Abstract
The activity of the alternative pathway is affected by a number of factors, including the level and reduction state of the alternative oxidase (AOX) protein, and the reduction state of the ubiquinone pool. To investigate the significance of these factors for the rate of alternative respiration in vivo, we studied root respiration of six wild monocotyledonous grass species that were grown under identical controlled conditions. The activity of the alternative pathway was determined using the oxygen isotope fractionation technique. In all species, the AOX protein was invariably in its reduced (high activity) state. There was no correlation between AOX activity and AOX protein concentration, ubiquinone (total, reduced, or oxidized) concentration, or the reduction state of the ubiquinone pool. However, when some of these factors are combined in a linear regression model, a good fit to AOX activity is obtained. The function of the AOX is still not fully understood. It is interesting that we found a positive correlation between the activity of the alternative pathway and relative growth rate; a possible explanation for this correlation is discussed. Inhibition of the AOX (with salicylhydroxamic acid) decreases respiration rates less than the activity present before inhibition (i.e. measured with the 18O-fractionation technique).
When herbaceous plants are grown with free access to nutrients, they exhibit inherent differences in relative growth rate (RGR) and rates of nutrient uptake (Poorter and Remkes, 1990; Garnier, 1991; Poorter et al., 1991; Van der Werf et al., 1992). For example, fast-growing species exhibit RGR values that are more than 3-fold higher than those of slow-growing species (Poorter and Remkes, 1990). In a similar manner, the rate of net NO3− uptake is 4- to 6-fold higher in fast-growing species than in slow-growing ones (Poorter et al., 1991). Rates of root respiration are expected to be higher because more respiratory energy is needed for growth and ion uptake. Although the measured rates of root respiration are higher (approximately 1.7-fold) in fast-growing species than in slow-growing ones, they are not as high as predicted from their high rates of growth and ion uptake. Calculations about the specific respiratory costs for energy-requiring processes (Poorter et al., 1991) suggested that fast-growing species should exhibit 3-fold higher rates of respiration than their slow-growing counterparts. Scheurwater et al. (1998) concluded that the major cause of the relatively low rates of root respiration in fast-growing grasses is the lower specific costs for nitrate uptake in fast-growing grasses compared with their slow-growing counterparts.
Another possible explanation for the relatively low rate of root respiration in fast-growing species might be the occurrence of lower relative activities of the non-phosphorylating alternative pathway, compared with that in slow-growing species. The cytochrome (cyt) pathway and the alternative pathway constitute the respiratory electron-transport pathways of plant mitochondria. In contrast to the cyt pathway, the alternative pathway does not contribute to the generation of a proton-motive force beyond the branch point (ubiquinone; Vanlerberghe and McIntosh, 1997). The alternative oxidase (AOX) protein is found in every examined plant species and in almost every plant organ. The AOX gene is encoded by a small gene family that exhibits highly conserved regions (Whelan et al., 1996; Ito et al., 1997; Vanlerberghe and McIntosh, 1997). Taken together, these findings suggest that the alternative pathway plays a vital role in plant functioning, but a clear function for the alternative pathway has yet to be established.
If the relative activity of the alternative pathway in fast-growing species were lower than that in slow-growing species, the production of respiratory energy (ATP) per unit oxygen consumption would be higher. To test if fast-growing species have a lower relative alternative pathway activity, we studied six monocotyledonous grass species, all grown under the same controlled conditions. The six wild grass species differ in their RGR (mg g−1 d−1): Poa annua (272), Poa alpina (166), Poa compressa (188), Poa pratensis (182), Poa trivialis (255), and Holcus lanatus (268; Van Arendonk and Poorter, 1994; Atkin et al., 1996). The same differences in RGR between these species have been found by various authors (Scheurwater et al., 1999). There are three fast-growing and three slower growing species. The RGR of a whole plant is closely related to the separate RGR of the roots or that of the shoot in vegetative herbaceous species (Hunt and Lloyd, 1987; Hunt and Cornelissen, 1997). P. annua is an annual species, whereas the others are all perennials. All the species occur naturally in lowland regions, except P. alpina, which is a subalpine species (Van Arendonk and Poorter, 1994; Atkin et al., 1996).
A determination of the activity of the alternative pathway in six wild grass species also raises questions about the main factors that control this activity. The total concentration of the protein is certainly important, but no correlation between activity and concentration can be expected in the six species if there were large differences in substrate concentration or AOX reduction state. Therefore, the reduction state of the AOX protein and the substrate concentration (reduction state of the ubiquinone pool [Qr/Qt]) were also determined. In addition, we explored the relationships between the root AOX protein concentration, alternative pathway activity, and other respiratory parameters (e.g. cyt c oxidase and ubiquinone concentration).
RESULTS
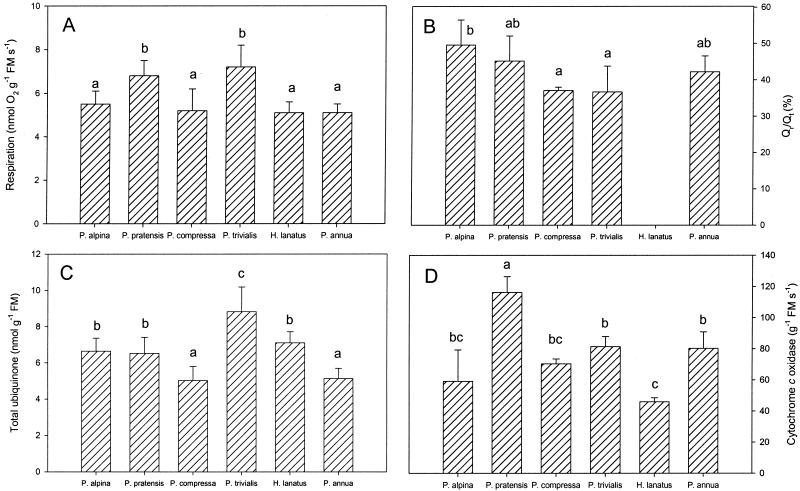
Total respiration rates differed for the six species (between 5.1–7.2 nmol O2 g−1 fresh mass [FM] s−1); P. pratensis and P. trivialis had a significantly faster rate of root respiration than the other four species (Fig. 1A). Root respiration was either stimulated (6%) or inhibited (up to 21%) by salicylhydroxamic acid (SHAM), and the KCN-resistant SHAM-sensitive respiration was between 25% and 40% (Table I).
Figure 1.
Respiration (A), Qr/Qt (B), ubiquinone concentration (C), and cyt c oxidase activity (D) in P. alpina, P. pratensis, P. compressa, P. trivialis, H. lanatus, and P. annua. Error bars represent sd. The number of replicates was at least four; columns with a different letter are significantly different (per measured parameter).
Table I.
RGR (mg g−1 d−1; Van Arendonk and Poorter, 1994; Atkin et al., 1996)
| Species | RGR | SHAM Sensitive | AOX “Capacity” |
|---|---|---|---|
| P. alpina | 166 | 5.3 ± 5.2 b | 30.6 ± 5.0 ab |
| P. pratensis | 182 | 18.2 ± 2.5 c | 40.8 ± 7.2 b |
| P. compressa | 188 | −5.9 ± 7.8 a | 30.3 ± 3.0 ab |
| P. trivialis | 255 | 8.7 ± 0.4 bc | 26.8 ± 7.4 ab |
| H. lanatus | 268 | 20.6 ± 1.8 c | 25.1 ± 9.7 a |
| P. annua | 272 | 11.2 ± 4.6 b | 38.6 ± 5.9 ab |
Inhibition of respiration (percentage) after a treatment in six monocotyledonous species in the presence of SHAM or KCN. The SHAM-sensitive respiration is corrected with the control and the AOX “capacity” is the KCN-insensitive, SHAM-sensitive respiration (see “Materials and Methods”). Mean and sd are presented. Means with the same character per treatment are not significantly different (P = 0.05, n ≥ 4). See Figure 1A for the respiration rate.
The 18O-fractionation technique was used to measure the activity of the alternative pathway. Fractionation values for the alternative pathway (with KCN) and for the cyt pathway (with SHAM) in all five Poa spp. were similar to values reported previously for mitochondria isolated from nongreen tissue (Ribas-Carbo et al., 1997; Table II).
Table II.
Fractionation (Δ in %), as defined by Farquhar and Richards (1984), of the alternative oxidase (Δalt; with KCN), cytochrome oxidase (Δcyt; with SHAM), and control (no additions) measurements (Δ)
| Species | Δalt | Δcyt | Δ | % Alt Path | Alt Path |
|---|---|---|---|---|---|
| % | |||||
| P. alpina | 25.34 ± 0.15(3) | 19.16 ± 0.28(3) | 20.49 ± 0.23(4) | 22 ± 4 | 0.65 ± 0.10 |
| P. pratensis | 26.33 ± 0.73(2) | 20.10 ± 0.06(2) | 20.93 ± 0.16(4) | 13 ± 3 | 0.63 ± 0.17 |
| P. compressa | 25.17 ± 0.38(3) | 19.60 ± 0.22(3) | 20.06 ± 0.50(3) | 11 ± 7 | 0.36 ± 0.24 |
| P. trivialis | 26.06 ± 0.64(2) | 18.69 ± 0.19(2) | 22.29 ± 0.48(4) | 49 ± 7 | 2.02 ± 0.48 |
| H. lanatus | 19.84 ± 0.27(2) | – | – | – | – |
| P. annua | 26.55 ± 0.10(3) | 19.51 ± 0.32(3) | 21.61 ± 0.44(6) | 30 ± 6 | 1.18 ± 0.25 |
The values are given both as percentage alternative path (% alt path) and absolute activity (alt path in nmol O2 g−1 FM s−1). Mean and se; no. in parentheses is the no. of replicates.
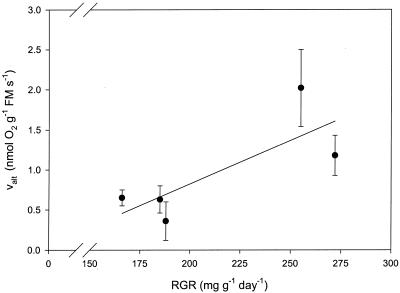
We were not able to measure the activity of the alternative pathway in H. lanatus because the fractionation by the AOX (in the presence of KCN) did not give consistent results. There was a positive trend (Pearson one-tailed correlation coefficient 0.78, P = 0.061) between the RGR (measured by Van Arendonk and Poorter, 1994; Atkin et al., 1996, under the same conditions as used before in our laboratory) and alternative pathway activity (Fig. 2). There was no correlation between the life history trait (annual/perennial) or the natural habitat (lowland/subalpine) and alternative or cyt pathway activity.
Figure 2.
Activity of the AOX versus RGR (mg g−1 d−1). RGR was measured previously in our laboratory under the same conditions used to grow plants for the alternative pathway measurements (Van Arendonk and Poorter, 1994; Atkin et al., 1996). From left to right, P. alpina, P. pratensis, P. compressa, P. trivialis, and P. annua. Pearson one-tailed correlation coefficient 0.78, P = 0.061.
AOX Activation State and Activity
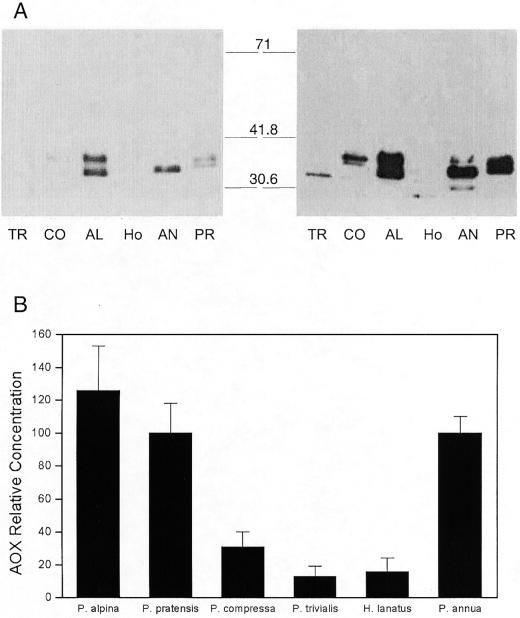
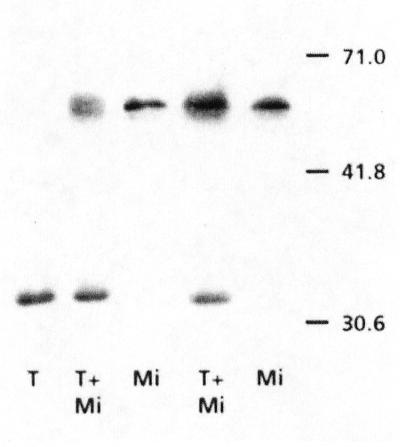
In an effort to understand the reason for the observed differences in alternative pathway activity in the various species, several parameters associated with AOX and respiratory biochemistry were assayed. There were large differences (almost 10-fold) in AOX protein concentration between the species (Fig. 3). Not one of the six species had an oxidized (covalently bound) form of the AOX, which should appear around 66 kD (Umbach and Siedow, 1995). When mitochondrial extracts (with oxidized AOX) were added to tissue just before the extraction, there was no change between the oxidized and reduced form of the AOX protein from the whole tissue or from the isolated mitochondria (Fig. 4).
Figure 3.
A, Immunoblots of AOX (detected with monoclonal antibodies) in whole root tissue extracts from P. trivialis (TR), P. compressa (CO), P. alpina (AL), H. lanatus (HO), P. annua (AN), and P. pratensis (PR) roots. One example of a blot with a short (left) and a long (right) exposure. B, Relative AOX concentration (intensity of the bands) for P. annua is set as 100%. Average concentration (%) and se; samples are from two different batches, n ≥ 5.
Figure 4.
Immunoblots of AOX (detected with monoclonal antibodies) isolated from whole tissue (T) or isolated mitochondria (Mi, 2×) and from whole tissue where a mitochondrial extract was added (T + Mi, 2×) to the tissue just before the extraction (P. annua roots).
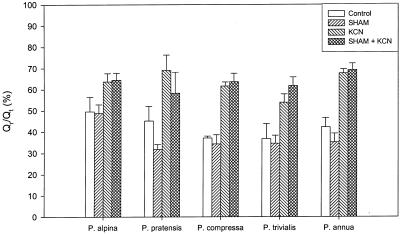
The total ubiquinone concentration was between 5.1 and 8.8 nmol g−1 FM (Fig. 1C). Substrate concentration (Qr/Qt) for both oxidizing pathways was different (between 37%–49%) for the species used (Fig. 1B). It was not possible to obtain reliable Qr/Qt measurements in H. lanatus; the measured values varied and were much more oxidized compared with the other five species. The Qr/Qt increased after addition of KCN and KCN with SHAM, but SHAM alone did not increase Qr/Qt. In P. annua and P. pratensis there was even a decrease of the Qr/Qt after SHAM addition (Fig. 5). Possibly because of an inactive ubiquinone pool (Millenaar et al., 2000), the Qr/Qt does not increase to 1 after addition of a combination of KCN and SHAM. It has been found before (in isolated mitochondria) that a fraction of the ubiquinone pool is not redox active (Van den Bergen et al., 1994; Ribas-Carbo et al., 1995). This inactive component of the ubiquinone pool cannot accept electrons from the miochondrial dehydrogenases and hence cannot act as a substrate for the cyt and alternative pathways.
Figure 5.
Qr/Qt, without inhibitors (control) or in the presence of SHAM, KCN, or SHAM + KCN in P. alpina, P. pratensis, P. compressa, P. trivialis, H. lanatus, and P. annua. Error bars represent sd and the number of replicates was at least three.
Carbonyl cyanide-m-chlorophenyl hydrazone (CCCP) alone (18% ± 4.6%) and in combination with valinomycin (22% ± 3.6%) stimulated respiration, but valinomycin alone (7% ± 3.2%) did not significantly stimulate respiration rate (n > 6, averages with ses). After addition of uncouplers (CCCP and/or valinomycin), the Qr/Qt did not change compared with the control in P. annua roots (Table III). Qr/Qt decreased or did not change after addition of SHAM and uncouplers together.
Table III.
Qr, Qt after treatment with CCCP, valinomycin, SHAM, and KCN in roots of P. annua
| Treatment | Qr/Qt |
|---|---|
| Control | 45.0 ± 5.0 c |
| SHAM | 36.2 ± 3.8 ab |
| KCN + SHAM | 67.0 ± 6.7 d |
| CCCP | 43.5 ± 2.7 bc |
| CCCP + SHAM | 37.8 ± 1.0 a |
| Valinomycin | 42.6 ± 4.6 bc |
| Valinomycin + SHAM | 37.1 ± 4.0 abc |
| Valinomycin + CCCP | 38.8 ± 1.6 abc |
| Valinomycin + CCCP + SHAM | 39.7 ± 4.8 bc |
Average values and sds are shown. Averages with a different letter are significantly different (Tukey B, P < 0.05), n is 4 to 8.
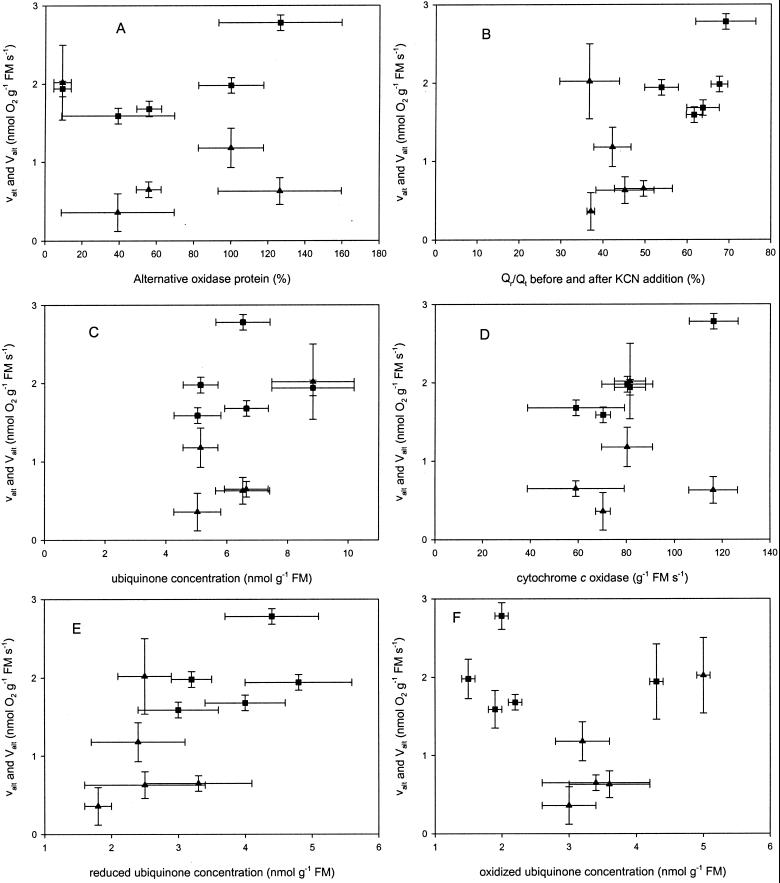
There was no relation between the activity of the AOX or KCN resistance and the concentration of AOX protein, Qr/Qt, or total ubiquinone concentration (Fig. 6). There was no relation between the activity of the AOX and the cyt c oxidase concentration; however, there was a correlation between KCN-resistant respiration and cyt c oxidase.
Figure 6.
Relation between activity (▴, valt) and “capacity” (▪, Valt, KCN-resistant, SHAM-sensitive respiration) with: A, AOX concentration; B, Qr/Qt before (activity) or after (“capacity”) addition of KCN; C, total ubiquinone concentration; D, cyt c oxidase capacity; E, reduced; and F, oxidized ubiquinone concentration. Error bars represent sd.
DISCUSSION
RGR and AOX Activity
Fast-growing species have a relatively low rate of root respiration when compared with slow-growing ones, considering their high rates of growth and ion uptake. A possible explanation might be that fast-growing species have a lower relative activity of the non-phosphorylating alternative pathway (Scheurwater et al., 1998).
Total respiration rate measured with the gas chromatography (GC) during the activity measurements (18O fractionation) at Duke University was 22% to 44% lower as compared with the respiration rates measured with the oxygen electrode in previous experiments at Utrecht University. The growing conditions may have been slightly different; moreover, the duration of the measurements was about twice as long with the GC as compared with the measurements with the oxygen electrode. During the measurements the roots are detached from the plant, and the respiration decreases during the measurement. After correction for the duration of the measurement the difference in total respiration is much smaller; the GC measurements are 11% to 26% lower as compared with the oxygen electrode. Any decrease in total respiration does not influence the partitioning between the two pathways because the regression lines obtained to calculate the partitioning have r2 values higher than 0.995. The lines would, in fact, have been curvilinear if the partitioning had changed during the measurement.
All the measurements where taken after 5 to 6 weeks, and not all species may have been at exactly the same developmental stage. Millar et al. (1998) found differences in AOX activity at different developmental stages of soybean (Glycine max) roots, but only in very young roots (d 4) was the AOX activity lower compared with than in older roots (d 7 and 17). The RGR can also differ during plant development; however, the difference in RGR between species will remain (Poorter and Pothmann, 1992). There was no difference in respiration rate between the species used. One reason is the smaller range in RGR among the grasses used here, compared with those by Poorter et al. (1991). However, on the basis of the theoretically calculated respiration rates differences in respiration rate were expected (Poorter et al., 1991).
There was no positive correlation, but actually a positive trend (Pearson one-tailed correlation coefficient 0.78, P = 0.061), between the RGR and the activity of the alternative pathway (Fig. 2). Therefore, contrary to what might be expected, fast-growing species have a relatively higher alternative pathway activity compared with slow-growing ones. As a consequence, the low root respiration rate of the fast-growing species compared with that of the slow-growing ones is probably not related to more efficient ATP production.
The flux of carbohydrates to the roots of a fast-growing species is about 3-fold higher than that in a slow-growing one (Poorter et al., 1990). This could result in those plants having a higher carbohydrate production also having a higher alternative pathway activity. However, 1 or 24 h after sugar addition and after 4 d of sugar starvation, no effect on the activity of the alternative pathway activity was observed (Millenaar et al., 2000; F.F. Millenaar, M.A. Gonzàlez-Meler, J.N. Siedow, A.M. Wagner, and H. Lambers, unpublished data). Therefore, it is unlikely that plants with a higher carbohydrate level have a higher alternative pathway activity.
The question still remains whether the relatively greater contribution of respiration via the alternative pathway is a prerequisite for fast growth, or whether plants grow faster despite the apparent larger waste of energy (and carbohydrates) via the AOX. In several papers, a role of the AOX in the protection against oxidative stress has been suggested (Purvis and Shewfelt, 1993; Wagner and Moore, 1997). The AOX can stabilize the Qr/Qt in vivo (Millenaar et al., 1998), which may prevent damage by reactive oxygen species, because radical formation is linked to the relative reduction state of the respiratory chain (Forman and Boveris, 1982). It was shown recently that plant cells that have a genetically low concentration of AOX show an increase in radical production, and cells with higher amounts of AOX protein have less oxygen free radical production (Maxwell et al., 1999). In a similar manner, maize (Zea mays) plants that overproduce iron superoxide dismutase from Arabidopsis cope better with oxidative stress, and their RGR was also faster as compared with the control plants (Van Breusegem et al., 1999). Combining these results with the present findings, it is tempting to suggest that a high alternative pathway activity leads to less oxygen free radical production, and hence allows faster growth.
AOX Activity and KCN Resistance
There were large differences in AOX activity (measured with the 18O-fractionation technique) between the investigated species. In the recent past, our understanding of the mechanisms that account for activity of the alternative pathway in isolated mitochondria has increased substantially. We now know that the AOX can become more activated when the AOX protein is reduced or when α-keto acids, e.g. pyruvate, are present in sufficiently high concentration (Millar et al., 1993; Umbach and Siedow, 1993; Umbach et al., 1994; Hoefnagel et al., 1995; Millar et al., 1996). There might be an unknown activation mechanism to activate AOX as suggested by Vanlerberghe et al. (1998) because a mutated AOX protein missing a regulatory cystine residue showed an unexpected activity.
During the isolation of mitochondria the reduction state of the AOX changes to a more oxidized (less active) form (Umbach and Siedow, 1997; Millenaar et al., 1998). However, when mitochondrial extracts (with oxidized AOX) were added to tissue just before the extraction, there was no change between the oxidized and reduced form of the AOX protein from the whole tissue or from the isolated mitochondria (Fig. 4). Therefore, the procedure that we used for whole tissue extracts does not change the reduction state of the protein.
We have previously shown that the AOX protein invariably occurs in its reduced form during the light period in roots of P. annua (Millenaar et al., 1998). In a similar manner, both in control leaves of Arabidopsis and in leaves infected with Pseudomonas syringae no oxidized form of the AOX protein was observed (Simons et al., 1999). There is also no oxidized form of the AOX protein in roots of P. annua after an exposure of the plants to 4 d of low light or complete darkness (Millenaar et al., 2000). In a similar manner, none of the six species used in this study had an oxidized form (less active, covalently bound dimer) of AOX (Fig. 3). Although these studies showed that the AOX protein is mainly in the active form, this does not necessary mean that the inactive form of the protein does not exists in other species or under different growing conditions.
The present species were also chosen on the basis of their different SHAM sensitivity. Several studies have revealed a correlation between the rate of respiration that is sensitive to inhibition by SHAM in the presence of cyanide and the amount of the AOX. Hilal et al. (1997) found a correlation between the immunolocalization of the AOX in soybean roots and hypocotyls and SHAM-sensitive O2 uptake in the presence of KCN. It was also shown that the SHAM-sensitive O2 uptake in the presence of KCN depends on AOX levels in transgenic plants (Vanlerberghe et al., 1994; Hiser et al., 1996). However, no data are available on the occurrence of this relationship in different species and in vivo. We expected that the chosen species also differed in the concentration of AOX protein. There were large differences (almost 10-fold) in the signal observed in immunoblots (Fig. 3). The AOX antibody binds to a highly conserved region of the protein (Finnegan et al., 1999); therefore, the signal obtained on immunoblots is probably a reflection of the AOX concentration.
In all of the present wild monocotyledonous species the AOX is in the active form, and also the pyruvate concentration is probably sufficiently high to fully activate AOX in vivo (Millar et al., 1998; Millenaar et al., 1998), although there is no conclusive evidence. Until now, it was not possible to directly assess the influence of pyruvate on the AOX activity in vivo. Therefore, one might expect a correlation between the concentration of the AOX protein and its activity; such a correlation, however, was not found (Fig. 6A). In the literature there is also no clear relationship between the AOX concentration and the AOX activity (18O fractionation). During a variety of stresses the concentration of the AOX increases. Infection of tobacco (Nicotiana tabacum) leaves with tobacco mosaic virus resulted in an increased concentration of AOX; however, no change in the activity of the alternative pathway was observed (Lennon et al., 1997). In mung bean (Vigna radiata) grown at 19°C, the concentration of the AOX increased over 2-fold in both hypocotyls and leaves compared with plants grown at 28°C. The plants grown at 19°C maintained a higher activity of the alternative pathway compared with the ones grown at 28°C. This response, however, was not observed in soybean cotyledons, despite the increased concentration of AOX (Gonzàlez-Meler et al., 1999). There is no clear relation between the AOX concentration and activity, despite the lack of differences in the activation state (reduction state of the AOX protein and pyruvate concentration).
The substrate concentration (Qr/Qt) also influences the activity of the AOX. The different species had different reduction states (Qr/Qt) as well as different total ubiquinone concentrations (Qt). It should be noted that it is not valid to compare Qr/Qt values if the Qt is different; however, there was no relation between the total ubiquinone concentration or Qr/Qt and the activity of the AOX (Fig. 6, B and C). Moreover, we found no relation between the concentration of reduced or oxidized ubiquinone and the activity of the AOX (Fig. 6, E and F). Ribas-Carbo et al. (1997) came to the conclusion that the AOX concentration is limiting the AOX activity in etiolated soybean cotyledons and the ubiquinone concentration is limiting the AOX activity in soybean roots.
The different species have different activities of the AOX, and the two changing factors are concentration of AOX and the substrate concentration (Qr/Qt and Qt or reduced ubiquinone [Qr] and oxidized fraction of the ubiquinone pool [Qox]); however, none of these factors alone can explain the activity in these species. It is apparent that no one of the known factors has a crucial role in determining the activity because there is no correlation between any of the known factors and the activity (Fig. 6). Assuming that these factors are important, and hence can counteract each other, e.g. when large amounts of substrate are present, but concentration of AOX is low, the activity will still not be very high. Therefore, it is reasonable to suggest that a combination of two or more factors is determining the AOX activity.
An Attempt to Model the AOX Activity and Capacity
We analyzed if more factors can be combined in a multiple linear regression model to explain the AOX activity, e.g. activity or capacity = [AOX] × a1 + Qr/Qt × a2 + Qtot × a3 or [AOX] × a1 + Qr × a4 + Qox × a5. The coefficients (a1–a5) are estimated via the least-square method (SPSS, Inc., Chicago). The outcome of the model predicts activities. However, because it is an additive experimental model, it is only possible to use values between the minimum and maximum values that are used to calculate the coefficients. For instance, calculating the activity with an AOX concentration of 0% will obviously not result in a proper activity.
If AOX concentration and Qr/Qt and Qt are combined in a multiple linear regression, the activity can be explained with an r2 of 0.91 (Table IV). If the AOX concentration, Qr, and Qox are used as parameters, then the activity is explained with an r2 of 0.90. The AOX concentration has little influence because if the AOX concentration is taken out in the activity model, then the regression becomes significant (Table IV).
Table IV.
The coefficients of several models (multiple linear regression) to explain the activity and “capacity” (KCN-resistant respiration, corrected for residual respiration) with the alternative oxidase concentration, reduction state of the ubiquinone pool before or after addition of KCN, and the total ubiquinone concentration; or with the reduced and oxidized ubiquinone pool instead of the Qr/Qt and Qt e.g. activity model 1 = [AOX] × a1 + Qr/Qt × a2 + Qt × a3
| Model | Coefficients
|
Statistics
|
|||||
|---|---|---|---|---|---|---|---|
| AOX (a1) | Qr/Qt (a2) | Qt (a3) | Qr (a4) | Qox (a5) | r2 | p | |
| Activity 1 | 0.003 | −0.039 | 0.372 | – | – | 0.91 | n.s.a |
| Activity 2 | – | −0.027 | 0.328+ | – | – | 0.90 | * |
| Activity 3 | −0.001 | – | – | −0.287 | 0.493 | 0.90 | n.s. |
| Activity 4 | – | – | – | −0.369 | 0.528 | 0.90 | * |
| Capacity 1 | 0.001 | 0.023 | 0.079 | – | – | 0.97 | ** |
| Capacity 2 | – | 0.024 | 0.072 | – | – | 0.97 | ** |
| Capacity 3 | −0.009 | – | – | 1.325* | −1.061+ | 1.00 | ** |
| Capacity 4 | – | – | – | 0.807* | −0.488 | 0.99 | ** |
Capacity model 1 = [AOX] × a1 + Qr × a4 + Qox × a5. The a1 through a5 coefficients correspond to the respective coefficients listed in the table. Note that some models do not contain the AOX concentration and all models go through the origin. The fit of the linear model is represented by the r2 and the p value shows the significance of the model; the significance of the individual coefficients is also shown; +, p < 0.1; *, p < 0.05; and **, p < 0.01.
n.s., Not significant.
Adding cyt c oxidase or cyt pathway activity hardly improves the model, and replacing it with one of the three factors decreases the accuracy of the model. In a similar manner, there is no relation between KCN resistance (corrected for residual respiration) and the AOX concentration, Qr/Qt, and Qt (Fig. 6). If all these factors are combined, then again, the KCN-resistant respiration can be fitted with an r2 of 0.97 (Table IV). If AOX concentration, Qr, and Qox are used as parameters, then the KCN-resistant respiration is explained with an r2 of 1.00.
When combining AOX concentration, Qr/Qt, and Qt (or Qr and Qox) it is possible to explain both the activity and the KCN-resistant respiration (“capacity”). The individual coefficients are not discussed because most of the coefficients are not significant, although the regressions are, which is due to the low number of replicates.
Future experiments where one or more factors (concentration of the AOX, cyt c oxidase, ubiquinone, and Qr/Qt) can be modified should resolve if our results reflect causal relations or not. However, we conclude that there does not seem to be one single most important factor that explains the AOX activity, but a combination of factors. It will be interesting to examine more plants whereby the several factors can be changed more independently from each other.
SHAM Inhibition and the Effect of Uncouplers
Addition of SHAM alone to the roots of the grass species had different effects on their root respiration, from a slight stimulation (5%) to inhibition (20%) of respiration. The activity of the AOX was 10% to 50% of the respiration (measured with 18O fractionation). With the exception of P. pratensis (possibly caused by peroxidases), the SHAM inhibition of respiration (absolute or in percentage) is less than the activity as estimated with the 18O fractionation. Therefore, the cyt pathway has become more active after SHAM addition compared with the situation before SHAM addition. The cyt pathway can become more active if Qr/Qt increases (Van den Bergen et al., 1994; Wagner and Krab, 1995) or if there is a shift from closer to state 4 to closer to state 3. Addition of SHAM did not cause any increase in Qr/Qt; there was even a decrease in some species. Qr/Qt increased or did not further change when KCN was applied first, before SHAM was added. Therefore, the cyt pathway became more active without an increase in Qr/Qt after SHAM addition. This suggests a shift from nearer to state 4 toward state 3 after SHAM addition. The flux through the cyt pathway is apparently not only affected by the Qr/Qt.
To establish whether state 4 to state 3 transitions are an issue here, we measured the reaction upon addition of SHAM in the presence of uncoupler. The proton gradient across the inner mitochondrial membrane can be removed by adding an uncoupler, and hence the control of ADP/ATP ratios will be impaired. The cyt pathway cannot change from state 4 to state 3 if there is no proton gradient. Addition of CCCP and/or valinomycin did not change Qr/Qt compared with the control (Table III). Addition of SHAM after CCCP and/or valinomycin resulted in no change or a decrease in Qr/Qt. If we assume that the activity of the AOX is not decreased after addition of uncouplers, then the activity of the cyt pathway appears to increase without a change from closer to state 4 toward state 3 and without an increase in Qr/Qt. Also, Wagner and Wagner (1995) found no change in Qr/Qt after benzhydroxamic acid addition, independent of the presence of an uncoupler in a Petunia hybrida cell suspension. At present, the only possible explanation is that, when the rate of respiration changes with no change in Qr/Qt, the dehydrogenase activity changes as much as the oxidase activity.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Roots of 5- to 6-week-old plants were used for all measurements. Seeds were germinated on moistened filter paper for 1 week. The seedlings were then transferred to sand for 1 week, after which they were placed in 30-L containers (24 plants per container) and grown on an aerated nutrient solution (Poorter and Remkes, 1990; with the exception that the Fe concentration was doubled). The nutrient solution was replaced every week and the pH was adjusted every 2nd d to 5.8. Plants grew at 20°C, 60% (v/v) relative humidity, with a photoperiod of 14 h at 450 μmol m−2 s−1 (photosynthetically active radiation).
Respiration of Intact Roots
Roots (1.5–2.0 g FM) were severed and transferred to an airtight cuvette containing nutrient solution without Fe, and respiration was measured as a decrease of the oxygen concentration using a Clark-type electrode (Yellow Springs Instrument Co., Yellow Springs, OH; Lambers et al., 1993). The alternative pathway was inhibited with 2 mm (Poa alpina), 3 mm (Poa compressa, Poa annua, Poa trivialis, and Holcus lanatus), or 10 mm (Poa pratensis) SHAM (1 m stock solution in methoxyethanol). To inhibit the cyt pathway, KCN was used at a concentration of 0.5 mm {0.5 m stock solution in 20 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], pH 8}. To uncouple respiration, 0.5 μm CCCP and/or 1.5 μm valinomycin (both 1 mm stock in methanol) were used. The rate of respiration in the time interval between 10 to 15 min after addition of the inhibitors was used to calculate the percentage inhibition. Because the control respiration rate decreased somewhat during the measurement period (3%–15%), the rates of respiration after addition of SHAM, CCCP, or valinomycin were corrected for this decline. The KCN-resistant respiration (AOX “capacity”) was corrected for a residual component of respiration (measured in the presence of both KCN and SHAM). The true maximum capacity cannot be reached easily in vivo because the capacity should then be measured with a Qr/Qt of 1.0, which cannot be reached under normal conditions in vivo because the dehydrogenases decrease in activity at high Qr/Qt. Therefore, the term “capacity” is placed between quotes.
AOX Protein
The total protein content of root extracts was determined according to Lowry et al. (1951). Mitochondria were isolated according to Umbach and Siedow (1997). For AOX protein detection, root extracts were prepared from 100 mg (FM) of frozen root material that was homogenized in liquid nitrogen using a mortar and pestle and then suspended in a total volume of 400 μL of protein sample mix (62.5 mm Tris-HCl [pH 6.8], 2% [v/v] SDS, 10% [v/v] glycerol, and 0.001% [v/v] bromphenol blue). After centrifugation for 10 min at 16,000g in an Eppendorf centrifuge to precipitate cell debris, the proteins were separated by SDS/PAGE according to Laemmli (1970), and subsequently electrotransferred to nitrocellulose filters using blot transfer buffer (25 mm Tris, 192 mm Gly, and 20% [v/v] methanol). Immunodetections of the AOX protein were carried out according to the product protocol of the AOX monoclonal antibody (GT Monoclonal Antibodies, Lincoln, NE). Antibodies from Dr. Thomas E. Elthon (Elthon et al., 1989) were used as the primary antibody (1:100). Anti-mouse IgG Fab fragments conjugated to peroxidase (Boehringer Mannheim, Mannheim, Germany) were used as the secondary antibody (1:25,000), using SuperSignal ULTRA Chemiluminescent Substrate according to the protocol supplied by Pierce (Rockford, IL). There was no difference in the total protein concentration in the samples between the species; therefore, the same amount of protein per gram FM was loaded onto the gels.
To quantify the intensity of the bands in the autoradiograms an image analysis system (Kontron/Zeiss, Eching, Germany) was used. Scanning was performed with a Panasonic black and white CCD camera (WC-CD50), digitized four times, and averaged to improve the signal to noise ratio (frame size 640 × 512 pixels; 256 gray levels). The band intensities were corrected for the background.
Cyt c Oxidase Capacity
Root extracts were prepared from 300 mg (FM) of frozen root material that was homogenized in liquid nitrogen using a mortar and pestle and then suspended in a total volume of 1.2 mL with 0.1 m KH2PO4 (pH 7.5) and 0.1% (w/v) Triton X-100. The extract was centrifuged at 13,000g for 5 min, and the supernatant was used for a spectrophotometric assay. Cyt c oxidase was measured at 550 nm in the presence of 12 μm reduced Cyt c (5 μL) and 0.3 mL extract in the cuvette with 1 mL KH2PO4 buffer. Cyt c (in KH2PO4 buffer) was reduced with sodium dithionite. Excess dithionite was removed by a gentle flow of normal air in the solution for a few minutes. The assay was performed at 25°C and the first-order rate constant was calculated (g−1 FM s−1; Smith, 1961). The extinction coefficient of cyt c was measured by adding K3Fe(CN)6 (3 μL of a 0.1-mm solution) at a final concentration of 0.23 μm (whereby the volume changes only by 0.2%), which completely oxidizes the reduced cyt c. Addition of 0.5 mm KCN or bubbling with CO inhibited the reaction to 6 ± 1 and 16% ± 4%, respectively (average and se). The activity measured, should represent the maximal activity in the extract, and therefore is related to the concentration of cyt c oxidase present.
Measurement of Ubiquinone Reduction Levels in Intact Roots
The ubiquinone assays were done according to Wagner and Wagner (1995). Root systems were vertically split in two, and treated with or without KCN+SHAM. Root extracts were prepared from 0.8 g of fresh root material that was ground in liquid nitrogen, using a mortar and pestle, suspended in 15 mL methanol and 15 mL petroleum ether (boiling point 40°C–60°C), and vortexed for 30 s. The mixture was centrifuged (1,500g, 1 min) and the upper petroleum ether phase was removed, transferred to a test tube, and evaporated to dryness under a flow of nitrogen. Another 15 mL of petroleum ether was added to the lower phase, and the vortex and centrifugation steps were repeated. The upper phase was added to the one obtained previously. The extracted ubiquinones were resuspended with a glass rod in 75 μL of nitrogen-purged ethanol and analyzed by HPLC (HP 1050 series, Hewlett Packard, Amstelveen, The Netherlands). A reversed-phase Lichrosorb 5 RP 18 column (Chrompack, Bergen op Zoom, The Netherlands) with an ethanol-methanol mixture as the mobile phase (1 mL min−1) was used (starting with 10 min of 20% [v/v] ethanol, followed by a gradient to 70% [v/v] ethanol at 40 min). Detection was performed at 290 and 275 nm for Qr and Qox, respectively. Commercially obtained ubiquinone 10 and ubiquinone 9 were used as standards (Sigma, Zwijndrecht, The Netherlands, and Fluka, Zwijndrecht, The Netherlands, respectively). The extinction of Qr measured at 290 nm was multiplied by 3.56 according to Crane (1963) because of the lower extinction coefficient for Qr as compared with that of Qox. The measured Qr/Qt is an average Qr/Qt for all cells in the roots.
Oxygen Fractionation and Gas Phase Respiration Measurements
Root samples (0.5–1.2 g FM) were kept in the dark for 25 min before gas phase respiratory measurements were taken in a 4.96-mL stainless-steel closed cuvette at 20°C. A CO2 absorber (ascarite II) was present during measurements to avoid inhibition of respiration as a consequence of build up of CO2 in the closed cuvette during the course of the experiment (Gonzàlez-Meler et al., 1996). Oxygen extraction and isotope analysis were carried out as described in Robinson et al. (1995) with modifications of Gonzàlez-Meler et al. (1999). Roots were carefully surface dried prior to measurements to minimize diffusion resistance to tissue gas exchange. Over the course of the experiment, each sample consumed at least 30% but no more than 50% of the initial oxygen. The r2 values for all unconstrained linear regressions of the fractionation values (with a minimum of five data points) were greater than the value of 0.995 considered minimally acceptable (Ribas-Carbo et al., 1995, 1997; Lennon et al., 1997; Gonzàlez-Meler et al., 1999). During inhibitor treatments, either 0.5 mm KCN {in 1 mm TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid], pH 8.0} and 2 mm (P. alpina), 3 mm (P. compressa, P. annua, P. trivialis, and H. lanatus), or 10 mm (P. pratensis) SHAM (in water from a 1-m stock in dimethyl-d6 sulfoxide) were applied by sandwiching the roots between medical wipes soaked with the corresponding inhibitor and incubating in the dark for at least 25 min (Lennon et al., 1997). All stock solutions were freshly prepared before use. The CO2 absorber was not present in experiments requiring KCN to avoid recovery from the inhibitor. Calculations of oxygen isotope fractionation were made as described by Guy et al. (1989) with modifications (Gonzàlez-Meler et al., 1999). Electron partitioning between the two pathways in the absence of inhibitors was calculated as described by Guy et al. (1989).
Statistics
SPSS for Windows 8.0 was used for statistical analysis. One-way analysis of variance with a Tukey B post hoc test was used for the statistical analysis. The correlations were calculated with the Pearson correlation test.
ACKNOWLEDGMENTS
We thank Beth Guy for growing the plants for the 18O measurements and Larry Giles for his assistance with the gas-phase mass-spectrometer system.
Footnotes
This work was supported in part by the U.S. Department of Agriculture National Research Initiative (grant no. CPG 94–37306–0352 to J.N.S.), by the National Science Foundation Division of Environmental Biology (grant no. DEB–94–15541 to the Duke University Phytotron), and by the Netherlands Organization for the Advancement of Science (grant no. SIR 14–2309).
LITERATURE CITED
- Atkin OK, Botman B, Lambers H. The causes of inherently slow growth in alpine plants: an analysis based on the underlying carbon economies of alpine and lowland species. Funct Ecol. 1996;10:698–707. [Google Scholar]
- Crane FL. The coenzyme Q group (ubiquinones): progress in the chemistry of fats and other lipids. Prog Chem Fats Lipids. 1963;7:267–289. [Google Scholar]
- Elthon TE, Nickels RL, McIntosh L. Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol. 1989;89:1311–1317. doi: 10.1104/pp.89.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Richards RA. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol. 1984;11:539–552. [Google Scholar]
- Finnegan PM, Wooding AR, Day DA. An alternative oxidase monoclonal antibody recognises a highly conserved sequence among alternative oxidases subunits. FEBS Lett. 1999;447:21–24. doi: 10.1016/s0014-5793(99)00259-8. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Boveris A. Superoxide radical and hydrogen peroxide in mitochondria. In: Pryor WA, editor. Free Radicals in Biology. V. New York: Academic Press; 1982. pp. 65–90. [Google Scholar]
- Garnier E. Resource capture, biomass allocation and growth in herbaceous plants. Tree. 1991;6:126–131. doi: 10.1016/0169-5347(91)90091-B. [DOI] [PubMed] [Google Scholar]
- Gonzàlez-Meler MA, Ribas-Carbo M, Giles L, Siedow JN. The effect of growth and measurement temperature on the activity of the alternative respiratory pathway. Plant Physiol. 1999;120:765–772. doi: 10.1104/pp.120.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzàlez-Meler MA, Ribas-Carbo M, Siedow JN, Drake BG. Direct inhibition of plant mitochondrial respiration by elevated CO2. Plant Physiol. 1996;112:1349–1355. doi: 10.1104/pp.112.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy RD, Berry JA, Fogel ML, Hoering TC. Differential fractionation of oxygen isotopes by cyanide-resistant and cyanide-sensitive respiration in plants. Planta. 1989;177:483–491. doi: 10.1007/BF00392616. [DOI] [PubMed] [Google Scholar]
- Hilal M, Castagnaro A, Moreno H, Massa EM. Specific localization of the respiratory alternative oxidase in meristematic and xylematic tissues from developing soybean roots and hypocotyls. Plant Physiol. 1997;115:1499–1503. doi: 10.1104/pp.115.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser C, Kapranov P, McIntosh L. Genetic modification of respiratory capacity in potato. Plant Physiol. 1996;110:277–286. doi: 10.1104/pp.110.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefnagel MHN, Millar AH, Wiskich JT, Day DA. Cytochrome and alternative respiratory pathways compete for electrons in the presence of pyruvate in soybean mitochondria. Arch Biochem Biophys. 1995;318:394–400. doi: 10.1006/abbi.1995.1245. [DOI] [PubMed] [Google Scholar]
- Hunt R, Cornelissen JHC. Components of relative growth rate and their interrelations in 59 temperate plant species. New Phytol. 1997;135:395–417. [Google Scholar]
- Hunt R, Lloyd PS. Growth and partitioning. New Phytol. 1987;106:235–249. [Google Scholar]
- Ito Y, Saisho D, Nakazone M, Tsutsumi N, Hirai A. Transcript levels of tandem-arranged alternative oxidase genes in rice are increased by low temperature. Gene. 1997;203:121–129. doi: 10.1016/s0378-1119(97)00502-7. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambers H, Van der Werf A, Bergkotte M. Hendry GF, Grime JP, eds, Methods in Comparative Plant Ecology. London: Chapman & Hall; 1993. Respiration: the alternative pathway; pp. 140–144. [Google Scholar]
- Lennon AM, Neuenschwander UH, Ribas-Carbo M, Giles L, Ryals JA, Siedow JN. The effects of salicylic acid and tobacco mosaic virus infection on the alternative oxidase of tobacco. Plant Physiol. 1997;115:783–791. doi: 10.1104/pp.115.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall R. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Atkin OK, Menz RI, Henry B, Farquhar G, Day DA. Analysis of respiratory chain regulation in roots of soybean seedlings. Plant Physiol. 1998;117:1083–1093. doi: 10.1104/pp.117.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Hoefnagel MHN, Day DA, Wiskich JT. Specificity of the organic acid activation of alternative oxidase in plant mitochondria. Plant Physiol. 1996;111:613–618. doi: 10.1104/pp.111.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Wiskich JT, Whelan J, Day DA. Organic acid activation of the alternative oxidase of plant mitochondria. FEBS Lett. 1993;329:259–262. doi: 10.1016/0014-5793(93)80233-k. [DOI] [PubMed] [Google Scholar]
- Millenaar FF, Benschop JJ, Wagner AM, Lambers H. The role of the alternative oxidase in stabilizing the in vivo reduction state of the ubiquinone pool and the activation state of the alternative oxidase. Plant Physiol. 1998;118:599–607. doi: 10.1104/pp.118.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenaar FF, Roelofs R, Gonzàlez-Meler MA, Siedow JN, Wagner AM, Lambers H. The alternative oxidase in roots of Poa annua after transfer from high-light to low-light conditions. Plant J. 2000;23:623–632. doi: 10.1046/j.1365-313x.2000.00832.x. [DOI] [PubMed] [Google Scholar]
- Poorter H, Pothmann P. Growth and carbon economy of a fast-growing and slow-growing grass species as dependent on ontogeny. New Phytol. 1992;120:159–166. [Google Scholar]
- Poorter H, Remkes C. Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia. 1990;83:553–559. doi: 10.1007/BF00317209. [DOI] [PubMed] [Google Scholar]
- Poorter H, Remkes C, Lambers H. Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiol. 1990;94:621–627. doi: 10.1104/pp.94.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, van der Werf A, Atkin OK, Lambers H. Respiratory energy requirement of roots vary with the potential growth rate of a plant species. Physiol Plant. 1991;83:469–475. [Google Scholar]
- Purvis AC, Shewfelt RL. Does the alternative pathway ameliorate chilling injury in sensitive plant tissues? Physiol Plant. 1993;88:712–718. doi: 10.1111/j.1399-3054.1993.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Ribas-Carbo M, Berry JA, Yakir D, Giles L, Robinson SA, Lennon AM, Siedow JN. Electron partitioning between the cytochrome and alternative pathways in plant mitochondria. Plant Physiol. 1995;109:829–837. doi: 10.1104/pp.109.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Lennon AM, Robinson SA, Giles L, Berry JA, Siedow JN. The regulation of electron partitioning between the cytochrome and alternative pathways in soybean cotyledon and root mitochondria. Plant Physiol. 1997;113:903–911. doi: 10.1104/pp.113.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SA, Ribas-Carbo M, Yakir D, Giles L, Reuveni Y, Berry JA. Beyond SHAM and cyanide: opportunities for studying the alternative oxidase in plant respiration using oxygen isotope discrimination. Aust J Plant Physiol. 1995;22:487–496. [Google Scholar]
- Scheurwater I, Cornelissen C, Dictus F, Welschen R, Lambers H. Why do fast- and slow-growing grass species differ so little in their rate of root respiration, considering the large differences in rate of growth and ion uptake. Plant Cell Environ. 1998;21:995–1005. [Google Scholar]
- Scheurwater I, Koren M, Lambers H, Atkin OK. Variation in Specific Respiratory Costs in the Roots of Fast- and Slow-Growing Grass Species. PhD Thesis. Utrecht, The Netherlands: Utrecht University; 1999. The contribution of roots and shoots to whole plant nitrate reduction in fast- and slow-growing grass species; pp. 77–96. [Google Scholar]
- Simons BH, Millenaar FF, Mulder L, Van Loon LC, Lambers H. Enhanced expression and activation of the alternative oxidase during infection of Arabidopsis with Pseudomonas syringae pv tomato. Plant Physiol. 1999;120:529–538. doi: 10.1104/pp.120.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. Spectrophotometric assay of cytochrome c oxidase. Methods Biochem Anal. 1961;2:427–434. doi: 10.1002/9780470110188.ch13. [DOI] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN. Covalent and noncovalent dimers of the cyanide-resistant alternative oxidase protein in higher plant mitochondria and their relationship to enzyme activity. Plant Physiol. 1993;103:845–854. doi: 10.1104/pp.103.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN. Changes in the redox state of the alternative oxidase regulatory sulfhydryl/disulfide system during mitochondrial isolation: implication for inferences of activity in vivo. Plant Sci. 1997;123:19–28. [Google Scholar]
- Umbach AL, Wiskich JT, Siedow JN. Regulation of alternative oxidase kinetics by pyruvate and intermolecular disulfide bond redox status in soybean seedling mitochondria. FEBS Lett. 1994;348:181–184. doi: 10.1016/0014-5793(94)00600-8. [DOI] [PubMed] [Google Scholar]
- Van Arendonk JJCM, Poorter H. The chemical composition and anatomical structure of leaves of grass species differing in relative growth rate. Plant Cell Environ. 1994;17:963–970. [Google Scholar]
- Van Breusegem F, Sloote L, Stassart J-M, Moens T, Van Botterman J, Van Montagu M, Inzé D. Overproduction of Arabidopsis thaliana FeSOD confers oxidative stress tolerance to transgenic maize. Plant Cell Physiol. 1999;40:515–523. doi: 10.1093/oxfordjournals.pcp.a029572. [DOI] [PubMed] [Google Scholar]
- Van den Bergen CWM, Wagner AM, Krab K, Moore AL. The relationship between electron flux and the redox poise of the quinone pool in plant mitochondria: interplay between quinol-oxidizing and quinone-reducing pathways. FEBS Lett. 1994;226:1071–1078. doi: 10.1111/j.1432-1033.1994.01071.x. [DOI] [PubMed] [Google Scholar]
- Van der Werf W, Welschen R, Lambers H. Respiratory losses increase with decreasing inherent growth rate of a species and with decreasing nitrate supply: a search for explanations for these observations. In: Lambers H, van der Plas LHW, editors. Molecular, Biochemical and Physiological Aspects of Plant Respiration. The Hague, The Netherlands: Academic Publishing; 1992. pp. 421–432. [Google Scholar]
- Vanlerberghe GC, McIntosh L. Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:703–734. doi: 10.1146/annurev.arplant.48.1.703. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L, Yip JYH. Molecular localization of a redox-modulated process regulating plant mitochondrial electron transport. Plant Cell. 1998;10:1551–1560. doi: 10.1105/tpc.10.9.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Vanlerberghe AE, McIntosh L. Molecular genetic alteration of plant respiration: silencing and overexpression of alternative oxidase in transgenic tobacco. Plant Physiol. 1994;106:1503–1510. doi: 10.1104/pp.106.4.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM, Krab K. The alternative respiration pathway in plants: role and regulation. Physiol Plant. 1995;95:318–325. [Google Scholar]
- Wagner AM, Moore AL. Structure and function of the plant alternative oxidase: its putative role in the oxygen defense mechanism. Biosci Rep. 1997;17:319–333. doi: 10.1023/a:1027388729586. [DOI] [PubMed] [Google Scholar]
- Wagner AM, Wagner MJ. Measurements of in vivo ubiquinone reduction levels in plant cells. Plant Physiol. 1995;108:277–283. doi: 10.1104/pp.108.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan J, Millar AH, Day DA. The alternative oxidase is encoded in a multigene family in soybean. Planta. 1996;198:197–201. doi: 10.1007/BF00206244. [DOI] [PubMed] [Google Scholar]