig. 2). As cell migration and proliferation share common elements of molecular pathways, there might be competition in determining which process prevails in a given cell. Indeed, the mathematical modeling predicts that the increase in cell proliferation occurs at the expense of cell migration and vice versa [73]. This popular concept of the dichotomy between proliferation and migration is discussed in the literature as the “Go-or-Grow” or “Divide or conquer” concept.
Fig. 2.
An overview of “Go-or-Grow” concept. Main orchestrators of CDK activity namely p27KIP1, p21CIP1, and p57KIP2 inhibit proliferation (”Grow”) and activate migration (”Go”) via direct/indirect interactions with the components of cell cycle machinery and EMT-TFs network (see text for details)
The Go-or-Grow hypothesis was studied in normal physiological conditions. C. elegans anchor cells (AC) represent a model allowing to study molecular mechanisms of invasion in embryonic development. AC, specialized uterine cells, play a role in the development of the nematode reproductive system; they breach the basement membrane separating the uterine and vulval tissues to form a uterine-vulval connection. AC invasion depends on the fos-1a gene, whose loss prevents the disintegration of the basement membrane [74]. Of note, mammalian orthologues of fos-1a, AP-1 family members cooperate with EMT-TFs at different levels and are potent EMT inducers in various cancers [75–77]. Mechanistically, a nematode p21CIP1 homolog was required for the G1/S cell cycle arrest and, at the same time, essential for AC invasion [78].
An association between embryonic EMT programmes and cell cycle control was studied in the course of the formation of neural crest cells (NCC). This population of multipotent cells is generated via EMT during the closure of the neural tube in the embryonic development of vertebrates. The population of NCC gives rise to various cell lineages, including peripheral neurons, Schwann cells, melanocytes, and craniofacial structures, among others. Delamination of NCC from neural folds and their migration to new embryonal territories is regulated by distinct EMT-TFs in distinct species of vertebrates [79, 80]. It has been reported that different NCC utilize different mechanisms of EMT upon delamination, and their migration may or may not be associated with the exit from the cell cycle. While cranial NCC exhibit various distributions over the cell cycle phases, delaminating cells at the sites of neural tube closure within the trunk are accumulated in the G0/G1 phases (reviewed in [80, 81].
A mechanistic link between reversible cell cycle arrest (cell quiescence) and EMT was established in vitro in studies analyzing gene expression signatures of serum-deprived quiescent primary fibroblasts. In addition to a number of genes associated with the cell cycle regulation, serum deprivation activated several genes implicated in EMT, namely TGFb1, IL6, IL11, N-cadherin encoding gene CDH2, EMT-TF-encoding gene ZEB2, and others [82].
Go-or-Grow: EMT and cell cycle control in cancer
The applicability of the Go-or-Grow concept to cancer is of particular interest. Indeed, on the one hand, a central feature of cancer cells is their ability to overcome growth-inhibiting stimuli and sustain uncontrolled proliferation [66]. On the other hand, most conventional or targeted anti-cancer agents are effective against cells passing through the S and G2/M phases of the cell cycle, and cancer cells residing in G1/G0 are therapy-resistant. Therefore, the proliferative plasticity or the ability of cancer cells to transit between proliferative and quiescent states poses an obstacle to therapy, and understanding underpinning mechanisms is critically important [83, 84].
EMT substantially impacts drug resistance largely because it is commonly associated with slow proliferation or cell cycle arrest. Indeed, one of the most potent EMT inducers, TGFβ, is a classical tumour suppressor and canonical inhibitor of the cell cycle in the G1 phase. TGFβ-mediated cell cycle arrest involves SMAD-mediated activation of CDKN2B and CDKN1A genes encoding CDK inhibitors p15INK1 and p21CIP1 [85]. In squamous cell carcinoma, TGF-β/SMAD signalling induced quiescence via activation of p21CIP1 in the proportion of cells. In contrast to the bulk of the tumour, these cells exhibited resistance to DNA damage and increased tumorigenic potential [84]. This study did not address the implication of EMT-TFs, which act in concert with TGFβ/SMAD. However, in earlier reports, the ability of EMT-TFs to attenuate G1/S cell cycle progression has been shown in vitro. In particular, SNAIL- and ZEB2-induced EMT in cultured carcinoma cells went along with the delays in G1- to S-phase transition caused by the transcriptional repression of CCND2 and CCND1, respectively [86, 87]. In addition, CDK inhibitors are transcriptional targets of EMT-TFs; SNAIL activated p21CIP1-encoding gene CDKN1A [86]; and SLUG induced both CDKN1A and CDKN1B (codes for p27KIP1) in cervical carcinoma cells [88]. Concomitant with the attenuated cell cycle progression, EMT-TFs stimulate individual cell motility and invasion. To our knowledge, the impact of EMT-TFs-induced CDK inhibitors on the motility of cells during EMT has not been addressed. Activation of the G1/S checkpoint represents a common EMT feature; ERK2 induces EMT, which is associated with enhanced expression of CDKN1A and CDKN1B [89]. Emerging evidence indicates a negative feedback mechanism links EMT-TF ZEB1 with the G1/S checkpoint regulation. CDK4/6 kinases phosphorylate and stimulate deubiquitinase USP51, which stabilises ZEB1 [90] (Fig. 2). This regulatory circuit may fine-tune the balance between “Go” and “Grow” types of cell behaviour, and generate proliferating cells which an intermediate epithelial-mesenchymal phenotype (see following sections).
Experimental data supporting the “Go-or-Grow” hypothesis in cancer cells in vitro align with the analyses of patients’ samples. In several cancer types, high cell proliferation indices are typical for the central areas of solid tumours, whereas cells at the tumour periphery are often non-proliferative and highly invasive. According to some reports, enhanced expression of D- and E-type cyclins in breast cancer correlated with a less malignant phenotype and a more favourable prognosis [91, 92]. EMT at the invasive front of colorectal cancer is associated with the enhanced expression of the CDKN2A gene product p16INK4A, which correlates with low survival rates [93]. EMT programs at the tumour-stroma boundary are induced by ZEB1 and lead to the transient loss of the basement membranes due to proteolytic cleavage of their components and down-regulated synthesis of laminin-5, a basement membrane component secreted by tumour cells [94]. These data can be interpreted as proof of the dichotomy between the growth of primary tumours versus invasion, with the latter being responsible for poor prognosis. The “Go-or-Grow” phenotypic switch in cancer was modeled in vivo by knocking out the p21CIP1-coding gene CDKN1A in the polyoma virus middle T mammary tumour model [95]. Depletion of this potent CDK inhibitor stimulated tumour growth and resulted in the suppressed cell invasion in vitro and reduced metastatic spread. These results reflect the function of the nematode p21CIP1 homologous protein in the invasion of AC cells in C. elegans. They suggest that metastatic cancer cells can hijack and utilize the evolutionarily conserved mechanisms regulating the “Go-or-Grow” switches. On the other hand, there is no correlation between cell motility and proliferation in established cancer cell lines in vitro [96]. Therefore, mechanisms coupling cell cycle regulation and cell motility can be circumvented in cancer. Alternatively, the mechanisms maintaining “Go-or-Grow” switches do exist; but in a single cell, the “Go” and “Grow” functions are separated in time. On the level of cell populations, rapidly dividing cells can be highly invasive. These cell populations may exist in brain tumours, malignant mesothelioma, or cutaneous melanoma. Although the death of patients with these cancer types is caused mainly by metastatic disease, the unfavorable prognosis is closely associated with high mitotic activity [96]. This contrasts with the above-cited reports on the presence of slowly proliferating cells on the invasive front predicting poor survival of ER + breast or colorectal cancer patients [91–93]. If proliferative capacity and invasive potential within a given cell are not separated in time, this may have important implications for tumour biology. Cells that underwent TGFβ-induced EMT but were resistant to TGFβ-imposed growth inhibition displayed genetic instability and defects in cytokinesis. These abnormalities were associated with SNAIL-dependent suppression of Lamin B1 and other nuclear envelope proteins implicated in mitotic control. Thus, breaching the Go-or-Grow principle in cancer cells may trigger heritable genetic defects, subsequently contributing to tumor evolution [97].
Quiescence, adult stem cells, and EMT
Quiescence is a characteristic of subpopulations of adult stem cells (ASC), a specialised type of tissue-resident cells responsible for tissue homeostasis and repair. In the epidermis, intestinal epithelium, and regenerating tissues, quiescent ASC co-exist with the stem cells repeatedly undergoing asymmetric divisions. The latter subpopulation maintains tissue integrity by differentiating into parenchymal cells and producing new stem cells (self-renewal) [98, 99]. Unlike irreversibly arrested senescent and terminally differentiated cells, quiescent ASC can be reversibly activated by environmental triggers [100, 101].
Stem cells are located in specialised niches formed by stem cell progeny, and supportive stromal cells. The niche determines a balance between quiescent and proliferating stem cells. Disruption of this equilibrium in a healthy tissue may result in deregulated cell proliferation, compromised tissue repair, impaired homeostasis, and regeneration processes [100]. For example, failure to maintain quiescence in hematopoietic stem cells (HSC) leads to the extended expansion of the stem cell population and its exhaustion leading to the depletion of adaptive immunity and myeloproliferative disorders [102]. The importance of quiescence was also demonstrated in the studies on NCC, the decrease in the hippocampal neural stem cell population resulted in cognitive defects due to decreased amounts of new neurons [103].
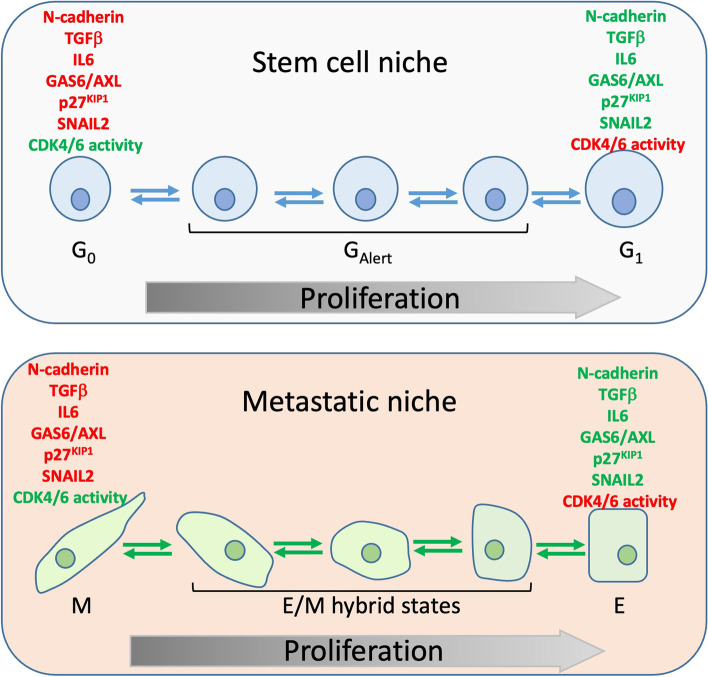
To maintain quiescence/proliferation balance, stem cells communicate with each other and other components of a niche via cell–cell, cell-soluble factors, and cell-extracellular matrix (ECM) interactions [100, 101]. In healthy tissues, lack of niche-derived soluble factors is a determinant of quiescence, whereas secretion and release of growth factors from ECM enforce stem cells to enter the cell cycle. For instance, the presence of the hepatocyte growth factor (HGF) in injured muscles stimulates muscle cell proliferation via the c-MET/mTOR pathway and tissue repair [104]. Intercellular interactions are yet another factor supporting the quiescent state of the ASC. Quiescent ASC present in neural, muscle, hematopoietic, and endometrial stem cell niches are associated with the surrounding cells via AJ mediated by N-cadherin [105–108] and N-cadherin depletion leads to the dissolution of AJ, the release of stem cells from contact inhibition and their activation. In addition, niches maintain stem cell quiescence by producing growth-inhibitory factors, at least two of which, TGFβ1 and IL-6 family, are classical inducers of EMT programs [109, 110]. Therefore, at least two considerations, a role for the mesenchymal marker N-cadherin and the presence of the EMT triggers in the stem cell niches, indicate that in normal physiological conditions, EMT and stemness are associated (see Fig. 3).
Fig. 3.
A parallel between GAlert and E/M hybrid states of cells. The same factors such as N-cadherin, TGFβ, IL6, GAS6/AXL, p27KIP1 and SNAIL2 are implicated in both stem cell quiescence and EMT/MET equilibrium
The interconnection between EMT and cell stemness is apparent in embryonic tissues: NCCs, generated via EMT, represent a stem cell population that gives rise to various tissues in a developing embryo. In adult tissues, the impact of EMT programmes on stem cell biology has been studied in several types of epithelia. In the stratified epithelium of a mammary gland, stem cells are present within the outer basal layer. These stem cells express low levels of asymmetrically distributed E-cadherin that mediates their physical association with luminal cells. At the same time, adult mammary stem cells express some levels of mesenchymal markers N-cadherin and vimentin indicating that they reside in an intermediate phenotypic state in which epithelial and mesenchymal features are combined [111, 112]. This state is governed by the EMT-TF SLUG in conjunction with its interacting partner chromatin modifier Lysine-specific histone demethylase 1 (LSD1) [113]. Indeed, SLUG ablation in KO mice significantly delays mammary gland morphogenesis reflecting impaired lineage commitment, and the combined ectopic expression of SLUG and SOX9 results in the reprogramming of luminal cells into mammary stem cells with the renewal potential [114]. The role of a particular EMT-TF, SLUG, in stemness is not restricted to the mammary gland. In the stratified epithelium of other tissues, such as the prostate gland, upper airways of the lungs, and hair follicles, SLUG was shown to inhibit differentiation and support oligopotency [112, 115–117]. In addition to SLUG, other EMT-TFs, such as ZEB1, are expressed and sustain stemness in the subpopulations of basal cells in the prostate and mammary glands [118, 119].
As EMT-TFs are implicated in the biology of ASC, the Go-or-Grow principle might be applicable to these cell types. A recent study has shown that ZEB1 is required for branching morphogenesis and maintains quiescence in the subset of basal mammary stem cells via the upregulation of WNT pathway inhibitor Axin2 [120]. Likewise, experimental data support the existence of a Go-or-Grow dichotomy in HSC. The SDF-1 (stromal-derived factor-1) plays a crucial role in mobilisation and homing of hematopoietic stem cells via the interaction with cognate receptor CXCR4 [121]. Conditional ablation of either the receptor or the ligand stimulates HSC to enter the cell cycle [122], suggesting that this pathway is responsible for cell quiescence and motility. Several small Rho GTPases regulate cytoskeletal dynamics and cell motility downstream of SDF-1/CXCR4 signalling, while CDC42 is responsible for the retention of HSC in G1/G0 [123, 124]. Interestingly, CXCR4 is the effector of ZEB2 in some hematopoietic cells [125], and genetic ablation of ZEB2 in hematopoietic lineage resulted in an increase in HSC and progenitors in the bone marrow and other specific features resembling myeloproliferative diseases in humans [126].
As opposed to the broad implication of SLUG in cell stemness in stratified epithelia, neither this protein nor other EMT-TFs are associated with stem cells in most single-layered epithelial tissues, such as pancreatic ducts or intestinal epithelium [112]. This is, however, in stark contrast with the cancer stem cells (CSC), cells that harbour stem features and are present in cancers derived from the same types of simple epithelia.
EMT and cancer stem cells
CSC represent a distinct pool of tumour cells, which are highly tumourigenic but retain some features of normal epithelial stem cells. Like their normal counterparts, CSC are characterised by asymmetric division, self-renewal, and the ability to generate more differentiated progeny composing tumour mass. Because of their high tumourigenic potential, and resistance to various therapies, it is accepted that CSC represent a source for distant metastases and post-therapy cancer relapse. A causative link between EMT and cancer stemness was proposed theoretically and then demonstrated experimentally in a series of seminal works [127–131]. Expression of EMT-TFs SNAIL or TWIST1 in immortalised mammary epithelial cells generated cells with the CD44highCD24− phenotype. These cells were capable of forming mammospheres, which contained both basal and luminal precursors. Similar mammosphere-forming CD44high and CD24-negative cells with EMT gene expression signatures were isolated from normal and cancerous breast tissues [130].
The use of single-cell RNA sequencing technology for the analysis of the metastatic process in a PDX breast cancer mouse model has shown that EMT-derived stem-like cells appear at the early stages of metastatic dissemination [132]. These metastasis-inducing cells with reduced levels of E-cadherin and CD24 expressed EMT-TFs SLUG and TWIST1 and dormancy-associated gene signature including high CDKN1B (p27KIP1). Metastatic cells in PDX models with a high metastatic burden demonstrated higher expression of luminal differentiation genes, c-MYC, and proliferative signatures [132]. These data are in line with the hierarchical model of cancer metastasis. The model suggests that dormant CSC disseminate at the initial stages of the metastatic process, while extensive proliferation and differentiation occur at the advanced stages. The hierarchical model also applies to colorectal cancer, where Lgr5 (Leucine-rich repeat-containing G protein-coupled receptor 5) receptor is a marker of stem cells in the normal intestinal epithelium [133]. Lgr5+ cells isolated from normal tissues, primary tumours, or metastases do not show any EMT features but are characterised by stem cell gene signatures. After depletion of Lgr5+ cells from primary Apcmin/+;KrasG12D-induced tumours, Lgr5− cells displayed activated c-Myc pathway and overrepresentation of genes implicated in cell-cycle progression [134]. The hierarchical model is supported by the analyses of the pools of CTC (circulating tumour cells) isolated from blood samples of breast, colon, or lung cancer patients. These cells are derived from epithelial tumours, possess tumour-initiating potential, and express mesenchymal markers [135–137].
As cited above, compelling evidence collected in different cancer models indicates that epithelial traits are required for cancer cells to re-establish tumour growth in target organs and accomplish the metastatic process [132, 134]. Indeed, in the polyomavirus middle T antigen breast cancer mouse model, single metastatic tumor cells arriving at target organs were predominantly mesenchymal, but all micrometastases comprising three or more cells were E-cadherin-positive [138]. Irreversible loss of epithelial differentiation by enforced SNAIL expression led to the inhibition of tumorigenic and metastatic potentials in prostate and bladder cancer cells [139]. The role of E-cadherin was studied in mouse models of invasive ductal mammary adenocarcinoma. Although down-regulation of the CDH1 gene coding for E-cadherin enhanced the invasive capacity of tumour cells, their metastatic potential was diminished as the result of activated TGFβ signalling, decreased cell proliferation, and reduced stress resistance [140]. These and many other reports demonstrate the importance of the reversibility of EMT programmes (EMT/MET plasticity) in metastatic processes [4, 112, 141]. While the epithelial state mainly contributes to the increasing mass of primary and secondary tumours (“Grow” function), the mesenchymal state facilitates invasion and colonisation (“Go” function). Indeed, given the tiny diameter of microcapillary utilized by metastasizing cells, loss of epithelial adhesion would make intravasation much more efficient. However, research combining in vivo imaging and lineage tracking has shown that migration of epithelial cell clusters is often a common feature. In these clusters, cells retain intercellular junctions and form cohesive migrating groups (so-called collective migration) [142]. Regardless of their epithelial appearance, intercellular adhesion in these groups is weakened because of a partial EMT [143] (see next section). Some reports have shown that MET programs are essential not only for forming macrometastases but also capable of generating highly tumourigenic epithelial-like CSC with metastasis-seeding properties [144–146]. Moreover, in some cases, such as the syngeneic mouse model of breast cancer, epithelial CTCs (defined by high levels of Epcam, E-Cadherin, and Grhl2 expression) have the strongest metastatic ability as compared to the pool of mesenchymal CTCs (low EpCAM, E-cadherin expression, high levels of Vimentin, and EMT-TFs expression) [147].
Intermediate EMT stages and reconciliation of “Go” and “Grow” features
A concept reconciling findings that “Go” and “Grow” capabilities are combined in a single cell proposes that EMT and MET do not represent binary switches. This model suggests that both programs represent processes empowering cancer cells by the spectra of both epithelial and mesenchymal traits leading to the generation of so-called hybrid, quasi-mesenchymal, or E/M phenotypes. In vitro studies have shown that several distinct intermediate states can be delineated, which differ with regard to the expression of differentiation markers [148–150]. These distinct E/M states are characterised by particular chromatin landscapes and are epigenetically stabilized to a certain degree. However, cells in the early and late hybrid states were the most plastic and retained the capability to equally produce epithelial and mesenchymal subpopulations [151–153].
The in vivo existence of E/M cells was demonstrated by immunohistochemistry or by the analyses of gene expression signatures. Significantly, enrichment for hybrid EMT RNA signature has been associated with poor survival and resistance to therapy in several tumor types (reviewed in: [151]), including breast cancer [145]. Whereas quiescent CD44high/CD24− cells with EMT signatures were detected primarily at the invasive fronts of primary mammary tumours, proliferative epithelial cells expressed aldehyde dehydrogenase (ALDH+) and were located within central tumour areas. A pool of the cells with intermediate E/M features, CD44high/CD24−/ALDH+ cells, was also detected in tumour tissue and corresponded to less than 0.1% of cells within a tumour [145]. In later studies, the isolation of E/M cells from mammary carcinoma cell cultures was carried out using a combination of CD104 and CD44 antigens. These cells stably resided in the hybrid state, displayed CSC features, and expressed high levels of SNAIL, SLUG, and TWIST1. The canonical WNT signalling pathway drove this highly aggressive phenotype. Ectopic expression of ZEB1 in E/M cells induced a complete EMT, inhibited tumourigenicity, and triggered a switch from canonical to non-canonical WNT signalling [154]. These results show that although individual EMT-TFs are capable of inducing complete EMT in vitro, their functions in tumourigenesis are different in vivo. Indeed, ZEB1, but not TWIST1 or SNAIL, is a driver of tumour progression in the mouse model of pancreatic ductal adenocarcinoma KrasG12D;P53R172H/+ [155, 156]. A different series of cell surface markers (EpCAM, VCAM, ITGAV, and ITGB3) along with single-cell RNA sequencing (sc-RNAseq) was applied to identify E/M states in squamous cell carcinoma of the skin [152].
Application of unbiased single-cell RNA sequencing approach led to the identification of intermediate states in different cancer types, including glioblastoma, metastatic breast cancer, pancreatic ductal adenocarcinoma, squamous cell carcinoma of the skin, head and neck, and cutaneous melanoma [151, 157–160]. In particular, an intermediate state in malignant melanoma exhibited an NCC transcriptional program, which overlapped with the expression programs specifying proliferative, invasive and CSC phenotypes. These cells driven by the nuclear receptor RXRG were present in the pool of persister cells tolerant towards BRAFV600E and MEK inhibitors [158].
E/M cells were present in CTC pools isolated from the blood samples of patients with various cancer types, and E/M CTC, rather than fully differentiated epithelial or mesenchymal cells, are associated with short metastasis-free and overall survival [151]. Epithelial cells expressing mesenchymal markers are often detected in clusters of CTC. These clusters form microemboli via association with non-malignant cells, including the platelets, which maintain mesenchymal features of CTC by releasing TGFβ [161]. Circulating microemboli have up to 50 times more metastatic potential than single CTCs [162]. These clusters are oligoclonal in their origin and do not contain intravascular CTC aggregates [163]. In breast and pancreatic cancer modes, the CTC clusters are composed of highly motile E/M and proliferative epithelial cells. They are capable of Plakoglobin- and E-cadherin-dependent collective migration and enter the circulation as groups while retaining proliferative potential [143, 164].
Thus, analyses of animal models and patient samples show that combining “Go” and “Grow” capabilities in E/M hybrid states facilitates tumorigenesis. Stimulating one capability at the expense of the other locks cells in E or M states (for instance, via manipulation with the expression levels of ZEB1) [154, 155] and reduces tumorigenicity and metastasis.
E/M plasticity, exit from quiescence and metastasis
While most CTCs do not survive in circulation, a proportion extravasate into target organs, form pools of disseminated tumour cells (DTC), and survive therapy in a reversible dormant state. The presence of dormant cancer cells detectable in bone marrow reflects a condition termed the minimal residual disease that is a sensitive indicator of poor prognosis and a major clinical challenge. Understanding the mechanisms of the exit from dormancy is of tremendous importance also because the colonisation of secondary organs is the bottleneck in the metastatic process [165]. Three likely complementary mechanisms are implicated in maintaining single-cell or micrometastatic dormancy: angiogenic dormancy, immune-mediated dormancy, and cellular dormancy [166, 167]. Whereas the two first scenarios involve a balance between cell proliferation and death imposed by lack of nutrients or immune attacks, the third scenario suggests that DTC may enter deep quiescence. Cell cycle exit is associated with resistance to anti-proliferative drugs; quiescent DTCs evade immunosurveillance [168] and are insensitive to immunotherapies. Therefore this third type of dormancy can be particularly clinically relevant and will be discussed below.
By combining a sophisticated lineage-tracing approach with the scRNA-seq, Simeonov and colleagues investigated mechanisms of metastasis in PDAC and identified four E/M hybrid stages (H1-H4) in addition to the fully differentiated epithelial and mesenchymal cell pools [160]. In this KrasG12D;P53R172H/+ -driven cancer model, proliferative gene expression signatures were observed in cells with the E and H3 differentiation states. Based on the expression of cell surface markers, EpCAM-negative squamous carcinoma cells were separated into six discrete subpopulations of the E/M cells presenting different degrees of mesenchymal differentiation [151, 152]. In agreement with the Go-or-Grow conception, the proliferative capability gradually decreased while the invasive potential progressively increased along the epithelial-mesenchymal spectrum. In the experimental metastasis assay, early E/M cells (i.e., E/M cells positioned closer to the epithelial end of the spectrum) exhibited the highest metastatic potential that significantly exceeded the metastatic propensity of fully differentiated E or M cells [151]. Likewise, in the Cre+/-PtenL/L;KrasG12D/+ mouse model of prostate cancer, E/M hybrid cells exhibited increased proliferative and decreased invasive potential compared to highly invasive mesenchymal cells [169].
Cells corresponding to the mesenchymal end of the E/M spectrum are highly invasive and, due to their non-proliferative nature, apoptosis-resistant. Therefore, it has been accepted that these cells are the source of dormant DTCs, and supposedly they represent metastatic seeds. The ability to differentiate along the mesenchymal-epithelial axis leading to the exit from dormancy is the requirement for successful colonisation. This ability depends on the mutational landscape of tumour cells and is driven by microenvironmental cues.
DTCs reside in metastatic niches, specific locations in target organs, which provide anchorage and survival support [170]. There is an apparent analogy between metastatic niches occupied by DTCs possessing the CSC features and ASC niches, which are present in most normal tissues. The pre-existing ASC niches can attract metastatic tumour cells. For example, HSC niches in the bone marrow are targeted and occupied by DTCs derived from metastatic prostate cancer [171, 172]. Apparently, the dormant state of HSC and DTCs is controlled via the same pathways initiated by niche-produced ligands belonging to the TGFβ family [172]. Additionally, growth arrest specific-6 (GAS6), a ligand secreted by osteoblasts in the bone marrow environment, contributes to the dormancy of prostate cancer-derived DTCs and inhibits the proliferation of hematopoietic progenitors [172, 173]. GAS6 and the cognate receptor AXL, a TAM (TYRO3, AXL, MER) RTK family member, play a major role in EMT, therapy resistance, and metastasis in different tumour types [174]. Shifting the balance in HSC niches towards quiescence and more mesenchymal end of the E/M spectrum aligns with multiple reports describing the role of GAS6/AXL signalling in EMT and drug resistance in cancer [174, 175]. Interestingly, the analysis of neural stem cells showed that AXL and MER were part of the 1000 gene signature that is downregulated in cells, which were released from quiescence and entered the cell cycle [176]. These observations indicate that TAM receptors maintain quiescence in different lineages, which is in line with the widespread role of these molecules in drug resistance in cancer. In breast and ovarian cancer, AXL-induced EMT programmes are mediated by SNAIL family members [63, 177]. Remarkably, SLUG was shown to maintain quiescence also in HSC in bone marrow niches [178]. However, it remains unclear if, by analogy with EMT-inducing malignant signalling, SLUG is an effector of the GAS6/AXL pathway in normal stem cells as well.
The newly discovered facets of ASC reveal new aspects of their similarity with DTCs. Quiescence of ASC does not represent a deep static condition of dormancy but is highly dynamic. Quiescent ASC may transit between different states, characterised by various depths of dormancy [100]. For example, in addition to the canonical deep quiescence (G0), muscle ASC can acquire another form of quiescence, GAlert. Cells in the GAlert phase require much less time than canonical G0 cells to enter the cell cycle in injured muscle tissue. G0/GAlert transition is regulated by the mTORC1 pathway during the repair of injured tissue [179, 180]. Likewise, the phenomenon of fluctuating depths of quiescence was reported in hematopoietic ASC [181]. In contrast to G0/GAlert transitions, the balance between different phases of quiescence in hematopoietic ASC was independent of mTORC1 and regulated by the levels of CDK6. Apparently, Rb/E2F pathway regulates transitions within G0, as well as the transition from the quiescence to G1 [100, 182]. As the G0 phase is characterised by much higher levels of p27KIP1 than G1 [183], this CDK inhibitor is likely implicated in G0/G1 transition by regulating CDK4/6 activity. However, the detailed mechanism of this transition is not studied, definite G0 markers are unknown, and this area warrants further investigations.
The plastic nature of quiescent ASC is reminiscent of tumour cells which have the potential to adopt various differentiation states along the E/M spectrum. This plastic feature of both cell types is predetermined by characteristic epigenetic patterns. Trimethylated lysines 4 and 27 of histone 3 (H3K4me3 and H3K27me3) mark promoters of actively transcribed and silenced genes, respectively. In some populations of ASC identified in muscle and neuronal tissues, both repressive and activating marks are present in the same chromatin domains (so-called bivalent domains) [184, 185]. This organisation of the chromatin allows rapid activation or silencing of genes by removal of H3K27me3 or H3K4me3 marks, respectively, in response to the proliferation or differentiation-inducing stimuli. Remarkably, the same pattern of chromatin modifications was associated with E/M plasticity [186, 187]. The similarity in the epigenetic landscapes between ASC and disseminated tumour cells is an indication that common signalling mechanisms govern the exit from quiescence in stem cell and metastatic niches. This assumption can be tested by analyzing similarities in the chromatin accessibility landscapes in ASC and cancer cells derived from the same tissue and undergoing G0-GAlert-G1 and E-E/M-M transitions, respectively.
Concluding remarks and perspectives
Uncontrolled proliferation, enhanced invasiveness, and drug resistance of dormant cancer cells are critically important from a clinical perspective. In this review, we discussed the migration—proliferation dichotomy (“Go-or-Grow” conception) in light of EMT/MET plasticity. EMT and MET programs have been implicated in balancing invasive and proliferating states and also in the acquisition of stem cell traits in both homeostasis and cancer. In recent years, the identification of cells combining epithelial and mesenchymal features led to the re-evaluation of a concept considering EMT and MET as binary switches between fully differentiated states. Cells adopting intermediate states express both epithelial and mesenchymal markers. They were isolated from stem cell compartments of adult epithelial tissues, primary tumours, and populations of CTCs. Thus, EMT/MET plasticity is a characteristic feature of both DTC and ASC.
ASC often reside in the dynamic GAlert phase, which is important for accelerated regeneration of damaged tissues. It remains not studied whether DTC undergo a similar type of G0-GAlert-G1 transitions in the process of colonisation and during cancer relapse after therapy failure. Given the established role of EMT triggers in regulating ASC proliferation, it is reasonable to speculate that the molecular pathways controlling the transition across the E-M axis are also implicated in the dynamic shifts within the G0 phase. Then the interpretation of the signals in the niches will involve coordinated changes in signalling networks controlling cell motility and cell cycle entry leading to the “Go-or-Grow” decision.
Cell plasticity is responsible for cellular adaptations to microenvironmental cues during the metastatic process and for generating drug-tolerant persister cells. The presence of bivalent domains in the chromatin of cancer cells is critical for determining their multipotency. Therefore, targeting chromatin bivalency can limit the cellular ability to transit across the E/M axis and to adopt characteristics of GAlert states. There is a great hope that further research in cancer epigenetics and the development of tools for targeting bivalent chromatin will freeze cancer cells in the drug-susceptible state and prevent metastasis.
Acknowledgements
We apologize to the colleagues whose relevant studies were not cited in this paper.
Abbreviations
- EMT
Epithelial mesenchymal transition
- MET
Mesenchymal epithelial transition
- AJ
Adherens junctions
- EMT-TFs
Epithelial mesenchymal transition transcription factors
- MET-TFs
Mesenchymal epithelial transition transcription factors
- OVOL2
Ovo-Like Transcriptional Repressor 2
- GRHL2
Grainyhead-like protein
- LDL2
Lethal Giant Larvae 2
- KLF4
Krüppel-like factor 4
- CLDN4
Claudin-4
- CLDN5
Claudin-5
- lncRNAs
Long non-coding RNAs
- ceRNAs
Competing endogenous RNAs
- CKIs
Cyclin-dependent kinase inhibitors
- AC
Anchor cells
- NCC
Neural crest cells
- ASC
Adult stem cells
- HSC
Hematopoietic stem cells
- ECM
Cell-extracellular matrix
- HGF
Hepatocyte growth factor
- LSD1
Lysine-specific histone demethylase 1
- SDF-1
Stromal-derived factor-1
- CSC
Cancer stem cells
- Lgr5
Leucine-rich repeat-containing G protein-coupled receptor 5
- CTC
Circulating tumour cells
- ALDH +
Aldehyde dehydrogenase
- sc-RNAseq
Single-cell RNA sequencing
- DTC
Disseminated tumour cells
- GAS6
Growth arrest specific-6
Authors’ contributions
Writing the manuscript, AA, NA, DS, ET; conceptualization and supervision, ET; Figures, AA and ET. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Nazarbayev University Faculty Development Competitive Research Grants (240919FD3909 and 080420FD1908 to ET) and by the Ministry of Health of the Republic of Kazakhstan under the program-targeted funding of the Ageing and Healthy Lifespan research program (IRN: 51760/ПЦФ-MЗ PК-19 to AA and ET).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors approved on the contents of the manuscript to publish in Molecular Cancer.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 2.Horejs C-M. Basement membrane fragments in the context of the epithelial-to-mesenchymal transition. Eur J Cell Biol. 2016;95(11):427–440. doi: 10.1016/j.ejcb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Savagner P. Epithelial–mesenchymal transitions: from cell plasticity to concept elasticity. Curr Top Dev Biol. 2015;112:273–300. doi: 10.1016/bs.ctdb.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342(6159):1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 5.Goossens S, Vandamme N, Van Vlierberghe P, Berx G. EMT transcription factors in cancer development re-evaluated: Beyond EMT and MET. Biochim Biophys Acta Rev Cancer. 2017;1868(2):584–91. [DOI] [PubMed]
- 6.Stemmler MP, Eccles RL, Brabletz S, Brabletz T. Non-redundant functions of EMT transcription factors. Nat Cell Biol. 2019;21(1):102–112. doi: 10.1038/s41556-018-0196-y. [DOI] [PubMed] [Google Scholar]
- 7.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27(55):6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 8.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu J, Qin L, He T, Qin J, Hong J, Wong J, et al. The TWIST/Mi2/NuRD protein complex and its essential role in cancer metastasis. Cell Res. 2011;21(2):275–289. doi: 10.1038/cr.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manshouri R, Coyaud E, Kundu ST, Peng DH, Stratton SA, Alton K, et al. ZEB1/NuRD complex suppresses TBC1D2b to stimulate E-cadherin internalization and promote metastasis in lung cancer. Nat Commun. 2019;10(1):1–15. doi: 10.1038/s41467-019-12832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24(1):306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Sawada J-I, Sui G, Affar EB, Whetstine JR, Lan F, et al. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature. 2003;422(6933):735–8. doi: 10.1038/nature01550. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Tillo E, Lazaro A, Torrent R, Cuatrecasas M, Vaquero E, Castells A, et al. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;29(24):3490–3500. doi: 10.1038/onc.2010.102. [DOI] [PubMed] [Google Scholar]
- 14.Tong Z, Cai M, Wang X, Kong L, Mai S, Liu Y, et al. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene. 2012;31(5):583–594. doi: 10.1038/onc.2011.254. [DOI] [PubMed] [Google Scholar]
- 15.Skrypek N, Goossens S, De Smedt E, Vandamme N, Berx G. Epithelial-to-mesenchymal transition: epigenetic reprogramming driving cellular plasticity. Trends Genet. 2017;33(12):943–959. doi: 10.1016/j.tig.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Hamamori Y, Sartorelli V, Ogryzko V, Puri PL, Wu H-Y, Wang JY, et al. Regulation of histone acetyltransferases p300 and PCAF by the bHLH protein twist and adenoviral oncoprotein E1A. Cell. 1999;96(3):405–413. doi: 10.1016/s0092-8674(00)80553-x. [DOI] [PubMed] [Google Scholar]
- 17.Postigo AA, Depp JL, Taylor JJ, Kroll KL. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 2003;22(10):2453–2462. doi: 10.1093/emboj/cdg226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu DS-S, Wang H-J, Tai SK, Chou C-H, Hsieh C-H, Chiu P-H, et al. Acetylation of snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell. 2014;26(4):534–48. [DOI] [PubMed]
- 19.Li S, Yang J. Ovol proteins: guardians against EMT during epithelial differentiation. Dev Cell. 2014;29(1):1–2. doi: 10.1016/j.devcel.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subbalakshmi AR, Sahoo S, McMullen I, Saxena AN, Venugopal SK, Somarelli JA, et al. KLF4 induces mesenchymal–epithelial transition (MET) by suppressing multiple EMT-inducing transcription factors. Cancers. 2021;13(20):5135. doi: 10.3390/cancers13205135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung VY, Tan TZ, Ye J, Huang R-L, Lai H-C, Kappei D, et al. The role of GRHL2 and epigenetic remodeling in epithelial–mesenchymal plasticity in ovarian cancer cells. Commun Biol. 2019;2(1):272. doi: 10.1038/s42003-019-0506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakrabarti R, Hwang J, Andres Blanco M, Wei Y, Lukačišin M, Romano R-A, et al. Elf5 inhibits the epithelial–mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nat Cell Biol. 2012;14(11):1212–1222. doi: 10.1038/ncb2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subbalakshmi AR, Sahoo S, Manjunatha P, Goyal S, Kasiviswanathan VA, Mahesh Y, et al. The ELF3 transcription factor is associated with an epithelial phenotype and represses epithelial-mesenchymal transition. J Biol Eng. 2023;17(1):1–16. doi: 10.1186/s13036-023-00333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe K, Villarreal-Ponce A, Sun P, Salmans ML, Fallahi M, Andersen B, et al. Mammary morphogenesis and regeneration require the inhibition of EMT at terminal end buds by Ovol2 transcriptional repressor. Dev Cell. 2014;29(1):59–74. doi: 10.1016/j.devcel.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong T, Watanabe K, Ta CH, Villarreal-Ponce A, Nie Q, Dai X. An Ovol2-Zeb1 mutual inhibitory circuit governs bidirectional and multi-step transition between epithelial and mesenchymal states. PLoS Comput Biol. 2015;11(11):e1004569. doi: 10.1371/journal.pcbi.1004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mooney SM, Talebian V, Jolly MK, Jia D, Gromala M, Levine H, et al. The GRHL2/ZEB feedback loop—a key axis in the regulation of EMT in breast cancer. J Cell Biochem. 2017;118(9):2559–2570. doi: 10.1002/jcb.25974. [DOI] [PubMed] [Google Scholar]
- 27.Haensel D, Sun P, MacLean AL, Ma X, Zhou Y, Stemmler MP, et al. An Ovol2-Zeb1 transcriptional circuit regulates epithelial directional migration and proliferation. EMBO Rep. 2019;20(1):e46273. doi: 10.15252/embr.201846273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Coban B, Wu H, Chouaref J, Daxinger L, Paulsen MT, et al. GRHL2-controlled gene expression networks in luminal breast cancer. Cell Commun Signal. 2023;21(1):1–19. doi: 10.1186/s12964-022-01029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma J, Wang P, Liu Y, Zhao L, Li Z, Xue Y. Krüppel-like factor 4 regulates blood-tumor barrier permeability via ZO-1, occludin and claudin-5. J Cell Physiol. 2014;229(7):916–926. doi: 10.1002/jcp.24523. [DOI] [PubMed] [Google Scholar]
- 30.Nieto MA, Huang RY-J, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166(1):21–45. [DOI] [PubMed]
- 31.Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop—a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11(9):670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill L, Browne G, Tulchinsky E. ZEB/miR-200 feedback loop: at the crossroads of signal transduction in cancer. Int J Cancer. 2013;132(4):745–754. doi: 10.1002/ijc.27708. [DOI] [PubMed] [Google Scholar]
- 33.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9(6):582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braun J, Hoang-Vu C, Dralle H, Hüttelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29(29):4237–4244. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 35.Saydam O, Shen Y, Würdinger T, Senol O, Boke E, James MF, et al. Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/β-catenin signaling pathway. Mol Cell Biol. 2009;29(21):5923–5940. doi: 10.1128/MCB.00332-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su J, Zhang A, Shi Z, Ma F, Pu P, Wang T, et al. MicroRNA-200a suppresses the Wnt/β-catenin signaling pathway by interacting with β-catenin. Int J Oncol. 2012;40(4):1162–1170. doi: 10.3892/ijo.2011.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miquelajauregui A, Van de Putte T, Polyakov A, Nityanandam A, Boppana S, Seuntjens E, et al. Smad-interacting protein-1 (Zfhx1b) acts upstream of Wnt signaling in the mouse hippocampus and controls its formation. Proc Natl Acad Sci. 2007;104(31):12919–12924. doi: 10.1073/pnas.0609863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, et al. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011;30(4):770–782. doi: 10.1038/emboj.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siemens H, Jackstadt R, Hünten S, Kaller M, Menssen A, Götz U, et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. 2011;10(24):4256–4271. doi: 10.4161/cc.10.24.18552. [DOI] [PubMed] [Google Scholar]
- 40.Kaller M, Liffers S-T, Oeljeklaus S, Kuhlmann K, Röh S, Hoffmann R, et al. Genome-wide characterization of miR-34a induced changes in protein and mRNA expression by a combined pulsed SILAC and microarray analysis. Mol Cell Proteomics. 2011;10(8). [DOI] [PMC free article] [PubMed]
- 41.Yang S, Li Y, Gao J, Zhang T, Li S, Luo A, et al. MicroRNA-34 suppresses breast cancer invasion and metastasis by directly targeting Fra-1. Oncogene. 2013;32(36):4294–4303. doi: 10.1038/onc.2012.432. [DOI] [PubMed] [Google Scholar]
- 42.Nie D, Fu J, Chen H, Cheng J, Fu J. Roles of microRNA-34a in epithelial to mesenchymal transition, competing endogenous RNA sponging and its therapeutic potential. Int J Mol Sci. 2019;20(4):861. doi: 10.3390/ijms20040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao M, Liu L, Yang Y, Li M, Ma Q, Chang Z. LncRNA HCP5 induces gastric cancer cell proliferation, invasion, and EMT processes through the miR-186-5p/WNT5A axis under hypoxia. Front Cell Dev Biol. 2021;9:663654. doi: 10.3389/fcell.2021.663654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan H, Zhao L. lncRNA nuclear-enriched abundant transcript 1 promotes cell proliferation and invasion by targeting miR-186-5p/HIF-1α in osteosarcoma. J Cell Biochem. 2019;120(4):6502–6514. doi: 10.1002/jcb.27941. [DOI] [PubMed] [Google Scholar]
- 45.Yuan J-H, Yang F, Wang F, Ma J-Z, Guo Y-J, Tao Q-F, et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25(5):666–81. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Tao F, Tian X, Lu M, Zhang Z. A novel lncRNA, Lnc-OC1, promotes ovarian cancer cell proliferation and migration by sponging miR-34a and miR-34c. J Genet Genomics. 2018;45(3):137–145. doi: 10.1016/j.jgg.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Wang J, Yin Y, Meng Q, Lyu Y. The role of EMT-related lncRNA in the process of triple-negative breast cancer metastasis. Biosci Rep. 2021;41(2):BSR20203121. doi: 10.1042/BSR20203121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ming H, Li B, Zhou L, Goel A, Huang C. Long non-coding RNAs and cancer metastasis: Molecular basis and therapeutic implications. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188519. doi: 10.1016/j.bbcan.2021.188519. [DOI] [PubMed] [Google Scholar]
- 49.Brabletz S, Schuhwerk H, Brabletz T, Stemmler MP. Dynamic EMT: a multi-tool for tumor progression. EMBO J. 2021;40(18):e108647. doi: 10.15252/embj.2021108647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Postigo AA. Opposing functions of ZEB proteins in the regulation of the TGFβ/BMP signaling pathway. EMBO J. 2003;22(10):2443–2452. doi: 10.1093/emboj/cdg225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, et al. A SNAIL1–SMAD3/4 transcriptional repressor complex promotes TGF-β mediated epithelial–mesenchymal transition. Nat Cell Biol. 2009;11(8):943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayashi M, Nimura K, Kashiwagi K, Harada T, Takaoka K, Kato H, et al. Comparative roles of Twist-1 and Id1 in transcriptional regulation by BMP signaling. J Cell Sci. 2007;120(8):1350–1357. doi: 10.1242/jcs.000067. [DOI] [PubMed] [Google Scholar]
- 53.Tang Y, Feinberg T, Keller ET, Li X-Y, Weiss SJ. Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation. Nat Cell Biol. 2016;18(9):917–929. doi: 10.1038/ncb3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lehmann W, Mossmann D, Kleemann J, Mock K, Meisinger C, Brummer T, et al. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat Commun. 2016;7(1):10498. doi: 10.1038/ncomms10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feldker N, Ferrazzi F, Schuhwerk H, Widholz SA, Guenther K, Frisch I, et al. Genome-wide cooperation of EMT transcription factor ZEB 1 with YAP and AP-1 in breast cancer. EMBO J. 2020;39(17):e103209. doi: 10.15252/embj.2019103209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li S, Kendall SE, Raices R, Finlay J, Covarrubias M, Liu Z, et al. TWIST1 associates with NF-κ B subunit RELA via carboxyl-terminal WR domain to promote cell autonomous invasion through IL8 production. BMC Biol. 2012;10:1–16. doi: 10.1186/1741-7007-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stemmer VD, De Craene B, Berx G, Behrens J. Snail promotes Wnt target gene expression and interacts with β-catenin. Oncogene. 2008;27(37):5075–80. doi: 10.1038/onc.2008.140. [DOI] [PubMed] [Google Scholar]
- 58.Yu N, Hu T, Yang H, Zhang L, Song Q, Xiang F, et al. Twist1 contributes to the maintenance of some biological properties of dermal papilla cells in vitro by forming a complex with Tcf4 and β-catenin. Front Cell Dev Biol. 2020;8:824. doi: 10.3389/fcell.2020.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neumann DP, Goodall GJ, Gregory PA, editors. Regulation of splicing and circularisation of RNA in epithelial mesenchymal plasticity. Seminars in Cell & Developmental Biology; 2018: Elsevier. [DOI] [PubMed]
- 60.Lyu J, Cheng C. Regulation of alternative splicing during epithelial-mesenchymal transition. Cells Tissues Organs. 2022;211(2):238–251. doi: 10.1159/000518249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaul YD, Freinkman E, Comb WC, Cantor JR, Tam WL, Thiru P, et al. Dihydropyrimidine accumulation is required for the epithelial-mesenchymal transition. Cell. 2014;158(5):1094–1109. doi: 10.1016/j.cell.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei Q, Qian Y, Yu J, Wong CC. Metabolic rewiring in the promotion of cancer metastasis: mechanisms and therapeutic implications. Oncogene. 2020;39(39):6139–6156. doi: 10.1038/s41388-020-01432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antony J, Thiery JP, Huang RY-J. Epithelial-to-mesenchymal transition: lessons from development, insights into cancer and the potential of EMT-subtype based therapeutic intervention. Phys Biol. 2019;16(4):041004. [DOI] [PubMed]
- 64.Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19(11):1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vasaikar SV, Deshmukh AP, den Hollander P, Addanki S, Kuburich NA, Kudaravalli S, et al. EMTome: a resource for pan-cancer analysis of epithelial-mesenchymal transition genes and signatures. Br J Cancer. 2021;124(1):259–269. doi: 10.1038/s41416-020-01178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanahan D, Weinberg R. Hallmarks of cancer: the next generation. cell. 2011; 144: 646–74. Hanahan D Weinberg RA Hallmarks of cancer: the next generation cell. 2011;144(646):74. [DOI] [PubMed]
- 67.Duronio RJ, Xiong Y. Signaling pathways that control cell proliferation. Cold Spring Harb Perspect Biol. 2013;5(3):a008904. doi: 10.1101/cshperspect.a008904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14(2):159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 69.Jeannot P, Nowosad A, Perchey RT, Callot C, Bennana E, Katsube T, et al. p27Kip1 promotes invadopodia turnover and invasion through the regulation of the PAK1/Cortactin pathway. elife. 2017;6:e22207. doi: 10.7554/eLife.22207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bayarmagnai B, Perrin L, Esmaeili Pourfarhangi K, Graña X, Tüzel E, Gligorijevic B. Invadopodia-mediated ECM degradation is enhanced in the G1 phase of the cell cycle. J Cell Sci. 2019;132(20):jcs227116. doi: 10.1242/jcs.227116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18(8):862–876. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao D, Besser AH, Wander SA, Sun J, Zhou W, Wang B, et al. Cytoplasmic p27 promotes epithelial–mesenchymal transition and tumor metastasis via STAT3-mediated Twist1 upregulation. Oncogene. 2015;34(43):5447–5459. doi: 10.1038/onc.2014.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuznetsov M, Kolobov A. Investigation of solid tumor progression with account of proliferation/migration dichotomy via Darwinian mathematical model. J Math Biol. 2020;80(3):601–626. doi: 10.1007/s00285-019-01434-4. [DOI] [PubMed] [Google Scholar]
- 74.Lattmann E, Deng T, Hajnal A. To Divide or Invade: A look behind the scenes of the proliferation-invasion interplay in the caenorhabditis elegans anchor cell. Front Cell Dev Biol. 2021:1727. [DOI] [PMC free article] [PubMed]
- 75.Fuxe J, Vincent T, Garcia de Herreros A. Transcriptional crosstalk between TGFβ and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle. 2010;9(12):2363–74. doi: 10.4161/cc.9.12.12050. [DOI] [PubMed] [Google Scholar]
- 76.Dhillon AS, Tulchinsky E. FRA-1 as a driver of tumour heterogeneity: a nexus between oncogenes and embryonic signalling pathways in cancer. Oncogene. 2015;34(34):4421–4428. doi: 10.1038/onc.2014.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Talotta F, Casalino L, Verde P. The nuclear oncoprotein Fra-1: a transcription factor knocking on therapeutic applications’ door. Oncogene. 2020;39(23):4491–4506. doi: 10.1038/s41388-020-1306-4. [DOI] [PubMed] [Google Scholar]
- 78.Matus DQ, Lohmer LL, Kelley LC, Schindler AJ, Kohrman AQ, Barkoulas M, et al. Invasive cell fate requires G1 cell-cycle arrest and histone deacetylase-mediated changes in gene expression. Dev Cell. 2015;35(2):162–174. doi: 10.1016/j.devcel.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bruneel K, Verstappe J, Vandamme N, Berx G. Intrinsic balance between ZEB family members is important for melanocyte homeostasis and melanoma progression. Cancers. 2020;12(8):2248. doi: 10.3390/cancers12082248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao R, Trainor PA, editors. Epithelial to mesenchymal transition during mammalian neural crest cell delamination. Semin Cell Dev Biol; 2022: Elsevier. [DOI] [PubMed]
- 81.Kohrman AQ, Matus DQ. Divide or conquer: cell cycle regulation of invasive behavior. Trends Cell Biol. 2017;27(1):12–25. doi: 10.1016/j.tcb.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu H, Adler AS, Segal E, Chang HY. A transcriptional program mediating entry into cellular quiescence. PLoS Genet. 2007;3(6):e91. doi: 10.1371/journal.pgen.0030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marcucci F, Stassi G, De Maria R. Epithelial–mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discovery. 2016;15(5):311–325. doi: 10.1038/nrd.2015.13. [DOI] [PubMed] [Google Scholar]
- 84.Brown JA, Yonekubo Y, Hanson N, Sastre-Perona A, Basin A, Rytlewski JA, et al. TGF-β-induced quiescence mediates chemoresistance of tumor-propagating cells in squamous cell carcinoma. Cell Stem Cell. 2017;21(5):650–64. e8. doi: 10.1016/j.stem.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ikushima H, Miyazono K. TGFβ signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10(6):415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 86.Vega S, Morales AV, Ocaña OH, Valdés F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18(10):1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mejlvang J, Kriajevska M, Vandewalle C, Chernova T, Sayan AE, Berx G, et al. Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol Biol Cell. 2007;18(11):4615–4624. doi: 10.1091/mbc.E07-05-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cui N, Yang W-T, Zheng P-S. Slug inhibits the proliferation and tumor formation of human cervical cancer cells by up-regulating the p21/p27 proteins and down-regulating the activity of the Wnt/β-catenin signaling pathway via the trans-suppression Akt1/p-Akt1 expression. Oncotarget. 2016;7(18):26152. doi: 10.18632/oncotarget.8434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shin S, Buel GR, Nagiec MJ, Han M-J, Roux PP, Blenis J, et al. ERK2 regulates epithelial-to-mesenchymal plasticity through DOCK10-dependent Rac1/FoxO1 activation. Proc Natl Acad Sci. 2019;116(8):2967–2976. doi: 10.1073/pnas.1811923116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Z, Li J, Ou Y, Yang G, Deng K, Wang Q, et al. CDK4/6 inhibition blocks cancer metastasis through a USP51-ZEB1-dependent deubiquitination mechanism. Signal Transduct Target Ther. 2020;5(1):25. doi: 10.1038/s41392-020-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berglund P, Stighall M, Jirström K, Borgquist S, Sjölander A, Hedenfalk I, et al. Cyclin E overexpression obstructs infiltrative behavior in breast cancer: a novel role reflected in the growth pattern of medullary breast cancers. Can Res. 2005;65(21):9727–9734. doi: 10.1158/0008-5472.CAN-04-3984. [DOI] [PubMed] [Google Scholar]
- 92.Lehn S, Tobin NP, Berglund P, Nilsson K, Sims AH, Jirström K, et al. Down-regulation of the oncogene cyclin D1 increases migratory capacity in breast cancer and is linked to unfavorable prognostic features. Am J Pathol. 2010;177(6):2886–2897. doi: 10.2353/ajpath.2010.100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wassermann S, Scheel SK, Hiendlmeyer E, Palmqvist R, Horst D, Hlubek F, et al. p16INK4a Is a β-catenin target gene and indicates low survival in human colorectal tumors. Gastroenterology. 2009;136(1):196–205. e2. doi: 10.1053/j.gastro.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 94.Spaderna S, Schmalhofer O, Hlubek F, Berx G, Eger A, Merkel S, et al. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology. 2006;131(3):830–840. doi: 10.1053/j.gastro.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 95.Qian X, Hulit J, Suyama K, Eugenin E, Belbin T, Loudig O, et al. p21CIP1 mediates reciprocal switching between proliferation and invasion during metastasis. Oncogene. 2013;32(18):2292–2303. doi: 10.1038/onc.2012.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garay T, Juhász É, Molnár E, Eisenbauer M, Czirók A, Dekan B, et al. Cell migration or cytokinesis and proliferation?–revisiting the “go or grow” hypothesis in cancer cells in vitro. Exp Cell Res. 2013;319(20):3094–3103. doi: 10.1016/j.yexcr.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 97.Comaills V, Kabeche L, Morris R, Buisson R, Yu M, Madden MW, et al. Genomic instability is induced by persistent proliferation of cells undergoing epithelial-to-mesenchymal transition. Cell Rep. 2016;17(10):2632–2647. doi: 10.1016/j.celrep.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–5. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14(6):329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Velthoven CT, Rando TA. Stem cell quiescence: dynamism, restraint, and cellular idling. Cell Stem Cell. 2019;24(2):213–225. doi: 10.1016/j.stem.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marescal O, Cheeseman IM. Cellular mechanisms and regulation of quiescence. Dev Cell. 2020;55(3):259–271. doi: 10.1016/j.devcel.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jacob B, Osato M. Stem cell exhaustion and leukemogenesis. J Cell Biochem. 2009;107(3):393–399. doi: 10.1002/jcb.22150. [DOI] [PubMed] [Google Scholar]
- 103.Jones KM, Sarić N, Russell JP, Andoniadou CL, Scambler PJ, Basson MA. CHD7 maintains neural stem cell quiescence and prevents premature stem cell depletion in the adult hippocampus. Stem cells. 2015;33(1):196–210. doi: 10.1002/stem.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodgers JT, Schroeder MD, Ma C, Rando TA. HGFA is an injury-regulated systemic factor that induces the transition of stem cells into GAlert. Cell Rep. 2017;19(3):479–486. doi: 10.1016/j.celrep.2017.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Porlan E, Martí-Prado B, Morante-Redolat JM, Consiglio A, Delgado AC, Kypta R, et al. MT5-MMP regulates adult neural stem cell functional quiescence through the cleavage of N-cadherin. Nat Cell Biol. 2014;16(7):629–638. doi: 10.1038/ncb2993. [DOI] [PubMed] [Google Scholar]
- 106.Goel AJ, Rieder M-K, Arnold H-H, Radice GL, Krauss RS. Niche cadherins control the quiescence-to-activation transition in muscle stem cells. Cell Rep. 2017;21(8):2236–2250. doi: 10.1016/j.celrep.2017.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hosokawa K, Arai F, Yoshihara H, Iwasaki H, Nakamura Y, Gomei Y, et al. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood, The Journal of the American Society of Hematology. 2010;116(4):554–563. doi: 10.1182/blood-2009-05-224857. [DOI] [PubMed] [Google Scholar]
- 108.Nguyen HP, Xiao L, Deane JA, Tan K-S, Cousins FL, Masuda H, et al. N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Hum Reprod. 2017;32(11):2254–2268. doi: 10.1093/humrep/dex289. [DOI] [PubMed] [Google Scholar]
- 109.Batard P, Monier M-N, Fortunel N, Ducos K, Sansilvestri-Morel P, Phan T, et al. TGF-(beta) 1 maintains hematopoietic immaturity by a reversible negative control of cell cycle and induces CD34 antigen up-modulation. J Cell Sci. 2000;113(3):383–390. doi: 10.1242/jcs.113.3.383. [DOI] [PubMed] [Google Scholar]
- 110.Sampath SC, Sampath SC, Ho AT, Corbel SY, Millstone JD, Lamb J, et al. Induction of muscle stem cell quiescence by the secreted niche factor Oncostatin M. Nat Commun. 2018;9(1):1531. doi: 10.1038/s41467-018-03876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525(7568):256–260. doi: 10.1038/nature14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lambert AW, Weinberg RA. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer. 2021;21(5):325–338. doi: 10.1038/s41568-021-00332-6. [DOI] [PubMed] [Google Scholar]
- 113.Phillips S, Prat A, Sedic M, Proia T, Wronski A, Mazumdar S, et al. Cell-state transitions regulated by SLUG are critical for tissue regeneration and tumor initiation. Stem Cell Rep. 2014;2(5):633–647. doi: 10.1016/j.stemcr.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148(5):1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kahounová Z, Remšík J, Fedr R, Bouchal J, Mičková A, Slabáková E, et al. Slug-expressing mouse prostate epithelial cells have increased stem cell potential. Stem Cell Research. 2020;46:101844. doi: 10.1016/j.scr.2020.101844. [DOI] [PubMed] [Google Scholar]
- 116.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci. 2009;106(31):12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wilson MM, Weinberg RA, Lees JA, Guen VJ. Emerging mechanisms by which EMT programs control stemness. Trends Cancer. 2020;6(9):775–780. doi: 10.1016/j.trecan.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 118.Wang X, Xu H, Cheng C, Ji Z, Zhao H, Sheng Y, et al. Identification of a Zeb1 expressing basal stem cell subpopulation in the prostate. Nat Commun. 2020;11(1):706. doi: 10.1038/s41467-020-14296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nguyen QH, Pervolarakis N, Blake K, Ma D, Davis RT, James N, et al. Profiling human breast epithelial cells using single cell RNA sequencing identifies cell diversity. Nat Commun. 2018;9(1):2028. doi: 10.1038/s41467-018-04334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Han Y, Villarreal-Ponce A, Gutierrez G, Nguyen Q, Sun P, Wu T, et al. Coordinate control of basal epithelial cell fate and stem cell maintenance by core EMT transcription factor Zeb1. Cell Rep. 2022;38(2):110240. doi: 10.1016/j.celrep.2021.110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suárez-Álvarez B, López-Vázquez A, López-Larrea C. Mobilization and homing of hematopoietic stem cells. Stem Cell Transplantation. 2012:152–70. [DOI] [PubMed]
- 122.Tzeng Y-S, Li H, Kang Y-L, Chen W-C, Cheng W-C, Lai D-M. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood, The Journal of the American Society of Hematology. 2011;117(2):429–439. doi: 10.1182/blood-2010-01-266833. [DOI] [PubMed] [Google Scholar]
- 123.Yang L, Wang L, Geiger H, Cancelas JA, Mo J, Zheng Y. Rho GTPase Cdc42 coordinates hematopoietic stem cell quiescence and niche interaction in the bone marrow. Proc Natl Acad Sci. 2007;104(12):5091–5096. doi: 10.1073/pnas.0610819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Williams DA, Zheng Y, Cancelas JA. Rho GTPases and regulation of hematopoietic stem cell localization. Methods Enzymol. 2008;439:365–393. doi: 10.1016/S0076-6879(07)00427-2. [DOI] [PubMed] [Google Scholar]
- 125.Soen B, Vandamme N, Berx G, Schwaller J, Van Vlierberghe P, Goossens S. ZEB proteins in leukemia: friends, foes, or friendly foes? Hemasphere. 2018;2(3):e43. doi: 10.1097/HS9.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li J, Riedt T, Goossens S, Carrillo García C, Szczepanski S, Brandes M, et al. The EMT transcription factor Zeb2 controls adult murine hematopoietic differentiation by regulating cytokine signaling. Blood, The Journal of the American Society of Hematology. 2017;129(4):460–472. doi: 10.1182/blood-2016-05-714659. [DOI] [PubMed] [Google Scholar]
- 127.Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, et al. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and β-catenin. Cells Tissues Organs. 2005;179(1–2):56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- 128.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5(9):744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 129.M Sa 2008 guo W, liao Mj, eaton en, ayyanan a, zhou ay Brooks M, reinhard f, zhang cc, Shipitsin M, campbell ll, Polyak K, Brisken c, yang j and Weinberg ra: the epithelialmesenchymal transition generates cells with properties of stem cells cell 133 704 715 [DOI] [PMC free article] [PubMed]
- 130.Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morel A-P, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE. 2008;3(8):e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526(7571):131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou Y, Xia L, Wang H, Oyang L, Su M, Liu Q, et al. Cancer stem cells in progression of colorectal cancer. Oncotarget. 2018;9(70):33403. doi: 10.18632/oncotarget.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.de Sousa e Melo F, Kurtova AV, Harnoss JM, Kljavin N, Hoeck JD, Hung J, et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature. 2017;543(7647):676–80. doi: 10.1038/nature21713. [DOI] [PubMed] [Google Scholar]
- 135.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–4. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Okabe T, Togo S, Fujimoto Y, Watanabe J, Sumiyoshi I, Orimo A, et al. Mesenchymal characteristics and predictive biomarkers on circulating tumor cells for therapeutic strategy. Cancers. 2020;12(12):3588. doi: 10.3390/cancers12123588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Visal TH, den Hollander P, Cristofanilli M, Mani SA. Circulating tumour cells in the-omics era: how far are we from achieving the ‘singularity’? Br J Cancer. 2022;127(2):173–184. doi: 10.1038/s41416-022-01768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Beerling E, Seinstra D, de Wit E, Kester L, van der Velden D, Maynard C, et al. Plasticity between epithelial and mesenchymal states unlinks EMT from metastasis-enhancing stem cell capacity. Cell Rep. 2016;14(10):2281–2288. doi: 10.1016/j.celrep.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Celià-Terrassa T, Meca-Cortés Ó, Mateo F, De Paz AM, Rubio N, Arnal-Estapé A, et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Investig. 2012;122(5):1849–1868. doi: 10.1172/JCI59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS, et al. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. 2019;573(7774):439–444. doi: 10.1038/s41586-019-1526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol. 2020;30(10):764–776. doi: 10.1016/j.tcb.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nat Cell Biol. 2012;14(8):777–783. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- 143.Aiello NM, Maddipati R, Norgard RJ, Balli D, Li J, Yuan S, et al. EMT subtype influences epithelial plasticity and mode of cell migration. Dev Cell. 2018;45(6):681–95. e4. doi: 10.1016/j.devcel.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stankic M, Pavlovic S, Chin Y, Brogi E, Padua D, Norton L, et al. TGF-β-Id1 signaling opposes Twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transition. Cell Rep. 2013;5(5):1228–1242. doi: 10.1016/j.celrep.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014;2(1):78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schmidt JM, Panzilius E, Bartsch HS, Irmler M, Beckers J, Kari V, et al. Stem-cell-like properties and epithelial plasticity arise as stable traits after transient Twist1 activation. Cell Rep. 2015;10(2):131–139. doi: 10.1016/j.celrep.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 147.Liu X, Li J, Cadilha BL, Markota A, Voigt C, Huang Z, et al. Epithelial-type systemic breast carcinoma cells with a restricted mesenchymal transition are a major source of metastasis. Sci Adv. 2019;5(6):eaav4275. doi: 10.1126/sciadv.aav4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jordan NV, Johnson GL, Abell AN. Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell Cycle. 2011;10(17):2865–2873. doi: 10.4161/cc.10.17.17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang J, Tian X-J, Zhang H, Teng Y, Li R, Bai F, et al. TGF-β–induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Sci Signal. 2014;7(345):ra91-ra. doi: 10.1126/scisignal.2005304. [DOI] [PubMed] [Google Scholar]
- 150.Jolly MK, Tripathi SC, Jia D, Mooney SM, Celiktas M, Hanash SM, et al. Stability of the hybrid epithelial/mesenchymal phenotype. Oncotarget. 2016. [DOI] [PMC free article] [PubMed]
- 151.Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 152.Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, et al. Identification of the tumour transition states occurring during EMT. Nature. 2018;556(7702):463–468. doi: 10.1038/s41586-018-0040-3. [DOI] [PubMed] [Google Scholar]
- 153.Hari K, Ullanat V, Balasubramanian A, Gopalan A, Jolly MK. Landscape of epithelial–mesenchymal plasticity as an emergent property of coordinated teams in regulatory networks. Elife. 2022;11:e76535. doi: 10.7554/eLife.76535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kröger C, Afeyan A, Mraz J, Eaton EN, Reinhardt F, Khodor YL, et al. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc Natl Acad Sci. 2019;116(15):7353–7362. doi: 10.1073/pnas.1812876116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Krebs AM, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, Busch H, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19(5):518–529. doi: 10.1038/ncb3513. [DOI] [PubMed] [Google Scholar]
- 156.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178(4):835–49. e21. doi: 10.1016/j.cell.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rambow F, Rogiers A, Marin-Bejar O, Aibar S, Femel J, Dewaele M, et al. Toward minimal residual disease-directed therapy in melanoma. Cell. 2018;174(4):843–55. e19. doi: 10.1016/j.cell.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 159.Wouters J, Kalender-Atak Z, Minnoye L, Spanier KI, De Waegeneer M, Bravo González-Blas C, et al. Robust gene expression programs underlie recurrent cell states and phenotype switching in melanoma. Nat Cell Biol. 2020;22(8):986–998. doi: 10.1038/s41556-020-0547-3. [DOI] [PubMed] [Google Scholar]
- 160.Simeonov KP, Byrns CN, Clark ML, Norgard RJ, Martin B, Stanger BZ, et al. Single-cell lineage tracing of metastatic cancer reveals selection of hybrid EMT states. Cancer Cell. 2021;39(8):1150–62. e9. doi: 10.1016/j.ccell.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tao J, Zhu L, Yakoub M, Reißfelder C, Loges S, Schölch S. Cell–cell interactions drive metastasis of circulating tumor microemboli. Can Res. 2022;82(15):2661–2671. doi: 10.1158/0008-5472.CAN-22-0906. [DOI] [PubMed] [Google Scholar]
- 162.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lüönd F, Sugiyama N, Bill R, Bornes L, Hager C, Tang F, et al. Distinct contributions of partial and full EMT to breast cancer malignancy. Dev Cell. 2021;56(23):3203–21. e11. doi: 10.1016/j.devcel.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 165.Vanharanta S, Massagué J. Origins of metastatic traits. Cancer Cell. 2013;24(4):410–421. doi: 10.1016/j.ccr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7(11):834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Risson E, Nobre AR, Maguer-Satta V, Aguirre-Ghiso JA. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat Cancer. 2020;1(7):672–680. doi: 10.1038/s43018-020-0088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165(1):45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ruscetti M, Quach B, Dadashian EL, Mulholland DJ, Wu H. Tracking and functional characterization of epithelial–mesenchymal transition and mesenchymal tumor cells during prostate cancer metastasis. Can Res. 2015;75(13):2749–2759. doi: 10.1158/0008-5472.CAN-14-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Celià-Terrassa T, Kang Y. Metastatic niche functions and therapeutic opportunities. Nat Cell Biol. 2018;20(8):868–877. doi: 10.1038/s41556-018-0145-9. [DOI] [PubMed] [Google Scholar]
- 171.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Investig. 2011;121(4):1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cackowski FC, Taichman RS. Parallels between hematopoietic stem cell and prostate cancer disseminated tumor cell regulation. Bone. 2019;119:82–86. doi: 10.1016/j.bone.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12(2):116–IN4. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Auyez A, Sayan AE, Kriajevska M, Tulchinsky E. AXL receptor in cancer metastasis and drug resistance: when normal functions go askew. Cancers. 2021;13(19):4864. doi: 10.3390/cancers13194864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Antony J, Huang RY-J. AXL-Driven EMT State as a Targetable Conduit in CancerTargeting AXL in Cancer. Cancer Res. 2017;77(14):3725–32. doi: 10.1158/0008-5472.CAN-17-0392. [DOI] [PubMed] [Google Scholar]
- 176.Shin J, Berg DA, Zhu Y, Shin JY, Song J, Bonaguidi MA, et al. Single-cell RNA-seq with waterfall reveals molecular cascades underlying adult neurogenesis. Cell Stem Cell. 2015;17(3):360–372. doi: 10.1016/j.stem.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Leconet W, Chentouf M, Du Manoir S, Chevalier C, Sirvent A, Aït-Arsa I, et al. Therapeutic Activity of Anti-AXL Antibody against Triple-Negative Breast Cancer Patient-Derived Xenografts and MetastasisAnti-AXL mAb Inhibits EMT and Metastasis Potential of TNBC. Clin Cancer Res. 2017;23(11):2806–2816. doi: 10.1158/1078-0432.CCR-16-1316. [DOI] [PubMed] [Google Scholar]
- 178.Sun Y, Shao L, Bai H, Wang ZZ, Wu W-S. Slug deficiency enhances self-renewal of hematopoietic stem cells during hematopoietic regeneration. Blood. 2010;115(9):1709–1717. doi: 10.1182/blood-2009-07-232934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, et al. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to GAlert. Nature. 2014;510(7505):393–396. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Relaix F, Bencze M, Borok M, Der Vartanian A, Gattazzo F, Mademtzoglou D, et al. Perspectives on skeletal muscle stem cells. Nat Commun. 2021;12(1):692. doi: 10.1038/s41467-020-20760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Laurenti E, Frelin C, Xie S, Ferrari R, Dunant CF, Zandi S, et al. CDK6 levels regulate quiescence exit in human hematopoietic stem cells. Cell Stem Cell. 2015;16(3):302–313. doi: 10.1016/j.stem.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kwon JS, Everetts NJ, Wang X, Wang W, Della Croce K, Xing J, et al. Controlling depth of cellular quiescence by an Rb-E2F network switch. Cell Rep. 2017;20(13):3223–3235. doi: 10.1016/j.celrep.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Susaki E, Nakayama K, Nakayama KI. Cyclin D2 translocates p27 out of the nucleus and promotes its degradation at the G0–G1 transition. Mol Cell Biol. 2007;27(13):4626–4640. doi: 10.1128/MCB.00862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cui K, Zang C, Roh T-Y, Schones DE, Childs RW, Peng W, et al. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell. 2009;4(1):80–93. doi: 10.1016/j.stem.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu L, Cheung TH, Charville GW, Hurgo BMC, Leavitt T, Shih J, et al. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep. 2013;4(1):189–204. doi: 10.1016/j.celrep.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, et al. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154(1):61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Malouf GG, Taube JH, Lu Y, Roysarkar T, Panjarian S, Estecio MR, et al. Architecture of epigenetic reprogramming following Twist1-mediated epithelial-mesenchymal transition. Genome Biol. 2013;14(12):1–17. doi: 10.1186/gb-2013-14-12-r144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.