Abstract
Chronic emesis (CE) is a poorly understood condition in human and nonhuman primates that negatively impacts the quality of life. Early identification of risk factors for the development of CE is likely to improve the ability to manage CE cases successfully and is, therefore, desirable. Using a case-control study, we reviewed the necropsy records of the California National Primate Research Center and identified 24 animals with recorded CE, defined as five or more incidents of emesis in 1 month. A group of 89 healthy rhesus macaques (Macaca mulatta), comparable in age and percent time housed indoors, was similarly identified. Next, we investigated the association between the occurrence of CE during later stages of life after infancy and the behavioral temperament scores attained in infancy, age, sex, birth location, rearing condition, history of self-injurious behavior (SIB), and the number of lifetime sedation events. Our analysis revealed that CE was associated with degrees of temperament constructs obtained in infancy (data was available for n = 113), such as Confidence (odds ratio (OR) = 0.45, 95% CI: 0.18, 1.08, p = 0.07), Gentleness (OR = 0.47, 95% CI: 0.23, 0.96, p = 0.03), Nervousness (OR = 2.04, 95% CI: 0.98, 4.23, p = 0.05), and Vigilance (OR = 0.36, 95% CI: 015, 0.87, p = 0.02), suggesting that CE is linked to behavioral phenomenon measured in early life, long before it becomes a medical concern. Our data suggest that CE was positively correlated with a history of SIB (OR 4.26, 95% CI: 0.98, 18.47, p = 0.04). Accurate prediction of CE can then assist behavioral and colony management professionals in making informed decisions regarding the care of animals at risk of developing CE. Moreover, the novel information we reported here could have valuable implications in human medicine, where gastrointestinal distress is a common complaint affecting a person’s quality of life.
Keywords: chronic emesis, early life biobehavioral temperament, rhesus macaque
1 |. INTRODUCTION
Among humans, chronic emesis (CE) is known to negatively impact the quality of life (Lacy et al., 2018), yet it remains poorly understood (Lacy et al., 2018; Pasricha et al., 2011). Emesis may be acute, associated with side effects of a pharmacological treatment or anesthesia, intestinal obstruction, and ingested toxins (Andrews, 1992; Andrews & Horn, 2006). Emesis may also be chronic, associated with primary disease, a structural pathology, pregnancy, and stress (Bayley et al., 2002; Otero-Regino et al., 2020). For example, Cyclic Vomiting Syndrome, an idiopathic condition in humans characterized by acute bouts of retching and vomiting, may be associated with a history of early adverse life events, mood disorders, and chronic stress (Levinthal & Bielefeldt, 2014). Similarly, emotional distress, food aversion, and sensitization to certain stimuli were found to be associated with CE, further establishing the connection between CE and poor welfare (Andrews, 1992; Koch, 1997).
Emesis has additionally been reported in nonhuman primate (NHP) species such as the rhesus macaque (Macaca mulatta) (Reinhardt et al., 1987). NHP’s have been used as models for the chemoreceptor trigger zone for emesis (Brizzee et al., 1955), enterotoxin-induced emesis and diarrhea (Hauschild et al., 1971), chemotherapy-induced emesis, anorexia, and weight loss (Breen et al., 2020), and radiation-induced emesis (Mattsson & Yochmowitz, 1980). NHPs diagnosed with CE are likely to experience reduced levels of welfare as compared to their healthy counterparts. CE may also affect the animal’s biological functioning and may inadvertently influence the outcome of the biomedical research (Guerrero-Martin et al., 2021). Thus, investigators who are unaware of an animal’s clinical condition may introduce confounds into their datasets and jeopardize their robustness (Pomerantz et al., 2022). On the other hand, informed investigators may find CE animals unsuitable for their studies, thereby introducing two additional challenges: avoiding animal selection bias (i.e., by excluding a specific sector of the population), and maximizing the efficiency of animal use to reduce the number of total animals (i.e., avoid developing psychological and/or clinical conditions that would deem animals unsuitable for research and require the use of alternative animals). Further, if animals are unresponsive to treatment, or comorbidities are present, veterinarians may elect to humanely euthanize them. Therefore, finding ways to mitigate these losses to improve animal health and welfare is critical, especially given the worsening shortage of rhesus macaques supply for biomedical research use (Subbaraman, 2021). Finally, the similarities in symptoms across humans and macaques suggest that rhesus macaques may serve as an appropriate research model to study CE in humans. Successful identification of risk factors is an important step toward offering additional treatment options, thereby advancing the animal’s well-being and care and the quality of research data collected from CE animals. For those reasons, in the current study, we aimed to identify reliable risk factors for developing CE among research rhesus macaques. The CNPRC’s database contains early life biobehavioral temperament (ELBT) scores obtained from the evaluation of temperament in infants between 90 and 120 days of age. We hypothesized that CE animals would score higher on the Nervous temperament scale, as previous studies have showed an association between Nervous temperament, immune function, and health outcomes (Capitanio et al., 2011), as well as an association between Nervous temperament and motor stereotypies (Vandeleest et al., 2011). We also analyzed Confidence, Vigilance, and Gentleness in an exploratory manner, to determine whether they had any association with CE development. Lastly, we expected that animals with a history of self-injurious behavior (SIB) would be more likely to also develop CE than individuals that do not exhibit this behavior.
2 |. METHODS
All aspects of management and research use conformed to applicable US federal regulations and the guidelines described in the Guide for the Care and Use of Laboratory Animals (Council et al., 2010) and the US Department of Agriculture’s Animal Welfare regulations United States Department of Agriculture, U. States Department of Agriculture, & Animal and Plant Health Inspection Service, A. and Plant Health Inspection Service. (2017), and adhered to the study’s protocol as approved by the California National Primate Research Center (CNPRC, Davis, California) Institutional Animal Care and Use Committee. In addition, methods adhered to the guidelines and principles of the American Society of Primatologists for the ethical treatment of nonhuman primates (American Society of Primatologists (n.d.).
2.1 |. Housing conditions
The animals in this study experienced various forms of social housing conditions throughout their life. In the last year of life, CE animals spent a mean of 198± 109 days in continuous full contact (pair), 154.6± 129.6 days in intermittent contact (pair), 210.2± 138.5 days singly housed, and 51.8± 82.1 days in continuous full contact in a social group. Control animals spent a mean of 231.4± 122.2 days in continuous full contact, 112± 1 days in intermittent contact, 99± 77 days in protected contact (pair), 215.7± 134.1 days singly housed, and 120.8 ± 160.8 days in continuous full contact in a social group.
2.2 |. Inclusion/exclusion criteria
2.2.1 |. Cases
Emesis incidents definition and identification
At the CNPRC, the clinical veterinary staff defined CE in rhesus macaques as five or more reported incidents of emesis within a 30-day period. As emesis was shown to be associated with poor welfare and/or certain clinical conditions, animal care staff was trained to identify and report it during routine daily health checks. Vomiting was then recorded in the CNPRC health records database for each NHP.
Medical records review
We searched the CNPRC health records database for necropsy reports from rhesus macaques that were not being actively studied for a research project at the time of euthanasia and had not spontaneously died. The initial search included records obtained between January 1, 2000–December 31, 2021, and returned 6019 reports matching these criteria. Following consultation with the center’s clinical veterinarians, the necropsy reports were further searched for the terms “emesis,” “vomit,” “esophagitis,” and “regurgitation.” Although regurgitation is functionally different from emesis, characterized by the passive movement of swallowed food back up to the mouth, this term was included in the search criteria to avoid missing potential cases of CE. Reports that included “regurgitation” in the context of heart disease (i.e., mitral regurgitation) were excluded from the subset. A board-certified veterinary pathologist (RJR) then reviewed these reports to validate inclusion/exclusion criteria. The thorough review and analysis of the necropsy reports and histology slides by the pathologist allowed for the identification and exclusion of any animals with significant disease states that could have potentially confounded the observed effects of the experimental intervention. Additionally, animals without a histology readout for the stomach were also excluded from the study to ensure the integrity and reliability of the results. These measures were implemented to enhance the rigor and precision of the study design and analysis.
The physical medical records were reviewed, and animals that had no reports of vomit, vomited in association with the sedation prior to before euthanasia, or were infants were also removed. This left a final 24 animals diagnosed with CE by veterinary staff in our case group ranging from 3 to 22 years of age.
2.2.2 |. Controls
Animal selection process
Animals for the control group were selected similarly via a search that yielded 2235 necropsy reports from January 2019–April 2022. To account for the lack of inter-rater reliability tests among the different pathologists who have worked at the CNPRC throughout the years and in order to minimize potential inter-rater disagreements, only the most recent necropsy logs were selected and searched. By doing so, we were able to include reports written by the same team of pathologists, deeming them more likely to be consistent in their ratings. These necropsy logs were filtered to include only animals that were euthanized and not on an active research project, and the probable cause of euthanasia was filtered to exclude colitis, gastritis, self-injurious behavior (SIB), trauma, typhlocolitis, gastrointestinal tract, diarrhea, phytobezoar, trichobezoar, liver disease, and kidney disease. Our goal was to compare our case group to a control group of animals with no history of CE. To that end, all gastrointestinal-related causes of euthanasia were excluded. Additionally, any cause of euthanasia with liver or kidney pathology was also excluded since both liver and kidney disease may both be associated with gastrointestinal clinical signs. SIB as a cause of euthanasia was excluded because of our suspicion that both SIB and CE might be related to stress. This means animals with severe cases of SIB that affected quality of life were excluded from the control group, but animals with relatively infrequent to moderate instances of SIB were still included. We can similarly assume that animals in the CE group exhibited infrequent to moderate SIB, as they were euthanized for CE rather than for SIB. We also excluded animals euthanized due to trauma to prevent bias as outdoor-housed animals are more likely to be susceptible to sustaining a life-threatening trauma injury than indoor-housed ones. Animals housed outdoors live in large groups with complex social structures maintained by affiliative and aggressive interactions, and while indoor-housed animals are not immune to pairmate-aggression, we thought it was statistically more likely that an animal euthanized due to trauma would be outdoor-housed. Animals housed outdoors are also generally born outdoors, as it is very difficult to introduce adult animals into an established group’s social structure. Therefore, the difference in the amount of time those animals would have spent outdoors as compared to our case group animals would have skewed our data, as animals that spend the majority of their life outdoors are less likely to be identified for incidents of emesis due to the large group housing structure. These animals are also generally subjected to fewer sedation events as compared to indoor-housed animals. The resulting group of animals was then sorted by age; those younger than 3 and older than 22 years of age were excluded to create an age group comparable to our cases. The percent of time spent indoors was calculated for each animal by dividing the number of days spent indoors by the total number of days in their life. The percent time of life spent indoors was then sorted from lowest to highest, and those that had spent less than 5% of their life indoors were excluded to make the control cohort comparable to the case cohort. Further evaluation of the cause of euthanasia led to the exclusion of those that had been euthanized because of seizures and neoplasms since these animals may also have exhibited gastrointestinal signs from the disease or the pharmaceutical interventions, resulting in a control group of 89 animals.
2.3 |. Demographic variables
The animals’ age in years at the time of euthanasia, sex (male vs. female), birth location (indoor vs. outdoor), and rearing condition (dam-reared vs. nursery-reared) were extracted from the CNPRC’s database. It was also noted whether the animals had a history of SIB. The medical record for each animal was examined to find the dates of reported emesis occurrences and the dates of sedation events. Incidents of emesis that were documented within 48 h of a sedation event was considered an acute incident of emesis. The number of sedations for each animal was also normalized by taking the total number of sedations in the animal’s life and dividing it by the animal’s age in years.
2.4 |. Early life biobehavioral temperaments (ELBT)
ELBT scores for those animals tested as infants were also extracted from the CNPRC database. This data was available for 11 of the 24 CE animals (46%), and for 38 of the 89 control animals (43%). Infants between 90 and 120 days of age were separated from their dams for a 25-hour period and relocated to standardized individual indoor cages. The infants underwent a series of standardized assessments, including a blood draw, their response to novel objects, and their response to a human intruder, at the end of which each animal’s temperament was rated. Animals were given temperament scores for Vigilant, Gentle, Confident, and Nervous traits. Within each year, scores for each trait were z-scored (Golub et al., 2009). Further details of the temperament scoring provided in Supporting Information.
2.5 |. Statistical analysis
The data were analyzed using JMP® Statistical Software (Version 16) R version 4.2.0 (R Core Team, 2022), and Rstudio (Rstudio Team, 2022). Pearson’s χ2 test was used to determine the odds ratio (OR) for sex, birth location, rearing condition, and SIB with a 95% confidence interval and alpha defined as 0.1. Univariate logistic regression was used to determine the OR and 95% confidence interval (CI) for the ELBT scores, and alpha was defined as 0.1. The Kruskal–Wallis test was also used to compare the difference in ages between the CE and control cohorts using a χ2 approximation.
3 |. RESULTS
The case and control group demographics were comparable in the ratio of males to females, indoor birth to outdoor births, dam-reared to nursery-reared, and the total number of sedations between the two groups (Table 1). While the control group was selected to include primarily healthy animals that were euthanized due to reasons unrelated to gastrointestinal clinical signs, there were still sporadic incidents of emesis in some members of that cohort. These incidents were generally secondary to a sedation event that occurred that day or the day prior or random incidents that could be attributed to eating too fast or to a cage-mate that had been recently sedated, suggesting they were incidents of acute emesis, rather than chronic emesis.
TABLE 1.
Demographic variables of rhesus macaques (Macaca mulatta) exhibiting chronic emesis compared to healthy animals.
| Variable | Chronic Emesis (n = 24) |
Healthy Animals (n = 89) |
|---|---|---|
| Average Age in Years (Range) | 9.7 (2.9–22.1) | 11.8 (3.0–20.50) |
| Males (%) | 42% | 34% |
| Females (%) | 58% | 66% |
| % Indoor Birth | 87% | 88% |
| % Outdoor Birth | 13% | 12% |
| % Dam-Reared | 79% | 83% |
| % Nursery-Reared | 21% | 17% |
| % SIB Flag | 17% | 4% |
| Average # Sedations (Range) | 53.7 (15–125) | 64.5 (10–140) |
| Average # Emesis (Standard Deviation) | 36.6 (25.27) | 1.2 (2.11) |
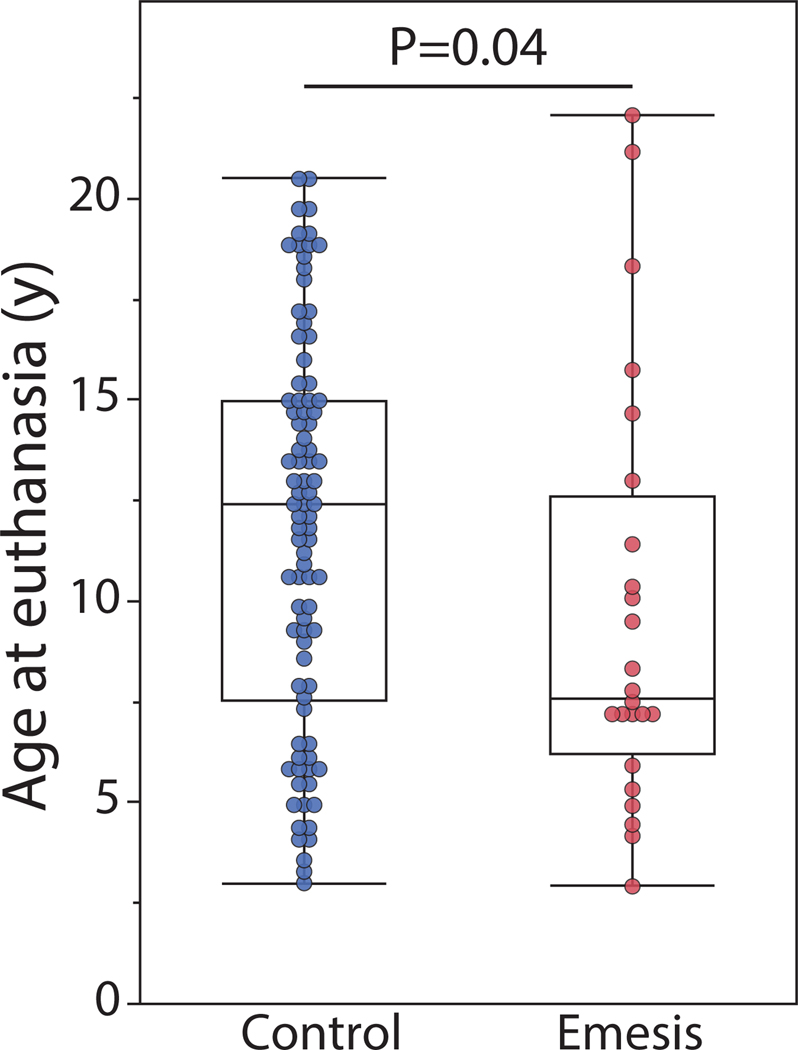
While the age range of the two groups is relatively comparable, it should be noted that the median age at the time of euthanasia in the CE group was 7.6 years, while the median age in the Control group was 12.4 years (Figure 1). This demonstrates that animals with CE were generally euthanized at an earlier age than the healthy control animals.
FIGURE 1.
The chronic emesis cohort was euthanized at an earlier age compared with healthy controls. Box and whisker plot for the age at euthanasia suggests that median of the age at euthanasia was significantly lower in the chronic emesis cases compared with healthy controls (Kruskal–Wallis Test, using χ2 Approximation; p = 0. 04). The upper and lower whiskers represent the minimum and maximum data. The length of the box is the difference between the 75th and 25th percentiles, and the line within the box represents the median.
It is also worth noting the age at which emesis started for the CE group and those animals in the Control group that had incidents of emesis. The median age at which emesis started in the CE group was 4.9 years, while the median age at which emesis started in the Control group was 8.6 years (p = 0.04; Supporting Information: - Figure S1). This suggests that emesis started significantly earlier in life for the CE group as compared to the control group.
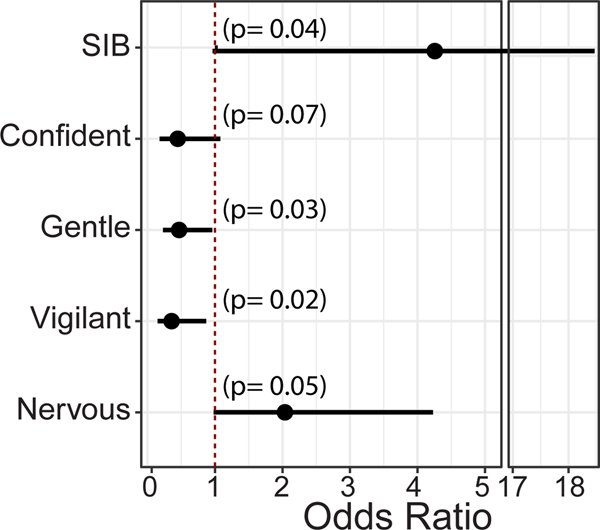
Data analysis showed the history of infrequent to moderate SIB to be significantly associated with emesis incidents. SIB was found to be significantly and positively associated with chronic emesis, with 17% of the CE group having a history of SIB compared to only 4% among the Control group. Pearson’s χ2 test was performed for a history of SIB, and CE, which produced an odds ratio of 4.26 (95% CI: 0.98, 18.47; p = 0.039, Figure 2), indicating that animals with a history of SIB were 4.26 times more likely to also develop CE in comparison to individuals without a history of SIB.
FIGURE 2.
Odds ratios for SIB, and ELBT scores show a significant relationship with CE. The Forest plot shows the odds ratio (black dot) point estimates and 95% confidence interval (black horizonal line on either side of the dot) for the association between chronic emesis and independent variables: SIB, and ELBT scores for Confidence, Gentleness, Vigilance, and Nervousness. The red dotted line represents the null hypothesis of OR = 1.0. p values are marked on the plot for each variable. ELBT, early life biobehavioral temperament; SIB, self-injurious behavior.
We analyzed the ELBT scores with univariate logistic regression. Our reasoning for using a univariate model is detailed in the Supplementary Materials. All four temperament scores, Confidence, Gentleness, Nervousness, and Vigilance, were significant predictors of the development of CE (Figure 2). Under the univariate model, we found that for every unit increase in Confidence score, there was a 55% decrease in the odds of the animal having chronic emesis (OR = 0.45, 95% CI: 0.18, 1.08, p = 0.07). For every unit increase in Gentleness score, there was a 53% decrease in the odds of the animal having chronic emesis (OR = 0.47, 95% CI: 0.23, 0.96, p = 0.03). For every unit increase in Vigilance score, there was a 64% decrease in the odds of the animal having chronic emesis (OR = 0.36, 95% CI: 015, 0.87, p = 0.02). Finally, for every unit increase in Nervousness score, the animal was 2 times as likely to have chronic emesis (OR = 2.04, 95% CI: 0.98, 4.23, p = 0.05).
Our data suggest that sex, birth location, rearing condition, and the normalized number of sedations were not significantly associated with emesis incidence (Table 2).
TABLE 2.
Pearson’s Chi-Square value and p-values for variables found to be statistically insignificant.
| Variable | Pearson’s Chi Square | p-value |
|---|---|---|
| Sex | 0.52 | 0.47 |
| Birth Location | 0.00 | 0.98 |
| Rearing Condition | 0.21 | 0.65 |
| Normalized # Sedations | 0.14 | 0.71 |
4 |. DISCUSSION
We demonstrated that the development of CE was associated with behavioral temperaments measured at a very early stage of life, and with history of SIB. These findings, particularly the relationship between ELBT scores (lower Confident, Gentle, and Vigilant scores, and higher Nervous scores) and the development of CE, suggest that CE may be predicted years before it develops clinically. Moreover, our results suggest that CE animals might be more prone to develop comorbidities that would require humane euthanasia earlier than the control animals.
4.1 |. CE and ELBT
It has previously been shown that behavioral traits present in early life may influence behaviors in the later stages of life. Similar associations between the ELBT and motor stereotypic behaviors (Gottlieb et al., 2013), the detrimental impact of nursery-rearing on the immune system development (Ardeshir et al., 2014), and the association between low birth weight and chronic adult disease in humans (Kajantie & Räikkönen, 2010) have been previously reported. Our results, however, suggest that temperament scores obtained in infancy are linked with CE, a clinical sign that develops years later. The ELBTs are measured when infant macaques are only 90 to 120-day-old (Golub et al., 2009). Based on the behavioral responses to various tests, the results were scored as Confidence, Gentleness, Nervousness, and Vigilance, and scores were normalized per cohort and presented as Z-scores. Confidence, Gentleness, and Vigilance were all negatively associated with the development of CE, and Nervousness was positively associated with the development of CE. Despite the young age at which these animals were tested, these four temperament scores were all significant predictors for the development of CE later in the animals’ lives.
Our findings provide strong support to the value of the ELBT scoring system, which has also been used to predict the quality of social interactions in rhesus macaques and improve quality of life through the formation of successful social pairings (Pomerantz & Capitanio, 2021). The ELBT scores can be used to identify animals that are at high risk for developing CE during infancy, and steps can be taken to ensure their welfare and longevity. It should be noted, however, that while our findings show a strong association between ELBT and CE, our small sample size for the CE group limits the predictive power of this study, and further studies should be done to validate it.
4.2 |. Age at euthanasia and at first emesis incident
The discrepancy in median ages between the two groups indicates that animals with CE were euthanized earlier than controls. This could be due to multiple factors, including the relatively small sample size of the CE group, secondary medical conditions related to chronic ongoing emesis nonresponsive to treatment, and/or other behavioral concerns. The difference in median ages at which animals started exhibiting emesis between the two groups suggests that animals in the CE group generally started exhibiting emesis at an earlier age than those in the Control group.
4.3 |. CE and SIB
Animals with a history of infrequent to moderate SIB were 4.26 times more likely to develop CE than animals without such a history. While establishing causality was beyond the scope of this study, we identified a positive association between SIB and CE. Because we excluded severe cases of SIB that led to euthanasia, the full extent to which CE and SIB are associated in this population is not fully captured in this study. Previous research suggests that the development of SIB is related to early and chronic exposure to stressful events (Novak, 2003). Therefore, this close association between SIB and CE could imply that the development of CE was similarly related to stressful events, consistent with human cyclic vomiting syndrome and its connection with early adverse life events, mood disorders, and chronic stress (Levinthal & Bielefeldt, 2014). Our findings also support previous research showing comorbidity in behavioral problems, such as floating limb and SIB (Rommeck et al., 2009), and that monkeys that are likely to develop motor stereotypic behaviors are also more likely to develop SIB (Gottlieb et al., 2013). Behavioral Management staff at the CNPRC was regularly consulted on CE cases. They collected historical data such as rearing and social history as well as BBA data to identify potential risk factors for low tolerance to stress and additionally collected behavioral data via video cameras under different contexts to identify proximate factors that might have triggered the behavior. The outcomes of the assessments guided the proposed treatment plan that could involve specific types of environmental enrichment, changing cage location, and reassessing behavioral compatibility with current social partners.
4.4 |. CE and number of sedations
Previous research showed an association between the number of lifetime veterinary/medical procedures and SIB (Novak, 2003). Accordingly, we hypothesized that the normalized number of sedations would be significantly associated with the development of CE. Our analysis, however, did not yield such an outcome. One possible explanation for this incongruity could be that animals exhibiting CE were less likely to be used in studies requiring sedation, while healthy animals not exhibiting CE were more likely to be deemed appropriate candidates for studies requiring repeated sedation, a possible case of Neyman’s bias, a form of selection bias. Further analysis could look at the number and type of research projects these animals were used in before and after chronic emesis was first reported.
4.5 |. Current study shortcomings and suggestions for future research
Given that this was a retrospective case–control study, our findings are limited by selection and recall bias. Our ability to identify CE cases was restricted to those necropsy reports that specifically mentioned emesis or vomiting. At the CNPRC, the case definition of CE was at least five incidents of emesis in a 1-month period, though medical interventions and the use of further diagnostics were at the discretion of the clinical veterinary staff, depending on the severity of the case. Further research should be done to look at monkeys with similar ELBT scores to those we found associated with our chronic emesis cases. These animals could be followed prospectively from infancy to adulthood to determine how many develop chronic emesis and what early life and chronic stressors they were exposed to before the development of chronic emesis. A multicenter study should also be considered to increase the sample size and validate these findings across primate centers.
Environmental factors such as early life microbial composition and type of rearing have been associated with gut health (Ardeshir et al., 2014) and the development of idiopathic chronic diarrhea (ICD) (Ardeshir et al., 2014). ICD is a common gastrointestinal issue and a significant cause of morbidity and mortality among young rhesus macaques (Ardeshir et al., 2013; Blackwood et al., 2008; Haertel et al., 2018). Other factors, such as genetics (Kanthaswamy et al., 2014) or viral infection (Wang et al., 2019) have also been linked to the development of ICD. Due to the rarity of CE we were not able to investigate the familial aggregation of CE. We also could not investigate the gut virome or microbiome’s association with CE because of the case-control study design and lack of access to the tissues and samples. We suggest that a multicenter prospective cohort study provides the foundation to answer the role of these systems in the development of CE.
5 |. CONCLUSION
By taking steps to reduce the risk of developing CE in these high-risk individuals, we can minimize the negative impacts on their well-being, improve the quality of the research data, and mitigate the loss of valuable research animals (Subbaraman, 2021).
Supplementary Material
ACKNOWLEDGMENTS
We wish to thank the IT team members at the CNPRC for their assistance with acquiring and organizing the data. We also thank the members of the Population and Behavioral Health Services for overseeing the behavior and welfare of the animals and the Primate Medicine Unit for keeping such thorough medical records and for always striving to maintain the clinical health and welfare of the animals under their care. We express our gratitude to John Capitanio and his team for developing the BBA database, which was utilized in our examination of early-life behavioral temperaments in this study. We also thank Maureen Touchstone and her team for gathering the medical records for our review. This work is in partial fulfillment of the MPVM degree for J. Y. Nakatani. Research in this publication was supported by the NIH Grant P51OD011107-60 to the CNPRC, Grant R24OD010962 to J. Capitanio and 2U42OD010990 to J. A. R., and the UC Davis, 2020 Innovative Developmental Award to A. A.
Funding information
University of California Davis, Grant/Award Number: 2020 Innovative Developmental Award; National Institute of Health, Grant/Award Numbers: P51OD011107-60, R24OD010962, U42OD010990
Abbreviations:
- CE
chronic emesis
- CI
confidence interval
- CNPRC
California National Primate Research Center
- ELBT
early life biobehavioral temperament
- NHP
nonhuman primate
- OR
odds ratio
- SIB
self-injurious behavior
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- American Society of Primatologists. (n.d.) Principles for the ethical treatment of non-human Primates. Retrieved October 31, 2022, from https://asp.org/2021/04/20/principles-for-the-ethical-treatment-of-non-human-primates/
- Andrews PLR (1992). Physiology of nausea and vomiting. British Journal of Anaesthesia, 69, 2S–19S. 10.1093/bja/69.supplement_1.2S [DOI] [PubMed] [Google Scholar]
- Andrews PLR, & Horn CC (2006). Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Autonomic Neuroscience, 125(1–2), 100–115. 10.1016/j.autneu.2006.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshir A, Narayan NR, Méndez-Lagares G, Lu D, Rauch M, Huang Y, Van Rompay KKA, Lynch SV, & Hartigan-O’Connor DJ (2014). Breast-fed and bottle-fed infant rhesus macaques develop distinct gut microbiotas and immune systems. Science Translational Medicine, 6(252). 10.1126/scitranslmed.3008791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshir A., Oslund KL., Ventimiglia F., Yee J., Lerche NW., & Hyd DM. (2013). Idiopathic microscopic colitis of rhesus macaques: Quantitative assessment of colonic mucosa. The Anatomical Record, 296(8), 1169–1179. 10.1002/ar.22727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshir A, Sankaran S, Oslund K, Hartigan-O’Connor D, Lerche N, Hyde D, & Dandekar S. (2014). Inulin treatment leads to changes in intestinal microbiota and resolution of idiopathic chronic diarrhea in rhesus macaques. Annals of the American Thoracic Society, 11(Suppl 1), S75. 10.1513/AnnalsATS.201306-208MG [DOI] [Google Scholar]
- Bayley TM, Dye L, Jones S, DeBono M, & Hill AJ (2002). Food cravings and aversions during pregnancy: Relationships with nausea and vomiting. Appetite, 38(1), 45–51. 10.1006/appe.2002.0470 [DOI] [PubMed] [Google Scholar]
- Blackwood RS, Tarara RP, Christe KL, Spinner A, & Lerche NW (2008). Effects of the macrolide drug tylosin on chronic diarrhea in rhesus macaques (Macaca mulatta). Comparative Medicine, 58(1), 81–87. [PMC free article] [PubMed] [Google Scholar]
- Breen DM, Kim H, Bennett D, Calle RA, Collins S, Esquejo RM, He T, Joaquim S, Joyce A, Lambert M, Lin L, Pettersen B, Qiao S, Rossulek M, Weber G, Wu Z, Zhang BB, & Birnbaum MJ (2020). GDF-15 neutralization alleviates platinum-based chemotherapy-induced emesis, anorexia, and weight loss in mice and nonhuman primates. Cell Metabolism, 32(6), 938–950. 10.1016/j.cmet.2020.10.023 [DOI] [PubMed] [Google Scholar]
- Brizzee KR, Neal LM, & Williams PM (1955). The chemoreceptor trigger zone for emesis in the monkey. American Journal of Physiology-Legacy Content, 180(3), 659–662. 10.1152/ajplegacy.1955.180.3.659 [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, & Cole SW (2011). Nervous temperament in infant monkeys is associated with reduced sensitivity of leukocytes to cortisol’s influence on trafficking. Brain, Behavior, and Immunity, 25(1), 151–159. 10.1016/j.bbi.2010.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council NR, Studies D. on E. and L., Research, I. for L. A., & Animals C. for the U. of the G. for the C. and U. of L. (2010). Guide for the care and use of laboratory animals (8th Ed.). National Academies Press. [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, & Capitanio JP (2009). Iron deficiency anemia and affective response in rhesus monkey infants. Developmental Psychobiology, 51(1), 47–59. 10.1002/dev.20345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Capitanio JP, & McCowan B. (2013). Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta): Animal’s history, current environment, and personality: Stereotypic behavior and self-biting. American Journal of Primatology, 75(10), 995–1008. 10.1002/ajp.22161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Martin SM, Rubin LH, McGee KM, Shirk EN, Queen SE, Li M, Bullock B, Carlson BW, Adams RJ, Gama L, Graham DR, Zink C, Clements JE, Mankowski JL, & Metcalf Pate KA (2021). Psychosocial stress alters the immune response and results in higher viral load during acute simian immunodeficiency virus infection in a pigtailed macaque model of human immunodeficiency virus. The Journal of infectious diseases, 224(12), 2113–2121. 10.1093/infdis/jiab252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haertel AJ, Prongay K, Gao L, Gottlieb DH, & Park B. (2018). Standard growth and diarrhea-associated growth faltering in captive infant rhesus macaques (Macaca mulatta). American Journal of Primatology, 80(9), e22923. 10.1002/ajp.22923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild AHW, Walcroft MJ, & Campbell W. (1971). Emesis and diarrhea induced by enterotoxin of Clostridium perfringens type A in monkeys. Canadian Journal of Microbiology, 17(8), 1141–1143. 10.1139/m71-180 [DOI] [PubMed] [Google Scholar]
- Kajantie E, & Räikkönen K. (2010). Early life predictors of the physiological stress response later in life. Neuroscience and Biobehavioral Reviews, 35(1), 23–32. 10.1016/j.neubiorev.2009.11.013 [DOI] [PubMed] [Google Scholar]
- Kanthaswamy S, Elfenbein HA, Ardeshir A, Ng J, Hyde D, Smith DG, & Lerche N. (2014). Familial aggregation of chronic diarrhea disease (CDD) in rhesus macaques (Macaca mulatta): Chronic diarrhea disease (CDD) genetics. American Journal of Primatology, 76(3), 262–270. 10.1002/ajp.22230 [DOI] [PubMed] [Google Scholar]
- Koch KL (1997). A noxious trio: Nausea, gastric dysrhythmias and vasopressin. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society, 9(3), 141–142. 10.1046/j.1365-2982.1997.d01-44.x [DOI] [PubMed] [Google Scholar]
- Lacy BE, Parkman HP, & Camilleri M. (2018). Chronic nausea and vomiting: Evaluation and treatment. The American Journal of Gastroenterology, 113(5), 647–659. 10.1038/s41395-018-0039-2 [DOI] [PubMed] [Google Scholar]
- Levinthal DJ, & Bielefeldt K. (2014). Adult cyclical vomiting syndrome: A disorder of allostatic regulation? Experimental Brain Research, 232(8), 2541–2547. 10.1007/s00221-014-3939-4 [DOI] [PubMed] [Google Scholar]
- Mattsson JL, & Yochmowitz MG (1980). Radiation-induced emesis in monkeys. Radiation Research, 82(1), 191–199. 10.2307/3575247 [DOI] [PubMed] [Google Scholar]
- Novak MA (2003). Self-injurious behavior in rhesus monkeys: New insights into its etiology, physiology, and treatment. American Journal of Primatology, 59(1), 3–19. 10.1002/ajp.10063 [DOI] [PubMed] [Google Scholar]
- Otero-Regino W, Lúquez-Mindiola A, & Otero-Parra L. (2020). Diagnostic investigation and treatment of chronic nausea and vomiting. NeuroGastroLATAM Reviews, 4(1), 4040. 10.24875/NGL.20000046 [DOI] [Google Scholar]
- Pasricha PJ, Colvin R, Yates K, Hasler WL, Abell TL, Ünalp–Arida A, Nguyen L, Farrugia G, Koch KL, Parkman HP, Snape WJ, Lee L, Tonascia J, & Hamilton F. (2011). Characteristics of patients with chronic unexplained nausea and vomiting and normal gastric emptying. Clinical Gastroenterology and Hepatology, 9(7), 567–576. 10.1016/j.cgh.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz O, Baker KC, Bellanca RU, Bloomsmith MA, Coleman K, Hutchinson EK, Pierre PJ, Weed JL, & National Primate Research Centers’ Behavioral Management Consortium. (2022). Improving transparency—A call to include social housing information in biomedical research articles involving nonhuman primates. American Journal of Primatology, 84(6). 10.1002/ajp.23378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz O, & Capitanio JP (2021). Temperament predicts the quality of social interactions in captive female rhesus macaques (Macaca mulatta). Animals, 11(8), 8. 10.3390/ani11082452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2022). R: A Language and Environment for Statistical Computing (4.2.0).
- Reinhardt V, Houser WD, Sadoff DA, Scheffler J, Eisele SG, & Hempel MJ (1987). Treatment of nonspecific diarrhea with metronidazole in rhesus macaques. Journal of Medical Primatology, 16(5), 311–316. 10.1111/j.1600-0684.1987.tb00339.x [DOI] [PubMed] [Google Scholar]
- Rommeck I, Anderson K, Heagerty A, Cameron A, & McCowan B. (2009). Risk factors and remediation of self-injurious and self-abuse behavior in rhesus macaques. Journal of Applied Animal Welfare Science, 12(1), 61–72. 10.1080/10888700802536798 [DOI] [PubMed] [Google Scholar]
- Rstudio Team. (2022). RStudio: Integrated development for R.
- Subbaraman N. (2021). The US is boosting funding for research monkeys in the wake of COVID. Nature, 595(7869), 633–634. 10.1038/d41586-021-01894-z [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture, U. States Department of Agriculture, & Animal and Plant Health Inspection Service, A. and Plant Health Inspection Service. (2017). USDA animal care: Animal welfare act and animal welfare regulations.
- Vandeleest JJ, McCowan B, & Capitanio JP (2011). Early rearing interacts with temperament and housing to influence the risk for motor stereotypy in rhesus monkeys (Macaca mulatta). Applied Animal Behaviour Science, 132(1–2), 81–89. 10.1016/j.applanim.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K-Y, Christe KL, Yee J, Roberts JA, & Ardeshir A. (2019). Rotavirus is associated with decompensated diarrhea among young rhesus macaques (Macaca mulatta). American Journal of Primatology, 81(1), e22948. 10.1002/ajp.22948 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.