Abstract
Cadaveric dissection is a unique and unrivalled educational tool that allows students in medicine and associated life sciences to explore spatial three-dimensional anatomy, principles of structure and related function, and anatomical variations, including pathological alterations. Human tissue dissection enables researchers to comprehend the variety that exists in life that cannot be appreciated through the literature or artificial specimens. Using cadavers is the best way to simulate surgical and anatomical teaching. A cadaver has been shown to imitate surgical and anatomical training better than any other existing method. By the use of soft embalming approaches, cadavers have become more realistic and training-friendly. The main aim of this review is to describe various innovative and recent cadaver preservation techniques in detail, which can help anatomists to modify the techniques in their institute for gross anatomy teaching and surgical training or workshops to get a lifelike cadaver.
Keywords:anatomy, cadaver dissection, formaldehyde, soft embalming, Thiel embalming.
INTRODUCTION
The term ”cadaver” is derived from the Latin word “cadere“, which means ”to fall”, and it refers to soldiers who died in battle. As a result, a cadaver is a deceased human body that is used in scientific or medical research. The term ”dissect” comes from the Latin word “dissecare“, which means ‘to cut apart’ or ‘to separate into pieces’ (1). In order to study the detailed anatomical structure of a deceased animal, plant, or human, dissection (cutting) is done as a part of the educational process. Anatomical dissection has been used in medical education since the third century BC in Greece. There was a time in history when dissection was illegal and considered offensive to the general public. However, over time, the discipline has gained worldwide acceptance as an essential teaching and learning tool in medicine (2).
For teaching human anatomy, donated or unclaimed dead bodies received by the department of anatomy forms the mainstay in gross anatomy education (3). People enrolled in medicine can peruse three-dimensional anatomy and variations and pathological changes using the unparalleled and exceptional instructional tool known as cadaveric dissection (4). Optimal preservation of cadavers from decomposition is one of the most important considerations for employing human bodies in educational contexts (5). For decades, the traditional method of preservation has been using ‘formaldehyde’ as a traditional embalming fluid. Edward C. Johnson, an embalming and funeral historian, has written that “embalming is a means of artificially preserving the dead human body and it is one of humankind’s longest practiced arts.” (6).
Unfortunately, formaldehyde has been classified as a mitogen. Studies suggest that staff handling cadavers in the form of corpse preparation or teaching personnel as well as students doing cadaveric dissection are at risk of adverse effects that include coughing, nausea, watery and burning eyes, or rashes on the skin. They are also at increased risk of bronchial asthma, myeloid leukaemia and other uncommon malignancies such as nasopharyngeal, paranasal sinus, and nasal tumors (7, 8). The conservation and maintenance of cadavers is necessary for imparting gross anatomy to students in medical fields, and this requires infection-free specimens with little to no mitogenic materials (9). Also, with the growing demand for surgical training, it was felt that formalin-based cadavers used as training models provided unrealistic handling and were deemed unsuitable due to significant changes in the strength, colour, and fragility of organs and tissues (10, 11).
Once Professor Walter Thiel introduced a new method of preserving the cadavers, called ”soft embalming”, which gave them a realistic aspect, a huge shift occurred in the techniques of embalming. However, due to the requirement of the large number of chemicals and the time-consuming process of producing and storing the bodies, its broad application has proven to be difficult (12). Likewise, many new cadaver preservation techniques are gradually developed in various institutes all over the world to replace the usage of only formaldehyde looking at the hazardous concerns from its prolonged use.
With this review, we are going to enumerate and describe in detail various innovative techniques developed for the preservation of cadavers and specimens till now, with their advantages and disadvantages. This review will probably assist anatomy educators and embalmers in changing their conventional embalming procedures and protecting both themselves and their students from repeated exposure to hazardous chemicals.
METHODOLOGY
Objective
This article aims to outline the large range of readily accessible chemicals which are employed to embalm cadavers across the world, along with a summary of their benefits and drawbacks.
Information sources and search strategy
Over a period of eight months, the authors performed a vast review of articles published between 1984 and August 30, 2022. According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1) for 2020, articles were searched in four medical databases (PubMed, Google Scholar, Cochrane central register of clinical trials and Scopus), using the following key terms: ‘cadaver dissection’, ‘formaldehyde for embalming’, ‘soft embalming’, ‘Thiel embalming’, ‘innovation in cadaver embalming’. Titles and abstracts were screened by each of the three authors independently and reviewed with inclusion/exclusion criteria. After data extraction, findings were summarised and reported in Table 1.
Inclusion and exclusion criteria
English articles (original, review, case series, case reports, published and preprints) that reported the use of chemicals for the embalming of cadavers for teaching or research purposes till August 2022 were included in the present study. Then, the eligible articles were critically analyzed. Those with incomplete information, unavailable full text, no target observations as well as other article types (letters, comments, news and so on) were all excluded. Institutional Ethical Committee approval and informed consent were not necessary as our study was a systematic review of the literature, which was therefore limited to published information, with no direct involvement of human subjects.
DISCUSSION
There have long been ways to keep the deceased safe, ranging from natural ones likemummification or freezing to artificial ones like immersion or arterial injection. Unlike funeral embalming, anatomical embalming prioritizes the long-term preservation of tissues over the preservation of a person's outward appearance. Soft embalming is a method for preserving tissues that uses a combination of salt compounds and extremely low levels of volatile formaldehyde and formalin to achieve a number of unique features. Procedures like intubation, lumbar puncture, thoracocentesis, and all disciplines of surgery, including pelvic floor repair and face lift, benefit from soft-embalmed cadavers over unembalmed cadavers or simulators. Given the adaptability and flexibility of the internal organs and peritoneal membranes, soft-embalmed cadavers are highly useful for teaching endoscopic surgical anatomy. For novel instructional applications in cadaver-based anatomy and clinical teaching, soft embalming offers a lot of potential. Here we present a comprehensive review on the new techniques developed in the recent decades for betterment of soft embalming.
Genelyn embalming
Genelyn is a novel embalming solution developed by an Australian-based organization. Formaldehyde, methanol, and 2-Butoxyethanol are some of the ingredients of the Genelyn embalming solution that have been documented in the literature. On the other hand, producers do not divulge the whole composition (13). In an attempt to use Genelyn embalmed cadavers for surgical skill training, the authors documented that cadavers had no odour and were able to provide appropriate insufflation for laparoscopic surgeries. They agreed that the solid organs and luminal structures of Genelyn embalmed cadavers seemed to have the appearance and feel authenticity of actual patients. They also postulated that immersion of embalmed cadavers was not required; instead, they could be stored at 4 °C and cadavers could be reused for about 6-8 months without moulds (14). In another pilot study assessing the lifting of the microvascular flap using Genelyn embalmed cadaver and formalin embalmed cadaver, it was found that cadavers embalmed with Genelyn provided better tissue identification, colour, and lifelike pliability of organs (15). Genelyn method has the advantages that it requires no expensive investments in new infrastructure and results are obtained more quickly because it is not necessary to keep cadavers submerged for extended periods of time. Nevertheless, Balta et al determined Genelyn to be a hard fixative by analysing just the range of joint movements without dissecting the cadavers to examine tissue adaptability, segregation of tissue planes, and tissue colors (16). When Jaung et al compared the effectiveness of Graz fluid, Dodge fluid and Genelyn preparation, they reported Genelyn only after Graz fluid fared well in parameters like fungicidal properties, joint flexibility and life-like appearance (13). One of the reasons for the documented inadequate tissue preservation could be due to the delay in placing a cadaver in cold storage, which leads to decomposition. Also, it is after the proper thawing, cadavers must be subjected to Genelyn embalming. The thawing time varies according to the cadaver's structure and climate zone. As such, the period between death and embalming has to be less than six hours without cold storage and less than 24 hours with cold storage for perfect preservation with Genelyn preparation (14).
Ethanol-glycerol-lysoformin embalming
In 1975, Tutsch developed an embalming fluid composition that substituted lysoformin for phenol. Lysoformin comprises 6.0 g of formaldehyde and 1.8 g of glutaraldehyde per 100 g, as per the manufacturer's product information sheet (Lysoform Dr. Hans Rosemann GmbH, Berlin, Germany); consequently, this embalming fluid is fully devoid of aromatic compounds (17). The use of lysoformin for laparoscopic procedures in cadavers has been documented in the literature. It is well established that Thiel's embalming procedure as well as fresh-frozen cadavers give excellent tissue characteristics and a realistic atmosphere for laparoscopic surgical procedures. Like Thiel’s, low odour, optional reuse and easy storage were similarly obtained utilizing the ethanol- glycerol-lysoformin fixation method. Furthermore, although Thiel's embalming procedure is technically complicated and uses very expensive chemical components, ethanol-glycerol-lysoformin fixation is relatively simple and cost-effective (18). Wedel et al evaluated the effectiveness of ethanol-glycerol-lysoformin (70% ethanol, 30% glycerol, 0.3% lysoformin; ca. 20l) embalmed cadavers for laparoscopic procedures training and documented it was a concise cost-effective risk-free method for producing tissue that was close to life in terms of consistency and elasticity, with minor discoloration and increased viscosity of organs. Over the course of a year, it was possible to reuse body donors many times (19).
Modified Larssen solution (MLS)
The 20% modified Larssen solution contains 2000 mL distilled water, 180 g sodium chloride 200 g sodium bicarbonate, 200 g sodium sulphate, 200 g chloral hydrate, 100 mL of 20% formalin, and 400 mL liquid glycerin. This formulation was further diluted with 5000 mL of distilled water. The 10% modified Larssen solution was prepared in a similar manner, but with 10% formalin and the subsequent addition of 3000 mL of distilled water. In a study that assessed the effectiveness of MLS, the color of muscles, fatty tissue, fascia, nerves and vessels in MLS embalmed cadavers were found to have a lifelike appearance; they were odorless with good joint flexibility. Also, its application does not require expensive equipments and the authors concluded it was an effective soft embalming technique (20). Celik et al used MLS fixed cadavers for a transoral endoscopic thyroidectomy vestibular approach and 94.5% of participants believed that MLS fixation gave a living feel appearance of the cadavers, which could be stored for 4.53 years and further used 6.27 times repeatedly for endoscopic workshops (21).
Imperial College London soft-preservation (ICL-SP) solution
The ICL-SP solution technique is used in the Imperial College, London, and is based on alcohol, water, glycerol and phenol (soft-preserved) rather than fixed in formalin. Cadavers embalmed by this method can be used for about six months. Balta et al compared the effectiveness of Genelyn, Thiel and ICL-SP solutions and concluded that the joints of cadavers embalmed with the ICL-SP method closely resembled those of living individuals (16). Balta et al reported that bacterial colonies in the ICL-SP solution-embalmed cadavers began to show up after two months, whereas formalin-embalmed cadavers did not develop bacteria after embalming, which made the ICL-SP solution ineffective if thorough disinfection was needed after embalming (22).
Phenoxyethanaol based embalming (Crosado technique)
Phenoxyethanol (PE) is a glycol ether having antibacterial and antifungal effects as well as a colourless oily appearance and a pleasing odour. It is a common preservative being used to inhibit fungal and bacterial contamination, particularly in the cosmetics and pharmaceutical industries and it is quite inexpensive (23). Over two decades of usage, phenoxyethanol-based embalming (the "Crosado" technique) has been proven to provide consistent outcome for anatomical preservation. Ethanol, glycerin, water and phenoxyethanol are the main ingredients of the embalming fluid (24). In studies showing that PE had the capacity to reduce exposure to formaldehyde and phenol it has been claimed that, by eliminating some of the phenol and formaldehyde from their fixatives, soft tissue pliability may be restored (25, 26). This approach was found to be suitable for embalming of cadavers for a number of dissections and prosections as well as histology and plastination. One of the technique's main advantages is its ability to minimise the quantity of formaldehyde used to a bare minimum while being sturdy enough to be used outside of the anatomical context. The tissues retain their attractive properties even after extended usage, providing an alternative to, or reduction in, the use of toxic chemicals (24).
N-vinyl-2-pyrrolidone (NVP) embalming
N-vinyl-2-pyrrolidone (NVP) is an organic compound with a five-membered lactam ring and a vinyl group. The ”preserve” solution, which included 100% NVP and 0.1% N,N'-dibutyl-phenylenediamine, was diluted to provide NVP concentrations of 10% of body weight. The arteries were ligated once the infusion was finished, and the corpse was submerged in 5 L of 5% ”Preserve” solution in a sealed plastic bag and preserved at 4°C (27). The endoscopic transnasal skull base approach was reported to be successful in cadaver dissection that had been embalmed with NVP, providing an atmosphere that was similar to something like a realistic surgical field (27). Another research revealed that cadavers fixed with NVP exhibited more range of motion than those preserved with formalin. Under the dermis, the amount of subcutaneous fat was greatly reduced and the connective tissues were translucent, making it possible to visualise the veins, cutaneous nerves, and ligaments vividly. The internal organs and abdominal wall were still supple and malleable, just like those in freshly deceased people. N-vinyl-2-pyrrolidone possesses bactericidal and preservation properties in addition to its fixative properties. It is formalinfree and does not necessitate the use of costly ventilation equipment. The use of NPV allowed for an extended preservation duration of up to 11 months (28). In another study, in cadavers embalmed with NVP, regular vibrations of the vocal folds using NVP-embalmed larynges, successfully created voiced sounds during experimental phonation (29). N-vinyl-2-pyrrolidone has a number of disadvantages. With increasing NVP concentration, flexibility decreased, and even at the maximum concentration of 20%, the brain tissue was too soft to dissect (27).
Zinc chloride embalming
In a study, four animal specimens were embalmed with a solution of 40% zinc chloride (400 g in one liter of tap water) combining with one liter of glycerin and 200 g thymol per 10 liters of solution (30). When dissected after a time interval of three weeks, muscle tissue and joints both remained supple and flexible. The identification and dissection of the arteries and nerves was simple. Drying of the tissue was not evident. Major organs and muscle fibers had the same colour as formalin-fixed specimens. The skin and internal organs lacked any signs of salt deposition. The authors came to the conclusion that a 40% ZnCl2 solution could embalm and preserve anatomical specimens without the use of formalin, alcohol, glycerin or thymol (30).
Thiel embalming
Since 2016, when formaldehyde was identified as a human 1B carcinogen, the European Commission on Toxic Chemicals has reduced the amount of formaldehyde to which employees were exposed at work. As a consequence, in 2018, the working group of the Anatomische Gesellschaft provided suggestions to limit the use of formaldehyde in anatomy centers (31). In the 1990s, W. Thiel developed the Thiel-embalming method as a soft-fix embalming technique. In this method, ethylene glycol is utilised to preserve tissue flexibility, along with 4-chloro-3-methylphenol, boric acid, potassium nitrate, and ammonium nitrate for disinfecting (32). The fixative's formalin content is considerably lowered, with a final concentration of 0.8% of the whole formula, resulting in a non-irritating, practically odourless product that retains the colour, suppleness, and plasticity of a living person, with enough joint mobility and tissue elasticity, making it ideal for surgical techniques and invasive clinical procedure training. The salts employed (ammonium nitrate, potassium nitrate) absorb the water in the tissues, and nitrates in the salts cause the muscles to have a crimson hue due to the activity of nitrosomyoglobin, which is generated by the muscles themselves. Antiseptic qualities are provided by formaldehyde. Ethylene glycol is responsible for the tissues' haptic characteristics. Thiel used bacteriologic tests to check the technique's disinfection efficiency, and none of the cadavers tested positive for mould (33). Following embalming, cadavers are kept for around six months in an immersion solution containing 3% boric acid, 10% mono-ethylene glycol, 10% ammonium nitrate, 5% potassium nitrate, 7% sodium sulfite and 2% formalin (33). Furthermore, Thiel-embalmed cadavers may be injected with a variety of dyes, resins, and natural latex, among other things, allowing even the tiniest vascular branches (less than 1 mm) to be detected and the whole vascularization to be seen (34, 35). Disadvantages of Thiel method mainly included the high prices of necessary materials. Furthermore, it prevented the detection of thrombogenic potential in the use of vascular sutures. To avoid mummification, the preparations were to be kept in hermetically sealed containers between 0° and 6°C (36). Thiel embalmed cadavers could not be used as a realistic evaluation model for radiofrequency ablation owing to the significant changes in electrical conductivity in embalmed tissue (37). Since Thiel embalmed tendons did not accurately imitate the biomechanical characteristics of fresh frozen tendons, they were also disregarded for biomechanical study. It may be linked to collagen denaturation caused by the high salt (boric acid) content in Thiel embalming solution (38). Thiel's approach drastically changed the plastic energy absorption of human and bovine specimens. Furthermore, when Thiel-embalmed human cadavers are examined utilising clinical magnetic resonance imaging (MRI) sequences, particularly those based on spin-echo MRI, there is a loss of signal and contrast (39).
Saturated salt solution
There is a lengthy history of embalming cadavers with sodium chloride salt to enhance surgical skills. Ambroise Paré (1510–1590), one of the founding fathers of surgery, a pioneer in surgical methods and anatomist, characterized his embalming solution as containing ”common salt” (6). Saturated salt solution (SSS) consisted of sodium chloride, 20% formaldehyde, phenol, glycerine, isopropyl alcohol, and water (40). Studies have shown that cadavers embalmed with SSS had sufficient antibiotic protection, their joints remained flexible and their soft tissue quality was acceptable for surgical skill training (41). Studies have also revealed that where Thiel soft embalmed cadavers may not be appropriate for central venous catheterization instruction, SSS-embalmed cadavers may be used instead (42). The SSS method is also cheap and simple with low risk of infection. The SSS solution can also be used to store organs that have been fixed in formalin (and washed) at room temperature. There is no formaldehyde evaporation, and no odour or harmful consequences are produced. Different organs (previously embalmed, dissected, and labelled) could also be displayed in glass containers filled with SSS. The drawback observed with storage of the cadaver in SSS is the corrosion of the metal by the salt. Metal containers should thus be avoided. Stainless steel surfaces, such as dissection tables and trays, can be protected against corrosion by thoroughly cleaning them with tap water after each usage (43).
Glutaraldehyde embalming
The main component of embalming fluid, glutaraldehyde, was first shown to be particularly successful in cadaver preservation for surgical dissections by Natekar and De Souza. The embalming fluid was modified somewhat from Natekar's technique by adding 2% glutaraldehyde, methanol, glycerine, cetrimide, eosin, eucalyptus oil and water. When compared to typical formalin-fixed cadavers, glutaraldehyde fixed cadavers showed more flexibility and colour retention. Cadavers were found to be exceedingly pliable, with a realistic aspect and no stiffness or hardness. The skin's elasticity was preserved, and all joints, including the metacarpophalangeal and interphalangeal ones, had lifelike flexibility. There was no evidence of fungal development or a foul odour. Cadavers could also be utilized several times over a five-year period without the requirement for defrosting or refreezing (44). In contrast to formaldehyde, glutaraldehyde is a slow diffuser that produces a fast and irreversible reaction with proteins. As a result, glutaraldehyde provides more permanent end point fixation while perfusing the tissue slowly, whereas formaldehyde perfuses the tissue quickly but only generates irreversible fixation. As glutaraldehyde perfuses slowly, after injecting glutaraldehyde into the vascular system, the body could be frozen for a month to allow for good fixing while reducing the risk of putrefaction due to the lower temperature (5).
Formaldehyde free embalming
This embalming solution comprised ethyl alcohol (25%), polyethylene glycol PEG400 (20%), chloroxylenol (0.1%), and sodium nitrate (10%) was made up to 100% by volume by adding tap water. The embalmed specimens (two cats, two dogs, two goats and two sheep) were periodically examined over a period of six months. First, the specimens were colorimetrically appraised, and then the texture profiles, including hardness, adhesiveness and distortion were examined. Moreover, tissue samples from the skeletal muscle, liver, lung, and gut were collected and microbiologically analysed at various time intervals. The embalmed specimens resembled their living counterparts nearly exactly. No microbial growth was observed in the tissue samples collected from the embalmed animals, excepting the colon tissue from one cat and the lung tissue of one sheep (45).
CONCLUSION
Irrespective of their chemical properties, ideal embalming fluids should guarantee adequate structural preservation of organs over through the long run, limit overhardening, and ensure tissue and organ colour retention. Furthermore, desiccation and bacterial or fungal growth has to be prevented. Also, it is important to lessen environmental chemical risks exposures and possible biohazards.
Conflict of interests: none declared.
Financial support: none declared.
FIGURE 1.
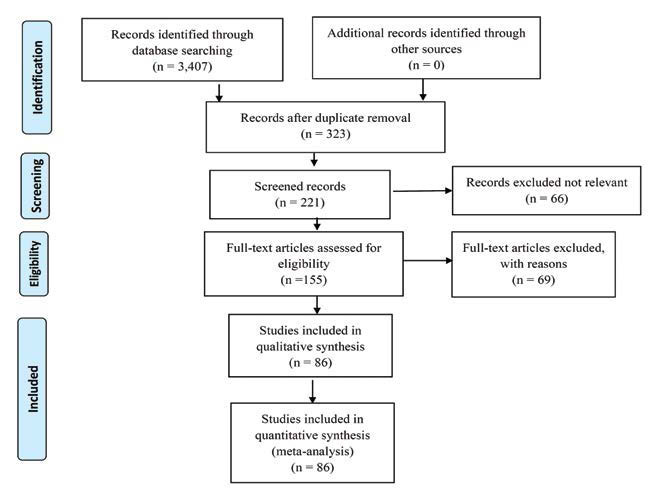
Flow diagram for the selection of studies using preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020
TABLE 1.
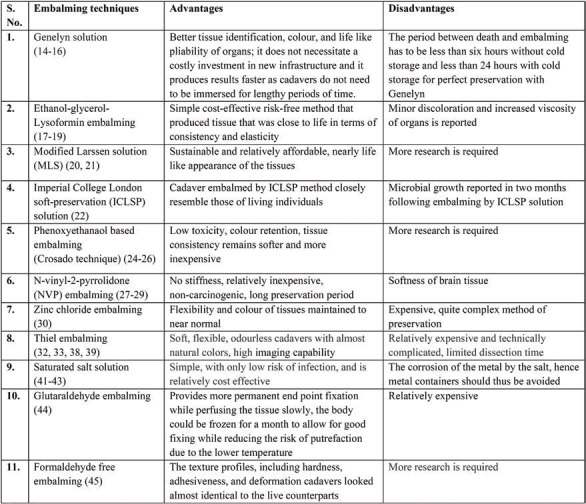
Comparison of various chemicals used for cadavers embalming
Contributor Information
Ariyanachi KALIAPPAN, Department of Anatomy, AIIMS Bibinagar, Hyderabad, India.
Rohini MOTWANI, Department of Anatomy, AIIMS Bibinagar, Hyderabad, India.
Tanu GUPTA, First year medical student, AIIMS Bibinagar, Hyderabad, India.
Mrudula CHANDRUPATLA, Department of Anatomy, AIIMS Bibinagar, Hyderabad, India.
References
- 1.Merriam-Webster’s collegiate dictionary. (Book, 2003) [WorldCat.org] [Internet]. [cited 2022 Jun 20] Available from: https://www.worldcat.org/title/merriam-websters-collegiate-dictionary/oclc/51764057.
- 2.Merriam-Webster’s collegiate dictionary. Human cadaveric dissection: a historical account from ancient Greece to the modern era [Internet]. [cited 2022 Jun 20] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4582158/
- 3.Ochs M, Mühlfeld C, Schmiedl A. Präparierkurs: Grundlage ärztlichen Handelns. Dtsch Arztebl. 2012;109:2126–2127. [Google Scholar]
- 4.Eisma R, Wilkinson T. From “Silent Teachers” to Models. PLoS Biol. 2014;12:e1001971. doi: 10.1371/journal.pbio.1001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner E. Human body preservation – old and new techniques. J Anat. 2014;224:316–344. doi: 10.1111/joa.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauptmann M, Stewart PA, Lubin JH, et al. Mortality from lymphohematopoietic malignancies and brain cancer among embalmers exposed to formaldehyde. J Natl Cancer Inst. 2009;101:1696–708. doi: 10.1093/jnci/djp416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein BD. Hematological and toxicological evaluation of formaldehyde as a potential cause of human leukemia. Hum Exp Toxicol. 2011;30:725–735. doi: 10.1177/0960327110381682. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald GJ, MacGregor DB. Procedures for embalming cadavers for the dissecting laboratory. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 1997;215:363–365. doi: 10.3181/00379727-215-44144. [DOI] [PubMed] [Google Scholar]
- 11.Prasad Rai B, Tang B, Eisma R, et al. A qualitative assessment of human cadavers embalmed by Thiel’s method used in laparoscopic training for renal resection. Anat Sci Educ. 2012;5:182–186. doi: 10.1002/ase.1267. [DOI] [PubMed] [Google Scholar]
- 13.Jaung R, Cook P, Blyth P. A comparison of embalming fluids for use in surgical workshops. Clin Anat N Y N. 2011;24:155–161. doi: 10.1002/ca.21118. [DOI] [PubMed] [Google Scholar]
- 14.Rajasekhar SSSN, Kumar VD, Raveendranath V, et al. Advanced training in laparoscopic gastrointestinal surgical procedures using Genelyn®-embalmed human cadavers: A novel model. J Minimal Access Surg. 2021;17:495. doi: 10.4103/jmas.JMAS_152_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng NYB, Loh CYY, Athanassopoulos T. A cost-effective cadaveric model for plastic surgery simulation. Indian J Plast, Surg Off Publ Assoc Plast Surg India, 2016. [DOI] [PMC free article] [PubMed]
- 16.Balta JY, Twomey M, Moloney F, et al. A comparison of embalming fluids on the structures and properties of tissue in human cadavers. Anat Histol Embryol. 2019;48:64–73. doi: 10.1111/ahe.12412. [DOI] [PubMed] [Google Scholar]
- 18.Ackermann J, Wedel T, Hagedorn H, et al. Establishment and evaluation of a training course in advanced laparoscopic surgery based on human body donors embalmed by ethanol-glycerol-lysoformin fixation. Surg Endosc. 2021;35:1385–1394. doi: 10.1007/s00464-020-07523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wedel T, Ackermann J, Hagedorn H, et al. Educational training in laparoscopic gynecological surgery based on ethanol-glycerol-lysoformin-preserved body donors. Ann Anat Anat Anz Off Organ Anat Ges. 2019;221:157–164. doi: 10.1016/j.aanat.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Balta JY, Cryan JF, O’Mahony SM. The antimicrobial capacity of embalming solutions: a comparative study. J Appl Microbiol. 2019;126:764–770. doi: 10.1111/jam.14191. [DOI] [PubMed] [Google Scholar]
- 24.Crosado B, Löffler S, Ondruschka B, et al. Phenoxyethanol‐Based Embalming for Anatomy Teaching: An 18 Years’ Experience with Crosado Embalming at the University of Otago in New Zealand. Anat Sci Educ. 2020;13:778. doi: 10.1002/ase.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frølich KW, Andersen LM, Knutsen A, Flood PR. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271–278. doi: 10.1002/ar.1092080214. [DOI] [PubMed] [Google Scholar]
- 26.Wineski LE, English AW. Phenoxyethanol as a nontoxic preservative in the dissection laboratory. Acta Anat, (Basel) 1989. [DOI] [PubMed]
- 27.Maruyama K, Yokoi H, Nagase M, et al. Usefulness of N-vinyl-2-pyrrolidone Embalming for Endoscopic Transnasal Skull Base Approach in Cadaver Dissection. Neurol Med Chir, (Tokyo) 2019. [DOI] [PMC free article] [PubMed]
- 28.Haizuka Y, Nagase M, Takashino S, Ket al. A new substitute for formalin: application to embalming cadavers. Clin Anat N Y N. 2018;31:9098. doi: 10.1002/ca.23011. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto M, Nagase M, Watanabe I, et al. Excised human larynx in N-vinyl-2-pyrrolidone-embalmed cadavers can produce voiced sound by pliable vocal fold vibration. Anat Sci Int, [Internet]. 2022. [DOI] [PubMed]
- 30.Goodarzi N, Akbari G, Razeghi Tehrani P. Zinc Chloride, A New Material for Embalming and Preservation of the Anatomical Specimens. Anat Sci J. 2018;15:25–30. [Google Scholar]
- 31.Waschke J, Bergmann M, Bräuer L, et al. Recommendations of the working group of the Anatomische Gesellschaft on reduction of formaldehyde exposure in anatomical curricula and institutes. Ann Anat Anat Anz Off Organ Anat Ges. 2019;221:179–185. doi: 10.1016/j.aanat.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Ottone NE, Vargas CA, Fuentes R, del Sol M. Walter Thiel’s Embalming Method: Review of Solutions and Applications in Different Fields of Biomedical Research. Int J Morphol, 2016.
- 34.Healy SE, Rai BP, Biyani CS, et al. Thiel embalming method for cadaver preservation: a review of new training model for urologic skills training. Urology. 2015;85:499–504. doi: 10.1016/j.urology.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Hubmer MG, Fasching T, Haas F, et al. The posterior interosseous artery in the distal part of the forearm. Is the term “recurrent branch of the anterior interosseous artery” justified? Br J Plast Surg. 2004;57:638–644. doi: 10.1016/j.bjps.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Odobescu A, Moubayed SP, Harris PG, et al. A new microsurgical research model using Thiel-embalmed arteries and comparison of two suture techniques. J Plast Reconstr Aesthetic Surg. 2014;67:389–395. doi: 10.1016/j.bjps.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 37.Liao P, Wang Z. Thiel-embalming technique: investigation of possible modification in embalming tissue as evaluation model for radiofrequency ablation. J Biomed Res. 2019;33:280–288. doi: 10.7555/JBR.32.20160148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fessel G, Frey K, Schweizer A, et al. Suitability of Thiel embalmed tendons for biomechanical investigation. Ann Anat Anat Anz Off Organ Anat Ges. 2011;193:237–241. doi: 10.1016/j.aanat.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Gueorguieva MJ, Yeo DTB, Eisma R, Melzer A. MRI of Thiel-embalmed human cadavers. J Magn Reson Imaging. 2014;39:576–583. doi: 10.1002/jmri.24210. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi S, Homma H, Naito M, et al. Saturated Salt Solution Method: A Useful Cadaver Embalming for Surgical Skills Training. Medicine, 2014. [DOI] [PMC free article] [PubMed]
- 43.Mani K. Modified saturated salt solution (MSSS) method for embalming. Indian J Clin Anat Physiol. 2019;2019:9882. [Google Scholar]
- 45.Preetha Menon, Adnan Aldarwich, Layaly Hamdan, et al. Novel formaldehyde-free embalming fluid formulation for long-term preservation of cadavers for anatomy teaching. Emirates Journal of Food and Agriculture. 2021;33:718–725. [Google Scholar]


