Abstract
In many plants, efflux of organic anions from roots has been proposed as one of the major Al resistance mechanisms. However it remains unknown how plants regulate efflux of organic anions in response to Al. In this study, the regulatory mechanisms of Al-responsive malate efflux in wheat (Triticum aestivum) were characterized focusing on the role of protein phosphorylation. Al-resistant wheat (cv Atlas) initiated malate efflux at 5 min after addition of Al, and this response was sensitive to temperature. K-252a, a broad range inhibitor of protein kinases, effectively blocked the Al-induced malate efflux accompanied with an increased accumulation of Al and intensified Al-induced root growth inhibition. A transient activation of a 48-kD protein kinase and an irreversible repression of a 42-kD protein kinase were observed preceding the initiation of malate efflux, and these changes were canceled by K-252a. Malate efflux was accompanied with a rapid decrease in the contents of organic anions in the root apex, such as citrate, succinate, and malate but with no change in the contents of inorganic anions such as chloride, nitrate, and phosphate. These results suggest that protein phosphorylation is involved in the Al-responsive malate efflux in the wheat root apex and that the organic anion-specific channel might be a terminal target that responds to Al signaling mediated by phosphorylation.
Al constitutes the most abundant metal in the earth's crust. At a low soil pH, solubilized Al ions (mainly in the phytotoxic form of Al3+) severely inhibit root elongation. Thus, Al toxicity is a serious problem decreasing plant growth on acid soils around the world. To improve biological production on acid soils with a low-input-manner, studies on the inherent functions of Al-resistance in plants are required for application in breeding and genetic engineering (Matsumoto, 2000).
One possible mechanism of Al-resistance is proposed as efflux of organic anions from root apexes (Ma, 2000). Organic anions, such as citrate, can bind Al ions and reduce phytotoxicity (Hue et al., 1986). Although the types of organic anions differ with the plant species, it has been demonstrated that Al-resistant plant species/cultivars can release larger amounts of organic anions than Al-sensitive ones. The major organic anion released in response to Al is malate in wheat (Triticum aestivum) (Delhaize et al., 1993; Ryan et al., 1995), citrate in leguminous crops (Miyasaka et al., 1991; Ma et al., 1997a; Yang et al., 2000), and oxalate in buckwheat (Ma et al., 1997b). A recent molecular approach established that citrate efflux is enhanced by overproduction of citrate in transgenic tobacco or papaya plants (De la Fuente et al., 1997). However, in genetically Al-resistant plants, organic anion efflux is highly specific to Al. Making a resistant plant, which can release organic anions only in the presence of Al, would be a more preferable strategy in preventing the excess carbon loss.
From the experiments with channel inhibitors or the patch-clamping technique, anion channels on the plasma membrane are suggested to be involved in the Al-responsive efflux of organic anions (Ryan et al., 1995; Papernik and Kochian, 1997; Ryan et al., 1997; Zheng et al., 1998; Piñeros and Kochian, 2001). Nevertheless, the anion channel responsible for permeation of organic anions is still not well characterized, because anion channels described so far pass inorganic anions in preference to organic ones (Hedrich and Marten, 1993; Schmidt and Schroeder, 1994; Frachisse et al., 1999). Moreover, since there are marked differences in the lag time required for the induction of efflux of organic anion between each plant species (Ma, 2000), the regulatory mechanism of organic anion efflux in response to Al stress is still lacking.
Protein phosphorylation plays an important role in the regulation of various biological activities in plants (Dixon et al., 1994; Hardie, 1999). It has also been demonstrated that protein phosphorylation provides a signal transduction pathway for mediating extracellular stimuli into cells (Hirt, 1997). The mitogen-activated protein (MAP) kinase cascade is one of the major pathways for transmitting signals such as wounding (Usami et al., 1995; Mizoguchi et al., 1996), pathogen elicitors (Suzuki and Shinshi, 1995), drought stress (Mizoguchi et al., 1996), and hormone signaling (Seo et al., 1999). Recent studies have shown that protein phosphorylation may play a significant role in the regulation of ion channels in guard cells (Pei et al., 1996; Pei et al., 1997; Li et al., 1998). Activation of ion channels induces simultaneous efflux of K+ and anions, resulting in the stomatal closure due to loss of turgor in guard cells. However, the role of protein phosphorylation on Al-induced efflux of organic anions in root cells is still unclear.
In this study, Al-induced malate efflux in wheat was further characterized to clarify the role of protein phosphorylation in Al-resistance mechanisms. Using various inhibitors of protein phosphorylation/dephosphorylation, we demonstrate that the initiation of Al-responsive malate efflux is associated with protein phosphorylation, possibly related to an organic anion-specific channel or its upstream signaling by a K-252a-sensitive protein kinase. We also provide evidence that Al activates a 48-kD MAP-like kinase and inactivates a 42-kD kinase before the induction of malate efflux. A possible role of protein phosphorylation in Al-responsive malate efflux is discussed.
RESULTS
Rapid Malate Efflux in Response to Al
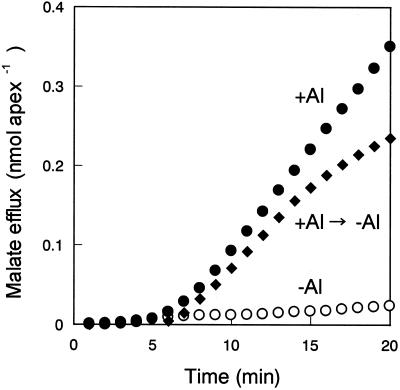
Al rapidly induces malate efflux from root apexes in Al-resistant wheat cultivars. Significant induction is detectable within 15 min (Ryan et al., 1995). To identify the regulatory system responsible for mediating Al-dependent induction of malate efflux, we further examined the time required for the induction. The solution containing 200 μm CaCl2 with or without 200 μm Al was passed through a column containing excised root apexes of cv Atlas at a flow rate of 0.5 mL min−1, and the malate concentration in the eluent was monitored.
The malate efflux started to increase 5 min after the addition of Al, and the efflux rate reached a maximum level at 10 min after Al treatment (Fig. 1). To check the significance of the lag time, we exposed root apexes to 200 μm Al for 1 min, and then rinsed three times with CaCl2 solution for 4 min. This treatment with a pulse of Al was sufficient to induce malate efflux (Fig. 1). This result suggested that a 5-min induction period after exposure to Al was prerequisite for malate efflux.
Figure 1.
Time course of malate efflux from root apexes of Al-resistant wheat (cv Atlas) after exposure to 200 μm AlCl3. Excised root apexes (2 mm in length from root apices) were exposed to 200 μm CaCl2 (○; Ca solution) or the Ca solution containing 200 μm Al (pH 4.2) (●; Ca+Al solution) passing through at a flow rate of 0.5 mL min−1. Root apexes were exposed to the Ca+Al solution for 1 min (♦) followed by three rinses with the Ca solution, and subsequent exposure to the Ca solution at a flow rate of 0.5 mL min−1 from 5 min after addition of the Ca+Al solution. Eluents passed through root apexes were collected every 1 min and assayed for determination of malate concentration. Treatments replicated three times gave similar results. Data from a representative experiment are shown.
The effect of low temperature on the malate efflux was examined. From Al-resistant (cv Atlas) seedlings grown either at 25°C or 4°C for 24 h, root apexes were excised and exposed to 200 μm Al solution at 25°C or 4°C. Treatment with Al at 4°C prevented the induction of malate efflux in root apexes excised from the plants grown at either 4°C or 25°C (Table I). The drastic change in temperature apparently did not damage the ability of the root to respond to Al since roots pretreated with Al at 4°C were able to release malate in response to Al treatment at 25°C (Table I). This finding indicated that the initiation of Al-induced malate release was dependent on temperature.
Table I.
Effect of low temperature on Al-induced malate efflux from root apexes of Al-resistant wheat (cv Atlas)
| Treatment
|
Malate Efflux
|
||
|---|---|---|---|
| Pretreatment | Treatment | 25°C Grown | 4°C Grown |
| nmol apex−1 30 min−1 | |||
| − | −Al at 25°C | 0.05 ± 0.02 | 0.05 ± 0.01 |
| − | +Al at 25°C | 0.90 ± 0.03 | 0.91 ± 0.02 |
| − | +Al at 4°C | 0.04 ± 0.01 | 0.15 ± 0.01 |
| +Al at 4°C | +Al at 25°C | 1.04 ± 0.02 | 0.90 ± 0.02 |
From seedlings grown at either 25°C or 4°C for 24 h before the treatment, root apexes were excised and exposed to 200 μm CaCl2 solution (Ca solution) or the Ca solution containing 200 μm Al (pH 4.2; Ca+Al solution) at 25°C or 4°C. Periods of pretreatment and treatment were 30 min each. Values are means ± se (n = 3).
A Protein Kinase Inhibitor Prevents Al-Induced Malate Efflux
Previous findings that a substantial time lag exists between the perception of Al and the initiation of malate efflux prompted us to investigate possible pathways for transmitting Al-signaling. In guard cells, gating of anion channels may be regulated by the modification of protein kinases and/or phosphatases (Pei et al., 1997). To examine the effect of inhibitors of protein kinases or protein phosphatases on the Al-responsive malate efflux, we treated excised root apexes of cv Atlas with various types of inhibitors for 30 min and then with 200 μm Al.
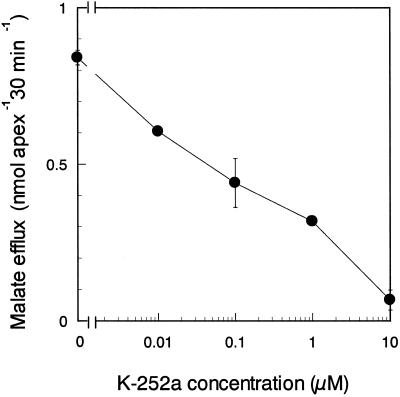
Among the protein kinase inhibitors tested here, K-252a, a broad range inhibitor of protein kinases, strongly prevented the Al-responsive efflux of malate (Table II). K-252a inhibited malate efflux in a concentration-dependent manner (Fig. 2). Compared with the control, the decrease of malate efflux was 92% in the root apexes pretreated with 10 μm K-252a and 62% in those pretreated with 1 μm K-252a. This suggested that a K-252a-sensitive protein kinase(s) in the root apex might be involved in the induction of malate efflux by Al. The amount of malate released from root apexes pretreated with 10 μm staurosporine, another broad range inhibitor of protein kinases, was 37% less than control. KN-62, a specific inhibitor of animal myosin light chain kinase, and calphostin C and chelerythrine, selective inhibitors of animal protein kinase C, had no effect on Al-dependent malate efflux (Table II).
Table II.
Effect of inhibitors of protein kinases or protein phosphatases on Al-induced malate efflux from root apexes of Al-resistant wheat (cv Atlas)
| Treatment | Malate Efflux |
|---|---|
| nmol apex−1 30 min−1 | |
| Control | 0.84 ± 0.02 |
| Protein kinase inhibitors: | |
| 50 μm KN-62 | 0.84 ± 0.04 |
| 10 μm Calphostin C | 0.91 ± 0.00 |
| 10 μm Chelerythrine | 0.90 ± 0.02 |
| 10 μm Staurosporine | 0.53 ± 0.04 |
| 10 μm K-252a | 0.07 ± 0.03 |
| Protein phosphatase inhibitors: | |
| 10 μm Cyclosporin A | 0.89 ± 0.03 |
| 10 μm Microsystin-LR | 0.93 ± 0.02 |
| 1 μm Calyculin A | 0.83 ± 0.03 |
| 1 μm Okadaic acid | 0.64 ± 0.05 |
Excised root apexes were pretreated with 200 μm CaCl2 solution (Ca solution) containing or not containing either type of inhibitor and then exposed to the Ca solution containing 200 μm Al (pH 4.2) (Ca+Al solution). The highest concentration of each inhibitor tested in the experiment is indicated. Dimethyl sulfoxide concentration in the Ca solution was less than 1%, and dimethyl sulfoxide at this concentration did not inhibit Al-induced malate efflux. After the pretreatment, root apexes were rinsed three times with the Ca solution to remove excess inhibitor. Periods of pretreatment and treatment were 30 min each. Values are means ± se (n = 3).
Figure 2.
Dose-response curve for Al-induced malate efflux in response to a protein kinase inhibitor, K-252a. Experimental procedures were the same as shown in Table II. Values are means ± se (n = 3).
Pretreatment of root apexes with 1 μm okadaic acid, an inhibitor of protein phospahatase type 1 and 2A, resulted in a slight decrease in Al-dependent malate efflux. Other protein phosphatase inhibitors were tested in this study, microcystin LR and calyculin A, inhibitors of protein phospahatase type 1 and 2A, and cyclosporin A, an inhibitor of protein phosphatase type 2B, did not or only slightly prevented malate efflux (Table II). These results indicated that protein phosphatases might have a minor role for the induction of malate efflux.
Malate Efflux Confers Al Resistance in Wheat
Complexes between Al and organic anions such as citrate and oxalate can ameliorate the toxicity of Al ions. Compared with citrate and oxalate, malate is suggested to bind to Al less effectively (Hue et al., 1986). This raises a question as to whether malate efflux is insufficient to explain the Al-resistance mechanism in wheat cultivars (Parker and Pedler, 1998). To examine the possible role of malate efflux in the Al-resistance mechanism, we asked if inhibition of malate efflux by K-252a would affect Al accumulation in root apexes and on Al-induced inhibition of root growth in cv Atlas.
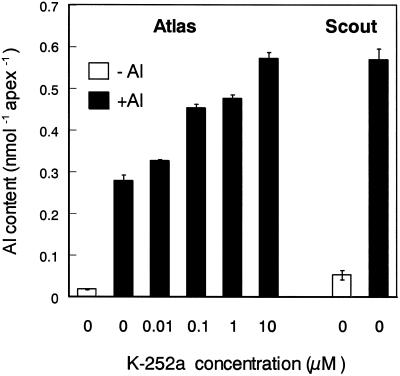
Pretreatment with K-252a increased the Al content in the root apex of cv Atlas, and the effect of K-252a was concentration-dependent (Fig. 3). Without the pretreatment, the amount of Al accumulated in root apexes was 2-fold lower in cv Atlas than in cv Scout, an Al-sensitive wheat. The root apex of cv Atlas pretreated with 10 μm K-252a accumulated Al at the same level as the root apex of cv Scout without the pretreatment. This suggested that malate efflux from root apexes contributed to the decrease in the Al accumulation in Al-resistant wheat.
Figure 3.
Effect of K-252a on the accumulation of Al in root apexes of Al-resistant wheat (cv Atlas). Excised root apexes were pretreated with the Ca solution containing 0, 0.01, 0.1, 1, or 10 μm K-252a and then exposed to the Ca solution (−Al) or Ca solution containing 200 μm Al (+Al, pH 4.2), each for 30 min. After Al treatment, root apexes were rinsed three times with the Ca solution to remove exchangeable Al. Accumulation of Al in root apex of Al-sensitive wheat (cv Scout) was shown in the right as a control. Values are means ± se (n = 3).
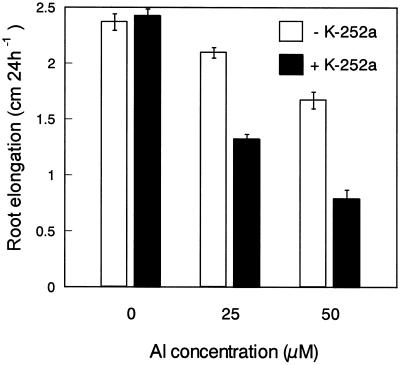
To study the possible role of malate efflux in root elongation in the presence of Al, intact roots of cv Atlas were pretreated with 1 μm K-252a for 30 min and then exposed to 200 μm CaCl2 solution containing 0, 25, and 50 μm Al for 1 d. Since the treatment of roots with 10 μm K-252a resulted in a severe inhibition of root elongation even in the absence of Al (in a preliminary experiment), the concentration of K-252a was set to 1 μm. In the absence of Al, pretreatment with 1 μm K-252a hardly affected root elongation (Fig. 4). In the presence of 25 or 50 μm Al, K-252a promoted the inhibition of root elongation (Fig. 4). Because K-252a is a broad range protein kinase inhibitor, it could be inhibiting many protein kinases that affect Al-resistance independently from malate efflux. However, pretreatment with 1 μm staurosporine, a broad range protein kinase inhibitor, which was less effective in preventing malate efflux than K-252a (Table II), did not enhance either Al accumulation or Al-induced inhibition of root elongation (data not shown). Thus, K-252a enhanced the accumulation of Al and intensified the Al-induced root growth inhibition, supporting the idea that the malate efflux from the root apex accounted for the Al-resistance in wheat. Furthermore, K-252a-sensitive protein kinase(s) has a key role in the Al-resistance mechanism caused by the efflux of malate.
Figure 4.
Effect of K-252a on Al-induced inhibition of root elongation in Al-resistant wheat (cv Atlas). Intact roots of 4-d-old seedlings were pretreated with the Ca solution containing 1 μm K-252a for 30 min and then exposed to the Ca solution containing 0, 25 or 50 μm Al for 1 d. All solutions were adjusted to pH 4.5 by the addition of 0.1 n HCl. Root length was measured with a ruler before and after Al treatment. Results represent means ± se (n = 8).
Al Activates 48-kD Protein Kinase
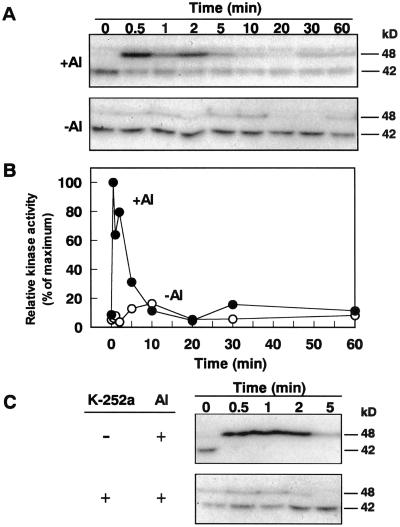
In a number of plants, induction of protein kinase activity by various extracellular stimuli has been demonstrated. Al-responsive malate efflux was under the control of K-252a-sensitive protein kinase(s) (Fig. 2). To identify the protein kinase(s) involved in Al-responsive malate efflux in the root apex of Al-resistant wheat, we investigated the changes in protein kinase activity in the root apexes of cv Atlas immediately after the exposure to Al.
Using in-gel kinase assay with myelin basic protein (MBP) as an artificial substrate, we observed activation of a 48-kD protein kinase in the root apex treated with 200 μm Al (Fig. 5, A and B). Activity of the 48-kD protein kinase was elevated from 0.5 to 5 min after the addition of Al, and it diminished after 5 min. This suggested that transient activation of the 48-kD protein kinase might be involved in the early physiological response to Al. We tested the effect of K-252a on the activation of the 48-kD protein kinase. Excised root apexes of cv Atlas were pretreated with 10 μm K-252a for 30 min and then exposed to 200 μm Al. Pretreatment of root apex with K-252a inhibited Al-induced activation of the 48-kD protein kinase (Fig. 5C). This suggested that K-252a-sensitive kinase(s) was a component of the Al-induced 48-kD protein kinase.
Figure 5.
Activation of a 48-kD kinase in response to Al. A, Excised root apexes of Al-resistant wheat (cv Atlas) were exposed to the Ca solution (−Al) or the Ca+Al solution (+Al; pH4.2) at indicated times. Crude protein extracts from the root apexes were assayed for in-gel kinase activity using MBP as a substrate. B, Forty-eight-kilodalton kinase activity (○, −Al; ●, +Al) was quantified from the intensity of bands on the x-ray-film digitized with an LAS 1000 image analyzer (Fuji-Film, Tokyo). The relative kinase activity was normalized according to a maximum band intensity of the Ca+Al solution at 0.5 min. C, K-252a blocked Al-induced activation of 48-kD kinase and Al-induced inactivation of 42-kD kinase. Excised root apexes of Al-resistant wheat (cv Atlas) were pretreated with the Ca solution without (−K-252a) or with 10 μm K-252a (+K-252a) for 30 min. After the K-252a treatment, root apexes were rinsed three times with the Ca solution to remove excess K-252a and then exposed to the Ca+Al solution at indicated times. Crude protein extracts from the root apex were assayed for in-gel kinase activity using MBP as a substrate.
In contrast, the activity of a 42-kD protein kinase decreased immediately after addition of Al (Fig. 5A), and this Al-induced suppression of the 42-kD activity was not observed with pretreatment with K-252a (Fig. 5C). Since the 42-kD activity was detected without embedding MBP in the gel (data not shown), it appeared that Al decreased the activity of the 42-kD protein kinase due to the decrease in autophosphorylation.
Malate Is Released through Organic Anion- Specific Channel
Protein phosphorylation is proposed to play a significant role in regulating the gating of anion channels in guard cells. In root apexes, the final pathway for the malate efflux is suggested to be the anion channel on the plasma membrane. However, the specific anion channels responsible for malate permeation in wheat are still unidentified. One difficulty in finding malate-permeable channels may be derived from the marked differences in the permeability of anion channels to inorganic and organic anions. For example, malate is suggested to be 10- to 100-fold less permeable than chloride and nitrate. To obtain valid information for the identification of a specific anion channel, we investigated the changes in anion contents of wheat root apexes treated or not treated with Al.
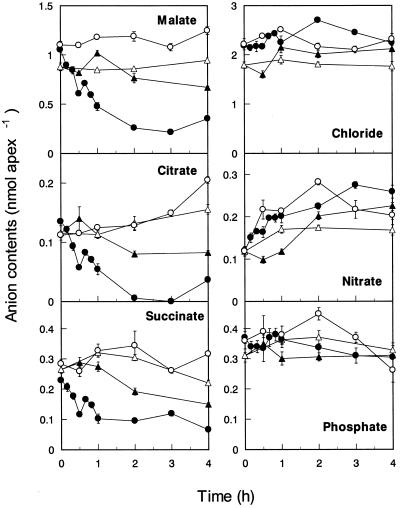
Using capillary ion electrophoresis, we were able to identify several anions in the root apexes of both cv Atlas and cv Scout. In the absence of Al, organic anions detected consistently in root apexes of both cultivars were malate, citrate, and succinate, even though the amount of citrate or succinate was 4- to 10-fold lower than that of malate (Fig. 6). In cv Atlas, the malate content in the root apex rapidly decreased immediately after addition of Al, and the lowest level was 0.2 nmol apex−1 after 2 h Al treatment (Fig. 6). Simultaneously, contents of both citrate and succinate in the root apex of cv Atlas decreased under Al stress, but the contents of inorganic anions such as chloride, nitrate, and phosphate were unaffected by Al. These results suggested that the pathway, which is specific to organic anions, might be involved in the Al-responsive efflux of malate in Al-resistant wheat.
Figure 6.
Effect of Al on anion contents in root apexes of Al-resistant (○, ●; cv Atlas) and Al-sensitive (▵, ▴; cv Scout) wheat. Excised root apexes were exposed to the Ca solution (○, ▵) or Ca+Al solution (●, ▴). The solution was renewed every 1 h when the treatment period exceeded 1 h. After the treatment, root apexes were rinsed three times with water to remove excess treatment solution. Anions in the roots were extracted in 80% (v/v) ethanol and separated by the capillary ion electrophoresis system. Values are means ± se (n = 3).
In Al-sensitive cv Scout, the decrease in the contents of organic anions, especially malate, after exposure to Al was much less than that in cv Atlas (Fig. 6). Before Al treatment, the contents of most of the anions in cv Scout were slightly less than those in cv Atlas, presumably due to slightly smaller diameter of the root in cv Scout than in cv Atlas. This result suggested that inability of cv Scout in releasing malate in response to Al was not derived from the shortage of malate contents in the root apex.
DISCUSSION
In many plants, the efflux of organic anions has been proposed as an Al-resistance mechanism. However, it is still not clear how the efflux of organic anions in response to Al is controlled in the plant. In this study, we focused our attention on the role of protein phosphorylation in the regulatory mechanism of Al-responsive malate efflux. K-252a, an inhibitor of protein kinases, effectively blocked Al-responsive malate efflux. Inhibition of malate efflux by K-252a was further supported by the promotion of Al accumulation and the intensification of Al-induced root-growth inhibition by K-252a. Activation of 48-kD protein kinase was observed preceding the initiation of malate efflux, and pretreatment of the root apexes with K-252a canceled this activation.
Our findings indicated that protein phosphorylation may be required for the signal transduction in Al-activated malate efflux. Existence of a significant time lag (Fig. 1) and requirement of temperature (Table I) for the induction of malate efflux imply that metabolic activity is necessary for the response. Although results from inhibitor studies need to be interpreted with caution, our results show that K-252a causes a specific, dose-dependent inhibition of malate efflux (Fig. 2). Roots of cv Atlas pretreated with K-252a showed increased sensitivity to Al, as indicated by the enhanced accumulation of Al (Fig. 3) and intensified Al-dependent inhibition of root elongation (Fig. 4). In contrast, pretreatment with staurosporine, which had less inhibitory effect on malate efflux (Table II), did not enhance either Al accumulation or Al-induced inhibition of root elongation (data not shown). These differences in the effects of broad range protein kinase inhibitors on the Al resistance indicate that protein kinase(s) specific for K-252a may play a significant role in the metabolic pathway for malate release. Because K-252a inhibits broad range of protein kinases, one may speculate whether increased Al accumulation was due to the repression of malate efflux or other Al exclusion mechanisms that are sensitive to K-252a. Although a question is still remaining whether malate efflux is fully accountable for the decrease in Al accumulation, the present results suggest the possibility that phosphorylation-dependent malate efflux is able to decrease the Al accumulation and to restrain Al-induced root growth inhibition. In plants, to our knowledge, this is the first indication that protein phosphorylation is requisite for the release of organic anions in response to Al.
It is possible that the protein phosphorylation plays a key role in some process involved in the transmission of the primal recognition of Al to the final release of malate, although we could not identify the specific pathway associated with malate efflux. Previous studies demonstrated that an anion channel on the plasma membrane may act as a final step of Al-dependent release of organic anions. Anion channel blockers such as niflumic acid and anthracene-9-carboxylic acid can inhibit the efflux of organic anions from root apex of wheat (Ryan et al., 1995; Papernik and Kochian, 1997) and buckwheat (Zheng et al., 1998). A patch-clamp study showed that the slow-type anion channel on the plasma membrane is activated by Al3+ not by La3+ in wheat (Ryan et al., 1997). Piñeros and Kochian (2001) recently characterized an Al-activated anion channel in maize root cells by excised membrane patch techniques, indicating that machinery required for Al-dependent activation of the anion channel is localized to the plasma membrane. In guard cells, protein kinases could play a significant role in the regulation of ion channels by phosphorylation. Gating of anion channels in tobacco cells could be modulated by phosphorylation (Zimmermann et al., 1994). A Ca2+-dependent protein kinase may be involved in the pathway of the activation of Cl− channel (Pei et al., 1996) or K+ channel (Li et al., 1998). Pei et al. (1997) found that abscisic acid-induced activation of anion channel is under the control of K-252a-sensitive protein kinases. In the present study, K-252a-sensitive protein kinase might be involved in a direct phosphorylation of an anion channel or phosphorylation of upstream protein(s), which finally leads to the regulation of channel activity.
We found that Al transiently activates a protein kinase quickly enough to precede the initiation of malate efflux. This protein kinase had a molecular mass of 48 kD and phosphorylated MBP, indicating that this protein kinase may be categorized in MAP kinase group. MAP kinase is known to respond to various stimuli, such as salt, drought, hormone, and pathogen infection (Suzuki and Shinshi, 1995; Mizoguchi et al., 1996; Seo et al., 1999). MAP kinase is also activated by mechanical stress and wounding (Usami et al., 1995; Mizoguchi et al., 1996). Hirt (1997) speculated that the difference in the induction level or the time of activation of MAP kinase according to various stimuli may lead to the difference in the subsequent pathway. Our results showed that the activity of the 48-kD kinase was approximately 10-fold higher after the treatment with Al than without Al, and the Al-induced activation was lost within 5 min (Fig. 5, A and B), which was fairly rapid compared with the changes in wounding-activated MAP kinases (Usami et al., 1995; Mizoguchi et al., 1996). This rapid disappearance in the activity suggested that activation of 48-kD kinase in response to Al might be discriminated from that in response to other stimuli. Three consecutive phosphorylation reactions in the MAP kinase cascade result in the activation of MAP kinase (Hirt, 1997; Hardie, 1999). In this study, the 48-kD protein kinase activity was sensitive to pretreatment with K-252a (Fig. 5C), suggesting that the transduction of the Al signal might involve upstream K-252a-sensitive protein kinase(s) that activate the 48-kD protein kinase. In yeast, expression of a MAP kinase gene complemented Al tolerance in an Al-sensitive mutant, indicating that the MAP kinase may be associated with the expression of physiological responses involved in Al-resistance (Schott and Gardner, 1997). Although it remains unknown whether the 48-kD protein kinase is directly involved in the pathway for malate efflux, it appears that this 48-kD MAP-like kinase plays a significant role in the transduction of the Al signal to express some physiological responses in the root apex of Al-resistant wheat.
Although there was significant difference in the repression level between individual experiments, the acute decrease in the 42-kD protein kinase activity induced by Al was consistent (Fig. 5, A and C). Since the 42-kD activity was detected in the absence of the substrate MBP in the gel (data not shown), activity of this 42-kD protein kinase would be due to autophosphorylation. As inactivation of the 42-kD kinase was quick enough to precede the malate efflux, we could not rule out the possibility that this kinase might negatively regulated the malate efflux. One possibility is that Al might affect upstream K-252a-sensitive kinase(s), which modulate the 42-kD protein kinase activity. Further research is required to determine whether modulation of the 42-kD kinase activity might be a trigger for Al-responsive malate release.
The final regulatory mechanism of malate efflux could be related to the permeability of the plasma membrane. To specify the channels that account for the permeation of malate in cells of root apexes, we examined the movement of anions in the root apex. We found that root apexes of Al-resistant wheat cv Atlas lost organic anions, citrate, and succinate as well as malate, immediately after exposure to Al (Fig. 6). However, inorganic anions such as chloride, nitrate, and phosphate were maintained at almost a constant level irrespective of Al. Anion channels in plants have specific permeability for each anion. In guard cells or hypocotyl cells, the permeability of anion channels for malate is 4- to 10-fold less than that for Cl−, and 40- to 100-fold less than that for NO3− (Hedrich and Marten, 1993; Schmidt and Schroeder, 1994; Frachisse et al., 1999). These findings sharply contrast with the present findings demonstrating the Al-specific induction of the release of organic anions from the roots of an Al-resistant cultivar. In guard cells, extracellular malate is speculated to work as a positive-feedback regulator for malate efflux through anion channels for drought-induced stomatal closing (Hedrich and Marten, 1993; Schroeder, 1995). However, in root apexes, it is unlikely that extracellular malate serves as a regulator for anion channel opening, since the released malate easily combines with extracellular Al3+, and Al-malate complex might not trigger further malate efflux. Anions are suggested to easily pass through activated anion channels depending on the anion gradient between the inside and outside of the cell and the negative membrane potential (Hedrich and Marten, 1993). Since the rapid decrease in organic anion contents are paralleled with the release in response to Al, we propose that malate may be released through an anion channel or a transporter specific for organic anions in root apex of Al-resistant wheat.
In this study, we showed that malate efflux started 5 min after the addition of Al. It is most likely that malate efflux in wheat is one of the earliest responses to Al. In plants, there is little research on Al signaling mediated by second messengers. Jones and Kochian (1995) showed that Al inhibited the inositol 1,4,5-triphosphate pathway in an Al-sensitive cultivar of wheat. Al-induced depolarization of the cell membrane in wheat root apex is not fully accountable for the induction of malate efflux (Papernik and Kochian, 1997). An increase in cytoplasmic Ca2+ level in wheat root apexes may be related to the expression of Al toxicity (Zhang and Rengel, 1999). In the present study, we provided new evidence suggesting that protein phosphorylation is required for the malate efflux and that malate might be passed through organic anion-specific channels. Because of its specificity to Al and rapidness, Al-induced malate efflux is a useful system for studying how the Al signal is transmitted into the cell that expresses physiological responses underlying Al-toxicity or tolerance.
In conclusion, we obtained findings indicating that protein phosphorylation is involved in Al-dependent malate efflux in wheat. For determination of the transduction of the Al-signal participating in the release of organic anions, further research is needed to identify the proteins associated with signal transduction and the anion channel functioning in the malate efflux.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Two wheat (Triticum aestivum) cultivars (cv Atlas 66, an Al-resistant cultivar, and cv Scout 66, an Al-sensitive cultivar) were used. Seeds were surface-sterilized for 20 min in a 0.5% (v/v) sodium hypochlorite solution and placed on wet filter paper. Seeds were kept in the dark in a cold room for 2 to 6 d to germinate. The germinated seeds were transferred to a plastic mesh placed on a 0.5 mm CaCl2 solution (pH 4.5) in a black 5-L plastic container. The seedlings, which were grown for an additional 2 to 3 d in a growth chamber under a photon flux density of 150 μmol m−2 s−1 (14 h/10 h, light/dark) at 25°C, were used for the following experiments.
Determination of Malate Efflux
The amounts of malate released from root apexes were determined according to the method of Ryan et al. (1995) with minor modification. From roots placed in a Petri dish containing 200 μm CaCl2 solution at pH 4.5 (referred to as the Ca solution in this study), 2-mm root apexes were excised with a razor blade. Forty root apexes for each measurement in three independent replicates were transferred into a 3.5 cm Petri dish and washed three times with the Ca solution to remove any malate derived from the cutting injury. During the treatment, the dishes were placed on a reciprocal shaker (80 rpm). After 30 min, the solution was collected for malate analysis. For the inhibitor experiment, root apexes were pretreated for 30 min in the 3.5-cm Petri dish containing 1.0 mL of the Ca solution with or without inhibitor.
Al treatment was started by addition of 1.0 mL of the Ca solution or the solution containing 200 μm CaCl2 and 200 μm Al (referred to as the Ca+Al solution in this study). As the exiting form of Al in solution is very complex, it is difficult to distinguish Al3+ from other phytotoxic forms of Al, such as Al(OH)2+, Al(OH2)+, and polymer Al13. However, Al3+ dominates in solution at pH less than 5.0 and is likely to be the most phytotoxic species (Kinraide, 1991). In our experimental condition, Al3+ is considered to be the most dominant ion, since Ca+Al solution was prepared using 20 mm AlCl3 stock solution, and adjusted to pH 4.2 by addition of only 0.1 n HCl to minimize the formation of other phytotoxic species.
To determine an initiation time of malate efflux, we used the following measurement system. Root apexes (200 for each measurement) were placed in a column and through which the Ca solution was continually passed at a flow rate of 0.5 mL min−1. After subtracting the Ca solution, the Ca+Al solution was applied from the top and the eluent was collected from the bottom at a flow rate of 0.5 mL min−1. Root apexes were stirred continuously with a plastic rod.
Malate concentration was determined enzymatically as described previously (Delhaize et al., 1993), according to the procedure of Gutmann and Wahlefeld (1974).
Determination of Al in Root Apexes
The root apexes, which were excised after Al treatment, were washed three times with the Ca solution. Then, 40 root apexes were placed in a microcentrifuge tube (1.5 mL) containing 1.0 mL of 2 n HCl. The tubes were mixed with an orbital shaker at 10 rpm for 24 h to release Al from the root apexes. The Al concentration in the HCl solution after dilution was determined by graphite furnace atomic absorption spectrophotometry (model Z-9000, Hitachi, Tokyo).
Determination of Anions in Root Apexes
Excised root apexes were washed three times with distilled water, placed in an 80% (v/v) ethanol solution in a microcentrifuge tube, and boiled for 5 min at 80°C. The root apexes were ground using a microhomogenizer (model NS-310E, Nitto, Tokyo), and centrifuged for 5 min at 10,000g. After collecting the supernatant, the pellet was re-extracted twice by the same procedure. The supernatant of three replicates were mixed, freeze-dried to remove excess reagent, and reconstituted in 100 μL of ultra pure water. Reconstituted samples were filtered on 0.45-μm sterilized filters (Millipore, Tokyo), and used for analysis of anions.
Capillary ion electrophoresis was performed with a PACE 5510 system (Beckman Instruments, Fullerton, CA) equipped with UV detector (254 nm). Anions in the solution were separated in a fused silica capillary (81 cm long × 75 μm i.d.; Beckman, CA) with a carrier electrolyte at a constant voltage of a −20 kV. The carrier electrolyte was 5.0 mm 2,6-pyridinedicarboxylic acid, and 2.5% (v/v) CIA-Pak OFM-Anion BT (Waters, Milford, MA) at pH 6.4. Samples were injected by pressure (5 psi s−1) into a capillary at 20°C. Prior to inject samples, the capillary was rinsed with 0.1 n NaOH and then with water each for 3 min before equilibration with the carrier electrolyte for 5 min.
Protein Extraction
Excised root apexes (100 for each treatment) were collected and suspended in 1.0 mL of the Ca or Ca+Al solution (pH 4.2) in a microcentrifuge tube. The tubes were inverted occasionally by hand. The treatment was terminated by the subtraction of the solution by aspiration. Root apexes in the tubes were quickly frozen in liquid nitrogen and stored at −80°C until use.
To prepare the crude extracts from the root apexes, we homogenized the sample using a plastic pestle in 2 volumes (w/v) of extraction buffer containing 50 mm HEPES-KOH, pH 7.5, 5 mm EDTA, 5 mm EGTA, 2 mm dithiothreitol, 50 mm glycerophosphate, 10 mm Na3VO3, 10 mm NaF, 1 mm phenylmethylsulfonyl fluoride, 5 μg mL−1 leupeptin and antipain, and 10% (v/v) glycerol. The homogenates were centrifuged at 10,000g for 15 min at 4°C. Supernatants were used for the detection of in-gel kinase activity. The protein concentration in the supernatant was determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA).
Assay of In-Gel Kinase Activity
The assay of in-gel kinase assay was performed as described previously (Suzuki and Shinshi, 1995; Usami et al., 1995). To be brief, 20 μg of total protein per each lane was separated on 12.5% (w/v) SDS-polyacrylamide gel containing 0.25 mg mL−1 MBP embedded in running gels. After electrophoresis, SDS was removed by washing twice in buffer A (50 mm Tris-HCl, pH 8.0, 20% [v/v] 2-propanol) followed by washing twice in buffer B (50 mm Tris-HCl, pH 8.0, 5 mm 2-mercaptoethanol), each for 30 min at room temperature. The proteins in the gel were allowed to denature in buffer C (50 mm Tris-HCl, pH 8.0, 5 mm 2-mercaptoethanol, 6 m guanidine hydrochloride) for 1 h at room temperature and then overnight in buffer D (50 mm Tris-HCl, pH 8.0, 5 mm 2-mercaptoethanol, 0.05% [v/v] Tween 20) at 4°C.
The gel was incubated twice in buffer E (40 mm Tris-HCl, pH 8.0, 50 mm NaCl, 20 mm KCl, 10 mm MgCl2, 0.1 mm EGTA, 2 mm dithiothreitol) each for 30 min at room temperature and then incubated in 10 mL of buffer E containing 50 μm ATP plus 1.85 MBq (50 μCi) [γ-32P]ATP (3,000 Ci/mmol; Amersham-Pharmacia Biotech, Buckinghamshire, UK) for 90 min at room temperature. The gel was rinsed for at least 2 h with 5% (w/v) trichloroacetic acid and 1% (w/v) sodium pyrophosphate changing the solution four times to remove the unincorporated [γ-32P]ATP. The dried gel was exposed to x-ray film for autoradiography. The approximate molecular masses of protein kinases were estimated from the mobility of prestained size markers (Sigma-Aldrich, St. Louis).
Inhibitors
K-252a was purchased from Calbiochem (San Diego, CA). KN-62 [1-[N,Obis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenyl piperizine], and calphostin C, staurosporine, chelerythrine chloride, and cyclosporin A were purchased from Sigma-Aldrich. Calyculin A and okadaic acid were purchased from Wako Chemical (Osaka). All reagents except chelerythrine chloride were dissolved in dimethysulfoxide as a 1 mm stock solution, but chelerythrine chloride was dissolved in water.
Footnotes
This work was supported by the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) from the Ministry of Agriculture, Forestry and Fisheries of Japan (to H.M.); by a Grant-in-Aid for General Scientific Research (A) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 11306006 to H.M.); and by the Ohara Foundation for Agricultural Sciences.
LITERATURE CITED
- De la Fuente JM, Ramirez-Rodriguez V, Cabrera-Ponce JL, Herrera-Estrella L. Aluminum tolerance in transgenic plants by alteration of citrate synthase. Science. 1997;276:1566–1568. doi: 10.1126/science.276.5318.1566. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.): II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 1993;103:695–702. doi: 10.1104/pp.103.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Harrison MJ, Lamb CJ. Early events in the activation of plant defense responses. Annu Rev Phytopathol. 1994;32:479–501. [Google Scholar]
- Frachisse J-M, Thomine S, Colcombet J, Guern J, Barbier-Brygoo H. Sulfate is both a substrate and an activator of the voltage-dependent anion channel of Arabidopsis hypocotyl cells. Plant Physiol. 1999;121:253–262. doi: 10.1104/pp.121.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann I, Wahlefeld AW. L-(−)-malate: determination with malate dehydrogenase and NAD. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 3. New York: Academic Press; 1974. pp. 1585–1589. [Google Scholar]
- Hardie DG. Plant protein serine/threonine kinases: classification and functions. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:97–131. doi: 10.1146/annurev.arplant.50.1.97. [DOI] [PubMed] [Google Scholar]
- Hedrich R, Marten I. Malate-induced feedback regulation of plasma membrane anion channels could provide a CO2 sensor to guard cells. EMBO J. 1993;12:897–901. doi: 10.1002/j.1460-2075.1993.tb05730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt H. Multiple roles of MAP kinases in plant signal transduction. Trends Plant Sci. 1997;2:11–15. [Google Scholar]
- Hue NV, Craddock GR, Adams F. Effects of organic acids on aluminum toxicity in subsoils. Soil Sci Soc Am J. 1986;50:28–34. [Google Scholar]
- Jones DL, Kochian LV. Aluminum inhibition of the inositol 1,4,5-trisphosphate signal transduction pathway in wheat roots: a role in aluminum toxicity. Plant Cell. 1995;7:1913–1922. doi: 10.1105/tpc.7.11.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB. Identity of the rhizotoxic aluminium species. Plant Soil. 1991;134:167–178. [Google Scholar]
- Li J, Lee Y-RJ, Assmann SM. Guard cells possess a calcium-dependent protein kinase that phosphorylates the KAT1 potassium channel. Plant Physiol. 1998;116:785–795. doi: 10.1104/pp.116.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF. Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol. 2000;41:383–390. doi: 10.1093/pcp/41.4.383. [DOI] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H. Specific secretion of citric acid induced by Al stress in Cassia tora L. Plant Cell Physiol. 1997a;38:1019–1025. [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H, Hiradate S. Detoxifying aluminium with buckwheat. Nature. 1997b;390:569–570. [Google Scholar]
- Matsumoto H. Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol. 2000;200:1–46. doi: 10.1016/s0074-7696(00)00001-2. [DOI] [PubMed] [Google Scholar]
- Miyasaka SC, Buta JG, Howell RK, Foy CD. Mechanism of aluminum tolerance in snapbeans: root exudation of citric acid. Plant Physiol. 1991;96:737–743. doi: 10.1104/pp.96.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, Shinozaki K. A gene encoding a mitogen-activated protein kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:765–769. doi: 10.1073/pnas.93.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papernik LA, Kochian LV. Possible involvement of Al-induced electrical signals in Al tolerance in wheat. Plant Physiol. 1997;115:657–667. doi: 10.1104/pp.115.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DR, Pedler JF. Probing the “malate hypothesis” of differential aluminum tolerance in wheat by using other rhizotoxic ions as proxies for Al. Planta. 1998;205:389–396. [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Ward JM, Harper JF, Schroeder JI. A novel chloride channel in Vicia faba guard cell vacuoles activated by the serine/threonine kinase, CDPK. EMBO J. 1996;15:6564–6574. [PMC free article] [PubMed] [Google Scholar]
- Piñeros MA, Kochian LV. A patch-clamp study on the physiology of aluminum toxicity and aluminum tolerance in maize: identification and characterization of Al3+-induced anion channels. Plant Physiol. 2001;125:292–305. doi: 10.1104/pp.125.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Characterization of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta. 1995;196:103–110. [Google Scholar]
- Ryan PR, Skerrett M, Findlay GP, Delhaize E, Tyerman SD. Aluminum activates an anion channel in the apical cells of wheat roots. Proc Natl Acad Sci USA. 1997;94:6547–6552. doi: 10.1073/pnas.94.12.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Schroeder JI. Anion selectivity of slow anion channels in the plasma membrane of guard cells. Plant Physiol. 1994;106:383–391. doi: 10.1104/pp.106.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott EJ, Gardner RC. Aluminum-sensitive mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1997;254:63–72. doi: 10.1007/s004380050391. [DOI] [PubMed] [Google Scholar]
- Schroeder JI. Anion channels as central mechanisms for signal transduction in guard cells and putative functions in roots for plant-soil interactions. Plant Mol Biol. 1995;28:353–361. doi: 10.1007/BF00020385. [DOI] [PubMed] [Google Scholar]
- Seo S, Sano H, Ohashi Y. Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell. 1999;11:289–298. doi: 10.1105/tpc.11.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Shinshi H. Transient activation and tyrosine phosphorylation of a protein kinase in tobacco cells treated with a fungal elicitor. Plant Cell. 1995;7:639–647. doi: 10.1105/tpc.7.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami S, Banno H, Ito Y, Nishihama R, Machida Y. Cutting activates a 46-kilodalton protein kinase in plants. Proc Natl Acad Sci USA. 1995;92:8660–8664. doi: 10.1073/pnas.92.19.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZM, Sivaguru M, Horst WJ, Matsumoto H. Aluminum tolerance is achieved by exudation of citric acid from roots of soybean (Glycine max L. Merr.) Physiol Plant. 2000;110:72–77. [Google Scholar]
- Zhang WH, Rengel Z. Aluminum induces an increase in cytoplasmic calcium in intact wheat root apical cells. Aust J Plant Physiol. 1999;26:401–409. [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H. High aluminum resistance in buckwheat: I. Al-induced specific secretion of oxalic acid from root tips. Plant Physiol. 1998;117:745–751. doi: 10.1104/pp.117.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Thomine S, Guern J, Barbier-Brygoo H. An anion current at the plasma membrane of tobacco protoplasts shows ATP-dependent voltage regulation and is modulated by auxin. Plant J. 1994;6:707–716. [Google Scholar]