Abstract
Two major groups of specialized metabolites in maize (Zea mays), termed kauralexins and dolabralexins, serve as known or predicted diterpenoid defenses against pathogens, herbivores, and other environmental stressors. To consider the physiological roles of the recently discovered dolabralexin pathway, we examined dolabralexin structural diversity, tissue-specificity, and stress-elicited production in a defined biosynthetic pathway mutant. Metabolomics analyses support a larger number of dolabralexin pathway products than previously known. We identified dolabradienol as a previously undetected pathway metabolite and characterized its enzymatic production. Transcript and metabolite profiling showed that dolabralexin biosynthesis and accumulation predominantly occur in primary roots and show quantitative variation across genetically diverse inbred lines. Generation and analysis of CRISPR-Cas9-derived loss-of-function Kaurene Synthase-Like 4 (Zmksl4) mutants demonstrated dolabralexin production deficiency, thus supporting ZmKSL4 as the diterpene synthase responsible for the conversion of geranylgeranyl pyrophosphate precursors into dolabradiene and downstream pathway products. Zmksl4 mutants further display altered root-to-shoot ratios and root architecture in response to water deficit. Collectively, these results demonstrate dolabralexin biosynthesis via ZmKSL4 as a committed pathway node biochemically separating kauralexin and dolabralexin metabolism, and suggest an interactive role of maize dolabralexins in plant vigor during abiotic stress.
Introduction
Plant terpenoids are a structurally diverse metabolite class that serve critical functions in growth, defense, and environmental adaptation (Gershenzon and Dudareva 2007). Beyond widely conserved gibberellin (GA) phytohormones with key roles in plant growth, the vast majority of diterpenoids are often species-specific and serve specialized functions in stress responses during microbial attack, herbivory, as well as abiotic stress (Schmelz et al. 2014; Tholl 2015; Block et al. 2019; Murphy and Zerbe 2020; Li et al. 2021; Zhang et al. 2021).
In maize (Zea mays), two groups of bioactive diterpenoids, termed kauralexins (KX) and dolabralexins (DX), have been identified as serving defense-related functions (Murphy and Zerbe 2020). KX is acidic diterpenoids that feature an ent-iso-kaurene-derived backbone with distinct levels of oxidation and reduction (Ding et al. 2019). KX metabolites function as local defenses produced at nearly any site of challenge in response to pathogen infection by, for example, Fusarium verticillioides, F. graminearum, Rhizopus microsporus, Cochliobolus heterostrophus, and Colleototrichum graminicoloca, as well as in response to water deficit and below-ground oxidative stress (Schmelz et al. 2011, 2014; Christensen et al. 2018; Ding et al. 2019). Aligned with their stress-elicited formation, KX display potent antifungal activity in vitro and in planta, as well as antifeedant activity against the European corn borer (Ostrinia nubilalis) (Schmelz et al. 2011; Vaughan et al. 2015; Ding et al. 2019). Increased pathogen susceptibility of both the KX-deficient ent-copalyl pyrophosphate synthase (ent-CPS) mutant termed Anther Ear 2 (Zman2) and the Kaurene Synthase-Like 2 (Zmksl2) mutant demonstrated substantial impacts of diterpenoids on maize stress resilience (Vaughan et al. 2015; Christensen et al. 2018; Ding et al. 2019).
Recently, DX metabolites were discovered as an additional group of maize diterpenoids that are structurally distinct from KX, featuring a dolabradiene scaffold with oxygenations, often including a C-15,16 epoxide (Fig. 1) (Mafu et al. 2018). While the physiological functions of DX remain unknown, metabolite and transcript profiling of the DX pathway showed inducible DX formation in maize roots following infection by F. graminearum and F. verticillioides, as well as below-ground oxidative stress (Mafu et al. 2018). DX metabolites have been found to accumulate up to 225 µg g−1 fresh weight (FW) in mature maize roots infected with F. graminearum, compared to 9 µg g−1 FW KX in the same tissues (Mafu et al. 2018). Moreover, select DX to display potent antifungal efficacy in vitro against highly evolved Fusarium pathogens in maize (Mafu et al. 2018). Collectively, these findings are consistent with an important role of DX in below-ground stress responses.
Figure 1.
Maize diterpenoid biosynthetic network. Gibberellin (GA) biosynthesis (green) and specialized metabolite pathways for dolabralexins (DX) (blue) and kauralexins (KX) (purple) utilize the same central precursor. Dashed arrows indicate predicted crosstalk between GA and KX metabolic pathways. Known DX structures are shown in the bottom blue box. GGPP, geranylgeranyl diphosphate; KSL, Kaurene Synthase-Like; CPP, copalyl diphosphate; An, Anther Ear.
Despite structural and functional distinctions, GA, KX, and DX metabolites derive from a common precursor, ent-copalyl pyrophosphate (ent-CPP) that is formed by two catalytically redundant class II diterpene synthase (diTPS) enzymes, Anther ear 1 and 2 (ZmAN1 and ZmAN2), in the ent-CPS family (Fig. 1) (Bensen et al. 1995; Harris et al. 2005; Schmelz et al. 2011; Christensen et al. 2018; Mafu et al. 2018; Murphy et al. 2018). Gene expression, along with genetic and phenotypic studies of Zman1 mutants, demonstrated that ZmAN1 functions in GA biosynthesis, along with ZmKSL3 and to a lesser extent ZmKSL5 and ZmTPS1, all of which generate ent-kaurene (Fig. 1) (Bensen et al. 1995). In contrast, the Zman2 mutant displays no GA-deficiency phenotype (Harris et al. 2005). Multifunctional roles of ZmAN2 and derived KX and DX metabolites in biotic and abiotic stress responses are supported by Zman2 mutant phenotypes such as deficiencies in both KX and DX, increased susceptibility to fungal infection and water deficit, and altered rhizosphere microbiomes (Vaughan et al. 2014, 2015; Christensen et al. 2018; Mafu et al. 2018; Ding et al. 2019; Murphy et al. 2021). While the KX and DX levels are severely reduced in the Zman2 mutant (Vaughan et al. 2014, 2015; Christensen et al. 2018; Murphy et al. 2021), trace abundance of both metabolite groups can be detected, predictably derived from ent-CPP produced by ZmAN1 and siphoned away from the GA pathway. Downstream of ent-CPP, the KX and DX pathways bifurcate through the activity of two class I diTPSs, ZmKSL2, and Kaurene Synthase-Like 4 (ZmKSL4), respectively. ZmKSL2 converts ent-CPP into ent-iso-kaurene en route to KX, whereas ZmKSL4 transforms ent-CPP into the DX precursor dolabradiene (Mafu et al. 2018; Ding et al. 2019) (Fig. 1). Functional modification of these hydrocarbon scaffolds through cytochrome P450 monooxygenases (P450s) of the CYP701 (ZmCYP701A43) and CYP71 (ZmCYP71Z16, ZmCYP71Z18) families, along with a steroid 5α-reductase termed Kauralexin Reductase 2 (ZmKR2), then largely defines the structural diversity and bioactivity of KX and DX metabolites (Fig. 1, gene IDs listed in Supplemental Table S1). In addition, CYP71Z16 and CYP71Z18 exhibit expansive substrate promiscuity and function in the endogenous biosynthesis of diverse acidic sesquiterpenoid phytoalexins termed zealexins, highlighting an interconnected and modular pathway network for producing distinct defense metabolite families (Mao et al. 2016; Mafu et al. 2018; Ding et al. 2019; Ding et al. 2020). Two additional substrate-promiscuous P450s, Kaurene Oxidase (KO) 1 (ZmCYP701A26), and ZmKO2 (ZmCYP701A43), are tandemly arrayed on the same chromosome. While ZmKO1 exhibits activity in the GA pathway, ZmKO2 is biochemically responsible for highly oxidized KX (Mafu et al. 2016; Mao et al. 2017; Ding et al. 2019).
In this study, we expand the known diversity of DX metabolites with additional metabolite structure, biosynthesis characterizations and endogenously prove the first committed DX pathway branch point. Gene expression studies and metabolite profiling show DX accumulation primarily in roots with context-specific constitutive production when plants are grown in the field or field-collected soils. A defined Zmksl4 maize mutant displays a dramatic deficiency in DX metabolites, reduced plant fitness under water-deficit conditions, and an altered root system architecture, especially under water-deficit conditions. Current genetic evidence clarifies ZmKSL4 as the sole diTPS responsible for DX biosynthesis, as well as demonstrating previously unknown impacts on root system architecture.
Results
Zmksl4 mutant displays deficient DX production in planta
To investigate the physiological relevance of DX in maize that is independent of KX, we generated a CRISPR-Cas9-enabled Zmksl4 mutant through Agrobacterium-mediated transformation of the Hi-II maize hybrid line using a guide RNA targeting specifically ZmKsl4 (kaurene synthase-like 4) (Char et al. 2016). Seed of Zmksl4, homozygous for a one base pair insertion of either G or A in both alleles (Fig. 2A), and its isogenic wild-type (WT) sibling, both having the Cas9 removed through backcrossing to B73 and bulked before use in this study. Earlier studies showing abiotic stress inducibility of diterpenoid metabolism and increased drought susceptibility of the KX- and DX-deficient Zman2 mutant suggested interactive roles in the maize water-deficit response (Vaughan et al. 2014, 2015). To specifically consider this hypothesis in the context of DX, WT, and Zmksl4 plants were grown in fabric pots outdoors in maize field soil with controlled drip irrigation. Water deficit was imposed on half of the plants by limiting water for six consecutive days at either 1-month-old (vegetative stage 5, V5) or 2-month-old (vegetative stage 12, V12) at which time plants were assessed for root and shoot fresh weight, and leaf and root samples were collected for downstream analysis (Fig. 2B). No large-scale visual phenotypic differences were observed between WT and Zmksl4 under control or water-deficit conditions and all plants exhibited characteristic leaf curling upon water deficit (Fig. 2B).
Figure 2.
Zmksl4 maize mutant lacks DX metabolites. A)Zmksl4 contains a 1 bp insertion in the ZmKsl4 gene, causing a frameshift mutation. B) Experimental design used in this study. WT (dark gray) and Zmksl4 (light gray) plants were assessed for metabolite and transcript abundances at vegetative stage (V) V5 and V12. Water-deficit and well-watered were analyzed for each respective developmental stage and genotype. Photos are representative of plants from each sample type. Biological replicates (n) are listed for each sample type. Sample types (particular timepoint and water status) correspond to plot colors throughout this study. C) LC-MS/MS abundances of DXs for which standards were available. Letters represent significantly different abundance measured using an ANOVA and t-test with Sidak correction for multiple comparisons, P < 0.05. Box colors in C) correspond to sample types colored in B). The boundaries of the box in each boxplot represent the interquartile range; the edges of the box are the 25th and 75th percentiles, and the center line is the median. The upper and lower whiskers are the maximum and minimum values, respectively, that are within 1.5 times the interquartile range edges. Individual points are values greater than 1.5 times the interquartile range edges.
Targeted positive and negative mode electrospray ionization (ESI) liquid chromatography mass spectrometry (LC-MS/MS) analysis using enzymatically produced DX or tissue-purified standards, including the major DX metabolites epoxydolabranol and trihydroxydolabrene (THD), respectively, showed no detectable levels in roots or leaves of Zmksl4 mutant plants (Fig. 2C; metabolite atlas in Supplemental Fig. S1). Both epoxydolabranol and THD were found at low levels in WT V5 roots, and significantly greater in WT V12 roots (P < 0.01 for dolabradienol, P < 0.001 for epoxydolabranol or THD); there was no significant difference in response to water status (Fig. 2C). Epoxydolabrene was not detectable in any samples, including the purified standard, due to insufficient ionization for ESI LC-MS/MS analysis. THD could not be absolutely quantified because the enriched natural product standard was not of sufficient quantity or purity to determine an accurate mass, but it was the most abundant DX by LC peak area of its most dominant peak. Parallel analysis of available KX standards (kauralexin B1 and A3) showed overall low abundance in WT and Zmksl4 samples and no enrichment due to genotype. KA3 was found to be slightly enriched (1.7-fold) in V5 Zmksl4 roots compared to WT under well-watered conditions but was not significantly enriched in V5 water-deficit roots (P < 0.1), V12 roots, or any leaf tissue (Supplemental Dataset S1, respectively). The lack of key DX metabolites without significant variation in KX abundance (P > 0.1), with the except of KA3, in the Zmksl4 mutant, verifies that DX biosynthesis proceeds solely via ZmKSL4 without functional redundancies of known or yet uncharacterized maize diTPS.
Expansion of the DX metabolite family
Next, LC-MS/MS untargeted metabolomics of WT and DX-deficient Zmksl4 roots grown in the conditions described above was used to investigate the chemical diversity of maize DX metabolites. To identify possible previously unrecognized DXs, a generalized linear model was used to identify features enriched in WT and comparatively depleted in Zmksl4 roots (P < 0.1, fold change >2.5) at developmental stage V12, given that this was the stage when THD and epoxydolabranol were found to be most abundant in WT (Fig. 2C). Given that there was no statistically significant difference in epoxydolabranol or THD due to water status, samples were not separated by water status for this analysis. A total of 124 specific MS2 parent mass fragments at a particular retention time (RT), called “features”, were found to be enriched in WT but absent or substantially lower abundant in Zmksl4 (Supplemental Dataset S1), representing 0.3% of all detected features. Of these features, 91 had available MS2 spectra in at least one WT root sample at V12 (mass spectra and abundance plots of the most abundant feature for each predicted DX are in Supplemental Figs. S2 and S3, respectively).
As expected, both THD and epoxydolabranol were found among the enriched features; the major THD feature represented the most abundant in this selected list, with a log2 fold change of 8.9-fold greater in WT than Zmksl4. As individual metabolites may be represented by multiple features, all focal features were manually inspected for RT and possible presence in the available standards, for a total of 48 predicted uncharacterized DX metabolites. The list includes 32 metabolites in positive mode and 15 in negative mode. Several of these metabolites had peaks with the same parent ion and same MS2 at different RT (Supplemental Dataset S1)—these predictably represent either isomers or fragments of larger molecules that have the same structure. For features with parent masses less than 200 m/z, identical fragments are likely given that DX has the same backbone. Interestingly, DX-26 appeared to be an isomer of the parent mass of THD, not a matching fragment (Supplemental Fig. S2). The metabolites identified in positive mode range in their parent mass from m/z 95 to 391 and many contained characteristic diterpenoid mass ions such as m/z 81, 95, 119, and 145 that are also found in THD. All but eight of the positive mode, predicted DXs are within one minute of the THD retention time. While these metabolites were identified by their enrichment in all V12 WT roots compared to Zmksl4, 42 of the 48 predicted DXs were also enriched in V5 WT roots compared to Zmksl4 under either just well-watered or water-deficit conditions, or both, when samples were analyzed separately by water status.
To further investigate possible alterations in Zmksl4 metabolite profiles beyond DX, statistical analysis using PERMANOVA was used to determine the factors influencing the root and leaf metabolomes. Genotype was not a significant factor in V12 root feature abundance, nor in leaves at any stage (statistics available in Supplemental Dataset S2). Genotype was a significant factor for feature abundance in V5 roots when DX is in low abundance in WT (Supplemental Dataset S2). Outside of the 48 putative DX candidates, Zmkls4 mutants did not cause major shifts in other metabolite groups of V12 maize roots, making Zmksl4 plants suitable for investigating the diversity and biological relevance of DX metabolism in maize. No features were enriched in Zmksl4 roots compared to WT at V12 when samples are not separated by water status (P < 0.10, fold change >2.5); however, when separating by water status, some features were enriched, although not to the degree of DX enrichment in WT (Supplemental Dataset S1). Mass spectra and RTs for all features are available online (see Methods for details), and lists of features enriched in each sample type, as well as their abundance and statistics related to significance, are available in Supplemental Dataset S1.
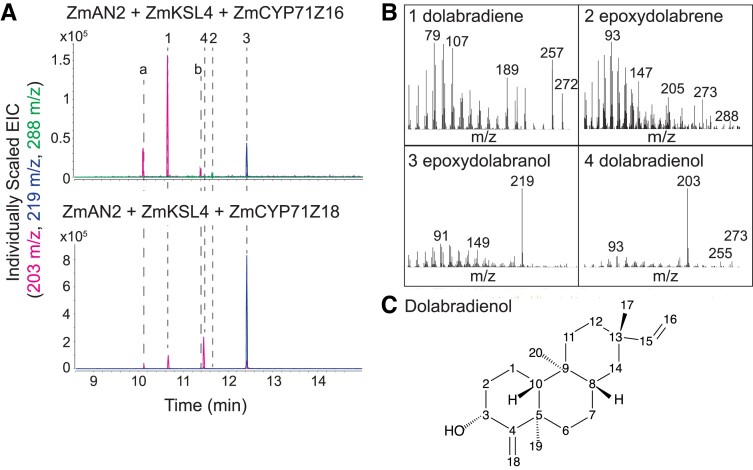
The complex co-occurrence of structurally related primary and specialized metabolites in maize tissue creates a challenge for DX purification for NMR structural elucidation. To circumvent this limitation, we used E. coli co-expression of ZmAN2, ZmKSL4, the maize cytochrome P450 reductase ZmCPR2, and ZmCYP71Z16 or ZmCYP71Z18 to produce previously unidentified pathway products to levels enabling purification (Fig. 3, A and B). Of the resulting metabolites, one ZmCYP71Z18 product featured a RT and mass fragmentation pattern similar to, but distinct from, the known DX metabolites epoxydolabranol and epoxydolabrene (Fig. 3, B and C). Purification of this compound using an optimized silica chromatography and semipreparative HPLC method (Murphy et al. 2019) enabled sufficient metabolite quantities to perform 1D and 2D NMR analysis, resulting in identification of the product as dolabradienol (Fig. 3C, Supplemental Fig. S4). Dolabradienol contains a single hydroxyl group in the C3 position and lacks the epoxy group of epoxydolabranol. In addition, comprehensive NMR analysis of DX products and select pathway intermediates refined previous structural assignments (Mafu et al. 2018), specifically defining a 5β, 10α rather than the previously reported 5α, 10β configuration of the DX skeleton (Supplemental Fig. S4). Notably, co-expression of ZmCYP71Z16 did not result in the formation of detectable amounts of dolabradienol, suggesting catalytic differences between ZmCYP71Z16 and ZmCYP71Z18 (Fig. 3B). Dolabradienol and epoxydolabrene—the two minor products of ZmCYP71Z18 and Z16, respectively—represent opposite single oxygenation positions, but a combination of both oxygenations results in the epoxydolabranol structure seen as the dominant product for both ZmCYP71Z enzymes (Fig. 1; Fig. 3B). Additional LC-MS/MS analysis against the enzymatically produced dolabradienol standard revealed dolabradienol to be one of the 48 putative DX candidates, with 2.7-fold enrichment in WT V12 roots (Fig. 2C). Although dolabradienol was formed as a lesser byproduct of the ZmCYP71Z18-catalyzed reaction, its enriched abundance in maize WT roots suggests that it is not entirely consumed as a substrate in DX biosynthesis. Instead, dolabradienol substantially contributes to the array of pathway end products (Fig. 2C).
Figure 3.
ZmCYP71Z18 makes a distinct dolabralexin (DX) product, dolabradienol. A) GC-MS extracted ion chromatograms (EIC) of E. coli co-expression of maize diterpenoid biosynthesis genes. Pink lines are EIC of 203 m/z; blue lines are EIC of 219 m/z; green lines are EIC of 288 m/z. Peaks labeled a and b are unknown metabolites. B) Mass spectra of ZmCYP71Z16/18 products, numbers correspond to peaks numbered in A). C) Dolabradienol structure determined by NMR, with detailed NMR analysis in Supplemental Fig. S4.
Transcriptome analyses place DX biosynthesis predominantly in maize roots
GA, KX, and DX biosynthetic pathways all utilize the common precursor, ent-CPP, suggesting a tight control of the downstream pathway branches to drive the production of diterpenoids with distinct functions. To investigate the spatiotemporal differences in the biosynthesis of GA, KX, and DX metabolites, four publicly available RNAseq datasets (Chen et al. 2014; Stelpflug et al. 2016; Kremling et al. 2018; Yi et al. 2019) were analyzed for key genes of the respective GA, KX, and DX pathway branches (gene list in Supplemental Table S1). FPKM (fragments per kilobase of exon per million mapped fragments) values for the genes of interest from all datasets used here are available in Supplemental Dataset S3, as well as the growing media used in each study (where available). It should be noted that roots used in the transcriptomics study by Stelpflug et al. (Stelpflug et al. 2016) were grown on germination paper or an unspecified greenhouse soil at various ages (Fig. 4), whereas roots used in the study by Kremling et al. (Kremling et al. 2018) were derived from seedlings grown in vermiculite (Fig. 5A). Details on the origin of root tissue in the remaining studies were unspecified (Chen et al. 2014; Yi et al. 2019). Across all four developmental atlases (Chen et al. 2014; Stelpflug et al. 2016; Kremling et al. 2018; Yi et al. 2019), GA pathway genes, including ZmAn1, ZmKo1, ZmKsl3 and to lesser extent ZmKsl5 and ZmTps1 (both of which generate ent-kaurene as does ZmKsl3, Fig. 1) expectedly showed patterns of co-expression across various primarily above-ground tissues and timepoints (Bensen et al. 1995; Fu et al. 2016; Mao et al. 2017) (Fig. 4). ZmCps3 and ZmCps4, two known class II diTPS in maize, are included for a complete comparison; however, their expression patterns do not match either GA, DX, or KX biosynthetic genes and their known metabolite products have not yet been reported in planta (Murphy et al. 2018).
Figure 4.
Spatiotemporal transcriptomes reveal tissue- and developmental-specificity of DX biosynthesis, as well as co-expression of KX and DX pathways. Data from Stepflug et al., sorted in descending order of ZmKsl4 expression. Co-expression of genes is determined by similar expression patterns, i.e. co-expressed genes are expressed in the same tissue, at the same time, and in similar abundance relative to other tissue types. DAS = days after sowing; DAP = days after pollinating; V = vegetative stage; dev. = development; Par. = parenchyma. Units are in FPKM (fragments per kilobase of exon per million mapped fragments); color is scaled from lowest (0 FPKM) in blue to the highest (215.86 FPKM) in red.
Figure 5.
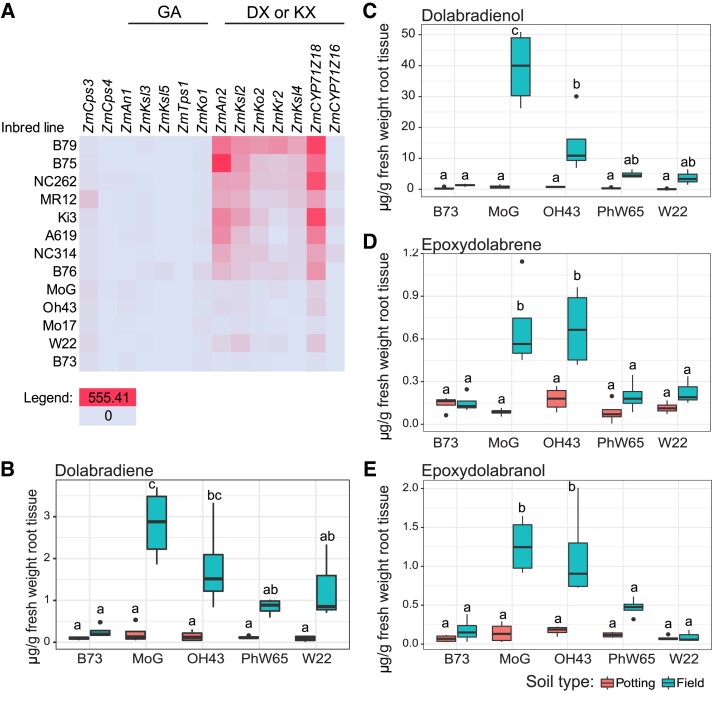
DX root transcript and metabolite abundances vary across maize inbred lines. A) Transcript data is from Kremling et al. (2018). Data is sorted in descending order by ZmKsl4 expression in root tissues; color is scaled from the lowest (0 FPKM) in blue to highest (555.41 FPKM) in red in units of FPKM (fragments per kilobase of exon per million mapped fragments). B73 has averaged over 17 separate replicates. B-E) Metabolite levels using GC-MS and authentic standards for major DX in a subset of available inbred roots grown in a greenhouse in field soil or potting soil. The boundaries of the box in each boxplot represent the interquartile range; the edges of the box are the 25th and 75th percentiles, and the center line is the median. The upper and lower whiskers are the maximum and minimum values, respectively, that are within 1.5 times the interquartile range edges. Individual points are values greater than 1.5 times the interquartile range edges. Letters represent statistically significant differences measured by ANOVA and a t-test with a Sidak correction for multiple comparisons (P < 0.05).
In contrast to GA pathway enzymes, ZmAn2, alongside ZmKsl2, ZmKo2, ZmCYP71Z16, ZmKr2, and ZmKsl4 of DX metabolism showed the highest expression in roots (Fig. 4). In agreement with previous studies (Mafu et al. 2018; Ding et al. 2019), expression of DX and KX pathway genes in roots at the tested timepoints suggests co-expression of these pathway genes. Of the root tissues, ZmKsl2 was most abundant in primary root tissues, whereas ZmKsl4 expression was lower and more broadly distributed in different root tissue types. Notably, ZmKsl4 showed the highest expression in primary roots seven days postgermination (Fig. 4). Analysis of an additional, dissected root transcriptome dataset showed highest expression of ZmAn2 and ZmKsl4 in seminal and crown roots, with only low expression in primary roots (Tai et al. 2016, roots grown in liquid solution), whereas another atlas (Kremling et al. 2018) showed highest ZmKsl4 expression in root tips of germinating seedlings. Together, despite differences in root age and growth conditions, these transcriptome data support the predominant expression of the ZmKsl4-mediated DX pathway in select root tissues, whereas ZmKsl2-derived KX pathway genes were expressed in both above- and below-ground tissues.
Given that ZmKsl4 expression predominates in roots, we sought to confirm the developmental and tissue-specificity of DX metabolites. Using the LC-MS/MS metabolite data for WT maize plants generated in this study (conditions detailed above), we found DX were most abundant in roots at the V12 stage and were of low abundance in stage V5 (Fig. 2C). DX were not detectable or lowly abundant (less than 1 × 105 peak height) in leaf tissue at all stages (Supplemental Fig. S3B), consistent with the gene expression patterns from the transcriptome tissue atlases. Previous work demonstrated stress-inducible transcript and metabolite accumulation in the KX pathway under drought stress (Vaughan et al. 2015), and both KX and DX under other stress conditions (Christensen et al. 2018; Mafu et al. 2018; Ding et al. 2019). Additional analysis of public datasets of above-ground tissue under heat and cold stress (Makarevitch et al. 2015, plants grown in a 1:1 mixture of autoclaved field soil and Metro Mix potting soil) and Cercospora zeina leaf infection (Swart et al. 2017, unspecified growth medium) showed no induction of ZmKsl4. In an analysis of a public dataset of 4-day-old B73 seedlings treated with PEG8000 as a water-deficit proxy, ZmAn2, ZmKsl2, ZmCYP71Z16, and to a lesser extent ZmKsl4 were expressed in maize root cortex cells, but not the meristem, elongation zone, or stele, under both water deficit and well-watered conditions (Opitz et al. 2016, plants grown on paper towel rolls). Here, our LC-MS/MS of WT roots identified predicted DX to be abundant in both water deficit and well-watered V5 and V12 roots (Fig. 2C), with no significant difference due to water status at either V5 or V12 (Supplemental Dataset S2).
Maize inbred lines vary substantially in their levels of DX pathway transcripts
To establish common patterns of DX abundance in maize roots, we next examined select genetically diverse maize lines. Prior studies demonstrated differential DX accumulation in roots of three field-grown maize varieties—B73, Mo17, and hybrid sweetcorn (variety Golden Queen) with B73 showing the lowest DX levels (Mafu et al. 2018). Using publicly available transcriptome data (Kremling et al. 2018), we assessed the transcript abundance in roots of germinated seedlings grown in vermiculite across a larger set of maize inbred lines; all public transcriptome data used in this study is available in Supplemental Dataset S3. Gene expression also varied across multiple replicates of B73 included in this study and was averaged across 17 replicates (Fig. 5A, Supplemental Dataset S3). As expected based on the tissue atlas (Fig. 4) (Stelpflug et al. 2016; Kremling et al. 2018), GA metabolism genes and the additional class II diTPS ZmCps3 and ZmCps4, whose roles and products in planta are yet unknown, do not show high transcript abundance in roots of any inbred line, nor do they co-express with DX or KX pathway genes, and were included for completeness of all known class II and class I diTPS in maize (Fig. 5A).
Expression of the DX and KX pathway genes, including ZmAn2, ZmKsl2, and ZmKsl4, varied substantially across maize inbred lines, with B73 and Mo17 showing comparatively low transcript abundance for KX- and DX-metabolic genes, whereas high gene expression was observed for B79 and B75 (Kremling et al. 2018) (Fig. 5A). Of the 274 lines tested, all but three had greater ZmKsl2 expression than ZmKsl4 in roots, and 8 had equal expression (Fig. 5A, Supplemental Dataset S3), consistent with the observed tissue-specific gene expression levels (Stelpflug et al. 2016; Kremling et al. 2018) (Fig. 4). Notably, DX and KX pathway genes showed patterns of co-expression with all major specialized diTPS genes in the network of most tested inbred lines—i.e. genes within either DX or KX pathway showed similar expression levels in the tested tissue in an inbred line compared to other inbred lines (Kremling et al. 2018). Only in select lines, such as W22, ZmKsl4 expression was not detected, whereas ZmAn2 and ZmKsl2 were expressed, suggesting that different lines may show distinct mixtures of the different metabolite groups (Kremling et al. 2018) (Fig. 5A).
To further investigate the influence of environmental and genetic variation, we grew four distinct maize inbred lines namely W22, PHW65, Oh43, and MoG, in the greenhouse using either commercial potting media comprised of peat moss or a 1:1 mixture of peat moss and soil from a maize field. In all cases, root DX levels measured using GC-MS were nearly undetectable in peat moss, whereas DX levels in roots from plants grown in field soil were always significantly higher in MoG and OH43 (P < 0.05) (Fig. 5, B to E). Moreover, total DX levels varied across inbred lines by more than 7-fold, with MoG being the highest-producing line among those tested (Fig. 5, B to E). The rank-order of highest ZmKsl4 expression matched the rank order of dolabradienol (the most abundant metabolite) production under field soil—MoG, Oh43, W22, followed by B73—despite differences in seed source, plant age, and growing media (Fig. 5C).
Zmksl4 exhibits altered changes in root/shoot ratios following water-deficit
Given the stress-inducibility of DX and their abundance in roots under field conditions (Mafu et al. 2018), we performed physiological studies on Zmksl4 mutant plants to understand the impact of DX-deficiency on Zmksl4 vigor, under well-watered and water-deficit conditions, on the same plants grown for metabolite analysis described above. To determine the effect of DX pathway products on the plant responses to water deficit, plants were analyzed for their fresh root and shoot weights at tissue harvest, using the same plants subsequently sampled for metabolite analysis described above. At V5, in which DX are only lowly abundant, genotype did not significantly impact root weight, shoot weight, or the root/shoot ratio, analyzed using analysis of variance (ANOVA), P < 0.05 (Fig. 6A). As expected, water stress significantly decreased root and shoot weights of both genotypes but did not affect the root/shoot ratio, as would have been expected (Fig. 6A). At V12, when DX were most abundant in WT samples, the root/shoot ratio significantly increased due to water deficiency in WT but did not increase in Zmksl4; Zmksl4 showed 68% of the WT root/shoot ratio under water-deficit conditions (Fig. 6B). Root and shoot weights were not impacted by either genotype or water status in V12 (Fig. 6B). Furthermore, while the WT root/shoot weight ratio average increased from 0.35 to 0.50 under water-deficit conditions, the Zmksl4 root/shoot ratio average ranged from 0.33 to 0.34 irrespective of the water condition (Fig. 6B).
Figure 6.
Zmksl4 exhibits an altered root/shoot ratio. Letters represent statistical significance based on the Tukey HSD t-test, with right-tailed values for water status and left-tailed values for genotype for root and shoot weight and left-tailed values for both genotype and water status for root/shoot ratio (P < 0.05). Error bars represent standard error. A) Measurements based on fresh weight roots of all above-ground tissue of V5 roots used for metabolomics analysis. B) Measurements based on fresh weight roots of all above-ground tissue of V12 roots used for metabolomics and transcriptomics analysis. C) Measurements based on dry weight roots of all above-ground tissue of V5 roots used for root architecture analysis. Statistics and raw data are available in Supplemental Dataset S2.
Given the altered Zmksl4 response to drought stress, in that its root/shoot ratio did not change under water deficit at either stage (Fig. 6), we sought to understand broader differential Zmksl4 response differences. To do so, LC-MS/MS untargeted metabolite profiles were used to comparatively analyze the metabolic response of WT and Zmksl4 to water deficit. At V12, 3% of WT metabolomic features were significantly altered in abundance due to water deficit, as determined with a linear model (P < 0.1) (statistics in Supplemental Dataset S2, metabolites and abundance in Supplemental Dataset S1). By comparison, 8% of Zmksl4 metabolites were changed. These features with altered abundance were dependent on genotype; only 9% of total features affected in all genotypes were commonly affected in both WT and Zmksl4, suggesting the genotypes have different metabolic responses to water deficit (Supplemental Fig. S5). Mass spectra and RTs for all features are available (see Methods for details), and features enriched in each sample type is available in Supplemental Dataset S1.
Zmksl4 has distinct root system architecture
To further investigate the water deficit susceptibility of the Zmksl4 mutant, the root system architecture of Zmksl4 compared to its WT sibling was measured using root imaging and quantification of root traits using the DiRT software package. Here, WT and Zmksl4 were grown in large pots of turface in a greenhouse until V5 (1 month after sowing), such that roots would not grow large enough to touch the insides of the pot. Turface was used, rather than field soil, because of its ability to easily wash away without damaging the root architecture. Water deficit was imposed by hydrating the media to a specific weight by volume before filling the pots; this level of hydration was then maintained by watering back to weight daily. Through this process, the plants experienced a diurnal cycle of soil matrix potentials between approximately −0.7 MPa to −1.3 Mpa, which was monitored hourly by matric potential sensors.
Measurements of dry tissue root and shoot weights recapitulated the previous experiment—genotype did not significantly impact root or shoot weight, but water had a significant impact (Fig. 6C). The difference between well-watered and water deficit weights for each genotype was greater than the previous experiment (Fig. 6, A and C). Unlike the previous experiment at V5, the root/shoot ratio was significantly impacted by both genotype and water status (Fig. 6C). Agreeing with the V12 experiment, WT and Zmksl4 plants have indistinguishable root/shoot ratios under well-watered conditions but are significantly different under water-deficit conditions (Fig. 6C). While both genotypes do show increased root/shoot ratios under water deficit, WT has a significantly greater increase than Zmksl4 (Fig. 6C).
Representative images and their corresponding image mask of WT and Zmksl4 roots used for root trait analysis are shown in Fig. 7A. PCA analysis of root architecture traits showed separation of sample types by water status, but not by genotype (Fig. 7B). Separation was largely driven by root system surface area and width, leaf and root tip number, nodal root length and diameter, root density, and minimum lateral root branching angle, among others (Fig. 7C). Several specific root traits were affected under water deficit in both genotypes: Root system surface area, median and maximum root system width, and root tip number are among some of the most heavily affected traits under water deficit (Fig. 7D). While Zmksl4 roots do have lower values for all these traits, it needs to be considered that Zmksl4 plants are also smaller under optimal water conditions (Fig. 7D). Similarly, the total rooting depth remained unchanged by genotype, as well as the average lateral root length, and average lateral root diameter.
Figure 7.
Zmksl4 has altered root system architecture in V5 roots grown in turface. A) Representative images, and their corresponding binary DIRT inputs, of wild-type and Zmksl4 root systems under well-watered and water-stressed conditions, scale bars are 5 cm. B) PCA analysis on DIRT traits shows that groups separate based on watering treatment but largely overlap between genotypes. C) The 20 of the most impactful DIRT trait vectors for PCA separation. D and E) Specific root system traits impacted by genotype and water status; D) many traits were affected to a similar degree relative to each well-watered control, E) while others were affected differently in Zmksl4 under water stress. Data are means ± SE (n = 17 to 21). Different lettering represents significant differences at the 95% level determined by one-way ANOVA analysis. ww = well-watered, ws = water-stressed.
Specific traits were, however, significantly changed in the mutant (Fig. 7E). Under water-deficit conditions, Zmksl4 exhibited a significant increase in root system density compared to well-watered conditions, and compared to WT of either water status, up to 140% of well-watered levels. WT did not show increased root density upon water deficit. The root top angle, defining the angle of axial root trajectory from the horizontal soil line, was significantly decreased in Zmksl4 plants, showing the roots grow more steeply downwards under both water conditions, resulting in a narrower root system. A similar observation was made for the root bottom angle, describing the angle of root branching from the horizontal soil line at depth. Zmksl4 plants also showed a larger reduction in nodal root length in response to water deficit compared to WT.
To next investigate the root architecture phenotype in fully grown plants under field conditions, WT and Zmksl4 plants were grown in the field until full maturity (i.e. seed harvest) and assessed by the DiRT software (Fig. 8A). Root crowns of 17 WT and 22 Zmksl4 plants grown in Stanford, CA to full maturity were removed by uniform pulling of the plants until they were released from the soil. Zmksl4 showed a severe reduction in root size (Fig. 8A) and analysis of root architecture traits substantially separated samples by genotype in PCA (Fig. 8B). Compared to WT, Zmksl4 shows a reduced stem diameter and smaller root area, a lower width of the entire root system, both median and maximum, and a lower number of total roots (Fig. 8D). Additionally, Zmksl4 showed a wider root diameter and a greater root density. The root top angle, however, was unaffected, and because these were crowns and not complete root systems, the root bottom angle could not accurately be quantified. The Drop 50, the measurement of the depth where 50% of the root tips have emerged, was larger for WT, depicting WT with deeper roots than Zmksl4 (Fig. 8, A and D).
Figure 8.
Zmksl4 root crowns have significantly denser root systems. A) Representative samples, and their corresponding binary DIRT inputs, of wild-type and Zmksl4 mature root crowns, scale bars are 5 cm. B) PCA analysis on DIRT traits shows a clear separation of genotypes. C) The 20 of the most impactful DIRT trait vectors for PCA separation. D) Several important root architectural traits were shown to be different between wild-type and Zmksl4. Data are averages ± SE (n = 17 to 21). Levels of significance were determined by student's t-test; *P ≤ 0.01, **P ≤ 0.001, ***P ≤ 0.0001, ******P ≤ 0.0000001.
Zmksl4 mutant transcriptome responds differently than WT to water deficit
Transcriptome analysis of the Zmksl4 mutant plants was performed using V12 roots grown in field soil, on the same plants previously assayed for metabolite content and root/shoot weights described above (Fig. 2B), with a focus on the developmental stage with highest DX abundance to investigate connections between DX production and plant stress responses. Principal component analysis (PCA) demonstrated that while water deficit accounted for 73% of sample variance, genotype had a minimal effect of 6% (Supplemental Fig. S6A), separating the genotype sample clusters under water-deficit conditions but not under well-watered conditions (Supplemental Fig. S6A). Furthermore, in a heat map of sample-to-sample distances, sample types predominantly cluster by water status, with limited clustering due to genotype (Supplemental Fig. S6B). Consistent with the genotype and environment effects on untargeted metabolite profiling described above, the mutation in Zmksl4 had less of an effect on gene expression than did water status, and there are a small but significant number of DEGs affected by the genotype by water status interaction (Supplemental Dataset S2).
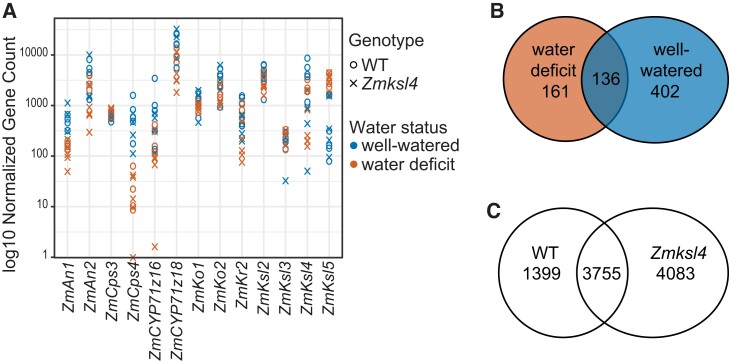
Next, differentially expressed genes (DEGs) were determined using the DESeq2 package in R (Supplemental Dataset S4). Among the analyzed diTPS and P450 genes, only ZmKsl4 was differentially expressed, as expected in the mutant (Fig. 9A, Supplemental Dataset S4). However, one known diterpenoid pathway gene, ZmKr2, was differentially expressed between WT and Zmksl4 under both well-watered and water-deficit conditions. None of the known GA-biosynthesis genes were significantly differentially expressed between WT and Zmksl4 under either water condition (P < 0.1) (Zhang et al. 2022). Of the currently known nine genes specifically impacting maize root architecture, RTH1 (ROOTHAIRLESS 1), RTH3, RTH, RTH5, RTH6, RUM1 (ROOTLESS WITH UNDETECTABLE MERISTEM 1), RTCS (ROOTLESS CONCERNING SEMINAL AND CROWN ROOTS), RTCL (RTCS-LIKE), RUL1 (RUM1-LIKE), and LRP1 (LATERAL ROOT PRIMORDIA 1) (Bray and Topp 2018), none showed differential expression between genotypes under water deficit or well-watered conditions. However, the gene Zm00001d033169, which is putatively orthologous to the Arabidopsis gene MORPHOGENESIS OF ROOT HAIR 6 (MRH6), was significantly down-regulated in Zmksl4 (P < 0.0001). Of the 45,808 genes with nonzero expression, 136 genes were differentially expressed between Zmksl4 and WT regardless of water status (Fig. 9B, Supplemental Dataset S4). Of these DEGs due to the mutation (Supplemental Dataset S4), there was no significant enrichment for any Gene Ontology (GO) terms. Genes down-regulated in Zmksl4 roots under both stress conditions included transcription factors C2C2-GATA-TRANSCRIPTION FACTOR 15 (GATA15), OPAQUE2 HETERODIMERIZING PROTEIN1 (OHP1), AND GNAT-TRANSCRIPTION FACTOR 7 (HAGTF7). INDOLE-3-GLYCEROL PHOSPHATE LYASE1 (IGL1), a gene involved in benzoxazinoid biosynthesis, was also down-regulated, along with NITRATE REDUCTASE 4 (NNR4), GRANULE-BOUND STARCH SYNTHASE-I which is more commonly known as WAXY1 (WX1), and PURPLE ACID PHOSPHATASE16 (PAP16) that is involved in plant phosphorous processes. Transcript levels of Zm00001d003716, putatively orthologous to the Arabidopsis gene USUALLY MULTIPLE ACIDS MOVE IN AND OUT TRANSPORTERS 25 (AT1G09380, UMAMIT25) were also strongly suppressed in Zmksl4 roots, suggesting broader alteration of transport processes.
Figure 9.
Root transcriptome analysis of Zmksl4 mutants reveals a complex core of 136 DEGs independent of plant water status, suggesting that the mutation impacts different processes and pathways yet without clear enrichments observed. A) Plot of normalized gene counts for diterpenoid pathways; only ZmKsl4 is a differentially expressed gene (DEG). B) Number of DEGs impacted by mutation for each water status; overlap is genes differentially expressed regardless of water status. C) Number of genes differentially expressed due to water status for each genotype, respectively; overlap is genes differentially expressed due to water status in both genotypes. Genes in B) and C) are listed, along with fold change values, in Supplemental Dataset S4.
As demonstrated for statistically significant enriched metabolites (P < 0.1), water status impacts each individual genotype differently: the number of genes commonly affected by water status in both genotypes was low, suggesting unique responses, and the number of genes affected in Zmksl4 was greater (Fig. 9C, Supplemental Dataset S4). Furthermore, GO terms enriched in the DEGs by water status in each genotype, independently, were largely different, supporting the different responses to water stress in the mutant (Supplemental Dataset S5).
Discussion
Diterpenoids have important functions in species-specific plant defense and environmental adaptation. In maize, KX and DX metabolites form two pathway branches with demonstrated or predicted roles in biotic and abiotic stress responses, as evidenced in previous work by the susceptibility of the Zman2 and Zmksl2 mutants to different stressors, the impact of KX and DX deficiency on the rhizosphere microbiome, the stress-inducibility of pathway transcripts and metabolites, and the in vitro antifungal activity of select metabolites (Schmelz et al. 2011; Christensen et al. 2018; Mafu et al. 2018; Ding et al. 2019; Murphy et al. 2021) (Fig. 1). In the current effort, we created a Zmksl4 knock-out mutant with selective DX-deficiency, enabling us to isolate the roles of DX from interconnections with ZmAN2 and KX metabolism. The lack of DX metabolites in Zmksl4 demonstrates that ZmKSL4 is the sole enzyme responsible for DX biosynthesis and that without other major impacts on the global metabolome or transcriptome, the mutant is suitable for analyzing the stress-protective relevance of DXs in maize (Fig. 2).
Comparative metabolite profiling of the Zmksl4 mutant and its WT sibling demonstrate that DX encompass a larger metabolite family than previously identified. Here we predict up to 48 additional metabolites (Supplemental Dataset S1, Supplemental Figs. S2 and S3) belonging to the DX family based on their absence in Zmksl4 roots and their abundance in WT roots at V12. Because multiple features may belong to the same metabolite, or multiple metabolites may co-elute at the same RT, this number represents an estimate to guide further study. Many of these enriched features had the same parent mass and similar MS2 spectra, but different RTs, suggesting they are either isomers or that some of the fragment ions are identical, which is expected of metabolites in a pathway based on the same hydrocarbon scaffold.
NMR structural analysis of additional products of the combined activity of ZmAN2, ZmKLS4, and the P450 ZmCYP71Z18 (Supplemental Fig. S4) identified one of these metabolites as the dolabradiene-derivative, dolabradienol, that we subsequently confirmed as highly abundant in roots of maize grown with field soil (Figs. 2C and 3). Absence of dolabradienol in enzyme assays using the closely related ZmCYP71Z16 support catalytic differences between these otherwise highly promiscuous P450s, both of which generate epoxydolabranol as the dominant product. The broader range of DX metabolites in mature maize roots is well aligned with the largely root-specific expression of relevant DX pathway genes as shown here and in prior studies (Mafu et al. 2018). This further supports a below-ground function of DX metabolism, contrasting the more tissue-wide distribution of the KX pathway despite their shared use of ZmAN2 (Schmelz et al. 2011; Christensen et al. 2018; Mafu et al. 2018; Ding et al. 2019).
Our observation that DX metabolites are produced in mature roots of several maize inbred lines grown in field soil, yet significantly less abundant in plants grown in peat moss (P < 0.05), suggests the presence of field soil factors plays a role in DX production, which we hypothesize to include microorganisms (Fig. 5). While select DX metabolites have been shown to be antibiotic in vitro, the number of additional potential roles in complex biotic and environmental interaction in the soil is myriad (Mafu et al. 2018; Ding et al. 2021; Murphy et al. 2021). Prior analyses of Zman2 mutants, now appreciated to be deficient in both KX and DX, demonstrated maize root diterpenoids collectively influence the rhizosphere microbiome, and support a collective role of root DX metabolites in plant-microbe interactions (Murphy et al. 2021). In contrast to earlier studies with young maize plants and root KX (Vaughan et al. 2015), our current study demonstrates that unlike KX, water deficit does not induce the accumulation of DX metabolites, but rather these metabolites are highly abundant in older plants grown in field soils under both well-watered and water-deficit conditions tested here (Fig. 2C and 5). Maize DX displays a high degree of root specificity and contrast KX metabolites, which are found both above and below ground at the local site of pathogen or pest attack in leaves, stems, and roots (Schmelz et al. 2011; Vaughan et al. 2015; Ding et al. 2019). Transcript and metabolite profiling of a range of maize inbred lines showed substantial diversity of DX formation across maize inbred lines (Fig. 5). For example, MoG roots produce >20-fold more DX than B73 roots at the same stage when grown in a greenhouse using field soils (Fig. 5). Select maize inbreds appear to produce and rely on different diterpenoid blends under different environmental conditions which provides an opportunity to investigate how distinct diterpenoids contribute to stress resilience and ultimately leverage such insights for understanding chemical defense traits and potential roles in breeding.
Although no increase in DX levels was detected in response to water deficit, phenotypic analysis of the DX-deficient Zmksl4 mutant revealed a reduced root/shoot ratio and reduced responsiveness to water deficit as compared to the WT sibling in both V5 and V12 roots, depending on the experiment (Fig. 6). Previous research at the seedling stage showed the Zman2 mutant to have a reduced root/shoot ratio under drought stress, and increased KX levels (DX were unknown at the time of the study and not detected) (Vaughan et al. 2015). In our current effort, Zmksl4 demonstrated a genotype by environment (GxE) interaction, with a different response to water deficit than WT in regard to its root/shoot ratio and changes in the metabolome and transcriptome. For example, the metabolites and transcripts that were differentially present under water deficit were largely different in Zmksl4 and WT, and Zmksl4 had a greater number of metabolites and unique transcripts changed (Supplemental Datasets S1 and S4). These changes could be causing the differences in root architecture or could be a downstream effect of the altered architecture.
Given the root predominance of DX metabolism, and the altered root/shoot ratio, analysis of the root architecture of WT and Zmksl4 plants in water-deficit and well-watered conditions was used to investigate how DX metabolites may impact plant vigor in Zmksl4 mutant plants. Even under optimal conditions, Zmksl4 in the early vegetative and fully mature stages demonstrated stunted growth in nearly all examined root traits, and mature root crowns of Zmksl4 were significantly denser than WT (Figs. 7 and 8). While both WT and Zmksl4 showed many classical water deficit responses, such as a reduction in stem diameter, root system surface area, and the total number of roots, only Zmksl4 showed distinct stress symptoms, including most notably an up to 140% increase in root system density (Figs. 7 and 8). This response was not seen in WT roots and is likely a result of the compounding effects of adopting a sharper rooting angle under the imposed stress while maintaining a similar level of root number to WT. However, it is important to note that many other root traits were affected in similar ways between genotypes when accounting for the change in the trait value between well-watered and water-deficit rather than the absolute difference between WT and Zmksl4 under stressed conditions. This suggests many of the differences observed in root architecture are due to overall environmental effects which may be more important than differential genotypic responses to water deficit specifically. The mutant phenotype suggests DX plays a role in root growth and biomass, which in turn impacts plant vigor with regards to the root/shoot ratio under water-deficit conditions (Fig. 6).
We hypothesize that observed differences in root architecture could result from altered layers of biochemical immunity, gene expression levels, and/or changes in the rhizosphere microbiome assembly, which includes plant-pathogen interactions. A modest level of significant changes in the Zmksl4 transcriptome compared to WT, 136 genes independent of water status (Fig. 9), speak against large-scale changes in the regulation of root development. However particular DEGs could be directly impacting the root architecture, such as the transcription factors GATA15, OHP1, and HAGTF7, all of which were significantly down-regulated in Zmksl4 in both water conditions. Another candidate of interest is the Zmksl4 suppression of Zm00001d033169, putatively orthologous to Arabidopsis MRH6, which has been implicated in root hair development. Changes in plant nutrient acquisition, processing, and transport could also be a cause or effect of the altered root architecture; both NNR4 and PAP16 were down-regulated and played roles in nitrate and phosphorous nutrition, respectively. Displaying opposite transporter responses, Zmksl4 mutants demonstrated a suppression of Zm00001d003716 (Arabidopsis putatively ortholog, UMAMIT25) transcripts and increased accumulation of Zm00001d019131 encoding an ATP-binding cassette protein (Supplemental Dataset S4). Given that most genes in the dataset remain unannotated, however, the precise causal effects of root architecture changes in Zmksl4 remain to be investigated in future work. Complex interactions such as compositional microbiome changes in the DX- and KX-deficient Zman2 mutant (Murphy et al. 2021) are supportive of DX roles in microbiome interactions which could further impact root architecture as an important component of water stress resilience, as plants navigate the soil for optimum water and nutrient uptake. Altered GA production is an alternative hypothesis for changes in the root architecture and water deficit response in the Zmksl4 mutant, considering that the GA biosynthesis derives from a shared upstream pathway. However, no GA-biosynthetic transcripts were up- or down-regulated in the Zmksl4 mutant, and GA metabolites were not detected in our LC-MS/MS dataset. Whether changes in DX production impact maize gene expression directly, or indirectly via impacts on the rhizosphere microbiome, and whether the root architecture is a cause or product of either changes remains to be investigated in detail.
The mechanisms underlying DX-mediated changes in root development and overall plant vigor can be further understood following identification of the larger and more complex network of terminal DX pathway products made by roots and void from Zmkls4 mutants (Supplemental Dataset S1). Future robust functional studies require defined mutants and sufficient purified pathway products. DX metabolites exist as important metabolites in the roots of mature maize plants grown in field soils yet are uncommonly observed in standard greenhouse conditions. To date, pathogen-induced regulation of the DX pathway only partially matches established patterns for the KX pathway activation (Mafu et al. 2018; Ding et al. 2019) which collectively implies biological roles for DX beyond antifungal defenses. Our collective findings create a foundation to elucidate the tissue- and pathway-specific functions of DXs that ultimately could inform stress resilience breeding and engineering strategies in maize.
Materials and methods
Generation of a maize Zmksl4 loss-of-function mutant
Zmksl4 mutant plants and isogenic WT sibling plants were generated as previously described (Char et al. 2016). In short, guide RNA uniquely targeting two genes, ZmKsl4 and ZmCYP71Z16, was inserted into the pGW plasmid (based on the pMCG1005 backbone) containing the rice codon-optimized Streptococcus pyogenes Cas9 under control of the maize ubiquitin 1 promoter, and the bar gene under control of the 4xCaMV35S promoter as a selectable marker. The resulting construct was transformed into the Hi-II maize hybrid line using Agrobacterium tumefaciens, strain EHA101 (Char et al. 2016). To generate the gene-specific Zmksl4 mutant, transgenic plants were then outcrossed to B73 and genotyped to select for plants that lacked the Cas9 gene, were heterozygous for a one base pair insertion in ZmKsl4, and contained no mutation in ZmCYP71Z16. Genotyping was performed using PCR for Cas9, ZmKsl4, and ZmCYP71Z16 using gene-specific primers and Phusion polymerase (New England BioLabs) according to manufacturer instructions. The presence or absence of Cas9 was used for genotyping. For ZmKsl4 and ZmCYP71Z16, PCR products were analyzed using PCR clean-up and sanger sequencing for the region of interest. An insertion of G or A at position 354 bp in the first exon of ZmKsl4 was verified, and a WT allele for ZmCYP71Z16 without any mutations, resulting in a mutant featuring two different alleles (insertion of G or A) that cause a frameshift rendering the protein truncated and predictively nonfunctional. Additional ZmCYP71Z16 mutants were not pursued in this study, and all alleles were WT for this gene, as determined by gene sequencing. Two plants, each without Cas9 and heterozygous for a G or A insertion in one allele and no insertion in the second allele, were crossed and genotyped, generating a 1:2:1 segregating population for homozygous-mutant:heterozygous:homozygous-WT (selfing was not possible because of developmental delays between ears and tassels, thus two separate ones were crossed). Two homozygous-mutant plants, each containing a G insertion in one allele and an A insertion in the other, were crossed (selfing was not possible due to developmental delays) to generate seed used in this study. All seed used in this study was homozygous for an insertion of an A or G in both alleles. One WT plant was selfed for use as a WT sibling. LC-MS/MS of the mutant and WT sibling described in this study confirm the absence of DX in Zmksl4 and its presence in its WT sibling.
Plant growth and materials for drought elicitation
Maize plants were grown at UC Davis field site (Davis, CA, USA). To control water-deficit conditions, field soil used for maize cultivation the prior year was sieved and placed into 3-gallon fabric pots on the field site. Seeds were sterilized by incubation with 15% bleach for 15 min and rinsed thrice with sterile water. One seed per pot was planted by hand. Plants were irrigated using controllable irrigation drip tape. For timepoint 1, plants were grown for 30 d before half of the plants were subjected to 6 d of water deficit, consisting of half the water provided via irrigation. At the end of timepoint 1, well-watered plants were at the V5 stage. For timepoint 2, plants were grown for 61 d before half of the plants were subjected to 6 d of severe water deficit, in which they were provided half of the water via irrigation. Water-deficit conditions for both timepoints were confirmed by a characteristic leaf-rolling phenotype. Well-watered plants were at the V12 stage for timepoint 2. Plants were fertilized twice during the growing season using solubilized ammonium phosphate, potassium nitrate, and ammonium sulfate through the irrigation system so that each plant received, in total, 14.5 g of NaCl, 2.5 g of N, 0.5 g P2O5, and 2.5 g of K2O.
On the day of harvest (day 36 for V5; day 67 for V12), plants were removed from their pots and roots were washed gently with water to minimize root damage and photographed. Plants were cut at the root-shoot junction and weighed for plant fresh weights. Washed roots and the uppermost leaf with a leaf collar were collected separately, frozen immediately in liquid N2, and stored at −80 °C for downstream analysis. For each sample collection, the biological replicates were as follows: V5: n = 4 (WT, well-watered), n = 7 (WT, water deficit), n = 7 (Zmksl4, well-watered), n = 6 (Zmksl4, water deficit). V12: n = 5 (WT, well-watered), n = 4 (WT, water deficit), n = 5 (Zmksl4, well-watered), n = 6 (Zmksl4, water deficit).
To consider the interactions of maize genotypes and soil types on resulting DX production, all maize inbreds (PHW65, Oh43, MoG, B73, W22) were grown in the greenhouses at the UCSD Biology Field Station for 40 d. Plants were grown in either commercial peat moss-based potting soil (BM2; Berger Corp) or a 1:1 mixture of local maize field soil and potting soil. Potted plants were removed from the soil, root systems were washed with H2O, frozen with liquid N2, ground in liquid N2 using a mortar and pestle to a fine powder, and stored at −80 °C. Working with liquid N2 and −80 °C is essential to halt all enzyme activities and maintain the stability of potentially labile analytes.
P450 functional analysis
Functional characterization of ZmCYP71Z18 was performed using E. coli co-expression assays as previously described (Murphy et al. 2019). In brief, the following plasmids were transformed into BL21DE3-C41 cells (Lucigen): codon-optimized pET-DUET1:ZmCPR2/ZmCYP71Z18 or ZmCYP71Z16; pET28b:ZmKSL4Δ106; pCOLA-DUET:GGPP-synthase:ZmAN2; pIRS plasmid of upstream pathway as described previously (Morrone et al. 2010; Mafu et al. 2018). Transformed E. coli was grown in 50 mL of Terrific Broth at 37 °C until an OD600 of ∼0.6 was reached. Cultures were cooled to 16 °C and protein production was induced with 1 mM isopropyl-thiogalactoside and supplemented with 25 mM sodium pyruvate, 4 mg/L riboflavin, and 75 mg/L δ-aminolevulinic acid. Enzyme products were extracted with 50 mL of 1:1 hexanes:ethyl acetate and a separatory funnel, and concentrated under N2 stream. Enzyme products were analyzed using GC-MS and NMR.
Root metabolite extraction
Collected leaf and root samples from the Davis field site were frozen in liquid N2 and homogenized twice in 30-sec increments with freezing in liquid N2 in between using a tissue homogenizer (Retsch). The frozen, pulverized tissue was weighed, and methanol with 0.01% formic acid was added at 10:1 volume:tissue weight for metabolite extraction. Samples were gently shaken at room temperature for 24 h, after which 2 mL of extract were removed to a glass vial and stored at −80 °C. A total of 200 µL of extract was then filtered into a 96-well plate using a filter plate to remove any possible particulates. V5: n = 4 (WT, well-watered), n = 7 (WT, water deficit), n = 7 (Zmksl4, well-watered), n = 6 (Zmksl4, water deficit). V12: n = 5 (WT, well-watered), n = 4 (WT, water deficit), n = 5 (Zmksl4, well-watered), n = 6 (Zmksl4, water deficit).
GC-MS analysis
GC-MS analysis of E. coli generated metabolites was performed on an Agilent 7890B gas chromatograph with a 5977 Extractor XL MS detector at 70 eV and 1.2 mL/min He flow, using an Agilent HP5-MS column (30 m, 250 mm i.d., 0.25 mm film) as previously described (Mafu et al. 2018; Murphy et al. 2019). Samples were suspended in 1 mL of hexanes, with 1 µL injection volume, pulsed splitless injection at 250 °C inlet temperature. The oven temperature was ramped from 50 °C to 300 °C at 20 °C/min and held for 3 min. MS data from 90 to 600 m/z were collected after a 10-min solvent delay.
For analysis of maize plants grown in different soil types, a simple approach to sample analysis relied on Vapor Phase Extraction (VPE) to remove high molecular weight analytes otherwise uncompatible with gas chromatography (GC) (Schmelz et al. 2004). In this modified VPE procedure, 50 mg sample aliquots finely ground in liquid nitrogen were weighed, extracted with 1 ml 1-propanol:hexane (3:10) in a 4 mL glass vial, and the resulting organic phase was derivatized using trimethylsilyl diazomethane (Schmelz et al. 2004). Following 20 min for derivatization, all liquids were dried under a N2 stream with care taken to avoid over-drying which could result in a loss of diterpenoid analytes (Schmelz et al. 2004). The modified sample preparation avoids the use of plasticware at all steps before VPE thus minimizing all sources of plasticizer contamination. Final analytical samples were eluted from the VPE traps using 150 ml of 1:1 hexane:ethyl acetate (Schmelz et al. 2004). GC/MS analyses were conducted using an Agilent 6890 series gas chromatograph joined to an Agilent 5973 mass selective detector (mass temperature, 150 °C; interface temperature, 250 °C; electron energy, 70 eV; source temperature, 230 °C). DB-35 MS column (Agilent; 30 m × 250 μm × 0.25 μm film) was used for gas chromatography. Samples were introduced with an initial oven temperature of 45 °C, as a splitless injection. The temperature was held for 2.25 min, then increased to 300 °C with a gradient of 20 °C min−1 and held at 300 °C for 5 min. A solvent delay of 4.5 min was selected to prevent ethyl acetate present in the sample from damaging the EI-filament. GC-MS-based quantification of DX utilized an external standard curve of dolabradienol that was spiked into similar root tissue samples lacking DX metabolites. Product identification of previously known analytes was conducted using authentic standards. Agilent Mass Hunter Qualitative and MS Quantitative Analysis software alongside Agilent ChemStation qualitative programs were used to generate and analyze the GC-MS generated chromatograms and spectra. Replicated experiments were summarized with peak areas captured in MassHunter Qualitative Navigator B.08.00, and MS Quantitative Analysis B.08.00, quantified in Excel, and statistically evaluated in JMP. MassHunter MS Quantitative program peak selection methods were manually produced taking into account RT shifts. Program-automated peak selections/integrations were instead substituted for manual selections/integrations of every target compound in every sample.
For purifying dolabradienol (2R,4bS,7S,10aR)-4b,7,10a-trimethyl-1-methylene-7-vinyltetradecahydrophenanthren-2-ol for use as a standard to quantify metabolite levels in testing maize plants in different soil types, 300 g of 35-d-post-pollination field-grown MoG root tissue was ground to a fine powder in liquid N2, extracted first with 500 mL of methanol then secondarily extracted with 500 mL of ethyl acetate (EA), filtered and dried using a rotary evaporator. The resulting oily residue was then allowed to partition and redissolve in 300 mL EA. The EA fraction was then dried down and the resulting oily residue was then separated by preparative flash chromatography (CombiFlashRf; Teledyne ISCO) on a 5 g C18, reverse phase, (RediSepRf High Performance GOLD) column. The mobile phase consisted of solvent A (100% H2O) and solvent B (100% Acetonitrile), with a continuous gradient of 0% to 100% B from 1 to 81 min using a flow rate of 18 mL min−1. 100 × L aliquots of these fractions were then derivatized using trimethylsilyldiazomethane, dried, then resolubilized in 200 mL of 1:1 hexane:EA and analyzed by GC/MS. One fraction spanning 42 to 43 min contained an enrichment of dolabradienol. Select concentrated samples were further purified by high performance liquid chromatography HPLC using repeated methylated/derivatized (using trimethylsilyldiazomethane) and 1 mL injections (passed through silica to filter out precipitate following derivatization) on a Zorbax RX-silica (250 × 4.6 mm, 5 µm; Agilent) column and a mobile phase consisting of solvent A (100% hexanes) and solvent B (100% EA) with a continuous gradient of A–B from 2 min to 37 min using a flow rate of 1 ml min−1. The recollected HPLC fractions spanning 11 min to 12 min retention times (RT) yielded dolabradienol at ∼95% purity.
LC-MS/MS analysis
Plant metabolites were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) with UHPLC reverse phase chromatography performed using an Agilent 1290 LC coupled with a QExactive Orbitrap MS (QE = 139) (Thermo Scientific, San Jose, CA). Samples were kept at 4 °C before injection. Chromatography was performed using a C18 column (Waters Acquity UPLC BEH, 1.7 µm, with VanGuard Pre-Column, 2.1 × 5 mm) at a flow rate of 0.4 mL/min and injection volume of 5 µL, with a column temperature of 60 °C. The column was equilibrated with 100% buffer A (100% LC-MS water w/0.1% formic acid) for 2 min, followed by a linear dilution of buffer A down to 0% with buffer B (100% acetonitrile w/0.1% formic acid) over 7 min, and followed by isocratic elution in 100% buffer B for 1.5 min. Full MS spectra were collected ranging from m/z 80–2,000 at 60,000 to 70,000 resolution in both positive and negative mode, with MS/MS fragmentation data acquisition using an average of stepped 10-20-40 and 20-50-60 eV collision energies at 17,500 resolution. For targeted analysis, product identification by comparison to standards was performed where authentic standards were available. When possible, standards were weighed and serially diluted to generate standard curves for quantification.
For untargeted analysis, exact mass and retention time coupled with MS/MS fragmentation spectra were used to identify compounds. Features—high intensity signals narrowly contained at a given retention time and m/z—were detected using the MZMine software version 2.24 (http://dx.doi.org/10.1093/bioinformatics/btk039). First, in MZMine, peaks were detected using a centroid method, removing background peaks below 1 × 105 for MS1 and 1 × 103 for MS2. Chromatograms were built using the ADAP Chromatogram builder, with a minimum of 5 scans per peak, a 10 ppm m/z tolerance, and group intensity threshold of 3 × 105. Peaks were deconvoluted, isotopes were grouped, and peaks were aligned between different samples. The resulting feature list was filtered such that a minimum of 2 samples contained the identified feature, and gaps were filled at 10% intensity tolerance. Features with a maximum peak height out of all samples less than 3 times the peak height of the corresponding feature in the blank control were discarded as background.
Generalized linear models were used to find features that showed a significantly different abundance (measured by peak height). These models were calculated using custom R scripts and the lm() function (R Core Team 2019), with statistical analysis results in Supplemental Dataset S2 and significantly enriched or depleted features listed in Supplemental Dataset S1. Generalized linear models are linear regression models used to determine if a particular feature is significantly different in abundance between two genotypes. All features were annotated using global natural products social molecular networking (GNPS) (Katajamaa et al. 2006; Pluskal et al. 2010; Ono et al. 2014; Wang et al. 2016; Nothias et al. 2019). In short, a feature-based molecular networking workflow was used to assign features to a molecular network with a cosine score above 0.7 and more than six matched peaks. The maximum size of a molecular family was 100, and low-scoring edges were removed to meet this threshold. The spectra were then searched against GNPS spectral libraries and annotated with the top hit if there was one. Complete annotations, features present, and Cytoscape visualization networks are available online for positive mode (https://gnps.ucsd.edu/ProteoSAFe/status.jsp? task=b55e139a9644496aa65aed179858ada7) and negative mode (https://gnps.ucsd.edu/ProteoSAFe/status.jsp? task=417de81fa3e04daf8e72d3465fe208d6). All scripts are available on GitHub (https://github.com/kmurphy61/ksl4/tree/main). Lists of significantly different features enriched or depleted in each sample type are available in Supplemental Dataset S1.
NMR analysis
For structural elucidation of dolabralexin metabolites, compounds were produced in E. coli as described above but scaled to 1 L cultures and concentration of hexane extracts using a rotary evaporator. Approximately 12 cultures were used to produce and purify sufficient compound amounts, followed by purification using silica column chromatography with a hexane:ethyl acetate gradient as previously described (Murphy et al. 2019). Products were controlled for purity using GC-MS, and high-quality samples were pooled and again concentrated by rotary evaporation. Compounds were then further purified to ≥98% purity by HPLC (Agilent 1100 Series) equipped with a diode array UV detector, and an Agilent ZORBAX Eclipse C18 semipreparative column. A 4 mL/min flow-rate of an acetonitrile:water gradient as mobile phase was used. Purified products were confirmed via GC-MS, concentrated under N2 stream, and dissolved in 0.5 mL of deuterated chloroform. NMR spectra were acquired on a Bruker Avance III 800 spectrometer with a 5-mm triple resonance cry probe at 25 °C. Acquired spectra, including 1D 1H, 2D HSQC, correlation spectroscopy, HMBC, and 1D 13C (201 MHz) were performed using TopSpin 3.2 software and default parameters. Deuterated chloroform was used as a reference for chemical shift calculations (13C 77.23 ppm, 1H 7.24 ppm).
Root architecture analysis
Zmksl4 and WT maize seeds were planted in potting mix filled germination trays and at four days old were transplanted into 18-inch tall 6-inch-wide tree pots. The pots were filled with turface MVP pre-calibrated to either well-watered status or to a matric water potential of −0.7 MPa. Calibration was accomplished by mixing the turface with water to a specific water content in a separate tub before filling the pots. Following this, each pot was weighed, and the hydration level was maintained throughout the experiment by watering each pot up to weight daily. Well-watered plants were watered to the drip point and could freely drain. Matric potential was monitored hourly using TEROS21 sensors (METER Group, Inc., Pullman, WA, USA) to assure acceptable levels of stress were being imposed. Four pots per treatment were equipped with TEROS21 sensors. At harvest, each root system was carefully removed from the pots and all the turface was gently washed off using water. Roots were then imaged using the DIRT analysis software (Das et al. 2015).
Mature root crowns were harvested from Zmksl4 and WT maize plants grown to maturity (i.e. seed harvest) at a maize field site in Stanford, CA. Root crowns were harvested by manually pulling plants from the soil, and removing the plant 6 inches from the root/shoot junction and above. Roots were shaken manually to remove large soil clumps, washed clean in a bucket of water, and air-dried at room temperature. As above, roots were then imaged using the DIRT analysis software (Das et al. 2015). PCA, ANOVA, and t-test analyses for all root architectures were conducted in OriginLab 2020.
RNAseq analysis
Total maize RNA was extracted from frozen, homogenized plant tissue prepared as outlined above using a Qiagen RNEasy Plant Mini Kit and associated instructions, including an on-column DNAse treatment (Qiagen RNAse-free DNAse set). These were the same plants used for LC-MS/MS V12: n = 5 (WT, well-watered), n = 4 (WT, water deficit), n = 4 (Zmksl4, well-watered), n = 5 (Zmksl4, water deficit). Library preparation and sequencing were performed by the Vincent J. Coates Genomics Sequencing Laboratory and Functional Genomics Laboratory (UC Berkeley, CA, USA) according to standard protocols. First, RNA was quantified and quality-tested on a Femto Pulse; RNA quality number (RQN) was between 4.6 and 8.2. Kapa Biosystems library preparation kits and custom Unique Dual Indexes were used for library preparation. Sequencing was performed on Illumina NovaSeq 6000 150PE Flow Cell S4 (1% PhiX control run) with paired-end sequencing. Raw sequencing data is available via National Institutes of Health Sequence Read Archive (SRA), SRA archive submission number BioProjectID: PRJNA810958. The percent quality score of 30 or higher (%QC30) was between 90.44% and 93.8%. The mean quality scores ranged from 35.15 to 35.91, and yield was between 7,299 and 62.282 Mbases. The clusters passing filter (%PF) was 100% for every sample, and the percent perfect barcode was between 87.57% and 98.21%. For RNAseq analysis, the raw reads were paired and adapters were trimmed using the trim_galore function and low-quality reads were removed based on standard parameters. Specifically, reads were mapped to the maize v4 reference (B73 RefGen_v4) genome using a Burrows-Wheeler alignment (mem) alignment, with a mapping quality cutoff of 40 to reduce multimapping, with samples ranging between 15,941,503 and 145,800,405 high-quality mapped reads per sample. Reads were counted using htseq for a total of 47,289 aligned genes (Dobin et al. 2013; Li 2013; Portwood et al. 2019). Downstream analysis was performed using the DESeq2 package in R (Anders et al. 2013), with all code available in GitHub (https://github.com/kmurphy61/ksl4/tree/main). Specifically, the DESeq2 package with “ashr” was used for calculating log fold change using shrinkage (Stephens 2017). Resulting DEG lists were aligned with B73 v3 gene IDs and, where available, GO terms, classical gene names, and putative orthologs were annotated. All resulting lists of DEGs and associated statistics are listed in Supplemental Dataset S4.
For Gene Ontology (GO) term enrichment analysis, GO terms were analyzed using GO Ontology database (DOI: 10.5281/zenodo.4735677 Released 2021-05-01) and the PANTHER Overrepresentation Test (Released 20210224) for up- and down-regulated DEG v4 IDs, separately. The Zea mays (all genes in database) were used as a reference list and GO biological processes (complete) were used as the annotation data set (Supplemental Dataset S5). Fisher's exact test with a Bonferroni correction for multiple testing was employed.
Analysis of publicly available transcriptomes
RNAseq data were accessed from published datasets, detailed in the respective publications (Chen et al. 2014; Stelpflug et al. 2016; Tai et al. 2016; Walley et al. 2016; Kremling et al. 2018; Yi et al. 2019). Custom R scripts, available at https://github.com/kmurphy61/ksl4/tree/main with all R scripts for this study, were used to filter data for the pathways of interest, with Gene IDs available in Supplemental Table S1. FPKM or RPKM values were plotted using the ggplot2 package in R.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers listed in Supplemental Table S1.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Dr. Reuben Peters (Iowa State University) for providing the pIRS construct.
Contributor Information
Katherine M Murphy, Department of Plant Biology, University of California-Davis, Davis, CA 95616, USA.
Tyler Dowd, Donald Danforth Plant Science Center, St. Louis, MO 63132, USA.
Ahmed Khalil, Section of Cell and Developmental Biology, University of California San Diego, La Jolla, CA 92093, USA.
Si Nian Char, Division of Plant Science and Technology, University of Missouri, Columbia, MO 65211, USA.
Bing Yang, Donald Danforth Plant Science Center, St. Louis, MO 63132, USA; Division of Plant Science and Technology, University of Missouri, Columbia, MO 65211, USA.
Benjamin J Endelman, Department of Plant Biology, University of California-Davis, Davis, CA 95616, USA.
Patrick M Shih, Environmental Genomics and Systems Biology Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA; Department of Plant and Microbial Biology, UC Berkeley, Berkeley, CA 94720, USA.
Christopher Topp, Donald Danforth Plant Science Center, St. Louis, MO 63132, USA.
Eric A Schmelz, Section of Cell and Developmental Biology, University of California San Diego, La Jolla, CA 92093, USA.
Philipp Zerbe, Department of Plant Biology, University of California-Davis, Davis, CA 95616, USA.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Table S1 . Zea mays V4 gene IDs used in this study and their abbreviations.
Supplemental Dataset S1 . Metabolite features for enriched LC-MS/MS peaks.
Supplemental Dataset S2 . Statistics and raw weight data associated with this study.
Supplemental Dataset S3 . Publicly available transcriptomic data of diterpenoid-related genes were used in this study.
Supplemental Dataset S4 . DEGs (differentially expressed genes) enriched or depleted in each sample type.
Supplemental Dataset S5 . GO (gene ontology) terms enriched for DEGs (differentially expressed genes) in this study.
Supplemental Figure S1 . Metabolite atlas for LC-MS/MS with available standards.
Supplemental Figure S2 . LC-MS/MS spectra of DX metabolites (i.e. not Supplemental Figure S1 for which standards were available) enriched in WT roots, but absent in Zmksl4, at V12 and for which MS/MS spectra were available in at least one WT V12 sample.
Supplemental Figure S3 . Boxplots of feature abundance of DX candidates (i.e. the features commonly enriched in V12 WT root samples for which MS2 were available) in root samples or leaf samples.
Supplemental Figure S4 . NMR analysis of dolabradienol.
Supplemental Figure S5 . Root metabolic features (LC-MS/MS) affected by water status in Zmksl4 and WT.
Supplemental Figure S6 . Global transcriptome changes in WT and Zmksl4.
Funding
Financial support for this work was provided by the National Science Foundation (NSF) Plant-Biotic Interactions Program (Award# 1758976 to EAS and PZ), the NSF Graduate Research Fellowship Program (to KMM), the UC Davis Innovation Institute for Food and Health (IIFH) Fellowship Program (to KMM), and the USDA National Institutes for Food and Agriculture Predoctoral Fellowship Program (Award# 2019-67011-29544 to KMM).
References
- Anders S, McCarthy DJ, Chen Y, Okoniewski M, Smyth GK, Huber W, Robinson MD. Count-based differential expression analysis of RNA sequencing data using R and bioconductor. Nat Protoc. 2013:8(9):1765–1786. 10.1038/nprot.2013.099 [DOI] [PubMed] [Google Scholar]
- Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS, Meeley RB, Briggs SP. Cloning and characterization of the maize An1 gene. Plant Cell 1995:7(1): 75–84 10.1105/tpc.7.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AK, Vaughan MM, Schmelz EA, Christensen SA. Biosynthesis and function of terpenoid defense compounds in maize (Zea mays). Planta 2019:249(1):21–30. 10.1007/s00425-018-2999-2 [DOI] [PubMed] [Google Scholar]
- Bray AL, Topp CN. The quantitative genetic control of root architecture in maize. Plant Cell Physiol. 2018:59(10):1919–1930. 10.1093/pcp/pcy141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Char SN, Neelakandan AK, Nahampun H, Frame B, Main M, Spalding MH, Becraft PW, Meyers BC, Walbot V, Wang K, et al. An Agrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize. Plant Biotechnol J. 201615(2): 257–268. 10.1111/pbi.12611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zeng B, Zhang M, Xie S, Wang G, Hauck A, Lai J. Dynamic transcriptome landscape of maize embryo and endosperm development. Plant Physiol. 2014:166(1):252–264. 10.1104/pp.114.240689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SA, Sims J, Vaughan MM, Hunter C, Block A, Willett D, Alborn HT, Huffaker A, Schmelz EA. Commercial hybrids and mutant genotypes reveal complex protective roles for inducible terpenoid defenses in maize. J Exp Bot. 2018:69(7):1693–1705. 10.1093/jxb/erx495 [DOI] [PubMed] [Google Scholar]
- Das A, Schneider H, Burridge J, Ascanio AK, Wojciechowski T, Topp CN, Lynch JP, Weitz JS, Bucksch A. Digital imaging of root traits (DIRT): a high-throughput computing and collaboration platform for field-based root phenomics. Plant Methods 2015:11(1):51. 10.1186/s13007-015-0093-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Murphy KM, Poretsky E, Mafu S, Yang B, Char SN, Christensen SA, Saldivar E, Wu M, Wang Q, et al. Multiple genes recruited from hormone pathways partition maize diterpenoid defences. Nat Plants 2019:5(10):1043–1056. 10.1038/s41477-019-0509-6 [DOI] [PubMed] [Google Scholar]
- Ding Y, Northen TR, Khalil A, Huffaker A, Schmelz EA. Getting back to the grass roots: harnessing specialized metabolites for improved crop stress resilience. Curr Opin Biotechnol. 2021:70:174–186. 10.1016/j.copbio.2021.05.010 [DOI] [PubMed] [Google Scholar]
- Ding Y, Weckwerth PR, Poretsky E, Murphy KM, Sims J, Saldivar E, Christensen SA, Char SN, Yang B, Tong AD, et al. Genetic elucidation of interconnected antibiotic pathways mediating maize innate immunity. Nat Plants 2020:6(11):1375–1388. 10.1038/s41477-020-00787-9 [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013:29(1):15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Ren F, Lu X, Mao H, Xu M, Degenhardt J, Peters RJ, Wang Q. A tandem array of ent-Kaurene synthases in maize with roles in gibberellin and more specialized metabolism. Plant Physiol. 2016:170(2):742–751. 10.1104/pp.15.01727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007:3(7):408–414. 10.1038/nchembio.2007.5 [DOI] [PubMed] [Google Scholar]
- Harris LJ, Saparno A, Johnston A, Prisic S, Xu M, Allard S, Kathiresan A, Ouellet T, Peters RJ. The maize An2 gene is induced by Fusarium attack and encodes an ent-copalyl diphosphate synthase. Plant Mol Biol. 2005:59(6):881–894. 10.1007/s11103-005-1674-8 [DOI] [PubMed] [Google Scholar]
- Katajamaa M, Miettinen J, Orešič M. MZmine: toolbox for processing and visualization of mass spectrometry based molecular profile data. Bioinformatics 2006:22(5):634–636. 10.1093/bioinformatics/btk039 [DOI] [PubMed] [Google Scholar]
- Kremling KA, Chen S-Y, Su M-H, Lepak NK, Romay MC, Swarts KL, Lu F, Lorant A, Bradbury PJ, Buckler ES. Dysregulation of expression correlates with rare-allele burden and fitness loss in maize. Nature 2018:555(7697):520–523. 10.1038/nature25966 [DOI] [PubMed] [Google Scholar]
- Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv preprint arXiv:1303.3997, 2013.
- Li J, Halitschke R, Li D, Paetz C, Su H, Heiling S, Xu S, Baldwin IT. Controlled hydroxylations of diterpenoids allow for plant chemical defense without autotoxicity. Science 2021:371(6526):255–260. 10.1126/science.abe4713 [DOI] [PubMed] [Google Scholar]
- Mafu S, Ding Y, Murphy KM, Yaacoobi O, Addison JB, Wang Q, Shen Z, Briggs SP, Bohlmann J, Castro-Falcon G, et al. Discovery, biosynthesis and stress-related accumulation of dolabradiene-derived defenses in maize. Plant Physiol. 2018:176(4):2677–2690. 10.1104/pp.17.01351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafu S, Jia M, Zi J, Morrone D, Wu Y, Xu M, Hillwig ML, Peters RJ. Probing the promiscuity of ent-kaurene oxidases via combinatorial biosynthesis. Proc Natl Acad Sci U S A. 2016:113(9):2526–2531. 10.1073/pnas.1512096113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarevitch I, Waters AJ, West PT, Stitzer M, Hirsch CN, Ross-Ibarra J, Springer NM. Transposable elements contribute to activation of maize genes in response to abiotic stress. PLoS Genet. 2015:11(1):e1004915. 10.1371/journal.pgen.1004915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Liu J, Ren F, Peters RJ, Wang Q. Characterization of CYP71Z18 indicates a role in maize zealexin biosynthesis. Phytochemistry 2016:121:4–10. 10.1016/j.phytochem.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Mao H, Shen Q, Wang Q. CYP701A26 Is characterized as an ent-kaurene oxidase with putative involvement in maize gibberellin biosynthesis. Biotechnol Lett. 2017:39(11):1709–1716. 10.1007/s10529-017-2401-7 [DOI] [PubMed] [Google Scholar]
- Morrone D, Lowry L, Determan MK, Hershey DM, Xu M, Peters RJ. Increasing diterpene yield with a modular metabolic engineering system in E. coli: comparison of MEV and MEP isoprenoid precursor pathway engineering. Appl Microbiol Biotechnol. 2010:85(6):1893–1906. 10.1007/s00253-009-2219-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Chung S, Fogla S, Minsky HB, Zhu KY, Zerbe P. A customizable approach for the enzymatic production and purification of diterpenoid natural products. J Vis Exp. 2019. 10.3791/59992-v [DOI] [PubMed] [Google Scholar]
- Murphy KM, Edwards J, Louie KB, Bowen BP, Sundaresan V, Northen TR, Zerbe P. Bioactive diterpenoids impact the composition of the root-associated microbiome in maize (Zea mays). Sci Rep. 2021:11(1):333. 10.1038/s41598-020-79320-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Ma LT, Ding Y, Schmelz EA, Zerbe P. Functional characterization of two class II diterpene synthases indicates additional specialized diterpenoid pathways in maize (Zea mays). Front Plant Sci. 2018:9:1542. 10.3389/fpls.2018.01542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Zerbe P. Specialized diterpenoid metabolism in monocot crops: biosynthesis and chemical diversity. Phytochemistry 2020:172:112289. 10.1016/j.phytochem.2020.112289 [DOI] [PubMed] [Google Scholar]
- Nothias LF, Petras D, Schmid R, Dührkop K, Rainer J, Sarvepalli A, Protsyuk I, Ernst M, Tsugawa H, Fleischauer M, et al. Feature-based molecular networking in the GNPS analysis environment. bioRxiv: 812404, 2019. [DOI] [PMC free article] [PubMed]
- Ono K, Demchak B, Ideker T. Cytoscape tools for the web age: D3.js and Cytoscape.js exporters. F1000Res. 2014:3:143. 10.12688/f1000research.4510.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz N, Marcon C, Paschold A, Malik WA, Lithio A, Brandt R, Piepho H-P, Nettleton D, Hochholdinger F. Extensive tissue-specific transcriptomic plasticity in maize primary roots upon water deficit. J Exp Bot. 2016:67(4):1095–1107. 10.1093/jxb/erv453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluskal T, Castillo S, Villar-Briones A, Oresic M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics 2010:11(1):395. 10.1186/1471-2105-11-395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portwood JL, Woodhouse MR, Cannon EK, Gardiner JM, Harper LC, Schaeffer ML, Walsh JR, Sen TZ, Cho KT, Schott DA, et al. MaizeGDB 2018: the maize multi-genome genetics and genomics database. Nucl Acids Res. 2019:47(D1):D1146–D1154. 10.1093/nar/gky1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019 [Google Scholar]
- Schmelz EA, Engelberth J, Tumlinson JH, Block A, Alborn HT. The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. Plant J 2004:39(5):790–808. 10.1111/tpj.2004.39.issue-5 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Huffaker A, Sims JW, Christensen SA, Lu X, Okada K, Peters RJ. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 2014:79(4):659–678. 10.1111/tpj.12436 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Kaplan F, Huffaker A, Dafoe NJ, Vaughan MM, Ni X, Rocca JR, Alborn HT, Teal PE. Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc Natl Acad Sci U S A. 2011:108(13):5455–5460. 10.1073/pnas.1014714108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelpflug SC, Sekhon RS, Vaillancourt B, Hirsch CN, Buell CR, de Leon N, Kaeppler SM. An expanded maize gene expression atlas based on RNA sequencing and its use to explore root development. Plant Genome 2016:9(1):1–16. 10.3835/plantgenome2015.04.0025 [DOI] [PubMed] [Google Scholar]
- Stephens M. False discovery rates: a new deal. Biostatistics 2017:18(2):275–294. 10.1093/biostatistics/kxw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart V, Crampton BG, Ridenour JB, Bluhm BH, Olivier NA, Meyer JJM, Berger DK. Complementation of CTB7 in the maize pathogen Cercospora zeina overcomes the lack of in vitro cercosporin production. Mol Plant Microbe Interact. 2017:30(9):710–724. 10.1094/MPMI-03-17-0054-R [DOI] [PubMed] [Google Scholar]
- Tai H, Lu X, Opitz N, Marcon C, Paschold A, Lithio A, Nettleton D, Hochholdinger F. Transcriptomic and anatomical complexity of primary, seminal, and crown roots highlight root type-specific functional diversity in maize (Zea mays L.). J Exp Bot. 2016:67(4):1123–1135. 10.1093/jxb/erv513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholl D. Biosynthesis and biological functions of terpenoids in plants. Adv Biochem Eng Biotechnol. 2015:148:63–106 10.1007/10_2014_295 [DOI] [PubMed] [Google Scholar]
- Vaughan MM, Christensen S, Schmelz EA, Huffaker A, McAuslane HJ, Alborn HT, Romero M, Allen LH, Teal PE. Accumulation of terpenoid phytoalexins in maize roots is associated with drought tolerance. Plant Cell Environ. 2015:38(11):2195–2207. 10.1111/pce.12482 [DOI] [PubMed] [Google Scholar]
- Vaughan MM, Huffaker A, Schmelz EA, Dafoe NJ, Christensen S, Sims J, Martins VF, Swerbilow J, Romero M, Alborn HT, et al. Effects of elevated [CO2] on maize defence against mycotoxigenic Fusarium verticillioides. Plant Cell Environ. 2014:37(12):2691–2706. 10.1111/pce.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley JW, Sartor RC, Shen Z, Schmitz RJ, Wu KJ, Urich MA, Nery JR, Smith LG, Schnable JC, Ecker JR, et al. Integration of omic networks in a developmental atlas of maize. Science 2016:353(6301):814–818. 10.1126/science.aag1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, Nguyen DD, Watrous J, Kapono CA, Luzzatto-Knaan T, et al. Sharing and community curation of mass spectrometry data with global natural products Social molecular networking. Nat Biotechnol. 2016:34(8):828–837. 10.1038/nbt.3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Gu W, Chen J, Song N, Gao X, Zhang X, Zhou Y, Ma X, Song W, Zhao H. High temporal-resolution transcriptome landscape of early maize seed development. Plant Cell 2019:31(5):974–992. 10.1105/tpc.18.00961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li R, Xu M, Hoffmann RI, Zhang Y, Liu B, Zhang M, Yang B, Li Z, Peters RJ. A (conditional) role for labdane-related diterpenoid natural products in rice stomatal closure. New Phytol. 2021:230(2):698–709. 10.1111/nph.17196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Nie X, Kong W, Deng X, Sun T, Liu X, Li Y. Genome-wide identification and evolution analysis of the gibberellin oxidase gene family in six Gramineae crops. Genes (Basel) 2022:13(5):863. 10.3390/genes13050863 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.