Abstract
Objectives/Hypothesis:
Patients with Down syndrome have a high incidence of obstructive sleep apnea (OSA) and limited treatment options. Hypoglossal stimulation has shown efficacy but has not yet been approved for pediatric populations. Our objective is to characterize the therapy response of adolescent patients with down syndrome and severe OSA who underwent hypoglossal stimulation.
Study Design:
Prospective longitudinal trial.
Methods:
We are conducting a multicenter single-arm trial of hypoglossal stimulation for adolescent patients with Down syndrome and severe OSA. Interim analysis was performed to compare objective sleep and quality of life outcomes at 12 months postoperatively for the first 20 patients.
Results:
The mean age was 15.5 and baseline AHI 24.2. Of the 20 patients, two patients (10.0%) had an AHI under 1.5 at 12 months; nine patients of 20 (45.0%) under five; and 15 patients of 20 (75.0%) under 10. The mean decrease in AHI was 15.1 (P < .001). Patients with postoperative AHI over five had an average baseline OSA-18 survey score of 3.5 with an average improvement of 1.7 (P = .002); in addition, six of these patients had a relative decrease of apneas compared to hypopneas and seven had an improvement in percentage of time with oxygen saturation below 90%.
Conclusions:
Patients with persistently elevated AHI 12 months after hypoglossal implantation experienced improvement in polysomnographic and quality of life outcomes. These results suggest the need for a closer look at physiologic markers for success beyond reporting AHI as the gold standard.
Level of Evidence:
4
Keywords: Down syndrome, pediatric obstructive sleep apnea, hypoglossal nerve stimulation
INTRODUCTION
Children with Down syndrome have a high incidence of obstructive sleep apnea (OSA), with as many as 80% of patients with Down syndrome having OSA compared to less than 5% of the general pediatric population.1 Untreated OSA can have serious and lifelong consequences.2 OSA contributes to worsened quality of life and adverse cardiopulmonary findings.2–4 There is also some evidence that untreated OSA impacts neurocognition in children; children with Down syndrome and untreated OSA, defined by a conservative cutoff of an apnea-hypopnea index (AHI) greater than 1.5, had intelligence quotient (IQ) scores nine points lower on average than children with Down syndrome without OSA.5 This finding suggests the need for aggressive OSA management in this population.
There are limited treatment options for OSA for children with Down syndrome. Adenotonsillectomy is the first line treatment, but residual disease is common after surgical options have been exhausted.6 Only 16% to 33% of children with Down syndrome have resolution of OSA after adenotonsillectomy alone.7–9 Many require subsequent continuous positive airway pressure (CPAP) support, which can be poorly tolerated due to coincident sensory integrative disorders.10,11 Hypoglossal nerve stimulation is a novel therapy that has shown efficacy in treating adults with OSA, but the device has not been approved by the Food and Drug Administration for the pediatric population.12, 13 We hypothesize that this therapy can be beneficial for patients with Down syndrome and OSA.
The efficacy and therapy response rates of hypoglossal nerve stimulation in a pediatric population have not yet been described. Prognostic factors like body-mass index have been investigated in other settings, with some evidence suggesting that lower body-mass index (BMI) is associated with improved therapy response after sleep surgery.14, 15 In adults, high BMI and complete concentric palatal collapse on drug-induced sleep endoscopy have been established as contraindications to hypoglossal nerve stimulation, but the prognostic factors associated with therapy response in pediatric population are unknown.
We are conducting an FDA-approved Phase 1 clinical trial of hypoglossal nerve implants in adolescents with Down syndrome who have persistent severe OSA after adenotonsillectomy and are not able to tolerate CPAP. We present the outcomes for the first 20 patients who underwent hypoglossal nerve stimulation. The objective of our study is to present planned interim results describing outcomes for therapy responders and non-responders and evaluating the association between BMI percentile and therapy response, in addition to exploring other prognostic characteristics.
MATERIALS AND METHODS
Study Design
We are conducting a Phase 1 single-arm multi-center clinical trial of the safety and efficacy of hypoglossal nerve stimulation in adolescent patients with Down syndrome and severe OSA. Patients were identified as candidates by participating physicians in institutional otolaryngology, sleep, and Down syndrome clinics. Verbal assent was obtained from all patients with Down syndrome, and written consent obtained from both parents/legal guardians and from patients with Down syndrome 18 years of age or older. Age, gender, and BMI percentile (adjusted for age and gender) were measured at baseline. If patient did not have a polysomnogram (PSG) in the last 6 months, they underwent a baseline PSG to confirm eligibility. Eligible patients then underwent a drug-induced sleep endoscopy (DISE) under sedation with propofol and/or dexmedetomidine. The velum, oropharynx, tongue base, epiglottis (VOTE) classification scheme was used to score the DISE exam and patients were excluded if they had circumferential palatal collapse.16
Patients with Down syndrome were included if they were between 10 and 21 years old, were English-speaking, and had persistent severe OSA. Persistent severe OSA was defined as AHI ≥10 after adenotonsillectomy and either inability to tolerate CPAP or dependence on a tracheotomy at night. Patients were excluded if they had a central apnea contribution over 25%, BMI over 95th percentile, a medical condition that would require future need for magnetic resonance imaging, DISE consistent with circumferential palatal collapse, or AHI ≥50. Sensory integrative disorders were not an exclusion criteria.
Study patients meeting inclusion criteria then underwent hypoglossal nerve stimulator implantation using standard techniques. One month after implantation, the nerve stimulators were activated in clinic then turned off. That evening, patients underwent titration of their devices during an overnight PSG, after which they were discharged to use the therapy nightly. Follow-up titration PSGs were performed at 2, 6, and 12 months. PSGs were scored using American Academy of Sleep pediatric standards.17 The majority of sleep centers reported percentage of time with oxygen saturation below 90%, but the studies for five of the patients reported percentage of time with oxygen saturation below 88%; these were combined for the purposes of data analysis. 12-month postoperative outcomes were measured at goal voltage titration.
Subjective caregiver-reported outcomes were obtained at baseline, 2, 6, and 12 months. The two surveys administered were the OSA-18 and the Epworth Sleepiness Scale (ESS). The OSA-18 is a validated quality of life survey for OSA that includes questions in five domains (sleep disorders, physical distress, emotional distress, diurnal problems, and caretaker occupation).18 The survey also has an additional question asking caregivers to rate their child’s quality of life on a scale of 0 to 10 where higher scores correspond to better quality of life. The OSA-18 survey score was calculated as the mean of the domain questions, excluding surveys where less than half of the questions were completed. The change in survey score was calculated by subtracting the follow-up from baseline survey score in order to quantify the effect of the change.19 The change in survey score ranged from −6 to 6 where a change <0.5 indicates a trivial change; 0.5 to 0.9 a small change; 1.0 to 1.4 a moderate change; and ≥ 1.5 a large change. The total OSA-18 score was calculated as the sum of all domain questions for surveys without any missing questions. The total score ranges from 18 to 126 where a total score <60 indicates a small impact on quality of life; 60 to 80 indicates a moderate impact; and >80 indicates a large impact. The ESS is a validated survey of daytime sleepiness.20 The ESS is scored as the sum of all items with scores ranging from 0 to 24 and higher scores indicating worse symptoms. Two patients did not complete the OSA-18 at 12 months and one patient did not complete the ESS at 12 months.
Patients were recruited from three academic centers with one surgeon performing the surgery at each site. The study was approved by the institutional review boards at each institution, as well as by the United States Food and Drug Administration (FDA), which issued an investigational device exemption (IDE) with a target enrollment of 42 patients to demonstrate device safety. Prior to this report, 37 patients were screened, of which seven were ineligible (one due to age, two due to BMI percentile, three due to PSG findings, and one due to DISE findings) and 10 declined. Planned interim analysis was performed for the first 20 consecutive patients implanted between April 2015 and August 2018.
Data Analysis
The pre-specified primary outcome was change in AHI. Prespecified secondary outcomes were OSA-18 and ESS scores, obstructive AHI, hypopnea proportion, and oxygen saturation. “Therapy responders” were defined as having AHI <5 after goal titration of the hypoglossal nerve stimulator. This threshold was identified because CPAP therapy would need to be continued in pediatric patients with moderate OSA (AHI ≥ 5). The 12-month postoperative outcomes were measured at goal voltage titration.
Paired t-tests were used to compare baseline and 12-month post-operative normally distributed variables. Sign tests were used for data that did not appear normally distributed or symmetric. The Shapiro–Wilk test was used to test normality. The chi-square test was used to compare therapy response rates for patients with high BMI percentile (85th percentile or higher based on age and gender). To compare characteristics of therapy responders to non-responders, t-tests were used for normally distributed variables and Wilcoxon tests for variables that were not normally distributed. A significance level of 0.05 was used for all tests.
RESULTS
Overall Outcomes
Patient demographics for the first 20 patients who completed the study are described in Table I. The majority of patients were female (65%) with a mean age of 15.5 and a mean BMI percentile of 73.5%. The mean baseline AHI was 24.2 with standard deviation (SD) 8.2, and the mean 12-month AHI was 9.1 (mean change: −15.1; SD 12.5; P < .0001). All patients tolerated implantation well, and there were no serious complications.
TABLE I.
Patient Demographics (n = 20).
| Characteristic | Frequency (n = 20) |
|---|---|
|
| |
| Gender | |
| Male | 7 (35%) |
| Female | 13 (65%) |
| Age | |
| 10–12 | 3 (15%) |
| 13–17 | 12 (60%) |
| 18–21 | 5 (25%) |
| BMI Percentile | |
| Normal (<85th percentile) | 9 (45%) |
| Overweight (≥85th percentile) | 11 (55%) |
For the overall group of 20 patients, there were significant improvements in subjective outcomes (Fig. 1). The mean average baseline OSA-18 survey score was 3.5 (SD 1.3), and the mean 12-month post-operative OSA-18 score was 1.7 (SD 0.7). The mean change in OSA-18 was −1.9 (SD 1.3, P < .0001). There were significant improvements in all the OSA-18 domains except for emotional distress (Table II). At baseline, the mean ESS was 8.9 (SD 6.5) and eight patients (42.1%) had abnormally elevated ESS scores above 10. In comparison, the mean 12-month post-operative ESS was 5.4 (SD 5.3). In addition, there was a significant decrease in abnormal ESS scores with three patients (15.8%) who had ESS scores above 10 at 12 months (P = .027). The mean change was −3.3 (SD 4.3; P = .004). Lower scores on the OSA-18 survey and total scores and the ESS reflect better quality of life and less symptoms.
Fig. 1.
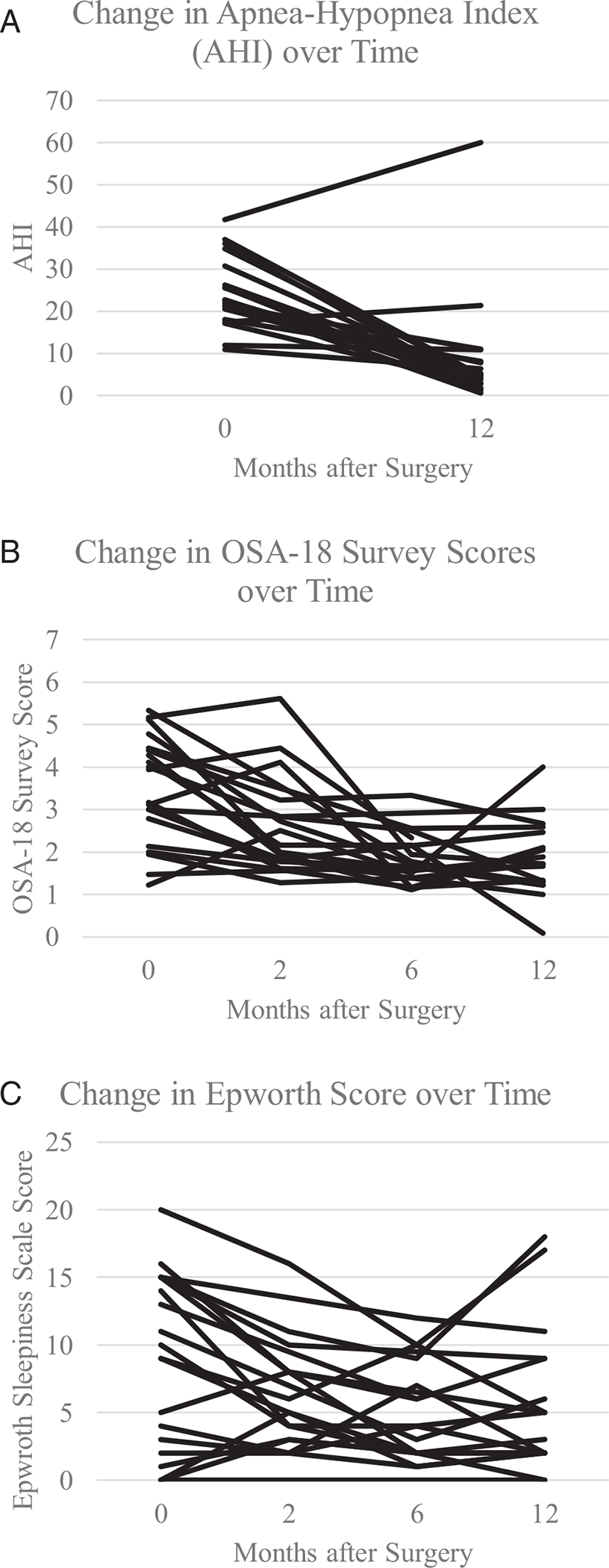
Postoperative change in a) apnea-hypopnea index (AHI) b) OSA-18 survey scores c) Epworth Sleepiness (ESS) scores.
TABLE II.
OSA-18 Survey Outcomes in All Patients, n = 20.
| Characteristic | Baseline (SD) | 12-Month (SD) | Change (SD) | P Value |
|---|---|---|---|---|
|
| ||||
| Survey score | 3.5 (1.3) | 1.7 (0.7) | −1.9 (1.3) | <.0001 |
| Total score | 68.6 (20.9) | 30.2 (8.8) | −42.8 (20.0) | <.0001 |
| Domain average scores | ||||
| Sleep disorders | 4.6 (1.7) | 1.9 (1.0) | −2.8 (1.6) | <.0001 |
| Physical distress | 2.4 (1.3) | 1.7 (0.7) | −0.8 (1.3) | .02 |
| Emotional distress | 2.8 (1.7) | 1.8 (0.8) | −1.0 (1.7) | .06 |
| Diurnal problems | 3.7 (1.4) | 2.2 (1.0) | −1.7 (1.3) | <.0001 |
| Caretaker preoccupation | 3.9 (1.7) | 1.5 (0.7) | −2.6 (1.7) | <.0001 |
| Overall quality of life* | 4.7 (1.8) | 8.1 (1.3) | +3.3 (1.7) | <.0001 |
Scored on a scale of 0 to 10 where higher scores correspond to better quality of life.
Therapy Response
Of the 20 patients, two patients (10.0%) did not experience a decrease in AHI and 15 patients (75%) had a 50% or greater decrease in AHI 12 months after surgery when at the goal titration for hypoglossal nerve stimulation (Fig. 1). There were nine patients (45.0%) who were “therapy responders” (12-month post-operative AHI under 5). Two (10.0%) out of 20 patients had a 12-month post-operative AHI under 1.5, and 15 patients (75.0%) had 12-month post-operative AHI under 10.
There were 11 patients (55.0%) with BMI in the 85th percentile or greater. Of these 11 patients, five patients (45.5%) responded to therapy, compared to 44.4% of patients with BMI under the 85th percentile (P = .96). There were also not significant differences between therapy responders and non-responders in gender, age, baseline polysomnographic features, or baseline caregiver-reported scores (Table III).
TABLE III.
Baseline Characteristics of Therapy Responders (12-month Post-operative AHI <5, n = 9) Compared to Therapy Non-responders (12-month Post-operative AHI ≥5, n = 11).
| Characteristic | Therapy Responders, n = 9 (95% CI) | Therapy Non-responders, n = 11 (95% CI) | P Value |
|---|---|---|---|
|
| |||
| Male gender | 66.7% (28.2–100%) | 63.6% (29.7–97.5%) | .895 |
| Age | 15.2 (12.5–17.9) | 15.6 (13.6–17.7) | .785 |
| Body-mass index percentile | 78.7% (61.8–95.5%) | 69.2% (46.0–92.3%) | .964 |
| Baseline caregiver-reported scores | |||
| Epworth sleepiness Scale | 10.3 (4.5–16.2) | 7.6 (4.0–11.3) | .387 |
| OSA-18 survey Score | 3.4 (2.2–4.5) | 3.5 (2.8–4.3) | .800 |
| Baseline polysomnogram | |||
| AHI | 26.8 (22.0–31.7) | 22.1 (18.9–28.3) | .188 |
| Obstructive AHI | 24.2 (19.0–29.3) | 19.9 (13.9–25.9) | .256 |
| Central apnea index | 2.6 (0.7–4.6) | 2.2 (1.2–3.2) | .651 |
| Hypopnea proportion* | 67.0% (47.2–86.9%) | 70.7% (53.8–87.7%) | .655 |
| Supine AHI* | 37.6 (12.2–63.0) | 23.4 (13.0–33.8) | .231 |
| Percentage of time SpO2 < 90%* | 0.5% (0–1.3%) | 5.5% (0–13.7%) | .235 |
| Sleep efficiency | 80.3% (72.1–88.5%) | 67.3% (56.4–78.2%) | .055 |
| REM percentage | 12.2% (6.7–8.7%) | 13.6% (8.3–19.0%) | .672 |
AHI = apnea-hypopnea Index; OSA-18 = Obstructive Sleep Apnea-18; REM = rapid eye movement; SpO2 = oxygen saturation.
Missing for 1 patient.
Outcomes in Therapy Non-responders
Among the 11 patients who had persistent AHI of five or greater, improvements were noted in other domains. With regard to subjective outcomes, the therapy non-responders had an average baseline OSA-18 survey score of 3.5 (SD 1.1) with an average change of −1.7 (SD 1.4; P = .002). Similarly, the average total OSA-18 score at baseline was 70.7 (SD 13.4) with an average change in total OSA-18 score of −38.5 (P = .005). There were significant improvements in the OSA-18 domains of sleep disorders, diurnal problems, and caretaker preoccupation in the therapy non-responders (Table IV). The average change in ESS was −2.1 (SD 3.3; P = .074). One of the two patients with ESS over 10 in this group had normalization of ESS.
TABLE IV.
Outcomes in Therapy Non-responders, n = 11 (12-month Post-operative AHI ≥5).
| Characteristic | Baseline (SD) | 12-Month (SD) | Change (SD) | P Value |
|---|---|---|---|---|
|
| ||||
| Parent-reported outcomes | ||||
| OSA-18 | ||||
| Survey score | 3.5 (1.1) | 1.8 (0.9) | −1.7 (1.4) | .002 |
| Total score | 70.7 (13.4) | 31.5 (9.5) | −38.5 (20.1) | .005 |
| Domain average scores | ||||
| Sleep disorders | 4.6 (1.4) | 2.1 (0.9) | −2.5 (1.4) | .0003 |
| Physical distress | 2.1 (0.9) | 1.9 (0.8) | −0.2 (0.8) | .46 |
| Emotional distress | 3.2 (1.8) | 1.8 (1.0) | −1.2 (1.9) | .07 |
| Diurnal problems | 3.6 (1.1) | 2.3 (1.0) | −1.3 (1.4) | .02 |
| Caretaker | 4.2 (1.7) | 1.6 (0.8) | −2.6 (2.0) | .003 |
| Preoccupation | ||||
| Overall quality of Life* | 4.1 (1.8) | 7.7 (1.6) | +3.5 (1.7) | .0001 |
| Epworth sleepiness score mean score | 7.6 (5.4) | 5.0 (5.2) | −2.1 (3.3) | .07 |
| Polysomnographic outcomes | ||||
| Respiratory events | ||||
| AHI | 22.1 (9.2) | 13.8 (16.0) | −8.2 (12.4) | .05 |
| Obstructive AHI | 19.9 (8.9) | 12.4 (15.2) | −7.5 (12.1) | .07 |
| Central apnea index | 2.2 (1.5) | 1.5 (1.5) | −0.65 (2.1) | .32 |
| Hypopnea proportion | 70.7% (25.3%) | 80.9% (15.0%) | 10.2% (28.9%) | .27 |
| Supine AHI | 23.4 (15.5) | 17.8 (18.6) | −5.6 (14.1) | .22 |
| Oxygenation | ||||
| Percentage of time | 5.5% (12.2%) | 0.84% (2.8%) | −4.7% (12.8%) | .11 |
| SpO2 < 90% | ||||
| SpO2 Nadir | 85.2% (6.1%) | 87.6% (5.2%) | +2.4% (5.3%) | .05 |
| Baseline SpO2 | 96.4% (1.5%) | 95.0% (1.5%) | −1.3% (1.5%) | .01 |
| Sleep fragmentation | ||||
| Sleep efficiency | 67.3% (16.2%) | 69.2% (19.9%) | +1.9% (23.7%) | .80 |
| Stage N1 percentage | 7.8% (8.7%) | 4.6% (5.0%) | −3.2% (7.4%) | .07 |
| REM percentage | 13.6% (8.0%) | 12.6% (0.1%) | −1.0% (10.3%) | .75 |
AHI = apnea-hypopnea index; SD = standard deviation; REM = rapid eye movement; SpO2 = oxygen saturation.
Scored on a scale of 0 to 10 where higher scores correspond to better quality of life.
With regard to PSG findings in the 11 therapy non-responders, 6 patients (54.5%) had a relative decrease of apneas compared to hypopneas, and one patient (9.1%) started with apnea-dominant OSA at baseline that became hypopnea-dominant OSA after implantation. In non-responders, the baseline proportion of hypopnea events out of total respiratory events was 70.7% (SD 25.3%) compared to 80.9% at 12 months (SD 15.0%; P = .27).
Three of the patients with persistent AHI of five or greater did not have baseline oxygen saturation below 90%. Of the remaining eight patients, seven of them (87.5%) had a decrease in the percentage of time with oxygen saturation below 90%. The mean baseline change in percentage of time with oxygen saturation below 90% was −4.7% (P = .11). These results were not changed when excluding sleep studies performed at sleep labs that report percentage of time with oxygen saturation below 88% instead of below 90%. Other PSG findings are described in Table IV.
DISCUSSION
In this interim analysis of the outcomes after hypoglossal nerve stimulation for adolescents with Down syndrome and severe persistent OSA, we found overall high rates of AHI improvement for 20 patients. At 12 months after surgery, 15 patients (75%) had at least a 50% decrease in AHI. However, 55% of patients still had 12-month post-operative AHI of 5 or greater. In a small study of three adult patients with severe OSA and Down syndrome, all patients experienced substantial reductions in AHI even though titrated AHI continued to be >5 in two of these patients.21 In comparison, two large studies in the general adult population have reported therapy response rates of 63% to 69%.15, 22
Studies have identified different predictors of OSA treatment response after tonsillectomy in children. Factors like BMI, baseline AHI, race, and age have been identified as prognostic indicators of treatment success after tonsillectomy.9, 14, 23, 24 For instance, in the Childhood Adenotonsillectomy Trial, 464 children were randomized to adenotonsillectomy or watchful waiting for OSA. Black race, obesity, and higher baseline AHI were all poor prognostic indicators for polysomnographic normalization after tonsillectomy.14
It is unclear whether these are also important factors for hypoglossal nerve stimulation. In a study of 71 adults who underwent hypoglossal nerve stimulation, lower oxygen desaturation index was associated with improved treatment response.22 Another study identified female gender as predictive of greater improvement in AHI.15 There have been mixed results regarding the effect of BMI on therapy response.13, 15, 25 We did not find any significant association between age, gender, BMI percentile, baseline polysomnographic features, or baseline quality of life scores and pediatric hypoglossal stimulation therapy response. To our knowledge, there is no other data about treatment response rates after pediatric hypoglossal nerve implantation.
Our findings raise the question of how best to define success after OSA treatment. AHI is the most commonly used measure of OSA severity and therapy response. The advantages of AHI include universal reporting, ease of comparison, responsiveness to treatment, and correlation with cardiovascular and other health end points. Yet some criticize AHI as a uni-dimensional and crude metric.26 One limitation of AHI is that it places equal emphasis on apnea and hypopnea events. In our study, 54.5% of patients who had 12-month post-operative AHI of five or greater had a favorable decrease in apneas relative to hypopneas, and a decrease in complete breathing cessation may still be beneficial.
Another concern is that AHI may not adequately capture the outcomes that are of importance to patients, such as quality of life scores and neurobehavioral outcomes. Some have found a poor correlation between AHI and subjective quality of life scores.27, 28 For instance, in the Childhood Adenotonsillectomy Trial, ESS score improved more after adenotonsillectomy than in the control arm, and reported sleepiness was only weakly associated with improvement in AHI.28
One of the challenges is that there is not a consistent definition for therapy response. We used a threshold of AHI ≥5 since CPAP is typically recommended in pediatric patients with moderate or severe OSA, and we found that 55% of patients still had AHI of 5 or greater 12 months after surgery. However, there was a high baseline AHI of 24.2, and all but three patients had a decrease in AHI of five or greater 12 months postoperatively. Excluding those three patients, there was a mean decrease in AHI of 19.0, representing substantial improvements even though a majority of the patients were not fully cured.
As AHI is a single value that may not capture the physiologic complexity of the disease, other outcomes should also be considered in defining successful treatment. These include other polysomnographic features of improved sleep, such as oxygenation and sleep fragmentation, as well as patient-reported outcomes and neurobehavioral outcomes. In our study, we found improvements in OSA-18 even in our therapy non-responders. ESS scores also improved, even though pediatric OSA is often associated with hyperactivity more than daytime sleepiness. Our next steps include an investigation into expressive language and neurocognitive outcomes, which are other domains that are important to patients and their families. Sleep-disordered breathing is associated with worse neurobehavioral morbidity in children without Down syndrome, with improvements after treatment like adenotonsillectomy but weak association with change in AHI.14, 29–31 Breslin et al. also found that children with Down syndrome and OSA had an average IQ 9 points lower compared to children with Down syndrome without OSA.5 Such other domains may not correlate with AHI but are nevertheless important to patients, and a multidisciplinary approach is needed to consider these outcomes holistically in forming patient-centered treatment plans.
These data must be interpreted in the context of the study design. Our results represent planned interim analysis, which can provide a preliminary report of safety and efficacy but should be considered in the framework of the larger study. Given the small number of patients, there is limited power with regard to comparing outcomes for therapy responders and non-responders. In the interpretation of our results, it is also important to differentiate between a statistically significant and a clinically significant result by taking into consideration the magnitude of the effect. In particular, there was a significant difference in the mean oxygenation, but the magnitude of the difference was only 1.3% and both the baseline and follow-up values were in the normal range. There was also some site variation in the sleep study reports from different sites, particularly with regard to percentage of time with oxygen saturation below 88% versus 90%; results were unchanged when excluding sites that reported oxygen saturation below 88%. An additional consideration is that some of the patients were young adults over 21 years old, and there are different scoring criteria for adolescents compared to young adults. Only three of the patients were over 21 years old, so all patients were assessed using the same criteria in order to maintain consistency across the study. Sensory integrative disorders were not in the exclusion criteria but no children with autism were implanted, so the results may not be generalizable to that population. Another limitation is the absence of a control arm consisting of patients who did not undergo surgery, though spontaneous resolution of OSA would be less likely to occur in an adolescent population with severe disease.32–34 At the trial completion, we plan to investigate neurobehavioral outcomes for a subset of patients as well as analyze additional characteristics like VOTE classification for the final sample of 42 patients. Pending FDA approval of hypoglossal stimulation in a pediatric population, future plans would also include a controlled trial comparing outcomes in children who did and did not receive the intervention.
CONCLUSIONS
In conclusion, hypoglossal nerve stimulation was able to be safely performed for 20 adolescents with Down syndrome with high efficacy rates. Patients with persistently elevated AHI at 12 months after surgery still experienced improvement in caregiver-reported outcomes. These results suggest the need for a closer look at multifaceted markers for success other than simply reporting AHI values as the gold standard.
ACKNOWLEDGMENTS
This trial received funding for a research coordinator from Inspire Medical Systems and for neurocognitive testing from the LuMind Foundation. Dr. Yu received research support from the American Thoracic Society Academic Sleep Pulmonary Integrated Research/Clinical Fellowship. Dr. Skotko occasionally consults on the topic of Down syndrome through Gerson Lehrman Group. He receives remuneration from Down syndrome non-profit organizations for speaking engagements and associated travel expenses. Dr. Skotko receives annual royalties from Woodbine House, Inc., for the publication of his book, Fasten Your Seatbelt: A Crash Course on Down syndrome for Brothers and Sisters. Within the past two years, he has received research funding from F. Hoffmann-La Roche, Inc. and LuMind Research Down Syndrome Foundation to conduct clinical trials for people with Down syndrome. Dr. Skotko is occasionally asked to serve as an expert witness for legal cases where Down syndrome is discussed. Dr. Skotko serves in a non-paid capacity on the Honorary Board of Directors for the Massachusetts Down Syndrome Congress and the Professional Advisory Committee for the National Center for Prenatal and Postnatal Down Syndrome Resources. Dr. Skotko has a sister with Down syndrome.
Footnotes
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Contributor Information
Phoebe K. Yu, Department of Otolaryngology, Massachusetts Eye and Ear Infirmary, Boston, Massachusetts, U.S.A..
Asitha D. L. Jayawardena, Department of Otolaryngology, Massachusetts Eye and Ear Infirmary, Boston, Massachusetts, U.S.A..
Matthew Stenerson, Department of Otolaryngology, Massachusetts Eye and Ear Infirmary, Boston, Massachusetts, U.S.A..
Margaret B. Pulsifer, Department of Otolaryngology, Massachusetts Eye and Ear Infirmary, Boston, Massachusetts, U.S.A.; Department of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts, U.S.A.
Julie A. Grieco, Department of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts, U.S.A..
Leonard Abbeduto, MIND Institute and Department of Psychiatry and Behavioral Sciences, University of California Davis, Sacramento, California, U.S.A..
Raj C. Dedhia, CPAP Alternatives Clinic and Division of Sleep Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, U.S.A..
Ryan J. Soose, Department of Otolaryngology and Division of Sleep Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, U.S.A..
Allison Tobey, Department of Otolaryngology and Division of Sleep Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, U.S.A..
Nikhila Raol, Department of Otolaryngology, Children’s Healthcare of Atlanta, Atlanta, Georgia, U.S.A..
Stacey L. Ishman, Department of Otolaryngology—Head and Neck Surgery, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, U.S.A..
Sally R. Shott, Department of Otolaryngology—Head and Neck Surgery, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, U.S.A..
Michael S. Cohen, Department of Otolaryngology, Massachusetts Eye and Ear Infirmary, Boston, Massachusetts, U.S.A..
Brian G. Skotko, Department of Pediatrics, Massachusetts General Hospital, Boston, Massachusetts, U.S.A.; Down Syndrome Program, Division of Medical Genetics and Metabolism, Department of Pediatrics, Massachusetts General Hospital, Boston, Massachusetts, U.S.A.
Thomas B. Kinane, Department of Pediatrics, Massachusetts General Hospital, Boston, Massachusetts, U.S.A..
Donald G. Keamy, Jr, Department of Otolaryngology, Massachusetts Eye and Ear Infirmary, Boston, Massachusetts, U.S.A..
Christopher J. Hartnick, Department of Otolaryngology, Massachusetts Eye and Ear Infirmary, Boston, Massachusetts, U.S.A..
BIBLIOGRAPHY
- 1.Shott SR. Down syndrome: common otolaryngologic manifestations. Am J Med Genet C Semin Med Genet 2006;142C:131–140. [DOI] [PubMed] [Google Scholar]
- 2.Knauert M, Naik S, Gillespie MB, Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J Otorhinolaryngol Head Neck Surg 2015;1:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horne RS, Wijayaratne P, Nixon GM, Walter LM. Sleep and sleep disordered breathing in children with down syndrome: effects on behaviour, neurocognition and the cardiovascular system. Sleep Med Rev 2019;44:1–11. [DOI] [PubMed] [Google Scholar]
- 4.Ingram DG, Singh AV, Ehsan Z, Birnbaum BF. obstructive sleep apnea and pulmonary hypertension in children. Paediatr Respir Rev 2017;23:33–39. [DOI] [PubMed] [Google Scholar]
- 5.Breslin J, Spano G, Bootzin R, Anand P, Nadel L, Edgin J. Obstructive sleep apnea syndrome and cognition in Down syndrome. Dev Med Child Neurol 2014;56:657–664. [DOI] [PubMed] [Google Scholar]
- 6.Nation J, Brigger M. the efficacy of adenotonsillectomy for obstructive sleep apnea in children with down syndrome: a systematic review. Otolaryngol Head Neck Surg 2017;157:401–408. [DOI] [PubMed] [Google Scholar]
- 7.Ingram DG, Ruiz AG, Gao D, Friedman NR. Success of tonsillectomy for obstructive sleep apnea in children with down syndrome. J Clin Sleep Med 2017;13:975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nehme J, LaBerge R, Pothos M, et al. Treatment and persistence/recurrence of sleep-disordered breathing in children with Down syndrome. Pediatr Pulmonol 2019;54:1291–1296. [DOI] [PubMed] [Google Scholar]
- 9.Tauman R, Gulliver TE, Krishna J, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr 2006;149:803–808. [DOI] [PubMed] [Google Scholar]
- 10.Amaddeo A, Frapin A, Touil S, Khirani S, Griffon L, Fauroux B. Outpatient initiation of long-term continuous positive airway pressure in children. Pediatr Pulmonol 2018;53:1422–1428. [DOI] [PubMed] [Google Scholar]
- 11.Trucco F, Chatwin M, Semple T, Rosenthal M, Bush A, Tan HL. Sleep disordered breathing and ventilatory support in children with down syndrome. Pediatr Pulmonol 2018;53:1414–1421. [DOI] [PubMed] [Google Scholar]
- 12.Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014;370:139–149. [DOI] [PubMed] [Google Scholar]
- 13.Woodson BT, Gillespie MB, Soose RJ, et al. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg 2014;151:880–887. [DOI] [PubMed] [Google Scholar]
- 14.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013;368:2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thaler E, Schwab R, Maurer J, et al. Results of the ADHERE upper airway stimulation registry and predictors of therapy efficacy. Laryngoscope 2020;130:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol 2011;268:1233–1236. [DOI] [PubMed] [Google Scholar]
- 17.Iber C, American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Franco RA Jr, Rosenfeld RM, Rao M. First place–resident clinical science award 1999: quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg 2000;123:9–16. [DOI] [PubMed] [Google Scholar]
- 19.Sohn H, Rosenfeld RM. Evaluation of sleep-disordered breathing in children. Otolaryngol Head Neck Surg 2003;128:344–352. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Boon M, Ishman SL, Suurna MV. Hypoglossal nerve stimulation in three adults with down syndrome and severe obstructive sleep apnea. Laryngoscope 2019;129:E402–E406. [DOI] [PubMed] [Google Scholar]
- 22.Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg 2018;159:194–202. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med 2010;182:676–683. [DOI] [PubMed] [Google Scholar]
- 24.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130:576–584. [DOI] [PubMed] [Google Scholar]
- 25.Huntley C, Steffen A, Doghramji K, Hofauer B, Heiser C, Boon M. Upper airway stimulation in patients with obstructive sleep apnea and an elevated body mass index: a multi-institutional review. Laryngoscope 2018;128:2425–2428. [DOI] [PubMed] [Google Scholar]
- 26.Punjabi NM. COUNTERPOINT: is the apnea-hypopnea index the best way to quantify the severity of sleep-disordered breathing? no. Chest 2016;149:16–19. [DOI] [PubMed] [Google Scholar]
- 27.Baldassari CM, Alam L, Vigilar M, Benke J, Martin C, Ishman S. Correlation between REM AHI and quality-of-life scores in children with sleep-disordered breathing. Otolaryngol Head Neck Surg 2014;151:687–691. [DOI] [PubMed] [Google Scholar]
- 28.Paruthi S, Buchanan P, Weng J, et al. Effect of adenotonsillectomy on parent-reported sleepiness in children with obstructive sleep apnea. Sleep 2016;39:2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep 2006;29:1115–1134. [DOI] [PubMed] [Google Scholar]
- 30.Song SA, Tolisano AM, Cable BB, Camacho M. Neurocognitive outcomes after pediatric adenotonsillectomy for obstructive sleep apnea: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol 2016;83:205–210. [DOI] [PubMed] [Google Scholar]
- 31.Taylor HG, Bowen SR, Beebe DW, et al. cognitive effects of adenotonsillectomy for obstructive sleep apnea. Pediatrics 2016;138:e20154458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan KC, Au CT, Hui LL, Ng SK, Wing YK, Li AM. How OSA evolves from childhood to young adulthood: natural history from a 10-year follow-up study. Chest 2019;156:120–130. [DOI] [PubMed] [Google Scholar]
- 33.Chervin RD, Ellenberg SS, Hou X, et al. Prognosis for spontaneous resolution of OSA in children. Chest 2015;148:1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spilsbury JC, Storfer-Isser A, Rosen CL, Redline S. Remission and incidence of obstructive sleep apnea from middle childhood to late adolescence. Sleep 2015;38:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]


