Abstract
To provide an extensive review on the associations between knee inflammation and altered pain perception mechanisms in people with knee osteoarthritis (OA). MEDLINE, Web of Science, EMBASE and Scopus were searched up to 13 December 2022. We included articles reporting associations between knee inflammation (measured by effusion, synovitis, bone marrow lesions (BMLs) and cytokines) and signs of altered pain processing (assessed by quantitative sensory testing and/or questionnaire for neuropathic-like pain) in people with knee OA. Methodological quality was evaluated using the National Heart, Lung and Blood Institute Study Quality Assessment Tool. Level of evidence and strength of conclusion were determined using the Evidence-Based Guideline Development method. Nine studies were included, comprising of 1889 people with knee OA. Signs of greater effusion/synovitis may be positively associated with lower knee pain pressure threshold (PPT) and neuropathic-like pain. Current evidence could not establish an association between BMLs and pain sensitivity. Evidence on associations between inflammatory cytokines and pain sensitivity or neuropathic-like pain was conflicting. There are indications of a positive association between higher serum C reactive protein (CRP) levels and lower PPT and presence of temporal summation. Methodological quality varied from level C to A2. Signs of effusion/synovitis may be positively associated with neuropathic-like pain and pain sensitivity. There are indications of a possible positive association between serum CRP levels and pain sensitivity. Given the quality and the small amount of included studies, uncertainty remains. Future studies with adequate sample size and follow-up are needed to strengthen the level of evidence.PROSPERO registration number: CRD42022329245.
Keywords: Osteoarthritis, Knee; Inflammation; Synovitis; Cytokines
WHAT IS ALREADY KNOWN ON THIS TOPIC
Knee osteoarthritis is characterised by low-grade chronic inflammation.
Inflammatory signs (eg, effusion, synovitis and proinflammatory cytokines) are weakly or moderately associated with pain intensity.
Pain in osteoarthritis is complex and characterised by nociceptive and neuropathic components along with possible pain sensitivity (peripheral and/or central sensitisation).
WHAT THIS STUDY ADDS
Signs of effusion/synovitis may be positively associated with neuropathic-like pain and signs of pain sensitivity.
There are indications of a possible positive association between serum C reactive protein levels and pain sensitivity.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Understanding how different inflammatory markers are related to symptoms is critical to devise new treatments and will ultimately lead to improvement of therapeutic approaches and better pain management.
Introduction
Osteoarthritis (OA) is a leading cause of chronic disability and carries a serious health and economic burden.1 The knee is the most affected joint accounting for approximately 85% of the burden of OA worldwide2 (about 365 million people globally).3 Knee OA is a multifactorial disease of the whole joint organ that presents a multitude of pathological changes involving cartilage degradation, osteophyte formation, osteochondral unit remodelling and joint inflammation.4 Clinically, patients with OA experience stiffness, pain and loss of function.5 Chronic pain is the dominant symptom and the main drive for patients to seek medical help.6 Unfortunately, to date, there is no effective therapy that guarantees an effective and long-lasting symptomatic relief nor a stop to the progression of OA.7
Traditionally, OA pain was considered and investigated as purely nociceptive arising from tissue damage. However, evidence on associations between tissue damage and pain is conflicting.8 Furthermore, a substantial portion of patients (~20%) suffer from persistent pain after the removal of damaged tissue by undergoing total knee arthroplasty (TKA).9 These observations led to an emerging growing body of evidence showing how various pain mechanisms contribute to pain.10 Pain in OA is characterised by nociceptive and neuropathic components along with possible sensitisation (peripheral and/or central).11 12 A subgroup of patients with knee OA (~40%) describe their pain as a sensation of burning, electric shock, numbness and itch, attributable to a neuropathic cause.13 Neuropathic pain is defined as ‘pain caused by a lesion or disease of the somatosensory system’.14 As sensitisation may occur in the absence of actual tissue damage of the somatosensory system (requirement to fulfil the definition of neuropathic pain),14 the new term ‘nociplastic’ pain was recently introduced.15 Modern screening tools can identify these pain characteristics attributable to features of altered pain perception mechanism.16 Neuropathic-like pain symptoms can be identified using patient-reported outcome measures, such as the painDETECT Questionnaire,17 the Neuropathic Pain Questionnaire (NPQ),18 the Douleur Neuropathique-4 (DN4)19 and the Leeds Assessment of Neuropathic Symptoms and Signs.20 Altered pain processing mechanisms can be evaluated by quantitative sensory testing (QST), often reported as the gold standard for quantification of somatosensory abnormalities.21 22 QST involves a series of psychophysical techniques that quantify somatosensory-evoked responses to noxious or innocuous stimuli in experimental settings to identify possible dysfunction of the somatosensory system.23 Localised pain, triggered by experimental stimuli on the affected joint, is associated with peripheral sensitisation, whereas regional or widespread pain at a site adjacent to or at a distance from the site of origin suggests a combination of peripheral and central sensitisation.12 24 The most common QST modalities include pain pressure threshold (PPT) (to test for widespread hyperalgesia) and/or temporal summation (TS) (to assess spinal hyperexcitability) and/or conditioned pain modulation (CPM) (to assess a descending inhibitory pathway).25
Due to the complexity of OA pain, the underlying mechanisms have long been unclear, but it is now known that the typical low-grade chronic inflammation of the arthritic joint might be involved in pain generation or altered pain perception.26 27 Inflammation of richly innervated structures, such as the synovial membrane (termed synovitis) or lesions in the subchondral bone (termed bone marrow lesions (BMLs)), can contribute to or has been associated with pain.28 29 Both synovitis30 and BMLs31 32 are sites of proinflammatory mediators expression. Evidence suggests that proinflammatory cytokines, such as interleukin (IL)-6, IL-1β and tumour necrosis factor-α (TNF-α), are not only mediators of joint inflammation and further degeneration, they can also activate nociceptors leading to increased responsiveness to stimulation.33 34 A permanent state of proinflammatory environment within the joint may facilitate a continuous activation of nociceptors, which may result in sensitisation and/or neuropathic-like pain.34
In a previous systematic review, we concluded that different inflammatory aspects are associated with pain in patients with knee OA but the strength of this association did not exceed the moderate level and the level of evidence ranged from limited to moderate.35 However, we mainly focused on pain intensity (typically measured with self‐reported pain tools such as Visual Analogue Scale, Numerical Rating Scale or questionnaires). With this work, we intend to go beyond the mere pain intensity aspect by focusing on studies presenting data on experimental measures of pain (QST) or neuropathic-like pain. In the last years, several studies attempted to better understand the relationship between inflammation and altered pain processing in patients with knee OA. However, no extensive synthesis is available in the literature. The aim of this work is to systematically review and summarise the scientific literature on the association between markers of inflammation and altered pain processing in patients with knee OA.
Methods
Design
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).36 The PRISMA checklist can be found in the online supplemental file. The protocol for this review was registered on PROSPERO (registration number: CRD42022329245). Papers reporting associations between inflammation (exposure) and experimental measures of pain (QST) and/or neuropathic-like pain (outcome) in patients with knee OA (population) were included.
rmdopen-2022-002945supp001.pdf (1.8MB, pdf)
Data sources and study selection
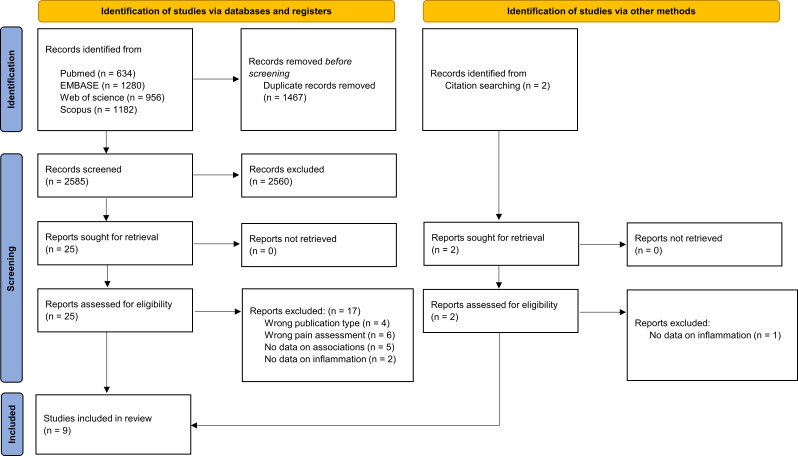
Four different databases were consulted (from inception until 13 December 2022): MEDLINE, Web of Science, EMBASE and Scopus. The full search strategy is reported in the online supplemental material. After de-duplication, two reviewers (PD and HM) independently screened the papers based on title and abstract (percentage of agreement 97%) using the Rayyan online tool.37 Conflicts were resolved by discussion and in case of remaining disagreement, a third reviewer (PC) was consulted. The remaining records were independently screened by the same two reviewers (PD and HM) on full text and for unresolved disagreements, the third reviewer (PC) was consulted. Additionally, the reference lists of eligible studies were hand searched for possible relevant records. The flow chart (figure 1) provides more details on the full screening process.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram documenting the search and screening process.
rmdopen-2022-002945supp002.pdf (108.1KB, pdf)
Eligibility criteria
Papers were eligible if the following inclusion criteria were met: (1) original observational studies (cross-sectional, case–control and cohort studies); (2) written in English, Dutch, French, German or Italian; (3) investigating people with knee OA; (4) the results report an association between (i) inflammation investigated through (a) medical imaging (ie, evaluation of effusion/synovitis, BMLs assessed by MRI and/or ultrasound), (b) synovial biopsy (evaluation of synovitis by synovial tissue analysis), (c) circulating biomarkers (biomarkers—cytokines, C reactive protein (CRP) found in blood samples, synovial fluid or urine); (ii) altered pain processing assessed by QST (ie, PPT, CPM and TS) and/or (iii) neuropathic-like pain assessed by questionnaires (ie, painDETECT, DN4 and NPQ). Interventional studies were included if associations between inflammation and pain sensitivity were reported from analysis of baseline data. Other study designs were excluded. Studies including patients with other inflammatory joint diseases (eg, rheumatoid arthritis), presence of other comorbidities per inclusion criteria or multiple OA cohorts (eg, including patients with knee OA and patients with hip OA) were excluded if data from patients with knee OA could not be pooled separately.
Data extraction
From included papers, two reviewers (PD and HM) extracted the following information: (1) author, publication year and country; (2) study design (when a cohort study only provided baseline data on the association between inflammation and pain sensitivity, the paper was considered to be a cross-sectional); (3) patient characteristics (sex, age and body mass index (BMI)); (4) sample size, definition of knee OA, symptoms duration and OA duration; (5) inflammation assessment; (6) assessment of experimental measures of pain (QST) or neuropathic-like pain; (7) statistical analysis; (8) potential confounders; (9) overall quality based on risk of bias (RoB) assessment; and (10) results of the association between inflammation and experimental measures of pain and/or neuropathic-like pain.
Risk of bias
The RoB assessment was performed independently by the same reviewers (PD and HM) (percentage of agreement 92%) using the National Heart, Lung and Blood Institute (NHLBI) Study Quality Assessment Tool.38 The NHLBI is a study design-depending tool, consisting of 14 items that evaluate potential methodological flaws (eg, patient selection, adjustment for confounders, study power), and it was previously used in systematic reviews on OA.37 39 The reviewers answered each question with yes, no or other (ie, cannot determine, not applicable, not reported). Differences in scoring were resolved by consulting the third reviewer (PC). A summary score was calculated based on the number of ‘yes’ selected divided by the total amount of items assessed (excluding the not applicable). Final judge of each study was determined according to the following standardisation: <7=high RoB (poor quality), 7–10=moderate RoB (fair quality), 11–14=low RoB (good quality).40
Best evidence synthesis
Due to high heterogeneity across papers, it was not indicated to perform a meta-analysis and instead a qualitative evidence synthesis was performed. Level of evidence and strength of conclusion were determined using the Evidence-Based Guideline Development method.41 Each included study was graded for a level of evidence ranging from D to A1, depending on evidence-based medicine domain, study design and individual methodological quality. The strength of conclusion (levels 1–4) was determined to different outcome levels, taking into account the composition of the respective levels of evidence and consistency of their results (table 1).
Table 1.
EBRO classification of the study results according to the level of evidence and definition of level of conclusion assigned per outcome
| Level of evidence | |
| A1 | Systematic reviews and meta-analyses, based on minimally 2 independent A2 studies |
| A2 | Prospective cohort studies with sufficient sample size and follow-up; adequately controlled for confounding factors and precluding selective loss to follow-up |
| B | Prospective cohort studies, but lacking the quality criteria of A2. Retrospective cohort studies and case–control studies |
| C | Non-comparative studies |
| D | Expert opinion |
| Level of conclusion | |
| 1 | One A1 or at least two independent A2 studies that support each other’s conclusions and do not show conflicting evidence |
| 2 | One A2 or at least two independent B studies that support each other’s conclusions and do not show conflicting evidence |
| 3 | One B or at least two C studies that support each other’s conclusions and do not show conflicting evidence |
| 4 | Research at level C or two or more independent studies at higher level that do not support each other’s conclusions and show conflicting evidence |
| Consensus | There is no evidence, but consensus (level D) |
| No conclusions | There is no evidence and no consensus |
EBRO, Evidence-Based Richtlijn (Guideline) Ontwikkeling (Development).
Results
Literature search and characteristics of included studies
The initial search strategy resulted in 4052 records of which 1467 duplicates were removed and 2560 excluded based on the screening on title and abstract. After screening 25 on full text, 8 studies remained and another 1 was included through citation hand searching. Finally, a total of 9 studies with 1889 participants were included in this review.
The included studies consisted of seven cross-sectional studies,42–48 one case–control49 and one study reporting longitudinal cohort data.50 Patients’ characteristics, assessments of inflammation and pain sensitivity, and statistical analysis are reported in detail in table 2. Overall, the majority of the patients in the included studies are represented by women, with one study reporting data of only female patients. Mean age ranges between 65 and 75 years and consists of mainly overweight patients with a mean BMI ≥28 kg/m2. Different definitions of OA were reported. Six papers used the American College of Rheumatology (ACR) criteria42 43 45–48 and five of these also included the Kellgren-Lawrence radiographic grading system to diagnose knee OA.42 43 45 47 48 Only one study46 specified which ACR criteria were used (ie, clinical, clinical/radiographic or clinical/laboratory). One study included patients scheduled for TKA49 and one study used data from individuals with knee OA (31% with radiographic evidence of knee OA at baseline) or with specific risk factors for developing knee OA.50
Table 2.
Characteristics of included studies (n=9)
| Study 1st author, year, country, design | Participants’ characteristics | Marker(s) of inflammation | Experimental pain assessment | Statistical analysis | Quality |
| Arendt-Nielsen,42 2014, Denmark, CS |
Patients grouped according to experimental pain CPM, knee Most sensitised: n=42 (52% female); mean age 64.57±7.63 years; BMI=28.98±5.64 kg/m2; KL=1.95±0.94 Medium sensitised: n=193 (30% female); mean age 67.71±3.66 years; BMI=27.46±3.93 kg/m2; KL=1.54±0.69 Least sensitised: n=46 (30% female); mean age 63.40±7.32 years; BMI=27.68±4.03 kg/m2; KL=1.82±0.85 TS, knee Most sensitised: n=46 (49% female); mean age 65.37±5.87 years; BMI=26.82±3.75 kg/m2; KL=1.77±0.72 Medium sensitised: n=187 (55% female); mean age 65.80±7.28 years; BMI=28.20±4.64 kg/m2; KL=1.74±0.83 Least sensitised: n=48 (37% female); mean age 63.37±7.95 years; BMI=30.14±4.78 kg/m2; KL=2.04±0.77 OA definition: ACR Symptoms duration: N.R. OA duration: N.R. |
Blood samples Serological markers: hsCRP |
PPT (8 knee locations, muscle tibialis anterior, muscle extensor carpi radialis) TS (pressure pain) CPM |
Repeated measures ANOVA and post-hoc test (t-test with Bonferroni correction) | Good |
| Imamura,43 2015, Brazil, CS |
OA group: n=53 (100% female); mean age 71.23±7.62 years; BMI=28.33±5.95 kg/m2 OA definition: ACR, KL grade 2–4 and VAS >4 (averaged daily pain for the past month and lasting ≥3 months) Symptoms duration: at least 24 months; median 96 months (range 36–150) OA duration: N.R. |
Blood samples Proinflammatory cytokines: IL-6, IL-8, TNF-α Anti-inflammatory cytokine: IL-10 |
PPT over vastus medialis, adductor longus, rectus femoris, vastus lateralis, tibialis anterior, peroneus longus, iliacus, sartorius, gracilis, quadratus lumborum and popliteus PPT over supraspinous ligaments (L1–L2, L2–L3, L3–L4, L4–L5, L5–S1, S1–S2) Hyperalgesia: pinch and roll manoeuvre at the L1, L2, L3, L4, L5, S1 and S2 dermatomes |
Spearman correlation Mann-Whitney U |
Fair |
| Lee,44 2011, USA, CS |
OA group: n=26 (76.9% female); mean age 59±7.5 years; BMI=27.5 (IQR: 24.8–32.6) kg/m2 OA definition: clinically diagnosed knee OA, documented by a physician Symptoms duration: N.R. OA duration: N.R. Controls: n=33 (69.7% female); mean age 57.7±10.3 years; BMI=27.9 (IQR: 24.3–30.0) kg/m2 |
Blood samples CRP, IL-6, IL-1β, TNF-α |
PPT at the trapezius, 1st metacarpophalangeal joint and quadriceps Heat pain threshold+suprathreshold heat pain ratings on ventral forearm Cold pressor task |
General linear mixed models | Fair |
| Li,45 2020, China, CS | n=83 (74.7% female); mean age 63.4±10.4 years; BMI=N.R. OA definition: ACR KL grade 1: 20 2: 48 3: 12 4: 3 Symptoms duration: N.R. OA duration: N.R. |
SF samples Inflammatory cytokines: IL-1β, IL-6, TNF-α |
painDETECT | Spearman correlation | Fair |
| Neogi,50 2016, USA, cohort | n=1111 with knee OA or at risk (62% female), mean age 66.9±7.5 years; BMI=29.7±4.8 kg/m2; 38% with radiographic knee OA Subjects free of TS at baseline n=716 (61% female); mean age 66.3±7.4 years; BMI=29.7±4.8; 37% with radiographic knee OA OA definition: sample from MOST Study KL grade 0–3 and frequent knee pain in the past 30 days or no frequent pain, but with at least one risk factor (history of knee injury/operation, overweight/obese) Symptoms duration: N.R. OA duration: N.R. |
Non-CE-MRI Hoffa-synovitis (0–3) (WORMS) Effusion (0–3) (WORMS) BMLs (0–3) (WORMS) |
PPT knee (peripheral sensitisation) PPT wrist (central sensitisation) TS mechanical (central sensitisation) |
Linear regression Logistic regression Conditional logistic regression |
Good |
| Petersen,49 2016, Denmark, CC |
Patients with KOA BLOKS 0 n=16 (75% female), mean age 67.9±5.75 years; BMI=30.5±3.0 kg/m2; KL grade: 3.9 (95% CI: 3.7 to 4.0) OA duration: 11.5 years (95% CI: 4.2 to 18.8) Symptoms duration: N.R. BLOKS 1 n=24 (46% female), mean age 67.5±4.9 years; BMI=30.7±2.65 kg/m2; KL grade: 4 (95% CI: 4.0 to 4.0) OA duration: 13.5 years (95% CI: 8.8 to 18.1) Symptoms duration: N.R. BLOKS 2 and 3 n=21 (57% female), mean age 69.1±4.1 years; BMI=29.1±2.7 kg/m2; KL grade: 3.9 (95% CI: 3.7 to 4.0) OA duration: 15.3 years (95% CI: 10.0 to 20.6) Symptoms duration: N.R. OA definition: patients with KOA scheduled for TKA Controls VAS <10 mm, KL <2, no long-lasting pain problems in the past year n=33 (55% female), mean age 66.3±1.8 years; BMI=27.0±1.4 k g/m2 |
Non-CE-MRI Degrees of synovitis: no (BLOKS 0), low (BLOKS 1), moderate (BLOKS 2) or severe (BLOKS 3) |
PPT (7 sites in the peripatellar region) | ANOVA | Fair |
| Radojčić,46 2017, Denmark, CS | n=104 (62% female); mean age 66.78±7.24 years; BMI=30.4±5.21 kg/m2 OA definition: ACR (clinical and radiological); patients scheduled for TKA Symptoms duration: ≥6 months before enrolment OA duration: N.R. |
non-CE-MRI Synovitis (MOAKS) Effusion/synovitis (MOAKS) CE-MRI Synovitis (11-point score on CE images) Blood and SF Plasma IL-6 SF IL-6 |
NPQ | Spearman correlation Linear regression Logistic regression |
Good |
| Sofat,47 2019, UK, CS |
Patients with OA (n=120) Advanced OA n=78 (64% female); mean age 68.9±7.7 years; BMI=32.3±5.6 kg/m2 Mild OA n=42 (71% female); mean age 64.1±9.6; BMI=29.2±4.7 kg/m2 OA definition: ACR; KL grade ≥2 Symptoms duration: N.R. OA duration: N.R. |
Non-CE-MRI Synovitis (MOAKS) BML load (MOAKS) |
PPT
|
Pearson correlation | Good |
| Tchetina,48 2020, Russia, CS | n=50 (74% female); mean age 67.6±7.5 years; BMI=30.5±9.05 kg/m2 KL 3: 37 4: 13 OA definition: ACR, KL Symptoms duration: permanent pain during the last 3 months before enrolment OA duration: 9.9 years (range 3–30) |
Blood samples TNF-α, IL-1β gene expression |
DN4 painDETECT |
Spearman correlation | Fair |
ACR, American College of Rheumatology; ANOVA, analysis of variance; BLOKS, Boston-Leeds Osteoarthritis Knee Score; BMI, body mass index; BML, bone marrow lesion; CC, case–control; CE-MRI, contrast-enhanced MRI; CPM, conditioned pain modulation; CS, cross-sectional; DN4, Douleur Neuropathique-4 Questions; hsCRP, high-sensitivity C reactive protein; IL, interleukin; KL, Kellgren-Lawrence score; KOA, knee osteoarthritis; MOAKS, MRI Osteoarthritis Knee Score; NPQ, Neuropathic Pain Questionnaire; N.R., not reported; OA, osteoarthritis; PPT, pressure pain threshold; SF, synovial fluid; TKA, total knee arthroplasty; TNF-α, tumour necrosis factor-α; TS, temporal summation; VAS, Visual Analogue Scale; WORMS, Whole Organ MRI Score.
Inflammation was assessed by either MRI, blood samples and/or synovial fluid. Four articles46 47 49 50 used non-contrast-enhanced MRI to semiquantitatively assess effusion/synovitis, with two of them reporting measurements of BMLs as well.47 50 Only one study assessed synovitis using contrast-enhanced MRI.46 A variety of inflammatory wet biomarkers were investigated in six studies42–46 48 of which TNF-α and IL-6 were most commonly reported. The majority of these studies obtained data from blood sample analysis with only two articles analysing synovial fluid.45 46
Altered pain processing was assessed by QST in six studies.42–44 47 49 50 Pain sensitivity was primarily assessed using PPT.42–44 47 49 50 To evaluate pain sensitisation, one study used TS50 and another study combined TS and CPM.42 Presence of neuropathic-like pain was assessed by questionnaires in three studies.45 46 48 The three studies assessing presence of neuropathic-like pain applied the painDETECT Questionnaire,45 48 neuropathic pain-related DN448 and NPQ.46
Quality assessment of included studies
Agreement was reached on 117 of 126 (93%) quality assessment items scored. Five studies43–46 48 were scored as having moderate RoB, resulting in fair overall quality. The remaining four studies42 47 49 50 were scored as having low RoB, resulting in good quality. Overall, the majority of the studies did not provide sample size justification or power description (criterion 5). Other two common sources of bias across the included studies were lack of blinded assessment (criterion 12) and analysis without including key potential confounders (criterion 14). Results of methodological quality assessment are outlined in table 3.
Table 3.
Quality assessment of included studies
| Study 1st author, year | Study design | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Overall RoB |
| Arendt-Nielsen,42 2014 | CS | Y | Y | Y | Y | N | NA | NA | Y | Y | NA | Y | N | NA | Y | 8/10=80% low |
| Imamura,43 2015 | CS | Y | Y | Y | N | N | NA | NA | Y | Y | NA | N | Y | NA | N | 6/10=60% moderate |
| Lee,44 2011 | CS | Y | N | Y | Y | N | NA | NA | Y | Y | NA | Y | NR | NA | N | 6/10=60% moderate |
| Li,45 2020 | CS | Y | Y | Y | Y | N | NA | NA | Y | Y | NA | Y | NR | NA | N | 6/10=60% moderate |
| Neogi,50 2016 | C | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | 12/14=86% low |
| Petersen,49 2016 | CC | Y | Y | Y | Y | N | Y | CD | Y | N | Y | Y | Y | / | / | 9/12=75% moderate |
| Radojčić,46 2017 | CS | Y | Y | Y | Y | N | NA | NA | Y | Y | NA | Y | NR | NA | Y | 8/10=80% low |
| Sofat,47 2019 | CS | N | Y | Y | Y | Y | NA | NA | Y | Y | NA | Y | Y | NA | Y | 9/10=90% low |
| Tchetina,48 2020 | CS | Y | Y | Y | Y | N | NA | NA | Y | Y | NA | Y | NR | NA | N | 7/10=70% moderate |
<7 (50%)→<7 (50%)→high RoB=poor quality; 7–10 (50%–75%)→moderate RoB=fair quality; 11–14 (>75%)→low RoB=good quality.
C, cohort; CC, case–control; CD, cannot determine; CS, cross-sectional; N, no; NA, not applicable; NR, not reported; RoB, risk of bias; Y, yes.
Associations
Results on the associations between inflammatory signs and measures of altered pain perception mechanisms are outlined in table 4. An overview of the level of evidence and level of conclusion is reported in table 5.
Table 4.
Associations between inflammatory signs and measures of altered pain perception mechanisms
| Study | Associations | Results | Confounders | Level of evidence |
| Effusion/synovitis and pain sensitivity | ||||
| Petersen et al49 | A significant BLOKS group effect in the ANOVA of the PPTs | Site 1 (ANOVA: F2=3.14, p<0.03) Site 2 (ANOVA: F2=6.59, p<0.001) Site 3 (ANOVA: F2=4.04, p<0.01) Site 4 (ANOVA: F2=3.43, p<0.02) Site 5 (ANOVA: F2=2.40, p<0.07) Site 6 (ANOVA: F2=4.49, p<0.006) Site 7 (ANOVA: F2=1.24, p<0.3) |
BMI, sex and age | B |
| Neogi et al50 | Hoffa-synovitis (baseline)—PPT patella (baseline) | β=−0.42 (95% CI: −0.67 to −0.18) | Age, sex, BMI, race, study site, KL, presence of patellofemoral OA, depressive symptoms, catastrophising, widespread pain, use of analgesics | A2 |
| Hoffa-synovitis (baseline)—PPT patella change (follow-up 24 months) | β=−0.30 (95% CI: −0.52 to −0.08) | |||
| Hoffa-synovitis (baseline)—PPT wrist (baseline) | β=−0.19 (95% CI: −0.35 to −0.03) | |||
| Hoffa-synovitis (baseline)—PPT wrist change (follow-up 24 months) | β=−0.10 (95% CI: −0.26 to 0.06) | |||
| Effusion (baseline)—PPT patella (baseline) | β=0.23 (95% CI: −0.03 to 0.49) | |||
| Effusion (baseline)—change PPT patella (follow-up 24 months) | β=−0.04 (95% CI: −0.28 to 0.19) | |||
| Effusion (baseline)—PPT wrist (baseline) | β=−0.24 (95% CI: −0.40 to −0.07) | |||
| Effusion (baseline)—PPT wrist change (follow-up 24 months) | β=−0.24 (95% CI: −0.41 to −0.08) | |||
| Hoffa-synovitis (baseline)—TS patella (baseline) (n=1111) | OR=0.83 (95% CI: 0.63 to 1.08) | |||
| Hoffa-synovitis (baseline)—TS patella incident (n=716) | OR=1.12 (95% CI: 0.75 to 1.66) | |||
| Hoffa-synovitis (baseline)—TS wrist (baseline) (n=1111) | OR=0.84 (95% CI: 0.75 to 1.66) | |||
| Hoffa-synovitis (baseline)—TS wrist incident (n=716) | OR=1.22 (95% CI: 0.85 to 1.75) | |||
| Effusion (baseline)—TS patella (baseline) (n=1111) | OR=0.89 (95% CI: 0.67 to 1.18) | |||
| Effusion (baseline)—TS patella incident (n=716) | OR=1.54 (95% CI: 1.01 to 2.36) | |||
| Effusion (baseline)—TS wrist (baseline) (n=1111) | OR=1.13 (95% CI: 0.85 to 1.51) | |||
| Effusion (baseline)—TS wrist incident (n=716) | OR=0.94 (95% CI: 0.66 to 1.35) | |||
| Sofat et al47 | Synovitis—PPT | r=−0.02, N.S. | N.A. | C |
| BMLs and pain sensitivity | ||||
| Neogi et al50 | BMLs—PPT patella (baseline) | β=0.14 (95% CI: −0.16 to 0.08) | Age, sex, BMI, race, study site, KL, presence of patellofemoral OA, depressive symptoms, catastrophising, widespread pain, use of analgesics | A2 |
| BMLs—change PPT patella (follow-up 24 months) | β=0.03 (95% CI: −0.25 to 0.31) | |||
| BMLs—PPT wrist (baseline) | β=0.05 (95% CI: −0.14 to 0.25) | |||
| BMLs—PPT wrist change (follow-up 24 months) | β=−0.10 (95% CI: 0.29 to 0.10) | |||
| BMLs sum—PPT patella (baseline) | β=0.03 (95% CI: −0.01 to 0.08) | |||
| BMLs sum—change PPT patella (follow-up 24 months) | β=−0.01 (95% CI: −0.05 to 0.04) | |||
| BMLs sum—PPT wrist (baseline) | β=0.00 (95% CI: −0.03 to 0.03) | |||
| BMLs sum—PPT wrist change (follow-up 24 months) | β=0.00 (95% CI: −0.03 to 0.03) | |||
| BMLs—TS patella (baseline) (n=1111) | OR=0.95 (95% CI: 0.68 to 1.32) | |||
| BMLs—TS patella incident (n=716) | OR=0.92 (95% CI: 0.56 to 1.49) | |||
| BMLs—TS wrist (baseline) (n=1111) | OR=1.04 (95% CI: 0.74 to 1.45) | |||
| BMLs—TS wrist incident (n=716) | OR=1.05 (95% CI: 0.68 to 1.62) | |||
| BMLs sum—TS patella (baseline) (n=1111) | OR=0.98 (95% CI: 0.93 to 1.03) | |||
| BMLs sum—TS patella incident (n=716) | OR=1.00 (95% CI: 0.92 to 1.07) | |||
| BMLs sum—TS wrist (baseline) (n=1111) | OR=0.98 (95% CI: 0.93 to 1.04) | |||
| BMLs sum—TS wrist incident (n=716) | OR=1.00 (95% CI: 0.94 to 1.07) | |||
| Sofat et al47 | BML_load—PPT | r=−0.069, N.S. | N.A. | C |
| nBML—PPT | r=−0.114, N.S. | |||
| Inflammatory cytokines and pain sensitivity | ||||
| Imamura et al43 | IL-6—PPT | N.S. | / | C |
| IL-10—PPT | N.S. | |||
| TNF-α—PPT | Significantly associated with the majority of PPT measures of the muscular, ligamentous or subcutaneous sources (p<0.05) | |||
| Lee et al44 | Log IL-6—PPT quadriceps | F=0.98, p=N .S. | BMI | C |
| Log IL-6—(PPT quadriceps)² | N.S. | |||
| Log IL-6—PPT thumb | F=0.02, N.S. | |||
| Log IL-6—(PPT thumb)² | N.S. | |||
| Log IL-6—PPT trapezius | F=0.97, N.S. | |||
| Log IL-6—(PPT trapezius)² | N.S. | |||
| Log IL-6—HPT | F=0.27, N.S. | |||
| Log IL-6—HPR at 49°C | F=4.51, p=0.04 | |||
| Log IL-6—(HPR at 49°C)² | F=4.27, p=0.05 | |||
| Log IL-6—HPR at 51°C | F=5.79, p=0.02 | |||
| Log IL-6—(HPR at 51°C)² | F=5.26, p=0.03 | |||
| Log IL-6—CPR | F=0.03, p=N .S. | |||
| Log IL-6—CPR² | N.S. | |||
| Log IL-6—CPT | F=5.96, p=0.02 | |||
| Log IL-6—CPT² | F=6.57, p=0.02 | |||
| IL-1β—pain measures | N.S., data N.R. | |||
| TNF-α—pain measures | N.S., data N.R. | |||
| C reactive protein and pain sensitivity | ||||
| Arendt-Nielsen et al42 | hsCRP—local/spreading sensitisation/TS | Association sign, data N.R. | Unadjusted | B |
| Lee et al44 | Log CRP—PPT quadriceps | F=8.13, p=0.009 | Unadjusted | C |
| Log CRP—(PPT quadriceps)² | F=6.58, p=0.02 | |||
| Log CRP—PPT thumb | F=7.44, p=0.01 | |||
| Log CRP—(PPT thumb)² | N.S. | |||
| Log CRP—PPT trapezius | F=9.99, p=0.004 | |||
| Log CRP—(PPT trapezius)² | F=9.36, p=0.006 | |||
| Log CRP—HPT | F=0.55, p=N .S. | |||
| Log CRP—HPR at 49°C | F=1.17, N.S. | |||
| Log CRP—(HPR at 49°C)² | N.S. | |||
| Log CRP—HPR at 51°C | F=1.96, N.S. | |||
| Log CRP—(HPR at 51°C)² | N.S. | |||
| Log CRP—CPR | F=5.20, p=0.03 | |||
| Log CRP—CPR² | F=5.11, p=0.03 | |||
| Log CRP—CPT | F=0.74, N.S. | |||
| Log CRP—CPT² | N.S. | |||
| Effusion/synovitis and neuropathic-like pain | ||||
| Radojčić et al46 | 11-point synovitis—NPQ | r=0.25, p=0.011 | BMI, sex and age | C |
| Inflammatory cytokines and neuropathic-like pain | ||||
| Li et al45 | IL-1β—painDETECT | r=−0.019, N.S. | N.A. | C |
| IL-6—painDETECT | r=−0.034, N.S. | |||
| TNF-α—painDETECT | r=−0.115, N.S. | |||
| Radojčić et al46 | Plasma IL-6—NPQ | β=4.029 (95% CI: −0.032 to 0.838) | BMI, sex and age | C |
| Synovial fluid IL-6—NPQ | β=0.043 (95% CI: 0.005 to 0.082) | |||
| Tchetina et al48 | TNF-α—DN4 | r=0.330, p=0.02 | N.A. | C |
| IL-1β—DN4 | r=0.496, p<0.01 | |||
| IL-1β—painDETECT | r=0.313, p=0.04 | |||
ANOVA, analysis of variance; BLOKS, Boston-Leeds Osteoarthritis Knee Score; BMI, body mass index; BMLs, bone marrow lesions; CPR, cold pain rating; CPT, cold pain tolerance; DN4, Douleur Neuropathique-4 Questions; HPR, heat pain rating; HPT, heat pain threshold; (hs)CRP, (high-sensitivity) C reactive protein; IL, interleukin; KL, Kellgren-Lawrence score; N.A., not applicable; NPQ, Neuropathic Pain Questionnaire; N.S., not significant; OA, osteoarthritis; PPT, pressure pain threshold; TNF-α, tumour necrosis factor-α; TS, temporal summation.
Table 5.
Level of conclusion for associations between inflammatory signs and altered pain perception (pain sensitivity and neuropathic-like pain)
| Associations | Level of evidence | Level of conclusion | ||||
| Inflammatory signs—pain sensitivity | A1 | A2 | B | C | D | |
| Effusion/synovitis | Neogi et al50[+] | Petersen et al49[+] | Sofat et al47[ns] | 2 | ||
| BMLs | Neogi et al50[ns] | Sofat et al47[ns] | 2 | |||
| Pro-inflammatory cytokines | 4 | |||||
| IL-1β | Lee et al44[ns] | |||||
| IL-6 | Imamura et al43[ns] | |||||
| Lee et al44[+] | ||||||
| TNF-α | Imamura et al43[ns] | |||||
| Lee et al44[+] | ||||||
| CRP | Arendt-Nielsen et al42[+] | Lee et al44[+] | 3 | |||
| Inflammatory signs—neuropathic-like pain | ||||||
| Effusion/synovitis | Radojčić et al46[+] | 4 | ||||
| Pro-inflammatory cytokines | 4 | |||||
| IL-1β | Li et al45[ns] | |||||
| Tchetina et al48[+] | ||||||
| IL-6 | Li et al45[ns] | |||||
| Radojčić et al46[ns] | ||||||
| TNF-α | Li et al45[ns] | |||||
| Tchetina et al48[+] | ||||||
Level of evidence: A1=systematic reviews and meta-analyses, based on minimally two independent A2 studies; A2=prospective cohort studies with sufficient sample size and follow-up; adequately controlled for confounding factors and precluding selective loss to follow-up; B=prospective cohort studies, but lacking the quality criteria of A2; retrospective cohort studies and case–control studies; C=non-comparative studies; D=expert opinion. [+] Significant positive association. [ns] Not significant association. [-] Significant negative association.
Level of conclusion: 1=one A1 or at least two independent A2 studies that support each other’s conclusions and do not show conflicting evidence; 2=one A2 or at least two independent B studies that support each other’s conclusions and do not show conflicting evidence; 3=one B or at least two C studies that support each other’s conclusions and do not show conflicting evidence; 4=research at level C or two or more independent studies at higher level that do not support each other’s conclusions and hence, show conflicting evidence; consensus=there is no evidence, but consensus (level D); no conclusions=there is no evidence and no consensus.
BMLs, bone marrow lesions; CRP, C reactive protein; IL, interleukin; TNF-α, tumour necrosis factor-α.
Effusion/synovitis and pain sensitivity
Three studies47 49 50 presented data on associations between effusion/synovitis and pain sensitivity. Although PPT was primarily used, the QST protocols differ significantly across studies (eg, different testing regions). Two studies revealed that patients with OA with MRI-detected presence of synovitis or effusion showed significantly lower PPT values at the knee when compared with those without49 50 or to those with lower grade of effusion or synovitis.49 Further, a longitudinal cohort study reported that individuals with synovitis exhibit worsening PPT at the patella over time.50 Moreover, presence of knee effusion resulted in an increased risk of developing TS at the patella and with a decrease in PPT at the wrist after 24 months.50 In contrast, Sofat et al found no significant associations between structural signs of synovitis on non-contrast-enhanced MRI and PPT at the knee in those with mild to advanced knee OA.47 According to conclusion level 2, inflammatory signs of effusion/synovitis may be associated with signs of pain sensitivity.
BMLs and pain sensitivity
According to conclusion level 2, the included studies could not establish an association between BMLs and pain sensitivity.47 50
Inflammatory cytokines and pain sensitivity
There is conflicting evidence on the association between inflammatory cytokines and pain sensitivity (conclusion level 4). One study showed that serum levels of TNF-α were positively correlated with lower pressure tolerance at areas located further beyond the painful knee joint.43 For other proinflammatory (IL-6 and IL-8) and anti-inflammatory (IL-10) cytokines, although heightened in patients with OA compared with controls, no correlation was found with PPT.43 Another study reported that serum levels of IL-6 were significantly associated with cold pain tolerance and heat pain ratings (heightened pain sensitivity was associated with elevated IL-6).44 IL-1β and TNF-α were not significantly associated with any experimental pain measures.44
According to conclusion level 3, two studies indicated a possible association between CRP levels and pain sensitivity.42 44 One study described a significant association between higher serum CRP levels and greater local/spreading pain sensitivity,42 and another study reported a significant association between serum CRP levels and PPT at multiple locations, indicating widespread hyperalgesia.44
Effusion/synovitis and neuropathic-like pain
Limited evidence suggested a possible association between synovitis and neuropathic-like pain (conclusion level 4). Synovitis on contrast-enhanced MRI (measured by a composite score obtained summing synovial thickness measured in millimetres at 11 anatomical knee regions) showed a significant positive correlation with neuropathic-like pain (measured by NPQ) in patients undergoing total knee replacement surgery.46 Other MRI findings including synovial volume, Hoffa-synovitis and whole-knee effusion were not significantly correlated with NPQ score.46
Inflammatory cytokines and neuropathic-like pain
No association was found between inflammatory cytokines and painDETECT score.45 The NPQ and IL-6 levels in synovial fluid, but not in plasma, were significantly correlated.46 In patients with end-stage OA, higher neuropathic-like pain scores on painDETECT and DN4 Questionnaires were found to be positively correlated with gene expression of TNF-α and IL-1β.48 Taking these results together, there is conflicting evidence on a possible association between inflammatory cytokines and neuropathic-like pain (conclusion level 4).
Discussion
The aim of this systematic review was to synthesise and evaluate the available literature on the associations between knee inflammation (as measured by effusion, synovitis, BMLs and cytokines) and altered pain processing (assessed by QST and/or questionnaires for neuropathic-like pain) in patients with knee OA.
The main findings indicate a probable positive association between knee inflammation and altered pain processing (pain sensitivity and neuropathic-like pain). Specifically, local inflammatory signs of effusion and synovitis assessed by non-contrast MRI were found to be positively associated with altered pain sensitivity (measured by PPT and TS). Synovitis measured on contrast-enhanced MRI was found to be positively associated with presence of neuropathic-like pain. However, this finding is based on one study only. At a systemic level, conflicting evidence was found on the association between serum levels of inflammatory cytokines (IL-1β, IL-6 and TNF-α) and pain sensitivity. Serum CRP levels, a measure of systemic inflammation, were found to be positively associated with lower PPT and presence of TS. However, the limited amount of included articles, most of which (56%) have moderate RoB along with some conflicting evidence, makes it difficult to draw strong conclusions and indicates the need for further research on these associations.
From our results, it emerges that knee local inflammatory lesions such as synovitis and effusion may be positively associated with pain sensitivity (conclusion level 2). Synovitis in knee OA is characterised by hyperplasia of synovial fibroblasts, which are a major source of proinflammatory cytokines51 that can sensitise nociceptors.52 Included studies of moderate to good quality suggest a positive association between signs of synovitis (effusion/synovitis measured on non-contrast-enhanced MRI) and hyperalgesia expressed by lower PPT (at the knee and at a remote site from the affected knee). Neogi et al showed that individuals with synovitis demonstrate a lower PPT at the knee and a decrease of this threshold over 2 years, reflecting an increased pain sensitivity in those participants.50 Effusion at baseline was associated with a higher risk of developing TS (an augmented response to repetitive equal-intensity noxious stimuli) and with a decrease in PPT at the wrist after 24 months.50 These results support the notion that in the course of joint inflammation, nociceptors experience increased stimulation and that persistent activation can contribute to the development of central sensitisation.33 This has also been demonstrated in rodent models of OA pain, in which synovitis is followed by evidence of central sensitisation.53 Therefore, effusion and synovitis are two attractive therapeutic targets for interventions aiming to prevent the occurrence of sensitisation which, in turn, may prevent pain to become chronic and more severe.
Recently, there has been a growing interest in the role of subchondral bone in OA pain. BMLs are considered to be (micro)traumatic lesions related to excessive mechanical load or remodelling related to tissue injury.54 In OA, BML changes may contribute to pain also due to a proinflammatory environment in which angiogenesis, along with new sensory nerves, occurs.55 However, the studies included in this review did not find an association between BMLs measured by non-contrast MRI and signs of pain sensitivity (conclusion level 2). This could be due to the limited amount of information as only two of the included studies investigated these possible associations.47 50 Furthermore, heterogeneity of included patients in both studies was high. Recent evidence based on animal models showed that pain induced by acute inflammatory response in early monoiodoacetate (MIA)-induced OA involves sensitisation of nerves innervating the joint capsule but not the underlying subchondral bone, while pain in late MIA-induced OA involves the additional recruitment of nerves that innervate the subchondral bone.56 These findings suggest that the role of BMLs in pain generation may differ according to the OA severity. However, the two included studies reporting data on the association between BMLs and pain sensitivity included patients with high heterogeneity ranging from early to late knee OA.47 50 This could be an additional reason for which an association between BMLs and pain sensitivity was not found.
At a molecular level, proinflammatory cytokines have been considered to play important roles in knee OA pain.57 In case of synovitis, the synovial membrane becomes hyperplastic and infiltrated with immune cells with subsequently increased levels of proinflammatory mediators in peripheral blood and synovial fluid.58 59 Proinflammatory cytokines such as IL-1β, IL-6 and TNF-α can directly activate nociceptive sensory neurons and contribute to sensitisation.26 33 Our results showed conflicting evidence regarding a possible significant association between cytokines and pain sensitivity (conclusion level 4). TNF-α was associated with lower PPTs at multiple anatomical regions. This suggests that higher serum levels of TNF-α may be linked to both local as well as widespread hyperalgesia. These results are congruent with experimental animal studies in which TNF-α could induce sensitisation.60 61 Similarly, in animal models, it was shown that a single injection of either IL-6 or IL-1β induced mechanical peripheral sensitisation.62 63 In the included studies, serum concentrations of IL-6 and IL-1β were not associated with pain sensitivity. Degradation of cartilage fragments causes production of inflammatory mediators in synovial cells, of which CRP is most discussed and considered to be an indicator of an acute inflammatory state.30 According to the results of two studies included in this review, there may be an association between CRP levels and pain sensitivity (TS and widespread hyperalgesia) (conclusion level 3). Patients with OA with elevated CRP levels experience lower pain thresholds on local and distant locations and less tolerance to cold-induced pain.
Apart from pain sensitivity, altered pain mechanisms in OA can be reflected by signs of neuropathic-like pain features.13 Inflammatory processes within the joint can cause continuous stimulation of nociceptors with subsequent sensitisation. Knee OA is characterised by chronic and low-grade inflammation64 in which nociceptors might be continuously stimulated with possible subsequent sensitisation and possibly the development of neuropathic-like pain.65–67 In our results, one cross-sectional study showed an association between local knee inflammation (synovitis) and neuropathic-like pain46 (conclusion level 4). No clear evidence was found regarding systemic inflammatory signs (cytokines) and their association with presence of neuropathic-like pain (conclusion level 4). Only one study reported consistent significant results on blood levels of proinflammatory cytokines which were found to be correlated with presence of neuropathic-like pain, but the correlation values did not go beyond the moderate level (ie, r values ≤0.5).48 However, the study sample included only patients with advanced knee OA and the analyses did not include potential confounders.48 Importantly, the results of Radojčić et al revealed that the association between synovial fluid IL-6 and neuropathic-like pain was explained by synovitis.46 However, further research is needed to strengthen the evidence. In addition to proinflammatory cytokines, biomarkers of extracellular matrix turnover (such as matrix metalloproteinases (MMPs) and derived degradation products of type I–II and III collagen) are also implicated in OA inflammation.30 One of the included studies measured serum levels of biomarkers of extracellular matrix turnover (C1M) and reported a positive significant association with neuropathic-like pain (β=0.229; 95% CI 0.036 to 0.422) depending on synovitis.46 This finding further supports the possibility of an association between inflammation and neuropathic-like pain. However, another study measured synovial fluid levels of MMP-3 and MMP-13 and found no correlation with neuropathic-like pain.45 These results were not included in the best-level synthesis as this review focused on proinflammatory cytokines which can directly trigger an increase in MMP synthesis68 as well as contribute to sensitisation of joint nociceptors.26
Our results need to be interpreted with caution as the included studies showed limitations. Statistical analyses varied widely between studies and most of these lack in inclusion of confounders. For example, out of three included studies presenting associations of data between inflammation and neuropathic-like pain, only one included potential confounders.46 Age, sex, gender, BMI, pain severity, depressive symptoms, pain catastrophising and use of antidepressants are important confounders to consider when investigating possible associations with neuropathic-like pain.69–71 In addition, only three (33%) of the included studies reported information on duration of symptoms. This is a limit as long history of symptoms may be associated with presence of sensitisation or neuropathic-like pain.72 Furthermore, the majority of the included studies measured biomarker levels in serum while, in line with the results of the current review, it has been proposed that local biomarkers (in synovial fluid) may be more promising to show the association with pain and pain mechanisms.73 The two studies investigating associations between cytokines and pain sensitivity have important limitations. The study of Imamura et al included only female participants, thus the results have limited generalisability.43 The study of Lee et al had a small sample size (n=26) with a majority of female participants (77%) and lack of covariates.44 Future studies are encouraged to measure biomarkers both in serum and synovial fluid. Moreover, nociceptive, nociplastic and neuropathic-like pain may occur in combination in some patients, making it challenging to differentiate the exact underlying pain mechanism.22 For example, results from screening questionnaires to measure neuropathic-like pain may be confounded by the concurrent presence and severity of nociceptive and/or nociplastic pain within the same subject.22 72 74 75 Thus, it is difficult to establish a clear association between markers of inflammation and specific altered pain mechanisms. Lastly, included studies had a cross-sectional design; therefore, no cause–effect relationship could be established. Future studies can strengthen the level of evidence by applying a longitudinal design with adequate sample size and are encouraged to implement appropriate confounders in the analyses.
This systematic review comprises strengths and limitations. We performed a systematic literature search on four major databases without any restriction on the publication date. We provided an extensive summary of the literature by including articles involving patients with knee OA diagnosed with different accepted criteria and investigated for inflammatory signs and altered pain perception with different methodologies. However, this approach has the disadvantage of increasing heterogeneity. In fact, due to the heterogeneity of the patients’ characteristics and outcome variables, we refrained from performing a meta-analysis. Lack of consistency across QST protocols remains a challenge within the field of OA.25 Because of different protocols of pain testing and a variety of inflammatory markers being used, combining and summarising data for developing an overview of current literature were challenging. However, we carefully evaluated the methodological quality of the included papers and performed the best level of evidence that takes into account both study design and RoB. Furthermore, due to the limited amount of included studies in each level of the best evidence synthesis, formal methods to assess potential publication bias (eg, funnel plots inspection) could not be performed. Lastly, including articles that assessed pain sensitivity by relying solely on QST had the drawback of excluding studies that investigated altered pain perception mechanisms using different methodologies. Alternative techniques, such as brain imaging (eg, functional MRI) or self-reported questionnaires (eg, central sensitisation inventory) may provide additional insights.
Conclusions
Local inflammatory signs of effusion/synovitis may be positively associated with neuropathic-like pain and signs of pain sensitivity. Included studies revealed a possible positive association between serum CRP levels and pain sensitivity. However, these results need to be interpreted with caution due to the limited amount of included studies along with the high heterogeneity in different methodologies. The associations between inflammatory signs and altered pain perception in patients with knee OA need to be explored further. Future high-quality studies with adequate sample size and long-term follow-up are needed to strengthen the level of evidence.
Footnotes
JS and PC contributed equally.
Contributors: PD was the primary person responsible for designing and conducting the study. PD and HM drafted the PROSPERO protocol and performed the systematic literature search. PC acted as the third reviewer to resolve conflicts. PD and HM extracted the data, and performed the descriptive analyses, the risk of bias assessment and the best evidence synthesis. PD drafted the article. All authors revised the manuscript critically for content. PD, JS and PC are the guarantors of this work and, as such, had full access to all study data and take responsibility for the integrity of the data and accuracy of the data analysis. All authors read and approved the final manuscript.
Funding: PD is funded by Research Foundation Flanders (FWO) (project grant no. G013318N).
Disclaimer: The funding source had no involvement in the study design, data extraction and interpretation, and writing of the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Not applicable.
References
- 1.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019;393:1745–59. 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. The Lancet 2016;388:1545–602. 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long H, Liu Q, Yin H, et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the global burden of disease study 2019. Arthritis Rheumatol 2022;74:1172–83. 10.1002/art.42089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers 2016;2:16072. 10.1038/nrdp.2016.72 [DOI] [PubMed] [Google Scholar]
- 5.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr Cartil 2013;21:1145–53. 10.1016/j.joca.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neill TW, Felson DT. Mechanisms of osteoarthritis (OA) pain. Curr Osteoporos Rep 2018;16:611–6. 10.1007/s11914-018-0477-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uivaraseanu B, Vesa CM, Tit DM, et al. Therapeutic approaches in the management of knee osteoarthritis (review). Exp Ther Med 2022;23:328. 10.3892/etm.2022.11257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finan PH, Buenaver LF, Bounds SC, et al. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum 2013;65:363–72. 10.1002/art.34646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wylde V, Beswick A, Bruce J, et al. Chronic pain after total knee arthroplasty. EFORT Open Rev 2018;3:461–70. 10.1302/2058-5241.3.180004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fayet M, Hagen M. Pain characteristics and biomarkers in treatment approaches for osteoarthritis pain. Pain Manag 2021;11:59–73. 10.2217/pmt-2020-0055 [DOI] [PubMed] [Google Scholar]
- 11.Güngör Demir U, Demir AN, Toraman NF. Neuropathic pain in knee osteoarthritis. Adv Rheumatol 2021;61:67. 10.1186/s42358-021-00225-0 [DOI] [PubMed] [Google Scholar]
- 12.Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage 2015;23:1043–56. 10.1016/j.joca.2015.02.163 [DOI] [PubMed] [Google Scholar]
- 13.Zolio L, Lim KY, McKenzie JE, et al. Systematic review and meta-analysis of the prevalence of neuropathic-like pain and/or pain sensitization in people with knee and hip osteoarthritis. Osteoarthritis and Cartilage 2021;29:1096–116. 10.1016/j.joca.2021.03.021 [DOI] [PubMed] [Google Scholar]
- 14.Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers 2017;3:17002. 10.1038/nrdp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosek E, Clauw D, Nijs J, et al. Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. Pain 2021;162:2629–34. 10.1097/j.pain.0000000000002324 [DOI] [PubMed] [Google Scholar]
- 16.Arendt-Nielsen L, Morlion B, Perrot S, et al. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain 2018;22:216–41. 10.1002/ejp.1140 [DOI] [PubMed] [Google Scholar]
- 17.Freynhagen R, Baron R, Gockel U, et al. PainDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. 10.1185/030079906X132488 [DOI] [PubMed] [Google Scholar]
- 18.Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain 2003;19:306–14. 10.1097/00002508-200309000-00004 [DOI] [PubMed] [Google Scholar]
- 19.Bouhassira D, Lantéri-Minet M, Attal N, et al. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136:380–7. 10.1016/j.pain.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 20.Bennett M. The LANSS pain scale: the leeds assessment of neuropathic symptoms and signs. Pain 2001;92:147–57. 10.1016/s0304-3959(00)00482-6 [DOI] [PubMed] [Google Scholar]
- 21.Gierthmühlen J, Schneider U, Seemann M, et al. Can self-reported pain characteristics and bedside test be used for the assessment of pain mechanisms? An analysis of results of neuropathic pain questionnaires and quantitative sensory testing. Pain 2019;160:2093–104. 10.1097/j.pain.0000000000001601 [DOI] [PubMed] [Google Scholar]
- 22.Bailly F, Cantagrel A, Bertin P, et al. Part of pain labelled neuropathic in rheumatic disease might be rather nociplastic. RMD Open 2020;6:e001326. 10.1136/rmdopen-2020-001326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arant KR, Katz JN, Neogi T. Quantitative sensory testing: identifying pain characteristics in patients with osteoarthritis. Osteoarthritis Cartilage 2022;30:17–31. 10.1016/j.joca.2021.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graven-Nielsen T, Arendt-Nielsen L. Peripheral and central sensitization in musculoskeletal pain disorders: an experimental approach. Curr Rheumatol Rep 2002;4:313–21. 10.1007/s11926-002-0040-y [DOI] [PubMed] [Google Scholar]
- 25.Rankin J, Rudy-Froese B, Hoyt C, et al. Quantitative sensory testing protocols to evaluate central and peripheral sensitization in knee oa: a scoping review. Pain Med 2022;23:526–57. 10.1093/pm/pnab285 [DOI] [PubMed] [Google Scholar]
- 26.Eitner A, Hofmann GO, Schaible HG. Mechanisms of osteoarthritic pain. studies in humans and experimental models. Front Mol Neurosci 2017;10:349. 10.3389/fnmol.2017.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Bosch MHJ. Inflammation in osteoarthritis: is it time to dampen the alarm(in) in this debilitating disease? Clin Exp Immunol 2019;195:153–66. 10.1111/cei.13237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roemer FW, Kassim Javaid M, Guermazi A, et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage 2010;18:1269–74. 10.1016/j.joca.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 29.Felson DT, Chaisson CE, Hill CL, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med 2001;134:541–9. 10.7326/0003-4819-134-7-200104030-00007 [DOI] [PubMed] [Google Scholar]
- 30.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis and Cartilage 2013;21:16–21. 10.1016/j.joca.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 31.Kuttapitiya A, Assi L, Laing K, et al. Microarray analysis of bone marrow lesions in osteoarthritis demonstrates upregulation of genes implicated in osteochondral turnover, neurogenesis and inflammation. Ann Rheum Dis 2017;76:1764–73. 10.1136/annrheumdis-2017-211396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Y, Chen X, Wang S, et al. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res 2021;9:20. 10.1038/s41413-021-00147-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaible H-G, von Banchet GS, Boettger MK, et al. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci 2010;1193:60–9. 10.1111/j.1749-6632.2009.05301.x [DOI] [PubMed] [Google Scholar]
- 34.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neuroscience Letters 2004;361:184–7. 10.1016/j.neulet.2003.12.007 [DOI] [PubMed] [Google Scholar]
- 35.Dainese P, Wyngaert KV, De Mits S, et al. Association between knee inflammation and knee pain in patients with knee osteoarthritis: a systematic review. Osteoarthritis Cartilage 2022;30:516–34. 10.1016/j.joca.2021.12.003 [DOI] [PubMed] [Google Scholar]
- 36.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Heart Lung and Blood Institute . Quality assessment tool for observational cohort and cross-sectional studies; 2021.
- 39.Iuamoto LR, Ito FLK, Tomé TA, et al. Effects of neuroplasticity in people with knee osteoarthritis: a systematic review of the literature. Medicine (Baltimore) 2022;101:e28616. 10.1097/MD.0000000000028616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hart MJ, Torres SJ, McNaughton SA, et al. Dietary patterns and associations with biomarkers of inflammation in adults: a systematic review of observational studies. Nutr J 2021;20:24. 10.1186/s12937-021-00674-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meeus M, Gebruers N. Health literacy: from reference to review. Leuven: Acco, 2016. [Google Scholar]
- 42.Arendt-Nielsen L, Eskehave TN, Egsgaard LL, et al. Association between experimental pain biomarkers and serologic markers in patients with different degrees of painful knee osteoarthritis. Arthritis Rheumatol 2014;66:3317–26. 10.1002/art.38856 [DOI] [PubMed] [Google Scholar]
- 43.Imamura M, Ezquerro F, Marcon Alfieri F, et al. Serum levels of proinflammatory cytokines in painful knee osteoarthritis and sensitization. Int J Inflam 2015;2015:329792. 10.1155/2015/329792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee YC, Lu B, Bathon JM, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res (Hoboken) 2011;63:320–7. 10.1002/acr.20373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Li Z, Li Y, et al. Profiling of inflammatory mediators in the synovial fluid related to pain in knee osteoarthritis. BMC Musculoskelet Disord 2020;21:99. 10.1186/s12891-020-3120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radojčić MR, Thudium CS, Henriksen K, et al. Biomarker of extracellular matrix remodelling C1M and proinflammatory cytokine interleukin 6 are related to synovitis and pain in end-stage knee osteoarthritis patients. Pain 2017;158:1254–63. 10.1097/j.pain.0000000000000908 [DOI] [PubMed] [Google Scholar]
- 47.Sofat N, Ejindu V, Heron C, et al. Biomarkers in painful symptomatic knee oa demonstrate that mri assessed joint damage and type ii collagen degradation products are linked to disease progression. Front Neurosci 2019;13:1016. 10.3389/fnins.2019.01016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tchetina EV, Glemba KE, Markova GA, et al. Development of postoperative pain in patients with end-stage knee osteoarthritis is associated with upregulation of genes related to extracellular matrix degradation, inflammation, and apoptosis measured in the peripheral blood before knee surgery. Life (Basel) 2020;10:224. 10.3390/life10100224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen KK, Siebuhr AS, Graven-Nielsen T, et al. Sensitization and serological biomarkers in knee osteoarthritis patients with different degrees of synovitis. Clin J Pain 2016;32:841–8. 10.1097/AJP.0000000000000334 [DOI] [PubMed] [Google Scholar]
- 50.Neogi T, Guermazi A, Roemer F, et al. Association of joint inflammation with pain sensitization in knee osteoarthritis: the multicenter osteoarthritis study. Arthritis Rheumatol 2016;68:654–61. 10.1002/art.39488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nanus DE, Wijesinghe SN, Pearson MJ, et al. Regulation of the inflammatory synovial fibroblast phenotype by metastasis-associated lung adenocarcinoma transcript 1 long noncoding RNA in obese patients with osteoarthritis. Arthritis Rheumatol 2020;72:609–19. 10.1002/art.41158 [DOI] [PubMed] [Google Scholar]
- 52.Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: the cytokine connection. Cytokine 2014;70:185–93. 10.1016/j.cyto.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mapp PI, Sagar DR, Ashraf S, et al. Differences in structural and pain phenotypes in the sodium monoiodoacetate and meniscal transection models of osteoarthritis. Osteoarthritis Cartilage 2013;21:1336–45. 10.1016/j.joca.2013.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alliston T, Hernandez CJ, Findlay DM, et al. Bone marrow lesions in osteoarthritis: what lies beneath. J Orthop Res 2018;36:1818–25. 10.1002/jor.23844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu W, Chen Y, Dou C, et al. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann Rheum Dis 2021;80:413–22. 10.1136/annrheumdis-2020-218089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgan M, Thai J, Nazemian V, et al. Changes to the activity and sensitivity of nerves innervating subchondral bone contribute to pain in late-stage osteoarthritis. Pain 2022;163:390–402. 10.1097/j.pain.0000000000002355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nees TA, Rosshirt N, Zhang JA, et al. Synovial cytokines significantly correlate with osteoarthritis-related knee pain and disability: inflammatory mediators of potential clinical relevance. J Clin Med 2019;8:1343. 10.3390/jcm8091343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu S, Deng Z, Chen K, et al. Cartilage tissue engineering: from proinflammatory and anti‑inflammatory cytokines to osteoarthritis treatments (review). Mol Med Rep 2022;25. 10.3892/mmr.2022.12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther 2017;19. 10.1186/s13075-017-1229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chakrabarti S, Hore Z, Pattison LA, et al. Sensitization of knee-innervating sensory neurons by tumor necrosis factor-α-activated fibroblast-like synoviocytes: an in vitro, coculture model of inflammatory pain. Pain 2020;161:2129–41. 10.1097/j.pain.0000000000001890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richter F, Natura G, Löser S, et al. Tumor necrosis factor causes persistent sensitization of joint nociceptors to mechanical stimuli in rats. Arthritis Rheum 2010;62:3806–14. 10.1002/art.27715 [DOI] [PubMed] [Google Scholar]
- 62.Brenn D, Richter F, Schaible HG. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum 2007;56:351–9. 10.1002/art.22282 [DOI] [PubMed] [Google Scholar]
- 63.Ebbinghaus M, Uhlig B, Richter F, et al. The role of interleukin-1β in arthritic pain: main involvement in thermal, but not mechanical, hyperalgesia in rat antigen-induced arthritis. Arthritis Rheum 2012;64:3897–907. 10.1002/art.34675 [DOI] [PubMed] [Google Scholar]
- 64.Robinson WH, Lepus CM, Wang Q, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2016;12:580–92. 10.1038/nrrheum.2016.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meng Y, Shen HL. Role of N-methyl-D-aspartate receptor NR2B subunit in inflammatory arthritis-induced chronic pain and peripheral sensitized neuropathic pain: a systematic review. JPR 2022;Volume 15:2005–13. 10.2147/JPR.S367982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDougall JJ. Osteoarthritis is a neurological disease - an hypothesis. Osteoarthr Cartil Open 2019;1:100005. 10.1016/j.ocarto.2019.100005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Q, Yaksh TL. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr Opin Anaesthesiol 2011;24:400–7. 10.1097/ACO.0b013e32834871df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molnar V, Matišić V, Kodvanj I, et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. IJMS 2021;22:9208. 10.3390/ijms22179208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fernandes GS, Valdes AM, Walsh DA, et al. Neuropathic-like knee pain and associated risk factors: a cross-sectional study in a UK community sample. Arthritis Res Ther 2018;20. 10.1186/s13075-018-1717-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hochman JR, Davis AM, Elkayam J, et al. Neuropathic pain symptoms on the modified paindetect correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage 2013;21:1236–42. 10.1016/j.joca.2013.06.023 [DOI] [PubMed] [Google Scholar]
- 71.Kim HJ, Ban MG, Yoon KB, et al. Neuropathic-like pain symptoms and their association with muscle strength in patients with chronic musculoskeletal pain. JCM 2022;11:5471. 10.3390/jcm11185471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lluch E, Nijs J, Courtney CA, et al. Clinical descriptors for the recognition of central sensitization pain in patients with knee osteoarthritis. Disabil Rehabil 2018;40:2836–45. 10.1080/09638288.2017.1358770 [DOI] [PubMed] [Google Scholar]
- 73.Felson DT. The current and future status of biomarkers in osteoarthritis. J Rheumatol 2014;41:834–6. 10.3899/jrheum.140094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szewczyk AK, Jamroz-Wiśniewska A, Rejdak K. Possible neuropathic pain in clinical practice-review on selected diagnostic tools and its further challenges. Diagnostics (Basel) 2022;13:108. 10.3390/diagnostics13010108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soni A, Wanigasekera V, Mezue M, et al. Central sensitization in knee osteoarthritis: relating presurgical brainstem neuroimaging and paindetect-based patient stratification to arthroplasty outcome. Arthritis Rheumatol 2019;71:550–60. 10.1002/art.40749 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002945supp001.pdf (1.8MB, pdf)
rmdopen-2022-002945supp002.pdf (108.1KB, pdf)