Abstract
Castor (Ricinus communis L.) is a dicotyledonous oilseed crop that can have either spineless or spiny capsules. Spines are protuberant structures that differ from thorns or prickles. The developmental regulatory mechanisms governing spine formation in castor or other plants have remained largely unknown. Herein, using map-based cloning in 2 independent F2 populations, F2-LYY5/DL01 and F2-LYY9/DL01, we identified the RcMYB106 (myb domain protein 106) transcription factor as a key regulator of capsule spine development in castor. Haplotype analyses demonstrated that either a 4,353-bp deletion in the promoter or a single nucleotide polymorphism leading to a premature stop codon in the RcMYB106 gene could cause the spineless capsule phenotype in castor. Results of our experiments indicated that RcMYB106 might target the downstream gene RcWIN1 (WAX INDUCER1), which encodes an ethylene response factor known to be involved in trichome formation in Arabidopsis (Arabidopsis thaliana) to control capsule spine development in castor. This hypothesis, however, remains to be further tested. Nevertheless, our study reveals a potential molecular regulatory mechanism underlying the spine capsule trait in a nonmodel plant species.
Genetic analysis of capsule spine formation and development in castor.
Introduction
Castor (Ricinus communis L.), a member of the spurge (Euphorbiaceae) family, is a tropical perennial shrub and vital oilseed crop (Chan et al. 2010). This species originated in Africa but is now widely cultivated in much of the world. Because castor seeds contain ∼46% to 55% oil, and ricinolate makes up the majority of castor oil (Scarpa and Guerci 1982), castor oil derivatives are being studied for use as replacements for petroleum-based fuels (Chauke et al. 2019), and as substrates for use in pharmaceutical (Darby et al. 2001) and other industries (Ogunniyi 2006). Because it is an oilseed crop, the majority of studies on castor have analyzed its oil content and other components of castor seeds, particularly ricin (Knight 1979; Lord et al. 1994). The release of the castor genome in recent years (Chan et al. 2010; Fan et al. 2019; Xu et al. 2021) has provided opportunities for the molecular and genetic analysis, breeding, and improvement of various important traits in castor.
The castor fruit is a capsule that is found in nature with either spiny or spineless phenotypes (Fig. 1A). Initially, these spine structures on castor capsules were thought to correspond to trichomes such as those found in Arabidopsis (Arabidopsis thaliana or Arabis alpina) (Oppenheimer et al. 1991; Chopra et al. 2019), thorns as found in Citrus (Citrus reticulata Blanco.) (Zhang et al. 2020), or prickles as found in rose (Rosa rugosa) (Zhou et al. 2020). Although different plant species possess apparently similar spine-like structures, these structures originate from different plant organs or tissues. During development, thorns develop specifically from shoot meristems (Bell and Bryan 1991; Barthelemy and Caraglio 2007), while spines develop from leaves or parts of leaves that contain vascular tissue (Armani et al. 2020), and prickles develop from epidermal tissue that does not contain vascular tissue (Bazely et al. 1991).
Figure 1.
Comparison of spine phenotypic characterization between castor (R. communis) DL01 and LYY5. A) Phenotypes of mature spiny capsule (DL01) and mature spineless capsule (LYY5). Scale bars, 1 cm. B) Spine phenotypes on capsules of DL01 and LYY5 (Stage I) before pollination. Early in development, DL01 capsules bear spines and those of LYY5 do not bear spines. Scale bars, 0.1 cm. C) Longitudinal sections of Stage I ovaries with bracts in castor varieties DL01 and LYY5, stained with Toluidine Blue O. The arrows indicate spines during early development. Scale bars, 1 mm. D) Transverse sections of Stage I ovaries with bracts in castor varieties DL01 and LYY5, stained with Toluidine Blue O. The arrows indicate spines during early development. Scale bars, 1 mm. E) Box plot of spine number on castor capsules at Stage I (SI) and Stage XI (SXI). The x-axis represents the developmental stages I through XI, while the y-axis represents spine numbers. The box for each stage depicts the median (center line) and upper and lower quartiles (box limits), and whiskers extend to the minimum and maximum values within 1.5 × interquartile range. ***P < 0.001 (Student's t-test).
Spines and other spine-like structures are physical defense structures that deter animals from eating plants (Johnson 1975; Cooper and Owen-Smith 1986; Mauricio and Rausher 1997; Serna and Martin 2006). One study has suggested that the spiny structures of some Euphorbiaceae serve as warnings to mammalian herbivores (Lev-Yadun 2001). However, glabrous (spineless) plant parts are also easier to harvest and process during agricultural production, suggesting that spinelessness might be a domesticated trait (Lim et al. 1984; Zhang et al. 2012). Thus, it is important to improve our understanding of the molecular genetic mechanisms and evolution of spine development in castor for reasons of both fundamental biological knowledge and practical agricultural application.
Previously, several R2R3-MYB (R2R3 myb domain protein) transcription factors (TFs) have been found to play key roles in the initiation or development of spine-like structures in many different plant species (Oppenheimer et al. 1991; Yang et al. 2018). The protein encoded by an R2R3-MYB gene (GLABRA 1 (GL1)) identified in Arabidopsis was designated AtMYB1 (Oppenheimer et al. 1991; Shinozaki et al. 1992). Basic helix-loop-helix proteins such as those encoded by GL3 (GLABRA 3) or EGL3 (ENHANCER OF GLABRA 3) (Payne et al. 2000; Szymanski et al. 2000) and the WD40 protein encoded by TTG1 (TRANSPARENT TESTA GLABRA 1) (Walker et al. 1999) can interact to form the TF complex MYB-bHLH (basic helix-loop-helix) -WD40 (WD-repeat protein 40) (Larkin et al. 2003). The protein encoded by MYB6 has been analyzed as a negative regulator of spine density in cucumber (Cucumis sativus) (Yang et al. 2018) and CsGL1 has also been shown to function in spine development but not initiation in cucumber (Li et al. 2015; Che and Zhang 2019; Wang et al. 2021).
MIXTA and MIXTA-like genes, which are not homologous to AtGL1, belong to the 9A R2R3-MYB gene family (Stracke et al. 2001; Brockington et al. 2013). MIXTA proteins can control the development of conical cell shape in petal epidermis (Noda et al. 1994) and positively regulate the development of epidermal conical cells (Perez-Rodriguez et al. 2005; Baumann et al. 2007; Jaffe et al. 2007) in snapdragon (Antirrhinum majus). In addition, the functions of proteins encoded by MIXTA-like genes have been analyzed in diverse species and shown to participate in the regulation of cell and cuticle development especially during epidermal cell differentiation (Noda et al. 1994; Folkers et al. 1997; Baumann et al. 2007; Jakoby et al. 2008), as do AtMYB16, AtMYB17, and AtMYB106 in Arabidopsis (Baumann et al. 2007; Gilding and Marks 2010; Pastore et al. 2011); GhMYB25 and GhMYB25-like in G. hirsutum (Gossypium hirsutum) (Machado et al. 2009; Walford et al. 2011); PtMYB186 gene in populus (Populus L.) (Plett et al. 2010); and MtMYBML3 in Medicago truncatula (Gilding and Marks 2010). Together, these studies indicate that the MIXTA and MIXTA-like proteins function to regulate the differentiation of plant epidermal cells into conical cells, trichomes, spines, or even root hairs.
A study of the regulatory cascade including MYB106 and WAX INDUCER1 (WIN1) to coordinately regulate cell differentiation and wax accumulation has been reported in Arabidopsis (Oshima et al. 2013). A further study showed that overexpression of WIN1 could change the structures of leaves and epidermal cells and alter the number and branching patterns of trichomes (Aharoni et al. 2004). Further, recent studies indicate that overexpression of WIN1 in Arabidopsis can activate the production of waxes and change their composition to regulate the permeability of the leaf cuticle and improve tolerance to drought, while repression of these functions can adversely affect the adhesion of plant organs and the formation of wax and cutin (Aharoni et al. 2004; Kannangara et al. 2007; Oshima et al. 2013).
In contrast to the research progress in several species mentioned above, the molecular mechanisms of capsule spine formation in castor have not yet been described. In the present study, we demonstrate that a deletion in a promoter or a single nucleotide polymorphism (SNP) leading to a premature stop codon in the third exon of RcMYB106 can cause a spineless phenotype in castor. We show that RcMYB106 can directly regulate transcription to control the expression of RcWIN1 during the development of capsule spines in castor. The results we present here provide insights into the molecular mechanisms and regulatory network underlying spine development. These results also provide further insights into the natural phenotypic variability of castor with spineless capsules, and into the artificial selection and recurrent evolution of genes involved in spine development during the evolution and post-domestication improvement of castor.
Results
Characterization of capsule spine development in castor
For the present study, we used the castor accession DL01, which is an elite cultivar with spiny capsules from the Inner Mongolia Autonomous Region in North China (102°10′N, 24°23′E) and LYY5, which is a castor accession with spineless, glabrous capsules from Yunnan Province in southwestern China (42°15′N, 119°15′E) (Fig. 1A). We have observed that the capsules of castor show a consistent phenotype throughout each panicle within a single plant, that is, either all spiny or all spineless (Supplemental Fig. S1A). To characterize castor capsule morphology in more detail, we divided its development process into 11 stages. The bracts completely enclose the ovary and female flower at Stage I; the female flower is just emerging at Stage II; the ovary phenotype is apparent at Stage II; the female flower has been pollinated at Stage IV; 1 d after pollination (DAP) denotes Stage V; 3 DAP denotes Stage VI; bract tissue has dehisced at Stage VII; 7 DAP denotes Stage VIII; 10 DAP denotes Stage IX; and 15 DAP denotes Stage X; and capsule and spine development cease at Stage XI (Supplemental Fig. S1B).
Through continuous observation, we recorded the dynamic development of capsules at different stages in the field and observed obvious differences between DL01 and LYY5 during capsule formation from Stages I to XI. During Stage I, when the ovary is tightly wrapped by the bracteoles, the female flower is unfolded and mature but not yet pollinated (Fig. 1B and Supplemental Fig. S1B). In DL01, the capsule and the spines stop developing at ∼20 DAP. At this time (Stage XI), the capsule diameter is about 1 cm and the spine length about 1.2 cm; the base of the spine is flattened and the apex of the spine is pointed (Fig. 1A and Supplemental Fig. S1B). At about 30 DAP, the capsules and spines start to become lignified, and then the seeds mature and are harvested at about 45 DAP.
To analyze the differences between DL01 and LYY5 in terms of the spine phenotypes in more detail, we stained longitudinal and transverse sections of Stage I capsules from both varieties with Toluidine Blue O and observed the development of spine tissue in DL01 but not in LYY5 (Fig. 1, C and D). Further, we counted the spines and found that the number of spines on DL01 capsules is much greater than that on LYY5 capsules at Stages I and XI (P < 0.001, Fig. 1E).
RcMYB106 has a key role in specifying spine identity in LYY5
To characterize the molecular mechanisms of spine development, we carried out genetic analysis using F1 progenies and an F2 segregating population derived from crosses between DL01 and LYY5. We observed fewer spines (P < 0.001, Fig. 2, A and B and Supplemental S1A) on the capsules of DL01×LYY5 F1 plants. Compared with DL01 (average 80 spines per capsule), the reciprocal hybrid LYY5×DL01 F1 exhibited significantly fewer spines (P < 0.001; an average of 10 spines per capsule or an average of 87.5% fewer spines). Because the F1 of reciprocal crosses exhibit the same spine number phenotype, the castor capsule spine phenotype is not likely to be maternally inherited. After selfing the F1 (DL01 as the male parent and LYY5 as the female parent), we obtained 3 capsule phenotypes including spiny, less spiny, and spineless in the segregating F2 population (Fig. 2, A and B). Evaluation of the phenotypes in the F2 population (434 spiny, 830 less spiny, and 441 spineless; c2 = 0.13, P = 0.9) and the F2:3 population (177 spiny, 349 less spiny, and 178 spineless; c2 = 0.019, P = 0.99) revealed that the capsule phenotype in this population is controlled by a partially recessive gene (Fig. 2A and Supplemental Table S1).
Figure 2.
Map- and sequencing-based cloning of Rc10G022841.A) Genetic analysis of the spine phenotypes between castor varieties DL01 and LYY5 in an F2 population of 1705 plants from the cross between DL01 as the male parent and LYY5 as the female parent, including 434 plants with spiny seed capsules, 830 plants with less spiny seed capsules, and 441 plants with spineless capsules. Scale bars, 0.5 cm. B) Box plot of the numbers of spines on seed capsules of different castor accessions. The x-axis represents the accessions DL01, LYY5, DL01×LYY5 F1, and LYY5×DL01 F1 representing spiny, less spiny, and spineless plants, respectively, in the F2 population. While the y-axis represents the number of spines on the capsules of different accessions. The plot box represents the median (center line) and upper and lower quartiles (box limits), and whiskers extend to the minimum and maximum values within 1.5 × interquartile range. ***P < 0.001 (Student's t-test). C) Fine mapping of the Rc10G022841 locus. The target gene was initially mapped to a region between the markers RC9-5.697 and RC9-13.219 on chromosome 10, and then to a narrower 126-kb region containing 5 open reading frames, 3 of which contained nonsynonymous SNP mutations. Numbers below the bar indicate the number of spineless recombinants corresponding to the marker. Spine phenotype is shown for each recombinant line (R1–R9). N, number of spineless phenotypes in the F2 population; Rec., recombinant line; “+” represents segregating progeny phenotype; “−” represents progeny with no phenotypic segregation; Black and white bars represent homozygous chromosomal segments for DL01 and LYY5, respectively.
To identify the candidate genes controlling spine development, we first mined the genomic sequences of castor (Xu et al. 2021) and the resequencing data of DL01 and LYY5 to identify insertion–deletion (InDel) polymorphisms. We then developed InDel markers to screen for polymorphisms between DL01 and LYY5 to identify candidate genes for capsule spine development by map-based cloning (Supplemental Figs. S2 and S3; Supplemental Table S2). We crossed DL01 and LYY5 to produce 44 recessive individuals with spineless capsule phenotypes and primary mapping results showed that the genotypes of most of the F2 individuals are consistent with that of LYY5 according to molecular markers located on chromosome 10. We then preliminarily mapped a region containing candidate genes for capsule spine development to chromosome 10 using flanking markers RC9-5.697 and RC9-13.219 (Supplemental Fig. S3; Fig. 2C). The locus of interest was then fine-mapped to an interval between the markers RC9-7.295 and RC9-7.411 using 441 F2 recessive spineless plants from the cross between DL01 and LYY5. The interval was further refined to within a 126-kb region by mapping at higher resolution in a population of 1,705 individuals (Fig. 2C). Five genes are located within this region, but only 3 nonsynonymous genes, Rc10G022840, Rc10G022841, and Rc10G022843, were identified within this region (Fig. 2C and Supplemental Table S3).
To further analyze candidate genes for capsule spine development, we annotated the protein functions of 3 genes using BLAST at UniProt (2019) (https://www.uniprot.org). Rc10G022840 was annotated as an uncharacterized protein, and Rc10G022843 was annotated as a nucleolar RNA-associated protein (Supplemental Table S3). However, one of the candidate genes in this 126-kb region, Rc10G022841, is putatively orthologous to Arabidopsis MYB106, which encodes a MIXTA-like R2R3-MYB family TF (UniProt; Fig. 2C and Supplemental S4). At its C-terminal amino acids 14 to 61 and 67 to 112, the predicted protein encoded by Rc10G022841 contains a conserved R2R3-MYB-like DNA-binding domain that is likely involved in DNA-binding and protein oligomerization (http://pfam.sanger.ac.uk/; Supplemental Fig. S4). The R2R3-MYB genes have been implicated in controlling some secondary metabolic functions in plants, in addition to the determination of plant cell development and tissue identity (Stracke et al. 2001). In Arabidopsis, the MIXTA-like gene MYB106, also known as NOECK or NOK, encodes a TF that is involved in leaf trichome development as a negative regulator of trichome branching (Folkers et al. 1997; Jakoby et al. 2008; Gilding and Marks 2010). In addition, R2R3-MYB also plays a role in trichome development in some woody perennial plants. For example, PtR2R3-MYB186 overexpression increases the density of trichomes on adaxial leaf surfaces in populus (Plett et al. 2010). Moreover, R2R3-MYB family members play essential roles in the morphology of epidermal cells and spine-like structures in snapdragon (Perez-Rodriguez et al. 2005; Baumann et al. 2007; Jaffe et al. 2007), tomato (Solanum lycopersicum, Solanaceae) (Schmitz et al. 2002; Deng et al. 2012; Yuan et al. 2021), sweet wormwood (Artemisia annua L., Asteraceae) (Matias-Hernandez et al. 2017; Shi et al. 2018; Qin et al. 2021), cucumber (C. sativus, Cucurbitaceae) (Li et al. 2015; Yang et al. 2018), and barrel medic (M. truncatula, Fabaceae) (Gilding and Marks 2010). Our phylogenetic analysis showed castor and Hevea brasiliensis clustering into the same clade as expected because both are members of the family Euphorbiaceae (Supplemental Fig. S5).
RcMYB106 is also required for spine identity in LYY9
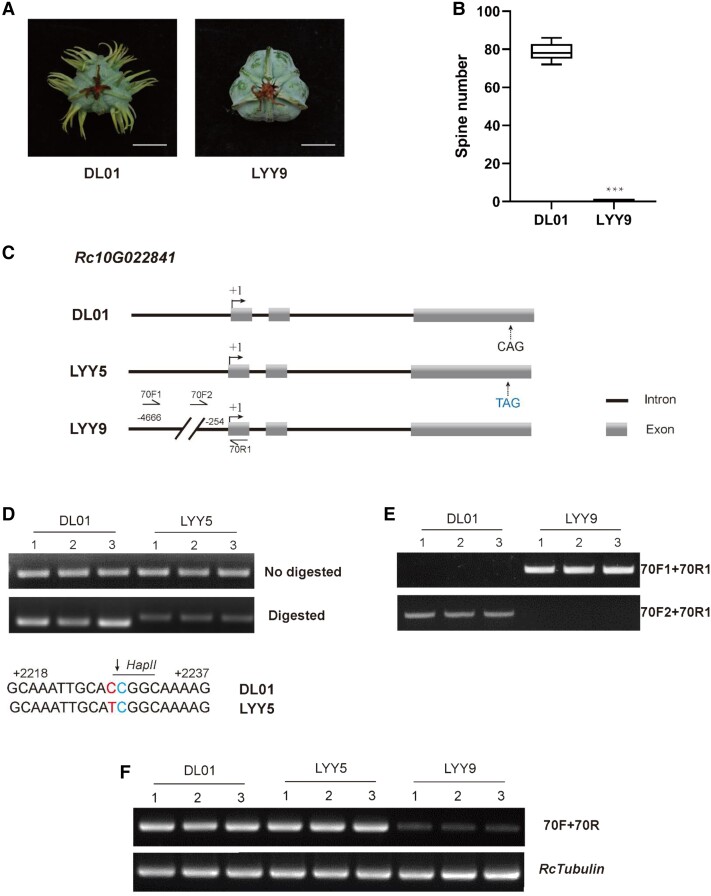
To further characterize the molecular genetic mechanisms controlling spine development, we constructed a population from a cross between the accessions DL01 and LYY9. LYY9 is an accession with spineless capsules (P < 0.001, Fig. 3A and B) that we collected from the Inner Mongolia Autonomous Region in North China (102°10′N, 24°23′E). The LYY9 accession is also a dwarf, which further facilitates phenotyping of the progenies resulting from crosses between DL01 and LYY9. Although the F1 phenotype of the DL01×LYY9 cross is less spiny, it has many more spines than does the LYY5×DL01 F1, which has an average of 51% fewer spines (Supplemental Figs. S6 and S7A). The average spine numbers of DL01, LYY9, and LYY9×DL01 F1 are shown as a box plot (P < 0.001, Supplemental Fig. S6B).
Figure 3.
Rc10G022841 as a candidate gene for the regulation of spine development in castor accession LYY9. A) Spine phenotypes of castor varieties DL01 and LYY9. Scale bars, 1 cm. B) Box plot of the number of spines on capsules of DL01 and LYY5. The x-axis represents the DL01 and LYY9 accessions, while the y-axis represents the number of spines on capsules of each accession. The plot box shows the median (center line) and upper and lower quartiles (box limits), and whiskers extend to the minimum and maximum values within 1.5 × interquartile range. ***P < 0.001 (Student's t-test). C) Sequence diagrams of the Rc10G022841 gene in DL01, LYY5, and LYY9. Compared with castor variety DL01, the variety LYY5 carries an SNP at position +2228 of the Rc10G022841 gene. However, accession LYY9 carries a 4353-bp deletion within the promoter region of Rc10G022841. D) Genotyping Rc10G022841 in LYY5. dCAPS analysis of the SNPs at nucleotide positions +2228 in Rc10G022841 in DL01 and LYY5. Alignment of the partial sequence of Rc10G022841 containing the SNP ("C" in DL01; "T" in LYY5) in the third exon that resulted in the creation of the HpaII restriction site. Base mismatches in the primer relative to the gene sequence occurred in HpaII restriction site. E) Genotyping Rc10G022841 in LYY9. PCR analysis shows the deletion in the Rc10G022841 genomic sequence in LYY9. F) RT-qPCR analysis of the expression pattern of Rc10G022841 in aril tissue of DL01, LYY5, and LYY9. Three biological replicates were sampled from each accession. The castor Tubulin gene was used as a control. G) Staining and detection of GUS activity in castor cotyledons expressed from pRcMYB106DL01:GUS and pRcMYB106LYY9:GUS. Agrobacterium carrying a 35S:GUS construct was transformed into cotyledons as a positive control; 10 mM MgCl2 was injected as a negative control. The images were digitally extracted for comparison and the samples were photographed at the same time. Scale bars, 0.3 cm. H) Quantitative analysis of GUS activity in the RcMYB106DL01 and RcMYB106LYY9 promoter-driven GUS reporter gene in DL01 cotyledons. MU, 4-methylumbelliferone. The results showed the means ± SD from 3 biological replicates that contain 3 technical repeats. Error bars denote SD. ***P < 0.001 (Student's t-test).
Genetic linkage analysis using 133 F2 recessive spineless individuals derived from the cross between DL01 and LYY9 showed that a gene controlling capsule spine number is located between the InDels RC9-6.861 and RC9-7.373 on castor chromosome 10. We further localized Rc10G022841 within a capsule spine phenotype-associated mapping region that has been repeatedly identified in other independent mapping populations (Supplemental Fig. S7). We then compared the mutation types of the Rc10G022841 gene based on the resequencing data from DL01, LYY5, and LYY9. Compared with the spiny DL01 accession, there is 1 nonsense SNP in the third exon of the Rc10G022841 DNA sequence in the spineless LYY5 accession. We determined that this SNP locus contains a C-to-T transversion resulting in a premature stop codon in its third exon (+2228 bp; Fig. 3C).
By comparing the genomic sequences of Rc10G022841 in DL01 and LYY9, we detected a 4353 bp deletion from −4,607 to −254 bp in its promoter region in LYY9, but no nonsynonymous mutations in its coding region (Fig. 3C). Therefore, we focused on Rc10G022841 as a candidate gene responsible for the castor capsule spine phenotype. Next, we searched for mutations in Rc10G022841 in the LYY5 and LYY9 accessions based on resequencing data. We designed a primer set for validation of the SNP in Rc10G022841 in the LYY5 genome by dCAPS enzyme digestion and clearly differentiated the DL01 amplicon(s) from LYY5 allele(s) (Fig. 3D). Next, we detected the genotype of the deleted region in the promoter of Rc10G022841 in LYY9 using 3 primer sets and obtained results are consistent with the resequencing data (Fig. 3E).
To further analyze whether the Rc10G022841 gene could be involved in the observed variation in spine phenotypes, we analyzed Rc10G022841 transcript expression in castor aril tissues at Stage I using reverse transcription-quantitative PCR (RT-qPCR). Our analysis revealed no differences in transcript expression of Rc10G022841 in DL01 and LYY5. However, the abundance of Rc10G022841 transcripts was lower in LYY9 compared with DL01 or LYY5 (Fig. 3F). We, therefore, hypothesized that the 4353-bp deletion in the promoter region of Rc10G022841 in LYY9 might have altered its transcript abundance and thereby its function. In addition, we detected the expression pattern of the Rc10G022841 gene in different tissues, female flower, male flower, leaf, and aril in DL01 using quantitative real-time PCR (qPCR) (Supplemental Fig. S8) and found that the abundance of Rc10G022841 transcripts varies in different tissues. The expression of Rc10G022841 in all 4 tissues was higher than that in the male flower. Further, our qPCR results also revealed that Rc10G022841 transcript abundances differ in the accessions DL01, LYY5, and LYY9 during different development stages (Supplemental Fig. S10, A to C). The Rc10G022841DL01 expression level was basically identical with Rc10G022841LYY5, but the abundance was substantially reduced of Rc10G022841LYY9. These results agree with our RT-qPCR results (Fig. 3F).
To determine whether the 253-bp Rc10G022841 promoter resulting from a 4353-bp deletion from −4,607 to −254 would affect the abundance of Rc10G022841 transcripts in LYY9, a modified GUS transactivation assay (Voinnet et al. 2003; Chen et al. 2018) was performed in castor cotyledons. GUS activity analysis demonstrated that a 2000-bp promoter sequence from Rc10G022841DL01 resulted in higher GUS activity than did the 253-bp promoter of Rc10G022841LYY9 (Fig. 3, G and H), suggesting that the 253-bp promoter of Rc10G022841LYY9 carries fewer cis-elements related to transcriptional activation.
Further, we found that Rc10G022840 is not detectably expressed and that the expression of Rc10G022843 does not differ among the accessions DL01, LYY5, and LYY9 (Supplemental Fig. S9). These results add further to the evidence suggesting the involvement of the Rc10G022841 gene in capsule spine development in castor.
Genetic analysis of RcMYB106 function in castor
To further analyze whether Rc10G022841 could be involved in the expression of castor capsule spine phenotypes, we examined the types of mutations present in the Rc10G022841 locus based on resequencing data from 225 spiny and 43 spineless castor varieties. Using the variants so far identified in Rc10G022841, we classified the allelic variants of this gene into 3 haplotypes: 1 associated with spiny capsules (haplotype A) and 2 others associated with spineless capsules (haplotype B and C) (Table 1). Thus, haplotype A might represent a functional Rc10G022841 allele while haplotypes B and C might represent some loss of function of this gene, a hypothesis consistent with the phenotypic variation observed in these varieties (Table 1). In our study, those castor varieties that carry haplotype A develop spines, whereas those varieties that carry a B or C haplotype do not form spines.
Table 1.
Nucleotide polymorphisms and their positions relative to ATG in 3 haplotypes of Rc10G022841 identified from 268 castor accessions.
| Haplotype | Phenotype | −4606 to −254 | +2228 | No. of acc. |
|---|---|---|---|---|
| Hap.A (DL01) | Spiny | – | – | 225 |
| Hap.B (LYY9) | Spineless | 4353 bp del. | – | 40 |
| Hap.C (LYY5) | Spineless | – | gC 2228 T/pE 307* | 3 |
Hap., Haplotype; del., deletion; acc., accession.
Next, to further examine the range of DNA variants that might control the phenotypes represented by these alleles, we compared the sequences of Rc10G022841 in the genomes of 268 castor accessions including spiny and spineless ones. Interestingly, 39 spineless accessions clustered together with LYY9, which originated from northern regions such as Shandong or Northeast China. Two other spineless accessions were more related to LYY5 from southern regions such as Yunnan Province (Table 1 and Supplemental Fig. S4). However, 224 accessions with spiny capsules consistently clustered together with DL01 (Supplemental Table S4).
To further examine whether mutations in the Rc10G022841 gene cause the capsule phenotypes characteristic of LYY5 and LYY9, we tested their alleles for complementation of function. From a cross between LYY5 as donor and LYY9 as recipient, F1 plants showed a spineless phenotype, which indicates that the mutations that cause these capsule phenotypes could complement each other as alleles (Fig. 4, A and B). Further analysis of the LYY9×LYY5 F1 genotype showed that the deletion in the promoter is heterozygous in this population (Fig. 4C). Our dCAPS analysis indicated that LYY9×LYY5 F1 is also heterozygous for the SNP at position +2228 (Fig. 4D).
Figure 4.
Genetic analysis of Rc10G022841.A) Phenotypic characterization of capsules of the castor varieties DL01, LYY5, LYY9, and LYY9×LYY5 F1. Scale bars, 1 cm. B) Box plot of the number of spines on capsules of the castor varieties DL01, LYY5, LYY9, and LYY9×LYY5 F1. The x-axis represents the different accessions, while the y-axis represents the total number of spines on all capsules in different accessions. For the plot box, the median (center line) and upper and lower quartiles (box limits), and whiskers extend to the minimum and maximum values within 1.5 × interquartile range. ***P < 0.001 (Student's t-test). C and D) PCR analysis of the deletion in the promoter region of Rc10G022841(C) and the SNP at position +2228 of Rc10G022841(D) showing the genotype of Rc10G022841 in LYY5, LYY9, and LYY9×LYY5 F1.
Further, the resequencing data of Rc10G022840 and Rc10G022843 in some spiny and spineless accessions revealed that the Rc10G022840 and Rc10G022843 genes contained consistent mutation sites across accessions, including in their promoter regions, CDSs (coding sequence), and 3¢-untranslated regions (3¢UTR; Supplemental Table S5). Together, these findings suggest that spine identity in castor requires Rc10G022841 function.
RcMYB106 regulates RcWIN1 expression for spine development in castor
To probe the regulatory networks that could underlie spine formation on castor capsules, we compared differentially expressed genes (DEGs) in young aril tissues of DL01, LYY5, and LYY9 plants by RNA sequencing (RNA-Seq) (Owens et al. 2019). Given that differences in the aril spine phenotype can be distinguished at Stage I, we chose to analyze the transcriptomes of Stage I arils from plants of the DL01, LYY5, and LYY9 accessions. We used the DESeq2 (Love et al. 2014) method to analyze the RNA-Seq data from Stage I DL01 and LYY5 arils and revealed the differential expression of 294 genes with log2-fold change in expression of ≥1 at P < 0.01 and q < 0.01. A total of 204 genes showed higher transcript abundance in DL01 relative to LYY5, while 90 genes showed lower transcript abundance in DL01 relative to LYY5 (Fig. 5A). Further, 84 genes showed higher transcript abundance and 20 showed lower transcript abundance in DL01 arils compared with those of LYY9. Comparing LYY5 and LYY9, 29 genes showed increased transcript abundance and 5 showed decreased transcript abundance in DL01 (Fig. 5A and Supplemental S11A). We found that a statistically significant overlap among DEGs in both sets suggested a strong association between different genotypes.
Figure 5.
RcMYB106 activates RcWIN1 expression during spine formation. A) Venn diagram showing the number of DEGs in different comparisons; B) RT-qPCR analysis of the expression patterns of RcWIN1 in the aril tissue of castor varieties DL01, LYY5, and LYY9. Three biological replicates were sampled for each accession. The castor Tubulin gene was used as a control. C) Diagram of the effector and reporter constructs used in dual-luciferase assays. REN, Renilla luciferase; LUC, firefly luciferase. D) Quantitation of luciferase (LUC)/Renilla (REN) activity in castor LYY5 cotyledons. Injection of Agrobacterium carrying only the RcWIN1 promoter-LUC reporter construct without an effector was used as control. The activity of the empty effector construct was set to 1. Data are mean ± SEM (n = 3). Error bars denote SD. ***P < 0.001 (Student's t-test). E) RT-qPCR analysis of the expression pattern of RcWIN1 in the young cotyledon tissue of castor varieties DL01, LYY5, and LYY9. Three biological replicates were sampled for each accession. The castor Tubulin gene was used as a control. F) Dual-luciferase assays in cotyledons of DL01, LYY5, and LYY9. Injection of Agrobacterium carrying only a promoter-LUC reporter construct without an effector was used as control (CK). The activity of the empty effector construct was set to 1. Data are mean ± SEM (n = 3). Error bars denote SD. *P < 0.05, ***P < 0.001 (Student's t-test). G) EMSA assay results showing that RcMYB106s from accessions DL01 and LYY5 could bind to the promoter of RcWIN1. The GST-RcMYB106 fusion protein and GST protein were incubated with CY5-labeled P8 or unlabeled probe, respectively. H) Interactions between RcMYB106DL01 or RcMYB106LYY5, and the RcWIN1 promoter. Interaction of RcMYB106DL01 or RcMYB106LYY5 and the RcWIN1 promoter in transformed EGY48 cells grown on SD/-Trp/-Ura medium containing X-gal. pPC86-241-4 + pLacZi-C53-106 as a positive control; pPC86 + pLacZi-proRcWIN1-P8 as a negative control.
Among these DEGs, we focused on the castor gene Rc07G016896, which is a putative ortholog of the Arabidopsis WIN1/SHN1 gene that encodes the TF WIN1, an ERF (ethylene response factor) in the B-6 subfamily of ERF/AP2 TFs (Supplemental Fig. S11, B and C). In Arabidopsis, the MIXTA-like MYB106 gene activates WIN1/SHN1, thereby regulating conical epidermal cell differentiation and cuticular ridge formation (Broun et al. 2004; Shi et al. 2011; Oshima et al. 2013). These functions make RcWIN1 an attractive candidate factor that might interact with RcMYB106 for controlling capsule spine development in castor. Ethylene-responsive TF WIN1 putative orthologs have been shown to participate in several functions in a variety of plant species. For example, WIN1 has been implicated in the regulation of trichome number and branching, cuticular wax synthesis, and lipid biosynthesis in a variety of contexts, as well as drought tolerance (Aharoni et al. 2004; Kannangara et al. 2007; Taketa et al. 2008; Al-Abdallat et al. 2014), but has not been previously implicated in spine outgrowth.
Our confirmation by RT-qPCR of our transcriptomic analyses showed that RcWIN1 transcripts are expressed at lower levels in LYY9 and are not detectably expressed in LYY5 compared with DL01 (Fig. 5B). Taken together, RcMYB106 might positively regulate the expression of the RcWIN1 gene. That is, a truncated protein expressed by RcMYB106 carrying the SNP at position +2228 might fail to activate the expression of RcWIN1 in LYY5. Further, if RcMYB106 is expressed at low levels, RcWIN1 expression would be activated less in LYY9. The expression patterns of RcMYB106 and RcWIN1 correspond to those of AtMYB106 and AtWIN1 in Arabidopsis, which suggests that RcMYB106 might also regulate RcWIN1 transcription in castor. In addition, we found 2 putative paralogs of RcWIN1, designated RcWIN2 and RcWIN3, in the castor genome. Protein sequence alignments of the putative proteins RcWIN1, RcWIN2, and RcWIN3 show that they each include a conserved AP2 domain (Supplemental Fig. S12, A to C). However, our RT-qPCR results indicate that RcWIN2 and RcWIN3 are not expressed in DL01, LYY5, or LYY9 arils (Supplemental Fig. S12D), consistent with the RNA-Seq results. Further, we also found that the expression of RcWIN1 transcripts was relatively stable in DL01, LYY5, and LYY9 during capsule development (Supplemental Fig. S10, D to F). The differential transcript abundance of RcWIN1 in different tissues is shown in Supplemental Fig. S8B.
To analyze whether RcMYB106 could regulate RcWIN1, we sequenced the 2000-bp promoter region of RcWIN1 to analyze its cis-acting elements (PLANTCARE; Supplemental Fig. S13). Because we identified many MYB TF binding sites as potential downstream targets of RcMYB106, we then performed a dual-luciferase assay. The CDSs of RcMYB106DL01 and RcMYB106LYY5 were cloned into the pGreenII62-SK vector to use as effectors with the RcWIN1 promoter cloned into the pGreenII0800 LUC vector as a reporter (Fig. 5C). We then measured LUC/REN fluorescence ratios after injecting the vectors carrying the TF CDSs and the RcWIN1 promoter into LYY5 cotyledons. A significantly higher ratio (2.5-fold) of LUC/REN expression was observed for the co-injection of RcMYB106DL01 and RcWIN1 compared with the corresponding control (P < 0.001) (Fig. 5D), which suggests that RcMYB106 can activate gene expression from the promoter of RcWIN1 in castor cotyledons.
RcMYB106 and RcWIN1 are consistently expressed in the arils and cotyledons of DL01, LYY5, and LYY9 (Fig. 5E), and after injection of the reporter vector carrying the promoter of RcWIN1 into DL01, LYY5, and LYY9 cotyledons, transient expression of LUC/REN could be detected. We observed higher ratios of LUC/REN expression for both the proRcWIN1DL01 and proRcWIN1LYY9 constructs compared with their corresponding controls and with proRcWIN1LYY5 (P < 0.001; Fig. 5F), suggesting that RcMYB106 can activate the expression of RcWIN1. The activation intensity of RcMYB106 in DL01 (8.6-fold) was stronger than that observed in LYY9 (P < 0.001; 2.3-fold), further indicating that RcMYB106 could positively regulate the transcription of RcWIN1.
We then carried out an electrophoretic mobility shift assay (EMSA) using the GST-RcMYB106 recombinant protein to verify whether the promoter of RcWIN1DL01 was bound by RcMYB106DL01 and RcMYB106LYY5 in vitro. Our results indicated that RcMYB106DL01 and RcMYB106LYY5 could bind to the P8 fragment among the 13 proRcWIN1DL01 subfragments (P1 through P13) tested (Fig. 5G and Supplemental S14). This suggests that the RcMYB106LYY5 truncated protein retained its binding function but lost its activation function. Further, our yeast one-hybrid (Y1H) assay also showed that RcMYB106DL01 and RcMYB106LYY5 could bind to the P8 fragment of the RcWIN1 (Fig. 5H). Therefore, RcMYB106 and RcWIN1 might together act as positive regulators of capsule spine development in castor in a similar manner to AtWIN1 for cuticle development in Arabidopsis (Oshima et al. 2013).
Genes encoding the ERF1 are responsive to the application of exogenous ethylene (Thomma et al. 1999; Lorenzo et al. 2003). We extracted the RcERF1 (Rc06G013376) that is homologous to AtERF1 (AT3G23240) from the castor genome data using TBtools software (Chen et al. 2020) and analyzed its response to the application of exogenous ethylene. RNA was extracted from leaf samples at 0, 6, 12, or 24 h after 4-mo-old plants were sprayed with different concentrations of ethephon (0, 100, 200, or 500 mg ml−1 in water). Plants were grown at a greenhouse temperature of 20 °C. Expression of RcERF1 (Rc06G013376) was induced upon treatment with 500 mg ml−1 ethylene at 24 h after treatment (Supplemental Fig. S15, A to C). Next, 4-mo-old DL01, LYY5, and LYY9 plants were treated with 500 mg ml−1 ethylene 3 times per week until inflorescences matured and the capsule spine phenotype could be observed. The capsules of LYY5 and LYY9 were still glabrous after plants were treated with 500 mg ml−1 ethylene liquid (Supplemental Fig. S15G). This indicates that RcWIN1 might not respond to ethylene directly and that treatment with only exogenous ethylene did not change the capsule spine phenotype. Further, we also found that the expression profiles of RcMYB106 and RcWIN1 did not differ from the control (Supplemental Fig. S15, D to F) upon treatment with 500 mg ml−1 ethephon. Our results further illustrate that the regulatory pathway controlling the castor capsule phenotype is likely intricate and might involve the effects of multiple hormones or pathways that could be the subjects of future studies.
Discussion
Although little is known about the evolution and breeding of many nonmodel crops, some studies have been undertaken to analyze the domestication of castor (Chan et al. 2010; Fan et al. 2019; Xu et al. 2021). Several loci potentially involved in capsule dehiscence, plant height, seed development, and other yield-associated traits have been detected using genome-wide association studies or sequencing technologies (Chan et al. 2010; Fan et al. 2019; Xu et al. 2021). In the present study, we used a map- and sequencing-based cloning strategy to identify RcMYB106 as a gene likely responsible for differences in the spine phenotypes of castor capsules. RcMYB106 represents a gene successfully cloned in castor using this kind of mapping strategy, which could also provide a way to further study important traits and their causative genes in other nonmodel plants.
MIXTA-like genes have evolved different functions in many plant species. For example, in Arabidopsis, MYB106 regulates the differentiation of epidermal cells and the appearance of cutin nanoridges (Oshima et al. 2013). In tomato pericarp, SlMIXTA-like controls the biosynthesis of cutin and patterning of epidermal cells (Lashbrooke et al. 2015). Further, in Mimulus lewisii flowers, a MIXTA-like gene influences the development of epidermal cells and the biosynthesis of carotenoids (Yuan et al. 2013). In Phalaenopsis “Aphrodite,” R2R3-MYB genes function to harmonize the development of conical cells in the epidermis with the biosynthesis of cuticular waxes (Lu et al. 2022). Although the mechanisms controlling trichome formation, epidermal cell differentiation, and cell fates have been analyzed before (Folkers et al. 1997; Baumann et al. 2007), studies of spine development have been relatively limited. By characterizing spine development in accessions that vary for this trait and by showing that RcMYB106 encodes an R2R3-MYB transcription factor that could be causally related to spine development, we have shown that the RcMYB106 could be a spine identity-determining gene.
We have provided compelling evidence of the biological function of RcMYB106 in spine development in castor, including a classical functional complementation test often used in the genetic analysis of model species (Chopra et al. 2019), although we do not yet have an excellent genetic transformation system. Next, we used transcriptomic analysis to identify RcWIN1 and experimentally verified that RcMYB106 could positively regulate RcWIN1 expression. As such, the function of the MYB106-WIN1 pathway in castor spine development is similar to that of AtWIN1 in Arabidopsis cuticle development (Oshima and Mitsuda 2013) in that the regulation of WIN1 transcript expression by an MYB TF is an essential aspect of both of these development pathways. As described in Fig. 6, when MYB106 functions correctly, WIN1 is normally expressed and the spiny phenotype of castor capsules is manifested. Without MYB106 function, WIN1 is not normally expressed and the capsule is spineless (Fig. 6).
Figure 6.
Model of RcMYB106 and RcWIN1 function in capsule spine development in castor. RcMYB106 can activate RcWIN1 expression. In the presence of functional RcWIN1, castor capsules are spiny. However, if RcMYB106 carries an SNP mutation resulting in a truncated protein or a deletion in its promotor region, and cannot activate RcWIN1 expression, the absence of functional RcWIN1 results in spineless capsules.
A previous study has shown that the TF encoded by MYB106 can coordinate with the WIN1 promoter to regulate the biosynthesis of cutin and the accumulation of wax on epidermal cells in Arabidopsis and Torenia fournieri (Oshima et al. 2013). Here, we confirmed a relationship between RcMYB106 and RcWIN1 in capsule spine development in castor, suggesting that MYB106 and WIN1 have evolved additional functions for spine formation in castor. Unfortunately, we do not yet have any win1 castor mutants for further genetic analysis of spine development.
The ability of the plant cuticle to protect plants from biotic or abiotic stress depends on its composition and permeability. Such properties have been demonstrated by the enhancement of drought tolerance in Arabidopsis (Aharoni et al. 2004) and tomato (Al-Abdallat et al. 2014) by the overexpression of WIN1. Our transcriptome analysis did result in the identification of some genes associated with stress tolerance in DL01, LYY5, and LYY9. However, any possible relationships of these genes to any processes related to spine development in castor need to be further explored in future work.
Changes in the expression or function of MYB106 or WIN1 can affect the morphology or number of spine-like structures in Arabidopsis (Aharoni et al. 2004; Jakoby et al. 2008). Functional analyses of MYB106 and WIN1 putative orthologs in different species have shown that they can display different but similar specific functions in castor bean, perennial woody plants, or an annual herb like Arabidopsis (Aharoni et al. 2004; Jakoby et al. 2008; Oshima and Mitsuda 2013). Thus, we overexpressed RcMYB106 in a myb106 mutant (SALK-025449), and the transgenic T3 homozygous plants show a branched trichome phenotype that is consistent with the myb106 genotype (Supplemental Fig. S16). These results indicate that the MIXTA-like gene might have retained some conserved functions yet still became functionally differentiated during evolution. This might further explain why the spine tissue of the castor capsule differs from the trichome tissue of Arabidopsis, which is in line with our hypothesis and reveals an interesting scientific phenomenon.
Further, the different phenotypes of myb106 mutants with different numbers of T-DNA insertions (Oshima et al. 2013) suggest that MYB106 function might be subject to gene dosage effects in Arabidopsis. Hence, it is easy to understand why spine traits in castor might differ between LYY5×DL01 F1 and LYY9×DL01 F1. We hypothesize that the different capsule spine phenotypes observed in LYY5×DL01 F1 and LYY9×DL01 F1 might be attributable to those populations carrying different alleles such as the 4353-bp deletion in the promoter region of Rc10G022841 or the SNP leading to a premature stop codon in Rc10G022841 in different accessions (Fig. 3B). It is noteworthy that the SNP resulting in the premature stop codon in Rc10G022841 does not reside in the conserved R2R3-MYB domain, suggesting that the truncated protein resulting from this premature stop codon still might play a crucial role in controlling differences in capsule spine phenotypes.
It will be interesting to test the function of the RcMYB106-RcWIN1 axis in other plant species. How the RcMYB106-RcWIN1 axis control spine formation remains to be further tested in other plant species that are more amenable to genetic transformation. Our identification of the RcMYB106-RcWIN1 axis in castor would be consistent with the hypothesis that the spine structures of aril are evolutionarily related to the trichome structures of leaves (Oshima et al. 2013). Additional studies of the genetic regulatory mechanisms underlying the development of spines or spine-like structures in other plant species are needed to test this hypothesis.
Materials and methods
Plant materials and growth conditions
Castor (R. communis L.) accessions DL01, LYY5, LYY9, and their cross progeny LYY5×DL01 F1, DL01×LYY5 F1, LYY9×DL01 F1, and LYY9×LYY5 F1 were grown in pots in the Northeast Forestry University research greenhouse at 18 to 25 °C under natural light supplemented with additional lighting resulting in at least a 14-h day (somewhat longer in summer). To improve germination, castor seeds were soaked in water at 4 °C for 2 d and were then sown in commercial soil (75% Turfy soil, 25% vermiculite). After the seeds had germinated and the cotyledons had expanded at approximately 7 d, the seedlings were then grown in the greenhouse as described above. From then on, the plants were watered 3 times per week with water and twice per month with water.
Because castor bean is a monoecious plant, its flowers can be self-pollinated simply by bagging the inflorescences to allow pollination to harvest seeds. For cross-pollination experiments, we artificially emasculated the flowers of the female parent, pollinated the stigma of the female parent with pollen from the male parent, and then bagged each pollinated flower. A total of 1,705 F2-LYY5/DL01 and 582 F2-LYY9/DL01 individuals were grown to evaluate capsule spine phenotypes in a field trial in the Inner Mongolia Autonomous Region in May 2020 and May 2021.
To prepare plant materials for transient expression assays, castor seeds were sown using the method described above and then grown on in the greenhouse. At approximately 12 d, after the first and second true leaves had emerged, the expanded cotyledons were suitable for transient transformation assays.
Col-0 wild-type Arabidopsis (A. thaliana) and myb106 mutant (SALK-025449) served as plant materials for transformation. All Arabidopsis plants were grown at 22 °C in the greenhouse. Seeds were grown on 1/2 MS medium for 10 d, and then transferred to soil (as described above) and grown with a 16/8-h light/dark photoperiod.
Histochemical analysis
We collected Stage I arils from plants of the castor accessions DL01 and LYY5. Samples were first soaked in a fixing solution of FAA (Free amino acids) Fixation Solution (Coolaber, Beijing, China) overnight at 4 °C and were then dehydrated in an alcohol series. We then embedded the samples in Paraplast (Sigma-Aldrich, St. Louis, MO, USA) and made 10-µm thick cross sections of embedded samples using a microtome. We then prepared and stained the sections in 0.01% (w/v) Toluidine Blue O prior to photographing them.
dCAPS analysis
Primers for dCAPS analyses were designed using the website dCAPS Finder 2.0 (dCAPS (wustl.edu)). Restriction enzymes HpaII and NcoI (New England Biolabs, Beijing, China) were used for the digestion of genomic DNA.
DNA extraction and PCR
We collected young true leaves from young castor plants and ground the samples. We then extracted genomic DNA from castor leaves using a modified cetyltrimethyl ammonium bromide method (Murray and Thompson 1980). PCR to amplify samples was performed in 20-μl reactions containing 1 μl of castor genomic DNA (50 μg ml−1), 0.5 μl of each primer (10 mM), 8.5 μl of dd H2O, and 10 μl of 2×Es Taq MasterMix (CoWin Biotech, Beijing, China).
Transcript expression analysis
Frozen tissue samples were ground to a powder in liquid nitrogen. Samples were pretreated with RNase-free DNase I (Invitrogen, Shanghai, China) as per the manufacturer's recommendations. Total RNA was extracted from arils at Stage I using the RNeasy Plant Mini Kit (CoWin Biotech, Beijing, China), following the manufacturer's recommendations. A Nanopro spectrophotometer (DHS Life Science Technology, Beijing, China) was used to determine RNA purity and concentration, and RNA integrity was verified by electrophoresis on 1% agarose gels. Three biological replicates were included in each experiment. We reverse-transcribed full-length cDNAs using the Prime Script RT Reagent Kit with gDNA Eraser (Takara, Beijing, China) following the manufacturer's protocols. We diluted cDNAs at least 4-fold before using them as templates for RT-qPCR. All RT-qPCR analyses were performed on an ABI 7500 PCR system using ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) following the manufacturer's protocols. The castor Tubulin gene was used as an expression reference. Primer sequences used here are shown in Supplemental Table S2.
Phylogenetic analysis
We performed BLASTP searches using the Rc10G022841 protein sequence as the query to identify orthologs in other plant species, then used ClustalX (Larkin et al. 2007) to conduct multiple amino acid sequence alignments. Next, we used the Neighbor-Joining method (Saitou and Nei 1987) in MEGA7 (Kumar et al. 2016) to infer phylogenetic relationships after using MUSCLE to perform alignments (Kumar et al. 2016).
GUS staining, imaging, and GUS activity analysis
The plasmids for expression studies (Rc10G022841DL01:GUS; Rc10G022841LYY9:GUS) were generated by cloning the promoter fragments of Rc10G022841DL01 (−1817 to +1) and Rc10G022841LYY9 (−253 to +1) into pBI121-GUS. pBI121-35S:GUS was used as a positive control and 10 mM MgCl2 was used as a negative control. We used a GUS staining kit (Coolaber, Beijing, China) to perform GUS staining per the manufacturer's protocols, and then used an ILCE-6400 M camera (Sony, Tokyo, Japan) to photograph samples, which speed, and offset were set up 100, 0, respectively.
For transient expression assays, we added 100 μl 0.5 M MES and 2 μl of 100 mM acetosyringone containing the appropriate antibiotics to LB medium and then 50 μl of Agrobacterium tumefaciens suspension was inoculated into the medium, which was incubated at 28 °C on a shaker at 250 rpm until OD600 = 1.0. Bacteria were pelleted by centrifuging at 4,000 rpm for 10 min at room temperature, then were resuspended with 10 mM MgCl2 to OD600 = 0.4. Next, 2 μl of 100 mM AS was added per milliliter of bacterial suspension, which was then allowed to stand at 28 °C for at least 3 h. The bacterial solution was drawn up in a syringe and the bacterial solution was injected into the underside of the cotyledons of castor seedlings that had grown for about 10 d. After 24 h of growth under dark conditions, GUS activity in samples was detected using a GUS Gene Quantitative Detection Kit (Coolaber, Beijing, China) according to the instructions.
RNA-Seq
Young arils (Stage I; with ovule removed) were collected from the inflorescences of the spiny variety DL01 and the spineless accessions LYY5 and LYY9. Three samples comprising 3 young arils per variety were collected for RNA extraction. We extracted and further purified the RNA using an Ominiplant RNA Kit (CoWin Biotech, Beijing, China). After enriching and purifying the mRNA, the quality and concentration of the rRNA-depleted total RNA were determined simultaneously. A total of 101 cycles of paired-end sequencing were performed on these 9 libraries on an Illumina HiSeq 2500 at Berry Genomics (Beijing, China).
The quality of raw sequence data from the 9 aril sample libraries was assessed and then adaptors and low-quality sequences were filtered out. The resulting clean, high-quality reads were then aligned to the reference castor bean genomes (http://oilplants.iflora.cn), and then the mapped reads were annotated against the castor reference genomes. The TPM values for each transcript (number of transcripts per Kilobase per million mapped reads) sequenced from the DL01, LYY5, and LYY9 libraries were calculated using TBtools software (Chen et al. 2020). We set the FDR (false discovery rate) at <0.01 and |log2 (ratio)| ≥ 1 for these TPM values in order to identify transcripts that are expressed differentially between the spiny and spineless phenotypes. The P-values for the t-tests were adjusted to control the FDR during multiple testing. We performed functional annotation of the DEGs using the UniProt website (https://www.uniprot.org).
Dual-luciferase assays
The CDSs of RcMYB106 were isolated from DL01 and LYY5 cDNAs and cloned into the pGreenII62-SK effector vector. The promoter sequence of RcWIN1 was isolated from DL0 genomic DNA and cloned into the pGreenII0800LUC reporter vector. We then transformed these effector and reporter vectors into the Agrobacterium strain GV3101 (pSoup-p19). The effector and reporter in Agrobacterium were injected together into LYY5 cotyledons following the method described in GUS staining assays. As a control, only the reporter vector was injected into DL01, LYY5, and LYY9 cotyledons following the same methods.
We detected firefly luciferase (LUC) and Renilla luciferase (REN) enzyme activities 24 h after infiltration using the Dual-Luciferase Reporter Assay System (E1910, Promega, Beijing, China) using a GloMax luminometer (Promega). We conducted 3 independent experiments for each effector/reporter combination with 3 technical replicates for all experiments and the promoter-LUC reporter construct as a control. The primers that were used for this study are listed in Supplemental Table S2.
Yeast one-hybrid assay
The CDSs of RcMYB106DL01 and RcMYB106LYY5 were cloned into the pPC86 vector. The P8 (probe 8) sequence of proRcWIN1 was inserted into the pLacZi vector. These constructs were designated as pPC86-RcMYB106DL01, pPC86-RcMYB106LYY5, and P178-proRcWIN1-P8, respectively. Additionally, pPC86-241-4 and P178-C53-106 were co-transformed into EGY48 competent cells as a positive control. However, pPC86 and P178-proRcWIN1-P8 were co-transformed into EGY48 competent cells as a negative control. Then transformants were grown on SD/-Trp/-Ura medium containing X-gal.
Electrophoretic mobility shift assay
EMSAs were performed by first generating the fusion proteins GST-RcMYB106DL01 and GST-RcMYB106LYY5. RcMYB106DL01-CDS and RcMYB106LYY5-CDS were cloned into the pGEX-4T-1 vector. proRcWIN1DL01 was further fragmented into 13 subfragments (P1 through P13) and each fragment was then inserted into the pEASY-Blunt Zero Cloning vector (TransGen Biotech, Beijing, China). It is worth noting that there were 30-bp overlaps between each 2 adjacent fragments. Probes were labeled with CY5 at their 5¢ end, and then the unlabeled probe was used as a competitor. GST protein served as a negative control. The primers details for this study are shown in Supplemental Table S2.
Exogenous ethylene treatment
Four-month-old castor seedlings were sprayed with one of the various concentrations of ethephon (100, 200, or 500 mg ml−1) before flowering and cultivated at 20°C. H2O was sprayed on other castor seedlings of the same age as a negative control. By detecting transcripts of ethylene response marker gene RcERF1 after treating plants for 0, 6, 12, or 24 h, we selected an appropriate ethephon concentration for subsequent studies and next observed the capsule spine phenotypes. We treated DL01, LYY5, and LYY9 with ethephon 3 times every week until castor inflorescences had matured and capsule phenotypes could be observed. While observing capsule traits, we also assayed the expression profiles of RcERF1, RcMYB106, and RcWIN1.
Statistical analyses
Statistical analyses and graph preparation were performed using Graphpad Prism (version 8.0). Comparisons of the significance between the 2 groups were performed by Mann–Whitney U tests. For comparison analysis experiments, the samples were randomly selected and assayed as biological replicates. Chi-square was used for assaying F2:3 population data in the research. P < 0.05 was considered statistically significant in all comparisons.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers RcMYB106 (Rc10G022841) and RcWIN1 (Rc07G0168961).
Supplementary Material
Acknowledgments
We thank Professor Chentao Lin for the revision of the article. We also thank Professor Hasi Yu and Professor Chang Liu for providing experimental guidance and suggestions. We hereby express our thanks to Tianxu Zhang for uploading data to NCBI. We extended our thanks to Yingbao Sun from the Institute of Botany, the Chinese Academy of Sciences to paint castor capsule. We would like to thank Zhibiao He, Wenchang Li, Deyun Tan, the Tongliao Agricultural and Animal Husbandry Research Institute, the Industrial Crops Institute, the Yunnan Academy of Agricultural Sciences, and the Zibo Academy of Agricultural Sciences for kindly providing castor materials. We would also like to thank Zhibiao He for again providing a planting area at the Tongliao Agricultural and Animal Husbandry Research Institute.
Contributor Information
Yueying Liu, State Key Laboratory of Tree Genetics and Breeding, College of Forestry, Northeast Forestry University, Harbin 150040, China; The Center for Basic Forestry Research, College of Forestry, Northeast Forestry University, Harbin 150040, China; College of Life Science, Northeast Forestry University, Harbin 150040, China.
Xinyu Wang, State Key Laboratory of Tree Genetics and Breeding, College of Forestry, Northeast Forestry University, Harbin 150040, China; The Center for Basic Forestry Research, College of Forestry, Northeast Forestry University, Harbin 150040, China.
Zongjian Li, State Key Laboratory of Tree Genetics and Breeding, College of Forestry, Northeast Forestry University, Harbin 150040, China; The Center for Basic Forestry Research, College of Forestry, Northeast Forestry University, Harbin 150040, China.
Jing Tu, State Key Laboratory of Tree Genetics and Breeding, College of Forestry, Northeast Forestry University, Harbin 150040, China; The Center for Basic Forestry Research, College of Forestry, Northeast Forestry University, Harbin 150040, China.
Ya-nan Lu, State Key Laboratory of Tree Genetics and Breeding, College of Forestry, Northeast Forestry University, Harbin 150040, China; The Center for Basic Forestry Research, College of Forestry, Northeast Forestry University, Harbin 150040, China.
Xiaohang Hu, Academy of Modern Agriculture and Ecology Environment, Heilongjiang University, Harbin 150080, China.
Qingzhu Zhang, State Key Laboratory of Tree Genetics and Breeding, College of Forestry, Northeast Forestry University, Harbin 150040, China; The Center for Basic Forestry Research, College of Forestry, Northeast Forestry University, Harbin 150040, China; College of Life Science, Northeast Forestry University, Harbin 150040, China.
Zhimin Zheng, State Key Laboratory of Tree Genetics and Breeding, College of Forestry, Northeast Forestry University, Harbin 150040, China; The Center for Basic Forestry Research, College of Forestry, Northeast Forestry University, Harbin 150040, China.
Author contributions
Z.Z. designed the research, analyzed data, and wrote the manuscript. Y.L. performed experiments, analyzed data, and wrote the manuscript. X.W., Z.L., J.T., and Y.-L. participated in some of the experiments. Q.Z. and X.H. provided experimental suggestions and revised the article.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1 . Characterization of castor spine phenotypes of the parental lines DL01 and LYY5 and their F1 offspring.
Supplemental Figure S2 . Genetic linkage map of DL01 and LYY5 showing the InDel markers across all 10 linkage groups of castor.
Supplemental Figure S3 . Electropherogram of PCR reactions for primary mapping of the Rc10G022841 gene associated with the spineless capsule phenotype of castor.
Supplemental Figure S4 . Analysis of conserved domains in the predicted protein sequence of Rc10G022841.
Supplemental Figure S5 . Phylogenetic tree of genes related to Rc10G022841 in different plant species.
Supplemental Figure S6 . Phenotypic characterization of panicles and capsules of castor DL01, LYY9, and F1 offspring.
Supplemental Figure S7 . Genetic and physical map of the target gene in a cross between DL01 and LYY9.
Supplemental Figure S8 . RT-qPCR analysis of the expression of RcMYB106 and RcWIN1 in various tissues in DL01.
Supplemental Figure S9 . Comparative RT-qPCR analysis of the expression in DL01 and LYY5 aril tissues of 2 genes Rc10G022840 and Rc10G022843 contained within the mapping interval between RC9-7.295 and RC9-7.411.
Supplemental Figure S10 . RT-qPCR analysis of the expression of RcMYB106 and RcWIN1 during capsule development.
Supplemental Figure S11 . Unique DEGs in spineless capsules compared with spiny capsules in castor.
Supplemental Figure S12 . Analysis of conserved domains in the predicted proteins encoded by RcWIN1 (Rc07G016896), RcWIN2 (Rc03G006091), and RcWIN3 (Rc03G005269).
Supplemental Figure S13 . Schematic analysis of cis-acting elements in the RcWIN1 promoter.
Supplemental Figure S14 . Screening the putatively bound probe of RcMYB106DL01 or RcMYB106LYY5 in the RcWIN1 promoter by EMSA.
Supplemental Figure S15 . Phenotypic analysis of castor capsule with application ethylene.
Supplemental Figure S16 . Trichome phenotypes of 35S:RcMYB106 in Arabidopsis.
Supplemental Table S1 . Genetic analysis of the capsule spine trait in segregating populations of castor.
Supplemental Table S2 . Primers used in this study.
Supplemental Table S3 . Position details of 3 genes carrying a nonsynonymous mutation between DL01 and LYY5 within the fine-mapped region on chromosome 10.
Supplemental Table S4 . Details regarding the castor accessions used in the present study.
Supplemental Table S5 . Major haplotypes of Rc10G022840 and Rc10G022843 in different castor accessions.
Funding
This work was supported by grants from the Opening Project of the State Key Laboratory of Tree Genetics and Breeding (K2021202) and the “5211” Research Initiation Funding of Northeast Forestry University (GCC2016-01).
References
- Aharoni A, Dixit S, Jetter R, Thoenes E, van Arkel G, Pereira A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell. 2004:16(9):2463–2480. 10.1105/tpc.104.022897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Abdallat AM, Al-Debei HS, Ayad JY, Hasan S. Over-expression of SlSHN1 gene improves drought tolerance by increasing cuticular wax accumulation in tomato. Int J Mol Sci. 2014:15(11):19499–19515. 10.3390/ijms151119499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armani M, Charles-Dominique T, Barton KE, Tomlinson KW. Developmental constraints and resource environment shape early emergence and investment in spines in saplings. Ann Bot. 2020:124(7):1133–1142. 10.1093/aob/mcz152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelemy D, Caraglio Y. Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Ann Bot. 2007:99(3):375–407. 10.1093/aob/mcl260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann K, Perez-Rodriguez M, Bradley D, Venail J, Bailey P, Jin H, Koes R, Roberts K, Martin C. Control of cell and petal morphogenesis by R2R3 MYB transcription factors. Development. 2007:134(9):1691–1701. 10.1242/dev.02836 [DOI] [PubMed] [Google Scholar]
- Bazely DR, Myers JH, Burke da Silva K. The response of numbers of bramble prickles to herbivory and depressed resource availability. Oikos. 1991:61(3):327–336. 10.2307/3545240 [DOI] [Google Scholar]
- Bell AD, Bryan A. Plant form: an illustrated guide to flowering plant morphology. Vol 40. London: Timber Press Inc; 1991. P. 534–535. [Google Scholar]
- Brockington SF, Alvarez-Fernandez R, Landis JB, Alcorn K, Walker RH, Thomas MM, Hileman LC, Glover BJ. Evolutionary analysis of the MIXTA gene family highlights potential targets for the study of cellular differentiation. Mol Biol Evol. 2013:30(3):526–540. 10.1093/molbev/mss260 [DOI] [PubMed] [Google Scholar]
- Broun P, Poindexter P, Osborne E, Jiang CZ, Riechmann JL. WIN1, a transcriptional activator of epidermal wax accumulation in Arabidopsis. Proc Natl Acad Sci. 2004:101(13):4706–4711. 10.1073/pnas.0305574101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AP, Crabtree J, Zhao Q, Lorenzi H, Orvis J, Puiu D, Melake-Berhan A, Jones KM, Redman J, Chen G, et al. Draft genome sequence of the oilseed species Ricinus communis. Nat Biotechnol. 2010:28(9):951–956. 10.1038/nbt.1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauke NP, Mukaya HE, Nkazi DB. Chemical modifications of castor oil: a review. Sci Prog. 2019:102(3):199–217. 10.1177/0036850419859118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che G, Zhang X. Molecular basis of cucumber fruit domestication. Curr Opin Plant Biol. 2019:47:38–46. 10.1016/j.pbi.2018.08.006 [DOI] [PubMed] [Google Scholar]
- Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. Tbtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020:13(8):1194–1202. 10.1016/j.molp.2020.06.009 [DOI] [PubMed] [Google Scholar]
- Chen XS, Li TT, Zhou SL, Zhao Y. Transient expression of exogenous protein in tobacco leaves. Bio Protoc. 2018:Bio-101:e1010127. 10.21769/BioProtoc.1010127. In Chinese. [DOI] [Google Scholar]
- Chopra D, Mapar M, Stephan L, Albani MC, Deneer A, Coupland G, Willing EM, Schellmann S, Schneeberger K, Fleck C, et al. Genetic and molecular analysis of trichome development in Arabis alpina. Proc Natl Acad Sci USA. 2019:116(24):12078–12083. 10.1073/pnas.1819440116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SM, Owen-Smith N. Effects of plant spinescence on large mammalian herbivores. Oecologia. 1986:68(3):446–455. 10.1007/BF01036753 [DOI] [PubMed] [Google Scholar]
- Darby SM, Miller ML, Allen RO. Forensic determination of ricin and the alkaloid marker ricinine from castor bean extracts. J. Forensic Sci. 2001:46(5):1033–1042. 10.1520/JFS15097J [DOI] [PubMed] [Google Scholar]
- Deng W, Yang Y, Ren Z, Audran-Delalande C, Mila I, Wang X, Song H, Hu Y, Bouzayen M, Li Z. The tomato SlIAA15 is involved in trichome formation and axillary shoot development. New Phytol. 2012:194(2):379–390. 10.1111/j.1469-8137.2012.04053.x [DOI] [PubMed] [Google Scholar]
- Fan W, Lu J, Pan C, Tan M, Lin Q, Liu W, Li D, Wang L, Hu L, Wang L, et al. Sequencing of Chinese castor lines reveals genetic signatures of selection and yield-associated loci. Nat Commun. 2019:10(1):3418. 10.1038/s41467-019-11228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkers U, Berger J, Hulskamp M. Cell morphogenesis of trichomes in Arabidopsis differential control of primary and secondary branching by branch initiation regulators and cell growth. Development. 1997:124(19):3779–3786. 10.1242/dev.124.19.3779 [DOI] [PubMed] [Google Scholar]
- Gilding EK, Marks MD. Analysis of purified glabra3-shapeshifter trichomes reveals a role for NOECK in regulating early trichome morphogenic events. Plant J. 2010:64(2):304–317. 10.1111/j.1365-313X.2010.04329.x [DOI] [PubMed] [Google Scholar]
- Jaffe FW, Tattersall A, Glover BJ. A truncated MYB transcription factor from Antirrhinum majus regulates epidermal cell outgrowth. J Exp Bot. 2007:58(6):1515–1524. 10.1093/jxb/erm020 [DOI] [PubMed] [Google Scholar]
- Jakoby MJ, Falkenhan D, Mader MT, Brininstool G, Wischnitzki E, Platz N, Hudson A, Hulskamp M, Larkin J, Schnittger A. Transcriptional profiling of mature Arabidopsis trichomes reveals that NOECK encodes the MIXTA-like transcriptional regulator MYB106. Plant Physiol. 2008:148(3):1583–1602. 10.1104/pp.108.126979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HB. Plant pubescence: an ecological perspective. Bot Rev. 1975:41(3):233–258. 10.1007/BF02860838 [DOI] [Google Scholar]
- Kannangara R, Branigan C, Liu Y, Penfield T, Rao V, Mouille G, Hofte H, Pauly M, Riechmann JL, Broun P. The transcription factor WIN1/SHN1 regulates cutin biosynthesis in Arabidopsis thaliana. Plant Cell. 2007:19(4):1278–1294. 10.1105/tpc.106.047076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B. Ricin—a potent homicidal poison. BMJ. 1979:1(6159):350–351. [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016:33(7):1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007:23(21):2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Larkin JC, Brown ML, Schiefelbein J. How do cells know what they want to be when they grow up? Lessons from epidermal patterning in Arabidopsis. Annu Rev Plant Biol. 2003:54(1):403–430. 10.1146/annurev.arplant.54.031902.134823 [DOI] [PubMed] [Google Scholar]
- Lashbrooke J, Adato A, Lotan O, Alkan N, Tsimbalist T, Rechav K, Fernandez-Moreno JP, Widemann E, Grausem B, Pinot F, et al. The tomato MIXTA-like transcription factor coordinates fruit epidermis conical cell development and cuticular lipid biosynthesis and assembly. Plant Physiol. 2015:169(4):2553–2571. 10.1104/pp.15.01145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Yadun S. Aposematic (warning) coloration associated with thorns in higher plants. J Theor Biol. 2001:210(3):385–388. 10.1006/jtbi.2001.2315 [DOI] [PubMed] [Google Scholar]
- Li Q, Cao C, Zhang C, Zheng S, Wang Z, Wang L, Ren Z. The identification of Cucumis sativus Glabrous 1 (CsGL1) required for the formation of trichomes uncovers a novel function for the homeodomain-leucine zipper I gene. J Exp Bot. 2015:66(9):2515–2526. 10.1093/jxb/erv046 [DOI] [PubMed] [Google Scholar]
- Lim HH, Domala S, Joginder S, Lee SH, Lim CS, Abu Bakar CM. Rice millers’ syndrome: a preliminary report. Br J Ind Med. 1984:41(4):445–449. 10.1136/oem.41.4.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JM, Roberts LM, & Robertus JD. Ricin: structure, mode of action, and some current applications. FASEB J. 1994:8(2):201–208. 10.1096/fasebj.8.2.8119491 [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell. 2003:15(1):165–178. 10.1105/tpc.007468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014:15(12):550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HC, Lam SH, Zhang D, Hsiao YY, Li BJ, Niu SC, Li CY, Lan S, Tsai WC, Liu ZJ. R2R3-MYB genes coordinate conical cell development and cuticular wax biosynthesis in Phalaenopsis aphrodite. Plant Physiol. 2022:188(1):318–331. 10.1093/plphys/kiab422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A, Wu Y, Yang Y, Llewellyn DJ, Dennis ES. The MYB transcription factor GhMYB25 regulates early fibre and trichome development. Plant J. 2009:59(1):52–62. 10.1111/j.1365-313X.2009.03847.x [DOI] [PubMed] [Google Scholar]
- Matias-Hernandez L, Jiang W, Yang K, Tang K, Brodelius PE, Pelaz S. AaMYB1 and its orthologue AtMYB61 affect terpene metabolism and trichome development ***in Artemisia annua and Arabidopsis thaliana. Plant J. 2017:90(3):520–534. 10.1111/tpj.13509 [DOI] [PubMed] [Google Scholar]
- Mauricio R, Rausher MD. Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evol Int J Org. 1997:51(5):1435–1444. 10.2307/2411196 [DOI] [PubMed] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980:8(19):4321–4325. 10.1093/nar/8.19.4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, Glover BJ, Linstead P, Martin C. Flower colour intensity depends on specialized cell shape controlled by a Myb-related transcription factor. Nature. 1994:369(6482):661–664. 10.1038/369661a0 [DOI] [PubMed] [Google Scholar]
- Ogunniyi DS. Castor oil: a vital industrial raw material. Bioresour Technol. 2006:97(9):1086–1091. 10.1016/j.biortech.2005.03.028 [DOI] [PubMed] [Google Scholar]
- Oppenheimer DG, Herman PL, Sivakumaran S, Esch J, Marks MD. A myb gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules. Cell. 1991:67(3):483–493. 10.1016/0092-8674(91)90523-2 [DOI] [PubMed] [Google Scholar]
- Oshima Y, Mitsuda N. The MIXTA-like transcription factor MYB16 is a major regulator of cuticle formation in vegetative organs. Plant Signal Behav. 2013:8(11):e26826. 10.4161/psb.26826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y, Shikata M, Koyama T, Ohtsubo N, Mitsuda N, Ohme-Takagi M. MIXTA-like transcription factors and WAX INDUCER1/SHINE1 coordinately regulate cuticle development in Arabidopsis and Torenia fournieri. Plant Cell. 2013:25(5):1609–1624. 10.1105/tpc.113.110783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens NDL, De Domenico E, Gilchrist MJ. An RNA-seq protocol for differential expression analysis. Cold Spring Harb Protoc. 2019:2019(6). 10.1101/pdb.prot098368 [DOI] [PubMed] [Google Scholar]
- Pastore JJ, Limpuangthip A, Yamaguchi N, Wu MF, Sang Y, Han SK, Malaspina L, Chavdaroff N, Yamaguchi A, Wagner D. LATE MERISTEM IDENTITY2 acts together with LEAFY to activate APETALA1. Development. 2011:138(15):3189–3198. 10.1242/dev.063073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CT, Zhang F, Lloyd AM. GL3 Encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics. 2000:156(3):1349–1362. 10.1093/genetics/156.3.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rodriguez M, Jaffe FW, Butelli E, Glover BJ, Martin C. Development of three different cell types is associated with the activity of a specific MYB transcription factor in the ventral petal of Antirrhinum majus flowers. Development. 2005:132(2):359–370. 10.1242/dev.01584 [DOI] [PubMed] [Google Scholar]
- Plett JM, Wilkins O, Campbell MM, Ralph SG, Regan S. Endogenous overexpression of Populus MYB186 increases trichome density, improves insect pest resistance, and impacts plant growth. Plant J. 2010:64(3):419–432. 10.1111/j.1365-313X.2010.04343.x [DOI] [PubMed] [Google Scholar]
- Qin W, Xie L, Li Y, Liu H, Fu X, Chen T, Hassani D, Li L, Sun X, Tang K. An R2R3-MYB transcription factor positively regulates the glandular secretory trichome initiation in Artemisia annua L. Front Plant Sci. 2021:12:657156. 10.3389/fpls.2021.657156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987:4(4):406–425. 10.1093/oxfordjournals.molbev.a040454 [DOI] [PubMed] [Google Scholar]
- Scarpa A, Guerci A. various uses of the castor oil plant (Ricinus communis L). J Ethnopharmacol. 1982:5(2):117–137. 10.1016/0378-8741(82)90038-1 [DOI] [PubMed] [Google Scholar]
- Schmitz G, Tillmann E, Carriero F, Fiore C, Cellini F, Theres K. The tomato blind gene encodes a MYB transcription factor that controls the formation of lateral meristems. Proc Natl Acad Sci USA. 2002:99(2):1064–1106. 10.1073/pnas.022516199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serna L, Martin C. Trichomes: different regulatory networks lead to convergent structures. Trends Plant Sci. 2006:11(6):274–280. 10.1016/j.tplants.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Shi P, Fu X, Shen Q, Liu M, Pan Q, Tang Y, Jiang W, Lv Z, Yan T, Ma Y, et al. The roles of AaMIXTA1 in regulating the initiation of glandular trichomes and cuticle biosynthesis in Artemisia annua. New Phytol. 2018:217(1):261–276. 10.1111/nph.14789 [DOI] [PubMed] [Google Scholar]
- Shi JX, Malitsky S, De Oliveira S, Branigan C, Franke RB, Schreiber L, Aharoni A. SHINE transcription factors act redundantly to pattern the archetypal surface of Arabidopsis flower organs. PloS Genet. 2011:7(5):e1001388. 10.1371/journal.pgen.1001388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K, Urao T, Koizumi M. Nucleotide sequence of a gene from Arabidopsis thaliana encoding a myb homologue. Plant Mol Biol. 1992:19(3):493–499. 10.1007/BF00023398 [DOI] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol. 2001:4(5):447–456. 10.1016/S1369-5266(00)00199-0 [DOI] [PubMed] [Google Scholar]
- Szymanski DB, Lloyd AM, Marks MD. Progress in the molecular genetic analysis of trichome initiation and morphogenesis in Arabidopsis. Trends Plant Sci. 2000:5(5):214–219. 10.1016/S1360-1385(00)01597-1 [DOI] [PubMed] [Google Scholar]
- Taketa S, Amano S, Tsujino Y, Sato T, Saisho D, Kakeda K, Nomura M, Suzuki T, Matsumoto T, Sato K, et al. Barley grain with adhering hulls is controlled by an ERF family transcription factor gene regulating a lipid biosynthesis pathway. Proc Natl Acad Sci. 2008:105(10):4062–4067. 10.1073/pnas.0711034105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Eggermont K, Tierens KF, Broekaert WF. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 1999:121(4):1093–1101. 10.1104/pp.121.4.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UniProt Consortium . Uniprot: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019:47(D1):D506–D515. 10.1093/nar/gky1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O, Rivas S, Mestre P, Baulcombe D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 2003:33(5):949–956. 10.1046/j.1365-313X.2003.01676.x [DOI] [PubMed] [Google Scholar]
- Walford SA, Wu Y, Llewellyn DJ, Dennis ES. GhMYB25-like: a key factor in early cotton fibre development. Plant J. 2011:65(5):785–797. 10.1111/j.1365-313X.2010.04464.x [DOI] [PubMed] [Google Scholar]
- Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC. The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell. 1999:11(7):1337–1349. 10.1105/tpc.11.7.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang L, Han L, Cheng Z, Liu X, Wang S, Liu L, Chen J, Song W, Zhao J, et al. HECATE2 Acts with GLABROUS3 and Tu to boost cytokinin biosynthesis and regulate cucumber fruit wart formation. Plant Physiol. 2021:187(3):1619–1635. 10.1093/plphys/kiab377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wu D, Yang T, Sun C, Wang Z, Han B, Wu S, Yu A, Chapman MA, Muraguri S, et al. Genomic insights into the origin, domestication and genetic basis of agronomic traits of castor bean. Genome Biol. 2021:22(1):113. 10.1186/s13059-021-02333-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Cai Y, Liu X, Dong M, Zhang Y, Chen S, Zhang W, Li Y, Tang M, Zhai X, et al. A CsMYB6-CsTRY module regulates fruit trichome initiation in cucumber. J Exp Bot. 2018:69(8):1887–1902. 10.1093/jxb/ery047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YW, Sagawa JM, Di Stilio VS, BradshawHD, Jr. Bulk segregant analysis of an induced floral mutant identifies a MIXTA-like R2R3 MYB controlling nectar guide formation in Mimulus lewisii. Genetics. 2013:194(2):523–528. 10.1534/genetics.113.151225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Xu X, Luo Y, Gong Z, Hu X, Wu M, Liu Y, Yan F, Zhang X, Zhang W, et al. R2r3 MYB-dependent auxin signalling regulates trichome formation, and increased trichome density confers spider mite tolerance on tomato. Plant Biotechnol J. 2021:19(1):138–152. 10.1111/pbi.13448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Rossignol P, Huang T, Wang Y, May A, Dupont C, Orbovic V, Irish VF. Reprogramming of stem cell activity to convert thorns into branches. Curr Biol. 2020:30(15):2951–2961.e2955. 10.1016/j.cub.2020.05.068 [DOI] [PubMed] [Google Scholar]
- Zhang H, Wu K, Wang Y, Peng Y, Hu F, Wen L, Han B, Qian Q, Teng S. A WUSCHEL-like homeobox gene, OsWOX3B responses to NUDA/GL-1 locus in rice. Rice. 2012:5(1):30. 10.1186/1939-8433-5-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou NN, Tang KX, Jeauffre J, Thouroude T, Arias DCL, Foucher F, Oyant LH. Genetic determinism of prickles in rose. Theor Appl Genet. 2020:133(11):3017–3035. 10.1007/s00122-020-03652-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.