ABSTRACT
Respiratory syncytial virus (RSV) fusion protein (F) is highly conserved between subtypes A and B (RSV/A and RSV/B). To become fully active, F precursor undergoes enzymatic cleavage to yield F1 and F2 subunits and releases a 27-amino-acid peptide (p27). Virus-cell fusion occurs when RSV F undergoes a conformational change from pre-F to post-F. Previous data show that p27 is detected on RSV F, but questions remain regarding if and how p27 affects the conformation of mature RSV F. Monoclonal antibodies against p27, site Ø (pre-F specific), and site II were used to monitor RSV F conformation by enzyme-linked immunosorbent assay (ELISA) and imaging flow cytometry. Pre-F to post-F conformational change was induced by a temperature stress test. We found that p27 cleavage efficiency was lower on sucrose-purified RSV/A (spRSV/A) than on spRSV/B. In addition, cleavage of RSV F was cell line dependent: HEp-2 cells had higher retention of p27 than did A549 cells when infected with RSV. Higher levels of p27 were also found on RSV/A-infected cells than on RSV/B-infected cells. We observed that RSV/A F with higher p27 levels could better sustain the pre-F conformation during the temperature stress challenge in both spRSV- and RSV-infected cell lines. Our findings suggest that despite F sequence similarity, p27 of RSV subtypes was cleaved with different efficiencies, which were also dependent on the cell lines used for infection. Importantly, the presence of p27 was associated with greater stability of the pre-F conformation, supporting the possibility that RSV has more than one mechanism for fusion to the host cell.
IMPORTANCE RSV fusion protein (F) plays an important role in entry and viral fusion to the host cell. The F undergoes proteolytic cleavages releasing a 27-amino-acid peptide (p27) to become fully functional. The role of p27 in viral entry and the function of the partially cleaved F containing p27 has been overlooked. p27 is thought to destabilize the F trimers, and thus, there is need for a fully cleaved F. In this study, we detected p27 on purified RSV virions and on the surface of virus-infected HEp-2 and A549 cells for circulating RSV strains of both subtypes. Higher levels of partially cleaved F containing p27 better sustained the pre-F conformation during the temperature stress challenge. Our findings highlight that the cleavage efficiency of p27 is different between RSV subtypes and among cell lines and that the presence of p27 contributes to the stability of the pre-F conformation.
KEYWORDS: respiratory syncytial virus, RSV, p27, peptide 27, F protein, cleavage, RSV subtypes, prefusion conformation
INTRODUCTION
Respiratory syncytial virus (RSV) is one of the major causes of acute lower respiratory infections (ALRI), imposing a significant impact on global public health (1–4). RSV primarily impacts children under the age of 5 years, older adults, and immunocompromised individuals (5–8), but all humans experience RSV reinfections throughout life (9). Prior to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, RSV outbreaks occurred predictably during the fall and winter months in temperate climates (10). Generally, during an outbreak, one of the two subtypes of RSV (RSV/A and RSV/B) predominates (11, 12). RSV subtypes are further subdivided into genotypes that can cocirculate at a given time (11, 13). Since 2011 and 2014, RSV/A Ontario (ON) and RSV/B Buenos Aires (BA) have been the two predominant circulating RSV genotypes around the globe (12).
RSV is an enveloped, negative-sense RNA virus belonging to the Pneumoviridae family. Of the 11 proteins encoded by its 15.2-kb genome, 3 are located on the viral envelope: the attachment (G), small hydrophobic (SH), and the fusion (F) proteins (14, 15). Epitope differences in the G protein—and to a lesser degree in the F protein—divide RSV into subtypes RSV/A and RSV/B (11–13). The F protein is highly conserved between RSV/A and RSV/B (16, 17), making it an excellent target for vaccine and therapeutic development. It is a class I transmembrane glycoprotein synthesized as a precursor (F0), becoming active, or fusogenic, upon enzymatic cleavage of an internal peptide of 27 amino acids (p27) flanked between RARR109 furin cleavage site 2 (FCS-2) and KKRKRR136 (FCS-1). The cleavage of F0 occurs intracellularly by furin-like enzymes, which yields two subunits of distinct sizes, F1 (50 kDa) and F2 (20 kDa), with the release of p27 (18, 19). The N terminus of the F1 subunit is a stretch of highly hydrophobic amino acids called the fusion peptide, responsible for anchoring the viral envelope to the host cell membrane.
The F protein plays a vital role in the early phase of infection, allowing the fusion of the virus membrane to the host cell membrane (15, 20). The RSV F protein can either remain as uncleaved F0 on the surface of RSV, as has been detected in viruses as well as infected cells (21), or retain p27 by undergoing partial cleavage on FCS-2, exposing the fusion peptide (21, 22). Complete cleavage of p27 is not a requirement for the F protein to execute its fusogenic function in vitro, although it might occur inefficiently (22). This is relevant because there are two concurrent observations pertaining to the mechanism of RSV fusion and entry into the host cell. The direct-fusion hypothesis first proposes that the F proteins on infectious RSV are fully cleaved (no p27), and RSV fuses directly with the cell membrane. The indirect-fusion hypothesis proposes that the F protein on RSV harbors partially cleaved p27 and it is internalized through actin-dependent macropinocytosis; a second enzymatic cleavage excises the remaining p27, and RSV gains access to the cytoplasm by fusing with the endosomal membrane (21).
The biological role of p27 has been gaining interest in the scientific community as we and others previously observed that p27 elicits a strong immune response in children and adults infected by both RSV/A and RSV/B (23–25), thus supporting the likelihood of partially cleaved F containing p27 during viral infection. A recent study by Lee et al. showed that p27 can be detected on the surface of RSV-infected cells and the lungs of infected mice (26), suggesting that p27 is broadly present in RSV-infected cells in vitro and in vivo. Groundbreaking studies using X-ray crystallography and cryo-electron microscopy (cryo-EM) have improved our understanding of the conformation-dependent function of the RSV F protein (15, 27, 28), showing that RSV F protein has a dynamic quaternary structure, existing in equilibrium between associated and dissociated trimers (29). Once triggered, the pre-F undergoes an irreversible rapid conformational transition to a highly stable post-F form (30). However, structures containing the p27 region in partially cleaved F proteins are challenging to obtain, likely due to the disordered nature of the region and possibly due to the low expression yields of the recombinant constructs containing the peptide (31–33).
To date, structural data of F have been obtained from prototypical strains of RSV/A (33–35) or RSV/B (36), requiring structural modifications to stabilize the metastable pre-F conformation. These studies led to identification of conformation-specific monoclonal antibodies (MAbs) targeting RSV F protein epitopes. Monoclonal antibodies targeting sites unique to pre-F conformation (sites Ø, III, and V) (29, 37, 38) and sites shared between pre-F and post-F forms (sites II and IV) have been used extensively. For this study, we chose D25 and palivizumab as the MAbs that target site Ø and site II, respectively. F protein binding by both D25 and palivizumab is consistent with a pre-F form, while loss of D25 binding to F protein is consistent with a post-F form (30).
Although not well understood, the F protein conformation rearrangement must happen near host cell membranes (via G and F proteins) for productive fusion (39). Due to the metastability of the pre-F, the conformational change may also happen spontaneously or be induced by thermodynamic factors—such as temperature and mechanical stress—hindering the fusogenic function of the F protein (40). Recent studies demonstrated that partially cleaved p27 disrupts the trimerization of the F protein (29, 31); however, Krueger et al. showed that a near-full-length recombinant F protein construct retaining p27 can naturally form trimers in lipid vesicles that adopt a quaternary structure conformation intermediate between pre-F and post-F, with p27 being positioned outside the trimeric arrangement (41). Thus, the relationship between p27 cleavage and F protein conformation is central to all proposed mechanisms of RSV entry in host cells.
Since p27 can elicit immune response after natural infection and may have a biological role in the conformation of F protein, in the present study, we wanted to examine if p27 is present in infectious sucrose-purified RSV (spRSV) particles of both RSV subtypes, as well as on RSV-infected HEp-2 and A549 cells. We also examined the effect of p27 on the resilience of the F protein conformation under a temperature stress test. We utilized MAbs specific to p27 (42), site Ø (unique to pre-F), and site II (shared by pre-F and post-F) (33, 43) to study the presence of p27 on the different conformations of the F protein.
Our findings demonstrate that p27 is present on spRSV, as well as on the surfaces of two cell lines infected with prototypic or contemporary strains of either RSV subtype (A or B). We observed that RSV/A isolates retained a higher proportion of p27 on their F proteins. We also observed that efficiency of enzymatic cleavage of p27 is not comparable between RSV subtypes and is also dependent on the cell line used for infection. Lastly, we show that the presence of p27 conferred improved thermal stability on the pre-F both in spRSV and on the surface of RSV-infected cells. Our overall findings support the view that the RSV F protein has evolved more than one mechanism to undergo fusion to the host cell.
RESULTS
Peptide 27 detected by Western blotting.
The prevailing dogma puts forth that infectious RSV only contains fully cleaved F protein primarily in the pre-F conformation, without p27. RSV immunogenicity data from natural infection and the presence of p27 in vitro are not consistent with this point of view (23, 25, 26). To determine if infectious RSV virions retained p27 on its F protein, we examined the F protein on infectious sucrose-purified RSV particles in which the F protein was subjected to ultrahigh centrifugal forces during the purification. We used both RSV A and B subtypes represented by prototypic (RSV/A/Bernett) and contemporaneous (RSV/B/BA) strains to demonstrate that p27 is retained in both subtypes and genotypes. We probed spRSV/A/Bernett (Fig. 1A) and spRSV/B/BA (Fig. 1B) for site II, site Ø, and p27 under reducing and denatured conditions by Western blotting.
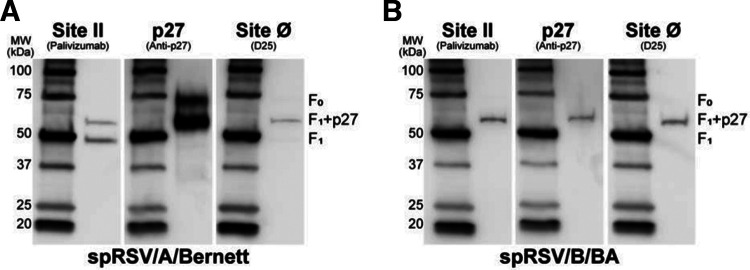
FIG 1.
p27 is present in the F protein of infectious sucrose-purified RSV (spRSV) A and B. Equal amounts of spRSV were resolved by reducing SDS-PAGE gel and analyzed by Western blotting. A representative blot of duplicates is shown. Membranes were probed with palivizumab (anti-site II), RSV7.10 (anti-p27), or D25 (anti-site Ø) monoclonal antibodies. F0, uncleaved F protein (~70 kDa); F1+p27, partially cleaved p27 remained on the N terminus of F1 subunit (~60 kDa); F1, fully cleaved subunit (~50 kDa).
Binding of spRSV/A/Bernett with palivizumab revealed a 50-kDa band corresponding to the F1 subunit, and a 60-kDa band corresponding to the F1 subunit retaining a partially cleaved p27 peptide (F1+p27) (Fig. 1A, site II). The bands on the nitrocellulose membrane probed with the p27 MAb confirmed the presence of the F1+p27 species (~60 kDa) and a 70-kDa band likely corresponding to an uncleaved F protein (F0), which was not detected by either palivizumab or D25 MAbs. Interestingly, D25 also detected a 60-kDa band likely corresponding to the F1+p27 species. The Western blot assay of spRSV/B/BA (Fig. 1B) showed a different distribution of F protein species. Binding with palivizumab and p27 MAbs revealed only a single 60-kDa band corresponding to the partially cleaved F1+ p27 species, whereas the band corresponding to the completely cleaved F1 subunit (50 kDa) was not detected. Similar to the finding for RSV/A/Bernett, D25 MAb detected a 60-kDa band in spRSV/B/BA. These findings show that p27 MAb detected a 60-kDa band in both RSV/A and RSV/B strains consistent with a partially cleaved F protein containing p27. In addition, the presence of uncleaved F protein (F0; 70 kDa) and fully cleaved F1 (50-kDa band) on spRSV/A/Bernett but not on spRSV/B/BA suggests that furin cleavage efficiency can differ between RSV subtypes (21).
Relative quantification of p27 and site Ø on the F protein of sucrose-purified RSV.
We estimated the relative proportion of F protein containing p27 and the F protein in the pre-F conformation by enzyme-linked immunosorbent assay (ELISA) by calculating the ratios of the area under the curve (AUC) of the signal intensity from spRSV/A/Bernett and spRSV/B/BA (Fig. 2). The AUC was calculated from the signal intensity generated by p27, site Ø (pre-F specific), and site II from serially diluted spRSV. To account for assay variability between different days, the AUC of site II was determined in every replicate (Fig. 2A and B). Site II was used as a surrogate measure of total F protein; the relative proportion of F protein containing p27 was calculated using the ratio of the AUC for p27 to the AUC for site II. Similarly, the proportion of pre-F was determined using the ratio of the AUC for site Ø to the AUC for site II.
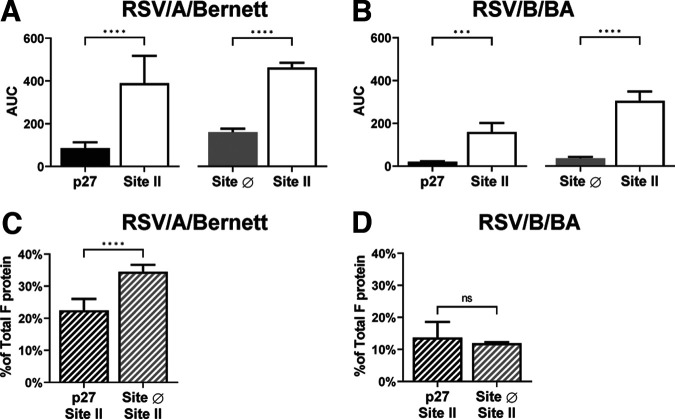
FIG 2.
p27 and F protein in the prefusion conformation can be detected at quantifiable amounts on the surface of spRSVs. Areas under the curve (AUC) of paired measures of p27 and site II or site Ø and site II probed with monoclonal antibody palivizumab (anti-site II), RSV7.10 (anti-p27), or D25 (anti-site Ø) for spRSV/A/Bernett (A) and spRSV/B/BA (B) are shown. Site II was used as a surrogate for total F protein, and site Ø is a pre-F-specific antigenic site. Ratios of the AUC of p27 to site II and site Ø to site II determined relative proportions of p27 and pre-F to total F protein on spRSV/A/Bernett (C) and spRSV/B/BA (D). Error bars are standard deviations (n = 4 replicates). Statistical significances were determined by unpaired parametric t test in GraphPad Prism, assuming Gaussian population distribution. Correlations were calculated with a two-tailed test. P values of <0.05 were considered significant, with a 95% confidence interval. ****, P < 0.0001. ns, not significant.
The relative proportion of p27 detected in spRSV/A/Bernett (Fig. 2C) was 22.5% and that for site Ø was 34.5%, indicating that approximately 22.5% and 34.5% of the F proteins in spRSV/A/Bernett contained p27 and were in a pre-F conformation, respectively. Lower values were detected for the F protein of RSV/B/BA, with AUC ratios of 13.7% and 11.9% of F proteins containing p27 and site Ø, respectively (Fig. 2D). Based on the AUC ratios, spRSV/A/Bernett appeared to have an approximately 1.6 times higher proportion of F protein containing p27 and an approximately 2.9 times higher proportion of F protein in a pre-F conformation (site Ø) than spRSV/B/BA.
F protein cleavage and site Ø stability in RSV-infected HEp-2 and A549 cells measured by flow cytometry.
Since we confirmed that p27 was present on spRSV for both RSV subtypes, we further wanted to validate these finding in in vitro studies on HEp-2 and A549 cells, where the F protein is in a minimally disturbed environment. Our prior study demonstrated significant differences in RSV growth kinetics and host response that were dependent on RSV strain and host cell type (44); we therefore speculated that the efficiency of p27 cleavage and the stability of the pre-F conformation could vary by RSV strain and host cell type. For these studies, we expanded the number of RSV strains used to include both prototypic (RSV/A/Tracy, RSV/A/Bernett, and RSV/B/18537) and contemporary (RSV/A/ON and RSV/B/BA) strains to evaluate if genetic variances within the same RSV subtype can affect p27 retention and its stability in the in vitro infection model. For these studies we used imaging flow cytometry, a powerful technique that collects single-cell images in real time and allows for collection of fluorescence data from specific regions of interest in the cell to measure p27, site Ø, and site II on virus-infected cells. During data analysis, we mitigated the contribution of fluorescence signal from any nonspecific intracellular staining using imaging-based masks to isolate signals to the surface of the cell membrane during data analysis (see Fig. S2 in the supplemental material).
At each day postinoculation (dpi), the same number of events was collected for analysis across all five RSV strains and we determined the percentage of cells that costained positive for site Ø and site II or p27 and site II, i.e., were double positive, to monitor the progression of infection and antigenic site expression in RSV-infected HEp-2 and A549 cells (Fig. 3).
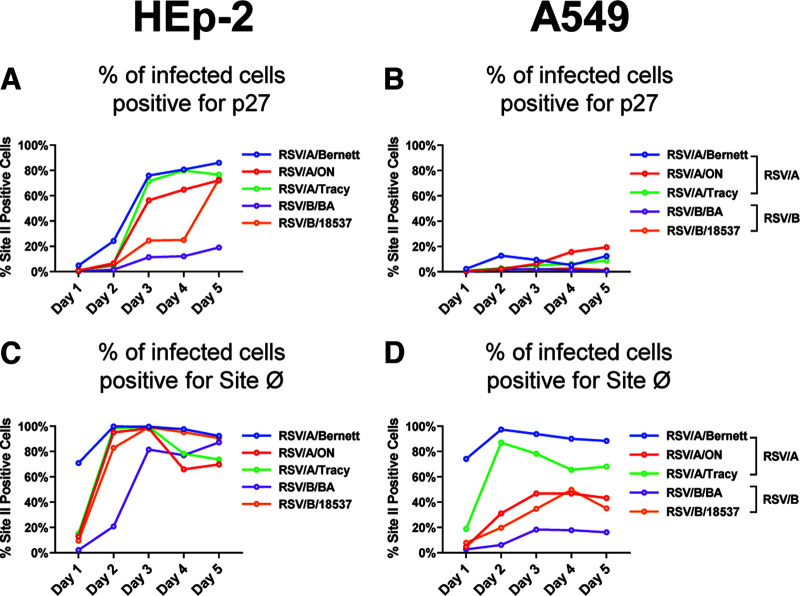
FIG 3.
Progressions of infection and F protein cleavage are different between HEp-2 and A549 cell lines infected with RSV/A or RSV/B. Percentage of double-positive RSV-infected HEp-2 (A and C) or A549 (B and D) cells per day postinoculation is shown. Cells were infected with RSV/A/Bernett, RSV/A/ON, RSV/A/Tracy, RSV/B/18537, or RSV/B/BA at an MOI of 0.07. Cells were costained with fluorescence-conjugated MAb (palivizumab [anti-site II], RSV7.10 [anti-p27], or D25 [anti-site Ø]). Antigenic site II is surrogate measure of total F protein, while antigenic site Ø is pre-F specific.
For RSV-infected HEp-2 cells, at 1 dpi, virtually no site II-positive cells were positive for p27 (Fig. 3A). At 3 dpi, between 60% and 80% HEp-2 cells infected with three different RSV/A strains exhibited site II-positive cells with F proteins containing p27 (Fig. 3A); HEp-2 cells infected with RSV/B/BA reached a maximum of approximately 18% site II-positive cells with detectable p27. Levels of site II-positive cells containing p27 among RSV/B/18537-infected HEp-2 cells were low through 4 dpi but spiked to 63% at 5 dpi, reaching levels similar to those of the RSV/A strains.
RSV-infected A549 cells also showed differences in the percentage of site II-positive cells with detectable p27 between the RSV/A and the RSV/B strains (Fig. 3B). Unlike the RSV-infected HEp-2 cells, the RSV/A-infected A549 cells exhibited a maximum of approximately 20% site II-positive cells presenting F proteins containing p27, and no more than 3% of RSV/B-infected A549 cells generated site II-positive cells with F proteins containing p27.
Higher levels of detectable p27 on either HEp-2 or A549 cells infected with the three RSV/A strains suggest that the cleavage efficiency of F proteins was lower in all three RSV/A strains than in the two RSV/B strains. Similarly, lower levels of site II-positive cells with detectable p27 among RSV-infected A549 cells suggest that the cleavage efficiency of F proteins was higher in virus-infected A549 cells than in virus-infected HEp-2 cells.
The number of cells expressing F proteins with detectable site Ø (pre-F-specific epitope) was different between HEp-2 and A549 cells (Fig. 3C and D). On RSV-infected HEp-2 cells (Fig. 3C), at 1 dpi, the proportion of site II-positive cells presenting F proteins with detectable site Ø (pre-F conformation) was below 20% for all RSV strains, except for RSV/A/Bernett, which had approximately 70% F proteins with site Ø. Between 2 and 3 dpi, all three RSV/A strains and RSV/B/18537 reached the maximum percentage of site II-positive cells expressing F proteins containing site Ø. The proportion of site II-positive cells with detectable site Ø slowly declined to approximately 80% by 5 dpi for the three RSV/A strains and RSV/B/18537, although it remained relatively stable for RSV/B/BA.
A549-infected cells showed a wider percent distribution of site II-positive cells containing F proteins with detectable site Ø than virus-infected HEp-2 cells (Fig. 3D). Of the five RSV strains tested, RSV/A/Bernett and RSV/A/Tracy had the highest percentages of site II-positive A549 cells with F protein containing site Ø, peaking at 2 dpi. The proportions of RSV/A/ON and RSV/B/18537 site II-positive infected A549 cells with F protein containing detectable site Ø peaked at approximately 45% between 3 and 4 dpi. A549 cells infected with RSV/B/BA had the lowest proportion of site II-positive cells expressing F proteins containing site Ø, less than 20%, throughout the 5 days of infection.
Temperature stress test to evaluate the stability of pre-F conformation in infectious sucrose-purified RSV and RSV-infected cells.
The F protein of RSV must maintain its pre-F quaternary structure to fuse with cell membranes. Even though elevated temperatures can irreversibly trigger the F protein conformation from pre-F (fusogenic) to post-F (nonfusogenic), its tertiary structure is highly thermally stable, retaining MAb binding to site II at temperatures as high as 90°C (45–47).
Since the presence of p27 is thought to destabilize trimerization of the F protein (31), we evaluated the effect of a temperature-induced conformational change on the F protein by monitoring the stability of site Ø and changes in p27 detection by ELISA and imaging flow cytometry. We hypothesized that if p27 destabilizes the pre-F form, then partially cleaved pre-F protein should undergo a conformational change to post-F at lower temperatures than fully cleaved F protein.
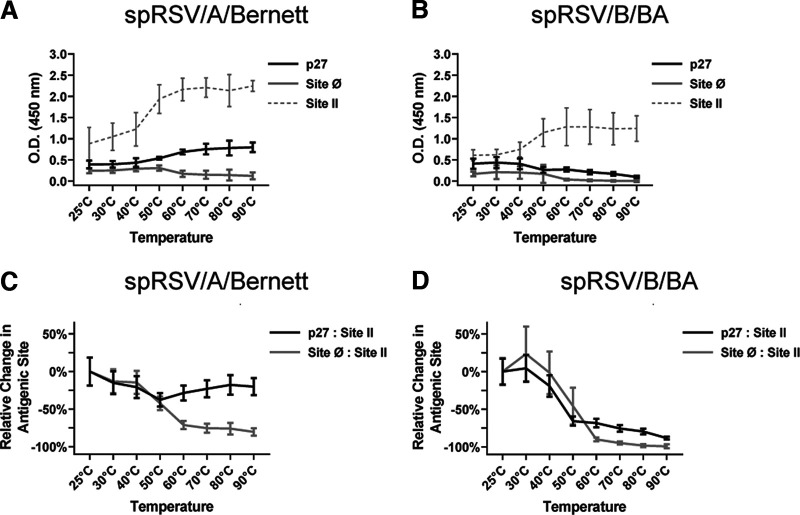
(i) F protein conformation on the surface of sucrose-purified RSV virions. The optical density (OD) signals generated for spRSV/A/Bernett (Fig. 4A) and spRSV/B/BA (Fig. 4B) with the MAbs against p27, site Ø (exclusive to pre-F), and site II (present in pre-F and post-F) are presented for the various temperature stress challenges. Site II was used as the surrogate measure for total F protein. Also, to facilitate comparisons between spRSVs, we monitored changes in MAb binding to antigenic sites as a function of temperature treatment by determining the ratios of p27 to site II and site Ø to site II and normalized the ratios to the corresponding value at 25°C (Fig. 4C and D). Transition in the conformation of F protein from pre-F to post-F was observed beginning at 50°C for both spRSV/A/Bernett (Fig. 4A) and spRSV/B/BA (Fig. 4B), demonstrated by a decrease in MAb binding to site Ø. Interestingly, an increase in MAb binding to both p27 and site II was detected when evaluating spRSV/A/Bernett. A similar increase in binding was detected for the MAb to site II in spRSV/B/BA but not against p27 in the temperature stress experiment. We attributed the increase in binding of site II to the “breathing” of the trimeric form, as Gilman et al. showed that the F protein from the A2 strain transitions between monomeric and trimeric folding states, like a breathing structure (29). To provide additional insight into the changing OD signals with increasing temperature challenges, the p27/site II and site Ø/site II ratios were calculated and then normalized against the respective ratios at 25°C (Fig. 4C and D). A clear distinction was observed for the relative changes in p27/site II ratio between spRSV/A/Bernett (Fig. 4C) and spRSV/B/BA (Fig. 4D). The p27/site II ratio remained relatively stable for spRSV/A/Bernett, while it dropped approximately 80% for spRSV/B/BA. Also, with increasing temperature, the site Ø/site II ratio dropped approximately 85% for spRSV/A/Bernett and almost completely for spRSV/B/BA.
FIG 4.
F proteins on spRSV harboring partially cleaved p27 show prefusion conformation with higher thermal stability. Changes in OD at 450 nm from indirect ELISA analysis of site II, site Ø, and p27 from spRSV/A/Bernett and spRSV/B/BA are shown. Individual spRSV aliquots were heated for 10 min at different temperatures (x axis) before ELISA (A and B). Differences in F protein content between spRSVs were adjusted by adopting site II as measure of total F protein. Changes in MAb binding to site Ø and p27 were determined at each temperature by the changes in site Ø/site II and p27/site II ratios relative to those at 25°C (C and D). Error bars are standard deviations (n = 3 replicates).
(ii) F protein conformation on the surface of RSV-infected cells. To further confirm that the differences we observed between spRSV/A/Bernett and spRSV/B/BA were not due to the sucrose purification process, we used imaging flow cytometry to detect p27, site II, and site Ø on the F protein expressed on the surface of virus-infected cells. We infected HEp-2 cells—the cell line used to generate the sucrose-purified RSV strains—as well as A549 cells to determine if host factors also contributed to the stability of the pre-F conformation under different temperature stress challenges (Fig. S3).
The virus-infected cells were harvested at 3 dpi, and individual aliquots with the same number of cells were diluted and incubated for 10 min at increasing temperatures to stress the system prior to staining and fixation. To make sure that heat did not inadvertently affect cell membrane integrity and mitochondrial function, uninfected cells were tested for lactate dehydrogenase (LDH) release extracellularly and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) activity. The cells maintained membrane integrity and mitochondrial function for 45 min at 65°C (data not shown). The number of site II-positive cells was comparable across all temperature points, cell lines, and RSV subtypes (Fig. S4), confirming that palivizumab bound to antigenic site II on the surface of infected cells after being incubated for 10 min at progressively elevated temperatures.
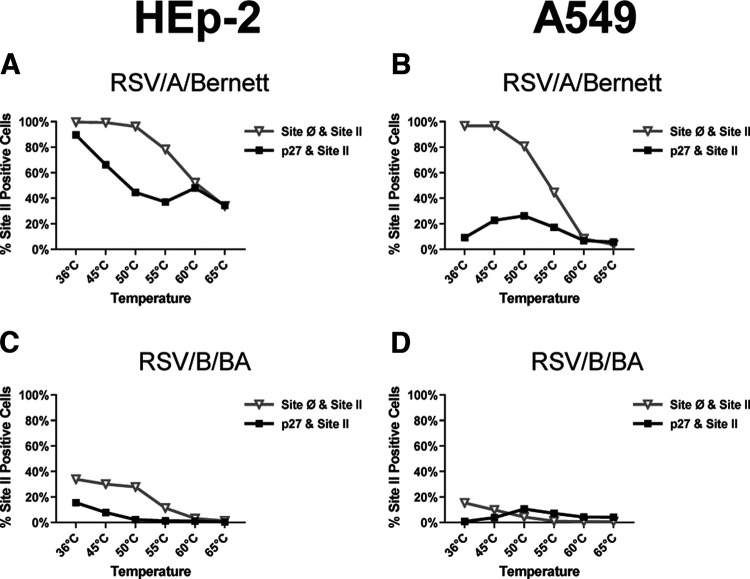
On the surface of RSV/A/Bernett-infected HEp-2 cells (Fig. 5A and Fig. S4A), at 36°C, virtually all cells expressing F protein had MAb binding to site Ø, consistent with F proteins in a pre-F conformation (site Ø+site II double positive). Similarly, approximately 90% of the same cells had MAb binding to p27 peptide (p27+site II double positive), suggesting that most infected cells expressed either uncleaved or partially cleaved F proteins on the surface. With increasing temperature, the percentage of RSV/A/Bernett-infected HEp-2 cells with MAb binding to p27 and site Ø declined (Fig. 5A and Fig. S4A). At the highest temperature treatment, 65°C, about 40% of HEp-2 cells infected with RSV/A/Bernett were positive for site Ø and p27.
FIG 5.
RSV F proteins on the surface of infected cells containing partially cleaved p27 have higher stability of the prefusion conformation. Percentages of RSV-infected cells with double-positive populations (p27+site II or site Ø+site II) after temperature-induced conformational change of F protein on the cell surface are shown. HEp-2 or A549 cells were infected with RSV/A/Bernett or RSV/B/BA (MOI = 0.07), incubated at 36°C, and harvested at 3 dpi. Aliquots of cell suspension were then heated for 10 min at increasing temperatures prior to staining and acquisition by imaging flow cytometry. (A) HEp-2 cells infected with RSV/A/Bernett; (B) HEp-2 cells infected with RSV/B/BA; (C) A549 cells infected with RSV/A/Bernett; (D) A549 cells infected with RSV/B/BA.
On the surface of A549 cells infected with RSV/A/Bernett (Fig. 5B and Fig. S4B), virtually all F proteins had detectable site Ø at 36°C, but p27 was detected in less than 10% of those cells. With increasing temperature, MAb binding to site Ø decreased, indicating transition to the post-F conformation. Interestingly, on cells heated at 50°C we observed an increase in MAb binding to p27, reaching a maximum of 23% of the A549 cells infected with RSV/A/Bernett. Further temperature elevation progressively decreased MAb binding to site Ø and p27, and at 65°C, less than 5% of the infected cells had detectable pre-F conformation or p27 peptide.
The F protein on the surface of RSV/B/BA infected HEp-2 or A549 cells (Fig. 5C and D and Fig. S4C and D) behaved in a similar manner to the F protein of RSV/A/Bernett under the same temperature conditions, except that the percentage of cells expressing F proteins with detectable p27 and site Ø was substantially lower. On the surface of RSV/B/BA-infected HEp-2 and A549 cells (Fig. 5C and D), at 36°C, 40% and 20% of the cells expressing F proteins, respectively, had detectable site Ø. On the other hand, p27 was detected in less than 15% of site II-positive HEp-2 cells (p27+site II double-positive cells) infected with RSV/B/BA at 36°C (Fig. 5C), and the proportion was below 5% for A549 cells (Fig. 5D). Again, an increase in MAb binding to p27 (p27+site II double-positive cells) was detected in RSV/B/BA-infected A549 cells at 50°C followed by a decline to below 5%. With increasing temperature stress, the proportion of cells expressing F proteins with detectable site Ø and p27 decreased to below 5% in both cell lines infected with RSV/B/BA (Fig. 5C and D).
Clear differences were observed in the detection of site Ø and p27 on the F protein between RSV/A/Bernett and RSV/B/BA (Fig. S3) and the stability of these antigenic sites to a temperature stress challenge in either HEp-2 or A549 RSV-infected cells.
DISCUSSION
The F protein is well conserved between RSV/A and RSV/B and is immunogenic, making it the lead candidate for vaccine development. RSV is unique among the negative-sense, single-stranded RNA viruses in that its F protein contains two furin cleavage sites. The RSV F protein requires cleavage at least at the R136 site to induce viral fusion to the host cell membrane, although fusion is less efficient than if cleaved in both furin sites, with release of the p27 peptide (22). The biological role of p27 in the F protein of RSV is largely unknown despite having the greatest amino acid variability between RSV/A and RSV/B strains and containing two or three of the five N-linked glycosylation sites on the F protein (16). Although it is now known that the F protein can exist partially cleaved on the surface of RSV/A-infected cells, retaining p27 (26), the efficiency of p27 cleavage and its effect on the F protein structure from different RSV subtypes and genotypes were unknown. To address this knowledge gap, we evaluated the presence of p27 on prototypic and contemporary strains of RSV/A and RSV/B using infectious spRSV and virus-infected HEp-2 and A549 cells. We used a novel anti-p27 MAb with high affinity for RSV/A (42, 48) and comparable binding activity against p27 for RSV/A and RSV/B strains (23, 25). We used Western blotting, ELISA, and imaging flow cytometry as orthogonal assays to verify the retention of p27 on the F protein.
Lee et al. demonstrated that p27 was detected on the surface of HEp-2 cells infected with a prototypical RSV/A strain, A2, as well on histology of lung tissues of RSV-infected BALB/c mice (26). We validated their observation with a prototypical RSV/A strain (RSV/A/Bernett) and further demonstrated that p27 was also detected on the F proteins expressed on the surface of HEp-2 and A549 cell lines infected with the contemporary strains RSV/A/ON and RSV/B/BA.
Western blot analysis of spRSVs revealed subgroup differences in the species of F protein, whereby F0 and F1 were both detected in spRSV/A/Bernett while only F1 was detected in spRSV/B/BA. Importantly, p27 was not detected on the F2 subunit, being preferentially cleaved on the FCS-2 site of F protein and remaining on the N terminus of the F1 subunit, capping the fusion peptide, as reported by Krzyzaniak et al. in 2013 (21).
Differences in posttranslational modifications and enzymatic activity are expected between cell lines. In vitro studies of HEp-2 and A549 cells infected with three prototypic and two contemporary RSV strains demonstrated that the p27 cleavage was more efficient on A549 than on HEp-2 cells. In addition, cleavage efficiency was less for the RSV/A strains in either cell line, as RSV/A strains retained more p27 than RSV/B strains over the course of 5 days. This implies that the processing of F proteins differs between cell lines and subtypes, despite the high sequence similarity of the cleavage sites among RSVs (16).
How p27 affects RSV infection is still unknown, but previous reports indicated that RSV/A could lead to greater severity of illness in children (49, 50) and generate a higher p27 antibody response in adults than RSV/B (23, 25), possibly linking higher levels of infectivity or viral fitness to the higher levels of p27 on the F protein.
Even though maintaining the pre-F conformation is important for the fusogenic activity of the RSV F protein, the process of sucrose purification of RSV by ultracentrifugation subjects the virions to mechanical stress, which can spontaneously trigger the pre- to post-F conformation. However, we observed that spRSV/A/Bernett had 1.6 and 2.9 times higher proportions of p27 and pre-F than spRSV/B/BA. It would also be expected that cells expressing F proteins with p27 would have less pre-F over time, as it is reported that p27 has a destabilizing effect on the F protein trimeric arrangement (31). But over the period of a 5-day infection, we observed higher levels of pre-F conformation on infected HEp-2 than on A549 cells and that F proteins with p27 showed greater stability of the pre-F form, contrary to a prior study (31).
Previous studies have shown that because of its bulky and disordered nature, uncleaved or partially cleaved p27 cannot fit in the hydrophobic cavities formed between F1 and F2 protomers and can disrupt the trimerization of F protein (31). But recent work by Krueger et al. demonstrated that recombinant F proteins retaining p27 can arrange themselves into stable trimers in solution, with the partially cleaved p27 positioning itself exposed to the solvent (41). Our data support the later observation where on the surface of RSV-infected HEp-2 or A549 cells and spRSV, higher proportions of the trimeric pre-F structure were present in F proteins harboring p27. Moreover, F proteins retaining p27 sustained the pre-F conformation at higher temperature treatments than those in which p27 was more efficiently cleaved. Heat has been previously shown to trigger from metastable pre-F to stable post-F (46). During elevated temperatures, RSV-infected HEp-2 cells retained more p27 and were more thermostable than A549 cells infected with the same RSV genotype. As expected, binding by the MAb D25 decreased significantly with elevated temperatures. This decrease in D25 binding was not due to denaturation of the F protein because palivizumab did not lose binding activity with elevated temperatures. Our premise is supported by a prior thermostability study with uncleaved and cleaved forms of F proteins in which Ruiz-Argüello et al. used MAbs that bind to site II and site IV. They demonstrated the F protein resistance to denaturation when heated up to 100°C, after which MAb binding was significantly reduced (45). Thus, the decrease in D25 binding was due to a loss of site Ø as the F protein transitioned to a post-F form. The higher resilience of pre-F conformation of RSV/A than RSV/B further supports the association between p27 and the stabilization of the pre-F conformation during stress conditions.
One of the shortcomings of our study is the use of a single anti-p27 antibody in the experimental design. In future studies, it would be good to include a second anti-p27-specific antibody. It would also be valuable to explore sucrose-purified RSV strains produced in A549 cells and perform infectivity studies to investigate the effects of partially cleaved p27 on viral fitness and infection mechanisms (for example, with RSV strains lacking the first or second p27 cleavage site). Our work supports the need for developing structural models that can reflect the effect of p27 on the F protein under biological conditions such as from continuous cell lines as well as human nose and lung organoids, as they better resemble the complexity of the native tissue (51, 52). Finally, new therapeutics and vaccines based on the RSV F protein should consider that F proteins from RSV/A or RSV/B strains may have different epitope stabilities and quaternary conformation biases depending on the cell line or host on which it is expressed.
In summary, we validate the results of previous studies that report on the cell surface expression of p27 and further expand on those findings that p27 is present on the surface of infectious sucrose-purified RSV virions from both subtypes (common process for purifying RSV produced in cell lines), as well on the surface of HEp-2 or A549 cells infected with 5 different RSV strains. Moreover, the presence of p27 helped the pre-F conformation to withstand both mechanical and higher temperatures stresses prior to converting to a post-F form. Altogether, our data suggest that in biologically relevant systems, p27 has a stabilizing effect on the pre-F quaternary structure, rather than a destabilizing effect, supporting the hypothesis that F protein can have multiple mechanism for entry in the host cell, such as direct or indirect fusion.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
Human epidermoid carcinoma larynx cells (HEp-2; ATCC) and adenocarcinomic human alveolar basal epithelial cells (A549; ATCC) were cultured at 36°C (5% CO2) in complete modified Eagle’s medium (MEM; Corning; 10-010-CM) supplemented with 10% fetal bovine serum (HyClone; SH30070.03), 2 mM l-glutamine (Gibco; 25030081), and antibiotic-antimycotic (100 U/mL of penicillin-streptomycin and 0.25 μg/mL of amphotericin B [Gibco; 15240062]).
Working pools of RSV/A/USA/BCM-Tracy/1989 (genotype GA1), RSV/A/WashingtonDC.USA/Bernett/1961 (genotype GA1), RSV/B/WashingtonDC.USA/18537/1962 (genotype GB1), RSV/A/USA/BCM813013/2013 (genotype ON), and RSV/B/USA/BCM80171/2010 (genotype BA) are referred to as RSV/A/Tracy, RSV/A/Bernett, RSV/B/18537, RSV/A/ON, and RSV/B/BA, respectively. RSV/A/Bernett and RSV/B/BA were purified by ultracentrifugation on a sucrose cushion (spRSV) as previously described (53, 54) and kept at −80°C until use. Each vial was thawed once. RSV/A/Tracy, RSV/A/Bernett, and RSV/B/18537 are prototypic strains, while RSV/A/ON and RSV/B/BA are contemporary strains.
RSV F specific monoclonal antibodies (MAbs) against antigenic site Ø (D25; Cambridge Biologics, LLC, Brookline, MA, USA), site II (palivizumab; MedImmune, LLC, Gaithersburg, MD), and p27 (RSV7.10, kindly provided by Gale Smith [Novavax, MD]) were used to assign the conformation of the F protein and to determine the presence of p27. Pre-F conformation was assigned by the detection of sites Ø and II. Post-F conformation was assigned by the absence of site Ø and the presence of site II.
Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Bio-Rad; number 172-1011) and HRP-conjugated goat anti-human IgG (Bio-Rad; number 172-1050) were used as secondary antibodies for ELISA and Western blot assays.
Western blotting.
The total protein concentration on sucrose-purified RSV/A/Bernett and RSV/B/BA was determined by micro-bicinchoninic acid (micro-BCA) assay following the manufacturer protocol (Thermo Scientific; 23235). Approximately 10 μg of each spRSV was mixed with SDS-Laemmli buffer under reducing conditions (50 μM β-mercaptoethanol [BME]) and incubated at 95°C for 5 min. The samples were run on a 4 to 20% Mini-PROTEAN TGX precast protein gels (Bio-Rad; 456-1095) at 200 V for 25 min and then transferred to a nitrocellulose membrane by semidry technique (Trans-Blot Turbo transfer system; Bio-Rad; 1704150). The membrane was blocked with 1% casein in Tris-buffered saline (TBS; Bio-Rad, 161-0782) for 1 h at room temperature, incubated with D25, palivizumab, or RSV7.10 MAb (anti-antigenic site Ø or II or p27, respectively) diluted in blocking buffer (1:1,000, 1:4,000, or 1:1,000, respectively) for 1 h at room temperature, and washed three times with PBST (phosphate-buffered saline with 0.1% Tween 20). Membranes were then incubated for 1 h at room temperature with HRP-conjugated goat anti-human IgG (membranes probed with D25 or palivizumab) or HRP-conjugated goat anti-mouse IgG (membranes probed with RSV7.10) at a 1:2,000 dilution in PBST. After four washes with PBST, the bands were developed with Clarity Western ECL substrate (Bio-Rad; 170-5060) and membranes were imaged with a ChemiDoc MP system (Bio-Rad; 12003154).
ELISA.
The relative proportions of site Ø, site II, and p27 in spRSVs were determined by a modified enzyme-linked immunosorbent assay (ELISA) protocol from Ye et al. (55). In short, 96-well microtiter plates (Immulon 2HB plates; Thermo Scientific) were coated with sequentially diluted spRSV/A/Bernett or spRSV/B/BA in PBS (starting at 10 μg/mL or 15 μg/mL of total protein, respectively) for 18 to 20 h at 4°C. PBS at pH 7.2 was used as coating buffer instead of carbonate/bicarbonate buffer at pH 9.6, as it preserved the binding of D25 to the pre-F conformation. Plates were then washed three times with 1× Kirkegaard and Perry Laboratories (KPL) wash buffer (SeraCare Life Sciences, Gaithersburg, MD) and blocked with 5% milk (Carnation instant nonfat dry milk) in KPL washing buffer for 1 h at 36°C. Monoclonal antibodies D25, palivizumab, and RSV7.10 (anti-antigenic sites Ø and II and p27, respectively) were diluted at 1.1 μg/mL in blocking buffer, added to the plate, and incubated for 1 h at 36°C. After three washes with KPL washing buffer, HRP-conjugated secondary antibodies goat anti-human IgG and goat anti-mouse IgG were diluted at 1:2,000 in KPL washing buffer and added to the plates for 1 h at 36°C. After six washes with KPL buffer, the signal was developed with 3,3′,5,5′-tetramethylbenzidine (TMB; catalog number 50-76-03; Kirkegaard and Perry Labs) for 18 min in the dark at room temperature. The developing reaction was stopped with 0.16 M sulfuric acid, and plates were promptly read at a 450-nm wavelength on a BioTek Synergy H1 microplate reader. Optical density (OD) values were plotted as a function of the spRSV dilution factor. Area under the curve (AUC) was calculated using the software GraphPad Prism v.9.0.
Temperature stress test to evaluate the stability of pre-F conformation on infectious sucrose-purified RSV.
spRSV/A/Bernett or spRSV/B/BA at a total protein concentration of 1.0 mg/mL or 0.72 mg/mL, respectively, was diluted 5-fold in distilled water, and aliquots were heated at various temperatures (25°C, 30°C, 40°C, 50°C, 60°C, 70°C, 80°C, and 90°C) for 10 min in a digital heat block. After each temperature treatment, samples were cooled to 25°C and diluted 16-fold in PBS. The wells of 96-well microtiter plates (Immulon 2HB plates; Thermo Scientific) were coated with 100 μL of each diluted aliquot in triplicate and incubated for 18 to 20 h at 4°C. The ELISA protocol was then executed as described above.
Imaging flow cytometry.
Fluorescently tagged antibodies were generated by covalently labeling monoclonal antibodies D25 (site Ø), palivizumab (site II), and RSV7.10 (p27) with Alexa Fluor 568 (AF568), Alexa Fluor 488, and Alexa Fluor 647, respectively, according to the manufacturer’s protocol (Invitrogen; catalog numbers A20184, A20181, and A20186). Each monoclonal antibody was titrated against uninfected and RSV-infected HEp-2 cells to give the optimal concentration at which maximum binding was achieved while maintaining the best separation between infected and uninfected cells.
HEp-2 and A549 cells were cultured to confluence as described above and infected at a multiplicity of infection (MOI) of 0.07. Uninfected cells were used to gate negative populations. At each determined time point—days 1, 2, 3, 4, and 5 postinoculation—the cells were washed with PBS and detached with Versene solution for 20 min at 36°C (0.48 mM EDTA in PBS; Thermo Fisher; catalog number A4239101). The cells (105 to 106 cells per staining condition) were resuspended in fluorescence-activated cell sorting (FACS) buffer (2% bovine serum albumin [BSA] in PBS, sterile), blocked with Fc block for 20 min at room temperature (Human TruStain FcX; Biolegend; catalog number 422302), and simultaneously stained for surface antigenic sites Ø and II and p27 with primary-conjugated antibodies (D25-AF568, palivizumab-AF488, and RSV7.10-AF647 at 8 μg/μL) for 30 min on ice. To minimize intracellular staining (we were interested on the F protein expressed on the cell surface), all staining steps and washes were performed without any permeation agent. After washing with FACS buffer, cells were stained with Hoechst 33342 at 5 ng/μL (Thermo Fisher; catalog number H3570) for 10 min on ice. Cells were washed with PBS and fixed in 4% paraformaldehyde (PFA), followed by acquisition by an Amnis Mark II ImageStream (405-nm, 488-nm, 561-nm, and 642-nm excitation lasers; magnification, ×40) using INSPIRE software (Luminex). Using the bright-field channel, a cell area-versus-cell aspect ratio plot was used to gate single cells from beads and cellular debris. Subsequently, out-of-focus objects were gated out from the data set using the gradient root-mean-square (RMS) measurement (<45%) on the same bright-field channel. For all RSVs, the same number of events was collected at each day postinoculation (dpi) based on the maximum number of cells at each dpi remaining attached to the culture dish (Fig. S1) (10,000 cells at days 1 to 3, 1,000 cells at day 4, and 500 cells at day 5). Single-stained cells were used for spectral compensation, while uninfected cells and fluorescence-minus-one (FMO) controls were used for gating strategy. Data analysis was performed using IDEAS software.
Temperature stress test to evaluate stability of pre-F conformation on RSV-infected HEp-2 or A549 cells.
HEp-2 and A549 cells were cultured to confluence as described above and infected with RSV at an MOI of 0.07. At 72 h postinoculation, the infected and uninfected cells were washed with PBS and detached with Versene solution for 20 min at 36°C (0.48 mM EDTA in PBS; Thermo Fisher; catalog number A4239101). Next, the cells were resuspended in PBS at 106 cells/400-μL aliquot and heated at various temperatures (36°C, 45°C, 50°C, 55°C, 60°C, and 65°C) for 10 min in a digital heat block. Cells were kept at 4°C until ready to stain with the same protocol as described above. After fixation, cells were analyzed on Amnis Mark II ImageStream (405-nm, 488-nm, 561-nm, and 642-nm excitation lasers; magnification, ×40; EMD-Millipore) and data analysis was performed using IDEAS software (Amnis).
Imaging flow cytometry data analysis.
Fluorescence intensity and image analyses were performed with the Amnis IDEAS software (Luminex), with all gate values determined empirically using unstained cells and FMO controls from each condition. The gating strategy is shown in Fig. S1. In short, in-focus cells were gated by an FSC gradient RMS histogram, followed by the selection of events containing one object per image (cell count mask). Nucleated and multinucleated cells (syncytia) were gated using a custom nucleus mask. A mask was created to isolate the fluorescence intensity signal from the cell membrane using the bright-field reference image to minimize the contribution of intracellular and nuclear background fluorescence. The percentage of RSV-infected cells (site II-positive events) expressing F proteins on the pre-F conformation (site Ø-positive events) was gated using a bivariate plot of pixel intensity for site Ø (568 nm) versus site II (488 nm). The percentage of RSV-infected cells with F proteins containing p27 was gated by a bivariate plot of pixel intensity for p27 (647 nm) versus site II (488 nm).
ACKNOWLEDGMENTS
We thank Gale Smith (Novavax, Maryland) for kindly providing the RSV7.10 anti-p27 antibody and the Texas Children’s Hospital William T. Shearer Center for Human Immunobiology for their generous support of this research.
This project was supported by the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the CPRIT Core Facility Support Award (CPRIT-RP180672), the NIH (CA125123 and RR024574), and the assistance of Joel M. Sederstrom.
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Pedro A. Piedra, Email: ppiedra@bcm.edu.
Kanta Subbarao, The Peter Doherty Institute for Infection and Immunity.
REFERENCES
- 1.Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, Alassani I, Ali A, Antonio M, Awasthi S, Awori JO, Azziz-Baumgartner E, Baggett HC, Baillie VL, Balmaseda A, Barahona A, Basnet S, Bassat Q, Basualdo W, Bigogo G, Bont L, Breiman RF, Brooks WA, Broor S, Bruce N, Bruden D, Buchy P, Campbell S, Carosone-Link P, Chadha M, Chipeta J, Chou M, Clara W, Cohen C, de Cuellar E, Dang D-A, Dash-Yandag B, Deloria-Knoll M, Dherani M, Eap T, Ebruke BE, Echavarria M, de Freitas Lázaro Emediato CC, Fasce RA, Feikin DR, Feng L, et al. 2017. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazur NI, Löwensteyn YN, Willemsen JE, Gill CJ, Forman L, Mwananyanda LM, Blau DM, Breiman RF, Madhi SA, Mahtab S, Gurley ES, El Arifeen S, Assefa N, Scott JAG, Onyango D, Tippet Barr BA, Kotloff KL, Sow SO, Mandomando I, Ogbuanu I, Jambai A, Bassat Q, CHAMPS Network the RSV GOLD Study Group, Caballero MT, Polack FP, Omer S, Momin Kazi A, Simões EAF, Satav A, Bont LJ. 2021. Global respiratory syncytial virus–related infant community deaths. Clin Infect Dis 73:S229–S237. doi: 10.1093/cid/ciab528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Reeves RM, Wang X, Bassat Q, Brooks WA, Cohen C, Moore DP, Nunes M, Rath B, Campbell H, Nair H, RSV Global Epidemiology Network, RESCEU investigators . 2019. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health 7:e1031–e1045. doi: 10.1016/S2214-109X(19)30264-5. [DOI] [PubMed] [Google Scholar]
- 4.Walsh EE. 2017. Respiratory syncytial virus infection. Clin Chest Med 38:29–36. doi: 10.1016/j.ccm.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glezen WP, Taber LH, Frank AL, Kasel JA. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 6.Ohuma EO, Okiro EA, Ochola R, Sande CJ, Cane PA, Medley GF, Bottomley C, Nokes DJ. 2012. The natural history of respiratory syncytial virus in a birth cohort: the influence of age and previous infection on reinfection and disease. Am J Epidemiol 176:794–802. doi: 10.1093/aje/kws257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curran D, Cabrera ES, Bracke B, Raymond K, Foster A, Umanzor C, Goulet P, Powers JH. 2022. Impact of respiratory syncytial virus disease on quality of life in adults aged ≥50 years: a qualitative patient experience cross-sectional study. Influenza Other Respir Viruses 16:462–473. doi: 10.1111/irv.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falsey AR, Walsh EE. 2005. Respiratory syncytial virus infection in elderly adults. Drugs Aging 22:577–587. doi: 10.2165/00002512-200522070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall CB, Walsh EE, Long CE, Schnabel KC. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 10.Tang JW, Loh TP. 2014. Correlations between climate factors and incidence—a contributor to RSV seasonality. Rev Med Virol 24:15–34. doi: 10.1002/rmv.1771. [DOI] [PubMed] [Google Scholar]
- 11.Peret TCT, Hall CB, Hammond GW, Piedra PA, Storch GA, Sullender WM, Tsou C, Anderson LJ. 2000. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J Infect Dis 181:1891–1896. doi: 10.1086/315508. [DOI] [PubMed] [Google Scholar]
- 12.Tabor DE, Fernandes F, Langedijk AC, Wilkins D, Lebbink RJ, Tovchigrechko A, Ruzin A, Kragten-Tabatabaie L, Jin H, Esser MT, Bont LJ, Abram ME, INFORM-RSV Study Group . 2020. Global molecular epidemiology of respiratory syncytial virus from the 2017−2018 INFORM-RSV study. J Clin Microbiol 59:e01828-20. doi: 10.1128/JCM.01828-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, Rodríguez-Tenreiro C, Sly P, Ramilo O, Mejías A, Baraldi E, Papadopoulos NG, Nair H, Nunes MC, Kragten-Tabatabaie L, Heikkinen T, Greenough A, Stein RT, Manzoni P, Bont L, Martinón-Torres F. 2018. Respiratory syncytial virus seasonality: a global overview. J Infect Dis 217:1356–1364. doi: 10.1093/infdis/jiy056. [DOI] [PubMed] [Google Scholar]
- 14.Pandya M, Callahan S, Savchenko K, Stobart C. 2019. A contemporary view of respiratory syncytial virus (RSV) biology and strain-specific differences. Pathogens 8:67. doi: 10.3390/pathogens8020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Battles MB, McLellan JS. 2019. Respiratory syncytial virus entry and how to block it. Nat Rev Microbiol 17:233–245. doi: 10.1038/s41579-019-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hause AM, Henke DM, Avadhanula V, Shaw CA, Tapia LI, Piedra PA. 2017. Sequence variability of the respiratory syncytial virus (RSV) fusion gene among contemporary and historical genotypes of RSV/A and RSV/B. PLoS One 12:e0175792. doi: 10.1371/journal.pone.0175792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan L, Coenjaerts FEJ, Houspie L, Viveen MC, van Bleek GM, Wiertz EJHJ, Martin DP, Lemey P. 2013. The comparative genomics of human respiratory syncytial virus subgroups A and B: genetic variability and molecular evolutionary dynamics. J Virol 87:8213–8226. doi: 10.1128/JVI.03278-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmer G, Budz L, Herrler G. 2001. Proteolytic activation of respiratory syncytial virus fusion protein. J Biol Chem 276:31642–31650. doi: 10.1074/jbc.M102633200. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Reyes L, Ruiz-Arguello MB, Garcia-Barreno B, Calder L, Lopez JA, Albar JP, Skehel JJ, Wiley DC, Melero JA. 2001. Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion. Proc Natl Acad Sci USA 98:9859–9864. doi: 10.1073/pnas.151098198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang A, Dutch RE. 2012. Paramyxovirus fusion and entry: multiple paths to a common end. Viruses 4:613–636. doi: 10.3390/v4040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krzyzaniak MA, Zumstein MT, Gerez JA, Picotti P, Helenius A. 2013. Host cell entry of respiratory syncytial virus involves macropinocytosis followed by proteolytic activation of the F protein. PLoS Pathog 9:e1003309. doi: 10.1371/journal.ppat.1003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmer G, Conzelmann K-K, Herrler G. 2002. Cleavage at the furin consensus sequence RAR/KR 109 and presence of the intervening peptide of the respiratory syncytial virus fusion protein are dispensable for virus replication in cell culture. J Virol 76:9218–9224. doi: 10.1128/jvi.76.18.9218-9224.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blunck BN, Aideyan L, Ye X, Avadhanula V, Ferlic-Stark L, Zechiedrich L, Gilbert BE, Piedra PA. 2022. Antibody responses of healthy adults to the p27 peptide of respiratory syncytial virus fusion protein. Vaccine 40:536–543. doi: 10.1016/j.vaccine.2021.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuentes S, Coyle EM, Beeler J, Golding H, Khurana S. 2016. Antigenic fingerprinting following primary RSV infection in young children identifies novel antigenic sites and reveals unlinked evolution of human antibody repertoires to fusion and attachment glycoproteins. PLoS Pathog 12:e1005554. doi: 10.1371/journal.ppat.1005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye X, Cabral de Rezende W, Iwuchukwu OP, Avadhanula V, Ferlic-Stark LL, Patel KD, Piedra F-A, Shah DP, Chemaly RF, Piedra PA. 2020. Antibody response to the furin cleavable twenty-seven amino acid peptide (p27) of the fusion protein in respiratory syncytial virus (RSV) infected adult hematopoietic cell transplant (HCT) recipients. Vaccines (Basel) 8:192. doi: 10.3390/vaccines8020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Lee Y, Klenow L, Coyle EM, Tang J, Ravichandran S, Golding H, Khurana S. 2022. Protective antigenic sites identified in respiratory syncytial virus fusion protein reveals importance of p27 domain. EMBO Mol Med 14:e13847. doi: 10.15252/emmm.202013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GBE, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, Zhou T, Graepel KW, Kumar A, Moin S, Boyington JC, Chuang G-Y, Soto C, Baxa U, Bakker AQ, Spits H, Beaumont T, Zheng Z, Xia N, Ko S-Y, Todd J-P, Rao S, Graham BS, Kwong PD. 2013. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Q, Wang Z, Ni F, Chen X, Ma J, Patel N, Lu H, Liu Y, Tian J-H, Flyer D, Massare MJ, Ellingsworth L, Glenn G, Smith G, Wang Q. 2019. Structure basis of neutralization by a novel site II/IV antibody against respiratory syncytial virus fusion protein. PLoS One 14:e0210749. doi: 10.1371/journal.pone.0210749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilman MSA, Furmanova-Hollenstein P, Pascual G, B van ‘t Wout A, Langedijk JPM, McLellan JS. 2019. Transient opening of trimeric prefusion RSV F proteins. Nat Commun 10:2105. doi: 10.1038/s41467-019-09807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLellan JS, Ray WC, Peeples ME. 2013. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol 372:83–104. doi: 10.1007/978-3-642-38919-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krarup A, Truan D, Furmanova-Hollenstein P, Bogaert L, Bouchier P, Bisschop IJM, Widjojoatmodjo MN, Zahn R, Schuitemaker H, McLellan JS, Langedijk JPM. 2015. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat Commun 6:8143. doi: 10.1038/ncomms9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swanson KA, Balabanis K, Xie Y, Aggarwal Y, Palomo C, Mas V, Metrick C, Yang H, Shaw CA, Melero JA, Dormitzer PR, Carfi A. 2014. A monomeric uncleaved respiratory syncytial virus F antigen retains prefusion-specific neutralizing epitopes. J Virol 88:11802–11810. doi: 10.1128/JVI.01225-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, Kumar A, Modjarrad K, Zheng Z, Zhao M, Xia N, Kwong PD, Graham BS. 2013. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLellan JS, Yang Y, Graham BS, Kwong PD. 2011. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol 85:7788–7796. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, Dormitzer PR, Carfi A. 2011. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci USA 108:9619–9624. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joyce MG, Bao A, Chen M, Georgiev IS, Ou L, Bylund T, Druz A, Kong WP, Peng D, Rundlet EJ, van Galen JG, Wang S, Yang Y, Zhang B, Chuang GY, McLellan JS, Graham BS, Mascola JR, Kwong PD. 2019. Crystal structure and immunogenicity of the ds-cav1-stabilized fusion glycoprotein from respiratory syncytial virus subtype b authors. Pathog Immun 4:294–323. doi: 10.20411/pai.v4i2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLellan JS. 2015. Neutralizing epitopes on the respiratory syncytial virus fusion glycoprotein. Curr Opin Virol 11:70–75. doi: 10.1016/j.coviro.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones HG, Battles MB, Lin C-C, Bianchi S, Corti D, McLellan JS. 2019. Alternative conformations of a major antigenic site on RSV F. PLoS Pathog 15:e1007944. doi: 10.1371/journal.ppat.1007944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farzan SF, Palermo LM, Yokoyama CC, Orefice G, Fornabaio M, Sarkar A, Kellogg GE, Greengard O, Porotto M, Moscona A. 2011. Premature activation of the paramyxovirus fusion protein before target cell attachment with corruption of the viral fusion machinery. J Biol Chem 286:37945–37954. doi: 10.1074/jbc.M111.256248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liljeroos L, Krzyzaniak MA, Helenius A, Butcher SJ. 2013. Architecture of respiratory syncytial virus revealed by electron cryotomography. Proc Natl Acad Sci USA 110:11133–11138. doi: 10.1073/pnas.1309070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krueger S, Curtis JE, Scott DR, Grishaev A, Glenn G, Smith G, Ellingsworth L, Borisov O, Maynard EL. 2021. Structural characterization and modeling of a respiratory syncytial virus fusion glycoprotein nanoparticle vaccine in solution. Mol Pharm 18:359–376. doi: 10.1021/acs.molpharmaceut.0c00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel N, Massare MJ, Tian J-H, Guebre-Xabier M, Lu H, Zhou H, Maynard E, Scott D, Ellingsworth L, Glenn G, Smith G. 2019. Respiratory syncytial virus prefusogenic fusion (F) protein nanoparticle vaccine: structure, antigenic profile, immunogenicity, and protection. Vaccine 37:6112–6124. doi: 10.1016/j.vaccine.2019.07.089. [DOI] [PubMed] [Google Scholar]
- 43.Melero JA, Mas V, McLellan JS. 2017. Structural, antigenic and immunogenic features of respiratory syncytial virus glycoproteins relevant for vaccine development. Vaccine 35:461–468. doi: 10.1016/j.vaccine.2016.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajan A, Piedra F-A, Aideyan L, McBride T, Robertson M, Johnson HL, Aloisio GM, Henke D, Coarfa C, Stossi F, Menon VK, Doddapaneni H, Muzny DM, Javornik Cregeen SJ, Hoffman KL, Petrosino J, Gibbs RA, Avadhanula V, Piedra PA. 2022. Multiple respiratory syncytial virus (RSV) strains infecting HEp-2 and A549 cells reveal cell line-dependent differences in resistance to RSV infection. J Virol 96:e01904-21. doi: 10.1128/jvi.01904-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruiz-Argüello MB, Martín D, Wharton SA, Calder LJ, Martín SR, Cano O, Calero M, García-Barreno B, Skehel JJ, Melero JA. 2004. Thermostability of the human respiratory syncytial virus fusion protein before and after activation: implications for the membrane-fusion mechanism. J Gen Virol 85:3677–3687. doi: 10.1099/vir.0.80318-0. [DOI] [PubMed] [Google Scholar]
- 46.Yunus AS, Jackson TP, Crisafi K, Burimski I, Kilgore NR, Zoumplis D, Allaway GP, Wild CT, Salzwedel K. 2010. Elevated temperature triggers human respiratory syncytial virus F protein six-helix bundle formation. Virology 396:226–237. doi: 10.1016/j.virol.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Connolly SA, Leser GP, Yin H-S, Jardetzky TS, Lamb RA. 2006. Refolding of a paramyxovirus F protein from prefusion to postfusion conformations observed by liposome binding and electron microscopy. Proc Natl Acad Sci USA 103:17903–17908. doi: 10.1073/pnas.0608678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel N, Tian J-H, Flores R, Jacobson K, Walker M, Portnoff A, Gueber-Xabier M, Massare MJ, Glenn G, Ellingsworth L, Smith G. 2020. Flexible RSV prefusogenic fusion glycoprotein exposes multiple neutralizing epitopes that may collectively contribute to protective immunity. Vaccines (Basel) 8:607. doi: 10.3390/vaccines8040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laham FR, Mansbach JM, Piedra PA, Hasegawa K, Sullivan AF, Espinola JA, Camargo CA. 2017. Clinical profiles of respiratory syncytial virus subtypes A and B among children hospitalized with bronchiolitis. Pediatr Infect Dis J 36:808–810. doi: 10.1097/INF.0000000000001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papadopoulos NG, Gourgiotis D, Javadyan A, Bossios A, Kallergi K, Psarras S, Tsolia MN, Kafetzis D. 2004. Does respiratory syncytial virus subtype influences [sic] the severity of acute bronchiolitis in hospitalized infants? Respir Med 98:879–882. doi: 10.1016/j.rmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Rijsbergen LC, Lamers MM, Comvalius AD, Koutstaal RW, Schipper D, Duprex WP, Haagmans BL, de Vries RD, de Swart RL. 2021. Human respiratory syncytial virus subgroup A and B infections in nasal, bronchial, small-airway, and organoid-derived respiratory cultures. mSphere 6:e00237-21. doi: 10.1128/mSphere.00237-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porotto M, Ferren M, Chen Y-W, Siu Y, Makhsous N, Rima B, Briese T, Greninger AL, Snoeck H-W, Moscona A. 2019. Authentic modeling of human respiratory virus infection in human pluripotent stem cell-derived lung organoids. mBio 10:e00723-19. doi: 10.1128/mBio.00723-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piedra PA, Cron SG, Jewell A, Hamblett N, McBride R, Palacio MA, Ginsberg R, Oermann CM, Hiatt PW, Purified Fusion Protein Vaccine Study Group . 2003. Immunogenicity of a new purified fusion protein vaccine to respiratory syncytial virus: a multi-center trial in children with cystic fibrosis. Vaccine 21:2448–2460. doi: 10.1016/s0264-410x(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 54.Piedra PA, Glezen WP, Kasel JA, Welliver RC, Jewel AM, Rayford Y, Hogerman DA, Hildreth SW, Paradiso PR. 1995. Safety and immunogenicity of the PFP vaccine against respiratory syncytial virus (RSV): the Western blot assay aids in distinguishing immune responses of the PFP vaccine from RSV infection. Vaccine 13:1095–1101. doi: 10.1016/0264-410x(95)00034-x. [DOI] [PubMed] [Google Scholar]
- 55.Ye X, Iwuchukwu OP, Avadhanula V, Aideyan LO, McBride TJ, Ferlic-Stark LL, Patel KD, Piedra F-A, Shah DP, Chemaly RF, Piedra PA. 2018. Comparison of palivizumab-like antibody binding to different conformations of the RSV F protein in RSV-infected adult hematopoietic cell transplant recipients. J Infect Dis 217:1247–1256. doi: 10.1093/infdis/jiy026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S4. Download jvi.00254-23-s0001.pdf, PDF file, 1.1 MB (1.1MB, pdf)