Abstract
Glandular secretory trichomes (GSTs) can secrete and store a variety of specific metabolites. By increasing GST density, valuable metabolites can be enhanced in terms of productivity. However, the comprehensive and detailed regulatory network of GST initiation still needs further investigation. By screening a complementary DNA library derived from young leaves of Artemisia annua, we identified a MADS-box transcription factor, AaSEPALLATA1 (AaSEP1), that positively regulates GST initiation. Overexpression of AaSEP1 in A. annua substantially increased GST density and artemisinin content. The HOMEODOMAIN PROTEIN 1 (AaHD1)-AaMYB16 regulatory network regulates GST initiation via the jasmonate (JA) signaling pathway. In this study, AaSEP1 enhanced the function of AaHD1 activation on downstream GST initiation gene GLANDULAR TRICHOME-SPECIFIC WRKY 2 (AaGSW2) through interaction with AaMYB16. Moreover, AaSEP1 interacted with the JA ZIM-domain 8 (AaJAZ8) and served as an important factor in JA-mediated GST initiation. We also found that AaSEP1 interacted with CONSTITUTIVE PHOTOMORPHOGENIC 1 (AaCOP1), a major repressor of light signaling. In this study, we identified a MADS-box transcription factor that is induced by JA and light signaling and that promotes the initiation of GST in A. annua.
A MADS-box transcription factor induced by jasmonate and light signaling positively regulates glandular secretory trichome initiation in Artemisia annua.
Introduction
Trichomes are specialized hair-like structures that extend from the surface of the leaves, stems, and inflorescences of nearly all land plants (Payne et al. 1978; Li et al. 2022), and have key roles in the defense against biotic and abiotic stresses (Szymanski et al. 2000; Chalvin et al. 2020). Trichomes are classified as nonglandular or glandular in accordance with their ability to secrete metabolites (Huchelmann et al. 2017). Unlike nonglandular trichomes, glandular trichomes possess a glandular head that can store and secrete large quantities of specialized metabolites such as alkaloids, polysaccharides, and terpenoids (Fridman et al. 2005; Schilmiller et al. 2008; Graham et al. 2010; Tissier 2012; Ma et al. 2016). Over the past years, considerable efforts have been made to determine the molecular mechanisms that underlie trichome initiation and development. Due to the advantages of the mutant library, the most comprehensive transcriptional regulatory network of nonglandular trichome initiation, regulated by the TRANSPARENT TESTA GLABRA1 (TTG1)-basic helix-loop-helix (bHLH)-MYB module, has been constructed in the model plant Arabidopsis (Arabidopsis thaliana) (Payne et al. 2000; Balkunde et al. 2010). The differentiation of trichomes from epidermal cells is regulated by this module in conjunction with over 30 transcription factors (Tian et al. 2017; Fambrini and Pugliesi 2019; Schuurink and Tissier 2020; Wang et al. 2021). According to recent research, the MADS-box gene AGAMOUS (AG), a key regulator of Arabidopsis flower development (Bowman et al. 1991), can inhibit the proliferation of trichomes in carpel valves (Ó’Maoiléidigh et al. 2013, 2018). The microarray data from early stage flowers of the ag mutant showed that AG was capable of downregulating the expression of trichome initiation activators such as GLABRA1 (GL1) and ZINC FINGER PROTEIN 8 (ZFP8), while upregulating the expression of trichome initiation repressors such as CAPRICE (CPC) and TRICHOMELESS1 (TCL1) (Larkin et al. 1994; Schellmann et al. 2002; Gan et al. 2007; Wang et al. 2007; Ó’Maoiléidigh et al. 2013). Further, the MADS-box gene FLOWERING LOCUS C (FLC) has been reported to inhibit abaxial trichome formation independently of its role in flowering (Willmann and Poethig 2011).
Although the formation model of nonglandular trichomes has been established clearly, knowledge about the developmental process of glandular trichomes is still limited (Schuurink and Tissier 2020). Accumulated evidence has shown that the initiation and patterning of glandular trichomes are regulated by distinct transcriptional regulatory networks, as compared with nonglandular trichomes (Kang et al. 2010; Yang et al. 2018; Xie et al. 2021). Recent studies using tomato (Solanum lycopersicum, Solanaceae), sweet wormwood (Artemisia annua, Asteraceae), and cucumber (Cucumis sativus, Cucurbitaceae) (Chalvin et al. 2020) as research objects have aided in the understanding of the molecular basis underlying glandular trichome initiation. Tomato has been proposed as a potential model for clarifying the molecular mechanisms involved in glandular trichome development (Tissier 2012). On tomato leaves, 8 types of trichomes have been identified, of which types I, IV, VI, and VII are glandular. (Fan et al. 2019). Woolly (Wo), which encodes a homeodomain-leucine zipper (HD-ZIP) IV transcription factor, is mainly involved in type-I trichome formation (Yang et al. 2011). Recent work reported that another HD-ZIP gene, SlHD8, acts as the downstream regulator of jasmonate (JA) signaling that promotes trichome elongation (Hua et al. 2021). Additionally, members of the R2R3-MYB subfamily of transcription factors, such as MIXTA-like 1 (SlMX1), are known to be key regulators of trichome formation (Ewas et al. 2016).
The plant A. annua, which is well known for its production of the anti-malarial drug artemisinin, provides another example for the study of glandular trichome initiation and development (Shen et al. 2016; Ma et al. 2018). Two types of trichomes are present on the aerial parts of A. annua: the peltate glandular secretory trichome (GST) and the T-shaped trichome (Xie et al. 2020). At present, many transcription factor families have been identified to play a role in the initiation of GST in A. annua, including R2R3-MYB (AaMIXTA1, AaMYB5, AaMYB16, and AaMYB17), AP2/ERF (AaWIN1), SPL (AaSPL9), HD-ZIP (AaHD1, AaHD8), and WRKY (AaGSW2) (Yan et al. 2017; Shi et al. 2018; Xie et al. 2020; Qin et al. 2021; He et al. 2022; Wang et al. 2023). Although many transcript factors have been extensively studied, the identification of genes controlling GST initiation is of high interest to researchers.
JA has been shown to play a crucial role in plant development as well as in the response of plants to biotic and abiotic stresses. A number of studies have shown that JA could promote the initiation of GSTs in A. annua and other plants (Li et al. 2004; Maes et al. 2011). In addition to plant hormones, environmental factors such as light signaling are crucial for plant life. Plants obtain as much information as possible from perceiving light quantity and quality throughout their entire life to regulate plant growth, architecture, and secondary metabolite biosynthesis (De Wit et al. 2016; Li et al. 2021). There is evidence that light quality plays a role in the process of GST initiation in A. annua (Lopes et al. 2020). However, the molecular mechanism of light regulating trichome initiation in A. annua remains unclear.
In this study, AaSEPALLATA1 (AaSEP1), a MADS-box transcription factor, which acts as a positive regulator of GST initiation in A. annua was functionally characterized. AaSEP1 interacts with AaMYB16 to activate the expression of AaGSW2, thereby promoting GST formation. Moreover, AaSEP1 is induced by JA and light signaling, interacting with jasmonate ZIM-domain 8 (AaJAZ8) and CONSTITUTIVE PHOTOMORPHOGENIC1 (AaCOP1), respectively. This work uncovers a molecular mechanism by which a MADS-box transcript factor promotes GST initiation in A. annua.
Results
AaSEP1 interacts with AaMYB16, a positive regulator of GST initiation in A. annua
In our previous study, AaMYB16 was known to promote GST initiation in A. annua by activating AaGSW2 expression (Xie et al. 2021). To identify putative regulators of GST initiation, we have used AaMYB16 as bait to screen the proteins interacting with it from the complementary DNA (cDNA) library of A. annua. Based on the yeast two-hybrid (Y2H) screening results, Aannua10729S694100, which encodes a MADS-box superfamily protein, was found to interact with AaMYB16. More recently, investigators have found preliminary evidence that MADS-box genes have an effect on carpel valve trichome proliferation in A. thaliana (Ó’Maoiléidigh et al. 2018), which suggests that MADS-box genes potentially regulate the formation of trichome in other plants. To further test the function of Aannua10729S694100, we compared the transcript level of all 76 MADS-box genes and 4 glandular trichome-specific key enzyme genes (amorpha-4, 11-diene synthase [ADS], cytochrome P450-dependent hydroxylase [CYP71AV1], double bond reductase 2 [DBR2], aldehyde dehydrogenase 1 [ALDH1]) in A. annua. Heatmap data showed that 4 MADS-box genes were clustered with key enzyme genes (Supplemental Fig. S1). Meanwhile, Aannua10729S694100 showed the highest expression level in trichome among these 4 MADS-box genes (Supplemental Fig. S2). Phylogenetic analyses showed that Aannua10729S694100 (AaSEP1) shares high sequence similarity with AT5G15800 (AtSEP1) and AT3G02310 (AtSEP2) in Arabidopsis (Supplemental Figs. S3 and S4). As a functionally redundant protein with AtSEP1 and AtSEP2, AtSEP3 may also be involved in the formation of trichomes on carpel valves via AG-dependent pathways, indicating that AaSEP1 may play a similar role in A. annua (Ó’Maoiléidigh et al. 2013). Subsequently, we used Y2H, co-localization, bimolecular fluorescence complementation assay (BiFC), and Co-immunoprecipitation (Co-IP) assays to confirm the interaction between AaSEP1 and AaMYB16 (Fig. 1). Taken together, AaSEP1 is a protein that interacts with AaMYB16 and is highly expressed in trichome. These data suggest that AaSEP1 could be associated with GST formation in A. annua.
Figure 1.
AaSEP1 interacts with AaMYB16. A) Y2H assay. pGADT7-AaSEP1 combined with pGBKT7-AaMYB16 conferred AH109 cell growth on SD/-Leu/-Trp/-His/-Ade plates. B) Co-localization of AaSEP1 and AaMYB16 in N. benthamiana epidermal cells. Leaves were infiltrated with A. tumefaciens strains harboring combinations of AaSEP1-CFP, and AaMYB16-YFP fusion protein constructs, CFP and AaMYB16-YFP, YFP and AaSEP1-CFP were co-transformed as negative controls. Scale bars, 10 μm. C) Co-IP assay showing AaMYB16-AaSEP1 interaction. The indicated YFP-tagged AaMYB16 or the control LUC (full length of luciferase) were co-expressed with Flag-tagged AaSEP1 in N. benthamiana leaves, respectively. Protein extracts before (Input) and after immunoprecipitation (IP), and anti-YFP antibody-conjugated beads were detected by western blot analysis with an anti-Flag antibody. D) BiFC assays showing the interactions of AaSEP1 with AaMYB16 in the epidermal cells of N. benthamiana leaves. AaSEP1 was fused to the N-terminal fragment of the YFP (AaSEP1-nYFP), AaMYB16 was fused to the C-terminal fragment of YFP (AaMYB16-cYFP). Bars, 50 μm.
AaSEP1 is a trichome highly expressed transcription factor in A. annua
To investigate the spatial pattern of AaSEP1, we detected its transcript level in different tissues of A. annua. RT-qPCR results showed that AaSEP1 was consistently expressed in all the tested organs/tissues, and with the highest transcript level in trichomes (Fig. 2A). The transcript level of AaSEP1 did not differ with the age of the leaves (Fig. 2B). To obtain more precise expression patterns of AaSEP1, a 2,338 bp promoter sequence of AaSEP1 was cloned. Transgenic A. annua line pCAMBIA 1391Z-pAaSEP1 was constructed. GUS staining in Fig. 2C showed that AaSEP1 was highly expressed in the GSTs of A. annua. YFP protein was fused to the C-terminus of AaSEP1 to explore its subcellular localization. As shown in Fig. 2D, YFP fluorescent was observed in the nucleus of Nicotiana benthamiana epidermal cells, which is consistent with AaSEP1's role as a transcription factor.
Figure 2.
Expression pattern and subcellular localization of AaSEP1. A, B) Relative expression levels of AaSEP1 in different tissues (A) and in different positions of leaves: GP (growing point), L0 (leaf 0), L1 (leaf 1), L2 (leaf 2), L3 (leaf 3), L4 (leaf 4), L5 (leaf 5), L6 (leaf 6) and (B) were measured by reverse transcription quantitative polymerase chain reaction. Actin (EU531837) was used as an internal reference. Data values are means ± Sd (n = 3). C) GUS expression in young leaves of plants transformed with 1391Z-GUS empty vector (control plant) (1) and 1391-proAaSEP1-GUS (2–3). GST, glandular secretory trichome. Bars, 20 μm. D) Subcellular localization of 35S:AaSEP1-YFP in N. benthamiana leaf epidermal cells. YFP, yellow fluorescent protein. DAPI, 4′,6-diamidino-2-phenylindole staining. Bars, 50 μm.
AaSEP1 positively regulates GST initiation in A. annua
To verify the function of AaSEP1 in GST formation, overexpression or downregulation of AaSEP1 in A. annua was performed. Three independent AaSEP1 overexpression lines (AaSEP1-OE-2, AaSEP1-OE-11, AaSEP1-OE-12) and AaSEP1 antisense lines (ANAaSEP1-5, ANAaSEP1-12, ANAaSEP1-17) were used for analysis (Fig. 3A). Remarkably, GST densities on fully expanded leaves of AaSEP1 overexpression lines were increased by up to 64.5%, while AaSEP1 antisense lines showed a significant reduction (26.5% to 35.3%) (Fig. 3, B and C). Furthermore, scanning electron microscopy (sem) observations of AaSEP1 transgenic plants demonstrated that GSTs were noticeably increased or decreased in density without phenotype changes (Fig. 3D). As artemisinin was synthesized and stored in GST, we further checked the artemisinin contents of AaSEP1 transgenic plants. According to high-performance liquid chromatography (HPLC) analysis, the artemisinin contents in overexpression lines were increased by up to 104.7%, whereas the artemisinin contents of AaSEP1 antisense lines decreased by up to 31.6% compared with that in the wild-type (Fig. 3E). These results indicated that AaSEP1 acts as a positive regulator of GST initiation in A. annua.
Figure 3.
AaSEP1 expression affects A. annua GST initiation. A) Expression levels of AaSEP1 in different AaSEP1 overexpression (OE) and antisense (AN) lines measured by RT-qPCR. WT (wild type) A. annua plants as CK. Actin (EU531837) was used as an internal reference. B) The GSTs on adaxial and abaxial sides of mature leaves derived from WT, AaSEP1-OE-2, ANAaSEP1-17 A. annua plants. The spots represent GSTs. The backgrounds represent Chl (bars, 500 μm). C) Densities of adaxial glandular trichomes on mature leaves collected from AaSEP1 overexpression and antisense lines. D)Sem analysis of the surface of transgenic A. annua leaves. The morphologies of trichomes on the leaves of WT (1–2), AaSEP1-OE-2 (3–4), ANAaSEP1–17 (5–6) A. annua plants. Scale bars, 100 μm. E) Artemisinin content in different AaSEP1 overexpression and antisense lines, measured by HPLC. All data are given as means ± Sd (n = 3) *P < 0.05; **P < 0.01; Student's t-test.
AaSEP1 promotes the activation of the AaHD1-AaMYB16 complex on AaGSW2
AaMYB16 enhances AaHD1 activation of AaGSW2, and recent studies have reported that AaMYB16 function in GST initiation is largely dependent on AaHD1 (Xie et al. 2021). AaSEP1 was the identified protein that interacted with AaMYB16 (Fig. 1). To further investigate whether AaSEP1 was involved in the AaHD1-AaMYB16 regulatory network to regulate the initiation of GST, we first tested the expression of AaGSW2 in AaSEP1 overexpression lines. The RT-qPCR results showed that the expression of AaGSW2 was significantly increased in AaSEP1 overexpression lines compared with that in the control (Fig. 4A). Then we performed dual-LUC assays to explore the activation of AaSEP1 on the AaGSW2 promoter. However, AaSEP1 did not significantly activate the AaGSW2 promoter in Nicotiana benthamiana (Fig. 4B). In addition, the AaHD1 protein did not interact with AaSEP1 (Fig. 4C). Based on these results, we hypothesized that the function of AaSEP1 on GST formation was associated with the function of AaMYB16.
Figure 4.
AaSEP1 further enhances AaGSW2 activation via the AaHD1–AaMYB16 complex. A) Expression levels of AaGSW2 in AaSEP1 overexpression lines and WT plants were measured by RT-qPCR. WT (wild type) A. annua plants as CK. Actin (EU531837) was used as an internal reference. B) Transient transcriptional activity assay showing the activation of the AaGSW2 promoter by AaSEP1. The AaGSW2 promoter was fused to the LUC reporter and promoter activity was determined using a transient dual-LUC assay in N. benthamiana. Relative LUC activity was normalized to the activity of the reference Renilla (REN) luciferase. C) Y2H assay. pGADT7-AaHD1 combined with pGBKT7-AaSEP1 conferred AH109 cell growth on SD/-Leu/-Trp/-His/-Ade plates. The combination of pGADT7-AaHD1 and pGBKT7-AaMYB16 was positive control. D) Transient transcriptional activity assay showing the activation of the AaGSW2 promoter by AaSEP1 with AaMYB16. The AaGSW2 promoter was fused to the LUC reporter and promoter activity was determined using a transient dual-LUC assay in N. benthamiana. Relative LUC activity was normalized to the activity of the reference REN luciferase. All data are given as means ± Sd (n = 3) *P < 0.05; **P < 0.01; Student's t-test.
To test this hypothesis, we carried out dual-LUC assays to evaluate the interaction between AaSEP1 and AaMYB16 on AaGSW2 promoter activity. As shown in Fig. 4D, AaMYB16 significantly enhanced the activation of the AaGSW2 promoter by AaHD1 (Xie et al. 2021), while the combination with AaSEP1 significantly reinforced this. Based on these findings, we conclude that AaSEP1 promotes the transcriptional activity of AaGSW2 by interacting with AaMYB16 and then strengthens the function of the AaHD1-AaMYB16 regulatory network, thereby promoting GST initiation.
AaSEP1 can be induced by JA and its downregulated lines are less sensitive to exogenous JA
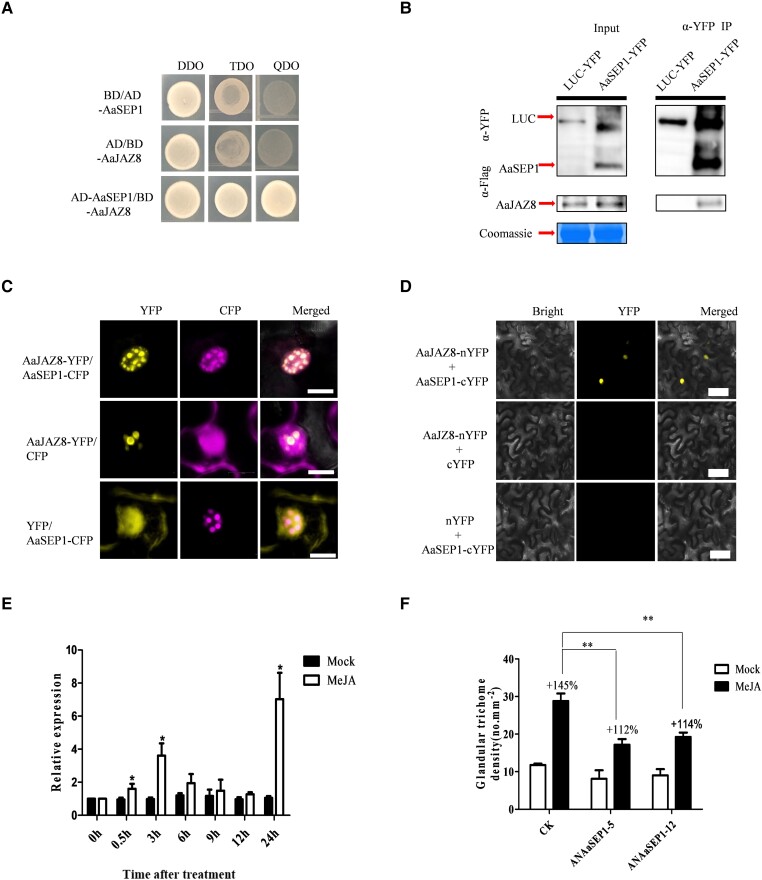
Previous studies have reported that JA can promote GST initiation in A. annua (Maes et al. 2011; Yan et al. 2017). We further explored whether GST formation, regulated by AaSEP1, was involved in the JA signaling pathway. We screened the cDNA library of A. annua young leaves for proteins interacting with AaSEP1. A number of proteins were identified, including the JA signaling negative regulator AaJAZ8, which has been proven to be involved in the initiation of GST as well as the content of artemisinin. Y2H, co-localization, BiFC, and Co-IP assays showed the protein interaction between AaSEP1 and AaJAZ8 (Fig. 5, A–D). Following JA treatment, the transcript levels of AaSEP1 in four-week-old A. annua plants significantly increased at 0.5, 3, and 24 h (Fig. 5E). To further test the hypothesis that AaSEP1 regulates trichome development by JA signaling, a six-week-long MeJA treatment was performed on AaSEP1 knockdown and wild-type lines. The GST density of MeJA-treated wild-type A. annua was found to be increased by 145%. Moreover, the GST densities of MeJA-treated AaSEP1 knockdown plants were only increased by 112% to 114% (Fig. 5F). Compared to the GST density of wild-type plants, AaSEP1 knockdown plants are less sensitive to MeJA treatment, indicating that AaSEP1 plays an essential role in JA-mediated GST initiation.
Figure 5.
AaSEP1 is regulated by MeJA. A) Y2H assay. pGADT7-AaSEP1 combined with pGBKT7-AaJAZ8 conferred AH109 cell growth on SD/-Leu/-Trp/-His/-Ade plates. B) Co-IP assay showing AaJAZ8–AaSEP1 interaction. The indicated YFP-tagged AaSEP1 or the control LUC (full length of luciferase) were co-expressed with Flag-tagged AaJAZ8 in N. benthamiana leaves, respectively. Protein extracts before (Input) and after immunoprecipitation (IP), and anti-YFP antibody-conjugated beads were detected by western blot analysis with an anti-Flag antibody. C) Co-localization of AaSEP1 and AaJAZ8 in N. benthamiana epidermal cells. Leaves were infiltrated with A. tumefaciens strains harboring combinations of AaSEP1-CFP, and AaJAZ8-YFP fusion protein constructs, and CFP and AaJAZ8-YFP, YFP and AaSEP1-CFP were co-transformed as negative controls. Scale bars, 10 μm. D) BiFC assays showing the interactions of AaSEP1 with AaJAZ8 in the epidermal cells of N. benthamiana leaves. AaJAZ8 was fused to the N-terminal fragment of YFP (AaJAZ8-nYFP), AaSEP1 was fused to the C-terminal fragment of YFP (AaSEP1-cYFP). Bars, 50 μm. E) The expression of AaSEP1 was induced by methyl-jasmonate (MeJA) treatments by RT-qPCR. 0.1% ethanol was used as a mock. Actin served as an internal reference. Data are given as means ± Sd (n = 3) (*P < 0.05; **P < 0.01; Student's t-test). F) ANAaSEP1 A. annua plants were less sensitive to MeJA. WT (wild type) A. annua plants as CK. The percentage increases in GST after JA treatment are presented above the bars. Data are given as means ± Sd (n = 3) (*P < 0.05; **P < 0.01; Student’s t-test).
AaSEP1 interacts with AaCOP1 and AaSEP1 is induced by light
Light quality has an influence on GST density in A. annua (Lopes et al. 2020), and AaSEP1 was a substantially upregulated gene in the transcriptome data of A. annua under light (Hao et al. 2017). To confirm that AaSEP1 was induced by light, the expression pattern of AaSEP1 in response to different light conditions was measured by RT-qPCR. As shown in Fig. 6A, the transcript level of AaSEP1 was drastically increased when shifting from darkness to light. On the contrary, the expression of AaSEP1 rapidly decreased after 0.5 h of darkness (Fig. 6B). In plants, the ubiquitin E3 ligase COP1 acts as a major repressor of light-dependent signaling (Ang et al. 1998). Y2H, BiFC, and co-localization studies all demonstrated an interaction between AaCOP1 and AaSEP1 (Fig. 6, C–E). In addition, the abundance of AaSEP1 protein in darkness was substantially decreased by the addition of AaCOP1 protein. However, the proteasome inhibitor MG132 (100 μM) was able to recover the abundance of AaSEP1 protein (Fig. 6F). In combination, these results demonstrate that AaSEP1 is a light-induced transcript factor and regulates GST formation in A. annua.
Figure 6.
AaSEP1 was regulated by light and interacted with AaCOP1. A, B) The seedlings of A. annua were transferred from darkness to light (A) and light to darkness (B). The expression level of AaSEP1 was measured by RT-qPCR. Actin (EU531837) was used as an internal reference. Data are given as means ± Sd (n = 3) (*P < 0.05; **P < 0.01; Student's t-test). C) Y2H assay. pGADT7-AaSEP1 combined with pGBKT7-AaCOP1 conferred AH109 cell growth on SD/-Leu/-Trp/-His/-Ade plates. D) Co-localization of AaSEP1 and AaCOP1 in N. benthamiana epidermal cells. Leaves were infiltrated with A. tumefaciens strains harboring combinations of AaSEP1-CFP, and AaCOP1-YFP fusion protein constructs, and CFP and AaCOP1- YFP, YFP, and AaSEP1-CFP were co-transformed as negative controls. Scale bars, 10 μm. E) BiFC assays showing the interactions of AaSEP1 with AaCOP1 in the epidermal cells of N. benthamiana leaves. AaSEP1 was fused to the N-terminal fragment of YFP (AaSEP1-nYFP), AaCOP1 was fused to the C-terminal fragment of YFP (AaCOP1-cYFP). Bars, 50 μm. F) Changes in the abundance of AaSEP1 in darkness with or without AaCOP1. AaSEP1-3×flag and AaCOP1-YFP were transiently co-transformed into N. benthamiana leaves. Proteins were extracted and visualized by immunoblotting with the anti-Flag and anti-GFP antibody. 100 μM proteasome inhibitor MG132 was used in this experiment. Actin was used as the loading control. The values in red represent the band intensities of AaSEP1 protein (ImageJ).
Discussion
Plant GSTs play a crucial role in the commoditization of natural pesticides, food additives, fragrance ingredients, and pharmaceuticals (Fridman et al. 2005; Schilmiller et al. 2008). Recently, the relationship between MADS-box TFs and nonglandular trichomes has been reported in Arabidopsis and cotton (Gossypium hirsutum) (Wang et al. 2019). A conditional knockdown of MADS-box gene AG led to the formation of trichomes on carpel valves in Arabidopsis (Ó’Maoiléidigh et al. 2013, 2018). Cotton GhMADS11 (Li et al. 2011) and GhMADS14 (Zhou et al. 2014) also play important roles in fiber formation. However, the roles played by MADS-box transcription factors in plant GST initiation are not well studied. In this study, we showed that the transcription of the MADS-box gene AaSEP1 was positively associated with GST initiation in A. annua. Overexpression of AaSEP1 in A. annua significantly increased the density of GST and artemisinin contents without changing the phenotype (Fig. 3).
We have previously identified an HD-ZIP-MYB complex AaHD1-AaMYB16/AaMYB5, in which AaMYB16 and AaMYB5 antagonistically affect the transcriptional activation of AaHD1 on AaGSW2 by interacting with AaHD1 (Xie et al. 2020). In this study, we showed that AaSEP1 interacted with GST-positive regulator AaMYB16 by Y2H, co-localization, BiFC, and Co-IP (Fig. 1). In addition, the expression of AaGSW2 was up-regulated 2 to 3 times in AaSEP1-overexpressing A. annua plants. Since AaMYB16 function in GST initiation is largely dependent on AaHD1, we hypothesize that the interaction between AaMYB16 and AaSEP1 has an impact on the AaHD1-AaMYB16 complex. Based on dual-LUC results, AaSEP1 significantly influenced the function of this complex on the downstream gene AaGSW2. Compared with the AaHD1-AaMYB16 complex regulator, the presence of AaSEP1 further enhanced the promoter activity of AaGSW2 (Fig. 4D).
JA and light are key factors that influence plant growth and development on multiple levels throughout the plant life cycle (Kazan and Manners 2011; Chen et al. 2018; Liu et al. 2023). Researchers have also revealed integrative hubs for light and JA signaling in some plants that modify their growth in more detail, including photomorphogenesis in seedlings, shade avoidance syndrome, and defense responses (Brendel et al. 2014; Liu et al. 2019; Liu and Wang 2020). The JA pathway component MYC2 integrates the JA and blue light signaling pathways to regulate photomorphogenic growth (Yadav et al. 2005). ELONGATED HYPOCOTYL 5 (HY5), a basic leucine-zipper (bZIP) transcription factor, has been identified as the major integrator of light and JA signaling pathways in plants (Lau and Deng 2010). Recent studies have shown that some TFs integrate JA and light signaling pathways to regulate artemisinin synthesis in A. annua (Zheng et al., 2023), such as AaHY5 (Hao et al. 2019), AaWRKY9 (Fu et al. 2021), and AaMYB108 (Liu et al. 2023). Here, we found that AaSEP1 transcription was significantly induced after JA treatment and that AaSEP1 interacted with AaJAZ8 (Fig. 5, A–E). In addition, after 5 wk of JA treatment, the GST density of AaSEP1 antisense plant lines increased less than that of wild-type A. annua (Fig. 5F). Based on these findings, AaSEP1 is involved in JA signaling that promotes the initiation of GST in A. annua, which is in agreement with previous research suggesting that JA plays a role in determining the density of glandular trichomes. Light signaling, however, was not well studied in A. annua in terms of its role in regulating GST initiation. In this study, we found that the transcript level of AaSEP1 was induced by light treatment (Fig. 6, A and B). AaSEP1 can also interact with AaCOP1 (Fig. 6, C–E). According to previous studies, plants have distinct sets of photoreceptors for different light spectrums, and COP1 is a critical light signaling component (Chen et al. 2021a). We then tested whether AaCOP1 can affect the stability of AaSEP1 protein in N. benthamiana leaves. As shown in Fig. 6F, the presence of AaCOP1 strongly enhanced the degradation of AaSEP1-Flag. While the addition of MG132, a commonly used inhibitor of proteasome-mediated degradation, reduced the effect of AaCOP1 on degrading AaSEP1.
Based on our current findings and previous studies, we propose a working model for GST initiation in A. annua (Fig. 7). Briefly, in the absence of JA or light, AaJAZ8 or AaCOP1 interacts with AaSEP1 and then attenuates the function of AaSEP1. In the presence of JA and light, AaJAZ8 and AaCOP1 are subsequently degraded by the 26S proteasome system, leading to the release of AaSEP1. Then AaSEP1 can interact with AaMYB16 to regulate the transcriptional activity of AaHD1 on the AaGSW2. Previous studies have reported that AaHD8-AaMIXTA1 complex regulates GST initiation by directly binding to the target gene AaHD1 (Yan et al. 2018). AaJAZ8 also interacts with AaHD1 and represses the transcriptional activity of AaHD1 (Yan et al. 2017). Taken together, we demonstrate a JA and light-responsive MADS-box gene, AaSEP1, which positively regulates the GST initiation in A. annua. In this study, we report that AaSEP1 interacts with AaJAZ8 and AaCOP1, regulating the GST initiation in A. annua. This study helps to enrich the understanding of the complex transcriptional regulation of GST initiation and development in other plant species.
Figure 7.
A regulatory model of A. annua GST initiation. In A. annua, AaJAZ8 represses AaHD1 transcriptional activity. AaHD8 positively regulates the expression of AaHD1. AaMYB16 strengthens the activation of the AaGSW2 promoter by AaHD1, while AaMYB5 substantially weakens the activation. AaSEP1 further promotes the function of the AaHD1-AaMYB16 complex on AaGSW2. Without JA and light, AaJAZ8 and AaCOP1 interact with AaSEP1, repressing the function of AaSEP1. In the presence of JA and light, AaJAZ8 and AaCOP1 are subsequently degraded by the 26S proteasome system, leading to the release of AaSEP1. Then AaSEP1 can interact with AaMYB16 to regulate the transcriptional activity of AaHD1 on AaGSW2, thereby promoting glandular trichome initiation.
Materials and methods
Plant materials and methyl jasmonate (MeJA) treatment
The A. annua cultivar used in this study was “Huhao 1” which has a high artemisinin content of 8–10 mg g−1. “Huhao 1” originated in Chongqing and was further subjected to several years of selection in Shanghai. N. benthamiana seeds were grown in pots and under a 16-h light photoperiod at 24 ± 2 °C. For MeJA treatment, two-week-old A. annua seedlings were sprayed with 100 μM MeJA. For the mock treatment, plants were sprayed with 0.1% (v/v) ethanol. Leaf samples were collected at 0, 0.5, 1.5, 3, 6, 12, and 24 h after each treatment.
RNA extraction and RT-qPCR
All expression of relative genes in A. annua was analyzed by reverse transcription quantitative PCR (RT-qPCR). Tissue samples were collected as previously described (Xie et al. 2020), and RNA was extracted using a plant RNA isolation reagent (Tiangen Biotech, Beijing, China). 0.5 μg aliquots of total RNA were used in the reverse transcriptase reaction to synthesize cDNA by using PrimeScriptRT Master Mix (Takara, Shiga, Japan). RT-qPCR was performed on a Roche lightercycler96 real-time PCR machine (Roche, Basel, Switzerland) and the 2−▴CT method was used for analysis (Livak and Schmittgen 2001). Three biological replicates were performed in all RT-qPCR experiments. All the primers used in RT-qPCR are listed in Supplemental Table S1.
A. annua transformation
The 765 bp full-length AaSEP1 was amplified by KOD DNA polymerase (Toyobo, Osaka, Japan) from a cDNA pool and then cloned into the pHB eventual binary vector that uses the 2 × 35S promoter (Wang et al. 2018). The 360 bp nonconservative AaSEP1 fragment was amplified and cloned into pHB vector to generate AaSEP1 antisense vector pHB-ANAaSEP1. The upstream 2,338 bp of AaSEP1 was amplified using PCR and inserted into the pCAMBIA 1391Z vector (Cambia). The constructs pHB-AaSEP1-YFP, pHB-ANAaSEP1, and pCAMBIA 1391Z-proAaSEP1 were introduced into the Agrobacterium tumefaciens strain EHA105 and then used to transform A. annua as described previously (Ma et al. 2018).
β-Glucuronidase (GUS) expression in 1391Z-proAaSEP1-GUS transgenic A. annua plants
Five-week-old 1391Z-proAaSEP1-GUS transgenic A. annua plants were stained to observe tissue distribution. Leaves and stems were stained in a GUS staining solution [1 mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid, 100 mM Na2HPO4, 50 mM KH2PO4, 10 mM Na2EDTA, 0.5 mM K3Fe(CN)6, 0.5 mM K4Fe(CN)6, and 0.1% (v/v) Triton X-100] and incubated at 37 °C for 12 h in the dark. Then samples were decolored with 70% ethanol at 65 °C.
Artemisinin content measurement
Leaves of five-month-old A. annua were gathered, dried at 50 °C in an oven, and then ground into powder. Subsequently, 0.1 g dried leaf powder was extracted twice with 2 mL methanol under ultrasound for 30 min (55 W, 30 °C). Then the samples were centrifuged (12,000 × g, 10 min) and the supernatants were filtered through nitrocellulose (0.22 μm). The artemisinin content was measured by HPLC as previously described (Chen et al. 2021b). Three repeats were measured in all samples.
Glandular trichome density counting
The mature leaves from three-month-old A. annua plants were collected. The images of leaves were obtained by fluorescence microscopy (Olympus, Tokyo, Japan) under 4× objective and excitation at 450 to 480 nm. ImageJ software (http://rsb.info.nih.gov/ij) was used to measure the area and count the GST number of leaves as previously described (Cheng et al. 2014). Three biological repeats were measured.
Sem
The mature leaves from three-month-old A. annua plants were collected and treated as previously described (Singh et al. 2016). Images were captured via extreme-resolution analytical field emission Sem (Jeol, Japan, and Thermo, USA). Three biological repeats were measured.
Y2H assays
Y2H assays were performed with the Matchmaker Gold Yeast Two-Hybrid System, according to the manufacturer's instructions (Takara, Japan). For Y2H analysis, the ORF of AaSEP1 and AaHD1 were cloned into the pGADT7 vector to create pGADT7-AaSEP1 and pGADT7-AaHD1. The ORFs of AaCOP1, AaMYB16, AaSEP1, and AaJAZ8 were cloned into the pGBKT7 vector to create pGBKT7-AaCOP1, pGBKT7-AaMYB16, pGBKT7-AaSEP1, and pGBKT7-AaJAZ8. The 2 constructs combinations, pGADT7-AaSEP1 and pGBKT7-AaCOP1, pGADT7-AaSEP1 and pGBKT7-AaMYB16, pGADT7-AaSEP1 and pGBKT7-AaJAZ8, pGADT7-AaHD1 and pGBKT7-AaSEP1, were co-transformed into yeast strain AH109. In addition, we also co-transformed pGADT7-AaSEP1/AaHD1 and pGBKT7, pGADT7 and pGBKT7- AaCOP1/AaJAZ8/AaMYB16/AaSEP1 as negative controls. The transformed yeast cells were cultivated on SD/-Leu/-Trp (DDO) medium at 30 °C for 3 d. The positive clones were tested on SD/-Leu/-His/-Trp (TDQ) and then on SD/-Leu/-His/-Trp/-Ade (QDO) medium. Yeast cells were photographed after 3 d of growth at 30 °C. Three independent repeats were performed to confirm these results. The primers are listed in Supplemental Table S1.
Subcellular localization and co-localization assays
For subcellular localization, the ORF of AaSEP1 was amplified by PCR using KOD plus DNA polymerase and then cloned into the plant expression vector pHB-YFP. The construct pHB-AaSEP1-YFP was transferred into A. tumefaciens strain GV3101 and then transiently transformed into five-week-old N. benthamiana leaves. YFP signals were observed by confocal laser microscopy (Leica TCS SP5-II) after 24-h dark and 24-h low light conditions. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). The empty vector pHB-YFP was used as a negative control.
For the two-protein co-localization assays, pHB-AaSEP1-CFP, pHB-AaJAZ8-YFP, pHB-AaMYB16-YFP, and pHB-AaCOP1-YFP were constructed and transferred into A. tumefaciens strain GV3101. The different combinations (pHB-AaSEP1-CFP + pHB-AaCOP1-YFP, pHB-AaSEP1-CFP + pHB-AaJAZ8-YFP, pHB-AaSEP1-CFP + pHB-AaMYB16-YFP, pHB-AaSEP1-CFP + pHB-YFP, pHB-CFP + pHB-AaCOP1-YFP, pHB-CFP + pHB-AaJAZ8-YFP, pHB-CFP + pHB-AaMYB16-YFP) were transiently co-transformed into five-week-old N. benthamiana leaves. After incubation at 22 °C for 24 h in the dark and 24 h in low light, YFP and CFP signals were observed as described previously (Ma et al. 2018). YFP fluorescence assay on a SP5 Meta confocal laser microscope (Leica, Germany) with a filter set of 514 nm for excitation and 530 nm for emission and CFP fluorescence assay with a filter set of 458 nm for excitation and 480 nm for emission were carried out. The DAPI stain is excited by UV light with a wavelength of 364 nm for excitation and 454 nm for emission.
BiFC assays
BiFC assays were performed as previously described (Xie et al. 2021). The full-length cDNAs of AaSEP1 and AaJAZ8 were cloned into pEarleyGate 201-YN (N terminus of YFP) to obtain AaSEP1-nYFP and AaJAZ8-nYFP. The full-length cDNAs of AaSEP1, AaMYB16, and AaCOP1 were cloned into pEarleyGate 202-YC (C terminus of YFP) to obtain AaMYB16-cYFP, AaSEP1-cYFP, and AaCOP1-cYFP.
These vectors were then transformed into A. tumefaciens strain GV3101. The different combinations were observed after incubation at 22 °C for 24 h in the dark and 24 h in low light. YFP signals were observed as previously described.
Co-IP assays
For the Co-IP assays, the ORFs of LUC, AaSEP1, and AaMYB16 were cloned into the pHB-YFP vector to generate pHB-AaSEP1-YFP and pHB-AaMYB16-YFP, and the ORFs of AaSEP1 and AaJAZ8 were cloned into the pHB-Flag vector to create pHB- AaSEP1-Flag and pHB-AaJAZ8-Flag. The 2 constructs (pHB-AaSEP1-YFP + pHB-AaJAZ8-Flag, pHB-AaMYB16-YFP + pHB-AaSEP1-Flag) were co-transformed into Agrobacterium strain GV3101 and then transiently transformed into four-week-old N. benthamiana leaves. Leaf samples were collected after 24-h dark and 24-h low light incubations and ground to powder in liquid nitrogen. Then the powder was re-suspended in extraction lysis buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 10% glycerol, 1 mM EDTA, and 0.5% Nonidet P-40, protease 100 μM Pefabloc (Sigma-Aldrich, USA), and 100 μM cocktail (Roche, Switzerland)). Samples were incubated for 10 min on ice and then centrifuged at 12,000 rpm for 10 min. The indicated GFP antibody (GeneScript, Nanjing, China) was incubated with 20 μL of Protein G Sepharose (GE Healthcare, Amersham, UK) for 2 h at 4 °C. And then the suspension samples were added to them for another 2 h at 4 °C. These immunoprecipitates were washed with lysis buffer 3 times. 45 μL supernatant of samples were dissolved with 15 μL 4×loading buffer and boiled at 100 °C for 10 min, then centrifuged at 12,000 rpm for 1 min before they were separated by 10% SDS-PAGE (polyacrylamide gel electrophoresis). Then proteins were transferred to polyvinylidene fluoride membranes and detected using anti-GFP (Abmart, China) or anti-Flag antibody (Sigma-Aldrich, USA) as described previously (Ma et al. 2018).
Dual-LUC assay
For the dual-LUC assays, the ORFs of AaSEP1, AaMYB16, and AaHD1 were cloned into pHB vector as effectors, and the promoter of AaGSW2 was cloned into pGREEN II 0800 vector as a reporter. The effectors were transformed into Agrobacterium strain GV3101, and pHB empty vector was used as a negative control. The reporter proAaGSW2-pGREEN II 0800 was transformed into Agrobacterium strain GV3101 with the helper plasmid pSoup 19, while the Renilla LUC gene driven by the constitutive 35S promoter was used as an internal reference. The effectors and reporters in different combinations were mixed in a 3:2 volume ratio to transform four-week-old N. benthamiana leaves.
Leaves were collected after 24-h dark and 24-h light incubation to measure LUC and REN activities using commercial dual-LUC reaction reagents (Promega). Three biological repeats were performed for each combination.
Light and dark treatments
Four-week-old A. annua seedlings were grown in the greenhouse under continuous white light. For the dark treatment, seedlings were transferred from white light to dark conditions, and leaves were collected after 0.5 h. The seedlings were then transferred to white light after 2 d in darkness, and leaves were collected after 0.5 h for light treatment. Leaf samples were collected from 6 randomly selected seedlings, and then frozen in liquid nitrogen for RNA extraction.
Protein degradation assay
The constructs pHB-AaSEP1-Flag and pHB-AaCOP1-YFP were transferred to A. tumefaciens strain GV3101. To evaluate the degradation of AaSEP1 by AaCOP1, five-week-old N. benthamiana leaves were transformed by injection of A. tumefaciens strain GV3101 cells harboring AaSEP1-Flag and AaCOP1-YFP. After incubation at 25 °C for 48 h of dark treatment, the leaves were pretreated with or without 100 µM MG132 (Calbiochem, USA) for 4 h and then were collected for western blot. AaSEP1 and AaCOP1 abundance were determined with the anti-Flag and anti-GFP monoclonal antibodies, with β-actin used as an internal control. The analysis was completed with 3 replicates.
Accession numbers
AaSEP1 (PWA45892.1), AaJAZ8 (KU744604), AaMYB16 (KX465136.1), AaHD1 (KU744599), AaGSW2 (KX465129.1), AaCOP1 (PWA73220.1).
Supplementary Material
Contributor Information
Tian-Tian Chen, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China.
Hang Liu, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China.
Yong-Peng Li, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China; Laboratory of Medicinal Plant Biotechnology, School of Pharmaceutical Sciences, Academy of Chinese Medical Science, Zhejiang Chinese Medical University, Hangzhou 310053, China.
Xing-Hao Yao, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China.
Wei Qin, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China.
Xin Yan, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China.
Xiu-Yun Wang, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China.
Bo-Wen Peng, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China.
Yao-Jie Zhang, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China.
Jin Shao, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China.
Xin-Yi Hu, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China.
Xue-Qing Fu, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China.
Ling Li, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China.
Yu-Liang Wang, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China.
Ke-Xuan Tang, Frontiers Science Center for Transformative Molecules, Joint International Research Laboratory of Metabolic & Developmental Sciences, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China; Integrative Science Center of Germplasm Creation in Western China (CHONGQING) Science City & Southwest University, School of Life Sciences, Southwest University, Chongqing 400715, China.
Author Contributions
T.C., Y.W., and K.T. designed the project; T.C., H.L., Y.L., W.Q., X.F., X.Y., X.W., and Y.Z. performed most of the experiments; B.P., L.L., X.-H.Y., J.S., X.H., and K.T. analyzed the data and discussed the article; T.C. wrote the manuscript. All the authors have read and approved the manuscript.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1 . Global expression profile of MADS transcription factors in A. annua.
Supplemental Figure S2 . Relative expression levels of candidate MADS transcription factors in trichome.
Supplemental Figure S3 . Phylogenetic analysis between different MADS transcription factors from various plant species.
Supplemental Figure S4 . The protein sequence alignment of AaSEP1, AtSEP1, and AtSEP2.
Supplemental Table S1 . Primers used in this study.
Funding
This work was supported by the National Key R&D Program of China (2018YFA0900600), the Bill & Melinda Gates Foundation (OPP1199872 and INV- 027291), SJTU Trans-med Awards Research (20190104), and the SJTU Global Strategic Partnership Fund (2020 SJTU-CORNELL).
Data availability
The authors confirm that the data underlying this article are available in the article and in its online supplementary material.
References
- Ang LH, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, Deng XW. Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell. 1998:1(2): 213–222. 10.1016/S1097-2765(00)80022-2 [DOI] [PubMed] [Google Scholar]
- Balkunde R, Pesch M, Hülskamp M. Trichome patterning in Arabidopsis thaliana. From genetic to molecular models. Curr Top Dev Biol. 2010:91: 299–321. 10.1016/S0070-2153(10)91010-7 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Drews GN, Meyerowitz EM. Expression of the Arabidopsis floral homeotic gene AGAMOUS is restricted to specific cell types late in flower development. Plant Cell. 1991:3(8): 749–758. 10.1105/tpc.3.8.749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel R, Svyatyna K, Jikumaru Y, Reichelt M, Mithöfer A, Takano M, Kamiya Y, Nick P, Riemann M. Effects of light and wounding on jasmonates in rice phyAphyC mutants. Plants. 2014:3(1): 143–159. 10.3390/plants3010143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalvin C, Drevensek S, Dron M, Bendahmane A, Boualem A. Genetic control of glandular trichome development. Trends Plant Sci. 2020:25(5): 477–487. 10.1016/j.tplants.2019.12.025 [DOI] [PubMed] [Google Scholar]
- Chen Q, Bai L, Wang W, Shi H, Ramón Botella J, Zhan Q, Liu K, Yang HQ, Song CP. COP1 promotes ABA-induced stomatal closure by modulating the abundance of ABI/HAB and AHG3 phosphatases. New Phytol. 2021a:229(4): 2035–2049. 10.1111/nph.17001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Fu TY, Yang SL, Hsieh HL. FIN219/JAR1 and cryptochrome1 antagonize each other to modulate photomorphogenesis under blue light in Arabidopsis. PLoS Genet. 2018:14(3): e1007248. 10.1371/journal.pgen.1007248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Li Y, Xie L, Hao X, Liu H, Qin W, Wang C, Yan X, Wu-Zhang K, Yao X, et al. AaWRKY17, a positive regulator of artemisinin biosynthesis, is involved in resistance to Pseudomonas syringae in Artemisia annua. Hortic Res. 2021b:8(1):217. 10.1038/s41438-021-00652-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Cao L, Wang S, Li YP, Zhou Y. Analyses of plant leaf cell size, density and number, as well as trichome number using cell counter plugin. Bio Protoc. 2014:4(13): e1165. 10.21769/BioProtoc.1165 [DOI] [Google Scholar]
- De Wit M, Galvão VC, Fankhauser C. Light-mediated hormonal regulation of plant growth and development. Annu Rev Plant Biol. 2016:67(1): 513–537. 10.1146/annurev-arplant-043015-112252 [DOI] [PubMed] [Google Scholar]
- Ewas M, Gao Y, Wang S, Liu X, Zhang H, Nishawy EME, Ali F, Shahzad R, Ziaf K, Subthain H, et al. Manipulation of SlMXl for enhanced carotenoids accumulation and drought resistance in tomato. Sci Bull. 2016:61(18): 1413–1418. 10.1007/s11434-016-1108-9 [DOI] [Google Scholar]
- Fambrini M, Pugliesi C. The dynamic genetic-hormonal regulatory network controlling the trichome development in leaves. Plants. 2019:8(8): 253. 10.3390/plants8080253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P, Leong BJ, Last RL. Tip of the trichome: evolution of acylsugar metabolic diversity in Solanaceae. Curr Opin Plant Biol. 2019:49: 8–16. 10.1016/j.pbi.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman E, Wang J, Iijima Y, Froehlich JE, Gang DR, Ohlrogge J, Pichersky E. Metabolic, genomic, and biochemical analyses of glandular trichomes from the wild tomato species Lycopersicon hirsutum identify a key enzyme in the biosynthesis of methylketones. Plant Cell. 2005:17(4): 1252–1267. 10.1105/tpc.104.029736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Peng B, Hassani D, Xie L, Liu H, Li Y, Chen T, Liu P, Tang Y, Li L, et al. AaWRKY9 contributes to light- and jasmonate-mediated to regulate the biosynthesis of artemisinin in Artemisia annua. New Phytol. 2021:231(5): 1858–1874. 10.1111/nph.17453 [DOI] [PubMed] [Google Scholar]
- Gan Y, Liu C, Yu H, Broun P. Integration of cytokinin and gibberellin signalling by Arabidopsis transcription factors GIS, ZFP8 and GIS2 in the regulations of epidermal cell fate. Development. 2007:134(11): 2073–2081. 10.1242/dev.005017 [DOI] [PubMed] [Google Scholar]
- Graham LA, Besser K, Blumer S, Branigan CA, Czechowski T, Elias L, Guterman I, Harvey D, Isaac PG, Khan AM, et al. The genetic map of Artemisia annua L identifies loci affecting yield of the antimalarial drug artemisinin. Science. 2010:327(5963): 328–331. 10.1126/science.1182612 [DOI] [PubMed] [Google Scholar]
- Hao X, Zhong Y, Fu X, Lv Z, Shen Q, Yan T, Shi P, Ma Y, Chen M, Lv X, et al. Transcriptome analysis of genes associated with the artemisinin biosynthesis by jasmonic acid treatment under the light in Artemisia annua. Front Plant Sci. 2017:8: 971. 10.3389/fpls.2017.00971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X, Zhong Y, Nützmann HW, Fu X, Yan T, Shen Q, Chen M, Ma Y, Zhao J, Osbourn A, et al. Light-induced artemisinin biosynthesis is regulated by the bZIP transcription factor AaHY5 in Artemisia annua. Plant Cell Physiol. 2019:60(8): 1747–1760. 10.1093/pcp/pcz084 [DOI] [PubMed] [Google Scholar]
- He Y, Fu X, Li L, Sun X, Tang K, Zhao J. AaSPL9 affects glandular trichomes initiation by positively regulating expression of AaHD1 in Artemisia annua L. Plant Sci. 2022:317: 111172. 10.1016/j.plantsci.2021.111172 [DOI] [PubMed] [Google Scholar]
- Hua B, Chang J, Xu Z, Han X, Xu M, Yang M, Yang C, Ye Z, Wu S. HOMEODOMAIN PROTEIN8 mediates jasmonate-triggered trichome elongation in tomato. New Phytol. 2021:230(3): 1063–1077. 10.1111/nph.17216 [DOI] [PubMed] [Google Scholar]
- Huchelmann A, Boutry M, Hachez C. Plant glandular trichomes: natural cell factories of high biotechnological interest. Plant Physiol. 2017:175(1): 6–22. 10.1104/pp.17.00727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Shi F, Jones AD, Marks MD, Howe GA. Distortion of trichome morphology by the hairless mutation of tomato affects leaf surface chemistry. J Exp Bot. 2010:61(4): 1053–1064. 10.1093/jxb/erp370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K, Manners JM. The interplay between light and jasmonate signalling during defence and development. J Exp Bot. 2011:62(12): 4087–4100. 10.1093/jxb/err142 [DOI] [PubMed] [Google Scholar]
- Larkin JC, Oppenheimer DG, Lloyd AM, Paparozzi ET, Marks MD. Roles of the GLABROUS1 and TRANSPARENT TESTA GLABRA genes in Arabidopsis trichome development. Plant Cell. 1994:6(8): 1065–1076. 10.2307/3869885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Deng XW. Plant hormone signaling lightens up: integrators of light and hormones. Curr Opin Plant Biol. 2010:13(5): 571–577. 10.1016/j.pbi.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Li P, Fu J, Xu Y, Shen Y, Zhang Y, Ye Z, Tong W, Zeng X, Yang J, Tang D, et al. CsMYB1 integrates the regulation of trichome development and catechins biosynthesis in tea plant domestication. New Phytol. 2022:234(3): 902–917. 10.1111/nph.18026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ning H, Zhang Z, Wu Y, Jiang J, Su S, Tian F, Li X. A cotton gene encoding novel MADS-box protein is preferentially expressed in fibers and functions in cell elongation. Acta Biochim Biophys Sin (Shanghai). 2011:43(8): 607–617. 10.1093/abbs/gmr055 [DOI] [PubMed] [Google Scholar]
- Li Y, Qin W, Fu X, Zhang Y, Hassani D, Kayani SI, Xie L, Liu H, Chen T, Yan X, et al. Transcriptomic analysis reveals the parallel transcriptional regulation of UV-B-induced artemisinin and favonoid accumulation in Artemisia annua L. Plant Physiol Biochem. 2021:163: 189–200. 10.1016/j.plaphy.2021.03.052 [DOI] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, Pichersky E, Howe GA. The tomato homolog of coronatine-insensitive1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell. 2004:16(1): 126–143. 10.1105/tpc.017954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Li L, Fu X, Li Y, Chen T, Qin W, Yan X, Wu Z, Xie L, Kayani S, et al. AaMYB108 is the core factor integrating light and jasmonic acid signaling to regulate artemisinin biosynthesis in Artemisia annua. New Phytol. 2023:237(6): 2224–2237. 10.1111/nph.18702 [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang H. JA Modulates phytochrome a signaling via repressing FHY3 activity by JAZ proteins. Plant Signal Behav. 2020:15(3): 1726636. 10.1080/15592324.2020.1726636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wei H, Ma M, Li Q, Kong D, Sun J, Ma X, Wang B, Chen C, Xie Y, et al. Arabidopsis FHY3 and FAR1 regulate the balance between growth and defense responses under shade conditions. Plant Cell. 2019:31(9): 2089–2106. 10.1105/tpc.18.00991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−▴▴CT method. Methods. 2001:25(4): 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lopes EM, Guimarães-Dias F, Gama T do SS, Macedo AL, Valverde AL, de Moraes MC, de Aguiar-Dias ACA, Bizzo HR, Alves-Ferreira M, Tavares ES, et al. Artemisia annua L. and photoresponse: from artemisinin accumulation, volatile profile and anatomical modifications to gene expression. Plant Cell Rep. 2020:39(1): 101–117. 10.1007/s00299-019-02476-0 [DOI] [PubMed] [Google Scholar]
- Ma D, Hu Y, Yang C, Liu B, Fang L, Wan Q, Liang W, Mei G, Wang L, Wang H, et al. Genetic basis for glandular trichome formation in cotton. Nat Commun. 2016:7(1): 10456. 10.1038/ncomms10456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YN, Xu DB, Li L, Zhang F, Fu XQ, Shen Q, Lyu XY, Wu ZK, Pan QF, Shi P, et al. Jasmonate promotes artemisinin biosynthesis by activating the TCP14-ORA complex in Artemisia annua. Sci Adv. 2018:4(11): eaas9357. 10.1126/sciadv.aas9357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes L, Van Nieuwerburgh FCW, Zhang Y, Reed DW, Pollier J, Vande Casteele SRF, Inzé D, Covello PS, Deforce DLD, Goossens A. Dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytol. 2011:189(1): 176–189. 10.1111/j.1469-8137.2010.03466.x [DOI] [PubMed] [Google Scholar]
- Ó’Maoiléidigh DS, Stewart D, Zheng B, Coupland G, Wellmer F. Floral homeotic proteins modulate the genetic program for leaf development to suppress trichome formation in flowers. Development. 2018:145(3): dev157784. 10.1242/dev.157784 [DOI] [PubMed] [Google Scholar]
- Ó’Maoiléidigh DS, Wuest SE, Rae L, Raganelli A, Ryan PT, Kwaśniewska K, Das P, Lohan AJ, Loftus B, Graciet E, et al. Control of reproductive floral organ identity specification in Arabidopsis by the C function regulator AGAMOUS. Plant Cell. 2013:25(7): 2482–2503. 10.1105/tpc.113.113209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne WW, Galsley JD, Bowles ML, Brunken JN, Engle LM, Guhardja E, Kurmarohita B, Parker AD, Peterson KM, Robbins R, et al. A glossary of plant hair terminology. Brittonia. 1978:30(2): 239–255. 10.2307/2806659 [DOI] [Google Scholar]
- Payne CT, Zhang F, Lloyd AM. GL3 Encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics. 2000:156(3): 1349–1362. 10.1093/genetics/156.3.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Xie L, Li Y, Liu H, Fu X, Chen T, Hassani D, Li L, Sun X, Tang K. An R2R3-MYB transcription factor positively regulates the glandular secretory trichome initiation in Artemisia annua L. Front Plant Sci. 2021:12: 657156. 10.3389/fpls.2021.657156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, Beerman A, Thumfahrt J, Jürgens G, Hülskamp M. TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J. 2002:21(19): 5036–5046. 10.1093/emboj/cdf524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilmiller AL, Last RL, Pichersky E. Harnessing plant trichome biochemistry for the production of useful compounds. Plant J. 2008:54(4): 702–711. 10.1111/j.1365-313X.2008.03432.x [DOI] [PubMed] [Google Scholar]
- Schuurink R, Tissier A. Glandular trichomes: micro-organs with model status? New Phytol. 2020:225(6): 2251–2266. 10.1111/nph.16283 [DOI] [PubMed] [Google Scholar]
- Shen Q, Lu X, Yan T, Fu X, Lv Z, Zhang F, Pan Q, Wang G, Sun X, Tang K. The jasmonate-responsive AaMYC2 transcription factor positively regulates artemisinin biosynthesis in Artemisia annua. New Phytol. 2016:210(4): 1269–1281. 10.1111/nph.13874 [DOI] [PubMed] [Google Scholar]
- Shi P, Fu X, Shen Q, Liu M, Pan Q, Tang Y, Jiang W, Lv Z, Yan T, Ma Y, et al. The roles of AaMIXTA1 in regulating the initiation of glandular trichomes and cuticle biosynthesis in Artemisia annua. New Phytol. 2018:217(1): 261–276. 10.1111/nph.14789 [DOI] [PubMed] [Google Scholar]
- Singh ND, Kumar S, Daniell H. Expression of β-glucosidase increases trichome density and artemisinin content in transgenic Artemisia annua plants. Plant Biotechnol J. 2016:14(3): 1034–1045. 10.1111/pbi.12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski DB, Lloyd AM, Marks MD. Progress in the molecular genetic analysis of trichome initiation and morphogenesis in Arabidopsis. Trends Plant Sci. 2000:5(5): 214–219. 10.1016/S1360-1385(00)01597-1 [DOI] [PubMed] [Google Scholar]
- Tian H, Wang X, Guo H, Cheng Y, Hou C, Chen JG, Wang S. NTL8 regulates trichome formation in Arabidopsis by directly activating R3 MYB genes TRY and TCL1. Plant Physiol. 2017:174(4): 2363–2375. 10.1104/pp.17.00510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier A. Glandular trichomes: what comes after expressed sequence tags? Plant J. 2012:70(1): 51–68. 10.1111/j.1365-313X.2012.04913.x [DOI] [PubMed] [Google Scholar]
- Wang C, Chen T, Li Y, Liu H, Qin W, Wu Z, Peng B, Wang X, Yan X, Fu X, et al. AaWIN1, an AP2/ERF protein, positively regulates glandular secretory trichome initiation in Artemisia annua. Plant Sci. 2023:329: 111602. 10.1016/j.plantsci.2023.111602 [DOI] [PubMed] [Google Scholar]
- Wang S, Kwak SH, Zeng Q, Ellis BE, Chen XY, Schiefelbein J, Chen JG. TRICHOMELESS1 regulates trichome patterning by suppressing GLABRA1 in Arabidopsis. Development. 2007:134(21): 3873–3882. 10.1242/dev.009597 [DOI] [PubMed] [Google Scholar]
- Wang S, Li L, Xu P, Lian H, Wang W, Xu F, Mao Z, Zhang T, Yang H. CRY1 interacts directly with HBI1 to regulate its transcriptional activity and photomorphogenesis in Arabidopsis. J Exp Bot. 2018:69(16): 3867–3881. 10.1093/jxb/ery209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Shen C, Meng P, Tan G, Lv L. Analysis and review of trichomes in plants. BMC Plant Biol. 2021:21(1): 70. 10.1186/s12870-021-02840-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yang Z, Li F. Updates on molecular mechanisms in the development of branched trichome in Arabidopsis and nonbranched in cotton. Plant Biotechnol J. 2019:17(9): 1706–1722. 10.1111/pbi.13167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann MR, Poethig RS. The effect of the floral repressor FLC on the timing and progression of vegetative phase change in Arabidopsis. Development. 2011:138(4): 677–685. 10.1242/dev.057448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Yan T, Li L, Chen M, Hassani D, Li Y, Qin W, Liu H, Chen T, Fu X, et al. An HD-ZIP-MYB complex regulates glandular secretory trichome initiation in Artemisia annua. New Phytol. 2021:231(5): 2050–2064. 10.1111/nph.17514 [DOI] [PubMed] [Google Scholar]
- Xie L, Yan T, Li L, Chen M, Ma Y, Hao X, Fu X, Shen Q, Huang Y, Qin W, et al. The WRKY transcription factor AaGSW2 promotes glandular trichome initiation in Artemisia annua. J Exp Bot. 2020:72(5): 1691–1701. 10.1093/jxb/eraa523 [DOI] [PubMed] [Google Scholar]
- Yadav V, Mallappa C, Gangappa SN, Bhatia S, Chattopadhyay S. A basic helix-loop-helix transcription factor in Arabidopsis, MYC2, acts as a repressor of blue light-mediated photomorphogenic growth. Plant Cell. 2005:17(7): 1953–1966. 10.1105/tpc.105.032060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Chen M, Shen Q, Li L, Fu X, Pan Q, Tang Y, Shi P, Lv Z, Jiang W, et al. HOMEODOMAIN PROTEIN 1 is required for jasmonate-mediated glandular trichome initiation in Artemisia annua. New Phytol. 2017:213(3): 1145–1155. 10.1111/nph.14205 [DOI] [PubMed] [Google Scholar]
- Yan T, Li L, Xie L, Chen M, Shen Q, Pan Q, Fu X, Shi P, Tang Y, Huang H, et al. A novel HD-ZIP IV/MIXTA complex promotes glandular trichome initiation and cuticle development in Artemisia annua. New Phytol. 2018:218(2): 567–578. 10.1111/nph.15005 [DOI] [PubMed] [Google Scholar]
- Yang S, Cai Y, Liu X, Dong M, Zhang Y, Chen S, Zhang W, Li Y, Tang M, Zhai X, et al. A CsMYB6-CsTRY module regulates fruit trichome initiation in cucumber. J Exp Bot. 2018:69(8): 1887–1902. 10.1093/jxb/ery047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Li H, Zhang J, Luo Z, Gong P, Zhang C, Li J, Wang T, Zhang Y, Lu Y, et al. A regulatory gene induces trichome formation and embryo lethality in tomato. Proc Natl Acad Sci U S A. 2011:108(29): 11836–11841. 10.1073/pnas.1100532108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Fu X, Shao J, Tang Y, Yu M, Li L, Huang L, Tang K. Transcriptional regulatory network of high-value active ingredients in medicinal plants. Trends Plant Sci. 2023:69: 387. 10.1016/j.tplants.2022.12.007 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Li BY, Li M, Li XJ, Zhang ZT, Li Y, Li XB. A MADS-box gene is specifically expressed in fibers of cotton (Gossypium hirsutum) and influences plant growth of transgenic Arabidopsis in a GA-dependent manner. Plant Physiol Biochem. 2014:75: 70–79. 10.1016/j.plaphy.2013.12.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data underlying this article are available in the article and in its online supplementary material.