Abstract
Objectives
REVEAL-CKD aims to estimate the prevalence of, and factors associated with, undiagnosed stage 3 chronic kidney disease (CKD).
Design
Multinational, observational study.
Setting
Data from six country-specific electronic medical records and/or insurance claims databases from five countries (France, Germany, Italy, Japan and the USA [two databases]).
Participants
Eligible participants (≥18 years old) had ≥2 consecutive estimated glomerular filtration rate (eGFR) measurements (calculated from serum creatinine values, sex and age) taken from 2015 onwards that were indicative of stage 3 CKD (≥30 and <60 mL/min/1.73 m2). Undiagnosed cases lacked an International Classification of Diseases 9/10 diagnosis code for CKD (any stage) any time before, and up to 6 months after, the second qualifying eGFR measurement (study index).
Main outcome measures
The primary outcome was point prevalence of undiagnosed stage 3 CKD. Time to diagnosis was assessed using the Kaplan-Meier approach. Factors associated with lacking a CKD diagnosis and risk of diagnostic delay were assessed using logistic regression adjusted for baseline covariates.
Results
The prevalence of undiagnosed stage 3 CKD was 95.5% (19 120/20 012 patients) in France, 84.3% (22 557/26 767) in Germany, 77.0% (50 547/65 676) in Italy, 92.1% (83 693/90 902) in Japan, 61.6% (13 845/22 470) in the US Explorys Linked Claims and Electronic Medical Records Data database and 64.3% (161 254/250 879) in the US TriNetX database. The prevalence of undiagnosed CKD increased with age. Factors associated with undiagnosed CKD were female sex (vs male, range of odds ratios across countries: 1.29–1.77), stage 3a CKD (vs 3b, 1.81–3.66), no medical history (vs a history) of diabetes (1.26–2.77) or hypertension (1.35–1.78).
Conclusions
There are substantial opportunities to improve stage 3 CKD diagnosis, particularly in female patients and older patients. The low diagnosis rates in patients with comorbidities that put them at risk of disease progression and complications require attention.
Trial registration
Keywords: NEPHROLOGY, EPIDEMIOLOGY, Chronic renal failure, Adult nephrology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
REVEAL-CKD uses large, contemporary, country-specific databases to provide robust estimates of the prevalence of undiagnosed stage 3 chronic kidney disease (CKD).
The study uses a strict, consistent and internationally recognised definition of stage 3 CKD to ensure accuracy when calculating the prevalence of diagnosed/undiagnosed CKD.
Data from the countries and databases examined may not be representative of other countries with substantially different healthcare systems or CKD screening policies.
There is a risk of misclassification of undiagnosed CKD if diagnoses were made in environments that did not contribute to the databases used or if diagnosing physicians did not use International Classification of Diseases 9/10 codes appropriately.
Introduction
Chronic kidney disease (CKD) is an established global public health concern.1 CKD has a significant effect on patients, attributable to direct mortality and morbidity, as well as elevated risk of cardiovascular diseases.2 The global prevalence of CKD is rising,3 owing to ageing populations and increased prevalence of CKD-associated risk factors including type 2 diabetes (T2D) and hypertension.4
Early intervention and appropriate management of CKD is recommended in the internationally recognised Kidney Disease: Improving Global Outcomes (KDIGO) guidelines5 to help delay disease progression and reduce the incidence of complications. Furthermore, in 2019, KDIGO held a controversies conference on the topic of early identification and intervention in CKD. The consensus statement from this conference urged action, including the implementation of screening programmes and interventions for high-risk individuals.6 Early-stage CKD is primarily asymptomatic,7 therefore, CKD is primarily diagnosed at later disease stages and the initiation of effective interventions is delayed or missed.5 Previous studies have demonstrated low levels of diagnosis of early-stage CKD in Italy,8 Sweden9 and the USA.10–15 However, these previous studies have been limited to single countries or databases, or at-risk groups such as patients with T2D, and did not assess the prevalence of CKD diagnosis across various subgroups (eg, patients with or without comorbidities). There is a need for contemporary information on the prevalence of, and factors associated with, undiagnosed stage 3 CKD, as well as a need to understand factors associated with diagnostic delay in these patients.
REVEAL-CKD (NCT04847531) is a multinational, observational study designed to fill this evidence gap. REVEAL-CKD aims to quantify the prevalence of, and factors associated with, undiagnosed stage 3 CKD in large populations across several countries.16 Here, we present data on the prevalence of, and factors associated with, undiagnosed stage 3 CKD in France, Germany, Italy, Japan and the USA.
Methods
Study design
The study design for REVEAL-CKD has been reported in detail elsewhere16 and is summarised below.
Existing secondary data were extracted from established, verified relevant databases containing electronic medical records and/or insurance claims in the countries of interest. Data for France were extracted from The Health Improvement Network, a large database of anonymised electronic medical records.17 Data for Germany were extracted from the German Disease Analyzer, a database of anonymised longitudinal data on drug prescriptions, diagnoses and medical and demographic data contributed by a panel of more than 2500 physicians in Germany.18 Data for Italy were extracted from the IQVIA Longitudinal Patient Database, a computerised network of over 900 family physicians, which includes anonymised data on patient consultations and treatments.19 Data for Japan were extracted from Japan Real World Data, an integrated database of medical information including both electronic medical records and claims data.20 Data for the USA were extracted from two separate databases: Explorys Linked Claims and Electronic Medical Records Data (LCED), a database of inpatient and outpatient medical records and claims data from commercially insured individuals,21 and TriNetX, a database of integrated electronic medical records and claims data from 35 healthcare organisations, which provides clinical patient data from both inpatient and outpatient encounters.22 The coverage of each database used is described in online supplemental table 1.
bmjopen-2022-067386supp001.pdf (605.6KB, pdf)
Patients aged ≥18 years were included in the analyses if they had at least two consecutive estimated glomerular filtration rate (eGFR) measurements that fell within the range indicative of stage 3 CKD (≥30 and <60 mL/min/1.73 m2) and were recorded >90 and ≤730 days apart, taken on or after 1 January 2015. The decision to require at least two eGFR measurements with a gap of at least 90 days between each measurement was made to ensure that patients met the requirements for the KDIGO definition of CKD.5 In order to investigate the potential impact of requiring two eGFR measurements to classify patients, a sensitivity analysis was performed on data from the TriNetX database that included all patients with at least one eGFR measurement within the range of stage 3 CKD, taken within the same date range used for the main analysis. All patients had at least 12 months of continuous presence in the database before the first qualifying eGFR measurement. Full inclusion and exclusion criteria are shown in online supplemental table 2. eGFR was calculated from serum creatinine values, sex and age, using the CKD Epidemiology Collaboration (CKD-EPI) equation.23 In line with current trends among physicians24 25 and guidance from expert recommendations,26 race modifiers were not used in the calculation of eGFR.
To account for potential delays in recording of diagnostic codes, undiagnosed CKD was defined as lacking an International Classification of Diseases (ICD) 9/10 diagnosis code corresponding to CKD (any stage), any time before and up to 6 months after index (date of second qualifying eGFR measurement). The ICD coding system varied by country depending on what was available in each database; the full list of ICD-9/10 codes used to determine diagnosed cases can be found in online supplemental table 3. A sensitivity analysis was performed to calculate the overall prevalence of undiagnosed stage 3 CKD using a broader definition of CKD adapted from Winkelmayer et al.27 This sensitivity analysis included diagnostic codes for several additional manifestations of renal disease (online supplemental table 4).
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Statistical analysis
Overall prevalence of undiagnosed stage 3 CKD and patient demographic and clinical characteristics at index are presented descriptively. Comorbidities at index were identified using ICD-9/10 codes. Medication use at index was identified by the presence of at least one prescription for a given medication at or in the 12 months before index. Odds ratios (ORs) for factors associated with being undiagnosed any time before and up to 6 months after index were calculated using logistic regression analysis, adjusted for covariates at index. Hazard ratios for diagnostic delay among patients undiagnosed at index were calculated using Cox regression analysis, adjusted for covariates at index. The Kaplan-Meier method was used to estimate the time to diagnosis among patients undiagnosed at index. Statistical analysis was performed by using Python V.3.7 and R V.4.0.2.
Results
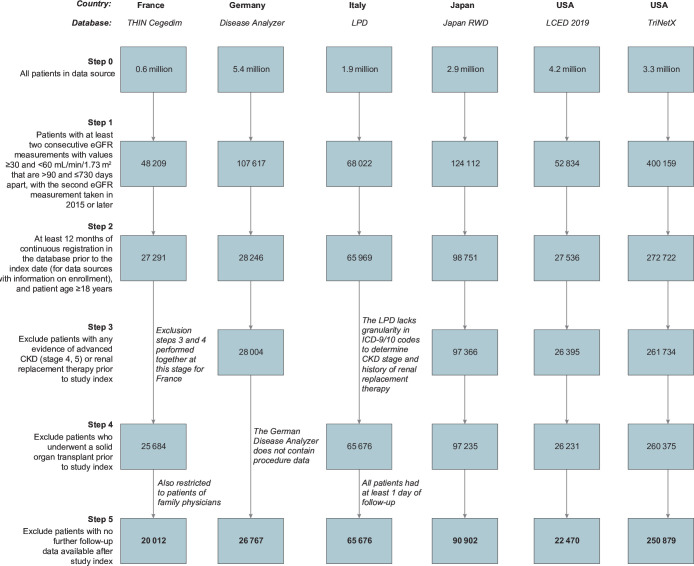
This analysis of patients with stage 3 CKD included 20 012 patients from France, 90 902 patients from Germany, 65 676 patients from Italy, 26 767 patients from Japan, 22 470 patients from the LCED database in the USA and 250 879 patients from the TriNetX database in the USA (figure 1). The characteristics of these patients at index are shown in table 1.
Figure 1.
Cohort selection. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ICD, International Classification of Diseases; LCED, Explorys Linked Claims and Electronic Medical Records Data; LPD, Longitudinal Patient Database; RWD, Real World Data; THIN, The Health Improvement Network.
Table 1.
Overall patient characteristics at study index (date of second eGFR measurement) according to country and database
| Country | France | Germany | Italy | Japan | USA | |
| Database | THIN cegedim n=20 012 | Disease analyzer n=26 767 | LPD n=65 676 | Japan RWD n=90 902 | LCED n=22 470 | TriNetX n=2 50 879 |
| CKD status*, n (%) | ||||||
| Diagnosed | 892 (4.5) | 4210 (15.7) | 15 129 (23.0) | 7209 (7.9) | 8625 (38.4) | 89 625 (35.7) |
| Undiagnosed | 19 120 (95.5) | 22 557 (84.3) | 50 547 (77.0) | 83 693 (92.1) | 13 845 (61.6) | 161 254 (64.3) |
| Age, y, median (IQR) | 80 (72–86) | 79 (72–84) | 80 (74–85) | 76 (69–83) | 74 (64–82) | 71 (64–78) |
| Age groups, y (%) | ||||||
| <45 | 67 (0.3) | 66 (0.2) | 188 (0.3) | 791 (0.9) | 243 (1.1) | 5523 (2.2) |
| 45–64 | 1677 (8.4) | 2431 (9.1) | 3780 (5.8) | 13 286 (14.6) | 5991 (26.7) | 63 726 (25.4) |
| 65–74 | 4641 (23.2) | 6032 (22.5) | 14 264 (21.7) | 25 627 (28.2) | 5592 (24.9) | 87 880 (35.0) |
| ≥75 | 13 627 (68.1) | 18 238 (68.1) | 47 444 (72.2) | 51 198 (56.3) | 10 644 (47.4) | 93 750 (37.4) |
| Male, n (%) | 9091 (45.4) | 11 216 (41.9) | 27 728 (42.2) | 48 123 (52.9) | 10 051 (44.7) | 105 112 (41.9) |
| eGFR, mL/min/1.73 m2, median (IQR) | 52 (45–56) | 52 (44–56) | 49 (42–55) | 52 (46–56) | 51 (44–56) | 51 (44–56) |
| CKD stage, n (%) | ||||||
| CKD stage 3a | 15 101 (75.5) | 19 492 (72.8) | 43 937 (66.9) | 70 668 (77.7) | 16 320 (72.6) | 183 618 (73.2) |
| CKD stage 3b | 4911 (24.5) | 7275 (27.2) | 21 739 (33.1) | 20 234 (22.3) | 6150 (27.4) | 67 261 (26.8) |
| Baseline UACR available, n (%) | 450 (2.2) | 0 (0.0)† | 9 (<0.1)‡ | 4992 (5.5) | 899 (4.0) | 4604 (1.8) |
| HDL, mmol/L, median (IQR) | 1.37 (1.11–1.65) | 1.34 (1.10–1.63) | 1.32 (1.09–1.58) | 1.40 (1.14–1.71) | 1.22 (0.98–1.50) | 1.22 (0.98–1.50) |
| Missing, n | 6514 | 8232 | 17 513 | 35 305 | 10 022 | 138 798 |
| LDL, mmol/L, median (IQR) | 2.89 (2.24–3.61) | 2.84 (2.17–3.65) | 2.69 (2.07–3.36) | 2.74 (2.30–3.31) | 2.38 (1.84–3.05) | 2.38 (1.81–3.05) |
| Missing, n | 6676 | 7087 | 19 475 | 33 589 | 8936 | 125 474 |
| Comorbidities, n (%) | ||||||
| Hypertension | 12 412 (62.0) | 13 679 (51.1) | 51 324 (78.1) | 53 022 (58.3) | 20 061 (89.3) | 203 155 (81.0) |
| Type 2 diabetes | 3532 (17.6) | 6935 (25.9) | 21 300 (32.4)§ | 18 989 (20.9) | 9288 (41.3) | 95 441 (38.0) |
| Established CVD¶** | 1449 (7.2) | 1904 (7.1) | 6937 (10.6) | 25 637 (28.2) | 6292 (28.0) | 49 744 (19.8) |
| Heart failure | 986 (4.9) | 4364 (16.3) | 6378 (9.7) | 30 063 (33.1) | 5314 (23.6) | 47 002 (18.7) |
| Atrial fibrillation | 2161 (10.8) | 4217 (15.8) | 11 105 (16.9) | 11 991 (13.2) | 4627 (20.6) | 41 214 (16.4) |
| Medication use, n (%) | ||||||
| ACE inhibitor | 4634 (23.2) | 9635 (36.0) | 25 098 (38.2) | 4501 (5.0) | 8783 (39.1) | 57 806 (23.0) |
| ARB | 6530 (32.6) | 10 573 (39.5) | 26 198 (39.9) | 21 422 (23.6) | 6302 (28.0) | 37 946 (15.1) |
| SGLT2 inhibitor | 0 (0.0) | 0 (0.0) | 353 (0.5) | 1363 (1.5) | 22 (0.1) | 2149 (0.9) |
| GLD (any) | 3489 (17.4) | 8319 (31.1) | 17 363 (26.4) | 13 431 (14.8) | 9400 (41.8) | 60 259 (24.0) |
| Antiplatelets | 5964 (29.8) | 6597 (24.6) | 31 151 (47.4) | 18 796 (20.7) | 2476 (11.0) | 16 308 (6.5) |
| Loop diuretic | 2924 (14.6) | 10 508 (39.3) | 22 160 (33.7) | 11 979 (13.2) | 5563 (24.8) | 43 470 (17.3) |
| Anticoagulants | 3018 (15.1) | 8182 (30.6) | 16 197 (24.7) | 14 486 (15.9) | 6347 (28.2) | 54 986 (21.9) |
Unless otherwise stated, percentages represent the proportion of patients in a specific group (eg, age) or with a specific variable (eg, medical history).
*Percentages represent the proportion of diagnosed/undiagnosed cases in the overall cohort for each country/database.
†UACR testing data not available in the German Disease Analyzer database.
‡Direct measurements of UACR were not available in the IQVIA Longitudinal Patient Database in Italy, however, UACR was calculated as urine albumin (mg/dL) divided by urine creatinine (g/dL) if patients had records for both of these variables on the same day.
§Owing to a lack of granularity for ICD-9 diagnostic codes in the database used, type of diabetes could not be determined in patients from Italy.
¶ Established CVD includes patients with a history of myocardial infarction, unstable angina, stroke, transient ischemic attack, coronary artery bypass graft and percutaneous coronary intervention.
** Owing to a lack of granularity for ICD-9 diagnostic codes in the database used, established CVD does not include coronary artery bypass graft and percutaneous coronary intervention in patients from Italy.
ACE, angiotensin-converting enzyme; ARB, angiotensin-II receptor blocker; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; GLD, glucose-lowering drug; HDL, high-density lipoprotein; ICD-9, International Classification of Diseases ninth revision; IQR, interquartile range; LCED, Explorys Linked Claims and Electronic Medical Records Data; LDL, low-density lipoprotein; LPD, Longitudinal Patient Database; RWD, Real World Data; SGLT2, sodium-glucose cotransporter-2; THIN, The Health Improvement Network; UACR, urinary albumin-creatinine ratio.
At index, median age was 71–80 years, median eGFR was 49–52 mL/min/1.73 m2, 66.9%%–77.7% of patients had CKD stage 3a (eGFR ≥45 and <60 mL/min/1.73 m2) and 22.3%–33.1% of patients had CKD stage 3b (eGFR ≥30 and <45 mL/min/1.73 m2). The overall prevalence of urinary albumin-creatinine ratio (UACR) testing was very low and ranged from 1.8% (US, TriNetX) to 5.5% (Japan).
Overall prevalence of undiagnosed stage 3 CKD
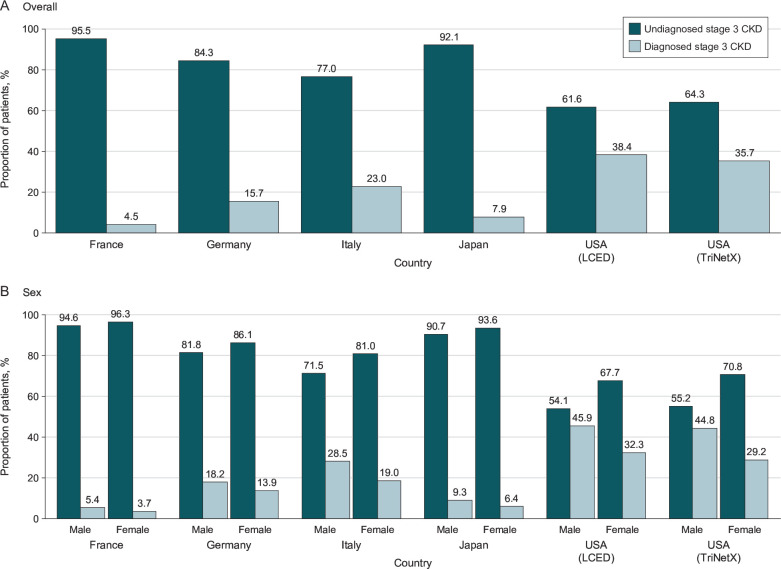
The proportion of patients with stage 3 CKD without a diagnosis at or within 6 months after index varied by database and was 95.5% in France, 84.3% in Germany, 77.0% in Italy, 92.1% in Japan, 61.6% in the US LCED database and 64.3% in the US TriNetX database (figure 2A). In the sensitivity analysis using a broader set of ICD-9/10 codes to identify CKD diagnoses, the prevalence of undiagnosed CKD was 53.6%–89.9% (online supplemental table 5). In the sensitivity analysis of 532 921 patients in the TriNetX database who had at least one qualifying eGFR measurement, the prevalence of undiagnosed stage 3 CKD was 82.2% (online supplemental table 6).
Figure 2.
Prevalence of undiagnosed stage 3 CKD according to country and database (A) overall and (B) by sex. Undiagnosed cases are those that lack a diagnosis code for CKD (any stage), any time before and up to 6 months after study index. CKD, chronic kidney disease; LCED, Explorys Linked Claims and Electronic Medical Records Data.
The proportion of patients with undiagnosed CKD per calendar year at index is shown in online supplemental figure 1. Overall, there were no prevailing trends in the proportion of patients with undiagnosed CKD per calendar year, except in Italy, where the proportion of undiagnosed CKD tended to increase over time (68.2% undiagnosed in 2015 to 83.1% in 2020).
Demographics and clinical characteristics of patients with diagnosed and undiagnosed stage 3 CKD
Characteristics for patients with diagnosed and undiagnosed stage 3 CKD at index are presented in online supplemental table 7.
Patients with undiagnosed CKD tended to have slightly higher eGFR values than those with diagnosed CKD. A greater proportion of patients with stage 3a CKD were undiagnosed than patients with stage 3b CKD. There were fewer comorbidities such as hypertension, T2D and established cardiovascular disease in patients who were undiagnosed than in those who were diagnosed. Similarly, the proportion of patients taking medicines such as glucose-lowering drugs, loop diuretics, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers tended to be lower in undiagnosed patients than in those who were diagnosed. In the sensitivity analysis of 532 921 patients in the US TriNetX database who had at least one qualifying eGFR measurement, the prevalence of comorbidities was lower than in the main cohort (online supplemental table 6). In all databases, a greater proportion of stage 3 CKD cases were undiagnosed in female patients than in male patients (figure 2B). In addition, in all databases, patients aged less than 45 years had the lowest proportion of undiagnosed CKD; the prevalence of undiagnosed CKD increased in older age groups in France, Germany, Italy and in the US TriNetX database (online supplemental figure 2).
Factors associated with undiagnosed CKD
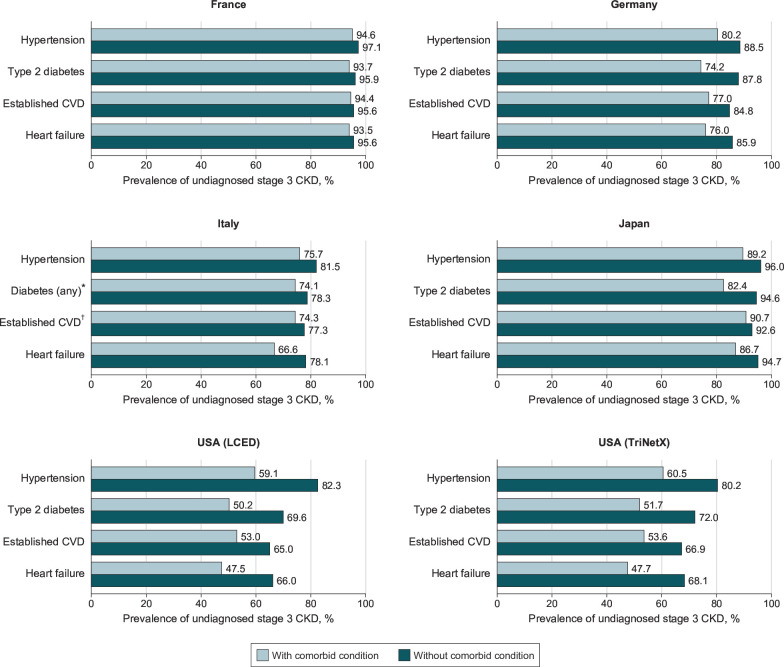
The proportion of undiagnosed CKD tended to be higher in those without comorbidities at study index versus those with such comorbidities (figure 3). When adjusting for baseline covariates, female patients (vs male patients), patients with CKD stage 3a (vs 3b) and patients without a diagnosis of diabetes or hypertension (vs those with a diagnosis) were consistently more likely to lack a CKD diagnosis before and up to 6 months after index (online supplemental figure 3).
Figure 3.
Prevalence of undiagnosed stage 3 CKD according to the presence of comorbidities at study index, by country and database. Established CVD includes patients with a history of myocardial infarction, unstable angina, stroke, transient ischaemic attack, coronary artery bypass graft and percutaneous coronary intervention. Study index is defined as the date of a patient’s second qualifying eGFR measurement. *Owing to a lack of granularity for ICD-9 diagnostic codes in the database used, type of diabetes could not be determined in patients from Italy. †Owing to a lack of granularity for ICD-9 codes in the database used, established CVD does not include coronary artery bypass graft and percutaneous coronary intervention in patients from Italy. CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ICD-9, International Classification of Diseases 9; LCED, Explorys Linked Claims and Electronic Medical Records Data.
Time to CKD diagnosis
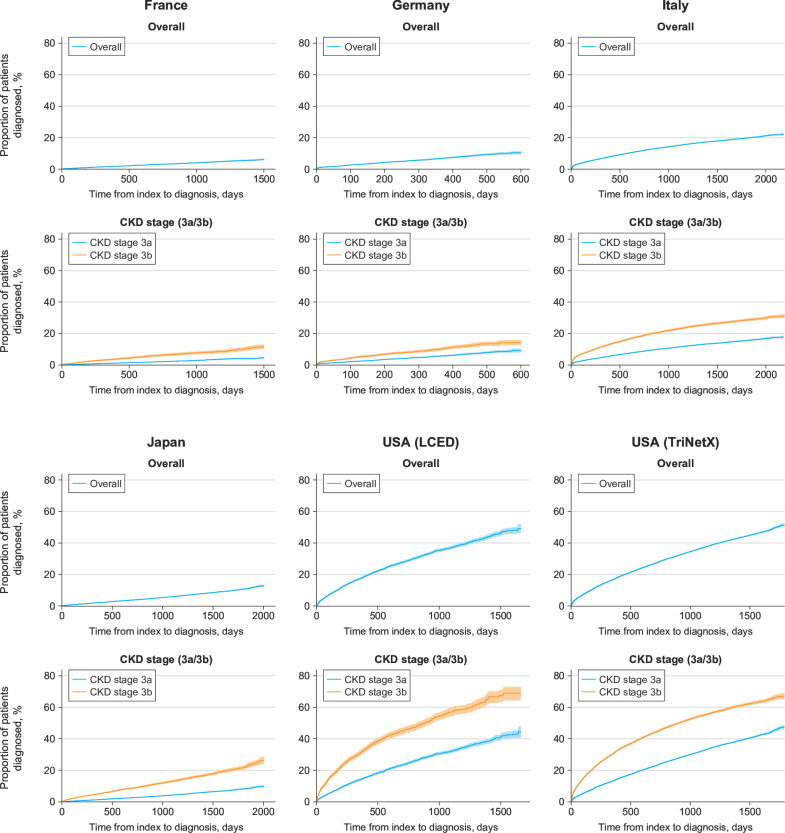
Among patients who lacked a diagnosis of stage 3 CKD at or before study index, the median (interquartile range [IQR]) follow-up duration was 2.22 (1.18–3.64) years in France, 0.61 (0.27–1.03) years in Germany, 3.64 (2.08–4.88) years in Italy, 1.96 (0.84–3.41) years in Japan, 1.28 (0.53–2.34) years in the US LCED database and 1.19 (0.44–2.32) years in the US TriNetX database. In patients undiagnosed at index, only a small proportion received a diagnosis during follow-up: 686/19 293 patients (3.6%) in France, 1157/23 302 patients (5.0%) in Germany, 8152/52 533 patients (15.5%) in Italy, 3855/84 603 patients (4.6%) in Japan, 3987/15 376 patients (25.9%) in the US LCED database and 44 007/178 410 patients (24.7%) in the US TriNetX database.
Among patients undiagnosed at index, diagnoses tended to accrue slowly over the whole duration of follow-up (figure 4). The proportion of patients with initial eGFR values indicative of stage 3b CKD (≥30 and <45 mL/min/1.73 m2) who received a diagnosis during follow-up was consistently higher than patients with initial eGFR values indicative of stage 3a CKD (≥45 and <60 mL/min/1.73 m2; figure 4).
Figure 4.
Kaplan-Meier estimates of time to CKD diagnosis according to country and database in patients undiagnosed at index, overall and by CKD stage (3a/3b). Shaded areas represent 95% confidence intervals. CKD, chronic kidney disease; LCED, Explorys Linked Claims and Electronic Medical Records Data.
Among all patients undiagnosed at index (regardless of whether they received a diagnosis during follow-up), median time to diagnosis was only calculable using the Kaplan-Meier method for the US TriNetX database, because more than half of the patients in the other databases remained undiagnosed at the end of the study period. In this database, the overall median (IQR) time to diagnosis was 4.75 (4.68–4.82) years.
After adjusting for selected baseline covariates, in all countries, female patients (vs male patients) and patients with stage 3a CKD at index (vs 3b) were more likely to be diagnosed later during follow-up (online supplemental figure 4). Although less pronounced, patients without a history of comorbidities such as diabetes, heart failure or hypertension had a slightly elevated likelihood of delayed diagnosis (vs patients with a history of these conditions). Older patients also typically had a greater likelihood of delayed diagnosis than patients aged less than 45 years.
Discussion
REVEAL-CKD is a large, multinational, observational study that uses a consistent, strict definition for undiagnosed CKD based on internationally recognised guidelines. By extracting data from contemporary, country-specific databases, the study provides a robust estimate of the prevalence of undiagnosed CKD in countries across the globe. The results from this analysis of six databases from five countries (France, Germany, Italy, Japan and the USA) demonstrate severe shortcomings in the diagnosis of stage 3 CKD. Although there was some variability among countries, the consistently high proportions of undiagnosed stage 3 CKD despite clinical evidence of the disease are highly concerning, as are the low levels of UACR testing. Of note, except in Japan, the prevalence of UACR testing did not appear to be substantially higher even in patients with a diagnosis of stage 3 CKD. UACR testing, however, is necessary for assessing the risk of future progression to kidney failure.28 Missing opportunities for early diagnosis, prognostic assessment and management leaves patients at greater risk of further disease progression and complications, including end-stage renal disease and cardiovascular events.6 29–31 Early interventions in CKD have been shown to improve outcomes by slowing CKD progression and reducing cardiovascular risk,6 32 and healthcare costs associated with the disease increase substantially as CKD stage advances.33 It is, therefore, vital for clinicians to seize the opportunity to diagnose and manage the condition as early as possible to minimise the impact of the disease, both in terms of financial burden and effects on health-related quality of life.
It is reassuring that the patients who have comorbidities that are established risk factors for CKD, such as hypertension and T2D, had higher rates of diagnosis and tended to be diagnosed sooner than patients without these conditions. However, even in patients with these comorbidities, the prevalence of undiagnosed CKD remained high. In the US databases, which had the lowest rates of undiagnosed CKD, approximately 50% of patients with comorbidities in addition to CKD still lacked a CKD diagnosis. Alarmingly, this was the case for patients with hypertension, T2D and established cardiovascular disease: groups in which KDIGO recommends screening for CKD,6 owing to their elevated risks of CKD progression and associated complications.34–36 Without an appropriate CKD diagnosis, opportunities may also be missed to prescribe newer therapies such as sodium-glucose cotransporter-2 inhibitors which have been shown to improve cardiorenal outcomes in patients with CKD.37 38
We observed that the prevalence of undiagnosed CKD tended to rise with age, and older patients tended to have a higher risk of increased diagnostic delay than younger patients. In elderly patients, physicians may assume that eGFR values indicative of stage 3 CKD are caused by age-related decline of kidney function.39 40 However, large population-based studies indicate that even in older adults at lower risk for kidney failure, stage 3 CKD is associated with an elevated risk of mortality, cardiovascular events and acute kidney injury.41 Accordingly, KDIGO guidelines support the use of a single threshold value to define CKD across age subgroups consistent with criteria for other chronic non-communicable diseases.5 In elderly patients, the effects of late-stage CKD are likely to have a substantial influence on physical and cognitive abilities, medication safety and cardiovascular prognosis.2 6 41 It is therefore important that physicians do not underestimate the burden and effects of CKD in elderly patients and initiate guideline-appropriate management in a timely manner. Existing clinical tools (such as confirmatory cystatin C testing in suspected cases of CKD) can help mitigate the risk of overdiagnosis, although these remain underutilised.6 CKD management in elderly patients should be adapted taking into consideration factors such as their age, frailty, comedications and comorbidities.
In line with previous studies that suggest CKD is more prevalent in women than in men,42 43 the proportion of female patients with stage 3 CKD was higher than in male patients in all countries except Japan. Despite the higher prevalence of CKD in female patients, after adjusting for potential confounding factors, female patients had a higher likelihood of being undiagnosed than male patients in all countries. It has been suggested that the rate of progression of CKD is slower in women than in men,44–47 and physicians may, therefore, be less likely to diagnose the condition at early stages in women. However, the inequality demonstrated in this study is substantial and suggests a need for elevated awareness to minimise this gender disparity.
REVEAL-CKD used the internationally recognised CKD-EPI equation to calculate eGFR values from available serum creatinine measurements.23 Multiple consecutive eGFR measurements indicative of stage 3 CKD were required to confirm the presence of CKD, in line with KDIGO recommendations suggesting a threshold of >90 days to consider the condition to be chronic.5 This decision was made to conform to these widely used guidelines, and to avoid overestimating the prevalence of undiagnosed stage 3 CKD by including patients who had isolated eGFR measurements within the threshold of inclusion for stage 3 CKD (as a result of, for example, transient dehydration or acute kidney injury). To investigate the potential impact of requiring two qualifying eGFR measurements for inclusion in REVEAL-CKD, a sensitivity analysis was performed using the TriNetX database that included patients with at least one eGFR measurement indicative of stage 3 CKD. Among these patients, the prevalence of undiagnosed stage 3 CKD was higher than in the main REVEAL-CKD cohort (82.2% vs 64.3%, respectively), whereas the prevalence of comorbidities was lower. This suggests that the requirement of multiple eGFR measurements may have biased the sample to select for patients with inherently poorer health status, because they may have been receiving more frequent healthcare visits than those with a single measurement, and therefore, may have had more eGFR measurements taken. Although it is difficult to confirm which patients in this sensitivity analysis truly had stage 3 CKD and which patients were included as a result of transient eGFR dips, it should be noted that these findings suggest that the true prevalence of undiagnosed stage 3 CKD may be even higher than identified in this study.
When calculating eGFR, race was not included as a modifier in line with recent trends among physicians24 25 and guidance from expert recommendations.26 Inclusion of the race modifier may have been expected to inflate eGFR in Black patients. Indeed, in a sensitivity analysis performed on the US TriNetX database which included data on race (online supplemental table 8), we saw that a substantial proportion of Black patients (46.1%, corresponding to 9.2% of the overall TriNetX cohort) were reclassified as having stage 2 CKD (eGFR between 60 and 89 mL/min/1.73 m2) when the race modifier was included in the calculation of eGFR. The inclusion of this modifier may, therefore, allow CKD to progress further in Black patients before they receive appropriate diagnosis and intervention. The decision to use the CKD-EPI equation without race was made in part to facilitate comparisons among countries and databases in which race was not available, and also to provide a consistent method of calculating eGFR for measurements taken across a time period where the inclusion of the race modifier was being actively debated.48–52
Some limitations must be kept in mind when interpreting these data. Results from the included countries may not be generalisable to other countries, which could have significantly different diagnostic coding practices, healthcare systems and screening policies; conclusions regarding the observed differences between countries cannot be drawn for similar reasons. The TriNetX and LCED databases contained a high proportion of commercially insured patients, and therefore, may not be representative of the overall US population. Furthermore, data licensing issues prevented the pooling of data from multiple databases to provide an overall estimate of the prevalence of undiagnosed CKD. Confirmatory UACR testing was not necessary to meet the study definition of stage 3 CKD owing to the extremely low levels of UACR testing in most of the cohorts. For the same reason, UACR testing was not included in the multivariate analyses which assessed factors associated with a lack of CKD diagnosis and factors associated with time to CKD diagnosis. The proportion of inpatient versus outpatient encounters was unavailable for many of the databases used, and therefore comparisons between diagnoses in these two settings could not be made. Because many of the databases used did not include data on race, variability in the prevalence of undiagnosed CKD according to race could not be assessed. Because data were collected from between 2015 and 2020, physicians may have still been using the race modifier for Black patients. Therefore, some Black patients may have been classified as having stage 2 CKD and have been less likely to receive a diagnosis as a result. It is important to note that this study focused on underdiagnosis for stage 3 CKD; low levels of UACR testing in all countries studied suggest that the prevalence of undiagnosed stage 1 and 2 CKD may be even higher. Lastly, there is a risk of misclassification if CKD diagnoses were made in clinical settings that do not contribute to the databases, or if patients had CKD that was recognised by their healthcare providers but was not recorded with an appropriate ICD-9/10 code in the databases. Although a lack of such codes may not always indicate that a patient’s CKD is undiagnosed, this definition of CKD diagnosis has been validated by previous real-world studies,8 11 12 27 and provides an appropriate surrogate measure for rates of diagnosis in large epidemiological studies such as REVEAL-CKD.
In conclusion, this analysis of six large, secondary databases from five countries demonstrates that most cases of stage 3 CKD are not diagnosed in a timely manner despite clinical evidence of the disease. Furthermore, although patients with existing risk factors for, or complications from, CKD were typically more likely to receive a CKD diagnosis, the prevalence of undiagnosed CKD in these patients remained alarmingly high. Clear opportunities exist for improved diagnosis of stage 3 CKD, particularly in female patients, elderly patients and patients at high risk of CKD progression and complications. Future research will help to quantify the impact of early diagnosis and initiation of effective therapies on the risk of CKD progression, complications and long-term patient outcomes.
Supplementary Material
Acknowledgments
Medical writing support was provided by Bobby Thompson, MSc(Res), of Oxford PharmaGenesis, Oxford, UK, and was funded by AstraZeneca. Analysis of data from the IQVIA Longitudinal Patient Database was conducted by Claudio Ripellino and Franca Heiman of IQVIA Solutions S.r.l., Milan, Italy, and was funded by AstraZeneca.
Footnotes
Contributors: NT, SB, EP, EW, HC, KJ and PK were responsible for the study concept and design. EP had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. MA, EP and HC developed and conducted the statistical analysis plan. NT, TM, MPS, JBV, LDN, MA, SB, EP, EW, HC, KJ and PK were involved in review and editing of manuscript drafts, as well as critical revision of the content during its development. All authors approved the final version of the manuscript before its submission. As the guarantor, NT accepts full responsibility for the work and conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author (NT) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: REVEAL-CKD is funded by AstraZeneca (grant number N/A). It is a non-interventional observational study, and as such, no drugs are supplied or funded. AstraZeneca designed the REVEAL-CKD study with input and guidance from the external authors. AstraZeneca provided funding for data collection, management and analysis. An AstraZeneca team reviewed this manuscript for scientific accuracy during its development and was allowed to make suggestions. However, the final content, analysis and interpretation of the data was determined by the authors. The decision to submit the data for publication was determined by the authors.
Competing interests: SB, EP, HC, KJ, and EW are employees of AstraZeneca and hold stock options. MA is an employee of AstraZeneca. NT has received grants from AstraZeneca, Boehringer 21 Ingelheim/Eli Lilly and Company, Janssen Pharmaceuticals, Otsuka Pharmaceutical Co, Ltd and Tricida, has received honoraria from AstraZeneca, Boehringer Ingelheim/Eli Lilly and Company, Janssen Pharmaceuticals, Otsuka Pharmaceutical and Tricida and holds stock options from Mesentech, Rénibus Therapeutics, pulseData and Tricida. MPS has received advisory board fees and honoraria from AstraZeneca, Bayer AG, Vifor Pharma Group and Boehringer Ingelheim/Eli Lilly and Company. LDN has received fees for scientific consultation and/or lectures by Astellas Pharma, AstraZeneca, Mundipharma and Vifor Pharma Group. PK has received speaker’s bureau and advisory board fees from AstraZeneca, Eli Lilly and Company and Novo Nordisk A/S, speaker’s fees from Bayer AG and honoraria from AstraZeneca and Eli Lilly and Company. TM and JBV have no conflicts of interest to disclose.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data used in this study were obtained from a third party and may not be publicly available. Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives – a position statement from Kidney Disease: Improving Global Outcomes. Kidney Int 2007;72:247–59. 10.1038/sj.ki.5002343 [DOI] [PubMed] [Google Scholar]
- 2.Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 2020;395:709–33. 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie Y, Bowe B, Mokdad AH, et al. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018;94:567–81. 10.1016/j.kint.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 4.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013;382:339–52. 10.1016/S0140-6736(13)60595-4 [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) CKD work group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 6.Shlipak MG, Tummalapalli SL, Boulware LE, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 2021;99:34–47. 10.1016/j.kint.2020.10.012 [DOI] [PubMed] [Google Scholar]
- 7.Fraser SD, Blakeman T. Chronic kidney disease: identification and management in primary care. Pragmat Obs Res 2016;7:21–32. 10.2147/POR.S97310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravera M, Noberasco G, Weiss U, et al. CKD awareness and blood pressure control in the primary care hypertensive population. Am J Kidney Dis 2011;57:71–7. 10.1053/j.ajkd.2010.08.022 [DOI] [PubMed] [Google Scholar]
- 9.Gasparini A, Evans M, Coresh J, et al. Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant 2016;31:2086–94. 10.1093/ndt/gfw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan TP, Sloand JA, Winters PC, et al. Chronic kidney disease prevalence and rate of diagnosis. Am J Med 2007;120:981–6. 10.1016/j.amjmed.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 11.Diamantidis CJ, Hale SL, Wang V, et al. Lab-based and diagnosis-based chronic kidney disease recognition and staging concordance. BMC Nephrol 2019;20:357. 10.1186/s12882-019-1551-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakris G. Prevalence and factors associated with undiagnosed chronic kidney disease in diabetes mellitus. National Kidney Foundation 2019 Spring Clinical Meetings; Boston, MA, USA, 2019 [Google Scholar]
- 13.Centers for Medicare and Medicaid Services . Chronic kidney disease often undiagnosed in Medicare beneficiaries. 2020. Available: https://www.cms.gov/files/document/ckd-data-highlight102020-2.pdf [Accessed Nov 2022].
- 14.Tuttle KR, Alicic RZ, Duru OK, et al. Clinical characteristics of and risk factors for chronic kidney disease among adults and children: an analysis of the CURE-CKD registry. JAMA Netw Open 2019;2:e1918169. 10.1001/jamanetworkopen.2019.18169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szczech LA, Stewart RC, Su H-L, et al. Primary care detection of chronic kidney disease in adults with type-2 diabetes: the ADD-CKD study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease). PLoS One 2014;9:e110535. 10.1371/journal.pone.0110535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kushner P, Peach E, Wittbrodt E, et al. Investigating the global prevalence and consequences of undiagnosed stage 3 chronic kidney disease: methods and rationale for the REVEAL-CKD study. Clin Kidney J 2022;15:738–46. 10.1093/ckj/sfab235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cegedim Health Data . THIN: The Health Improvement Network. 2021. Available: https://www.cegedim-health-data.com/cegedim-health-data/thin-the-health-improvement-network [Accessed Nov 2022].
- 18.Rathmann W, Bongaerts B, Carius H-J, et al. Basic characteristics and representativeness of the German Disease Analyzer database. Int J Clin Pharmacol Ther 2018;56:459–66. 10.5414/CP203320 [DOI] [PubMed] [Google Scholar]
- 19.Health Search . XV report healthsearch [Italian]. 2022. Available: https://report.healthsearch.it/ [Accessed Nov 2022].
- 20.Ono Y, Taneda Y, Takeshima T, et al. Validity of claims diagnosis codes for cardiovascular diseases in diabetes patients in Japanese administrative database. Clin Epidemiol 2020;12:367–75. 10.2147/CLEP.S245555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hensley Alford S, Piccone J, Sexton M, et al. Watson health: a new approach to population health and research. JPCRR 2016;3:201. 10.17294/2330-0698.1346 [DOI] [Google Scholar]
- 22.TriNetX . TriNetX research. 2021. Available: https://trinetx.com/ [Accessed Mar 2021].
- 23.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duggal V, Thomas I-C, Montez-Rath ME, et al. National estimates of CKD prevalence and potential impact of estimating glomerular filtration rate without race. J Am Soc Nephrol 2021;32:1454–63. 10.1681/ASN.2020121780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diao JA, Wu GJ, Taylor HA, et al. Clinical implications of removing race from estimates of kidney function. JAMA 2021;325:184–6. 10.1001/jama.2020.22124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol 2021;32:2994–3015. 10.1681/ASN.2021070988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkelmayer WC, Schneeweiss S, Mogun H, et al. Identification of individuals with CKD from Medicare claims data: a validation study. Am J Kid Dis 2005;46:225–32. 10.1053/j.ajkd.2005.04.029 [DOI] [PubMed] [Google Scholar]
- 28.Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011;305:1553–9. 10.1001/jama.2011.451 [DOI] [PubMed] [Google Scholar]
- 29.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011;79:1331–40. 10.1038/ki.2010.550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011;79:1341–52. 10.1038/ki.2010.536 [DOI] [PubMed] [Google Scholar]
- 31.Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015;3:514–25. 10.1016/S2213-8587(15)00040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smart NA, Titus TT. Outcomes of early versus late nephrology referral in chronic kidney disease: a systematic review. Am J Med 2011;124:1073–80. 10.1016/j.amjmed.2011.04.026 [DOI] [PubMed] [Google Scholar]
- 33.Dieguez G, Smith R. The impact of earlier CKD detection and delayed disease progression. 2021. Available: https://www.milliman.com/-/media/milliman/pdfs/2021-articles/7-13-21-the_impact_of_earlier_ckd_detection_and_delayed.ashx [Accessed Nov 2022].
- 34.Rahman M, Xie D, Feldman HI, et al. Association between chronic kidney disease progression and cardiovascular disease: results from the CRIC study. Am J Nephrol 2014;40:399–407. 10.1159/000368915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cabrera CS, Lee AS, Olsson M, et al. Impact of CKD progression on cardiovascular disease risk in a contemporary UK cohort of individuals with diabetes. Kidney Int Rep 2020;5:1651–60. 10.1016/j.ekir.2020.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovesdy CP, Isaman D, Petruski-Ivleva N, et al. Chronic kidney disease progression among patients with type 2 diabetes identified in US administrative claims: a population cohort study. Clin Kidney J 2021;14:1657–64. 10.1093/ckj/sfaa200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fontes-Carvalho R, Santos-Ferreira D, Raz I, et al. Protective effects of SGLT-2 inhibitors across the cardiorenal continuum: two faces of the same coin. Eur J Prev Cardiol 2022;29:1352–60. 10.1093/eurjpc/zwab034 [DOI] [PubMed] [Google Scholar]
- 38.Rangaswami J, Bhalla V, de Boer IH, et al. Cardiorenal protection with the newer antidiabetic agents in patients with diabetes and chronic kidney disease: a scientific statement from the American Heart Association. Circulation 2020;142:e265–86. 10.1161/CIR.0000000000000920 [DOI] [PubMed] [Google Scholar]
- 39.Glassock RJ, Rule AD. Aging and the kidneys: anatomy, physiology and consequences for defining chronic kidney disease. Nephron 2016;134:25–9. 10.1159/000445450 [DOI] [PubMed] [Google Scholar]
- 40.Schmitt R, Melk A. Molecular mechanisms of renal aging. Kidney Int 2017;92:569–79. 10.1016/j.kint.2017.02.036 [DOI] [PubMed] [Google Scholar]
- 41.De Nicola L, Minutolo R, Chiodini P, et al. The effect of increasing age on the prognosis of non-dialysis patients with chronic kidney disease receiving stable nephrology care. Kidney Int 2012;82:482–8. 10.1038/ki.2012.174 [DOI] [PubMed] [Google Scholar]
- 42.Carrero JJ, Hecking M, Chesnaye NC, et al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 2018;14:151–64. 10.1038/nrneph.2017.181 [DOI] [PubMed] [Google Scholar]
- 43.Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS ONE 2016;11:e0158765. 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silbiger S, Neugarten J. Gender and human chronic renal disease. Gend Med 2008;5 Suppl A:S3–10. 10.1016/j.genm.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 45.Jafar TH, Schmid CH, Stark PC, et al. The rate of progression of renal disease may not be slower in women compared with men: a patient-level meta-analysis. Nephrol Dial Transplant 2003;18:2047–53. 10.1093/ndt/gfg317 [DOI] [PubMed] [Google Scholar]
- 46.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int 2006;69:375–82. 10.1038/sj.ki.5000058 [DOI] [PubMed] [Google Scholar]
- 47.Swartling O, Rydell H, Stendahl M, et al. CKD progression and mortality among men and women: a nationwide study in Sweden. Am J Kidney Dis 2021;78:190–9. 10.1053/j.ajkd.2020.11.026 [DOI] [PubMed] [Google Scholar]
- 48.Diao JA, Inker LA, Levey AS, et al. In search of a better equation-performance and equity in estimates of kidney function. N Engl J Med 2021;384:396–9. 10.1056/NEJMp2028243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA 2019;322:113–4. 10.1001/jama.2019.5774 [DOI] [PubMed] [Google Scholar]
- 50.Norris KC, Eneanya ND, Boulware LE. Removal of race from estimates of kidney function: first, do no harm. JAMA 2021;325:135–7. 10.1001/jama.2020.23373 [DOI] [PubMed] [Google Scholar]
- 51.Powe NR. Black kidney function matters: use or misuse of race? JAMA 2020;324:737–8. 10.1001/jama.2020.13378 [DOI] [PubMed] [Google Scholar]
- 52.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight-reconsidering the use of race correction in clinical algorithms. N Engl J Med 2020;383:874–82. 10.1056/NEJMms2004740 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067386supp001.pdf (605.6KB, pdf)
Data Availability Statement
Data used in this study were obtained from a third party and may not be publicly available. Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.