Abstract
Background:Oral lichen planus (OLP) is a chronic inflammatory disease that affects the oral mucosa. This disorder has been suggested to be related to an impairment of lipid metabolism and profile. A number of studies indicate a higher incidence of dyslipidemia in OLP patients compared to not-affected individuals.
Objectives: The aim of this study was to investigate the correlations between lipid profile alterations and clinical features of oral lichen planus.
Patients and methods:A total of 52 patients diagnosed with OLP were enrolled in this study. Data regarding the demography, symptoms, severity of lesions assesed by Thongprasom score and lipid profile status were collected from the medical charts. The study group was divided into two sub-cohorts: Group 1, which included OLP patients with lipid profile within the normal range, and Group 2 comprising OLP patients with alterations of the lipid status.
Results:The comparative analysis between the two groups found a statistically significant association between the lipid profile and OLP symptoms. Thus, the most frequent symptom was pain, in OLP patients with normal lipid status (Group 1), and burning, in those with altered lipid status (Group 2) (p=0.050). Moreover, the presence of symptoms was reported by a higher percentage of patients from Group 2 (75%) than Group 1 (68.25%). Patients reporting burning symptomatology showed higher triglyceride levels than those who reported pain (p=0.032). Furthermore, we found that male OLP patients have higher levels of LDL compared with female subjects (p=0.021).
Conclusion:Lipid profile changes are not associated with an increased severity of OLP lesions. A statistically significant association was found between burning sensation and higher lipid profile parameters.
Keywords:oral lichen planus, lipid profile alterations, LDL, oral diseases.
INTRODUCTION
Oral lichen planus (OLP) is a chronic inflammatory disease of the oral mucosa that occurs more frequently in middle-aged and elderly people, with a higher prevalence in women (1). Previous studies indicate that OLP is an autoimmune disease induced by T-cell dysfunction. The OLP pathogenesis includes a dysregulated immune response and the initial events are still not fully understood (2). Long-term release of cytokines in chronic inflammatory conditions is thought to induce disturbances in lipid and/or carbohydrate metabolism resulting in an increase in serum triglycerides (TGs) and a decrease in high-density lipoprotein (HDL) (1, 3).
Lichen planus (LP) is a chronic inflammatory dermatosis involving the skin, limbs and mucous membranes. Although the exact etiopathogenesis of LP is not fully understood, the current literature shows that, similar to psoriasis, a T-cell-mediated inflammatory process plays an essential role in the development of the disease. The prevalence of LP has been found to range between 0.22% and 1.2% of the adult population worldwide, depending on the explored geographic region (4). Regarding the OLP global prevalence, a systematic review reported geographic differences, with Europe having the highest prevalence (1.43%) (5).
There is also growing evidence of an increased risk of metabolic syndrome(combination of diabetes, hypertensions and obesity) and dyslipidemia in some skin conditions such as seborrheic dermatitis and psoriasis. Among the various elements of the metabolic syndrome, insulin resistance has been found to be more commonly associated with LP, but recently, dyslipidemia has also been reported to be associated with LP in some studies (3).
Patients with LP are more likely to have a variety of systemic comorbidities than those without LP (6). There have been few studies in the literature showing the correlation of LP with obesity, hypertension, hyperlipidemia, liver disorders and diabetes mellitus. All these pathologies carry an increased risk of cardiovascular disease (6).
Studies have shown that the lipid profile, particularly in the initial stages of T cell receptor stimulation, has an impact on signaling receptors and lymphocyte activation. Both high levels of cholesterol and TGs are major causes of hyperlipidemia (7). Moreover, malignancy has been associated with changes in the lipid profile because of the key role that lipids play in maintaining cell membrane integrity (8).
It is well known that OLP is recognized as a potentially malignant oral condition with a greater potential for malignant change than normal oral mucosa (7). A number of patients develop oral carcinoma on initial clinical keratosis and atrophy, although the overall transformation rate of OLP counts for 0.05% in ten years (7).
Prolonged dyslipidemia caused by chronic inflammatory conditions supports the formation of atherosclerotic plaques associated with an increased cardiovascular risk. Some of the wellknown autoimmune skin diseases, such as alopecia and psoriasis, are linked to abnormalities in lipid structure and increased risk for cardiovascular disease (CVD) (9). There is also evidence of an association between LP and significant dyslipidemia. A case-control study showed that LP patients, especially those with mucosal involvement, had a higher incidence of metabolic syndrome, which was associated with CVD risk (9). According to previous research, OLP patients have a more impaired lipid metabolism than those with LP or healthy controls (7, 10, 11). However, there is insufficient evidence to establish the CVD risk in patients with OLP. Early detection and treatment of dyslipidemia could reduce mortality and improve the quality of life in these patients.
The aim of the present study was to investigate the correlations between lipid profile alterations and clinical features of olp.
MATERIAL AND METHODS
We performed an observational retrospective study to determine whether an altered lipid profile was associated with the severity of OLP symptoms and clinical lesions. The study was carried out in the Oral Pathology Department of the Faculty of Dentistry, ”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania. Patients were admitted between 2018 and 2020.
The selection criteria for our research included the presence of the following data in the medical chart: detailed clinical description of the oral lesions (type of lesions, location, dimensions), OLP diagnosed by histopathological criteria, results of blood tests, lipid profile and informed consent signed by each patient. We excluded patients undergoing lipid-lowering therapy.
For the present study, we also collected data on patients' age, smoking status, presence or absence of stress symptoms, other associated diseases, and skin lesions. The anamnestic data regarding the presence and type of symptoms (asymptomatic, pain or burning) and onset of the lesions were also recorded. The sixth classical clinical forms of OLP comprise reticular, papular, plaque lesions, atrophy, erosions/ulcers and bullous types (12). In this study we categorized the clinical forms of OLP in four patterns: keratotic type, which included white reticular, papular or plaques lesions, atrophic type, which included erythematous lesion surrounded by white components, erosive/ulcerative and bullous forms (13). The location of the lesion was categorized in buccal mucosa, tongue, gingiva and lips. In OLP, lesions are generally located symmetrically or involve multiple sites (12).
To provide a consistent indication of OLP severity, we scored the lesions using the Thongprasom score, a commonly used tool by experienced oral pathologists (14). This score has a scale from 0 to 5: score 0 – no lesions, score 1 – mild white striae, score 2 – white striae and atrophic area less than 1 cm², score 3 – white striae and atrophic area more than 1 cm², score 4 – white striae and erosive area less than 1 cm², and score 5 – white striae with erosive area more than 1 cm² (15) .
For an accurate diagnosis we used the histopathological evaluation of OLP lesions in all patients, as recommended by the American Academy of Oral and Maxillofacial Pathology (12).
The lipid profile included the serum levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c) and triglycerides (TGs).
This study was performed according to the recommendations of the Declaration of Helsinki and was approved by the Committee of Ethics in Research of “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania (No. 1140). Written informed consent was obtained from all selected patients.
Study design
The study group comprising 52 OLP patients was divided into two sub-cohorts: Group 1, which included OLP subjects with lipid profile within the normal range, and Group 2, which included OLP patients with lipid status alterations.
Statistical analysis
The difference between the mean age of participants in the two groups was assessed using the Student’s t-test. Differences between the groups for categorical variables were assessed using the chi-squared test (χ² test). Differences in TG, TC, LDL and HDL levels among the three groups (asymptomatic, pain, burning) were evaluated using the non-parametric test Kruskal–Wallis, followed by pairwise comparison (n > three groups) and Mann-Whitney U test when comparing two groups. Results were considered statistically significant with p <0.050.
RESULTS
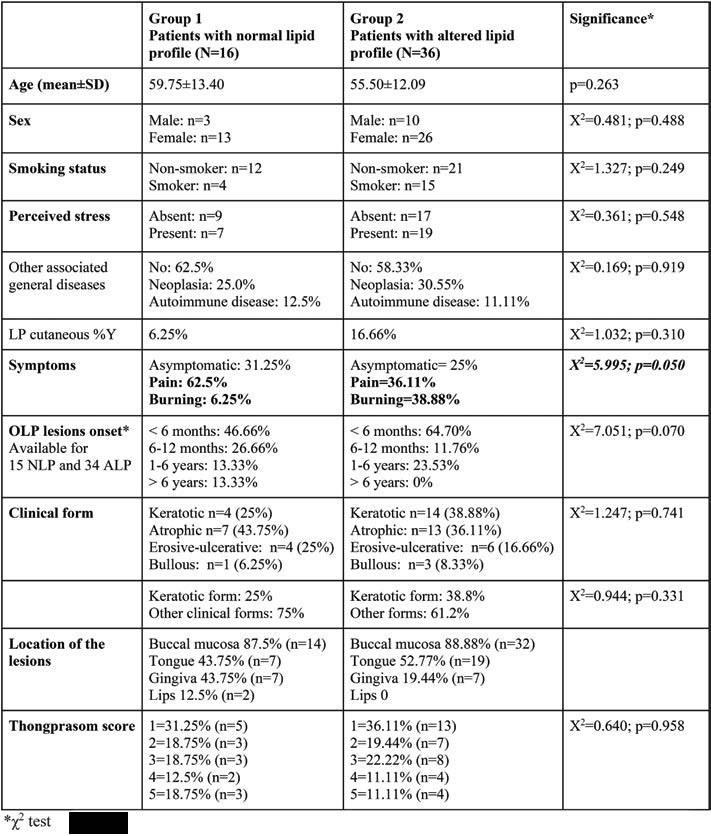
This study included 52 OLP patients (39 women and 13 men). Subjects’ mean age was 56.81±12.53 years. The demographic, clinical features and comparative analysis of OLP patients in both groups are summarized in Table 1. The comparative analysis detected no statistically significant differences in age, sex distribution, smoking status, the presence of LP skin lesions between the two groups. Also, the duration of the lesions or the lesion score (Thongprasom score) between the two groups were not statistically significant. Symptoms were reported in 38 patients (73.07% of all OLP patients).
The atrophic clinical form was the most common in Group 1, unlike Group 2, where keratotic pattern was predominant, closely followed by the atrophic type. By dividing the clinical forms in keratotic OLP and other forms, no statistical as sociation with the altered lipid profile was detected. In both groups, buccal mucosa was the most frequently affected site, followed by the tongue. There was no statistical association regarding the clinical Thongprasom score and lipid profile alterations.
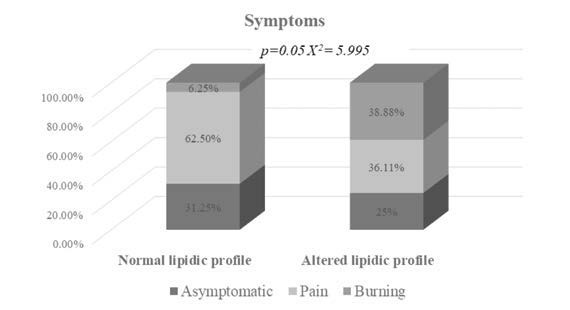
A statistically significant association between lipid status and OLP symptoms (pain in Group 1 and burning in Group 2; p=0.050) was observed (Figure 1), suggesting that the burning sensation in OLP patients could be related to an alteration of their lipid profile. Moreover, the presence of symptoms was reported by a higher percentage of patients with lipid profile alteration (75%) compared to those with a normal lipid profile (68.25%). The mean values of TC, TG, LDL and HDL for both study groups are summarised in Table 2. Considering the entire cohort of OLP patients, we found that TG levels were higher in subjects with burning symptoms compared to those who reported pain (Figure 2).
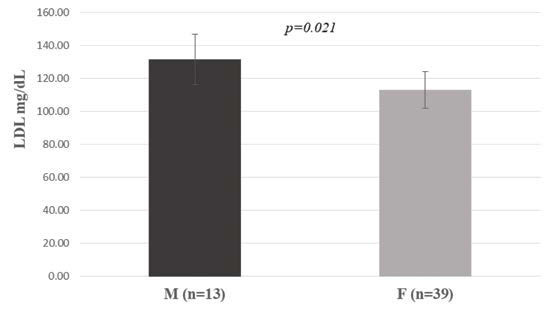
The comparison of TG, TC, HDL and LDL levels between OLP patients categorized by sex showed that male subjects reported higher levels of LDL than female patients (p=0.021) (Figure 3).
DISCUSSION
In the present study we investigated the lipid status of OLP patients with the aim to identify correlations between lipid impairment and OLP clinical features. The link between OLP and dyslipidemia may be associated with chronic inflammation, which is a component of both disorders (11). Furthermore, LP is a chronic mucocutaneous disease and it has been associated over the years with hypertension, diabetes and dyslipidemia (6).
The average age of our study participants (56.81 years) – higher than that reported by Rahmani et al (16) (49.92 years) and lower than that reported by Radic et al (7) (62.66 years) – was included in the assessment of OLP prevalence worldwide (patients’ age range 50-60 years) (5).
As OLP is an autoimmune disease, women are more likely to develop it, as also noticed in our sample (75%) and in earlier studies by Rahmani et al (16) (females: 63%) and Lopez et al (11) (females: 84%).
The association of OLP with dyslipidemia has been mentioned in previous studies to emphasize the importance of a higher atherogenic index in these patients (7, 10, 11, 17). However, there are still missing data on this topic regarding the influence of dyslipidemia on OLP lesions.
We found that an alteration of the lipid profile in OLP patients could be associated with their symptoms, given that the percentage of subjects with altered lipid profile reporting a burning sensation was higher that that of those who had a normal lipid profile (p=0.05). Moreover, patients with burning symptomatology showed higher levels of TGs than those who reported pain (p=0.032).
In a different prospective study (7) on 63 OLP patients and an equal number of healthy subjects, the analysis of the lipid profile revealed that OLP subjects had elevated lipid profile parameters without being statistically significant (higher values of cholesterol and TGs).
Aniyan et al (17) compared a group of 30 OLP patients with a group of healthy individuals and found that the average of VLDL cholesterol was higher in OLP patients than in healthy subjects. In contrast to controls, more OLP patients had serum dyslipidemia but this difference was not statistically significant. The correlation between dyslipidemia and OLP symptoms has been shown to be significant statistically in the same study (17).
When comparing OLP patients by gender and lipid profile, López-Jornet et al (11) reported that OLP male subjects had significantly lower HDL values, while we found that male patients had higher levels of LDL (p=0.021). In their analysis of 100 individuals with lichen planus, investigators from Spanish dermatology clinics found a statistically significant association between men's LDL levels and the cutaneous condition (18).
Moreover, regarding the OLP clinical form, López-Jornet etal (11) found significantly higher TG levels in patients with atrophic-erosive types than reticular-papular types, while other authors (17) as well as those of the present study detected no statistical correlation. In our study, the atrophic type was the most frequently encountered clinical form. This is a limitation of our research, which can be explained by the selection of patients (lipid profile registered in the medical chart and histological confirmation of OLP).
Mehdipour et al (19) showed that the lipid profile had higher value in patients with erosive OLP, without significant differences between those with erosive and non-erosive lichen planus.
A significant prevalence of dyslipidemia in patients with cutaneous lichen planus was reported by Ilves et al (20), Arias-Santiago et al (18), Mehdipour et al (19), Dreiher et al (21). In a casecontrol study, Baykal et al (22) detected dyslipidemia in 90% of LP patients with mucosal involvement, despite the fact that there were no significant differences in prevalence of dyslipidemia between LP patients and healthy controls. Moreover, the latter authors (22) considered mucosal involvement of LP to be a risk factor for dyslipidemia.
Some authors explain the connection between OLP and dyslipidemia by the presence of a reduced degree of chronic inflammation induced by oxidized LDL (23).
The absence of a control group in our research is another limitation of the present study. While previously published case-control studies (7, 10, 11) reported the presence of an altered lipid profile in OLP patients, we mainly explored the clinical differences associated with a modified lipid status.
Therefore, more research is needed to identify the link between OLP and lipid status and to determine whether lipid profile alterations may contribute to disease development.
CONCLUSION
Lipid profile changes are not associated with an increased severity of OLP lesions. A statistically significant association was found between burning sensation and higher lipidic profile parameters.
Conflicts of interest: none declared.
Financial support: none declared.
Authors’ contributions: All authors have read and agreed to the published version of the manuscript.
Institutional Review Board statement: The study was approved by the Committee of Ethics in Research of ”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania (No. 1140).
Informed consent: Informed consent was obtained from all patients involved in the present study.
Data availability: Datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
FIGURE 1.
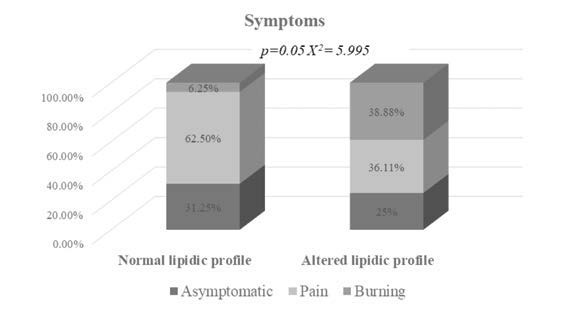
Symptoms reported by OLP patients with normal (n=16) and altered lipid profile (n=36)
TABLE 1.
Descriptive and comparative analysis of relevant variables between Groups 1 and 2
FIGURE 2.
Triglyceride levels in asymptomatic patients and those with pain and burning symptoms. Bars represent mean values±SEM (standard of error mean).
TABLE 2.
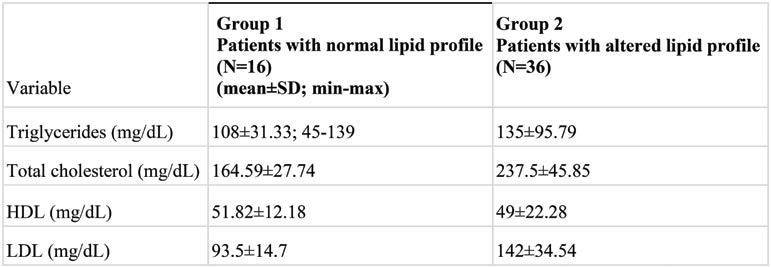
Liver disease severity based on etiology
FIGURE 3.
The relation between Stroop test results and age in the control group
FIGURE 3.
The relation between Stroop test results and age in the patient group
Contributor Information
Corina Andreea SELARU, “Doctoral School of “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Ioanina PARLATESCU, “Faculty of Dentistry, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Elena MILANESI, “Victor Babes” National Institute of Pathology, Bucharest, Romania.
Maria DOBRE, “Victor Babes” National Institute of Pathology, Bucharest, Romania.
Serban TOVARU, Faculty of Dentistry, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
References
- 1.Kennedy S, Bergqvist A, Chapron C, et al. ESHRE guideline on the diagnosis and management of endometriosis. Hum Reprod. 2005;20:2698–2704. doi: 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- 2.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 3.Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 4.Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab. 1996;81:174–179. doi: 10.1210/jcem.81.1.8550748. [DOI] [PubMed] [Google Scholar]
- 5.Brosens I, Gordts S, Benagiano G. Endometriosis in adolescents is a hidden, progressive and severe disease that deserves attention, not just compassion. Hum Reprod. 2013;28:2026–2031. doi: 10.1093/humrep/det243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vercellini P, Somigliana E, Vigano P, et al. “Blood on the Tracks” from corpora lutea to endometriomas. BJOG. 2009;116:366–371. doi: 10.1111/j.1471-0528.2008.02055.x. [DOI] [PubMed] [Google Scholar]
- 7.Khan KN, Fujishita A, Kitajima M, et al. Occult microscopic endometriosis: undetectable by laparoscopy in normal peritoneum. Hum Reprod. 2014;29:462–472. doi: 10.1093/humrep/det438. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzaki S, Canis M, Pouly JL, et al. Cyclooxygenase-2 expression in deep endometriosis and matched eutopic endometrium. Fertil Steril. 2004;82:1309–1315. doi: 10.1016/j.fertnstert.2004.03.059. [DOI] [PubMed] [Google Scholar]
- 9.Vercellini P, Eskenazi B, Consonni D, et al. Oral contraceptives and risk of endometriosis: a systematic review and meta-analysis. Hum Reprod Update. 2011;17:159–170. doi: 10.1093/humupd/dmq042. [DOI] [PubMed] [Google Scholar]
- 10.Bulun SE, Monsavais D, Pavone ME, et al. Role of estrogen receptor-β in endometriosis. Semin Reprod Med. 2012;30:39–45. doi: 10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruner KL, Matrisian LM, Rodgers WH, et al. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997;99:2851–2857. doi: 10.1172/JCI119478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langoi D, Pavone ME, Gurates B, et al. Aromatase inhibitor treatment limits progression of peritoneal endometriosis in baboons. Fertil Steril. 2013;99:656–662. doi: 10.1016/j.fertnstert.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attia GR, Zeitoun K, Edwards D, et al. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85:2897–2902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- 14.Lee DK, Nguyen T, O’Neill GP, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446:103–107. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 15.d’Anglemont de Tassigny X, Ackroyd KJ, Chatzidaki EE, Colledge WH. Kisspeptin Signaling Is Required for Peripheral But Not Central Stimulation of Gonadotropin-Releasing Hormone Neurons by NMDA. J Neurosci. 2010;30:8581–8590. doi: 10.1523/JNEUROSCI.5486-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shahab M, Mastronardi C, Seminara SB, et al. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cejudo Roman A, Pinto FM, Dorta I, et al. Analysis of the expression of neurokinin B, kisspeptin, and their cognate receptors NK3R and KISS1R in the human female genital tract. Fertil Steril. 2012;97:1213–1219. doi: 10.1016/j.fertnstert.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Abdelkareem AO, Alotaibi FT, AlKusayer GM, et al. Immunoreactivity of Kisspeptin and Kisspeptin Receptor in Eutopic and Ectopic Endometrial Tissue of Women With and Without Endometriosis. Reprod Sci. 2020;27:1731–1741. doi: 10.1007/s43032-020-00167-w. [DOI] [PubMed] [Google Scholar]


