Abstract
Arsenate [As(V)] is a metalloid with heavy metal properties and is widespread in many environments. Dietary intake of food derived from arsenate-contaminated plants constitutes a major fraction of the potentially health-threatening human exposure to arsenic. However, the mechanisms underlying how plants respond to arsenate stress and regulate the function of relevant transporters are poorly understood. Here, we observed that As(V) stress induces a significant Ca2+ signal in Arabidopsis (Arabidopsis thaliana) roots. We then identified a calcium-dependent protein kinase, CALCIUM-DEPENDENT PROTEIN KINASE 23 (CPK23), that interacts with the plasma membrane As(V)/Pi transporter PHOSPHATE TRANSPORTER 1;1 (PHT1;1) in vitro and in vivo. cpk23 mutants displayed a sensitive phenotype under As(V) stress, while transgenic Arabidopsis plants with constitutively active CPK23 showed a tolerant phenotype. Furthermore, CPK23 phosphorylated the C-terminal domain of PHT1;1, primarily at Ser514 and Ser520. Multiple experiments on PHT1;1 variants demonstrated that PHT1;1S514 phosphorylation is essential for PHT1;1 function and localization under As(V) stress. In summary, we revealed that plasma-membrane-associated calcium signaling regulates As(V) tolerance. These results provide insight for crop bioengineering to specifically address arsenate pollution in soils.
The calcium-dependent protein kinase CPK23 regulates the subcellular localization of the plasma membrane As(V)/Pi transporter PHT1;1 via phosphorylation to enhance arsenate tolerance in Arabidopsis.
Introduction
Arsenic (As), a metalloid ubiquitously found in soil, can easily accumulate in the human body and cause organ damage through its metabolism, including cancers and a wide range of clinical complications (Chen et al. 2019). The accumulation of ROS caused by arsenic in plants will also affect the growth of crops (Singh et al. 2006; Tang et al. 2022). Arsenate mainly exists in various sulfides or other rock-forming minerals (Smedley and Kinniburgh 2002). The dissolution of arsenate-containing minerals can ultimately penetrate and pollute groundwater (Matschullat 2000; Polizzotto et al. 2006). Irrigation of crops with contaminated groundwater has been shown to lead to the substantial accumulation of arsenate in cultivated foods (Fayiga and Saha 2016). In view of the impact of arsenate on food safety, understanding the absorption of arsenate by plants, including relevant regulatory mechanisms, is of great importance.
Three main forms of As in soil are available to plants: arsenate [As(V)], arsenite [As(III)], and methylated As (monomethyl arsinic acid and dimethyl arsinic acid) (Zhu et al. 2014). As(V) and As(III) are each respectively the dominant form of As in aerobic and anaerobic environments. Plant roots selectively take up specific As species via distinct pathways and transporters. As the oxyanion’s chemical structure of [As(V)] is similar to that of phosphate (Pi), it can readily enter plant roots via Pi transporters. To date, quite a few characterized high-affinity Pi transporters have been identified in the PHOSPHATE TRANSPORTER 1 (Pht1) family, as some of them are also involved in As(V) transport in plants (Li et al. 2015). In Arabidopsis (Arabidopsis thaliana), AtPht1;1 and AtPht1;4 were initially found to mediate Pi and As(V) acquisition from soils with both low and high levels of phosphate (Shin et al. 2004). CASEIN KINASE II (CK2) is a casein kinase family member that has been found to regulate PHT1 trafficking in rice (Chen et al. 2015), while recent research has revealed its function in response to aluminum stress (Wei et al. 2021). These findings suggest that kinases are involved in heavy metal responses by influencing transporters trafficking.
Other members of the PHT1 family also participate in the response to arsenic stress (Nagarajan et al. 2011; Remy et al. 2012; Leblanc et al. 2013). In addition, some Pi transport-related regulatory mechanisms affect As(V) tolerance (González et al. 2005; Castrillo et al. 2013; Wang et al. 2014; Navarro et al. 2021). Arsenite uptake mainly relies on plant aquaporins (Bienert et al. 2008; Jian et al. 2008; Li et al. 2014; Mukhopadhyay et al. 2014; Modareszadeh et al. 2021) or plasma membrane intrinsic proteins (Mosa et al. 2012), in contrast to arsenate uptake.
Ca2+ is a common second messenger in abiotic stress signaling (Luan and Wang 2021; Dong et al. 2022). Specific Ca2+ signals are generally detected by different Ca2+ sensors, like CALMODULIN (CaM), CALMODULIN-LIKE-PROTEINS, CALCIUM-DEPENDENT PROTEIN KINASES (CPKs), and CALCINEURIN B-LIKE PROTEINS (CBLs) and their interacting kinases, CBL INTERACTING PROTEIN KINASES (Defalco et al. 2010; Mohanta et al. 2019), and translates into transcriptional and metabolic responses (Perochon et al. 2011). In response to As stress, differential expression of CaMs suggests the possible involvement of Ca2+-dependent signaling in As tolerance of plants (Chakrabarty et al. 2009). Understanding how Ca2+ signals are translated into phosphorylation signals is essentially a problem in understanding how signals are decoded and processed. In this regard, CPKs, as important calcium regulatory proteins, are usually involved in decoding Ca2+ signals caused by stress or stimuli in plants (Hamel et al. 2014; Liu et al. 2017). Arabidopsis contains 34 CPKs that are widely involved in plant growth and development and responses to biotic, abiotic, and nutritional stresses (Harmon et al. 2001; Demir et al. 2013; Valmonte et al. 2014; Zhou et al. 2014; Wang et al. 2016; Liu et al. 2017). CPK4/5/6/11 have been reported to be involved in the regulation of biotic stress, drought, and high Mn stress responses (Zhu et al. 2007; Luo et al. 2014; Yang et al. 2020; Zhang et al. 2020; Zhou et al. 2020; Zhang et al. 2021b; Ju et al. 2022). CPK21/23 participate in responding to drought, salt, abscisic acid (ABA), Mn deficiency stress, and cadmium stress (Ma and Wu 2007; Franz et al. 2011; Geiger et al. 2011; Demir et al. 2013; Van Kleeff et al. 2018; Fu et al. 2022; Zhang et al. 2022). Additionally, CPK activity levels were markedly enhanced under As(V) stress in rice (Huang et al. 2012) which implies CPK participation in arsenate stress response. In this regard, CPK31 has been found to interact with As(III) transporter NIP1;1 and to determine the As(III) uptake and tolerance of Arabidopsis. However, how CPK31 regulates NIP1;1 requires further investigation (Ji et al. 2017). Nevertheless, the molecular mechanism underpinning the As(V) stress response remains poorly understood.
In the present study, we found that As(V) stress can induce clear Ca2+ signaling in the roots of Arabidopsis. On this basis, we screened out a cpk23 mutant with the most significant sensitive phenotype from among 31 CPKs single mutants under As(V) stress. By using co-immunoprecipitation mass spectrometry (IP-MS) with immunoprecipitated CPK23-FLAG from transgenic seedlings, we identified a peptide corresponding to PHT1;1, a phosphate transporter related to arsenic transport. After a series of protein interaction and phosphorylation experiments, we determined that CPK23 interacts with PHT1;1 and phosphorylates the S514 site at its C-terminus under arsenic stress. Multiple physiological experiments on PHT1;1 variants under As(V) stress demonstrated that PHT1;1S514 phosphorylation was essential for PHT1;1 function in As(V) tolerance. Fluorescence detection showed that CPK23 regulates the delocalization of PHT1;1 from plasma membranes under As(V) stress. Altogether, our results suggest that CPK23 regulates As(V) tolerance by affecting PHT1;1 subcellular localization in Arabidopsis via phosphorylation.
Results
As(V) stress induces a significant Ca2+ signal in Arabidopsis
Ca2+ is a well-known ubiquitous secondary messenger in plants (Chen et al. 2021; Zhang et al. 2021a; Dong et al. 2022). It has been broadly demonstrated that As(V) stress increases cytoplasmic Ca2+ concentrations in plants (Sharma et al. 2020). However, such a conclusion relies on the changes in transcription level and kinase activity of Ca2+ sensors (Chakrabarty et al. 2009; Huang et al. 2012). There is a lack of direct experimental evidence supporting a relationship between arsenate stress and calcium signals. Thus, we first explored whether As(V) stress-induced Ca2+ signals with wild-type (WT) Arabidopsis plants expressing the cytosolic fluorescence resonance energy transfer (FRET)-based ratiometric calcium indicator Yellow Cameleon 3.6 (YC3.6) (Behera et al. 2015), which specifically detects cytosolic free calcium (Miyawaki et al. 1997; Palmer and Tsien 2006). The seedlings were treated with 300 μ M Na2HAsO4 As(V) stress. We used both 600 μ M NaCl as a negative control to exclude the possibility that calcium signals are triggered by Na+ in Na2HAsO4 and 50 μ M LaCl3 treatment as the inhibition group in this experiment because of the high affinity of lanthanum ion with calcium channels. A clear Ca2+ spike was detected under the As(V) stress treatment, whereas the negative control did not elicit an increased fluorescent signal (Fig. 1, A and B). To further validate the As(V) stress-induced Ca2+ signal, we used the GCaMP imaging technique which was developed to detect Ca2+ dynamics in response to different stresses (Chen et al. 2013). We used 5-d-old Arabidopsis seedlings expressing GCaMP6 to monitor changes in Ca2+ levels ([Ca2+]cyt) in response to 300 μ M Na2HAsO4 and found that As(V) stress triggers a rapid increase in [Ca2+]cyt in root cells (Fig. 1, C and D). The fluorescence signals indicating As(V) stress-induced calcium fluctuation appeared in the root maturation zone, and gradually increased with a more substantial enhancement of such fluorescence signals in the middle column zone (Supplemental Videos 1–3). In addition, we used YC3.6 and GCaMP6 seedlings to test the induction of root calcium signals by phosphate stimuli and found that it could not induce calcium signals under As(V) stress (Fig. 1, and Supplemental Video 4). These results suggest that As(V) stress can induce a specific increase in [Ca2+]cyt in the root system of Arabidopsis.
Figure 1.
As(V) stress induces a rapid Ca2+ signal in Arabidopsis roots. A) Fluorescence images of Ca2+ signaling in the roots of YC3.6 transgenic plants. Four-day-old seedlings were soaked in ddH2O for 2 h and assessed after treatment with 300 μ M Na2HAsO4 (As(V) stress), 600 μ M NaCl (negative control), 300 μ M Na2HAsO4 with 50 μ M LaCl3 (inhibiting group), and 300 μ M Na2HPO4 (phosphate treatment). Scale bars, 100 μm. B) Quantitative determination of average Ca2+ signal in the roots of (A). Results are presented as the means ± Sds (n = 3). Statistical differences were calculated by two-way ANOVA. Different letters indicate means that were significantly different according to Tukey's multiple testing method (P < 0.05) for all the treatments. C) Fluorescence image of Ca2+ signaling in roots of GCaMP6s transgenic plants. Four-day-old seedlings were soaked in ddH2O for 2 h and assessed after treatment with 300 μ M Na2HAsO4 (As(V) stress), 600 μ M NaCl (negative control), 300 μ M Na2HAsO4 with 50 μ M LaCl3 (inhibiting group), and 300 μ M Na2HPO4 (phosphate treatment). Scale bars, 100 μm. D) Traces of average Ca2+ signals stimulated by different treatments in the roots of GCaMP6 transgenic plants are shown in (C). (F−F0)/F0, relative fluorescence intensity. The results are presented as the means ± Sds (n = 3).
CPK23 is required for As(V) tolerance
CPKs are a large protein family in plants (OsCPK1-OsCPK29 in rice and AtCPK1-AtCPK34 in Arabidopsis), and they participate in a variety of physiological processes and stress responses (Boudsocq et al. 2010; Takayuki et al. 2012; Boudsocq and Sheen 2013; Kudla et al. 2018). The concentrations of As(V) used in experiments are usually higher than those used in As(III) experiments (Sharma et al. 2020). Col-0 is not an Arabidopsis ecotype that is sensitive to arsenic stress (Fu et al. 2014). For lines within the Col-0 background, concentrations of As(V) at low levels may not induce phenotypic differences. Thus, we treated 5-d-old seedlings of 31 T-DNA insertion single mutants (Supplemental Table S2) of CPK genes with 300 μ M Na2AsO4 and identified cpk23 as having the most sensitive phenotype under As(V) stress (Supplemental Fig. S1). On this basis, quantitative PCR (qPCR) was conducted to test the As(V) stress-induced changes in the CPK23 expression level. The expression level of CPK23 showed no difference under As(V) stress (Supplemental Fig. S2A). Thus, we constructed transgenic lines overexpressing CPK23 for further study (Supplemental Fig. S2B). Additionally, because the kinase activity of CPKs has an important impact on its function and the CPK23 expression level was not induced by As(V) stress, we also constructed transgenic lines with constitutively active CPK23 (CPK23-VK) to determine whether As(V) stress directly affects the kinase activity of CPK23 (Supplemental Fig. S2B).
To confirm whether As(V) stress affects CPK23 function at the transcriptional level or the protein level, we treated CPK23 overexpression plants (CPK23-OE #9, CPK23-OE #36) and constitutively active CPK23 plants (CPK23-VK #3, CPK23-VK #7) under different levels of As(V) stress. However, CPK23-OE plants did not show a tolerance phenotype under As(V) stress (Fig. 2A). In contrast, all CPK23-VK plants showed a tolerance phenotype under 300 , 400, and 500 μ M As(V) stress, while 100 and 200 μ M did not induce significant differences among the mutants (Fig. 2A). The primary root length of CPK23-VK plants was significantly longer than that of WT plants, and its fresh weight was increased relative to that of WT plants under As(V) stress (Fig. 2, B and C). Corresponding to the physiological phenotype, the arsenic content in the roots of cpk23 mutants was increased significantly, which in CPK23-VK plants was decreased significantly(Fig. 2D).
Figure 2.
Phenotypic analysis of mutants and overexpression plants under As(V) stress. A) As(V) stress phenotypes of CPK23 mutant, overexpression, and constitutively active plants. The plants were grown under control and As(V) stress conditions (with 100, 200, 300, 400, and 500 μ M Na2HAsO4). B) Statistical analysis of the root lengths of the plants shown in (A). The results are presented as the means ± Sds (n = 15). Statistical differences were calculated by two-way ANOVA. Different letters indicate means that were significantly different according to Tukey's multiple testing method (P < 0.05) for genotypes within a given growth condition. C) Statistical analysis of the fresh weight of the plants shown in (A). The results are presented as the means ± Sds (n = 15). Statistical differences were calculated by two-way ANOVA. Different letters indicate means that were significantly different according to Tukey's multiple testing method (P < 0.05) for genotypes within a given growth condition. D) Statistical analysis of As concentrations of Col-0, cpk23, and CPK23-VK. Results are presented as the means ± Sds (n = 12). Statistical differences were calculated by two-way ANOVA. Asterisks indicate statistically different (*) and significantly different (**) compared with the wild type according to Student's t-tests method.
To verify whether CPK23 also participates in phosphate signal response, we detected the phosphate deficiency phenotype of the above mutants. We found that there was no difference in growth between WT plants and these mutants, indicating that CPK23 did not participate in the phosphate deficiency response (Supplemental Fig. S3). These results suggested that CPK23 and its kinase activity are specifically required for As(V) tolerance.
CPK23 interacts with Pi transporter PHT1;1
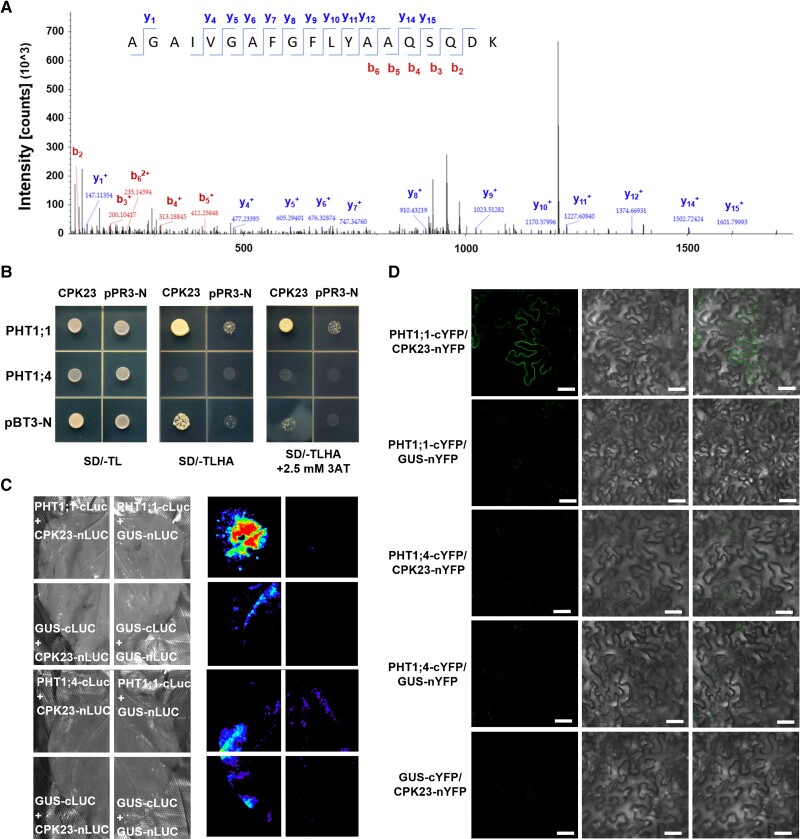
To identify the downstream protein phosphorylated by CPK23, we performed IP-MS using CPK23-FLAG transgenic seedlings. We identified a variety of 14-3-3 proteins, a series of calcium receptors, and some transporters for heavy metals including arsenic, cadmium, and copper transporters (Supplemental Table S1). One peptide of PHT1;1, a phosphate transporter involved in As(V) tolerance, was identified (Fig. 3A).
Figure 3.
CPK23 physically interacts with PHT1;1. A) IP-MS was conducted using immunoprecipitated CPK23-FLAG from transgenic seedlings. B) A split-ubiquitin yeast membrane system Y2H assay showing the interaction between CPK23 and both PHT1;1 and PHT1;4. C) LCI assays demonstrated the interaction between CPK23 and both PHT1;1 and PHT1;4. N. benthamiana leaves were co-infiltrated with A. tumefaciens cells containing different pairs of constructs. Luciferase assay images were captured using a cooled charge-coupled device imaging apparatus. D) BiFC assay showing the interaction between CPK23 and PHT1;1, PHT1;4. CPK23 is fused to YFPN, and PHT1;1 is fused to YFPC. Different pairs of constructs were co-expressed in N. benthamiana. YFP fluorescence was detected by confocal microscopy. Scale bars, 50 μm.
To verify the results of the IP-MS assays, we performed Yeast Two-Hybrid (Y2H) assays based on the split-ubiquitin yeast (Saccharomyces cerevisiae) membrane system to analyze the interaction between CPK23 and PHT1;1, as well as its homologue, PHT1;4. CPK23 was fused to a vector containing the N-terminal fragment of ubiquitin (pPR3-N), while PHT1;1 and PHT1;4 were fused to a vector containing the C-terminal fragment of ubiquitin (pBT3-N). Yeast colonies were detected in amino acid-deficient synthetic dropout medium when CPK23-pPR3-N was co-expressed with PHT1;1-pBT3-N. By contrast, yeast growth could not be detected when CPK23-pPR3-N was co-expressed with PHT1;4-pBT3-N (Fig. 3B).
To further verify these results in plant cells, we performed luciferase complementation imaging (LCI) assays. CPK23 was fused to the N-terminal fragment of luciferase (nLUC), and PHT1;1 was fused to the C-terminal fragment of luciferase (cLUC). LUC activity was detected when CPK23-nLUC was co-expressed with PHT1;1-cLUC in Nicotiana benthamiana leaves. By contrast, co-expression of CPK23-nLUC and PHT1;4-cLUC produced no LUC activity (Fig. 3C, and Supplemental Fig. S4A). Similar to the results of the LCI assays, bimolecular fluorescence complementation (BiFC) assays also support that CPK23 specifically interacts with PHT1;1. We observed intense yellow fluorescent protein (YFP) fluorescence signals in leaves with co-expression of CPK23-nYFP and PHT1;1-cYFP (Fig. 3D, and Supplemental Fig. S4B).
Based on the results of the interaction experiments, we then explored the genetic relationship between PHT1;1 and CPK23 by constructing double knockout mutants through hybridization. PHT1;1 deletion was able to significantly restore the sensitive phenotype caused by CPK23 knockout under As(V) stress, indicating that CPK23 acts upstream of PHT1;1 and plays a negative regulatory role (Supplemental Fig. S5). Altogether, these results indicated that CPK23 specifically interacts with the Pi transporter PHT1;1 and negatively regulates its function under As(V) stress.
CPK23 phosphorylate PHT1;1 at Ser514 and Ser520
As the full length of PHT1;1 could not be purified, we created constructs with its N-terminal domain (amino acid residues 1 to 23), central loop (amino acid residues 233 to 340), and C-terminal domain (amino acid residues 504 to 524) fused with GST-tag to obtain PHT1;1-N, PHT1;1-M and PHT1;1-C, respectively. To investigate whether CPK23 directly phosphorylates PHT1;1-N PHT1;1-M or PHT1;1-C, we performed in vitro kinase assays using purified CPK23-His and PHT1;1-N/M/C-GST, respectively. CPK23 phosphorylated GST-PHT1;1-C but not the constructs containing the other domains (Supplemental Fig. S6). To identify the phosphorylation sites in PHT1;1-C, we first determined some potential phosphorylation sites (Ser509, Ser514, Ser520) by referring to former research (Bayle et al. 2011) and the Group-based Prediction System webtool (http://gps.biocuckoo.org/links.php#l1) (Fig. 4A). We generated the identified Ser (S) to Ala (A) point mutations in PHT1;1-C fused with GST tags, obtaining the following constructs: PHT1;1-CS509A-GST, PHT1;1-CS514A-GST, and PHT1;1-CS520A-GST. To determine which phosphorylation sites on PHT1;1-C were the target of CPK23, we performed in vitro kinase assays. Notably, phosphorylation levels of PHT1;1-CS520A and especially PHT1;1-CS514A were lower than that of PHT1;1-C (Fig. 4B). These results suggested that CPK23 mainly phosphorylates the C-terminal domain of PHT1;1 at residue Ser514.
Figure 4.
CPK23 phosphorylated PHT1;1. A) The structure of PHT1;1 is shown with the positions of phosphorylation sites. Residues T104, S206, S258, S509, S514, and S520 are highlighted. B) The phosphorylation target sites of CPK23 were identified to be in PHT1;1-C. CPK23 was fused to His, while PHT1;1-C variants were fused to GST. The amount of protein loaded onto the gel was visualized with Coomassie brilliant blue (CBB), and phosphorylation was visualized with autoradiography (Autorad). C) A protein kinase assay of CPK23 with PHT1;1-C was used to characterize the response to As(V) stress. Ten-day-old seedlings were treated with 60 μ M Na2HAsO4 for the indicated time periods. The protein kinases were quantified by western blot, as shown at the bottom.
To elucidate how the phosphorylation of PHT1;1 was induced by As(V) stress, transgenic plants overexpressing CPK23 fused FLAG tags were grown under As(V) stress for 0, 3, 6, 12, and 24 h. CPK23 was extracted, enriched with FLAG beads, and then incubated with PHT1;1-C-GST purified by prokaryotic and [γ-32P] ATP (Fig. 4C, and Supplemental Fig. S7). We observed that the phosphorylation level of PHT1;1-C by CPK23 was induced by As(V) stress. The phosphorylation level of PHT1;1-C by CPK23 increased and peaked quickly at 1 h, and then decreased to a lower level compared to control conditions at 6 h, at which it was maintained thereafter. These findings indicated that the phosphorylation of PHT1;1 was induced by As(V) stress.
PHT1;1S514 phosphorylation is essential for PHT1;1 function in As uptake
To determine whether PHT1;1S514 and PHT1;1S520 phosphorylation affects the As(V) tolerance of plants, we transformed the constructs containing PHT1;1 and PHT1;1 variants under the control of its own promoter into the pht1;1 mutant. The transgenic plants were grown in 1/2 Murashige and Skoog (MS) medium for 5 d and transferred into 1/2 MS medium supplemented with 300 , 400 , and 500 μ M Na2HAsO4 for another 10 d. We found sensitive phenotypes in ProPHT1;1:PHT1;1S514A (#7 and #11) transgenic plants, while ProPHT1;1:PHT1;1S514D (#1 and #3) plants showed tolerance phenotypes partly similar to those of pht1;1 plants (Fig. 5A). In contrast, mutations on the S520 site of PHT1;1 appeared to not affect the As(V) tolerance (Supplemental Fig. S8). The root length and fresh weight of ProPHT1;1:PHT1;1S514A transgenic plants decreased significantly (Fig. 5, B and C). Correspondingly, the arsenic contents exhibited the same trend as the physiological phenotype (Fig. 5D). These results suggested that PHT1;1S514 phosphorylation is essential for PHT1;1 to function in As(V) tolerance.
Figure 5.
Phenotypic analysis of ProPHT1;1:PHT1;1S514A and ProPHT1;1:PHT1;1S514D transgenic plants under As(V) stress. A) As(V) stress phenotypes of ProPHT1;1:PHT1;1S514A and ProPHT1;1:PHT1;1S514D transgenic plants. These plants were grown under control and As(V) stress condition (with 300, 400, and 500 μ M Na2HAsO4). B) Statistical analysis of root lengths of plants shown in (A). Results are presented as the means ± Sds (n = 15). Statistical differences were calculated by two-way ANOVA. Different letters indicate means that were significantly different according to Tukey's multiple testing method (P < 0.05) for genotypes within a given growth condition. C) Statistical analysis of fresh weight of plants shown in (A). Results are presented as the means ± Sds (n = 15). Statistical differences were calculated by two-way ANOVA. Different letters indicate means that were significantly different according to Tukey's multiple testing method (P < 0.05) for genotypes within a given growth condition. D) Statistical analysis of As concentrations of Col-0, pht1;1 and ProPHT1;1:PHT1;1S514A. Results are presented as means ± Sds (n = 12). Statistical differences were calculated by two-way ANOVA. Asterisks indicate statistically different (*) and significantly different (**) compared with the wild type according to Student's t-tests method.
CPK23 regulates PHT1;1 subcellular localization under As(V) stress
The localization of the PHT1;1 transporter in the root cell has previously been shown to be controlled by As(V) (Castrillo et al. 2013; Navarro et al. 2021). As the Ser514 residue of PHT1;1 has been demonstrated to be a phosphorylation target of CPK23 under As(V) stress (Fig. 4), it is reasonable that CPK23 regulates PHT1;1 subcellular localization under As(V) stress via phosphorylation. To determine whether CPK23 is involved in As(V) tolerance through regulating PHT1;1 subcellular localization via phosphorylation, we first expressed PHT1;1 phosphorylation site mutants with green fluorescent protein (GFP) in WT plants. The transgenic plants were grown on the 1/2 MS medium for 7 d before fluorescence observation. Confocal laser scanning micrographs of fluorescence emitted by root cells of transgenic plants confirmed that the phosphorylation of the Ser514 site decisively influenced the localization of PHT1;1, while PHT1;1S514A-GFP plants showed more substantial co-localization signals with the plasma membrane than PHT1;1S514D-GFP plants (Fig. 6A).
Figure 6.
CPK23 regulates PHT1;1 subcellular localization under As(V) stress. A) A confocal laser scanning micrograph of fluorescence emitted by root cells of transgenic plants shows the expression of PHT1;1 phosphorylation site mutants with GFP-tag. PI dye staining mark cell membrane. Scale bars, 50 μm. B) A confocal laser scanning micrograph of fluorescence emitted by root cells of transgenic plants shows the expression of PHT1;1-GFP in WT Col-0 ecotype and cpk23 mutant A. thaliana with or without arsenate treatment. PI dye staining marks cell membrane. Scale bars, 50 μm. Arrows indicate the location and direction selected for the statistical analysis of relative intensity.
Correspondingly, on this basis, we further constructed the mutants with PHT1;1-GFP in both WT and cpk23 plants. The transgenic plants were grown on the 1/2 MS medium for 7 d and then transferred to a medium without phosphate (-Pi) for another 2 d in order to make PHT1;1-GFP accumulate on the plasma membrane. Seedlings under -Pi treatment were transferred to -Pi medium supplemented with 60 μ M As(V) for 12 h before fluorescence observation. Under As(V) exposure, PHT1;1-GFP in WT plants showed delocalization from the plasma membrane after 12 h of treatment. In contrast, PHT1;1-GFP in cpk23 plants remained at the plasma membrane (Fig. 6B). These results suggest that CPK23 regulates the subcellular localization of PHT1;1 under As(V) stress via phosphorylation of the Ser514 site.
Discussion
PHT1;1, a phosphate transporter belonging to the PHT1 family, also mediates As(V) uptake (Shin et al. 2004). PHT1;1 delocalized from the plasma membrane under As(V) stress to support tolerance (Castrillo et al. 2013; Navarro et al. 2021). The phosphorylation state of specific residues on the C-terminal domain of PHT1;1 affects its subcellular localization in A. thaliana root cells (Bayle et al. 2011). However, there is a lack of direct evidence confirming that the delocalization of PHT1;1 from the plasma membrane is regulated by a specific protein kinase. In this study, we identified a CPK23-PHT1;1 signaling cascade blocking arsenic uptake under As(V) stress to achieve As(V) tolerance in Arabidopsis. CPK23 was hypothesized to be activated by As(V) stress-induced Ca2+ signals which in turn interacted with and phosphorylated the As(V)/Pi transporter PHT1;1 under As(V) stress. Phosphorylation of specific sites enhanced delocalization of PHT1;1 from the plasma membrane under As(V) stress, ultimately decreasing arsenic uptake under As(V) stress and supporting As(V) tolerance in Arabidopsis (Fig. 7).
Figure 7.
Model for As(V) stress signaling and PHT1;1 As(V) uptake regulation in Arabidopsis. The model depicts the role of CPK23 in the plant response to As(V) stress. As(V) stress causes a change in intracellular Ca2+ concentration, and then activates CPK23. Next, CPK23 phosphorylates and delocalizes the plasma membrane-localized As(V)/Pi transporter PHT1;1. PHT1;1S514 phosphorylation enhances the delocalization of PHT1;1 and then decreases As(V) uptake and thus increases As(V) tolerance.
CPK23 is involved in Arabidopsis As(V) stress response
As a well-known secondary messenger in plants, calcium signals have been reported to be involved in several physiological and biochemical responses(Zhang et al. 2021a; Dong et al. 2022). Differences in CaMs expression and CDPK activity levels between control conditions and As(V) stress suggest the possible involvement of Ca2+ sensors in As tolerance in plants (Chakrabarty et al. 2009; Huang et al. 2012). We detected a rapid calcium signal spike in A. thaliana root cells under As(V) stress via calcium tracer techniques based on fluorescence confocal microscopy (Fig. 1), but phosphate treatment did not induce such signals within the same time range. This may imply the specificity of arsenic stress signals in activating calcium signals. Plants may coordinate the regulatory pathway between arsenic stress and phosphate absorption through temporal separation. Through a screening of 31 T-DNA insertion single mutants of the CPK family in A. thaliana, cpk23 plants were identified to have the most sensitive phenotype under As(V) stress (Supplemental Fig. S1). However, As(V) stress did not cause increased CPK23 expression (Supplemental Fig. S2A) which suggested that the calcium signals induced by arsenic stress might only affect CPK23 activity. Thus, we conducted further experiments to examine the phenotype and found that the overexpression of CPK23 did not lead to arsenic tolerance, while such a characteristic was observed in the transgenic CPK23-VK plants with constitutively active CPK23 (Fig. 2). These results demonstrate that CPK23 is specifically involved in the Arabidopsis As(V) stress response and promotes arsenic tolerance via its phosphorylation activity.
CPK23 positively regulates As(V) tolerance through phosphorylating PHT1;1
PHT1;1, a Pi transporter located on the plasma membrane, also mediates As(V) uptake (Shin et al. 2004). As(V) stress enhanced the delocalization of PHT1;1 from the plasma membrane (Castrillo et al. 2013; Navarro et al. 2021). CPK23 was also localized on the plasma membrane (Kawamoto et al. 2015), and we identified a peptide on PHT1;1 from immunoprecipitated CPK23-FLAG transgenic seedlings via IP-MS (Fig. 3A). We hypothesize that CPK23 participates in As(V) tolerance through PHT1;1. The interaction of CPK23 with PHT1;1 was identified in omics data obtained through IP-MS and further confirmed by Y2H, LCI, and BiFC assays (Fig. 3, B–D). The hybrid mutant of cpk23 and pht1;1 showed a tolerance phenotype similar to pht1;1 mutants but unlike that of cpk23 mutants (Supplemental Fig. S5).
Meanwhile, although statistically insignificant, some of the cpk23/pht1;1 double mutants were a little bit shorter than the pht1;1 mutants. This finding implied that PHT1;1 may not be the only element downstream of CPK23. The phenotype of pht1;1/pht1;4 double mutant showed that PHT1;4 contributed less to arsenic tolerance than PHT1;1 (Supplemental Fig. S9). Combined with the As(V)-sensitive phenotype of cpk23 and the related interaction results. PHT1;4 is unlikely to be directly downstream of CPK23. As PIP2;2, which is involved in arsenic tolerance (Modareszadeh et al. 2021), was identified by IP-MS as potentially interacting with CPK23, it is a notable candidate for further research. Additionally, CPK23 is involved in a variety of abiotic stress responses, including ABA responses (Geiger et al. 2010). As a metalloid element with heavy metal properties, arsenic also affects ABA levels in plants (Kumar et al. 2019). Therefore, CPK23 deletion may not only affect arsenate absorption through PHT1;1, but also affect other responses of plants to arsenic stress through the ABA pathway.
In addition, the C-terminus of PHT1;1 can be phosphorylated by CPK23 (Supplemental Fig. S6) and such activity is induced in vivo by As(V) stress (Fig. 4C). These results demonstrated that CPK23 positively regulates As(V) tolerance and that its phosphorylation activity towards PHT1;1 is required for its function in As(V) tolerance.
CPK23 participates in As(V) tolerance through regulating PHT1;1 delocalization under As(V) stress
Phosphorylation of the Ser514 residue in the C-terminal domain of PHT1;1 affects its plasma membrane localization in root cells under phosphate deficiency (Bayle et al. 2011). Coincidentally, through an in vitro phosphorylation experiment, we found that CPK23 can phosphorylate PHT1;1 at the same residue (Fig. 4B). CK2 has been found to regulate PHT1;1 trafficking in rice (Chen et al. 2015), it is possible for kinases to be involved in arsenate tolerance by regulating transporter localization. Therefore, we hypothesized that CPK23 regulates the abundance of PHT1;1 proteins on the plasma membrane by phosphorylating its C-terminal specific site and thus regulates the arsenic uptake of root cells. Through colocalization observation, we first confirmed that the phosphorylation of the S514 site reduced the localization of PHT1;1 on the plasma membrane (Fig. 6A). On this basis, we further found that loss of CPK23 blocks the delocalization of PHT1;1 from the plasma membrane under As(V) stress (Fig. 6B). Notably, the dephosphorylation of the PHT1;1Ser514 residue results in significant sensitive phenotypes (Fig. 5). Moreover, under As(V) exposure treatment, the arsenic concentration significantly increased in cpk23 and ProPHT1;1:PHT1;1S514A transgenic plants but decreased in pht1;1 and CPK23-VK plants (Figs. 2D and 5D). However, the phenotypes of CPK23-VK plants were similar to those of WT plants under phosphate deficiency, indicating that CPK23 did not participate in the phosphate deficiency response (Supplemental Fig. S3). Phosphorylation of the PHT1;1Ser514 residue mediates both phosphate deficiency and arsenate stress responses, while the regulation processes might occur in different parts of cells. PHF1 located in the ER regulates the detachment of newly synthesized PHT1;1 from the ER under phosphate deficiency (Bayle et al. 2011), while CPK23 protein, localized on the plasma membrane, mediates the separation of PHT1;1 from PM under arsenate stress. Different signals with different subcellular localization influence the subcellular localization of PHT1;1 by regulating the same phosphorylation site on PHT1;1. These results demonstrated that CPK23 specifically promotes As(V) tolerance by regulating arsenic uptake through influencing PHT1;1 delocalization under As(V) stress via phosphorylation.
Overall, our results suggest that CPK23 plays an essential role in As(V) tolerance. Under As(V) stress, plants reduce As(V) absorption from the surrounding environment into the cytoplasm by delocalizing plasma membrane-localized As(V)/Pi transporter PHT1;1. In this process, calcium signals are induced by high-level As(V) stress, which activates CPK23. Subsequently, CPK23 interacts with and phosphorylates the plasma membrane As(V)/Pi transporter PHT1;1 at the Ser514 residue. The delocalization from the plasma membrane of PHT1;1 enhanced by PHT1;1S514 phosphorylation, eventually decreases the As(V) uptake and increases the tolerance of plants to As(V) stress (Fig. 7).
Materials and methods
Plant materials and growth conditions
Arabidopsis (A. thaliana) Columbia (Col-0) was used as a control for all experiments. The T-DNA insertion lines were, cpk23 (SALK_007958) and pht1;1 (SALK_088586C). Arabidopsis seeds were surface sterilized and then stratified for 3 d at 4 °C. The seeds were sown in a nutrient medium consisting of 1% (w/v) agar (Sigma-Aldrich; A1296), 2% sucrose, and 1/2-strength MS at pH 5.70–5.75. To conduct As(V) stress treatments, plants were sown in 1/2 MS for 4 d, and then transferred to 1/2 MS with or without Na2HAsO4 (100 to 500 μ M) for 7 d. To conduct phosphate deficiency treatments, plants were sown in 1/2 MS, cultivated for 4 d and then transferred to 1/2 MS and 1/2-strength MS without phosphate but supplemented with 50 KH2PO4 and 25 µ M KH2PO4. All phenotypic experiments were repeated 3 times (n = 15 for each independent experiment). The root length was measured with ImageJ (Schneider et al. 2012). To grow plants in the soil, 7-d-old seedlings grown on 1/2 MS medium were transferred to nutrient-rich soil (Pindstrup substrate, Denmark) and grown in a greenhouse under controlled conditions (22 °C/19 °C, 16 h light/8 h dark, with light intensity adjusted to 120 µmol m−2 s−1).
Plasmid construction
To construct the overexpression vector, PCR was used to amplify the cDNA of WT plants, and products were then cloned into the pCAMBIA-1307FLAG vector with the 35S promoter using the SalI and SacI sites, with the FLAG tag preceding the coding sequence (CDS) of genes (Manishankar et al. 2018). To construct the constitutively active vector, PCR was used to amplify the CDS of CPK23, and products without calcium binding domain sequence (1 to 981 bp) were then cloned into the pCAMBIA-1307FLAG vector with the 35S promoter using the SalI and SacI sites, with the FLAG tag preceding the CDS. For genetic complementation analysis, full-length genomic DNA of PHT1;1 (containing 2,750 bp promoter and 1,725 bp genomic sequence) was amplified from WT genomic DNA and cloned into the pCAMBIA-1300 vector using the EcoRI and HindIII sites. Site mutagenesis of PHT1;1 was performed using the Tiangen Rapid Site-Directed Mutagenesis Kit. Agrobacterium tumefaciens strain GV3101 was used to transform WT or mutant plants. For BiFC analysis, the full-length CDS of CPK23 was amplified by PCR from WT cDNA and then cloned into the nYFP vector with the 35S promoter through the XbaI and BamHI sites. The full-length CDSs of PHT1;1 and PHT1;4 were cloned into the cYFP vector with the 35S promoter via the XbaI and BamHI sites. For the LCI assay, the full-length CDS of CPK23 was cloned into the pCAMBIA 1300-nLUC vector with the 35S promoter through the KpnI and SalI sites. The full-length CDSs of PHT1;1 and PHT1;4 were cloned into the pCAMBIA 1300-cLUC vector with the 35S promoter via the KpnI and SalI sites (Su et al. 2021). To construct recombinant protein vectors, CPK23 and PHT1;1 were amplified and cloned into the pET28a and pGEX4T-1 vectors to obtain CPK23-His, GST-PHT1;1-N, GST-PHT1;1-M, GST-PHT1;1-C. The GST tag precedes the CDS, while the His tag follows the CDS.
qPCR
Plant total RNA was extracted from 10-d-old seedlings using RNA simple total RNA kit (Tiangen), and the first-strand cDNA was synthesized by total RNA with FastKing RT kit (Tiangen). ACTIN2 was used as an internal control. qPCR was performed according to the instructions provided for the Real-time PCR instrument (CFX connect, BioRad, USA) using ChamQ SYBR qPCR Master Mix (Vazyme). Each sample was quantified at least in triplicate and normalized using ACTIN2 gene as an internal control. Statistical differences were calculated by two-way ANOVA. Different letters indicate means that were significantly different according to Tukey's multiple testing method (P < 0.05). The specific primers used are listed in Supplemental Table S3.
Elemental analysis
After 2 wk culture, WT seedlings as well as the transgenic seedlings by hydroponics were transferred to As(V) stress conditions for 2 wk. Plant samples and 5 mL of nitric acid were added to digestion tubes and digested at 120 °C for 6 h. Subsequently, 2 mL of H2O2 was added to the tubes twice and the temperature was maintained at 120 °C. Then, the temperature was increased to 170 °C, and the temperature was held at 170 °C until the digestion was complete. Finally, the samples were equalized in volume by the addition of ddH2O. The samples were analyzed by flame atomic absorption spectrometry (FAAS).
YC3.6 (GcaMP6)-based [Ca2+]cyt imaging
For Ca2+ imaging with the YC3.6 and GcaMP6 transgenic seedlings at the root tip and the partially elongated region of roots (near the root tip), seedlings were germinated and grown on a tissue culture plate with basal medium and 1% (w/v) phytoagar under long light at 22 °C for 4 d. To record YC3.6 fluorescence images, the excitation was provided at 438 nm and images were collected at emission 500 to 600 nm. To record GcaMP6 fluorescence images, the excitation was provided at 488 nm and images were collected at emission 500 to 600 nm. The scanning resolution was set at 1,024 × 1,024 pixels. Before time-lapse recording, the transgenic seedlings were transferred to a 1.5-mL tube with water and treated in the dark for 2 h. The seedlings were loaded onto the slide and imaged with a 20× objective lens after stimulation. Determine the average fluorescence intensity of the area in the root of each image through ImageJ. To make a video, individual images were cropped using Adobe Photoshop software and saved in PNG format and generated using ImageJ with the cropped images.
IP-MS
The full-length CDS of CPK23 was cloned into the pCAMBIA-1307FLAG vector with the 35S promoter for the construction of the CPK23-Flag lines while the empty pCAMBIA-1307FLAG vector with the 35S promoter was used for the construction of the Flag lines. The extracted total protein of A. thaliana CPK23-Flag and Flag lines was extracted with IP buffer, and enriched with anti-FLAG agarose beads (Proteintech, 20531-1-AP). Purified proteins were subjected to SDS-PAGE gel electrophoresis, and then the gel was cut into 1-mm3 pieces with a razor blade and placed into a 1.5-mL EP tube for intragel enzymolysis. The gel pieces were decolorized until transparent at room temperature with 600 μL of decolorizing solution (10% [v/v] glacial acetic acid and 40% [v/v] methyl alcohol) in each tube. After reduction and alkylation, the protein is enzymatically hydrolyzed with trypsin and incubated at 37 °C for more than 6 h. After an appropriate amount of acetonitrile was added to each tube, the supernatant was retained. This was repeated twice, and the supernatant was spun until dry at room temperature with a centrifugal concentrator. After 100 μL Pierce C18 Tip was used for desalting, the sample was dried with a centrifugal concentrator at room temperature and then re-dissolved in 0.1% (w/v) FA. The peptides were separated by liquid chromatography and analyzed by tandem mass spectrometry. The digestion peptides were separated by HPLC (Ultimate 3000 RSLCnano). RP-C18 analytical column (Acclaim PepMapTM RSLC 75 μm * 25 cm) was first saturated with Buffer A (0.1% [v/v] formic acid). Peptides were automatically loaded onto the Trap column (Acclaim PepMapTM 100 75 μm* 2 cm) and separated at a flow rate of 400 nL min−1. The following gradients of Buffer B (0.1% [v/v] formic acid and 80% [v/v] acetonitrile) were used for the indicated times: 8% to 32%, 124 min; 32% to 90%, 1 min; maintained at 90%, 1 min. The mass spectrum data were detected by the QExactive HF-X mass spectrometer with Nanospray Flex ESI ion source that was operated in data-dependent collection mode. The primary mass spectrum resolution was 60,000 (200 m z−1). Twenty parent ions with the highest ion strength in the full scan were selected for fragmentation using high-energy collision pyrolysis for secondary mass spectrometry detection. The secondary mass spectrum resolution was 15,000 (200 m z−1). The detection time was 140 min, using cation detection mode. The full scanning range of the mass spectrum was 350 to 1,800 m z−1. MS raw output data were obtained and imported into Proteome Discoverer (version 2.4) for software analysis, and Triticum_Aestivum database was used for the qualitative analysis of proteins. The differential proteins between CPK23-Flag and Flag lines were screened out for the CPK23-interacted protein discovery.
Y2H experiment
Yeast (S. cerevisiae) two-hybrid analysis was performed using a DUAL membrane kit3 (Dualsystems Biotech) according to the manufacturer's instructions. The coding sequences of CPK23 and PHT1s (PHT1;1, PHT1;4) genes were cloned into the vectors pPR3-N and pBT3-N as described previously (Su et al. 2021). Interactions were shown on SD/-LT, SD/-LTHA, and SD/-LTHA medium supplemented with 2.5 mM 3-AT.
BiFC assay
A BiFC assay was conducted according to a previously published method (Ju et al. 2022). In brief, resuspension buffer (10 mM MgCl2; 10 mM MES, pH = 5.6) was used to adjust the final concentration of the strain to a specific OD at 600 nm (OD600). The OD600 values of CPK23-nYFP and PHT1;1/PHT1;4-cYFP were adjusted to 1.5, and that of P19 was adjusted to 1.0. CPK23-nYFP and PHT1;1/PHT1;4-cYFP constructs were transiently expressed in N. benthamiana leaves. The YFP fluorescent signals in the N. benthamiana leaves were detected 48 h after transfection (Olympus IX83-FV3000). EYFP fluorescence was visualized by scanning with a 514 nm laser at 586 [V] PMT voltage and 530 to 630 nm spectral detection. CPK23-nYFP and GUS-cYFP, PHT1;1/PHT1;4-cYFP and GUS-nYFP were used as negative controls.
LCI assay
LCI was conducted according to a previously published method (Ju et al. 2022). In brief, the resuspension method of Agrobacterium tumefaciens is similar to the BiFC assay. The OD600 values of CPK23-nLUC and PHT1;1/PHT1;4-cLUC were adjusted to 1.5, and that of P19 was adjusted to 1.0. A. tumefaciens containing CPK23-nLUC or PHT1;1/PHT1;4-cLUC constructs were transformed into N. benthamiana leaves and expressed for 48 h, then the signals were detected by CCD (Princeton, 28 Lumazone Pylon 2048B). CPK23-nLUC and GUS-cLUC, PHT1;1/PHT1;4-cLUC and GUS-nLUC were used as negative controls.
Protein purification
For Escherichia coli protein extraction, the bacterial solution was induced overnight with the addition of an appropriate concentration of IPTG, and the cell wall was broken ultrasonically, the supernatant was obtained by centrifugation with 12,000×g and passed through agarose beads with the corresponding label. Finally, the target protein was eluted and collected with the corresponding elution buffer (100 mM reduced L-glutathione or 200 mM imidazole added to PBS). For protein purification, the following agarose beads were used: GST Settled Resin (Sangon Biotech, C600031) and Ni-NTA 6FF Settled Resin (Sangon Biotech, C600033). For plant protein extraction, the ground samples were added to IP buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 10% (v/v) glycerol, 5 mM MgCl2, 0.5% NP-40, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 1× protease inhibitor cocktail [Roche], and 1 mM DTT) and left to stand for 1 h, after which the supernatant was centrifuged with 12,000×g, and the polyclonal antibody corresponding to the label was added to the supernatant. After 2 h rotary incubation, agarose coagulation beads were added and rotated for another 2 h. Finally, the target protein was eluted and collected with the corresponding elution buffer.
After the target protein was extracted, protein loading buffer was added to the sample, and the protein was denatured by heating at 95 °C for 10 min. The samples were added to SDS-PAGE protein gel wells as needed for the experiment. PVDF membranes were cut to a suitable size, and the proteins were transferred at a constant current of 200 mA for 2 h. The membranes were incubated with 5% (w/v) nonfat dry milk for 2 h at room temperature, and an appropriate primary/secondary antibody was selected for incubation for 2 h at room temperature. The membranes were washed 3 times with TBST buffer at the end of each incubation. Finally, developed images were obtained using a chemiluminescence imager (Cell Signaling Technology).
In vitro and semi-in vivo phosphorylation analysis
An in vitro kinase assay was conducted according to a previously published method (Zhang et al. 2021b). Recombinant plasmids were transformed into E. coli BL21, and recombinant proteins were purified separately. First, kinase buffer containing 20 mM Tris-HCl (pH 7.4), 5 mM MgCl2, 0.01 mM CaCl2, 1 mM MnCl2, and 2 mM DTT, 1 μg of CPK23 kinase protein and 5 μg of PHT1;1-N/M/C protein was prepared. Then, the protein kinase assay was performed by adding 0.9 μL of 1 mM ATP and 1 μCi [γ-32P], and phosphorylation was initiated by adding 1 μCi [γ-32P]. After incubation for 30 min at 30 °C, the reactions were stopped by adding 5 μL of 5× SDS loading buffer and boiling for 10 min. Through SDS-PAGE of the reaction products, the phosphorylated proteins were visualized by autoradiography.
A semi-in vivo protein kinase assay was conducted according to a previously published method (Ju et al. 2022). Seven-day-old CPK23-OE transgenic seedlings growing in 1/2 MS medium were transferred to 60 μ M Na2HAsO4 stress medium (1/2 MS + 60 μ M Na2HAsO4) for the indicated times (0, 1, 6, 12, and 23 h). Total proteins were extracted with IP buffer and incubated with an anti-FLAG polyclonal antibody (Proteintech, 20531-1-AP) for 2 h. After that, Protein A/G PLUS-Agarose beads (Santa, D0116) were added and rotated for another 2 h. Finally, CPK23-Flag protein was eluted and collected with the PBS buffer containing 0.25 mg mL−1 3× FLAG peptide (SIGMA, SLCD1730).
For semi-in vivo phosphorylation assays, the protein kinase assay was performed with 2 μg of extracted protein and 5 μg of PHT1;1-C protein was prepared. The FLAG signals were detected with an anti-FLAG antibody (TransGen Biotech), and the procedures of kinase assay were similar to those of the in vitro kinase assay.
Subcellular localization of PHT1;1
Using T3 stable lines of PHT1;1-GFP transgenic plants and their variants, GFP fluorescence was observed with confocal microscopy (Olympus IX83-FV3000) The seeds were sown in a nutrient medium for 4 d and then transferred to 1/2 MS with or without 100 µ M Na2HAsO4 for 24 h. Seedlings were stained for 10 s with 10 μ M propidium iodide (PI) before imaging. Three lines were selected for each transgenic plant, and fluorescence was observed using a confocal microscope (Olympus IX83-FV3000). EGFP fluorescence was visualized by scanning with a 488 nm laser at 750 [V] PMT voltage and 500 to 540 nm spectral detection. And mCherry fluorescence for PI dye was visualized by scanning with a 561 nm laser at 655 [V] PMT voltage and 570 to 670 nm spectral detection. The relative signal content ratio of each fluorescence is obtained by ImageJ. Three independent replicates were selected for statistical analysis. The relative intensities of fluorescence were presented as the means.
Statistical analysis
The statistical significance of differences between mean values was determined using Student's t-tests or two-way ANOVA with Tukey's multiple comparison test. Different letters indicate means that were significantly different according to two-way ANOVA with Tukey's multiple testing method (P < 0.05). Different asterisks against the error bars of histograms indicate means that were statistically different (P < 0.05) or significantly different (P < 0.01) according to Student's t-tests. “n” indicates the number of biological replicates. For the analysis of plate experiments, the results were presented as the means ± Sd (n = 5 biological replicates, 15 seedlings). For the qPCR analysis, the results are presented as the mean ± Sd (n = 3 biological replicates, 4 technical replicates for each biological replicate). For the analysis of the relative fluorescence intensity of YC3.6-based and GCaMP6-based Ca2+ imaging, the results were presented as the means ± Sd (n = 4 biological replicates, 4 seedlings). For the protein kinase assay, 2 replicates were assessed. For the analysis of the colocalization, the plugin Coloc 2 (Arena et al. 2017; Stauffer et al. 2018) in Fiji (ImageJ) (Collins 2007; Schindelin et al. 2012) was used for making colocalization scatter plot and Plot Profile in Fiji (ImageJ) was used for measuring the relative fluorescence intensity at the white arrow, the results were presented as the means (n = 3 biological replicates, 3 seedlings), and a representative image is shown.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers: AT4G04740 (CPK23), AT5G43350 (PHT1;1), AT2G38940 (PHT1;4).
Supplementary Material
Acknowledgments
We thank Dr Kun-Hsiang Liu for providing GCaMP6 sensor lines, Dr Xueling Huang, Dr Fengping Yuan, and Hua Zhao (State Key Laboratory of Crop Stress Biology in Arid Areas, Northwest A&F University, Yangling, China) for instrument operation guidance, and Dr Xiaolin Shan and Dr Fenglian Lv (College of Natural Resources and Environment, Northwest A&F University, Yangling, China) for experiment assistance in FAAS.
Contributor Information
Yisong Liu, State Key Laboratory of Crop Stress Biology for Arid Areas and College of Life Sciences, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China.
Yanting Zhang, State Key Laboratory of Crop Stress Biology for Arid Areas and College of Life Sciences, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China.
Zhangqing Wang, State Key Laboratory of Crop Stress Biology for Arid Areas and College of Life Sciences, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China.
Shiyuan Guo, State Key Laboratory of Crop Stress Biology for Arid Areas and College of Life Sciences, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China.
Yanjun Fang, State Key Laboratory of Crop Stress Biology for Arid Areas and College of Life Sciences, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China.
Zhenqian Zhang, State Key Laboratory of Crop Stress Biology for Arid Areas and College of Life Sciences, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China.
Huiling Gao, State Key Laboratory of Crop Stress Biology for Arid Areas and College of Life Sciences, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China.
Huimin Ren, State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Hangzhou, Zhejiang 311300, China.
Cun Wang, State Key Laboratory of Crop Stress Biology for Arid Areas and College of Life Sciences, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China; Institute of Future Agriculture, Northwest Agriculture & Forestry University, Yangling, Shaanxi 712100, China.
Author contributions
Y.L. and C.W. designed the project. Y.L. performed the experiments. Y.L., Y.Z., and H.G. analyzed the data. Y.Z., Z.W., and S.G. constructed parts of the vectors. Y.L., Y.Z., and Z.W. wrote the manuscript. Y.F., Z.Z., H.G., and H.R. provide technological support and revised the manuscript. All authors are approved the contents of the manuscript.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1 . Phenotypic analysis of CPK single mutants.
Supplemental Figure S2 . CPK23 expression level detection.
Supplemental Figure S3 . Phenotypic analysis of mutants and overexpression plants under phosphorus deficiency.
Supplemental Figure S4 . Protein expression in N. benthamiana leaves in LCI and BiFC assays.
Supplemental Figure S5 . Genetic analysis between cpk23 and pht1;1 under As(V) stress.
Supplemental Figure S6 . CPK23 phosphorylate PHT1;1-C in vitro.
Supplemental Figure S7 . Semi-in vivo phosphorylation analysis of CPK23 with PHT1;1-C.
Supplemental Figure S8 . Phenotypic analysis of ProPHT1;1:PHT1;1S520A and ProPHT1;1:PHT1;1S520D transgenic plants.
Supplemental Figure S9 . Phenotypic analysis of PHT1;1 and PHT1;4 mutants under phosphorus deficiency.
Supplemental Table S1 . IP-MS data of CPK23 potential interacting proteins.
Supplemental Table S2 . T-DNA insertion mutants used in this study.
Supplemental Table S3 . Primers used in this study.
Supplemental Video S1 . Live imaging of cytosolic Ca2+ dynamics of Arabidopsis root exposed to NaCl.
Supplemental Video S2 . Live imaging of cytosolic Ca2+ dynamics of Arabidopsis root exposed to Na2HAsO4.
Supplemental Video S3 . Live imaging of cytosolic Ca2+ dynamics of Arabidopsis root exposed to Na2HPO4.
Supplemental Video S4 . Live imaging of cytosolic Ca2+ dynamics of Arabidopsis root exposed to Na2HAsO4 with LaCl3.
Funding
This research was funded by a grant from the National Natural Science Foundation of China (32222008 to C.W, 32100215 to PP.H, 31900236 to ZQ.Z), and partly supported by the open funds of the China Postdoctoral Science Foundation (2018M643740 to ZQ.Z), and Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2019JQ-150 to ZQ.Z). Supported by the open Project of the State Key Laboratory of Crop Stress Biology for Arid Areas (CSBAAKF2021008 to C.W) and the State Key Laboratory of Subtropical Silviculture (SKLSS-KF2022-08 to C.W).
Data availability
All the data and materials that support the findings of this study are available upon request from the corresponding author.
References
- Arena ET, Rueden CT, Hiner MC, Wang S, Yuan M, Eliceiri KW. Quantitating the cell: turning images into numbers with ImageJ. Wiley Interdiscip Rev Dev Biol. 2017:6(2):e260. 10.1002/wdev.260 [DOI] [PubMed] [Google Scholar]
- Bayle V, Arrighi J-F, Creff A, Nespoulous C, Vialaret J, Rossignol M, Gonzalez E, Paz-Ares J, Nussaume L. Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation. Plant Cell. 2011:23(4):1523–1535. 10.1105/tpc.110.081067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera S, Wang N, Zhang C, Schmitz-Thom I, Strohkamp S, Schültke S, Hashimoto K, Xiong L, Kudla J. Analyses of Ca2+ dynamics using a ubiquitin-10 promoter-driven Yellow Cameleon 3.6 indicator reveal reliable transgene expression and differences in cytoplasmic Ca2+ responses in Arabidopsis and rice (Oryza sativa) roots. New Phytol. 2015:206(2):751–760. 10.1111/nph.13250 [DOI] [PubMed] [Google Scholar]
- Bienert GP, Thorsen M, Schüssler MD, Nilsson HR, Wagner A, Tamás MJ, Jahn TP. A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol. 2008:6(26):1–15. 10.1186/1741-7007-6-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Sheen J. CDPKs in immune and stress signaling. Trends Plant Sci. 2013:18(1):30–40. 10.1016/j.tplants.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, Mccormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J, et al. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature. 2010:464(7287):418–422. 10.1038/nature08794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo G, Sánchez-Bermejo E, Lorenzo LD, Crevillén P, Fraile-Escanciano A, Mohan TC, Mouriz A, Catarecha P, Sobrino-Plata J, Olsson S, et al. WRKY6 Transcription factor restricts arsenate uptake and transposon activation in Arabidopsis. Plant Cell. 2013:25(8):2944–2957. 10.1105/tpc.113.114009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty D, Trivedi PK, Misra P, Tiwari M, Shri M, Shukla D, Kumar S, Rai A, Pandey A, Nigam D, et al. Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere. 2009:74(5):688–702. 10.1016/j.chemosphere.2008.09.082 [DOI] [PubMed] [Google Scholar]
- Chen QY, Desmarais T, Costa M. Metals and mechanisms of carcinogenesis. Annu Rev Pharmacol Toxicol. 2019:59:537–554. 10.1146/annurev-pharmtox-010818-021031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ding Y, Yang Y, Song C, Wang B, Yang S, Guo Y, Gong Z. Protein kinases in plant responses to drought, salt, and cold stress. J Integr Plant Biol. 2021:63(1):53–78. 10.1111/jipb.13061 [DOI] [PubMed] [Google Scholar]
- Chen J, Wang Y, Wang F, Yang J, Gao M, Li C, Liu Y, Liu Y, Yamaji N, Ma JF, et al. The rice CK2 kinase regulates trafficking of phosphate transporters in response to phosphate levels. Plant Cell. 2015:27(3):711–723. 10.1105/tpc.114.135335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013:499(7458):295–300. 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins TJ. ImageJ for microscopy. Biotechniques. 2007:43(1 Suppl):25–30.. 10.2144/000112517 [DOI] [PubMed] [Google Scholar]
- Defalco TA, Bender KW, Snedden WA. Breaking the code: Ca2+ sensors in plant signalling. Biochem J. 2010:425(1):27–40. 10.1042/BJ20091147 [DOI] [PubMed] [Google Scholar]
- Demir F, Horntrich C, Blachutzik JO, Scherzer S, Reinders Y, Kierszniowska S, Schulze WX, Harms GS, Hedrich R, Geiger D, et al. Arabidopsis nanodomain-delimited ABA signaling pathway regulates the anion channel SLAH3. Proc Natl Acad Sci U S A. 2013:110(20):8296–8301. 10.1073/pnas.1211667110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q, Wallrad L, Almutairi BO, Kudla J. Ca2+ signaling in plant responses to abiotic stresses. J Integr Plant Biol. 2022:64(2):287–300. 10.1111/jipb.13228 [DOI] [PubMed] [Google Scholar]
- Fayiga AO, Saha UK. Arsenic hyperaccumulating fern: implications for remediation of arsenic contaminated soils. Geoderma. 2016:284:132–143. 10.1016/j.geoderma.2016.09.003 [DOI] [Google Scholar]
- Franz S, Ehlert B, Liese A, Kurth J, Cazalé AC, Romeis T. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol Plant. 2011:4(1):83–96. 10.1093/mp/ssq064 [DOI] [PubMed] [Google Scholar]
- Fu SF, Chen PY, Nguyen QT, Huang LY, Zeng GR, Huang TL, Lin CY, Huang HJ. Transcriptome profiling of genes and pathways associated with arsenic toxicity and tolerance in Arabidopsis. BMC Plant Biol. 2014:14:94. 10.1186/1471-2229-14-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Zhang Z, Wallrad L, Wang Z, Höller S, Ju C, Schmitz-Thom I, Huang P, Wang L, Peiter E, et al. Ca2+-dependent phosphorylation of NRAMP1 by CPK21 and CPK23 facilitates manganese uptake and homeostasis in Arabidopsis. Proc Natl Acad Sci U S A. 2022:119(40):e2204574119. 10.1073/pnas.2204574119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Maierhofer T, Al-Rasheid KAS, Scherzer S, Mumm P, Liese A, Ache P, Wellmann C, Marten I, Grill E, et al. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci Signal. 2011:4(173):ra32. 10.1126/scisignal.2001346 [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al-Rasheid KAS, Grill E. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci U S A. 2010:107(17):8023–8028. 10.1073/pnas.0912030107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González E, Solano R, Rubio V, Paz-Ares LJ. PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 is a plant-specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidopsis. Plant Cell. 2005:17(12):3500–3512. 10.1105/tpc.105.036640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel LP, Sheen J, Séguin A. Ancient signals: comparative genomics of green plant CDPKs. Trends Plant Sci. 2014:19(2):79–89. 10.1016/j.tplants.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon AC, Gribskov M, Gubrium E, Harper JF. The CDPK superfamily of protein kinases. New Phytol. 2001:151(1):175–183. 10.1046/j.1469-8137.2001.00171.x [DOI] [PubMed] [Google Scholar]
- Huang TL, Nguyen QTT, Fu SF, Lin CY, Chen YC, Huang HJ. Transcriptomic changes and signalling pathways induced by arsenic stress in rice roots. Plant Mol Biol. 2012:80(6):587–608. 10.1007/s11103-012-9969-z [DOI] [PubMed] [Google Scholar]
- Ji R, Zhou L, Liu J, Wang Y, Yang L, Zheng Q, Zhang C, Zhang B, Ge H, Yang Y, et al. Calcium-dependent protein kinase CPK31 interacts with arsenic transporter AtNIP1; 1 and regulates arsenite uptake in Arabidopsis thaliana. PLoS One. 2017:12(3):e0173681. 10.1371/journal.pone.0173681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian FM, Yamaji N, Mitani N, Xu XY, Su YH, Mcgrath SP, Zhao FJ. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A. 2008:105(29):9931–9935. 10.1073/pnas.0802361105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju C, Zhang Z, Deng J, Miao C, Wang Z, Wallrad L, Javed L, Fu D, Zhang T, Kudla J, et al. Ca2+-dependent successive phosphorylation of vacuolar transporter MTP8 by CBL2/3-CIPK3/9/26 and CPK5 is critical for manganese homeostasis in Arabidopsis. Mol Plant. 2022:15(3):419–437. 10.1016/j.molp.2021.11.012 [DOI] [PubMed] [Google Scholar]
- Kawamoto N, Sasabe M, Endo M, Machida Y, Araki T. Calcium-dependent protein kinases responsible for the phosphorylation of a bZIP transcription factor FD crucial for the florigen complex formation. Sci Rep. 2015:5:8341. 10.1038/srep08341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J, Becker D, Grill E, Hedrich R, Hippler M, Kummer U, Parniske M, Romeis T, Schumacher K. Advances and current challenges in calcium signaling. New Phytol. 2018:218(2):413–431. 10.1111/nph.14966 [DOI] [PubMed] [Google Scholar]
- Kumar V, Vogelsang L, Seidel T, Schmidt R, Weber M, Reichelt M, Meyer A, Clemens S, Sharma SS, Dietz KJ. Interference between arsenic-induced toxicity and hypoxia. Plant Cell Environ. 2019:42(2):574–590. [DOI] [PubMed] [Google Scholar]
- Leblanc MS, Mckinney EC, Meagher RB, Smith AP. Hijacking membrane transporters for arsenic phytoextraction. J Biotechnol. 2013:163(1):1–9. 10.1016/j.jbiotec.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Santoni V, Maurel C. Plant aquaporins: roles in plant physiology. Biochim Biophys Acta. 2014:1840(5):1574–1582. 10.1016/j.bbagen.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Li N, Wang J, Won-Yong S. Arsenic uptake and translocation in plants. Plant Cell Physiol. 2015:57(1):4–13. [DOI] [PubMed] [Google Scholar]
- Liu KH, Niu Y, Konishi M, Wu Y, Du H, Chung HS, Li L, Boudsocq M, Mccormack M, Maekawa S, et al. Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature. 2017:545(7654):311–316. 10.1038/nature22077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S, Wang C. Calcium signaling mechanisms across kingdoms. Annu Rev Cell Dev Biol. 2021:37:311–340. 10.1146/annurev-cellbio-120219-035210 [DOI] [PubMed] [Google Scholar]
- Luo X, Chen Z, Gao J, Gong Z. Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis. Plant J. 2014:79(1):44–55. 10.1111/tpj.12534 [DOI] [PubMed] [Google Scholar]
- Ma SY, Wu WH. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol Biol. 2007:65(4):511–518. 10.1007/s11103-007-9187-2 [DOI] [PubMed] [Google Scholar]
- Manishankar P, Wang N, Köster P, Alatar AA, Kudla J. Calcium signaling during salt stress and in the regulation of ion homeostasis. J Exp Bot. 2018:69(17):4215–4226. 10.1093/jxb/ery201 [DOI] [PubMed] [Google Scholar]
- Matschullat J. Arsenic in the geosphere—a review. Sci Total Environ. 2000:249(1–3):297–312. 10.1016/S0048-9697(99)00524-0 [DOI] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, Mccaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997:388(6645):882–887. 10.1038/42264 [DOI] [PubMed] [Google Scholar]
- Modareszadeh M, Bahmani R, Kim D, Hwang S. Decreases in arsenic accumulation by the plasma membrane intrinsic protein PIP2; 2 in Arabidopsis and yeast. Environ Pollut. 2021:275:116646. 10.1016/j.envpol.2021.116646 [DOI] [PubMed] [Google Scholar]
- Mohanta TK, Yadav D, Khan AL, Hashem A, Abd Allah EF, Al-Harrasi A. Molecular players of EF-hand containing calcium signaling event in plants. Int J Mol Sci. 2019:20(6):1476. 10.3390/ijms20061476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosa KA, Kumar K, Chhikara S, Mcdermott J, Liu Z, Musante C, White JC, Dhankher OP. Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Res. 2012:21(6):1265–1277. 10.1007/s11248-012-9600-8 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay R, Bhattacharjee H, Rosen BP. Aquaglyceroporins: generalized metalloid channels. Biochim Biophys Acta. 2014:1840(5):1583–1591. 10.1016/j.bbagen.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan VK, Jain A, Poling M, Lewis AJ, Raghothama KG, Smith AP. Arabidopsis Pht1; 5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiol. 2011:156(3):1149–1163. 10.1104/pp.111.174805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro C, Mateo-Elizalde C, Mohan TC, Sánchez-Bermejo E, Urrutia O, Fernández-Muñiz MN, García-Mina JM, Muñoz R, Paz-Ares J, Castrillo G. Arsenite provides a selective signal that coordinates arsenate uptake and detoxificacion involving regulation of PHR1 stability in Arabidopsis thaliana. Mol Plant. 2021:14(9):1489–1507. 10.1016/j.molp.2021.05.020 [DOI] [PubMed] [Google Scholar]
- Palmer AE, Tsien RY. Measuring calcium signaling using genetically targetable fluorescent indicators. Nat Protoc. 2006:1(3):1057–1065. 10.1038/nprot.2006.172 [DOI] [PubMed] [Google Scholar]
- Perochon A, Aldon D, Galaud JP, Ranty B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie. 2011:93(12):2048–2053. 10.1016/j.biochi.2011.07.012 [DOI] [PubMed] [Google Scholar]
- Polizzotto ML, Harvey CF, Li G, Badruzzman B, Ali A, Newville M, Sutton S, Fendorf S. Solid-phases and desorption processes of arsenic within Bangladesh sediments. Chem Geol. 2006:228(1–3):97–111. 10.1016/j.chemgeo.2005.11.026 [DOI] [Google Scholar]
- Remy E, Cabrito TR, Batista RA, Teixeira MC, Sá-Correia I, Duque P. The Pht1;9 and Pht1;8 transporters mediate inorganic phosphate acquisition by the Arabidopsis thaliana root during phosphorus starvation. New Phytol. 2012:195(2):356–371. 10.1111/j.1469-8137.2012.04167.x [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012:9(7):676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012:9(7):671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SS, Kumar V, Dietz KJ. Emerging trends in metalloid-dependent signaling in plants. Trends Plant Sci. 2020:26(5):452–471. 10.1016/j.tplants.2020.11.003 [DOI] [PubMed] [Google Scholar]
- Shin H, Shin HS, Dewbre GR, Harrison MJ. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 2004:39(4):629–642. 10.1111/j.1365-313X.2004.02161.x [DOI] [PubMed] [Google Scholar]
- Singh N, Ma LQ, Srivastava M, Rathinasabapathi B. Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L and Pteris ensiformis L. Plant Sci. 2006:170(2):274–282. 10.1016/j.plantsci.2005.08.013 [DOI] [Google Scholar]
- Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem. 2002:17(5):517–568. 10.1016/S0883-2927(02)00018-5 [DOI] [Google Scholar]
- Stauffer W, Sheng H, Lim HN. Ezcolocalization: an ImageJ plugin for visualizing and measuring colocalization in cells and organisms. Sci Rep. 2018:8(1):15764. 10.1038/s41598-018-33592-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Wang T, Ju C, Deng J, Zhang T, Li M, Tian H, Wang C. Abscisic acid signaling negatively regulates nitrate uptake via phosphorylation of NRT1. 1 by SnRK2s in Arabidopsis. J Integr Plant Biol. 2021:63(3):597–610. [DOI] [PubMed] [Google Scholar]
- Takayuki A, Nagao H, Shoshi K, Ryu O. CDPK-mediated abiotic stress signaling. Plant Signal Behav. 2012:7(7):817–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Wang HQ, Chen J, Chang JD, Zhao FJ. Molecular mechanisms underlying the toxicity and detoxification of trace metals and metalloids in plants. J Integr Plant Biol. 2022:65(2):570–593. [DOI] [PubMed] [Google Scholar]
- Valmonte GR, Arthur K, Higgins CM, MacDiarmid RM. Calcium-dependent protein kinases in plants: evolution, expression and function. Plant Cell Physiol. 2014:55(3):551–569. [DOI] [PubMed] [Google Scholar]
- van Kleeff PJM, Gao J, Mol S, Zwart N, Zhang H, Li KW, de Boer AH. The Arabidopsis GORK K(+)-channel is phosphorylated by calcium-dependent protein kinase 21 (CPK21), which in turn is activated by 14-3-3 proteins. Plant Physiol Biochem. 2018:125:219–231. 10.1016/j.plaphy.2018.02.013 [DOI] [PubMed] [Google Scholar]
- Wang H, Xu Q, Kong YH, Chen Y, Duan JY, Wu WH, Chen YF. Arabidopsis WRKY45 transcription factor activates PHOSPHATE TRANSPORTER1; 1 expression in response to phosphate starvation. Plant Physiol. 2014:164(4):2020–2029. 10.1104/pp.113.235077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JP, Xu YP, Munyampundu JP, Liu TY, Cai XZ. Calcium-dependent protein kinase (CDPK) and CDPK-related kinase (CRK) gene families in tomato: genome-wide identification and functional analyses in disease resistance. Mol Genet Genomics. 2016:291(2):661–676. 10.1007/s00438-015-1137-0 [DOI] [PubMed] [Google Scholar]
- Wei P, Demulder M, David P, Eekhout T, Yoshiyama KO, Nguyen L, Vercauteren I, Eeckhout D, Galle M, De Jaeger G, et al. Arabidopsis casein kinase 2 triggers stem cell exhaustion under Al toxicity and phosphate deficiency through activating the DNA damage response pathway. Plant Cell. 2021:33(4):1361–1380. 10.1093/plcell/koab005 [DOI] [PubMed] [Google Scholar]
- Yang L, Zhang Y, Guan R, Li S, Xu X, Zhang S, Xu J. Co-regulation of indole glucosinolates and camalexin biosynthesis by CPK5/CPK6 and MPK3/MPK6 signaling pathways. J Integr Plant Biol. 2020:62(11):1780–1796. 10.1111/jipb.12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Fu D, Sun Z, Ju C, Miao C, Wang Z, Xie D, Ma L, Gong Z, Wang C. A tonoplast-associated calcium signaling regulates manganese homeostasis in Arabidopsis. Mol Plant. 2021b:14(5):805–819. 10.1016/j.molp.2021.03.003 [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu D, Yang B, Liu WZ, Mu B, Song H, Chen B, Li Y, Ren D, Deng H, et al. Arabidopsis CPK6 positively regulates ABA signaling and drought tolerance through phosphorylating ABA-responsive element-binding factors. J Exp Bot. 2020:71(1):188–203. 10.1093/jxb/erz432 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Z, Liu Y, Zhang T, Liu J, You Z, Huang P, Zhang Z, Wang C. Plasma membrane-associated calcium signaling modulates cadmium transport. New Phytol. 2022:238(1):313–331. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zhang C, Liu C, Fu A, Luan S. A Golgi-localized manganese transporter functions in pollen tube tip growth to control male fertility in Arabidopsis. Plant Commun. 2021a:2(3):100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lan W, Jiang Y, Fang W, Luan S. A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol Plant. 2014:7(2):369–376. 10.1093/mp/sst125 [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang X, He Y, Sang T, Wang P, Dai S, Zhang S, Meng X. Differential phosphorylation of the transcription factor WRKY33 by the protein kinases CPK5/CPK6 and MPK3/MPK6 cooperatively regulates camalexin biosynthesis in Arabidopsis. Plant Cell. 2020:32(8):2621–2638. doi: 10.1105/tpc.19.00971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YG, Yoshinaga M, Zhao FJ, Rosen BP. Earth abides arsenic biotransformations. Annu Rev Earth Planet Sci. 2014:42:443–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SY, Yu XC, Wang XJ, Zhao R, Li Y, Fan RC, Shang Y, Du SY, Wang XF, Wu FQ, et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell. 2007:19(10):3019–3036. 10.1105/tpc.107.050666 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data and materials that support the findings of this study are available upon request from the corresponding author.