ABSTRACT
Infectious bronchitis virus (IBV) infections are initiated by the transmembrane spike (S) glycoprotein, which binds to host factors and fuses the viral and cell membranes. The N-terminal domain of the S1 subunit of IBV S protein binds to sialic acids, but the precise location of the sialic acid binding domain (SABD) and the role of the SABD in IBV-infected chickens remain unclear. Here, we identify the S1 N-terminal amino acid (aa) residues 19 to 227 (209 aa total) of IBV strains SD (GI-19) and GD (GI-7), and the corresponding region of M41 (GI-1), as the minimal SABD using truncated protein histochemistry and neuraminidase assays. Both α-2,3- and α-2,6-linked sialic acids on the surfaces of CEK cells can be used as attachment receptors by IBV, leading to increased infection efficiency. However, 9-O acetylation of the sialic acid glycerol side chain inhibits IBV S1 and SABD protein binding. We further constructed recombinant strains in which the S1 gene or the SABD in the GD and SD genomes were replaced with the corresponding region from M41 by reverse genetics. Infecting chickens with these viruses revealed that the virulence and nephrotropism of rSDM41-S1, rSDM41-206, rGDM41-S1, and rGDM41-206 strains were decreased to various degrees compared to their parental strains. A positive sera cross-neutralization test showed that the serotypes were changed for the recombinant viruses. Our results provide insight into IBV infection of host cells that may aid vaccine design.
IMPORTANCE To date, only α-2,3-linked sialic acid has been identified as a potential host binding receptor for IBV. Here, we show the minimum region constituting the sialic acid binding domain (SABD) and the binding characteristics of the S1 subunit of spike (S) protein of IBV strains SD (GI-19), GD (GI-7), and M41 (GI-1) to various sialic acids. The 9-O acetylation modification partially inhibits IBV from binding to sialic acid, while the virus can also bind to sialic acid molecules linked to host cells through an α-2,6 linkage, serving as another receptor determinant. Substitution of the putative SABD from strain M41 into strains SD and GD resulted in reduced virulence, nephrotropism, and a serotype switch. These findings suggest that sialic acid binding has diversified during the evolution of γ-coronaviruses, impacting the biological properties of IBV strains. Our results offer insight into the mechanisms by which IBV invades host cells.
KEYWORDS: gammacoronavirus, infectious bronchitis virus, IBV, α-2, 6-sialic acid, sialic acid binding domain, SABD, receptor, serotype
INTRODUCTION
Coronaviruses (CoVs) are enveloped, single-stranded, positive-sense RNA viruses that are important infectious agents associated with respiratory and digestive diseases in animals and humans. Coronaviruses are phylogenetically divided into four genera, α-, β-, γ-, and δ-CoVs (1–3). Infectious bronchitis virus (IBV) is the prototype avian coronavirus belonging to the genus Gammacoronavirus, which is an important economic pathogen in the global poultry industry (4). Many different IBV strains circulate worldwide, and they have recently been classified into 32 phylogenetic lineages (GI-1 to -27 and GII to GVI) and dozens of serotypes, with pathologies varying from mild respiratory symptoms to severe renal and oviduct disease (4, 5). Since its first description in 1931, the Mass-type IBV (M41) in the GI-1 lineage has been circulating worldwide (6, 7). Several serotypes and genotypes of IBV cocirculate in poultry flocks (8). Previous epidemiologic studies have shown that the IBV-QX (GI-19) and IBV-TW (GI-7) strains, which belong to different serotypes (9), have become the dominant epidemic genotypes in Asia, with IBV-QX (GI-19) being prevalent worldwide (8, 10–14).
The binding and entry of avian coronaviruses to susceptible host cells requires interaction between cell surface receptors and the viral attachment protein spike (S). S protein binds to host receptors via its S1 subunit and then the virus fuses with the host cell membrane via its S2 subunit (15, 16). The sialic acid sugar is necessary for IBV infection of cells (17). Recent elucidation of the prototype IBV strain M41 spike protein structures by cryoelectron microscopy revealed the multidomain structure of the S1 subunit with two individually folded domains, designated S1-N-terminal domain (NTD) (amino acids [aa] 21 to 237) and S1-C-terminal domain (CTD) (aa 269 to 414), with sugar-binding sites located in the S1-NTD domain (15). Previous studies confirmed, using recombinantly expressed domains, that aa 19 to 272 of the IBV-M41 S protein were adequate for binding to tracheal tissue, as well as binding to the sialic acid moiety Neu5Ac(α2-3)Gal(β1-3)GlcNAc (18). Another study showed that aa 19 to 274 of the QX-IBV S protein were adequate for binding to the chicken trachea and kidney tissue, whereas this domain of QX-IBV shows no affinity for the known ligand of M41 [Neu5Ac(α2-3)Gal(β1-3)GlcNAc] (19). Thus, further studies are required to identify the exact location and binding features of the sialic acid-binding domain (SABD) of IBV.
Several CoVs use glycans present on the cell surface to mediate virus entry into cells. The S1-NTD in human CoV strains HKU1 and OC43 bind 9-O-acetylated (9-O-Ac) sialic acids (20). Evidence from IBV-M41 and other CoVs, such as Middle East respiratory syndrome CoV, suggests that viral attachment via the S1 N terminus to particular sialic acid glycans on the host cell surface is critical for viral entry and infection (18, 21). Consistent with this, the sialic acid glycan binding function in transmissible gastroenteritis virus is considered to affect viral pathogenicity (22, 23). Recent studies also suggested that the 9-O-Ac sialic acid of glycolipids and glycoproteins might be a host factor for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection (24). Sialic acid binding activity has been confirmed for many CoV S1-NTDs (25). Furthermore, unlike many other CoVs, the protein receptor for IBV has not been clarified and only α-2,3-linked sialic acids have been shown to be crucial for spike protein attachment (18, 19, 26) and subsequent infection of host cells, whereas the amino acid sequence identity of the conserved S2 domain is typically ≥90% between different serotypes. The S1 domain shows a high degree of sequence diversity, with 20 to 25% (even up to 50%) of the amino acids within the S1 subunit differing between IBV serotypes, especially in the S1-NTD (27). To date, the sialic acid-binding characteristics of IBV remain largely understudied, and the role of the variable IBV S1-NTD in infected chickens has not been demonstrated.
Here, we identified the minimal SABD of the S1 subunit of the QX-like IBV strain SD (GI-19), TW-like IBV strain GD (GI-7), and IBV strain M41 (GI-1). Then, we compared the difference in the sialic acid sugar-binding activity of the SABD and S1 proteins of these IBV strains. Next, we generated recombinant viruses in which the S1 or SABD in the genomes of strains SD and GD were replaced with the corresponding region from M41 by reverse genetics. The serotypes and pathogenicities of the recombinant strains were determined to illustrate the role of S1 and SABD in prevalent IBV strains.
RESULTS
The spike N-terminal S1-209 domain of three IBV strains contains a tissue-binding domain.
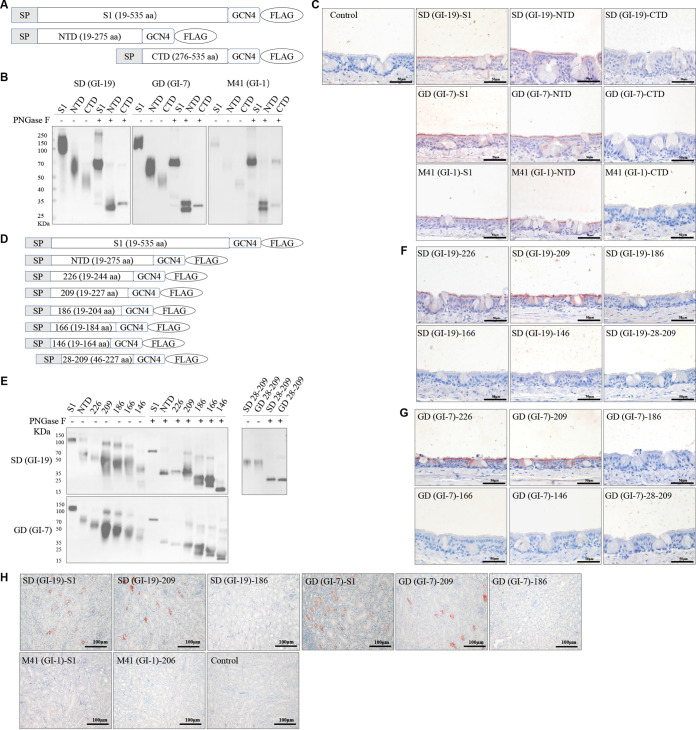
As shown in previous studies, the IBV-QX- and IBV-M41-S1-NTD (aa 19 to 272) were sufficient to bind to chicken tissue and sialic acid glycans (18, 19, 28). To further map the tissue-binding domain in the S1 subunit of three different IBV (SD/GD/M41) strains, sequences encoding these domains, including the S1, S1-NTD, and S1-CTD, were cloned and then expressed in mammalian HEK293 cells as soluble recombinant proteins (Fig. 1A). The proteins were analyzed by Western blotting after purification. A fractionated protein was pretreated with peptide-N-glycosidase F (PNGase F) to remove posttranslational glycosylation before loading. As expected, SD-, GD-, and M41-S1-NTD migrated at ~55 kDa (including glycosylation) and had a backbone of ~32 kDa after PNGase F treatment. Meanwhile, SD-, GD-, and M41-CTD migrated at ~60 kDa (including glycosylation) and had a backbone of ~35 kDa after PNGase F treatment (Fig. 1B). We demonstrated the binding activity of the SD-, GD-, and M41-truncated proteins by protein histochemistry on chicken trachea tissue slides. The SD-, GD-, and M41-S1-NTD proteins bound to the ciliated lining of epithelial cells but the SD-, GD-, and M41-S1-CTD proteins did not (Fig. 1C), confirming previous observations (18, 19) that SD- and GD-S1-NTD proteins, like M41-S1-NTD, contain a tissue-binding domain.
FIG 1.
Binding of IBV S1 and truncated proteins to paraffin-embedded healthy chicken tracheal and kidney tissues. (A and D) Schematic representation of the expression cassettes of S1 and truncated proteins, IBV-M41-S1 (aa 19 to 532), IBV-M41-NTD (aa 19 to 272), and IBV-M41-CTD (aa 273 to 532), with three aa insertions at the N terminus of S1, IBV-SD/GD-S1 (aa 19 to 535), IBV-SD/GD-NTD (aa 19 to 275), and IBV-SD/GD-CTD (aa 276 to 535), followed by a trimerization domain (GCN4) and a Flag tag (Flag) (A). IBV-SD/GD-S1-226 (aa 19 to 244), IBV-SD/GD-S1-209 (aa 19 to 227), IBV-SD/GD-S1-186 (aa 19 to 204), IBV-SD/GD-S1-166 (aa 19 to 184), IBV-SD/GD-S1-146 (aa 19 to 164), and IBV-SD/GD-S1-28-209 (aa 19 to 227) followed by a trimerization domain (GCN4) and Flag tag (Flag) (D). (B and E) Western blot analysis of proteins using anti-Flag HRP antibody. When indicated, the samples were treated with PNGase F prior to electrophoresis. (C, F, G, and H) Protein histochemistry of S1 and truncated proteins on paraffin-embedded chicken tracheal (C, F, and G) and kidney (H) tissues, as visualized by red staining. Equimolar concentrations of truncated proteins and S1 were used (S1 at 100 μg/mL and truncated proteins at 60 μg/mL).
To map the minimal tissue-binding domain, we cloned and expressed a panel of SD- and GD-S1 variants with deletions in the N- and/or C-terminal regions of the S1 domain in mammalian HEK293 cells (Fig. 1D). As expected, all of the S1 truncated proteins were highly glycosylated with migration patterns of approximately 60, 55, 50, 45, 40, 35, and 50 kDa for S1-NTD, S1-226, S1-209, S1-186, S1-166, S1-146, and S1-28-209, respectively (Fig. 1E). After PNGase F treatment, the S1-NTD, S1-226, S1-209, S1-186, S1-166, S1-146, and S1-28-209 truncated proteins migrated according to the predicted 33-, 25-, 23-, 20-, 18-, 16-, and 20-kDa sizes of their backbones, respectively (Fig. 1E). Then, we confirmed the binding activity of the SD- and GD-S1-truncated proteins by protein histochemistry on chicken tracheal tissue slides and revealed that SD- and GD-S1-226 and S1-209 but not SD- and GD-S1-186, S1-166, S1-146, and S1-28-209 could bind to chicken trachea tissue (Fig. 1F and G). We simultaneously expressed and confirmed that SD-S1-209 (SD-209), GD-S1-209 (GD-209), and M41-S1-206 (M41-206) were biologically active by protein histochemistry on chicken kidney tissue slides and found that SD-209 and GD-209, but not SD-S1-186, GD-S1-186, and M41-206 bound to chicken kidney tissue (Fig. 1H). The protein histochemistry data from the trachea tissue slides were consistent with the data from the kidney tissue slides, confirming that the N-terminal 209 aa of IBV-S1 (S1-209) are adequate for binding to host tissues and indicating that SD-209, GD-209, and M41-206 represent the minimal tissue-binding domain.
The S1-209 domain of the IBV S protein is dependent on sialic acids for tissue binding.
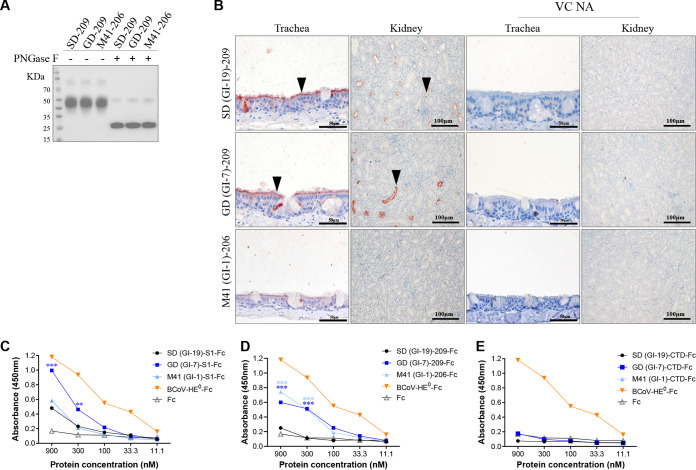
To reveal whether the binding of SD-209, GD-209, and M41-206 is exclusively dependent on sialic acids, trachea, and kidney tissue slides were pretreated with neuraminidase from Vibrio cholerae (VC NA) before applying SD-209, GD-209, and M41-206 proteins. Removal of sialic acids from the tracheal and renal tissues completely prevented the binding of these three proteins (Fig. 2A and B). Consistent with previous studies, the binding of M41-S1, M41-S1-NTD, and QX-S1-NTD appeared to be dependent on sialic acids, since binding was lost after pretreatment of the slides with VC NA to remove sialic acids (18, 19).
FIG 2.
Affinity of IBV S1 and S1 truncated proteins to host factors. (A) Western blot analysis of IBV-SD/GD/M41-209 proteins using anti-Flag HRP antibody; where indicated, the samples were treated with PNGase F prior to electrophoresis. (B) Histochemistry of IBV S1-209 truncated proteins on chicken tracheal and kidney tissues (left two columns) and for the tissues pretreated with neuraminidase from Vibrio cholerae (right two columns). (C, D, and E) IBV S1-Fc, S1-209-Fc, and S1-CTD-Fc bind to the glycan molecules in BSM with different affinities. The binding affinities of S1 and truncated proteins (in 3-fold serial dilutions, starting at 900 nM) to BSM.
To understand the sialic acid specificity and preference of IBV-S1 and truncated proteins, bovine submaxillary mucin (BSM), which is rich in sialic acid (29), was tested for binding with IBV-S1 truncated proteins by enzyme-linked immunosorbent assay (ELISA). As shown in Fig. 2C, the esterase inactive hemagglutinin-esterase (HE) proteins of bovine coronavirus (BCoV-HE0), which binds to BSM in a 9-O-Ac-Sia-dependent manner (29), was used as the positive control, and Fc protein was used as the negative control. Surprisingly, we found that none of the Fc and CTD-Fc proteins bound to BSM coated on the ELISA plate (Fig. 2E). The S1-Fc from SD, GD, and M41, as well as SD-209-Fc, GD-209-Fc, and M41-206-Fc, showed concentration-dependent binding to BSM, with varied affinities among different IBV strains (Fig. 2C and D). Taken together, these findings confirmed that SD-209, GD-209, and M41-206 proteins bind to host tissues in a sialic acid-dependent manner.
Not only α-2,3-linked but also α-2,6-linked sialic acids act as receptor determinants.
To identify sialic acid ligands that bind to SD-, GD-, and M41-S1-NTD, an ELISA was performed, in which Neu5Ac(α2-3)Gal(β1-3)GlcNAc and Neu5Ac(α2-6)Gal(β1-4)GlcNAc were used to coat the plates. As expected, no binding of SD-209 to Neu5Ac(α2-3)Gal(β1-3)GlcNAc and Neu5Ac(α2-6)Gal(β1-4)GlcNAc was observed at any of the protein concentrations, whereas GD-209 and M41-206 bound to both moieties in a concentration-dependent manner, and GD-209 showed lower affinity than M41-206 (Fig. 3A and B). Interestingly, all three IBV-S1 proteins can bind to both ligands in a concentration-dependent manner, with M41-S1 showing higher affinity than SD-S1, and M41-S1 showing lower affinity than GD-S1, while the SD-S1 protein exhibits a significantly higher affinity than the control SD-209 (Fig. 3C and D). Previous studies have shown that Neu5Ac(α2-3)Gal(β1-3)GlcNAc serves as an adsorbent receptor for IBV M41, but IBV-QX-S1-NTD does not bind to this particular glycan (19). Thus, the results indicated that not only Neu5Ac(α2-3)Gal(β1-3)GlcNAc but also Neu5Ac(α2-6)Gal(β1-4)GlcNAc can act as binding factors for S1 of the three IBV strains and SABD of IBV GD and M41.
FIG 3.
Detection of sialic acid glycan chains affecting IBV infection of CEK cells. (A and C) Affinity of S1-209-Fc (A) and S1-Fc (C) proteins for Neu5Ac(α2-3)Gal(β1-3)GlcNAc in ELISA. (B and D) Affinity of S1-209-Fc (B) and S1-Fc (D) proteins for Neu5Ac(α2-6)Gal(β1-4)GlcNAc in ELISA. (E) CEK cells were treated with either 50 mU neuraminidase from Vibrio cholerae (VC NA) or 50 mU α-2,3-sialidase (from Salmonella Typhimurium) or PBS at 37°C for 2 h before incubation with different IBV stocks. The data show the change in the virus genome copy number in the supernatants through time in each group compared to the PBS-treated control. (F) Immunofluorescence staining of IBV-infected CEK cells, with or without VC NA or α-2,3-sialidase pretreatment, with the IBV N protein at 36 hpi, as visualized in green (magnification, 20×). (G) Relative levels of immunofluorescence staining positive cell counts in IBV-infected CEK cells with or without pretreatment with VC NA or α-2,3-sialidase pretreatment; the mock-treated group was set as 1. All experiments were performed in duplicate and repeated twice. A representative result is shown. Error bars represent the means ± the SEM.
To study whether different sialic acid-binding activities may aid virus cell entry, we treated chicken embryonic kidney (CEK) cells with 50 mU of α-2,3-sialidase, 50 mU of VC NA, or a mock control for 2 h at 37°C and then infected the cells with different IBV strains. Infection levels were detected by measuring viral genome copies in the supernatants and immunostaining the cells. The viral titers in the supernatants of epidemic isolates IBV-SD and GD showed significantly reduced entry into neuraminidase-treated CEK cells, consistent with the IBV-M41-infected group and a previous study (17). As expected, the entry and replication efficiencies of the three IBV strains were significantly reduced after the CEK cells were treated with α-2,3-sialidase at 24 h postinfection (hpi). However, the viral titers of IBV-SD and M41 in the CEK cells treated with α-2,3-sialidase were significantly higher than CEK cells treated with VC NA at 24 hpi (Fig. 3E). Meanwhile, the immunostaining data from the cells were consistent with the viral copy numbers in the supernatants. Sialic acid depletion of CEK cells treated with α-2,3-sialidase and VC NA reduced IBV-SD, -GD, and -M41 entry by more than 50% compared to mock-treated cells. Higher levels of infection with IBV-SD and -M41 were detected in CEK cells treated with α-2,3-sialidase compared to those treated with VC NA (Fig. 3F and G). However, this phenomenon was not observed in IBV-GD (Fig. 3E and F). Collectively, our findings provide evidence that not only α-2,3-linked but also α-2,6-linked sialic acids on the surfaces of CEK cells can be used as a receptor determinant by the three IBV strains, thereby increasing the efficiency of infection.
Sialic acid 9-O-acetyl moiety prevents IBV S1 binding in BSM.
BSM is a heavily glycosylated protein rich in 7,9-O-Ac and 9-O-Ac sialic acids with only a minor population of unmodified Neu5Ac (30). The esterase-active HE protein from BCoV (BCoV-HE) preferentially cleaves 9-O-Ac groups, but shows lower activity against 7-O-acetyl groups (29). Pretreatment of BSM with HE results in a significant reduction in the ability of the positive-control BCoV-HE0 protein to bind to BSM, indicating that the esterases were enzymatically active (Fig. 4A) (29). The acetylated group of sialic acid at C-7 (Neu5,7Ac2) is unstable and will migrate to C-9 forming Neu5,9Ac2 under physiological conditions or upon pH and temperature changes (31, 32). We therefore investigated the effect of sialic acid 9-O-Ac or 7,9-O-Ac on the IBV spike–sialic acid interaction in BSM. To do this, we chemically transformed the sialosides into Neu5,9Ac2 by treating BSM with 100 mM Tris-HCl (pH 8.4) at 60°C for 30 min (29). Using BCoV-HE proteins to remove the 9-O acetylation group of sialic acids resulted in a dramatic reduction in the ability of the positive-control protein BCoV-HE0 to bind to BSM (Fig. 4A). This indicated that pretreatment of BSM with BCoV-HE enhanced binding of all three IBV-S1 and S1-NTDs to BSM compared to the mock-treated group (Fig. 4B to D). Meanwhile, pretreatment of BSM with 100 mM Tris-HCl (pH 8.4) at 60°C for 30 min, followed by treatment with BCoV-HE (pH 8.4-BCoV HE) also enhanced the binding of all three IBV-S1 and S1-NTDs to BSM compared to the pH 8.4-treated groups (Fig. 4B to D). However, no differences were observed in the binding affinities of all three IBV-S1 or S1-NTDs to BSM with 100 mM Tris-HCl (pH 8.4) at 60°C for 30 min compared to the mock-treated group (Fig. 4B to D). Taken together, we conclude that 9-O acetylation of the sialic acid glycerol side chain partially inhibits binding of the three IBV S1 and S1-NTDs to BSM.
FIG 4.
Esterase depletion assay. (A to D) BSM-coated ELISA plates were either mock treated, de-9-O-acetylated using BCoV-Mebus-HE-Fc (3 μg/well), or induced by migration of the Sia-7-O-acetylate group to C-9 by incubation at 60°C for 30 min with 100 mM Tris-HCl (pH 8.4) and then mock treated or treated with BCoV-Mebus-HE-Fc before incubation with IBV S1-Fc or S1-209-Fc. Relative binding was compared to BCoV-Mebus-HE0-Fc proteins in panels A, B, C, and D (30 μg/mL BCoV-Mebus-HE0-Fc protein was set at 100%). (E) Impact of BSM on IBV cell entry in CEK cells. IBV stocks were preincubated with 500 mg/mL or 50 mg/mL BSM or PBS at 37°C for 1 h before the infection of CEK cells. The data show the changes in virus genome copy number in the supernatant over time in each group. All experiments were performed in duplicate and repeated three times. A representative result is shown. Error bars represent the means ± the SEM.
To verify whether the glycans in BSM are involved in entry of the virus into cells, we carried out a BSM blockade assay by incubation of the virus with BSM before infection of CEK cells. The viral titers in the supernatants of the three IBVs showed no significant changes between the BSM-treated and mock-treated groups (Fig. 4E), indicating that the components of BSM bound by S1-NTDs may not be engaged in the viral entry process.
Recombinant rSD and rGD harboring the corresponding regions S1 and S1-206 of the M41 strain attenuated IBV infection in vivo.
The parental rSD and rGD strains, and four recombinant viruses (rSDM41-S1, rGDM41-S1, rSDM41-206, and rGDM41-206) were generated using reverse genetics (33, 34). All recombinant viruses could be rescued successfully (Fig. 5A). We next investigated the recombinant effects on viral replication in embryonated chicken eggs (ECEs). The four recombinant viruses and M41 replicated efficiently in ECEs and showed no changes in replication compared to the parental rSD or rGD viruses, but replicated slower than M41 (Fig. 5B). This indicated that IBV-S1 or S1-209 amino acids did not affect viral replication in ECEs.
FIG 5.
Rescue of rSD/rGD and recombinant replacement IBV strains. (A) Diagram showing the organization of the genomes in the recombinant IBVs. The genome structures of the IBV rSD and rGD strains are shown in the white rectangle. Regions coding for the recombinant regions in IBV strain M41 are shown in the green rectangle. (B) Growth kinetics of the wild-type and recombinant IBVs in ECEs. Allantoic fluids were collected from infected ECEs at the indicated time points. Viral copy numbers were determined by qPCR. A representative result is shown. Error bars represent the means ± the SEM.
We infected chickens with the virus to study the pathogenic ability and the nephrotropism of the recombinant mutants in vivo. Clinical symptoms were monitored and scored daily for each group over a period of 14 days. As shown in Fig. 6A, the chickens in the recombinant (r)SDM41-S1 and rSDM41-206 groups showed significantly lower clinical symptom scores at 7 to 14 days postinfection (dpi) with each strain (P < 0.05) compared to the rSD group. The chickens in the rGDM41-S1 group also showed significantly lower symptom scores at 5 to 14 dpi with each strain (P < 0.05) compared to the rGD group. Whereas, the chickens in the rGD M41-206 group exhibited significantly lower clinical symptom scores compared to those in the rGD group at 7 to 14 dpi (P < 0.05), and significantly higher clinical symptom scores compared to those in the rGDM41-S1 group at 9 to 14 dpi. In the M41-infected group, chickens only exhibited sneezing and slight listlessness at 6 to 10 dpi. There were no obvious clinical signs in the control group (Fig. 6A and B). During the observation period, rSD exhibited a 40% mortality rate; by contrast, mortality rates of 10 and 20% were obtained in the rSDM41-S1 and rSDM41-206 groups, respectively. Meanwhile, rGD exhibited a 70% mortality rate; by contrast, a mortality rate of 30% was observed in the rGDM41-206 group, and there were no deaths in the rGDM41-S1 and M41 groups. No mortality occurred in the control group (Fig. 6B). All strains caused severe tracheal ciliary injury at 5 and 7 dpi. At 14 dpi, the ciliary activity started to recover from the damage caused by all IBV strains (Fig. 6C).
FIG 6.
Infection of recombinant and parental IBV strains in vivo. (A) Clinical sign scores for each group after challenge with the parental and recombinant IBV strains. The chickens were monitored daily for 14 days and assigned clinical sign scores (0, normal; 1, slight nasal discharge, slight shaking, and slight lacrimation; 2, watery feces, depression, and coughing or sneezing; 3, heavy nasal discharge, heavy depression, mouth breathing, or tracheal rales; and 4, death). (B) Survival curve showing the percent survival in each group during the 14-day observation period. (C) Tracheal ciliostasis scores. The tracheal ciliary activity was assessed and scored at 5, 7, and 14 dpi using the scoring system described in Materials and Methods. (D to F) Histopathologic changes were detected in different tissues in different IBV-infected chickens. Chickens were euthanized at 7 dpi. Images show representative histopathological slides of the tracheas, lungs, and kidneys. Images were stained with hematoxylin and eosin. Black triangles indicate extensive loss of ciliated epithelial cells and inflammatory cellular infiltration (D); black arrows indicate inflammatory cell infiltration, black triangles indicate vascular congestion (E); open triangles indicate inflammatory cellular infiltration (F). (G) Mean lesion scores in the tracheas, lungs, and kidneys of chickens for each group after challenge with the parental and recombinant IBV strains. The indications for the scores were as follows: 0 to 1, no microscopic lesions, 1 to 3, mild lesions, 3 to 6, moderate lesions, 6 to 10, severe and extensive lesions (as described in reference 33). Error bars represent the means ± the SEM.
Histopathological analysis revealed microscopic lesions in the tracheas, lungs, and kidneys of all groups after hematoxylin and eosin staining. As shown in Fig. 6D, all infection groups exhibited mucosal thickening, mucosal epithelial cell desquamation, and cilial shedding, which were measured in the trachea tissue, and infiltration of inflammatory cells was also detected. The control group presented no obvious lesions. As shown in Fig. 6E, erythrocyte and inflammatory cell infiltration could also be seen in the lung bronchial tube in the rSD, M41, rGD, and rGDM41-206 groups. Compared to the rSD group, a small amount of erythrocyte and inflammatory cell infiltration was detected in the lung bronchial tube in the rSDM41-S1 and rSDM41-206 groups. There were no obvious lesions in the rGDM41-S1 and control groups. Meanwhile, we observed a large number of infiltrating inflammatory cells in the kidneys of the rSD and rGD groups. Compared to the rSD group, mild inflammatory cell infiltration was detected in the kidneys in the rSDM41-S1 and rSDM41-206 groups and also in the rGDM41-206 group compared to the rGD group. No obvious lesions were observed in the rGDM41-S1, M41, and control groups (Fig. 6F). As shown in Fig. 6G, mean lesion scores showed lesions in the trachea with no significant differences after each IBV infection. The lesions in the rSDM41-S1 and rGDM41-S1 groups were significantly lower than those from the rSD and rGD groups in the lungs and kidneys, respectively. Compared to the rSD and rGD groups, the lesions in the lungs in the rSDM41-206 and rGDM41-206 groups were lower than those from the rSD and rGD groups, respectively. Meanwhile, the lesions in the kidneys in the rSDM41-206 and rGDM41-206 groups were significantly lower than those from the rSD and rGD groups, respectively. These findings revealed that replacement with IBV-S1 of M41 resulted in strong attenuation and that replacement with S1-209 of M41 resulted in moderate attenuation in vivo, indicating that S1 and SABD are associated with the virulence of IBV.
Recombinant mutants of SD and GD harboring S1 or S1-206 amino acids from M41 showed decreased nephrotropism and an altered serotype.
To investigate the nephrotropism of the recombinant mutants in vivo, we used quantitative PCR (qPCR) to analyze the tissue distribution of the virus. As shown in Fig. 7A, the viral loads in the trachea and lung tissues in all infection groups presented no obvious difference at 5, 7, and 14 dpi. Compared to the rSD infection group, the viral loads in the kidneys of chickens inoculated with rSDM41-S1 and rSDM41-206 were significantly lower at 7 and 14 dpi; similarly, compared to the rGD infection group, the viral loads in the kidneys of chickens inoculated with rGDM41-S1 and rGDM41-206 were significantly lower at 5 and 7 dpi. Furthermore, the viral loads in the bursa in the rSDM41-S1 and rSDM41-206 groups were significantly lower than those in the rSD group only at 5 dpi; similarly, the viral loads in the bursa in the rGDM41-S1 and rGDM41-206 groups were significantly lower than those in the rGD group only at 5 dpi. These findings indicated that replacement with M41 S1 or S1-206 amino acids in rSD and rGD decreased the viral loads in the kidney and bursa.
FIG 7.
IBV loads in different tissues and immunohistochemical staining of infected chickens. (A) Viral loads in each group after challenge with the rSD/rGD and recombinant IBV strains, as measured for different tissues of chickens by RT-qPCR. (B and C) Immunohistochemical detection of IBV antigens in tracheal (B) and kidney (C) tissues. Black arrows indicate antigen immunoreactivity by mouse anti-IBV-N antibody. Error bars represent the means ± the SEM.
Next, we used immunohistochemistry to detect the distribution of IBV antigens. As shown in Fig. 7B, the presence of IBV antigen was detected in the mucosal layer of the tracheal tissues in all infection groups. No positive signals were detected in any tissues in the control group. The IBV antigen positive signals were lower in the rSDM41-S1 and rSDM41-206 groups than in the rSD group in kidney tissue; similarly, the positive signals were lower in the rGDM41-S1 and rGDM41-206 groups compared to the rGD group in kidney tissue, which was consistent with the qPCR results (Fig. 7C). This confirmed that replacement with M41 S1 or S1-206 amino acids in rSD and rGD decreased nephrotropism.
Since IBV-SD is closer phylogenetically to GD than M41 (33), we further detected the cross-reactivity of each group-positive antiserum with three S1-NTDs and the cross-neutralizing activity of each group-positive antiserum against IBVs. First, we carried out an ELISA to detect the cross-reactivity of SD-209 with each group-positive antiserum. As shown in Fig. 8A, SD-209 proteins reacted strongly with rSD antisera in a concentration-dependent manner with different affinities but did not react with other groups of antisera. Similarly, GD-209 proteins reacted strongly with rGD antisera but did not react with other groups of antisera (Fig. 8B). As expected, M41-206 proteins reacted strongly with M41, rSDM41-S1, rGDM41-S1, rSDM41-206, and rGDM41-206 antisera in a concentration-dependent manner with different affinities but not with rSD and rGD antisera (Fig. 8C). This indicated that the cross-binding activity of different serotype positive sera against S1-NTD was poor.
FIG 8.
Serum cross-binding ELISA. The cross-reactivity of IBV-specific antibodies with SD-209-Fc (A), GD-209-Fc (B), and M41-206-Fc (C) proteins was detected using recombinant IBV-positive sera. Error bars represent the means ± the SEM.
Then, we performed a sera cross-neutralization assay in ECEs. The neutralization titers and R-values are shown in Table 1. IBV-rSD, -rGD, and -M41 belonged to different serotypes, consistent with our previous study (35). The R value between rSDM41-S1 and rSD of 5.9% (i.e., below 11%) indicated that they belong to different serotypes; the R value between rSDM41-206 and rSD of 11.9% (i.e., higher than 11%) indicated that they belong to different serum subtypes. Analogously, the R value between rGDM41-S1 and rGD of 7.9% indicated that they belong to different serotypes; the R value between rGDM41-206 and rGD of 13% indicated that they belong to different serum subtypes. These data demonstrated that rSD and rGD, harboring the M41 S1 replacement, lost nephrotropism and showed an altered serotype, and that rSD and rGD, harboring the M41 S1-206 amino acid replacement, showed decreased nephrotropism and a partially changed serotype.
TABLE 1.
Serum cross-neutralization between parental IBV strains and rescued strains
| Strain | Serum titera |
Antigen relatedness value (R) to parental strain (%)b | ||||||
|---|---|---|---|---|---|---|---|---|
| rSD | rGD | M41 | rSDM41-S1 | rSDM41-206 | rGDM41-S1 | rGDM41-206 | ||
| rSD | 28.65 | 22.50 | 23.50 | 24.75 | 100 | |||
| rSDM41-S1 | 25.48 | 28.50 | 5.9 | |||||
| rSDM41-206 | 26.65 | 28.88 | 11.9 | |||||
| rGD | 29.50 | 25.50 | 24.75 | 26.5 | 100 | |||
| rGDM41-S1 | 26.29 | 28.88 | 7.9 | |||||
| rGDM41-206 | 26.50 | 29.375 | 13 | |||||
| M41 | 22.50 | 22.65 | 28.24 | 100 | ||||
Titers were obtained in reciprocal virus neutralization tests (diluted serum, constant virus).
R values were calculated according to a previously described method (49). The criteria used to classify antigenic relatedness were as follows: >70%, antigenic identity; 33 to 70%, minor subtype difference; 11 to 32%, major subtype difference; and <11%, no relatedness (distinct serotype).
DISCUSSION
In this study, we identified residues S1-19-227(209) as the minimal region constituting the SABD of IBV-SD(GI-19) and IBV-GD(GI-7), with the corresponding region of IBV-M41(GI-1) being residues S1-19-224(206). Using a neuraminidase assay and ELISA, we revealed that the S1 or SABD of the three IBV strains exhibited various capabilities for Neu5Ac(α2-3)Gal(β1-3)GlcNAc and Neu5Ac(α2-6)Gal(β1-4)GlcNAc biding. Moreover, reverse genetics and animal experiments demonstrated that IBV S1 and SABD amino acids affect the nephrotropism, virulence, and serotype of the three IBVs. Thus, the IBV SABD binds to various sialic acids with different affinities and affects the attenuation, nephrotropism, and serotypes of IBV strains.
The exact location of the SABD of IBV-M41 were the S1 N-terminal 19 to 224 aa residues (206 aa). Considering the presence of three amino acid insertions at the N terminus of S1, the corresponding region in IBV-SD and -GD was S1-19-227 amino acid (209 aa) residues. The minimal SABD was defined as six N-glycosylation sites (N33, N59, N85, N126, N160, and N194) that were crucial for NTD binding to tracheal tissue and sialic acid, which was consistent with previous studies (28, 36). Moreover, sites N219, N229, and N246 were not required for NTD binding to tracheal tissue and sialic acid and were located outside the SABD (18, 28, 36). Despite S1-28-209 containing six N-glycosylation sites required for binding to tracheal tissue and sialic acid, it was unable to bind indicating that the N-terminal truncation may influence the stability of the protein by removing residues required for proper folding. The binding of SABD and S1 proteins to tissue appeared to be entirely dependent on sialic acid, since no binding occurred when slides were preincubated with VC NA to remove sialic acids (Fig. 2B), and no trace levels of binding detected. However, we do not exclude the possibility that another host factor may play a role in IBV infection. Our results suggest that the minimal SABD of IBV is located at aa 19 to 227 of the S1 N terminus. The exact location of the SABD of other coronaviruses for binding to host tissues remains to be determined.
Viruses have evolved to select for specific interactions with particular sialic acid forms and linkages on different cells and tissues (37). The SD-SABD showed no affinity for the ligand Neu5Ac(α2-3)Gal(β1-3)GlcNAc in sialic acid glycans by ELISA, which was consistent with a previous study (19) and also showed no affinity for Neu5Ac(α2-6)Gal(β1-4)GlcNAc. Although GD-SABD presented low affinity for Neu5Ac(α2-3)Gal(β1-3)GlcNAc and Neu5Ac(α2-6)Gal(β1-4)GlcNAc, we found that M41-SABD, to our surprise, showed high-affinity binding to both Neu5Ac(α2-3)Gal(β1-3)GlcNAc and Neu5Ac(α2-6)Gal(β1-4)GlcNAc and that the M41-S1-NTD protein showed low affinity for Neu5Ac(α2-6)Gal(β1-4)GlcNAc in glycan arrays in a previous study (28). Instead of glycan arrays, we used ELISA to detect SABD binding to sialic acid, which might account for this difference. In contrast to IBV-SABD, we found that the three IBV-S1 proteins presented various affinities for Neu5Ac(α2-3)Gal(β1-3)GlcNAc and Neu5Ac(α2-6)Gal(β1-4)GlcNAc. IBV-S1 represents a complete protein structure, whereas the truncated versions may not represent the full structure, which may be the reason for the differences in affinity. Guinea fowl gammacoronaviruses bind to long linear or branched glycans capped with α-2,6-linked sialic acid (38). A previous study revealed that the five different clades of S1-NTD of SARS-CoV-2 presented different capabilities in glycan binding (25). In another study, the S1-NTD of two porcine epidemic diarrhea virus strains appeared to have distinct glycan-binding activity (39). In addition, no significant binding of SARS-CoV S1-NTD to sialic acid glycans was observed (25). Virus infection data suggest that α-2,3-sialidase and VC NA pretreatment decreases IBV entry into CEK cells to different degrees (Fig. 3). These results were generally consistent with a previous study (17), and the small difference in immunofluorescence may be caused by the different IBV strains and neuraminidase used in this study. We also observed a higher number of infected CEK cells with IBV-SD and M41 after pretreatment with α-2,3-sialidase compared to VC NA, while this phenomenon was not observed with IBV-GD. This indicated that sialic acids other than α-2,3-linked and α-2,6-linked sialic acids may also serve as receptors for IBV infection. Combined with the various degrees of binding revealed by ELISA, we suggest that α-2,6-linked sialic acid is also a receptor determinant during IBV infection of CEK cells. Thus, glycan binding appears to be a trait that has either weakened or strengthened during the evolution of coronaviruses.
Many coronaviruses bind to particular sialic acid glycans either as primary receptors for infection or as accessory receptors in host cell binding and uptake (37). It is well established that human CoV strains OC43 and HKU1, as well as BCoV, can utilize 9-O-Ac-sialic acid as an attachment factor or functional receptor (20, 40). However, an acetyl-group modification at position C-9 of sialic acid prevents MERS binding (21), whereas the acetyl-group modification at position C-9 of sialic acid does not affect SARS-CoV-2 binding (25). In this study, we showed that the S1 or S1-NTD of three IBVs bind to certain sialic acid glycans in the BSM to various degrees and the binding of IBV S1 and S1-NTD to sialic acid was inhibited by an acetyl group modification at position C-9 (Fig. 4). However, preincubating the virus with BSM has no obvious impact on IBV entry into CEK cells (Fig. 4E). Our results indicate that IBV may interact with sialic acid glycans without 9-O-Ac. In addition, unlike the sugar-binding human CoV strain OC43 and BCoV, IBV is not equipped with HE activity to separate the virus from glycans on host cells. Thus, further work is required to determine how IBV, without esterase enzymes, balances virion attachment and progeny release.
Reverse genetic techniques have been successfully applied to investigate the determinants of virulence and gene function and the relationship between genes and tissue tropism (33, 41, 42). Casais et al. constructed a recombinant IBV strain Beaudette in which the spike genes were replaced with the corresponding region of IBV M41-CK and showed that the recombinant strain had tropism for different cells compared to the parental strain (41). Subsequent studies showed that the recombinant IBV Beaudette strain, which harbored the S gene of M41 strain, retained avirulence (43). Shan et al. also used Beaudette strain as a backbone and replaced the hypervariable region of the S1 gene with the corresponding region from the QX-IBV strain; the results revealed that the pathogenicity of the recombinant virus remained avirulent (44). We exploited a similar reverse genetic system using IBV prevalent strains rSD and rGD as the backbone and replaced the corresponding regions with M41-S1 or SABD, respectively. We successfully constructed and rescued replacement recombinant strains, rSD, rSDM41-S1, rSDM41-206, rGD, rGDM41-S1, and rGDM41-206. Pathogenicity analysis in 1-day-old specific-pathogen-free (SPF) chickens revealed that the nephrotropism and virulence of rSDM41-S1, rSDM41-206, rGDM41-S1, and rGDM41-206 strains were significantly decreased to various degrees compared to their respective parental strains, rSD or rGD. A serum neutralization assay showed that rSDM41-S1 and rGDM41-S1 were different serotypes and that rSDM41-206 and rGDM41-206 were different subserotypes. The antigenicity change of rSDM41-206 and rGDM41-206 was smaller compared to rSDM41-S1 and rGDM41-S1, and the fact that S1-CTD was not replaced may be the reason for the incomplete change in antigenicity. Our previous study revealed that when the S gene was replaced by the corresponding region of attenuated GD, the pathogenicity was significantly decreased (33). This indicated that S1 and SABD were important for the nephrotropism, pathogenesis, and serotype of QX-like IBV and TW-like IBV.
In conclusion, we (i) identified the minimal SABD of IBV-SD, -GD, and -M41, (ii) revealed the binding characteristics of SABD and S1 to sialic acid, and (iii) demonstrated the role of SABD and S1 in the attenuation, nephrotropism, and serotypes of the three IBVs. Our results provide insight into IBV binding to host sialic acid receptors, which may aid the design of IBV vaccines. Thus, further work is required to determine how glycan binding in the SABD of IBV influences other viral phenotypes, for example, the mechanisms of virus entry and viral infectivity.
MATERIALS AND METHODS
Viruses and cells.
D980R, CV-1, and BHK-21 cells, as well as vaccinia virus vNotI/tk, were preserved in our laboratory. D980R and CV-1 were cultured in minimum essential medium (Thermo Fisher, Waltham, MA) with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY). BHK cells were electroporated to rescue the rSD and rGD strains with replacement of the corresponding regions S1 and S1-206 of the M41 strain. CEK cells were prepared from 19-day-old SPF chicken embryos and human embryonic kidney 293 (HEK293) cells were cultured in Dulbecco modified Eagle medium (DMEM) with 10% FBS, 100 mg/mL streptomycin, and 100 U/mL penicillin.
Construction, expression, and purification of recombinant protein.
To construct the plasmids expressing S1 and truncated proteins (Fig. 1A and D) of the three IBV strains, we first synthesized a full-length, codon-optimized IBV-M41 S1 gene (encoding aa 19 to 532; GenBank accession number AY851295), IBV-SD S1 gene (encoding aa 19 to 535; GenBank accession number MW351623.1), and IBV-GD S1 gene (encoding aa 19 to 535; GenBank accession number OQ117368). All of the IBV S1-truncated variants used in protein histochemistry were generated based on these templates and cloned into pCDNA3.1 mammalian expression vector with a CD5 signal peptide in the N terminus and a GCN4 trimerization motif and Flag tag in the C terminus, followed by a stop codon, as previously described with minor modifications (18, 30). The sequences were verified by automated nucleotide sequencing. The proteins were expressed in HEK293 cells. In brief, cells were transfected with pCDNA3.1 expression vectors using polyethyleneimine at a 1:2.5 (wt/wt) ratio. After 5 days, cell culture supernatants were harvested, and the recombinant proteins were purified using anti-Flag M2 affinity Sepharose beads according to the manufacturer’s procedures (Beyotime Technology, Beijing, China) and then stored at 4°C. Where indicated (Fig. 1B, 1E, and 2A), the S1 and truncated proteins were treated with N-glycosidase F (PNGase F; New England Biolabs, Inc.) to cleave off oligosaccharide side chains before electrophoresis according to the manufacturer’s procedures.
In addition, genes encoding IBV-M41-S1, S1-206, and S1-CTD (aa 19 to 532, 19 to 224, and 273 to 532, respectively); IBV-SD-S1, 209, and S1-CTD (aa 19 to 535, 19 to 227, and 276 to 535, respectively); IBV-GD-S1, 209, and S1-CTD (aa 19 to 535, 19 to 227, and 276 to 535, respectively) were amplified by PCR using codon-optimized IBV-M41 S1, IBV-SD S1, or IBV-GD S1 as respective templates, and were fused into the pFUSE-hIgG1-Fc2 expression vector (here designated Fc; InvivoGen, San Diego, CA). The BCoV-Mebus HE (spike aa 19 to 377; GenBank accession number AH010363) were synthesized and placed into the pFUSE-hIgG1-Fc2 expression vector. Site-directed mutagenesis was performed (aa 40 Ser to Ala), as described previously (45), to construct the enzymatically inactive form of BCoV-HE0-Fc with the same expression vector as described above. The location of the SABD of IBV-M41 was the S1 N-terminal aa 19 to 224 (206 aa) residues. Because of three amino acid insertions at the N terminus of S1, the similar regions of IBV-SD and -GD were residues S1-19-227 aa (209 aa). The recombinant proteins were produced in HEK293 cells and secreted into cell culture supernatants, then purified by protein affinity as per the manufacturer’s procedures (Sino Biological, Beijing, China).
Protein histochemistry.
Tracheal and kidney tissues from 6-week-old SPF chickens were fixed in formalin and embedded in paraffin before histochemical analysis of the binding of spike and truncated proteins as described previously (30). In brief, the tissue was sectioned at a thickness of 4 μm and deparaffinized and rehydrated; then, antigens were retrieved by boiling in 10 mM sodium citrate (pH 6.0) for 10 min. Endogenous peroxidase was inactivated with 1% hydrogen peroxide in methanol for 30 min at room temperature. After a washing step with phosphate-buffered saline–0.1% Tween (PBST), the slides were blocked with 10% normal goat serum for 30 min at room temperature. To detect the binding of IBV-S1 and truncated proteins to avian tissues, equimolar concentrations of chimeric S1-Flag and truncated proteins (S1 at 100 μg/mL and truncated proteins at 60 μg/mL) were precomplexed with anti-Flag-HRP (1:100; Abmart, Shanghai, China) for 30 min on ice prior to being applied to slides. After overnight incubation at 4°C, the slides were rinsed four times in PBS, and binding was visualized with 3-amino-9-ethyl-carbazole (AEC; ZSGB-BIO, Beijing, China). The tissues were counterstained with hematoxylin. To check for nonspecific staining, slides were incubated with comparable volumes of culture supernatants of HEK293 cells transfected with a pcDNA3.1 empty vector plasmid. Images were captured using light microscopy (CX41, Olympus Optical Co., Tokyo, Japan). Where indicated, before protein application, sialic acids were cleaved off from the tissues by incubation of the slides with 2 mU of neuraminidase from Vibrio cholerae (VC NA; Roche, Basel, Switzerland) at 37°C overnight.
Solid-phase ELISA and an on-the-plate O-Ac-Sia depletion assay on bovine submaxillary mucin.
A solid-phase enzyme-linked immunosorbent assay was performed as described previously (45–47) previously with minor adjustments. Bovine submaxillary mucin (BSM) was coated onto a 96-well ELISA plate (1 μg/well) at 4°C overnight. The plates were washed five times with PBST (PBS + 0.05% Tween 20) and subsequently blocked at 37°C for 2 h with 200 μL of blocking buffer (containing 5% skimmed milk + PBST). Then, the wells were washed five times and incubated with 3-fold serial dilutions of S1-Fc, S1-209-Fc, or BCoV-HE0-Fc proteins at 37°C for 1 h. After treatment with BCoV-HE-Fc or PBS (mock treatment) for 2 h at 37°C, the plates were incubated with S1-Fc, S1-209-Fc, or BCoV-HE0-Fc proteins. Anti-human IgG antibody-HRP (GenScript, Nanjing, China; 1:4,000) was used to assay the binding of S1-Fc, S1-209-Fc, or BCoV-HE0-Fc proteins with BSM (Aladdin, Shanghai, China). After incubation with peroxidase tetramethylbenzidine (TMB) substrate and termination solution, the optical density at 450 nm (OD450) was measured (1 M H2SO4).
Sialic acid binding ELISA.
Neu5Acα2-3Galb1-3GlcNAc-PAA-biotin and Neu5Ac(α2-6)Gal(β1-4)GlcNAcβsp3-PAA-biotin (Lectinity Holdings, Moscow, Russia) were coated onto a streptavidin matrix 96-well plate (Beaver Nano, Suzhou, China) at 0.5 μg/well overnight at 4°C as previously described (19) with minor adjustments. Plates were washed and blocked as described above. The proteins were then preincubated with mouse anti-human IgG Fc HRP antibody (GenScript; 1:4,000) for 30 min on ice. The proteins were diluted in PBS and applied onto the coated wells, followed by incubation for 3 h at room temperature. After incubation with peroxidase TMB substrate and termination solution (1 M H2SO4), the OD450 was measured.
Antiserum cross-binding ELISA.
An ELISA was performed as described above, with minor adjustments. In brief, a certain amount of SD-209-Fc, GD-209-Fc, or M41-206-Fc protein was coated onto a 96-well ELISA plate and incubated at 4°C overnight. Plates were washed and blocked as described above. The wells were washed four times, followed by incubation with chicken antisera diluted 1:200. Goat anti-chicken IgG/HRP antibody (Bioss, Beijing, China; 1:8,000) was used to detect cross-binding chicken antibodies. After incubation with peroxidase TMB substrate and termination solution (1 M H2SO4), the OD450 was measured.
Virus infection assay.
For the neuraminidase treatment test, CEK cells were treated with VC NA, α-2,3-sialidase (from Salmonella Typhimurium; TaKaRa, Otsu, Japan), or PBS (with Ca2+, Mg2+) for 2 h at 37°C before infection with different IBV stocks (108 viral copies) for 1 h at 37°C. For the BSM-blocking assay, the virus stocks (108 viral copies) were preincubated with different concentrations of BSM (diluted in PBS) or PBS at 37°C for 1 h before infecting the CEK cells at 37°C for 1 h. The inoculum was removed after absorption and washed four times with PBS and supplemented with fresh DMEM medium and 2% FBS. The viruses in the supernatants were harvested at 0, 12, 24, 36, 48, 60, and 72 hpi and stored at −80°C. At 36 hpi, cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and blocked with 5% bovine serum albumin. Next, IBV was detected using the anti-IBV N mouse primary antibody (3BN1; HyTest, Turku, Finland) at a 1:500 dilution and then a secondary antibody conjugated to Alexa Fluor 488-conjugated antibodies (Cell Signaling Technology). Fluorescence signals were captured under a microscope (Nikon, Tokyo, Japan). The infection experiments described above were performed in 24-well plates, using 0.5 mL of medium per well for cell culture, and repeated in triplicate with two technical replicates each time.
Real-time PCR.
Total RNA was extracted from samples using the HiPure Universal RNA minikit (Magen, Beijing, China) according to the manufacturer’s instructions. First-strand cDNA was transcribed using PrimeScript RT Master Mix (TaKaRa). The cDNA was used as the template for real-time qPCR (RT-qPCR) to quantify the viruses. The qPCR was performed with 2×M5 HiPer SYBR premix Ex Taq (with Tli RNase H) (Mei5bio, Beijing, China) according to the manufacturer and was performed as described previously (33, 34).
Virus rescue.
The S1 gene and S1-206 replaced mutants were constructed as previously described (33, 34). In brief, the four pGPT-rSD/rGD-S1/S1-SABD-positive plasmids were integrated into the vaccinia virus genome by homologous recombination. The verified viruses were reconstructed with pGPT-M41-S1/S1-206-negative plasmids. The replaced recombination mutations were introduced into rSD/rGD cDNA using the transient dominant selection system by homologous recombination (33, 34). After screening and transcription in vitro, the full-length RNAs of rSD, rGD, rSDM41-S1, rSDM41-206, rGDM41-S1, and rGDM41-206 were electroporated into BHK cells. The cell-supernatant mix was frozen and thawed three times and then inoculated into the allantoic cavities of 10-day-old SPF ECEs. The successfully rescued viruses were passaged in 10-day-old ECEs until the appearance of dwarf embryos, and then the recombinant viruses were sequenced to verify the replaced mutations. Then, 200 μL containing a 102 50% egg infectious doses (EID50) of rSD, rGD, M41, rSDM41-S1, rSDM41-206, rGDM41-S1, and rGDM41-206 viruses was inoculated into 10-day-old ECEs via the allantoic cavities at 12,18, 24, 36, 48, 60, 72, and 84 hpi. The allantoic fluid of five eggs per group was harvested and pooled for real-time PCR detection.
Animals and ethics statement.
SPF chickens and SPF embryonated eggs were purchased from Beijing Boehringer Ingelheim Vital Biotechnology Co., Ltd. (Beijing, China). The Beijing Administration Committee of Laboratory Animals approved the animal experimental protocols under the auspices of the Beijing Association for Science and Technology (approval SYXK [Jing] 2018-0038) and Ethical Censor Committee at China Agricultural University (approval 2022106).
Animal experiments.
A total of 160 1-day-old SPF chickens were divided into eight groups randomly and then inoculated via an eye dropper with 100 μL of 105 EID50 of IBV rSD, rGD, M41, rSDM41-S1, rSDM41-206, rGDM41-S1, rGDM41-206, or PBS as the negative control. Chickens were observed daily, and their clinical signs and mortality were recorded. Three birds per group were euthanized and necropsied at 5, 7, and 14 days postchallenge. Gross lesions were observed, and tracheas, kidneys, lungs, and bursas of Fabricius tissue samples were collected for viral detection via real-time PCR. The tracheal, kidney, and lung tissue samples were also collected for histopathological examination. The severity of lesions was assessed using the scoring criteria described elsewhere (33), the mean lesion scores were calculated for each group, and the tracheal and kidney tissue samples were also collected for immunohistochemical assays. The IBV N proteins were detected using the anti-IBV N antibody at a 1:1,000 dilution. The tracheal ciliary activity was assessed for the infected chickens as previously described (33, 34).
Virus cross-neutralization assay.
Neutralization antibodies were measured in infected chickens by a neutralization assay. In brief, serum samples were inactivated at 56°C for 30 min and then serially diluted 2-fold with PBS. A sample (0.1 mL) of the diluted sera was incubated with 0.1 mL IBV (200 EID50) at 37°C for 1 h. Then, 10-day-old SPF embryonated eggs were inoculated with the 0.2-mL virus-serum mixtures. After 6 days, the embryonated eggs were examined for IBV lesions, such as stunting or embryo dwarfing. The neutralizing titer of each serum sample against each virus was calculated as described previously (48). The cross-neutralization R values for each strain were calculated as previously described (35, 49). The difference in antigenicity (serotype) between two specific strains was defined as follows: an R value above 70% indicates identical antigenicity between the two viruses tested, an R value between 33 and 70% indicates a minor subtype difference, an R value between 11 and 32% indicates a major subtype difference, and an R value below 11% reflects a different serotype.
Statistical analysis.
Prism version 8.3 was used for statistical analysis (GraphPad, Inc., La Jolla, CA). Statistically significant differences were evaluated by performing a t test or two-way analysis of variance (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The data are presented in the figures as means ± the standard errors of the mean (SEM).
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Program of China (2021YFD1801103) and the 2115 Talent Development Program of China Agricultural University.
We thank Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing a draft of the manuscript.
Contributor Information
Guozhong Zhang, Email: zhanggz@cau.edu.cn.
Stacey Schultz-Cherry, St Jude Children’s Research Hospital.
REFERENCES
- 1.Fung TS, Liu DX. 2019. Human coronavirus: host-pathogen interaction. Annu Rev Microbiol 73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- 2.Woo PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, Bai R, Teng JL, Tsang CC, Wang M, Zheng BJ, Chan KH, Yuen KY. 2012. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol 86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulswit RJG, de Haan CAM, Bosch B-J. 2016. Coronavirus spike protein and tropism changes. Adv Virus Res 96:29–57. doi: 10.1016/bs.aivir.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackwood MW. 2012. Review of infectious bronchitis virus around the world. Avian Dis 56:634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- 5.Cavanagh D. 2007. Coronavirus avian infectious bronchitis virus. Vet Res 38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- 6.Schalk AF, Hawn MC. 1931. An apparently new respiratory disease of baby chicks. J Am Vet Med Assoc 78:413–422. [Google Scholar]
- 7.Valastro V, Holmes EC, Britton P, Fusaro A, Jackwood MW, Cattoli G, Monne I. 2016. S1 gene-based phylogeny of infectious bronchitis virus: an attempt to harmonize virus classification. Infect Genet Evol 39:349–364. doi: 10.1016/j.meegid.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji J, Gao Y, Chen Q, Wu Q, Xu X, Kan Y, Yao L, Bi Y, Xie Q. 2020. Epidemiological investigation of avian infectious bronchitis and locally determined genotype diversity in central China: a 2016-2018 study. Poult Sci 99:3001–3008. doi: 10.1016/j.psj.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng J, Huo C, Zhao J, Liu T, Li X, Yan S, Wang Z, Hu Y, Zhang G. 2018. Pathogenicity differences between QX-like and Mass-type infectious bronchitis viruses. Vet Microbiol 213:129–135. doi: 10.1016/j.vetmic.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Xu G, Cheng JL, Ma SH, Jia WF, Yan SH, Zhang GZ. 2018. Pathogenicity differences between a newly emerged TW-like strain and a prevalent QX-like strain of infectious bronchitis virus. Vet Microbiol 227:20–28. doi: 10.1016/j.vetmic.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Zhang H, Zhao J, Zhong Q, Jin JH, Zhang GZ. 2016. Evolution of infectious bronchitis virus in China over the past two decades. J Gen Virol 97:1566–1574. doi: 10.1099/jgv.0.000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mase M, Hiramatsu K, Watanabe S, Iseki H. 2022. Genetic analysis of the complete S1 gene in japanese infectious bronchitis virus strains. Viruses 14:716. doi: 10.3390/v14040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Chen W, Shen Y, Xia J, Fan S, Li N, Luo Y, Han X, Cui M, Zhao Y, Huang Y. 2022. Molecular characterization of infectious bronchitis virus in southwestern China for the protective efficacy evaluation of four live vaccine strains. Vaccine 40:255–265. doi: 10.1016/j.vaccine.2021.11.072. [DOI] [PubMed] [Google Scholar]
- 14.Vermeulen CJ, Dijkman R, de Wit JJS, Bosch B-J, Heesterbeek JAPH, van Schaik G. 2023. Genetic analysis of infectious bronchitis virus (IBV) in vaccinated poultry populations over a period of 10 years. Avian Pathol 2023:1–11. doi: 10.1080/03079457.2023.2177140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shang J, Zheng Y, Yang Y, Liu C, Geng Q, Luo C, Zhang W, Li F. 2018. Cryo-EM structure of infectious bronchitis coronavirus spike protein reveals structural and functional evolution of coronavirus spike proteins. PLoS Pathog 14:e1007009. doi: 10.1371/journal.ppat.1007009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wickramasinghe IN, van Beurden SJ, Weerts EA, Verheije MH. 2014. The avian coronavirus spike protein. Virus Res 194:37–48. doi: 10.1016/j.virusres.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winter C, Schwegmann-Wessels C, Cavanagh D, Neumann U, Herrler G. 2006. Sialic acid is a receptor determinant for infection of cells by avian infectious bronchitis virus. J Gen Virol 87:1209–1216. doi: 10.1099/vir.0.81651-0. [DOI] [PubMed] [Google Scholar]
- 18.Promkuntod N, van Eijndhoven RE, de Vrieze G, Grone A, Verheije MH. 2014. Mapping of the receptor-binding domain and amino acids critical for attachment in the spike protein of avian coronavirus infectious bronchitis virus. Virology 448:26–32. doi: 10.1016/j.virol.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouwman KM, Parsons LM, Berends AJ, de Vries RP, Cipollo JF, Verheije MH. 2020. Three amino acid changes in avian coronavirus spike protein allow binding to kidney tissue. J Virol 94:e01363-19. doi: 10.1128/JVI.01363-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulswit RJG, Lang Y, Bakkers MJG, Li W, Li Z, Schouten A, Ophorst B, van Kuppeveld FJM, Boons GJ, Bosch BJ, Huizinga EG, de Groot RJ. 2019. Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc Natl Acad Sci USA 116:2681–2690. doi: 10.1073/pnas.1809667116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Hulswit RJG, Widjaja I, Raj VS, McBride R, Peng W, Widagdo W, Tortorici MA, van Dieren B, Lang Y, van Lent JWM, Paulson JC, de Haan CAM, de Groot RJ, van Kuppeveld FJM, Haagmans BL, Bosch BJ. 2017. Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc Natl Acad Sci USA 114:E8508–E8517. doi: 10.1073/pnas.1712592114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwegmann-Wessels C, Herrler G. 2006. Sialic acids as receptor determinants for coronaviruses. Glycoconj J 23:51–58. doi: 10.1007/s10719-006-5437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwegmann-Wessels C, Zimmer G, Laude H, Enjuanes L, Herrler G. 2002. Binding of transmissible gastroenteritis coronavirus to cell surface sialoglycoproteins. J Virol 76:6037–6043. doi: 10.1128/jvi.76.12.6037-6043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petitjean SJL, Chen WZ, Koehler M, Jimmidi R, Yang JS, Mohammed D, Juniku B, Stanifer ML, Boulant S, Vincent SP, Alsteens D. 2022. Multivalent 9-O-acetylated-sialic acid glycoclusters as potent inhibitors for SARS-CoV-2 infection. Nat Commun 13:2564. doi: 10.1038/s41467-022-30313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo H, Li A, Lin HF, Liu MQ, Chen J, Jiang TT, Li B, Wang Y, Letko MC, Peng W, Shi ZL. 2022. The glycan-binding trait of the sarbecovirus spike N-terminal domain reveals an evolutionary footprint. J Virol 96:e0095822. doi: 10.1128/jvi.00958-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickramasinghe IN, de Vries RP, Grone A, de Haan CA, Verheije MH. 2011. Binding of avian coronavirus spike proteins to host factors reflects virus tropism and pathogenicity. J Virol 85:8903–8912. doi: 10.1128/JVI.05112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Britton P, Cavanagh D. 2007. Avian coronavirus diseases and infectious bronchitis vaccine development, p 161–181. In Thiel V (ed), Coronaviruses: molecular and cellular biology. Caister Academic Press, Norfolk, UK. [Google Scholar]
- 28.Parsons LM, Bouwman KM, Azurmendi H, de Vries RP, Cipollo JF, Verheije MH. 2019. Glycosylation of the viral attachment protein of avian coronavirus is essential for host cell and receptor binding. J Biol Chem 294:7797–7809. doi: 10.1074/jbc.RA119.007532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langereis MA, Bakkers MJ, Deng L, Padler-Karavani V, Vervoort SJ, Hulswit RJ, van Vliet AL, Gerwig GJ, de Poot SA, Boot W, van Ederen AM, Heesters BA, van der Loos CM, van Kuppeveld FJ, Yu H, Huizinga EG, Chen X, Varki A, Kamerling JP, de Groot RJ. 2015. Complexity and diversity of the mammalian sialome revealed by nidovirus virolectins. Cell Rep 11:1966–1978. doi: 10.1016/j.celrep.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wickramasinghe IN, Verheije MH. 2015. Protein histochemistry using coronaviral spike proteins: studying binding profiles and sialic acid requirements for attachment to tissues. Methods Mol Biol 1282:155–163. doi: 10.1007/978-1-4939-2438-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnard KN, Wasik BR, LaClair JR, Buchholz DW, Weichert WS, Alford-Lawrence BK, Aguilar HC, Parrish CR. 2019. Expression of 9-O- and 7,9-O-acetyl modified sialic acid in cells and their effects on influenza viruses. mBio 10:e02490-19. doi: 10.1128/mBio.02490-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamerling JP, Schauer R, Shukla AK, Stoll S, Van Halbeek H, Vliegenthart JF. 1987. Migration of O-acetyl groups in N,O-acetylneuraminic acids. Eur J Biochem 162:601–607. doi: 10.1111/j.1432-1033.1987.tb10681.x. [DOI] [PubMed] [Google Scholar]
- 33.Xu G, Ma S, Cheng J, Zhao Y, Zhang G. 2021. An attenuated TW-like infectious bronchitis virus strain has potential to become a candidate vaccine and S gene is responsible for its attenuation. Vet Microbiol 254:109014. doi: 10.1016/j.vetmic.2021.109014. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J, Sun L, Zhao Y, Feng D, Cheng J, Zhang G. 2021. Coronavirus endoribonuclease ensures efficient viral replication and prevents protein kinase R activation. J Virol 95:e02103-20. doi: 10.1128/JVI.02103-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan S, Liu X, Zhao J, Xu G, Zhao Y, Zhang G. 2017. Analysis of antigenicity and pathogenicity reveals major differences among QX-like infectious bronchitis viruses and other serotypes. Vet Microbiol 203:167–173. doi: 10.1016/j.vetmic.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouwman KM, Habraeken N, Laconi A, Berends AJ, Groenewoud L, Alders M, Kemp V, Verheije MH. 2020. N-glycosylation of infectious bronchitis virus M41 spike determines receptor specificity. J Gen Virol 101:599–608. doi: 10.1099/jgv.0.001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wasik BR, Barnard KN, Parrish CR. 2016. Effects of sialic acid modifications on virus binding and infection. Trends Microbiol 24:991–1001. doi: 10.1016/j.tim.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouwman KM, Delpont M, Broszeit F, Berger R, Weerts E, Lucas MN, Delverdier M, Belkasmi S, Papanikolaou A, Boons GJ, Guerin JL, de Vries RP, Ducatez MF, Verheije MH. 2019. Guinea fowl coronavirus diversity has phenotypic consequences for glycan and tissue binding. J Virol 93:e00067-19. doi: 10.1128/JVI.00067-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng F, Ye G, Liu Q, Navid MT, Zhong X, Li Y, Wan C, Xiao S, He Q, Fu ZF, Peng G. 2016. Identification and comparison of receptor binding characteristics of the spike protein of two porcine epidemic diarrhea virus strains. Viruses 8:55. doi: 10.3390/v8030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vlasak R, Luytjes W, Spaan W, Palese P. 1988. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc Natl Acad Sci USA 85:4526–4529. doi: 10.1073/pnas.85.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casais R, Dove B, Cavanagh D, Britton P. 2003. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J Virol 77:9084–9089. doi: 10.1128/jvi.77.16.9084-9089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keep S, Bickerton E, Armesto M, Britton P. 2018. The ADRP domain from a virulent strain of infectious bronchitis virus is not sufficient to confer a pathogenic phenotype to the attenuated Beaudette strain. J Gen Virol 99:1097–1102. doi: 10.1099/jgv.0.001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodgson T, Casais R, Dove B, Britton P, Cavanagh D. 2004. Recombinant infectious bronchitis coronavirus Beaudette with the spike protein gene of the pathogenic M41 strain remains attenuated but induces protective immunity. J Virol 78:13804–13811. doi: 10.1128/JVI.78.24.13804-13811.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shan D, Fang S, Han Z, Ai H, Zhao W, Chen Y, Jiang L, Liu S. 2018. Effects of hypervariable regions in spike protein on pathogenicity, tropism, and serotypes of infectious bronchitis virus. Virus Res 250:104–113. doi: 10.1016/j.virusres.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakkers MJ, Lang Y, Feitsma LJ, Hulswit RJ, de Poot SA, van Vliet AL, Margine I, de Groot-Mijnes JD, van Kuppeveld FJ, Langereis MA, Huizinga EG, de Groot RJ. 2017. Betacoronavirus adaptation to humans involved progressive loss of hemagglutinin-esterase lectin activity. Cell Host Microbe 21:356–366. doi: 10.1016/j.chom.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lang Y, Li W, Li Z, Koerhuis D, van den Burg ACS, Rozemuller E, Bosch BJ, van Kuppeveld FJM, Boons GJ, Huizinga EG, van der Schaar HM, de Groot RJ. 2020. Coronavirus hemagglutinin-esterase and spike proteins coevolve for functional balance and optimal virion avidity. Proc Natl Acad Sci USA 117:25759–25770. doi: 10.1073/pnas.2006299117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langereis MA, Zeng Q, Heesters BA, Huizinga EG, de Groot RJ. 2012. The murine coronavirus hemagglutinin-esterase receptor-binding site: a major shift in ligand specificity through modest changes in architecture. PLoS Pathog 8:e1002492. doi: 10.1371/journal.ppat.1002492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am J Epidemiol 27:493–497. doi: 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 49.Gravendyck M, Tritt S, Spenkoch-Piper H, Kaleta EF. 1996. Antigenic diversity of psittacine herpesviruses: cluster analysis of antigenic differences obtained from cross-neutralization tests. Avian Pathol 25:345–357. doi: 10.1080/03079459608419145. [DOI] [PubMed] [Google Scholar]