ABSTRACT
Clostridioides difficile produces an environmentally resistant dormant spore morphotype that infected patients shed to the hospital environment. C. difficile spores persist in clinical reservoirs that are not targeted by hospital routine cleaning protocols. Transmissions and infections from these reservoirs present a hazard to patient safety. This study aimed to assess the impact of patients acutely suffering from C. difficile-associated diarrhea (CDAD) on C. difficile environmental contamination to identify potential reservoirs. Twenty-three hospital rooms accommodating CDAD inpatients with corresponding soiled workrooms of 14 different wards were studied in a German maximum-care hospital. Additionally, four rooms that never accommodated CDAD patients were examined as negative controls. Stagnant water and biofilms from sinks, toilets, and washer disinfector (WD) traps as well as swabs from cleaned bedpans and high-touch surfaces (HTSs) were sampled. For detection, a culture method was used with selective medium. A latex agglutination assay and a Tox A/B enzyme-linked immunosorbent assay were performed with suspect colonies. Stagnant water and biofilms in hospital traps (29%), WDs (34%), and HTSs (37%) were found to be reservoirs for large amounts of C. difficile during the stay of CDAD inpatients that decreased but could persist 13 ± 6 days after their discharge (13%, 14%, and 9.5%, respectively). Control rooms showed none or only slight contamination restricted to WDs. A short-term cleaning strategy was implemented that reduced C. difficile in stagnant water almost entirely.
IMPORTANCE Wastewater pipes are microbial ecosystems. The potential risk of infection emanating from the wastewater for individuals is often neglected, since it is perceived to remain in the pipes. However, sewage systems start with siphons and are thus naturally connected to the outside world. Wastewater pathogens do not only flow unidirectionally to wastewater treatment plants but also retrogradely, e.g., through splashing water from siphons to the hospital environment. This study focused on the pathogen C. difficile, which can cause severe and sometimes fatal diarrheas. This study shows how patients suffering from such diarrheas contaminate the hospital environment with C. difficile and that contamination persists in siphon habitats after patient discharge. This might pose a health risk for hospitalized patients afterward. Since this pathogen’s spore morphotype is very environmentally resistant and difficult to disinfect, we show a cleaning measure that can almost entirely eliminate C. difficile from siphons.
KEYWORDS: reservoir, nosocomial infection, drains, trap, wastewater
INTRODUCTION
Clostridioides (formerly Clostridium) difficile is a leading cause of health care-associated infection worldwide and the major causative agent of health care-associated bacterial diarrhea in Germany. In 2020, Germany registered 1,595 severe clinical cases of C. difficile-associated diarrhea (CDAD), with case fatality rates of approximately 19% (1). Intestinal colonization is due to a dormant spore morphotype, which is shed in substantial amounts to the hospital environment by asymptomatically colonized and especially by symptomatic patients suffering from diarrhea (2). Those spores can easily survive aerobic conditions outside the host on inanimate surfaces for up to 5 months (3, 4), facilitating contamination, spreading, and finally transmission from patient to patient, to health care workers (HCWs), and others. Additionally, due to their structure, C. difficile spores are insensitive to many antibiotics, to physical stress (heat, radiation, pressure and drought), and chemical agents (5, 6).
Large amounts of C. difficile vegetative cells and its spores are released into the hospital sewage every day, accountable to defecation into the toilet, by showering of soiled patients, by tossing stools from bedpans inside washer disinfectors (WDs), and finally by HCW hand washing when giving care to CDAD patients. Therefore, the hospital sewage system, i.e., traps of showers, sinks, toilets, and WDs, has come under suspicion of functioning as a C. difficile reservoir in the clinical setting that is neither targeted by terminal sporicidal (i.e., room disinfection after CDAD patient release) nor routine daily room disinfection. In particular, sink traps have been confirmed as reservoirs for a large number of microorganisms and sources of patient infections, including highly antibiotic-resistant Acinetobacter baumannii (7), Pseudomonas aeruginosa (8, 9), Klebsiella spp. (10–13), and several others (14–18). Water stagnation, low water flow, the presence of sediments or nutrients, and the corrosion of water distribution systems render traps ideal environments for the gradual accumulation of bacteria and the formation of multispecies biofilms (8, 19–21). Pathogenic microorganisms in traps can be mobilized from biofilms into the stagnant water and escape via droplets once running water hits, thereby contaminating the surrounding areas, patient-related items, other objects stored close to the sink, the hands or materials washed, and even far-located areas via contaminated fomites (16, 22–26). In particular, WDs are disposal sites for heavily contaminated material, since they must decontaminate soiled bedpans, urinals, etc., from intestinal pathogens, thereby guaranteeing safe usage for the next patient. As a prerequisite, a so-called A0 value of 60, is recommended in DIN EN ISO 15883-3 for treating reusable bedpans in a ward, defining a temperature-time pair to be applied. Nevertheless, studies have shown that this value may be insufficient for eliminating experimentally applied C. difficile spores from bedpans (27, 28).
Unlike traps, high-touch surfaces (HTSs) are specifically targeted with sporicidal agents by hospital disinfection practices. Nevertheless, these often remain contaminated with C. difficile spores and thus can also contribute to nosocomial infection (28–31).
Several precaution measures, including strict compliance to hand hygiene, contact precautions (CDAD patient isolation), frequent environmental cleaning with sporicidal agents, and restricted use of antimicrobials have so far ameliorated but not contained nosocomial C. difficile infections to satisfactory levels (7, 8). Therefore, this study aimed to assess environmental contamination with C. difficile caused by patients acutely suffering from CDAD to identify potential reservoirs in the hospital sewage system (sinks, showers, toilets, and WD traps) as well as on HTSs and washed bedpans. Identified sites were resampled to draw conclusions on the persistence of C. difficile after CDAD patient discharge and indirectly on disinfection practices (i.e., terminal disinfection after patient discharge and routine cleaning with sporicidal agents). Finally, a sporicidal disinfection technique (cleaning intervention) was applied to decontaminate identified reservoirs in sink and shower traps.
RESULTS
All results are expressed as CFU displayed per total sampled surface (centimeters square) or 100-mL volumes in absolute numbers. In addition, a CFU range (low, 1 to 10; moderate, 11 to 100; high, >100) is given to highlight contamination levels. The samples were not pasteurized (or similar processing) prior to the culture procedure. Therefore, results cannot differentiate between C. difficile vegetative or spore forms.
Sampling sites were grouped into hospital traps (containing traps in patient en suite bathrooms, in soiled workrooms, and WDs with bedpans) and high-touch surfaces in patient rooms with en suite bathrooms.
Hospital traps.
Water samples and biofilms in traps of sinks and toilets of patient bathrooms were screened for the presence of C. difficile during the stay (T1) of CDAD patients and after their discharge (T2) (Fig. 1). Furthermore, traps in soiled workrooms were analyzed in wards that accommodated only one CDAD patient during the sampling period (Fig. 2).
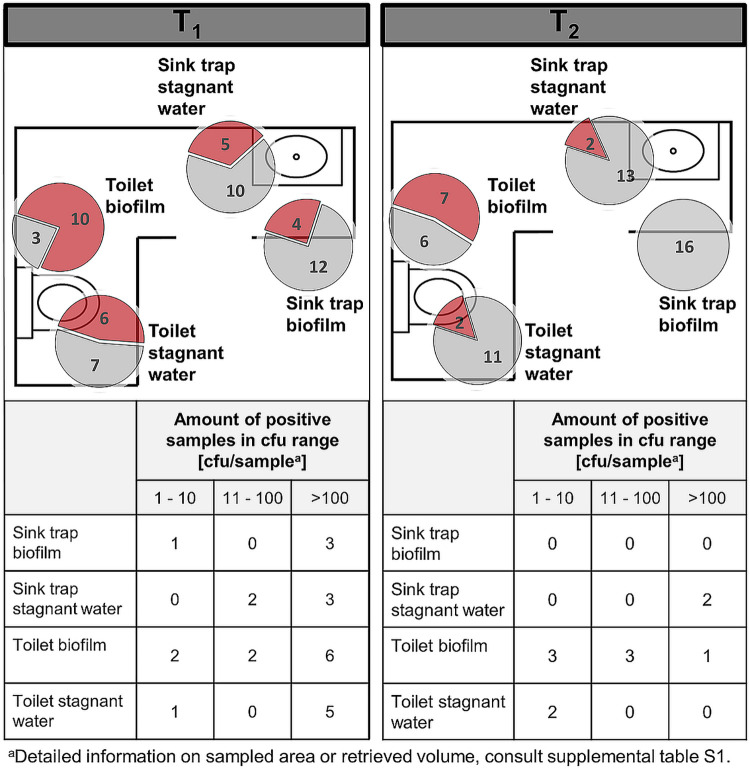
FIG 1.
Traps in patient rooms. Pie charts represent the amount of positive C. difficile findings (red) compared to negative findings (gray). The sum of red and gray portions per pie chart represents the number of total sampled sites in different rooms, during CDAD inpatient treatment (T1) (left) and after patient discharge (T2) (right). The floor plans are for illustrative purposes only and do not necessarily correspond to the actual room layout.
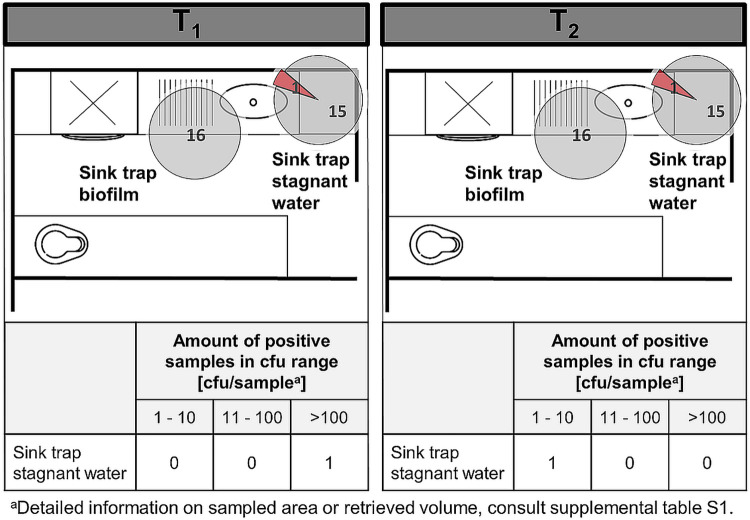
FIG 2.
Traps in soiled workrooms. Pie charts represent the amount of positive C. difficile findings (red) compared to negative findings (gray). The sum of red and gray portions per pie chart represents the number of total sampled sites in different rooms (and on different wards), during CDAD inpatient treatment (T1) in the respective ward (left) and after patient discharge (T2) (right). The floor plans are for illustrative purposes only and do not necessarily correspond to the actual room layout.
All sites apart from the soiled workroom sink and the patient bathroom sink trap biofilm tested positive for C. difficile during CDAD patient stay. After patient discharge, contamination was diminished but persisted. Contamination was generally higher in patient bathroom traps (sinks and toilets) than in the soiled workroom sinks (stagnant water and biofilm). Sink trap stagnant water was contaminated approximately twice as often as the associated biofilm. Highest contamination rates as well as the most CFU were found in the toilet biofilm (bowl swab) and the stagnant water. Those sites were contaminated in 77% and 46% of cases with very high bioburden. After patient discharge, biofilms inside the patient sink trap throughout sampled negative for C. difficile. The bathroom sink trap stagnant water also showed a decrease by 20%, but still, if C. difficile was found it was often present at high concentrations. In total, from 89 sampled traps, 26 traps were found to contain C. difficile during CDAD patient stay (29%). After discharge, a decrease in C. difficile was observed, with 12 positive traps (13%).
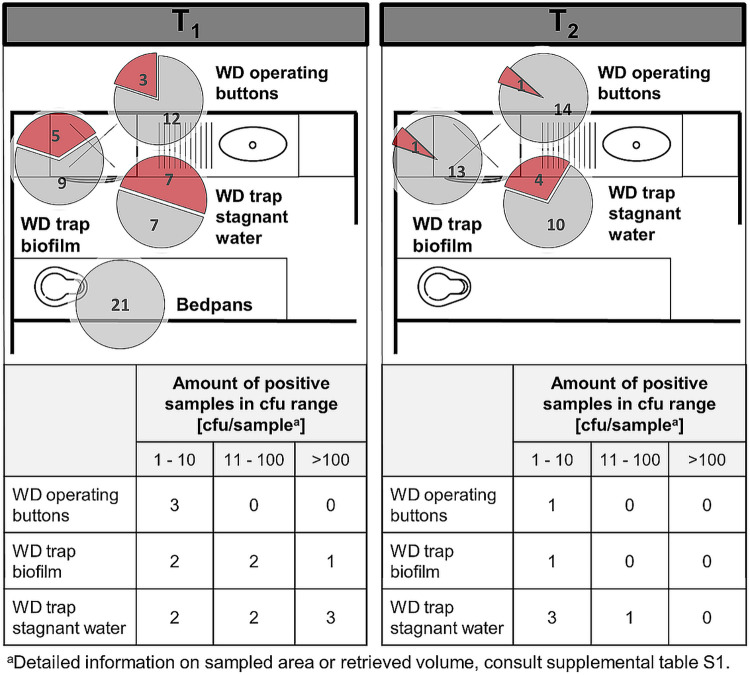
(i) Washer disinfectors and bedpans. WDs are disposal sites for fecal remains, especially when it comes to CDAD patients that cannot use the toilet. Sampling took place immediately after completion of a wash program (Fig. 3).
FIG 3.
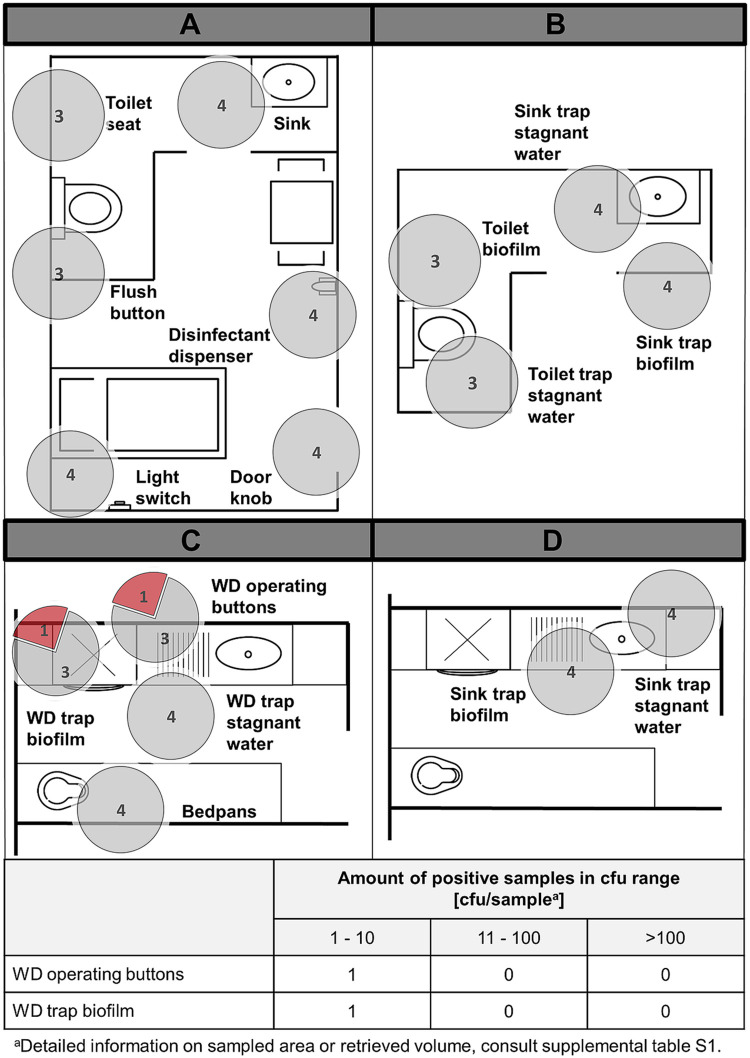
Washer disinfectors and bedpans in soiled workrooms. Pie charts represent the amount of positive C. difficile findings (red) compared to negative findings (gray). The sum of red and gray portions per pie chart represents the number of total sampled sites in different rooms, during CDAD inpatient treatment (T1) (left) and after patient discharge (T2) (right). The floor plans are for illustrative purposes only and do not necessarily correspond to the actual room layout.
The WD trap stagnant water was found to be heavily contaminated with C. difficile in 50% of cases during T1 and diminished to 29% after patient discharge. Biofilms in these traps contained C. difficile in 36% of cases in relatively high concentrations even after the wash program had been completed. Three of 15 WD operating buttons were found to be contaminated during patient stay. After patient release, only one WD operating buttons were contaminated. Finally, 21 bedpans were sampled immediately after exiting the clinic’s predefined WD wash program (A0 = 600). None was found to be contaminated with C. difficile.
In summary, excluding bedpans, during the stay of a single CDAD patient in the ward, 35% (15 of 43) sites linked to the ward WD were contaminated with large amounts of C. difficile, as opposed to 14% (6 of 43) contaminated sites after patient discharge.
(ii) Cleaning intervention. Since the study generated results that revealed toxigenic C. difficile isolates next to nontoxigenic isolates in several hospital traps (data not shown), an exemplary hygienic intervention was conducted to decontaminate two sink traps and one shower trap in patient en suite bathrooms by pouring a sporicidal product into the trap. Results are summarized in Table 1. One patient sink trap stagnant water and one shower trap stagnant water experienced a total elimination of floating C. difficile after the intervention. Another treated sink showed a >99% reduction, revealing only 2 CFU/100 mL after the treatment.
TABLE 1.
Cleaning intervention efficacy, based on C. difficile detected in patient bathroom before disinfection (T0) and after 15 min of disinfectant contact time (T15)
| Sample site | [CFU/100 mL] |
|
|---|---|---|
| T0 | T15 | |
| No. 1 sink trap stagnant water | >100 | 2 |
| No. 2 sink trap stagnant water | >100 | 0 |
| Shower trap stagnant water | >100 | 0 |
High-touch surfaces.
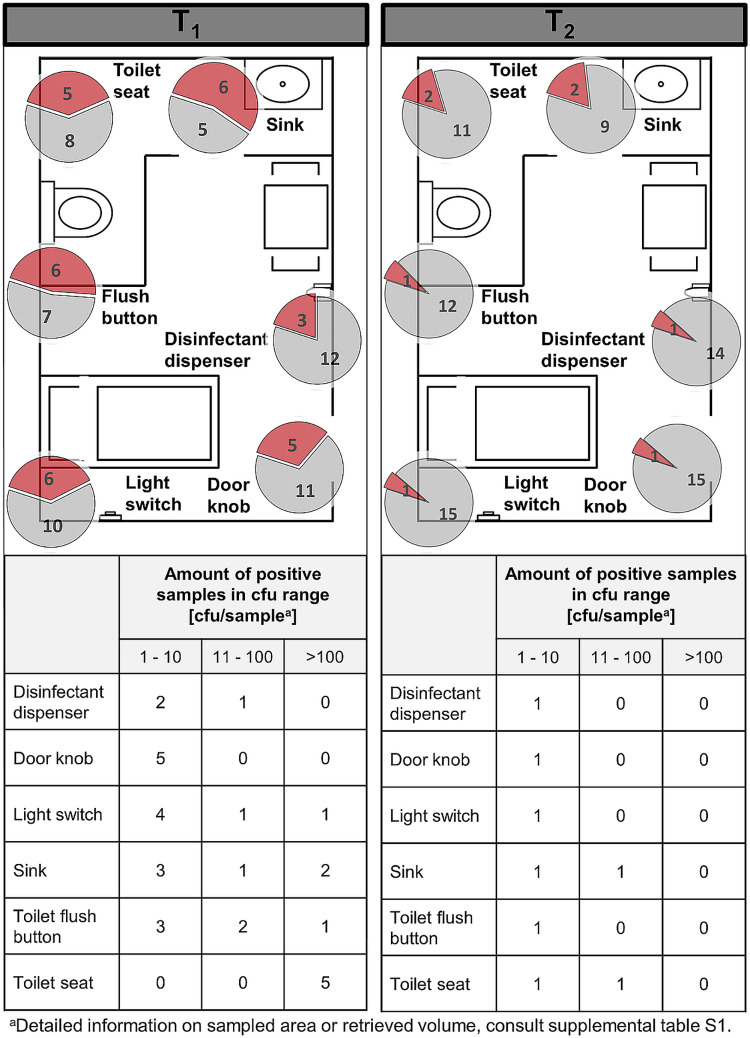
All sampling types tested positive for C. difficile occurrence at least once (Fig. 4) during CDAD inpatient presence, leading to an overall contamination frequency of 37%, and C. difficile persisted after patient discharge (approximately 10% positive). Patients’ sinks (55%) were found to be the most contaminated sites, followed by toilet flush buttons (46%), toilet seats (38%), door knobs (31%), light switches (37%) and disinfectant dispenser (20%). Sampling sites consistently decreased in their contamination amounts and rates after CDAD patient discharge (18%, 8%, 15%, 6%, 7%, and 7%). The highest C. difficile reduction was obtained for the flush button, which decreased by 38% after patient discharge. The disinfectant dispenser experienced the lowest reduction, by 13%.
FIG 4.
High-touch surfaces. Pie charts represent the amount of positive C. difficile findings (red) compared to negative findings (gray). The sum of red and gray portions per pie chart represents the number of total sampled sites in different rooms, during CDAD inpatient treatment (T1) (left) and after patient discharge (T2) (right). The floor plans are for illustrative purposes only and do not necessarily correspond to the actual room layout.
Control rooms.
As negative controls, four patient rooms with en suite bathrooms as well as four soiled workrooms in wards that never accommodated patients suffering from acute CDAD were sampled (Fig. 5).
FIG 5.
Control rooms. Pie charts represent the amount of positive C. difficile findings (red) compared to negative findings (gray). The sum of red and gray portions per pie chart represents the number of total sampled sites in different rooms on high-touch surfaces in patient rooms (A), trap stagnant water and biofilms in patient en suite bathrooms (B), sampled sites related to the washer disinfectors and bedpans in soiled workrooms (C), and sink trap stagnant water and biofilms in soiled workrooms (D). The floor plans are for illustrative purposes only and do not necessarily correspond to the actual room layout.
A biofilm sample inside a WD trap in one ward as well as another ward WD’s operating buttons were slightly contaminated with C. difficile at low levels. All other sites tested negative for the presence of C. difficile.
Statistical analysis results.
The Mann-Whitney U test showed that the positivity of sampling sites decreased significantly (P = 0.001; confidence interval [CI], 0.31 to ∞) between the two sampling time points T1 and T2. The proportion of positive sampling sites at T1 compared to control room sampling sites was also significant (P = 0.001, CI of 0.46 to ∞). When comparing T2 to the control rooms, reduction was not statistically significant (P = 0.047; CI, 0.21 to ∞).
DISCUSSION
In the framework of this study, we detected viable C. difficile in hospital traps of sinks, toilets, and showers in patient bathrooms as well as in ward WDs inside soiled workrooms while considering the effect of CDAD patient discharge on the environmental C. difficile concentration. Such infective reservoirs decreased significantly upon patient discharge but persisted inside the hospital wastewater system, presumably because they were not targeted during hospital disinfection procedures. Spores are environmentally resistant and are crucial for infection. They can tolerate aerobic conditions outside the host and also survive the acidic stomach passage to enter the intestine. The results shown here do not explicitly distinguish spores from vegetative forms because samples were not heat or alcohol shocked to kill the vegetative form. Nevertheless, it is expected that mainly spores were detected in this study, because vegetative forms survive only for a short time under aerobic conditions (15 min to 3 h, depending on the strain) (32, 33).
We found that patient bathroom sink-associated biofilms experienced a 100% C. difficile decrease after patient discharge, while trap stagnant water was still contaminated; this observation was unexpected and could possibly be attributed to the experimental procedure itself. The trap brush seemed to have released and loosened up the biofilm during sampling, eliminating C. difficile from the sampling site. Such a dramatic decline in C. difficile was not achieved when swabbing the biofilm, e.g., inside toilet bowls with a soft sponge. Therefore, biofilms inside toilets revealed C. difficile contamination in up to 77% of cases, with over 50% of the toilets remaining positive after CDAD patient discharge.
Traps constitute protected and humid environments that favor the formation of rich and diverse microbial communities (34). Frequent rinsing of nutritious liquid waste, such as soap, residual feeding supplements, beverages, and other waste nourishes microbial communities in biofilms and consequently promotes biofilm growth, where gene transfer can occur and consequently antibiotic resistance can emerge (35, 36). In the frame of this study, several toxin-producing C. difficile strains were detected (data not shown) along with nontoxigenic strains, which was an alarming finding since C. difficile can transfer its pathogenicity locus along with antibiotic resistance genes via conjugation to nontoxigenic strains, ultimately converting them to toxin producers (37) and potentially infecting patients. A molecular examination of the isolates would be useful from a scientific perspective in order to determine the clonal link between environmental isolates to patient strains and vice versa. In addition, a prospective examination of the patients who stayed in the rooms previously occupied by CDAD patients would be interesting.
Shear forces of running tap water might detach fragments of a biofilm lining the pipe, which may colonize other parts of the wastewater system, boosting spreading of C. difficile from one sink to another, as demonstrated by Kotay et al. (23). Therefore, traps cannot only become colonized with C. difficile by seeding spores from above, but also via retrograde by the spread from a neighboring sink, presumably even across rooms (23). The latter explanation might also give a reason for the occurrence of C. difficile in traps from rooms that never accommodated CDAD patients (e.g., control rooms), i.e., that C. difficile was shared or dispersed in the hospital plumbing infrastructure rather than only being excreted by asymptomatic C. difficile carriers in rooms that never accommodated CDAD patients. However, this hypothesis was not investigated in the hospital studied. As countermeasures, the German commission for hospital hygiene and infection prevention (KRINKO) demands that running tap water not be allowed to directly hit the trap, to minimize aerosol and droplet formation, since studies have found that contaminated trap water can be dispersed at a radius of 1.85 m around the strainer. Likewise, the insert of a plug to shield the trap has been proposed to avoid splashing and aerosolization (38). Those recommendations are not always implemented in clinical settings even if they help to reduce contamination of the environment.
Patients suffering from acute CDAD in hospital settings are usually under contact precautions and frequently use bedpans and commodes to defecate. The reprocessing of such reusable goods must ensure that no residual bacteria remain on the bedpan, so that potential harm to the subsequent user is prevented. To date, there is little literature on the efficacy of WDs under sustained clinical use in bedpan decontamination performance related to C. difficile and its spores. A study by Alfa and colleagues has long claimed that the A0 value of 60, as officially advised by the DIN EN ISO 15883-3:2009, is insufficient to reduce artificially applied C. difficile spores on bedpans, independent of the reprocessing procedure, i.e., the combination of cold and warm rinses (27). Complying with this norm, WDs in the studied hospital run at an A0 value of 600 (90°C and 60 s). As a result, even if WD traps stagnant water and biofilms had constantly large amounts of C. difficile in up to 50% of cases, bedpans sampled immediately after a wash run were never found to be positive for C. difficile, guaranteeing safe usage to patients. Presumably, the A0 value programmed to the WD is a temperature-time regimen targeting the surface of the bedpan and not the lower areas of the trap, explaining the finding of viable C. difficile spores in the WD trap stagnant water and biofilm. WD traps have large openings to allow easy passage of solid particles, and they are composed of a plastic material that additionally feeds biofilms with organic matter, and thus retrograde contamination of the bedpans by the heavily contaminated siphons can therefore theoretically not be completely ruled out, but this was not proven in this study. We conclude that the A0 concept should be revised to the current state of knowledge in terms of C. difficile elimination. Heat applied to the load inside WDs should also target the siphon to eliminate high C. difficile concentrations, since C. difficile spore transmission cannot be ruled out entirely and should ideally also only be introduced in the smallest possible quantities into the communal wastewater.
The cleaning intervention, as performed in this study, could potentially lay the foundation for further research on the suggestion of new cleaning regimens applied to hospital siphons. The protocol is inexpensive, easy, efficient, and could function as a short-term measure to rapidly combat C. difficile transmission from sink traps, e.g., in outbreak situations until further measures have been introduced. Disinfectants have already come into use to temporarily diminish bioburden of pathogens in sink traps; however, to the best of our knowledge, results with sporicidal agents, such as those chosen in the present study, have not been reported so far. The usage of peracetic acid-based disinfectants has been proven superior in combating biofilms compared to glutaraldehyde, for example, which has protein-fixing properties (39). It has also been argued that peracetic acid may be superior in eliminating thick, multispecies biofilms on a long-term basis, such as the ones occurring in siphons, as opposed to chlorine-based agents (40). Planktonic bacteria are constantly released from pipe-associated biofilms into the stagnant water, therefore making the sole decontamination of the water without removing the biofilm insufficient to destroy this bacterial reservoir (41).
We achieved a >99% elimination of C. difficile in the tested traps. Drawn samples were not treated with a neutralizing agent, in order to quench the residual disinfectant, and thus viable C. difficile concentrations might be slightly higher than those found with the culturing approach in this study. Long-term consequences of the intervention were not assessed and remain an interesting topic that should be addressed when proposing new hygiene guidelines.
Impeding biofilm formation in traps has been a topic of extensive research, and this has been mostly aimed at changing the design and materials of plumbing systems, but approaches have so far remained unsatisfactory or have only delivered temporary solutions (42). An interesting approach involves the installation of self-disinfecting sink traps (e.g., BIOREC, Lauta, Germany). These systems combine a thermal disinfection step (heating the stagnant water to ≥85°C) and an antimicrobial coating with further UV treatment and vibrations every time new water enters the trap (43), thereby impeding the development of biofilms and reducing trap-associated nosocomial infections. It certainly would be interesting to test whether this device is able to decontaminate C. difficile and its spores from contaminated sink traps.
By incorporating regular prophylactic decontamination of these so-far-overlooked C. difficile reservoirs in hospital traps into in-house routine cleaning guidelines as well as by official guidelines (e.g., German KRINKO recommendations), higher safety could be guaranteed in the clinical environment and ultimately to patients.
High-touch surfaces are known transmission vehicles for nosocomial infections and are specifically targeted by routine cleaning protocols and terminal disinfection procedures following CDAD patient discharge. The results indicate that C. difficile contamination on HTSs was significantly reduced after CDAD patient discharge, but it was never eliminated, leaving around 10% of sites still positive, which leads to the conclusion that high-touch surfaces of rooms that accommodate CDAD patients may always be contaminated with C. difficile to a certain extent. HTSs were contaminated in up to 54% (patient bathroom sink) of samples, with C. difficile still present on such sites in over 18% of cases even after CDAD patient discharge, when rooms were occupied anew. In comparison, rooms that never accommodated CDAD patients did not show any contamination with C. difficile. Statistical comparison between T1 and control rooms showed a statistically significant difference in contamination. The fact that the comparison of T2 samples to the control rooms did not show statistical significance was due to the small number of samples (or positive C. difficile findings). Therefore, reliability of statistical significance testing for this comparison is questionable. The findings are in accordance with those of Dubberke et al. (28) and Verity et al. (31), who found rooms accommodating CDAD patients to be frequently contaminated with substantially higher counts of C. difficile than other rooms (31). Disinfectant dispensers showed the lowest reduction of CFU after patient discharge; possibly, they were easily overlooked during cleaning practices or frequently contaminated anew. This study also detected a substantially higher C. difficile concentration on toilet seats and sinks compared to studies by Kim et al. (3). Ali and colleagues (2015) on the contrary showed higher contamination rates on toilet seats but found lower contamination frequencies on flush buttons (44). Since HTSs are frequently touched by clinic personnel and patients, contamination paths from there on must not only be interrupted by surface disinfection but also by appropriate hand hygiene. In addition to the use of gloves, hands should be washed rather than disinfected, since most disinfectants are ineffective against C. difficile spores. Hands should not only be washed after giving direct care to CDAD patients, but also after touching their surroundings (45, 46).
The disinfectant concentration necessary to achieve total sporicidal efficacy is sometimes so high that it is not tolerable or is acutely toxic to a patient in the room. Therefore, the studied hospital currently instructs cleaning staff to decontaminate surfaces in close proximity to a CDAD patient with an only low concentrated sporicidal agent (peracetic acid at a bactericidal or levurozidal concentration-time relation) according to the German KRINKO at least once per day (47). The low dose of the sporicidal agent may not be able to eliminate all C. difficile spores but will at least reduce them considerably, as shown by a study from the German Association for Applied Hygiene (48). Our findings strengthen the importance of terminal disinfection of rooms after patient discharge with an appropriately high concentration of sporicidal disinfectant. The efficiency of terminal disinfection with a sporicidal agent was not assessed in this study but might be an interesting outlook to evaluate efficacy of sporicidal cleaning procedures in the clinical setting.
Conclusions.
Occurrence of C. difficile reservoirs in hospitals plea for higher attention by authorities as well as hospital hygiene and infection control teams to eliminate reservoirs and ultimately to prevent C. difficile nosocomial infection. Finally, the study revealed and raised new questions with respect to C. difficile that can now be addressed.
MATERIALS AND METHODS
Room inclusion criteria.
All rooms accommodating CDAD inpatients in a German maximum-care hospital between mid-June and September 2018 were considered for investigation. A total of 23 single-occupancy (isolation) rooms and corresponding soiled workrooms of 14 different wards were selectively examined for the presence of C. difficile environmental contamination. A patient’s CDAD diagnosis was confirmed with the C. DIFF Quik Chek Complete test (Techlab, Blacksburg, VA, USA) and an Illumigene C. difficile test kit (Meridian Bioscience, Braine-l'Alleud, Belgium).
The studied hospital incorporates low-dose daily surface disinfection (with a disinfectant containing peracetic acid at a bactericidal and levurozidal concentration-time relation) in CDAD patient rooms. A fully sporicidal concentration is considered intolerable and even toxic to patients. For the terminal disinfection (i.e., after patient discharge), however, the concentration-time ratio with sporicidal effect should always be used, as validated by the German Association for Applied Hygiene (49) and advised by the German Commission for Hospital Hygiene and Infection Prevention (KRINKO) (47).
Test sites.
In patient rooms and adjacent soiled workrooms, sampling of test sites (see Table S1 in the supplemental material) took place the same day or latest 3 days after diagnosis while patients were suffering from acute CDAD (T1). Within 13 ± 6 days after patient discharge, patient rooms and soiled workrooms were resampled, only if contaminated sites were detected in the first examination and if patient rooms were not reoccupied by other CDAD inpatients in the meantime (T2). Four additional rooms that never accommodated CDAD patients were chosen as negative controls. As patient room design varies considerably between wards, not all sampling points intended by the study could be tested in each room.
Sample types and sampling.
Three sample types were collected for the detection of C. difficile in the hospital environment: stagnant water in traps, biofilms adhering to trap pipes, and environmental swabs. Several locations in the patient room area (high-touch surfaces), the patient bathroom (sinks, toilets, and showers), and the soiled workroom (washer disinfectors and bedpans) were chosen to be examined (Table S1). All samples were taken arbitrarily, without prior knowledge of time of cleaning or last usage. Bedpans were randomly selected in wards accommodating at least one CDAD inpatient, directly after exiting the washer disinfector, and were not traceable to individual patients.
(i) Stagnant water samples. Stagnant water from toilets, inside sink traps, and WD traps was collected by introduction of a flexible polytetrafluoroethylene hose (inner diameter of 2 mm, outer diameter of 3 mm; Bohlender, Grünsfeld, Germany) connected to a 50-mL syringe and pumping down 100 mL of the water without touching the pipe walls. In cases of WDs, water and biofilm samples were taken after the cleaning program. Remaining sampling sites were random grab samples, taken in a patient's room without prior knowledge of latest usage or cleaning of toilet or sink. To prevent contamination of the trap stagnant water by biofilm flocs, the water sample was always taken before the biofilm sample. Water samples were passed through a 30-μm MACS SmartStrainer (Miltenyi Biotec Inc., Auburn, CA, USA) to remove larger particles.
(ii) Biofilms. For trap biofilm withdrawal, a flexible, nylon-bristled trap brush (length of 505 mm, diameter of 12 mm; haug bürsten, Königsbrunn, Germany) was introduced into the pipe and scrubbed up and down five times, firmly scratching the luminal surface at the level of the trap as well as the vertical section above. Brushes were cut into three small chunks, placed in 100 mL of sterile demineralized water, and homogenized in a sterile container at 300 rpm for 30 min to loosen the biofilm from the brush. In case the brush was visually still covered with biofilm, containers were again shaken manually for 30 s. If released material was chunky and inhomogeneous, it was additionally passed through a homogenizer and subsequently filtered through a 30-μm MACS SmartStrainer with help of the backside of a sterile syringe that forced passage through the filter to remove larger particles.
(iii) Swabs. Surfaces were swabbed with sterile premoistened sponges (3M sponge sticks, Neuss, Germany) with constant high pressure using overlapping and multidirectional motions (see Table S1 for dimensions). Sponges were stored (maximum of 1 h) in sterile bags with 100 mL of sterile demineralized water until processing. Sample bags were shaken at 300 rpm for 30 min, vigorously massaged for 2 min, and then contents were passed through a 30-μm MACS SmartStrainer to remove large particles.
After the MACS SmartStrainer passage, all sample types were subjected to vacuum filtration through a 0.45-μm EZ-Fit filtration unit (MerckMillipore, Darmstadt, Germany), and the filter was placed onto ChromID agar (CID, bioMérieux, Craponne, France) and incubated anaerobically for 48 h at 37°C. C. difficile colonies grown on filters were counted to a maximum of 100 CFU per filter.
Characterization of isolates.
Since colony morphology on filter paper can be different from that with direct smears, at least 10 colonies of the same morphological type were streaked onto fresh CID plates alongside Columbia agar with 7% sheep blood (Thermo Fisher Scientific, Massachusetts, USA). After 48 h of anaerobic incubation at 37°C, plates were again screened for typical C. difficile morphology and characteristic horse barn odor. Colonies suspected of being C. difficile were analyzed with the latex agglutination assay C. difficile test kit (Oxoid Limited, Hampshire, United Kingdom). Positive isolates were subjected to a C. difficile Tox A/B enzyme-linked immunosorbent assay (Techlab, Blacksburg, VA, USA).
Cleaning intervention.
Two sink and one shower trap in patient en suite bathrooms were treated with the sporicidal agent Ultrasol active (Schumacher, Malsfeld, Germany) containing peracetic acid (made from a powder containing sodium percarbonate, citric acid, and sodium carbonate; a 1% solution [wt/vol] contains >0.075% peracetic acid). For this purpose, 1 liter of a 2% solution of the product was slowly poured into the sink trap and allowed a contact time of 15 min, in accordance with the manufacturer’s recommendations. Subsequently, the faucet was run on maximum flow with cold water for 2 min. One hundred milliliters of stagnant water was sampled and subjected to the isolation protocol mentioned above.
Statistical analysis.
Statistical analyses were carried out using R 4.2.2 (50). Normality testing was performed with a Shapiro-Wilk test, which rejected the null hypothesis. A paired, one-tailed Mann-Whitney U test was performed to compare the proportion of positive results per sampling site between T1 and T2, T1 and control rooms, and T2 and control rooms. Performance of three tests (multiple testing) resulted in a significance level of 5/3% (1.7%).
ACKNOWLEDGMENTS
We are grateful to the hygiene workers who accompanied us during sampling on the ward.
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Lia Freier, Email: lia.freier@ukbonn.de.
Christopher A. Elkins, Centers for Disease Control and Prevention
REFERENCES
- 1.Robert Koch Institut. 2021. Infektionsepidemiologisches Jahrbuch für 2020. Robert Koch Institut, Berlin, Germany. [Google Scholar]
- 2.Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, Goldman S, Wultańska D, Garlicki A, Biesiada G. 2019. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis 38:1211–1221. doi: 10.1007/s10096-019-03539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim KH, Fekety R, Batts DH, Brown D, Cudmore M, Silva J, Waters D. 1981. Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic-associated colitis. J Infect Dis 143:42–50. doi: 10.1093/infdis/143.1.42. [DOI] [PubMed] [Google Scholar]
- 4.Kramer A, Schwebke I, Kampf G. 2006. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards AN, Karim ST, Pascual RA, Jowhar LM, Anderson SE, McBride SM. 2016. Chemical and stress resistances of Clostridium difficile spores and vegetative cells. Front Microbiol 7:1698. doi: 10.3389/fmicb.2016.01698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheeldon LJ, Worthington T, Hilton AC, Elliott TSJ, Lambert PA. 2008. Physical and chemical factors influencing the germination of Clostridium difficile spores. J Appl Microbiol 105:2223–2230. doi: 10.1111/j.1365-2672.2008.03965.x. [DOI] [PubMed] [Google Scholar]
- 7.Hong KB, Oh HS, Song JS, Lim J, Kang DK, Son IS, Park JD, Kim EC, Lee HJ, Choi EH. 2012. Investigation and control of an outbreak of imipenem-resistant Acinetobacter baumannii infection in a pediatric intensive care unit. Pediatr Infect Dis J 31:685–690. doi: 10.1097/INF.0b013e318256f3e6. [DOI] [PubMed] [Google Scholar]
- 8.Breathnach AS, Cubbon MD, Karunaharan RN, Pope CF, Planche TD. 2012. Multidrug-resistant Pseudomonas aeruginosa outbreaks in two hospitals: association with contaminated hospital waste-water systems. J Hosp Infect 82:19–24. doi: 10.1016/j.jhin.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Hota S, Hirji Z, Stockton K, Lemieux C, Dedier H, Wolfaardt G, Gardam MA. 2009. Outbreak of multidrug-resistant Pseudomonas aeruginosa colonization and infection secondary to imperfect intensive care unit room design. Infect Control Hosp Epidemiol 30:25–33. doi: 10.1086/592700. [DOI] [PubMed] [Google Scholar]
- 10.Leitner E, Zarfel G, Luxner J, Herzog K, Pekard-Amenitsch S, Hoenigl M, Valentin T, Feierl G, Grisold AJ, Högenauer C, Sill H, Krause R, Zollner-Schwetz I. 2015. Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrob Agents Chemother 59:714–716. doi: 10.1128/AAC.04306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowe C, Willey B, O'Shaughnessy A, Lee W, Lum M, Pike K, Larocque C, Dedier H, Dales L, Moore C, McGeer A, Mount Sinai Hospital Infection Control Team . 2012. Outbreak of extended-spectrum β-lactamase–producing Klebsiella oxytoca infections associated with contaminated handwashing sinks. Emerg Infect Dis 18:1242–1247. doi: 10.3201/eid1808.111268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starlander G, Melhus Å. 2012. Minor outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in an intensive care unit due to a contaminated sink. J Hosp Infect 82:122–124. doi: 10.1016/j.jhin.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Vergara-López S, Domínguez MC, Conejo MC, Pascual Á, Rodríguez-Baño J. 2013. Wastewater drainage system as an occult reservoir in a protracted clonal outbreak due to metallo-β-lactamase-producing Klebsiella oxytoca. Clin Microbiol Infect 19:E490–498. doi: 10.1111/1469-0691.12288. [DOI] [PubMed] [Google Scholar]
- 14.Decraene V, Phan HTT, George R, Wyllie DH, Akinremi O, Aiken Z, Cleary P, Dodgson A, Pankhurst L, Crook DW, Lenney C, Walker AS, Woodford N, Sebra R, Fath-Ordoubadi F, Mathers AJ, Seale AC, Guiver M, McEwan A, Watts V, Welfare W, Stoesser N, Cawthorne J, TRACE Investigators’ Group . 2018. A large, refractory nosocomial outbreak of Klebsiella pneumoniae carbapenemase-producing Escherichia coli demonstrates carbapenemase gene outbreaks involving sink sites require novel approaches to infection control. Antimicrob Agents Chemother 62:e01689-18. doi: 10.1128/AAC.01689-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regev-Yochay G, Smollan G, Tal I, Zade NP, Haviv Y, Nudelman V, Gal-Mor O, Jaber H, Zimlichman E, Keller N, Rahav G. 2018. Sink traps as the source of transmission of OXA-48–producing Serratia marcescens in an intensive care unit. Infect Control Hosp Epidemiol 39:1307–1315. doi: 10.1017/ice.2018.235. [DOI] [PubMed] [Google Scholar]
- 16.Sib E, Voigt AM, Wilbring G, Schreiber C, Faerber HA, Skutlarek D, Parcina M, Mahn R, Wolf D, Brossart P, Geiser F, Engelhart S, Exner M, Bierbaum G, Schmithausen RM. 2019. Antibiotic resistant bacteria and resistance genes in biofilms in clinical wastewater networks. Int J Hyg Environ Health 222:655–662. doi: 10.1016/j.ijheh.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Sukhum KV, Newcomer EP, Cass C, Wallace MA, Johnson C, Fine J, Sax S, Barlet MH, Burnham C-AD, Dantas G, Kwon JH. 2022. Antibiotic-resistant organisms establish reservoirs in new hospital built environments and are related to patient blood infection isolates. Commun Med 2:1–15. doi: 10.1038/s43856-022-00124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kizny Gordon AE, Mathers AJ, Cheong EYL, Gottlieb T, Kotay S, Walker AS, Peto TEA, Crook DW, Stoesser N. 2017. The hospital water environment as a reservoir for carbapenem-resistant organisms causing hospital-acquired infections: a systematic review of the literature. Clin Infect Dis 64:1435–1444. doi: 10.1093/cid/cix132. [DOI] [PubMed] [Google Scholar]
- 19.Brooke JS. 2008. Pathogenic bacteria in sink exit drains. J Hosp Infect 70:198–199. doi: 10.1016/j.jhin.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Merlani GM, Francioli P. 2003. Established and emerging waterborne nosocomial infections. Curr Opin Infect Dis 16:343–347. doi: 10.1097/00001432-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Swan JS, Deasy EC, Boyle MA, Russell RJ, O'Donnell MJ, Coleman DC. 2016. Elimination of biofilm and microbial contamination reservoirs in hospital washbasin U-bends by automated cleaning and disinfection with electrochemically activated solutions. J Hosp Infect 94:169–174. doi: 10.1016/j.jhin.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Anaissie EJ, Penzak SR, Dignani MC. 2002. The hospital water supply as a source of nosocomial infections: a plea for action. Arch Intern Med 162:1483–1492. doi: 10.1001/archinte.162.13.1483. [DOI] [PubMed] [Google Scholar]
- 23.Kotay S, Chai W, Guilford W, Barry K, Mathers AJ. 2017. Spread from the sink to the patient: in situ study using green fluorescent protein (GFP)-expressing Escherichia coli to model bacterial dispersion from hand-washing sink-trap reservoirs. Appl Environ Microbiol 83:e03327-16. doi: 10.1128/AEM.03327-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotay SM, Donlan RM, Ganim C, Barry K, Christensen BE, Mathers AJ. 2019. Droplet- rather than aerosol-mediated dispersion is the primary mechanism of bacterial transmission from contaminated hand-washing sink traps. Appl Environ Microbiol 85:e01997-18. doi: 10.1128/AEM.01997-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landelle C, Verachten M, Legrand P, Girou E, Barbut F, Brun-Buisson C, Buisson CB. 2014. Contamination of healthcare workers’ hands with Clostridium difficile spores after caring for patients with C. difficile infection. Infect Control Hosp Epidemiol 35:10–15. doi: 10.1086/674396. [DOI] [PubMed] [Google Scholar]
- 26.Kommission für Krankenhaushygiene und Infektionsprävention beim Robert Koch-Institut. 2020. Anforderungen der Hygiene an abwasserführende Systeme in medizinischen Einrichtungen. Bundesgesundheitsbl 63:484–501. doi: 10.1007/s00103-020-03118-7. [DOI] [PubMed] [Google Scholar]
- 27.Alfa MJ, Olson N, Buelow-Smith L. 2008. Simulated-use testing of bedpan and urinal washer disinfectors: evaluation of Clostridium difficile spore survival and cleaning efficacy. Am J Infect Control 36:5–11. doi: 10.1016/j.ajic.2007.04.277. [DOI] [PubMed] [Google Scholar]
- 28.Dubberke ER, Reske KA, Noble-Wang J, Thompson A, Killgore G, Mayfield J, Camins B, Woeltje K, McDonald JR, McDonald LC, Fraser VJ. 2007. Prevalence of Clostridium difficile environmental contamination and strain variability in multiple health care facilities. Am J Infect Control 35:315–318. doi: 10.1016/j.ajic.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Exner M. 2007. Divergent opinions on surface disinfection: myths or prevention? A review of the literature. GMS Krankenhhyg Interdiszip 2:Doc19. [PMC free article] [PubMed] [Google Scholar]
- 30.Otter JA, Yezli S, French GL. 2011. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol 32:687–699. doi: 10.1086/660363. [DOI] [PubMed] [Google Scholar]
- 31.Verity P, Wilcox MH, Fawley W, Parnell P. 2001. Prospective evaluation of environmental contamination by Clostridium difficile in isolation side rooms. J Hosp Infect 49:204–209. doi: 10.1053/jhin.2001.1078. [DOI] [PubMed] [Google Scholar]
- 32.Buggy BP, Wilson KH, Fekety R. 1983. Comparison of methods for recovery of Clostridium difficile from an environmental surface. J Clin Microbiol 18:348–352. doi: 10.1128/jcm.18.2.348-352.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jump RLP, Pultz MJ, Donskey CJ. 2007. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob Agents Chemother 51:2883–2887. doi: 10.1128/AAC.01443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Withey Z, Goodall T, MacIntyre S, Gweon HS. 2021. Characterization of communal sink drain communities of a university campus. Environ DNA 3:901–911. doi: 10.1002/edn3.196. [DOI] [Google Scholar]
- 35.Madsen JS, Burmølle M, Hansen LH, Sørensen SJ. 2012. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med Microbiol 65:183–195. doi: 10.1111/j.1574-695X.2012.00960.x. [DOI] [PubMed] [Google Scholar]
- 36.Kotay SM, Parikh HI, Barry K, Gweon HS, Guilford W, Carroll J, Mathers AJ. 2020. Nutrients influence the dynamics of Klebsiella pneumoniae carbapenemase producing enterobacterales in transplanted hospital sinks. Water Res 176:115707. doi: 10.1016/j.watres.2020.115707. [DOI] [PubMed] [Google Scholar]
- 37.Brouwer MSM, Roberts AP, Hussain H, Williams RJ, Allan E, Mullany P. 2013. Horizontal gene transfer converts non-toxigenic Clostridium difficile strains into toxin producers. Nat Commun 4:2601. doi: 10.1038/ncomms3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kommission für Krankenhaushygiene und Infektionsprävention. 2016. Händehygiene in Einrichtungen des Gesundheitswesens. Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut (RKI). Bundesgesundheitsblatt 59:1189–1220. doi: 10.1007/s00103-016-2416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pineau L, Desbuquois C, Marchetti B, Luu Duc D. 2008. Comparison of the fixative properties of five disinfectant solutions. J Hosp Infect 68:171–177. doi: 10.1016/j.jhin.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C, Brown PJB, Miles RJ, White TA, Grant DG, Stalla D, Hu Z. 2019. Inhibition of regrowth of planktonic and biofilm bacteria after peracetic acid disinfection. Water Res 149:640–649. doi: 10.1016/j.watres.2018.10.062. [DOI] [PubMed] [Google Scholar]
- 41.Voigt AM, Faerber HA, Wilbring G, Skutlarek D, Felder C, Mahn R, Wolf D, Brossart P, Hornung T, Engelhart S, Exner M, Schmithausen RM. 2019. The occurrence of antimicrobial substances in toilet, sink and shower drainpipes of clinical units: a neglected source of antibiotic residues. Int J Hyg Environ Health 222:455–467. doi: 10.1016/j.ijheh.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 42.De Geyter D, Blommaert L, Verbraeken N, Sevenois M, Huyghens L, Martini H, Covens L, Piérard D, Wybo I. 2017. The sink as a potential source of transmission of carbapenemase-producing Enterobacteriaceae in the intensive care unit. Antimicrob Resist Infect Control 6:24. doi: 10.1186/s13756-017-0182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fusch C, Pogorzelski D, Main C, Meyer C-L, El Helou S, Mertz D. 2015. Self-disinfecting sink drains reduce the Pseudomonas aeruginosa bioburden in a neonatal intensive care unit. Acta Paediatr 104:e344–e349. doi: 10.1111/apa.13005. [DOI] [PubMed] [Google Scholar]
- 44.Ali S, Muzslay M, Wilson P. 2015. A novel quantitative sampling technique for detection and monitoring of Clostridium difficile contamination in the clinical environment. J Clin Microbiol 53:2570–2574. doi: 10.1128/JCM.00376-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deschênes P, Chano F, Dionne L-L, Pittet D, Longtin Y. 2017. Efficacy of the World Health Organization-recommended handwashing technique and a modified washing technique to remove Clostridium difficile from hands. Am J Infect Control 45:844–848. doi: 10.1016/j.ajic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Oughton MT, Loo VG, Dendukuri N, Fenn S, Libman MD. 2009. Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile. Infect Control Hosp Epidemiol 30:939–944. doi: 10.1086/605322. [DOI] [PubMed] [Google Scholar]
- 47.Bundesgesundheitsbl . 2019. Hygienemaßnahmen bei Clostridioides difficile-Infektion (CDI). Bundesgesundheitsbl 62:906–923. doi: 10.1007/s00103-019-02959-1. [DOI] [PubMed] [Google Scholar]
- 48.VAH. 2017. Empfehlung zur Auswahl sporizider Desinfektionsmittel bei Clostridium-difficile-Infektionen im human-medizinischen Bereich. Hyg Med 42–3. [Google Scholar]
- 49.Gemein S, Andrich R, Christiansen B, Decius M, Exner M, Hunsinger B, Imenova E, Kampf G, Koburger-Janssen T, Konrat K, Martiny H, Meckel M, Mutters NT, Pitten F-A, Schulz S, Schwebke I, Gebel J. 2022. Efficacy of five ‘sporicidal’ surface disinfectants against Clostridioides difficile spores in suspension tests and 4-field tests. J Hosp Infect 122:140–147. doi: 10.1016/j.jhin.2022.01.010. [DOI] [PubMed] [Google Scholar]
- 50.R Foundation for Statistical Computing. 2022. R: a language and environment for statistical computing. R Core Team, Vienna, Austria. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aem.00014-23-s0001.pdf, PDF file, 0.09 MB (90.9KB, pdf)