Abstract
Introduction
Intensive lifestyle intervention (ILI) has significantly reduced incidence of diabetes and improved many cardiovascular disease risk factors. We evaluated long-term effects of ILI on cardiometabolic risk factors, and microvascular and macrovascular complications among patients with diabetes in real-world clinical practice.
Research design and methods
We evaluated 129 patients with diabetes and obesity enrolled in a 12-week translational model of ILI. At 1 year, we divided participants into group A, who maintained <7% weight loss (n=61, 47.7%), and group B, who maintained ≥7% weight loss (n=67, 52.3%). We continued to follow them for 10 years.
Results
The total cohort lost an average of 10.8±4.6 kg (−9.7%) at 12 weeks and maintained an average weight loss of 7.7±10 kg (−6.9%) at 10 years. Group A maintained 4.3±9.5 kg (−4.3%) and group B maintained 10.8±9.3 kg (−9.3%) of weight loss at 10 years (p<0.001 between groups). In group A, A1c decreased from 7.5±1.3% to 6.7±0.9% at 12 weeks but rebounded to 7.7±1.4% at 1 year and 8.0±1.9% at 10 years. In group B, A1c decreased from 7.4±1.2% to 6.4±0.9% at 12 weeks then increased to 6.8±1.2% at 1 year and 7.3±1.5% at 10 years (p<0.05 between groups). Maintenance of ≥7% weight loss at 1 year was associated with a 68% lower risk of developing nephropathy for up to 10 years compared with maintenance of <7% weight loss (adjusted HR for group B: 0.32, 95% CI 0.11, 0.9, p=0.007).
Conclusions
Weight reduction in patients with diabetes can be maintained for up to 10 years in real-world clinical practice. Sustained weight loss is associated with significantly lower A1c at 10 years and improvement in lipid profile. Maintenance of ≥7% weight loss at 1 year is associated with decreased incidence of diabetic nephropathy at 10 years.
Keywords: weight management, lifestyle intervention(s), microvascular complications, diabetes complications
WHAT IS ALREADY KNOWN ON THIS TOPIC
Intensive lifestyle intervention (ILI) is known to improve cardiometabolic, macrovascular and microvascular risk factors in patients with diabetes and obesity.
Maintaining ≥7% weight loss after 1 year following an initial 12-week ILI may predict the ability to sustain long-term weight loss in patients with obesity and diabetes.
WHAT THIS STUDY ADDS
Significant weight reduction in patients with diabetes and obesity can be maintained for up to 10 years in real-world clinical practice.
Maintenance of weight loss is associated with lower incidence of developing diabetic nephropathy for up to 10 years.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study may affect practice guidelines of diabetes care and its complications as it highlights the importance of lifestyle modification in this population over long periods of time.
Our data support findings from the Action for Health in Diabetes (Look AHEAD) study and validate the benefits of lifestyle modification on renal outcomes in real-world clinical practice.
Introduction
Diabetes prevalence has grown rapidly to be a global public health issue.1 2 Diabetes-related complications incur heavy burdens on patients, families and healthcare systems.3 4 The USA spent nearly $404 billion on diabetes, pre-diabetes and gestational diabetes in 2017 accounting for almost $1240 per American.5 Lifestyle modification with diet and exercise is cornerstone to the management of patients with diabetes and overweight or obesity.6 Previous studies demonstrated that lifestyle intervention significantly reduced incidence of diabetes and improved many cardiovascular disease (CVD) risk factors.7–10 The landmark Action for Health in Diabetes (Look AHEAD) study demonstrated that weight loss through intensive lifestyle intervention (ILI) initially modified many CVD risk factors except for low-density lipoprotein cholesterol (LDL-C); however, there was no association between weight loss and reduction in rates of cardiovascular events or mortality.9 A more recent large nested control study linked postbariatric surgery weight reduction in patients with type 2 diabetes (T2D) to lower incidence microvascular complications including neuropathy, nephropathy, and retinopathy compared with usual care.11 Moreover, there is evidence that transient periods of improved glycemic control may confer benefits on long-term incidence of microvascular and macrovascular diseases despite recurrence of poor glycemic control.12 13 These prolonged benefits of short-term glycemic control were termed the ‘metabolic memory’ which may extend to beyond 10 years.14 We previously reported that ILI in real-world clinical practice can lead to long-term benefits in glycemia and lipidemia predicted by the ability to achieve ≥7% weight loss at 1 year.15–18 However, it is not fully understood if significant weight loss after ILI can be maintained beyond 5 years or influences the development of diabetes-related microvascular and macrovascular complications, and whether this relationship is independent of A1c worsening and/or weight regain. In this longitudinal study of the same cohort, we evaluated the 10-year follow-up outcomes of A1c, lipid profile, blood pressure (BP), and microvascular complications in response to whether participants lost or regained weight at 1 year after ILI.
Methods
The ILI program
A detailed description of the ILI program has been previously published.19 In brief, the Weight Achievement and Intensive Treatment (Why WAIT) is a 12-week multidisciplinary program for weight reduction and intensive diabetes management designed and implemented at the Joslin Diabetes Center in Boston, Massachusetts, since September 2005. Participants in the Why WAIT program were patients with body mass index (BMI) between 30 and 45 kg/m2 and with type 1 diabetes or T2D. Each participant underwent a comprehensive evaluation by the intervention team (diabetologist, registered dietician, clinical exercise physiologist, and psychologist or social worker). Eligible patients were enrolled in groups of 10–15 participants each.
Study subjects and design
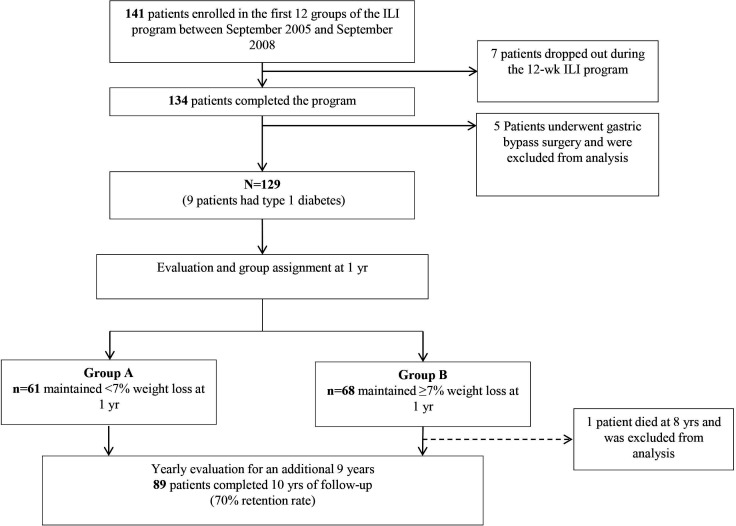
In the 5-year outcomes study of this cohort previously published by our group, we included detailed information on study design.15 A total of 128 participants (figure 1) were divided into group A, who maintained <7% weight loss (n=61, 47.7%), and group B, who maintained ≥7% weight loss (n=67, 52.3%). We selected 1 year as a starting point for group allocation to compare long-term weight loss because weight rebound is common directly after ILI. In this context, data at 1 year were collected retrospectively; however, all analyses up to 10 years were performed on prospective data. Microvascular and macrovascular outcomes were analyzed using retrospective data since those outcomes were not prespecified in our initial analysis plan.
Figure 1.
Flow chart of participants during the 10-year follow-up after initial 12-week intensive lifestyle intervention (ILI) using the Weight Achievement and Intensive Treatment (Why WAIT) model in clinical practice.
Cardiometabolic outcomes
Key cardiometabolic parameters were measured at baseline, after 12 weeks of ILI, then routinely at patients’ clinic visits over 10 years which occurred every 3–6 months. Body weight was captured weekly during the intervention then routinely during clinic visits over the following 10 years. Biochemical assessment was performed preoperatively or at annual clinic visits.
Microvascular and macrovascular complications
Data on nephropathy, retinopathy, and neuropathy development were derived retrospectively from electronic medical records. Nephropathy diagnosis was based on the definition of the Kidney Disease Improving Global Outcomes 2012 guidelines for chronic kidney disease (CKD) diagnosed by two separate measurements of urinary albumin excretion >30 µg/mg more than 3 months apart, sustained glomerular filtration rate (GFR) of less than 60 mL/min/1.73 m2 for more than 3 months and/or a urine albumin-creatinine ratio of 30 mg/g or greater for more than 3 months.20 Microalbuminuria was defined by a ratio between 30 and 300 µg/mg and macroalbuminuria as >300 µg/mg. Urine measurements of albumin/creatinine ratio were performed on a first-morning-void midstream urine sample. Retinopathy was defined based on International Classification of Diseases Ninth Revision (ICD-9) diagnosis codes 362.0x (diabetic retinopathy) and 250.5x (diabetes with ophthalmic manifestations). Neuropathy was defined based on first occurrence of ICD-9 diagnosis codes 250.6 (diabetes with neurological manifestations) and 357.2 (polyneuropathy in diabetes). Macrovascular disease was defined based on ICD-9 diagnosis codes 414.00 (coronary artery disease, unspecified), 412.00 (old myocardial infarction), 414.4 (coronary atherosclerosis due to calcified coronary lesion), 414.9 (chronic ischemic heart disease), 429.20 (CVD, unspecified), 428.00 (congestive heart failure), and 433.9 (peripheral vascular disease).
Study endpoints
Coprimary endpoints were changes in body weight and A1c at 10 years. Secondary endpoints included changes in lipid profile and BP at 10 years and incidence of nephropathy, retinopathy, neuropathy, CVD, and peripheral vascular disease during the 10 years of follow-up. All included variables were assessed at least every 6 months. If data were missing, last data carried forward were used.
Statistical analysis
This study was designed on an intent-to-treat principle and all available data were used for this analysis. There was no evidence that missing data were dependent on the study group. An unstructured covariance matrix was used to account for correlation between repeated outcomes. Χ2 test, Fisher’s exact test and two-sample t-tests were used to compare baseline characteristics and endpoints in the two study groups. Incident rates of diabetic nephropathy, retinopathy and neuropathy were calculated using the STATA (ir) procedure. Cox proportional hazards models were used to calculate HRs after dichotomizing the incidence of diabetic nephropathy, retinopathy, and neuropathy at each time point over the 10-year follow-up. We started with an unadjusted model. We then fitted a model with terms for five candidate covariates (sex, age, diabetes type, diabetes duration, and BMI) in addition to group assignment. Given the established sex disparities associated with incidence of diabetic nephropathy, we fitted a model with sex and group assignment only before fitting a fully adjusted model with the four remaining covariates. Data are expressed as mean (SD), mean (SEM) or n (%). All analyses were performed using STATA Special Edition V.15.0 for Windows (StataCorp, College Station, Texas, USA, 2017). In all tests, p<0.05 was considered statistically significant.
Results
Group A included 61 patients (47.7%) and group B included 67 patients (52.3%) (figure 1). Both groups were equally distributed within the 12-week ILI groups conducted over 3 years. At baseline, there were no differences between the two groups in A1c, BP, lipid profile, renal function (serum creatinine, blood urea nitrogen, estimated GFR (eGFR) and urinary microalbumin/creatinine ratio) or prevalence of CKD, current smoking status, or prevalence of macrovascular disease, nephropathy, retinopathy, neuropathy, or two or more microvascular complications. Group B had higher BMI compared with group A (39.3±5.1 kg/m2 vs 37.3±5.2 kg/m2) (table 1).
Table 1.
Baseline characteristics of the participants in the intensive lifestyle intervention
| All participants | Group A (n=61) | Group B (n=67) | P value | |
| Age (years) | 53.7±10.1 | 53.3±9.3 | 54.1±10.9 | 0.63 |
| Female sex, n (%) | 85 (66.4) | 44 (72.1) | 41 (61.2) | 0.2 |
| Duration of diabetes (years) | 9.6±9.4 | 9.0±9.1 | 10.0±9.7 | 0.61 |
| Body weight (kg) | 110.3±19 | 104.8±16.9 | 115.4±19.7 | 0.0014 |
| Body mass index (kg/m2) | 38.4±5.2 | 37.3±5.2 | 39.4±5.1 | 0.025 |
| HbA1c (%) | 7.43±1.23 | 7.47±1.33 | 7.41±1.14 | 0.8 |
| Systolic BP (mm Hg) | 128.3±14.6 | 126.3±14.1 | 130.1±15 | 0.13 |
| Diastolic BP (mm Hg) | 75.7±8.1 | 75.6±8.7 | 76.0±7.5 | 0.77 |
| Total cholesterol (mg/dL) | 167±32 | 165±31 | 168±34 | 0.6 |
| LDL-cholesterol (mg/dL) | 99.5±30 | 97.1±28.1 | 101.5±31.1 | 0.4 |
| HDL-cholesterol (mg/dL) | 43.4±10 | 44.6±10.2 | 42.3±9.9 | 0.2 |
| Triglycerides (mg/dL) | 137.4±73.2 | 128.2±60.3 | 145.6±82.7 | 0.17 |
| Serum creatinine (mg/dL) | 0.92±0.19 | 0.91±0.19 | 0.92±0.18 | 0.82 |
| Blood urea nitrogen (mg/dL) | 17.4±5.5 | 16.9±4.8 | 17.8±5.9 | 0.37 |
| Urinary microalbumin/creatinine ratio (μg/mg) | 30.1±56.7 | 22.5±26.4 | 36.7±73.8 | 0.17 |
| eGFR (mL/min/1.73 m2) | 83.6±25.4 | 83.6±24.6 | 83.5±26.2 | 0.97 |
| Smoker, yes, n (%) | 34 (26.6) | 14 (23) | 20 (29.9) | 0.37 |
| Macrovascular complications, n (%) | 13 (10.2) | 5 (8.2) | 8 (11.9) | 0.49 |
| Microvascular complications, n (%) | ||||
| Nephropathy | 35 (27.3) | 13 (21.3) | 22 (32.8) | 0.14 |
| Retinopathy | 22 (17.2) | 7 (11.5) | 15 (22.4) | 0.1 |
| Neuropathy | 16 (12.5) | 7 (11.5) | 9 (13.4) | 0.73 |
Data are mean (SD) or n (%). Total group, N=128. Group A, n=61 (participants maintained <7% weight loss at 1 year). Group B, n=67 (participants maintained ≥7% weight loss at 1 year). P value for between groups using Student’s t-test or Pearson’s χ2 test. Macrovascular complications were defined based on ICD-9 codes (414.00, 412.00, 414.4, 414.9, 429.20, 428.00, and 433.9).
BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Cardiometabolic outcomes
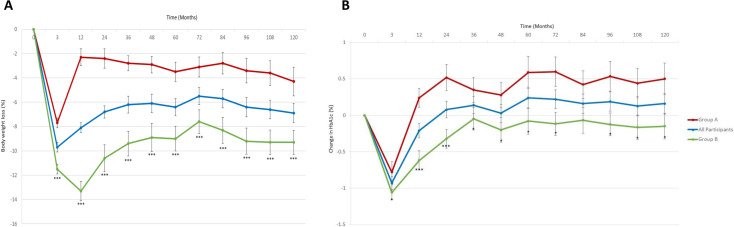
The total cohort lost an average of 10.8±0.4 kg (−9.7±0.4%) at 12 weeks and maintained an average weight loss of 7.7±0.9 kg (−6.9±0.8%) at 10 years (p<0.001 for both). Group A maintained 4.3±1.2 kg (−4.3±1.17%) and group B maintained 10.8±1 kg (−9.3±1.1%) of weight loss at 10 years (p<0.001 between groups) (table 2 and figure 2). In group A, A1c decreased from 7.5±1.3% to 6.7±0.9% at 12 weeks but rebounded to 7.7±1.4% at 1 year and 8.0±1.9% at 10 years (table 2 and figure 2). In group B, A1c decreased from 7.4±1.2% to 6.4±0.9% at 12 weeks and increased to 6.8±1.2% at 1 year and 7.3±1.4% at 10 years (p<0.05 between groups) (table 2 and figure 2). Despite weight regain, group A maintained significant improvements in LDL-C and high-density lipoprotein cholesterol, had no change in BP, but had significant worsening of serum triglycerides (TG) at 10 years. Group B maintained similar improvements in lipid profile and had a non-significant increase in serum TG at 10 years.
Table 2.
Changes from baseline in metabolic parameters after the 12-week intensive lifestyle intervention and at 1 year and 10 years of follow-up
| All participants | Group A | Group B | P value | |||||||
| 12 weeks | 1 year | 10 years | 12 weeks | 1 year | 10 years | 12 weeks | 1 year | 10 years | ||
| Body weight (kg) | −10.8±0.4*** | −9.1±0.8*** | −7.7±0.9*** | −8±0.4*** | −2.4±0.4*** | −4.3±1.2*** | −13.3±0.5*** | −15.2±0.9*** | −10.8±1.1*** | 0.0002 |
| HbA1c (%) | −0.93±0.08*** | −0.21±0.1* | 0.16±0.14 | −0.78±0.14*** | 0.24±0.12 | 0.5±0.22* | −1.06±0.1*** | −0.62±0.13*** | −0.15±0.17 | 0.035 |
| Systolic BP (mm Hg) | 121.8±13.5*** | 126.8±14.8* | 127.7±15.9 | 122.0±12.8* | 130.7±14.4 | 129.0±17.5 | 121.7±14.2** | 123.2±14.4* | 126.4±14.2 | 0.046 |
| Diastolic BP (mm Hg) | 73.2±9.3** | 74.0±8.3 | 74.0±9.1 | 74.1±7.4 | 76.0±7.4 | 74.3±8.9 | 72.3±10.7* | 72.1±8.8* | 73.6±9.4 | 0.56 |
| Total cholesterol (mg/dL) | 148.2±31.2*** | 165.2±34.4 | 160.1±35.2* | 150.7±28.6*** | 169.4±34.9 | 161.7±32.6 | 145.9±33.4** | 161.4±33.7 | 158.7±37.6* | 0.32 |
| LDL-cholesterol (mg/dL) | 87.9±28.9*** | 94.5±27.5* | 80.7±29.6*** | 87.8±26.5** | 94.8±27.2 | 81.2±28.9*** | 87.9±31.0*** | 94.3±28.0** | 80.2±30.4*** | 0.4 |
| HDL-cholesterol (mg/dL) | 42.2±10.1 | 48.1±12.5*** | 52.1±17.3*** | 44.1±10.8 | 50.0±13.8** | 53.0±17.1*** | 40.4±9.3 | 46.4±11.0** | 51.2±17.7** | 0.9 |
| Triglycerides (mg/dL) | 100.9±48.8*** | 143.2±84.6* | 162.6±88.8** | 101.2±46.9*** | 151.5±73.1** | 159.7±76.0** | 100.7±50.8*** | 135.6±93.7* | 165.2±99.5 | 0.48 |
| Serum creatinine (mg/dL) | 0.92±0.19 | 0.91±0.22 | 0.88±0.28 | 0.93±0.21 | 0.90±0.22 | 0.90±0.33 | 0.92±0.17 | 0.92±0.21 | 0.87±0.23 | 0.32 |
| Blood urea nitrogen (mg/dL) | 17.7±6.3 | 18.5±11.8 | 18.3±7.0 | 18.2±6.6 | 17.6±6.5 | 19.0±7.9 | 17.2±6.0 | 19.4±15.1 | 17.7±6.0 | 0.056 |
| Urinary microalbumin/creatinine ratio (μg/mg) | 18.2±26.8** | 26.6±46.6 | 94.8±282.9** | 16.8±22.4 | 26.4±46.1 | 124.1±329.7* | 19.4±30.6 | 26.7±47.5 | 67.3±229.9 | 0.16 |
| eGFR (mL/min/1.73 m2) | 81.9±23.6 | 83.6±25.5 | 88.1±37.1 | 81.5±24.2 | 83.9±23.7 | 89.4±43.3 | 82.3±23 | 83.4±27.2 | 87±31 | 0.053 |
| Antidiabetic medications | ||||||||||
| Metformin | 0.76±0.43 | – | 0.71±0.46 | 0.77±0.4 | – | 0.68±0.47 | 0.75±0.4 | – | 0.73±0.45 | 0.79 |
| Sulfonylurea | 0.19±0.4 | – | 0.18±0.39 | 0.18±0.39 | – | 0.16±0.37 | 0.2±0.4 | – | 0.19±0.4 | 0.66 |
| Dipeptidyl peptidase 4 (DPP-4) inhibitors | 0.05±0.23 | – | 0.03±0.17 | 0.05±0.22 | – | 0.05±0.22 | 0.06±0.24 | – | 0.01±0.12 | 0.28 |
| Glucagon-like peptide 1 (GLP-1) receptor agonists | 0.38±0.49 | – | 0.4±0.5 | 0.33±0.47 | – | 0.28±0.45 | 0.43±0.5 | – | 0.5±0.5 | 0.0077 |
| Thiazolidinediones (TZDs) | 0.12±0.32 | – | 0.04±0.19 | 0.08±0.28 | – | 0.03±0.18 | 0.15±0.36 | – | 0.04±0.2 | 0.73 |
| Insulin | ||||||||||
| Short acting | 20.6±36.6 | – | 37.6±62.9 | 19.6±33.6 | – | 44±70.4 | 21.5±39.3 | – | 31.8±55.2 | 0.29 |
| Long acting | 14±29.2 | – | 22.7±36.3 | 11.6±23.2 | – | 19.9±32.8 | 16.3±33.7 | – | 25±39.3 | 0.42 |
Data are mean±SEM. Total group, N=128. Group A, n=61 (participants maintained <7% weight loss at 1 year). Group B, n=67 (participants maintained >7% weight loss at 1 year).
*P<0.05; **p<0.01; ***p<0.001 compared with baseline. P value for group A vs group B at 10 years.
BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Figure 2.
Change in (A) body weight and (B) A1c over 10 years in response to a 12-week intensive lifestyle intervention in real-world clinical practice. Data are mean difference±SEM. All participants, N=128. Group A, n=61 (participants maintained <7% weight loss at 1 year). Group B, n=67 (participants maintained ≥7% weight loss at 1 year). ***P<0.001 (group A vs group B).
Microvascular complications
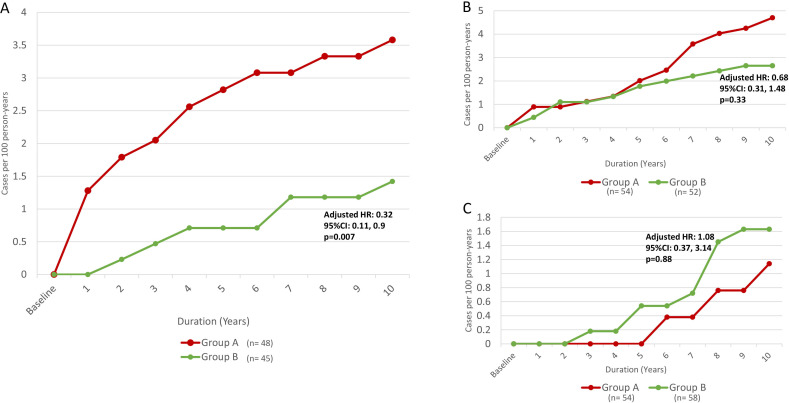
Figure 3 shows incidence rates for each microvascular complication (nephropathy, retinopathy, and neuropathy) during the 10-year follow-up. At 10-year follow-up, there were 14 new cases of nephropathy in group A compared with six cases in group B (3.58 vs 1.42 cases per 100 person-years, respectively; HR for group B: 0.4, 95% CI 0.15, 1.06, p=0.066). When adjusting for male sex, which was proportionally higher in group B versus group A (table 1), the group effect reached statistical significance (sex-adjusted HR for group B: 0.33, 95% CI 0.12, 0.94, p=0.039). Moreover, in a model fitted with terms for age, diabetes type, diabetes duration, BMI, plus group assignment and sex, the group effect retained independent significance (adjusted HR for group B: 0.32, 95% CI 0.11, 0.9, p=0.007) (figure 3).
Figure 3.
Incidence rate of (A) diabetic nephropathy, (B) diabetic retinopathy and (C) diabetic peripheral neuropathy in study groups over 10 years. Group A maintained <7% weight loss at 1 year following the 12-week intensive lifestyle intervention, meanwhile group B maintained ≥7% weight loss at 1 year following the 12-week intensive lifestyle intervention. Adjusted Cox proportional hazards models included terms for five candidate covariates (sex, age, diabetes type, diabetes duration and body mass index (BMI)).
During the 10-year follow-up there were 21 new cases of retinopathy in group A compared with 12 cases in group B (4.69 and 2.65 cases per 100 person-years, respectively; HR for group B: 0.57, 95% CI 0.27, 1.15, p=0.11), whereas six new cases of neuropathy occurred in group A compared with nine cases in group B (1.14 and 1.63 cases per 100 person-years, respectively; HR for group B: 1.45, 95% CI 0.51, 4.08, p=0.47). In fully adjusted models, the results for group effect were similar to those in the unadjusted models for incidence of retinopathy and neuropathy (figure 3), except for an effect for diabetes duration on incidence of retinopathy (HR: 1.08, 95% CI 1.03, 1.13, p=0.003). There was no difference in cumulative incidence of cardiovascular and peripheral vascular diseases between the two groups at 10 years.
Antidiabetic medications and insulin
During the 10-year follow-up period, the use of glucagon-like peptide 1 (GLP-1) receptor agonist significantly decreased in group A compared with group B (p=0.007). There were no significant differences in the use of other antidiabetic medications, short-acting or long-acting insulin between groups (table 2).
Discussion
Cardiometabolic outcomes
We evaluated the 10-year outcomes of cardiometabolic risk factors and incidence of microvascular complications in patients with diabetes after participation in an initial 12-week ILI program in real-world clinical practice. To date, our 5-year outcomes study reported the longest period maintaining weight loss after ILI, with the results of this study showing considerable weight loss in patients with diabetes is maintainable for up to 10 years. Despite the general assumption that weight loss is often difficult to maintain for patients with diabetes and obesity compared with the general population, this study demonstrated that participants were able to maintain an average of 6.9% weight loss after 10 years of an initial 12-week ILI program. Moreover, patients who lost and sustained ≥7% weight loss at 1 year were more likely to maintain a significant 9.3% weight loss after 10 years of follow-up. Although A1c and TG worsened with weight regain, sustained weight loss was associated with significantly lower A1c at 10 years and improvement in lipid profile. These results are consistent with our previous report of the 5-year outcomes in the same cohort.15 The decrease in GLP-1 receptor agonist use in group A participants is not surprising since this group maintained a significant weight loss even after the 10-year period so the need for weight-lowering medications is reduced.
Microvascular outcomes
In our cohort, maintenance of ≥7% weight loss at 1 year was associated with a 68% lower risk of developing nephropathy for up to 10 years compared with maintenance of <7% weight loss. This association was strengthened after adjusting for sex. It is well established that men are at a higher risk of developing diabetic nephropathy compared with women.21 Therefore, it is very possible that the numerically higher proportion of men in group B versus group A might have attenuated the group effect in the unadjusted model. We have previously published that participants in ILI sustained progressive improvement in serum creatinine and eGFR in the same cohort over 5 years irrespective of weight maintenance or weight regain.22 The study also showed that the improvement in eGFR is independent of the improvement in systolic BP or the improvement in glycemic control. We postulated that the improvement in renal function is probably related to the improvement in inflammation consequent to weight reduction.23 There was no significant association between maintenance of ≥7% weight loss at 1 year and incidence of retinopathy or peripheral neuropathy in our cohort regardless of adjustment. However, for each 1-year increase in diabetes duration, there was an 8% increased risk of diabetic retinopathy, independent of group assignment.
Various epidemiological studies have reported the role of cardiometabolic risk factors in the development and progression of diabetes complications.24–30 Poor glycemic control has been considered the major risk factor for the development of microvascular and macrovascular complications. Several large-scale clinical trials demonstrated that intensive use of antihyperglycemic medications could reduce the incidence of microvascular and macrovascular complications in patients with either type 1 diabetes or T2D.12 31 32 Moreover, prolonged tight metabolic control during early periods of diabetes (within 5 years of diagnosis) leads to sustained beneficial effects on latter incidence of microvascular and macrovascular complications.12 31–34 In our study, we found that even in patients with an average diabetes duration of 10±9.7 years at baseline, metabolic memory after short periods of ILI might extend for up to 10 years according to the observed reduced incidence of diabetic nephropathy. Our results are consistent with findings from a secondary analysis of the Look AHEAD study which found that the ILI group had significantly lower incidence rates of nephropathy after a median follow-up of 8 years compared with a diabetes support and education group (HR for ILI: 0.69, 95% CI 0.55 to 0.87, p=0.0016).35 Our findings may be explained by an extended metabolic memory for up to 10 years associated with sustained weight loss. We did not observe any significant differences in the incidence of retinopathy or neuropathy in our groups; however, there was an 8% increased risk of retinopathy with each 1-year increase in diabetes duration, irrespective of group assignment. Although it has been traditionally believed that CKD slowly progresses over years since diabetes diagnosis and that very limited tools are currently available in slowing or preventing deterioration of kidney function, this study provides preliminary data that ILI, even for short periods of time, may have a significant impact on preserving kidney function.
This study has several limitations. First, the lack of a control group that followed standard of diabetes care and support as used in the Look AHEAD study.8 However, the primary purpose of this study is to compare those who sustained weight loss versus those who regained weight, which did not exist in the Look AHEAD analysis. Second, this study was conducted at a single tertiary care center and had a relatively small number of participants that did not allow for survival analysis. Furthermore, this ILI program requires considerable time commitments and financial resources from participants, which may limit the generalizability of these results. Another limitation would be the fact that these data were collected in pre-sodium-glucose cotransporter-2 (SGLT-2) inhibitors era. Thus, it would not be possible to tell if our results can hold nowadays with the wide use of SGLT-2. Meanwhile, use of GLP-1 receptor agonist significantly decreased in group A compared with group B and might have contributed to weight regain in that group. Moreover, there is increased evidence that the use of GLP-1 receptor agonists for long periods may be associated with decreased incidence of cardiovascular and microvascular complications.36 37 Even though it was not widely used in our cohort, the use of GLP-1 receptor agonist might have confounded our results. Finally, data used to define diabetic nephropathy, retinopathy, and neuropathy were collected retrospectively from electronic health records, so missing data might have led to misclassification of microvascular and macrovascular complications.
In conclusion, we found that significant weight reduction in patients with diabetes can be maintained for up to 10 years in real-world clinical practice. A1c and TG worsened with weight regain, while other lipid improvements were maintained. Sustained weight loss is associated with significantly improved A1c at 10 years. Maintenance of ≥7% weight loss is significantly associated with decreased incidence of nephropathy, independent of A1c worsening and weight regain. There were no associations between sustained weight loss and incidences of retinopathy or neuropathy. Larger prospective and randomized clinical studies with longer follow-up periods are needed to confirm our observations.
Acknowledgments
The authors thank the clinical and administrative staff of the Why WAIT program at Joslin Diabetes Center.
Footnotes
Presented at: Data from this work were presented at the 79th and 81st Scientific Sessions of the American Diabetes Association in 2019 and 2021, respectively.
Contributors: ST, HZ, MA-B, and TS collected the data, conducted the statistical analysis, and drafted the manuscript. MWT, SD, and AK collected the data and reviewed and edited the manuscript. OH directs the Why WAIT program, designed the study, supervised the work, and reviewed and edited the manuscript. ST, HZ and OH are guarantors of this work and take responsibility for its integrity and the accuracy of data analysis. All authors approved the final version of the manuscript.
Funding: This is an investigator-initiated study funded internally at Joslin Diabetes Center.
Competing interests: OH receives research support from the National Dairy Council, Eli Lilly and Company, and Novo Nordisk; consults for Merck, Sanofi-Aventis and Abbott Nutrition; is on the advisory board of AstraZeneca; and is a shareholder of Healthimation. None of these entities supported this research in part or total.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Committee on Human Studies (CHS) at the Joslin Diabetes Center (ID: STUDY00000056). Each participant signed the study informed consent before enrollment in the 12-week ILI program.
References
- 1.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–53. 10.2337/diacare.27.5.1047 [DOI] [PubMed] [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14. 10.1016/j.diabres.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 3.Basit A, Hydrie MZI, Hakeem R, et al. Frequency of chronic complications of type II diabetes. J Coll Physicians Surg Pak 2004;14:79–83. [PubMed] [Google Scholar]
- 4.Basu S, Yudkin JS, Kehlenbrink S, et al. Estimation of global insulin use for type 2 diabetes, 2018-30: a microsimulation analysis. Lancet Diabetes Endocrinol 2019;7:25–33. 10.1016/S2213-8587(18)30303-6 [DOI] [PubMed] [Google Scholar]
- 5.Dall TM, Yang W, Gillespie K, et al. The economic burden of elevated blood glucose levels in 2017: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care 2019;42:1661–8. 10.2337/dc18-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association . Standards of medical care in diabetes-2022 abridged for primary care providers. Clin Diabetes 2022;40:10–38. 10.2337/cd22-as01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diabetes Prevention Program Research Group . Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the diabetes prevention program outcomes study. Lancet Diabetes Endocrinol 2015;3:866–75. 10.1016/S2213-8587(15)00291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wing RR, Look AHEAD Research Group . Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the look ahead trial. Arch Intern Med 2010;170:1566–75. 10.1001/archinternmed.2010.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Look AHEAD Research Group, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–54. 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomah S, Salah T, Al-Badri M, et al. Multidisciplinary intensive Lifestyle intervention improves markers of Nonalcoholic fatty liver disease (NAFLD) in patients with type 1 diabetes and obesity: a retrospective matched-cohort study. Clin Diabetes Endocrinol 2023;9:3.:3. 10.1186/s40842-023-00150-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Brien R, Johnson E, Haneuse S, et al. Microvascular outcomes in patients with diabetes after bariatric surgery versus usual care: a matched cohort study. Ann Intern Med 2018;169:300–10. 10.7326/M17-2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holman RR, Paul SK, Bethel MA, et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 13.Control D, Trial C. Epidemiology of diabetes interventions and complications (DCCT/EDIC) study Research Group intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jax TW. Metabolic memory: a vascular perspective. Cardiovasc Diabetol 2010;9:51. 10.1186/1475-2840-9-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamdy O, Mottalib A, Morsi A, et al. Long-Term effect of intensive lifestyle intervention on cardiovascular risk factors in patients with diabetes in real-world clinical practice: a 5-year longitudinal study. BMJ Open Diab Res Care 2017;5:e000259. 10.1136/bmjdrc-2016-000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhaver S, Al-Badri M, Salah T, et al. Hybrid model of intensive lifestyle intervention is potentially effective in patients with diabetes & obesity for post-COVID era. Front Endocrinol (Lausanne) 2022;13:1050527. 10.3389/fendo.2022.1050527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eldib AH, Dhaver S, Al-Badri M, et al. Magnitude of A1C improvement in relation to baseline A1C and amount of weight loss in response to intensive Lifestyle intervention in real-world diabetes practice: 13 years of observation. J Diabetes 16, 2023. 10.1111/1753-0407.13395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Badri M, Kilroy CL, Shahar JI, et al. In-person and virtual Multidisciplinary intensive Lifestyle interventions are equally effective in patients with type 2 diabetes and obesity. Ther Adv Endocrinol Metab 2022;13:20420188221093220.:20420188221093220. 10.1177/20420188221093220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamdy O, Carver C. The why wait program: improving clinical outcomes through weight management in type 2 diabetes. Curr Diab Rep 2008;8:413–20. 10.1007/s11892-008-0071-5 [DOI] [PubMed] [Google Scholar]
- 20.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members . Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–30. 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 21.Yu MK, Lyles CR, Bent-Shaw LA, et al. Risk factor, age and sex differences in chronic kidney disease prevalence in a diabetic cohort: the pathways study. Am J Nephrol 2012;36:245–51. 10.1159/000342210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Maradni A, Tomah S, Mottalib A, et al. The effect of intensive lifestyle intervention on renal function in patients with diabetes and obesity in real-world practice: a 5-years longitudinal study. Human Nutrition & Metabolism 2021;24:200119. 10.1016/j.hnm.2021.200119 [DOI] [Google Scholar]
- 23.Monzillo LU, Hamdy O, Horton ES, et al. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res 2003;11:1048–54. 10.1038/oby.2003.144 [DOI] [PubMed] [Google Scholar]
- 24.Bansal D, Gudala K, Esam HP, et al. Microvascular complications and their associated risk factors in newly diagnosed type 2 diabetes mellitus patients. Int J Chronic Dis 2014;2014:201423. 10.1155/2014/201423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agrawal RP, Ola V, Bishnoi P, et al. Prevalence of micro and macrovascular complications and their risk factors in type-2 diabetes mellitus. J Assoc Physicians India 2014;62:504–8. [PubMed] [Google Scholar]
- 26.Inassi J, Vijayalakshmy R. Role of duration of diabetes in the development of nephropathy in type 2 diabetic patients. Nat J Med Res 2013;3:5–8. [Google Scholar]
- 27.Mørkrid K, Ali L, Hussain A. Risk factors and prevalence of diabetic peripheral neuropathy: a study of type 2 diabetic outpatients in Bangladesh [International Journal of diabetes in developing countries 2010; 30:11]. Int J Diab Dev Ctries 2010;30:11. 10.4103/0973-3930.60004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Kaabi JM, Al Maskari F, Zoubeidi T, et al. Prevalence and determinants of peripheral neuropathy in patients with type 2 diabetes attending a tertiary care center in the United Arab emirates. J Diabetes Metab 2014;5:2. [Google Scholar]
- 29.Kasim K, Amar M, Sadek AAE, et al. Peripheral neuropathy in type-II diabetic patients attending diabetic clinics in al-azhar university hospitals, Egypt. Int J Diabet Mellit 2010;2:20–3. 10.1016/j.ijdm.2009.10.002 [DOI] [Google Scholar]
- 30.Jones CD, Greenwood RH, Misra A, et al. Incidence and progression of diabetic retinopathy during 17 years of a population-based screening program in England. Diabetes Care 2012;35:592–6. 10.2337/dc11-0943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathan D. For the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study Research Group. intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Group UPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–53. 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 33.Control D, Group CTR. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 34.White NH, Sun W, Cleary PA, et al. Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the diabetes control and complications trial. Arch Ophthalmol 2008;126:1707–15. 10.1001/archopht.126.12.1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Look AHEAD Research Group . Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the look ahead randomised clinical trial. Lancet Diabetes Endocrinol 2014;2:801–9. 10.1016/S2213-8587(14)70156-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muskiet MHA, Tonneijck L, Smits MM, et al. Glp-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol 2017;13:605–28. 10.1038/nrneph.2017.123 [DOI] [PubMed] [Google Scholar]
- 37.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–30. 10.1016/S0140-6736(19)31149-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.