Abstract
Objective
To compare the performance of a newly developed race-free kidney recipient specific glomerular filtration rate (GFR) equation with the three current main equations for measuring GFR in kidney transplant recipients.
Design
Development and validation study
Setting
17 cohorts in Europe, the United States, and Australia (14 transplant centres, three clinical trials).
Participants
15 489 adults (3622 in development cohort (Necker, Saint Louis, and Toulouse hospitals, France), 11 867 in multiple external validation cohorts) who received kidney transplants between 1 January 2000 and 1 January 2021.
Main outcome measure
The main outcome measure was GFR, measured according to local practice. Performance of the GFR equations was assessed using P30 (proportion of estimated GFR (eGFR) within 30% of measured GFR (mGFR)) and correct classification (agreement between eGFR and mGFR according to GFR stages). The race-free equation, based on creatinine level, age, and sex, was developed using additive and multiplicative linear regressions, and its performance was compared with the three current main GFR equations: Modification of Diet in Renal Disease (MDRD) equation, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2009 equation, and race-free CKD-EPI 2021 equation.
Results
The study included 15 489 participants, with 50 464 mGFR and eGFR values. The mean GFR was 53.18 mL/min/1.73m2 (SD 17.23) in the development cohort and 55.90 mL/min/1.73m2 (19.69) in the external validation cohorts. Among the current GFR equations, the race-free CKD-EPI 2021 equation showed the lowest performance compared with the MDRD and CKD-EPI 2009 equations. When race was included in the kidney recipient specific GFR equation, performance did not increase. The race-free kidney recipient specific GFR equation showed significantly improved performance compared with the race-free CKD-EPI 2021 equation and performed well in the external validation cohorts (P30 ranging from 73.0% to 91.3%). The race-free kidney recipient specific GFR equation performed well in several subpopulations of kidney transplant recipients stratified by race (P30 73.0-91.3%), sex (72.7-91.4%), age (70.3-92.0%), body mass index (64.5-100%), donor type (58.5-92.9%), donor age (68.3-94.3%), treatment (78.5-85.2%), creatinine level (72.8-91.3%), GFR measurement method (73.0-91.3%), and timing of GFR measurement post-transplant (72.9-95.5%). An online application was developed that estimates GFR based on recipient’s creatinine level, age, and sex (https://transplant-prediction-system.shinyapps.io/eGFR_equation_KTX/).
Conclusion
A new race-free kidney recipient specific GFR equation was developed and validated using multiple, large, international cohorts of kidney transplant recipients. The equation showed high accuracy and outperformed the race-free CKD-EPI 2021 equation that was developed in individuals with native kidneys.
Trial registration
ClinicalTrials.gov NCT05229939.
Introduction
Accurate prediction of glomerular filtration rate (GFR) is crucial for the management of patients with chronic kidney disease (CKD).1 2 It is particularly important for kidney transplant recipients, who are a high risk and rapidly growing population,3 require close monitoring, and place a huge strain on health systems worldwide. The GFR is the most predictive parameter of kidney graft failure4 and is therefore used to drive patient management and daily decision making,5 6 including putting patients back on the transplant waiting list or reinstating dialysis.
Historically, GFR equations that predict measured GFR (mGFR) were based on serum creatinine or cystatin C level, or both, as endogenous filtration markers.7 8 9 These equations were principally developed in people from North America, which has been regarded as “Americentrism” in GFR equations,10 and might limit generalisability worldwide.11 Importantly, these individuals had native kidneys and did not reach end stage kidney disease. The equations were later used in kidney transplant recipients worldwide,12 which has been criticised by the Kidney Disease: Improving Global Outcomes (KDIGO) consortium in its attempt to improve the management of kidney allografts.13 Indeed, studies have shown substantial heterogeneity in the performance of GFR equations when applied to kidney transplant recipients,14 which may be attributed to differences in this population15 and intrinsic characteristics specific to kidney transplant recipients,14 such as the use of immunosuppressive treatment and frequent antimicrobial treatments, as well as events such as rejection and tubular necrosis episodes.16 17
Overall, a need still exists for a specific and more accurate equation for kidney transplant recipients.18 According to our literature review (see supplementary table 1), only three GFR equations have been specifically developed in kidney transplant recipients, and validation of these equations was hampered by methodological shortcomings, such as a single centre setting, small cohort size, and lack of an external validation set.16 19 20 We thus hypothesised that GFR equations developed in a large, well phenotyped cohort of kidney transplant recipients could achieve good performance in predicting mGFR.
Recently, on the basis that use of race was sometimes associated with health inequalities,21 new race-free equations have been developed.22 However, some studies have shown potential for systematic misclassification when using a race-free approach.23 24 To date, the question of whether these new equations perform well on a large and international population of kidney transplant recipients remains unanswered.25 To address this unmet need, we constructed a multinational cohort of more than 15 000 kidney transplant recipients with 50 000 GFR measurements from 17 transplant cohorts in seven countries to develop a new race-free kidney recipient specific GFR equation and compared its performances with those of the three current main GFR equations, including the race-free Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2021 equation.
Methods
Study design and cohorts
Our study is a GFR equation development and validation study. Seventeen cohorts (that were either transplant centres or clinical trials) of adult kidney transplant recipients from seven countries were included. The development cohort (n=3622) was composed of consecutive patients prospectively included on the day of transplantation (living or deceased donation) at Necker Hospital (n=2737), Saint Louis Hospital (n=374), and Toulouse Hospital (n=511) in France between 1 January 2000 and 1 January 2021. The clinical data were anonymised and continuously entered into the Paris Transplant Group unified dataset (https://paristransplantgroup.com) using a standardised, shared protocol to ensure harmonisation (see supplementary methods 1.1 and the registered study protocol).
For external validation of the equation, we contacted 55 additional centres and trials worldwide: from Europe (n=17), the United States (n=21), South America (n=2), Canada (n=3), Asia (n=9), Africa (n=2), and Oceania (n=1). These cohorts were selected based on the transplant activity of the centre, research activity, and previous GFR related publications. The required data for being included in the study were based on previous publications on GFR equations7 8 26 and comprised mGFR, creatinine level, age, sex, and race. Fourteen cohorts had the required information and agreed to provide the data. External validation was finally carried out on datasets of kidney transplant recipients from Montpellier Hospital, France (n=1486); Tenon Hospital, France (n=469); Lyon Hospital, France (n=248); Saint Etienne Hospital, France (n=446); Mayo Clinic Hospital, MN, USA (n=4062); Mayo Clinic Hospital, FL, USA (n=709); Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial (BENEFIT) and Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial-EXTended criteria donors (BENEFIT-EXT) 27 28 (n=981); Angiotensin II Blockade for Chronic Allograft Nephropathy (ABCAN) trial29 (n=139); Bergamo Hospital, Italy (n=196); Zagreb Hospital, Croatia (n=883); Groningen Kidney Centre, the Netherlands (n=1738); Sydney Transplant Unit, Australia (n=430); and Aarhus University Hospital, Denmark (n=80), between 1 January 2000 and 1 January 2021 (see supplementary methods 1.2). Data from the two Mayo Clinic hospitals and from the two BENEFIT trials were each combined and therefore represent two unique cohorts in the results section. Supplementary tables 2-4 provide details about the organ allocation system and data collection.
Data collection and procedures
For each kidney transplant recipient, we collected information on age, sex, height, weight, body mass index (BMI), cause of end stage kidney disease, serum creatinine level (in mg/dL; 1 mg/dL=88.4 μmol/L), mGFR (mL/min/1.73m2), race (black v non-black), and treatment (calcineurin inhibitor based and mammalian target of rapamycin based). For each donor we collected information on age, sex, hypertension, diabetes mellitus, serum creatinine level (mg/dL), and donor type (living versus deceased). The transplant variables collected were previous kidney transplantation, cold ischaemia time, delayed graft function (defined as the need for dialysis in the first week after transplantation), and the number of human leucocyte antigen (HLA) A, B, and DR mismatches (supplementary methods 1.3). Five authors (MR, SAA, GD, OA, and AL) assessed and verified the data reported in this study.
Measurement and estimation of GFR
The outcome to be predicted—mGFR—was assessed using several measurement methods: 51Cr-EDTA, diethylenetriamine pentaacetic acid(99Tc-DTPA), inulin, iothalamate, or iohexol clearance. The choice of GFR measurement method was determined by the transplant centre’s preference, as markers differ in terms of availability, cost, and expertise.30 Studies have shown that these methods perform similarly for measuring GFR31 32 33 and that bias is low.30 Supplementary table 5 summarises the laboratory methods used.
The main centre in the development cohort used 51Cr-EDTA clearance to measure the GFR. Patients were administered 0.5 microCuries/kg of 51Cr-EDTA (GE Healthcare; Vélizy-Vil-Lacoublay, France) intravenously as a single primary bolus, followed by a constant 51Cr-EDTA infusion. Average urinary 51Cr-EDTA clearance, plasma 51Cr-EDTA, and urinary creatinine clearance were then assessed during six consecutive 30 minute clearance periods, and the measurements were averaged and standardised for body surface area (1.73m2), with body surface area calculated using the Du Bois formula.34
Serum and urinary creatinine levels were measured by the creatinine Jaffe Gen. 2 (CREJ2) colorimetric method (Roche) before 2011 and thereafter using the Multigent Creatinine (Enzymatic) assay (Sentinel Diagnostics) traceable to the National Institute of Standards and Technology (calibrated using isotope dilution mass spectrometry).
GFR was estimated using the Modification of Diet in Renal Disease equation,7 CKD-EPI 2009 equation,8 and race-free CKD-EPI 2021 equation,22 using creatinine level, recipient, age, sex, and race when needed. We used these equations because they are recommended by the latest guidelines on management of kidney disease and correspond to the most commonly used equations in transplant clinical practice.35 36
Statistical analysis
We followed the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD)37 for reporting the model we developed (see supplementary table 6). Continuous variables are presented as means and standard deviations (SDs) or medians and interquartile ranges (IQRs), as appropriate. Means and proportions between groups were compared with Student’s t test, an analysis of variance, or the χ2 test (or Fisher’s exact test if appropriate).
Development of kidney recipient specific GFR equation
To develop the kidney recipient specific GFR equation, we used linear regressions38 with mGFR as the outcome and recipient variables as explanatory variables. We developed additive models,39 and we also developed multiplicative models, assuming that the multiplication of the recipient variables might provide better performances than their addition. Overall, we ensured that our models followed the standard assumptions of regression, including an equal variation of the outcome, a normal or uniform distribution of explanatory variables, a linear association between the outcome and explanatory variables, and no collinearity of explanatory variables. Therefore, to ensure normality we log transformed creatinine values. To ensure that the association between mGFR and the log transformed creatinine value was linear, we also log transformed (natural logarithm) the mGFR. We applied a polynomial function to the log transformed creatinine value to identify the association between potential nth degree log transformed creatinine value and log transformed mGFR, which may inform the model. The variance inflation factor was computed for each parameter of each model to measure the collinearity of explanatory variables. We used the stepwise methods as well as a more pragmatic approach comprising testing many combinations of variables, even though they were not significantly associated with mGFR in univariate analysis, with P values <0.05. The combinations of parameters were based on the scientific literature,7 8 26 40 the literature search performed in the present study, and the experience of the numerous nephrologists and researchers involved in this study. According to these parameterisations, we selected the model showing the best performances and developed a race-free kidney recipient specific GFR equation based on the final list of parameters. Because of the low number of missing data, imputation was not performed. Supplementary tables 6-8 provide additional details on the development of the equation.
Evaluation of performances of race-free kidney recipient specific GFR equations
The model fit was assessed with five metrics: adjusted R2, root mean square error, mean absolute error, Akaike information criterion, and bayesian information criterion. The prediction performances of the equations developed were assessed with the P30, which is the proportion of the estimated GFR (eGFR) within 30% of the mGFR,22 and with correct classification,22 which is the agreement between the eGFR and the mGFR according to the KDIGO stages for GFR41 (GFR of >90, 60-89, 45-59, 30-44, 15-29, and <15 mL/min/1.73m2).
Validation of GFR equations
Firstly, we investigated the performances of the kidney recipient specific GFR equations in the development cohort. We then applied the equation to the external validation cohorts and assessed the performances. For each cohort, we also assessed the performances of the kidney recipient specific GFR equation based on the Modification of Diet in Renal Disease equation, the CKD-EPI 2009 equation, and the race-free CKD-EPI 2021 equation. We compared the performances of the different equations with a Wilcoxon test to identify the one that performed best.
Additional analyses
To explore other model selections, we performed Lasso regressions. We calculated the performances for the model using the coefficients of the Lasso regression, and for the standard linear regression with the parameters selected by Lasso. In addition, we investigated the performances of the GFR equations in several subpopulations: non-black patients (that is, patients of races other than black), black patients, male patients, female patients, older patients (median age 61.1 years (interquartile range 58.0-67.6), based on the overall median patients’ age), younger patients (median age 44.1 years (35.5-50.0)), underweight patients (BMI <18.5), patients with normal weight (BMI 18.5-25), overweight patients (BMI >25), obese patients42 (BMI >30), patients with creatinine values measured using the enzymatic method, patients with creatinine values measured using the colorimetric method, patients with GFR values measured using 99Tc-DTPA clearance, patients with GFR values measured using 51Cr-EDTA clearance, patients with GFR values measured using inulin clearance, patients with GFR values measured using iohexol clearance, patients with GFR values measured using iothalamate clearance, patients with an allograft from a living donor, patients with an allograft from a deceased donor, patients with an allograft from a younger donor, patients with an allograft from an older donor, patients whose age discrepancy with the donor is >10 years, patients whose age discrepancy with the donor is <10 years, patients treated with calcineurin inhibitor based regimens, patients treated with mammalian target of rapamycin inhibitor based regimens, patients treated with belatacept, patients with GFR measured before one year post-transplantation, patients with GFR measured after one year post-transplantation, patients with pre-emptive transplantation, and patients who received a transplant after initiation of dialysis.
We used R (version 3.2.1) and STATA (version 14) for the descriptive and prediction analyses. We considered P values <0.05 to be significant, and all tests were two tailed.
Patient and public involvement
No patients or members of the public were directly involved in the design, conduct, or reporting of this research.
Results
Description of cohorts
Overall, 15 489 participants were included in the study, in whom 50 464 GFR values were measured and estimated. The development cohort included a total of 3622 patients from three centres and 8827 GFR evaluations. The external validation cohort included a total of 11 867 patients from 11 centres and three trials, and 41 637 GFR evaluations. The median time from transplantation to GFR evaluation was 2.08 years (IQR 0.50-6.00 years). The mean GFR was 53.18 (SD 17.23) in the development cohort and 55.90 (19.69) in the external validation cohorts. GFR was normally distributed in all cohorts (see supplementary figures 1 and 2). Supplementary tables 3 and 4 outline the allocation systems, induction treatment, and methods to measure creatinine level and GFR in the development and external validation cohorts. Table 1 shows the baseline characteristics of the cohorts (see supplementary tables 9-12 for characteristics by centre).
Table 1.
Baseline characteristics of development and validation cohorts. Values are number (percentage) unless stated otherwise
| Development cohort (n=3622) | External validation cohorts | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| France (n=2649) | Europe and Oceania (n=3327) | North America (n=5891) | |||||||||
| No | Estimate | No | Estimate | No | Estimate | No | Estimate | ||||
| Recipient characteristics | |||||||||||
| Mean (SD) age (years) | 3622 | 51.41 (14.03) | 2649 | 52.55 (13.91) | 3327 | 49.25 (13.38) | 5891 | 52.34 (13.66) | <0.001 | ||
| Male patients | 3622 | 2171 (59.94) | 2649 | 1700 (64.18) | 3327 | 2012 (60.47) | 5891 | 3575 (60.69) | 0.008 | ||
| Mean (SD) BMI | 3622 | 24.76 (4.54) | 2649 | 25.08 (4.49) | 3327 | 25.57 (8.48) | 5793 | 28.42 (6.30) | <0.001 | ||
| Black race | 3622 | 333 (9.19) | 2649 | 150 (5.66) | 3327 | 0.0 (00.00) | 5891 | 448 (7.60) | <0.001 | ||
| Median (IQR) measured GFR (mL/min/1.73m2) | 3622 | 54 (43-66) | 2649 | 49 (36-62) | 3327 | 56 (43-70) | 5891 | 55 (43-68) | <0.001 | ||
| Cause of end stage kidney disease: | |||||||||||
| Glomerulonephritis | 3622 | 903 (24.93) | 1382 | 389 (28.15) | 1079 | 399 (36.98) | 4634 | 1045 (22.55) | <0.001 | ||
| Diabetes | 3622 | 358 (9.88) | 1382 | 138 (9.99) | 1079 | 54 (5.00) | 4634 | 960 (20.72) | |||
| Vascular | 3622 | 319 (8.81) | 1382 | 85 (6.15) | 1079 | 140 (12.97) | 4634 | 424 (9.15) | |||
| Other | 3622 | 2038 (56.27) | 1382 | 770 (55.72) | 1079 | 486 (45.04) | 4634 | 2207 (47.63) | |||
| Donor characteristics | |||||||||||
| Mean (SD) age (years) | 3622 | 51.01 (16.04) | 711 | 51.71 (15.72) | 2894 | 50.77 (14.08) | 5748 | 43.36 (14.76) | <0.001 | ||
| Male patient | 3087 | 1640 (53.13) | 1843 | 1027 (55.75) | 1155 | 639 (55.32) | 5749 | 2721 (47.33) | <0.001 | ||
| Hypertension | 2708 | 461 (17.02) | 929 | 155 (16.68) | 1079 | 424 (39.30) | 0 | NA | <0.001 | ||
| Diabetes mellitus | 2969 | 198 (6.67) | 783 | 40 (5.11) | 1079 | 66 (6.12) | 0 | NA | <0.001 | ||
| Creatinine >1.5 mg/dL | 3556 | 365 (10.26) | 1184 | 167 (14.10) | 862 | 126 (14.62) | 0 | NA | <0.001 | ||
| Deceased donor | 3610 | 2890 (80.06) | 1574 | 1402 (89.07) | 1079 | 1059 (98.15) | 5891 | 2349 (39.87) | <0.001 | ||
| Transplant baseline characteristics | |||||||||||
| Previous kidney transplant | 3106 | 551 (17.74) | 688 | 34 (4.94) | 1079 | 80 (7.41) | 4910 | 698 (14.22) | <0.001 | ||
| Delayed graft function | 3564 | 881 (24.72) | 1101 | 154 (13.99) | 963 | 223 (23.16) | 5649 | 689 (12.20) | <0.001 | ||
| Mean (SD) cold ischaemia time in deceased donors (hours) | 3111 | 16.13 (10.20) | 1765 | 15.85 (7.15) | 749 | 19.8 (13.40) | 4279 | 7.62 (10.39) | <0.001 | ||
| Mean (SD) No of HLA-A, B, or DR mismatches | 3111 | 4.30 (1.39) | 2168 | 3.54 (1.31) | 1065 | 3.05 (1.12) | 5624 | 3.41 (1.71) | <0.001 | ||
BMI=body mass index; GFR=glomerular filtration rate; HLA=human leucocyte antigen; IQR=interquartile range; NA=not available; SD=standard deviation.
1 mg/dL creatinine=88.4 μmol/L.
Performances of the current GFR equations in kidney transplant recipients
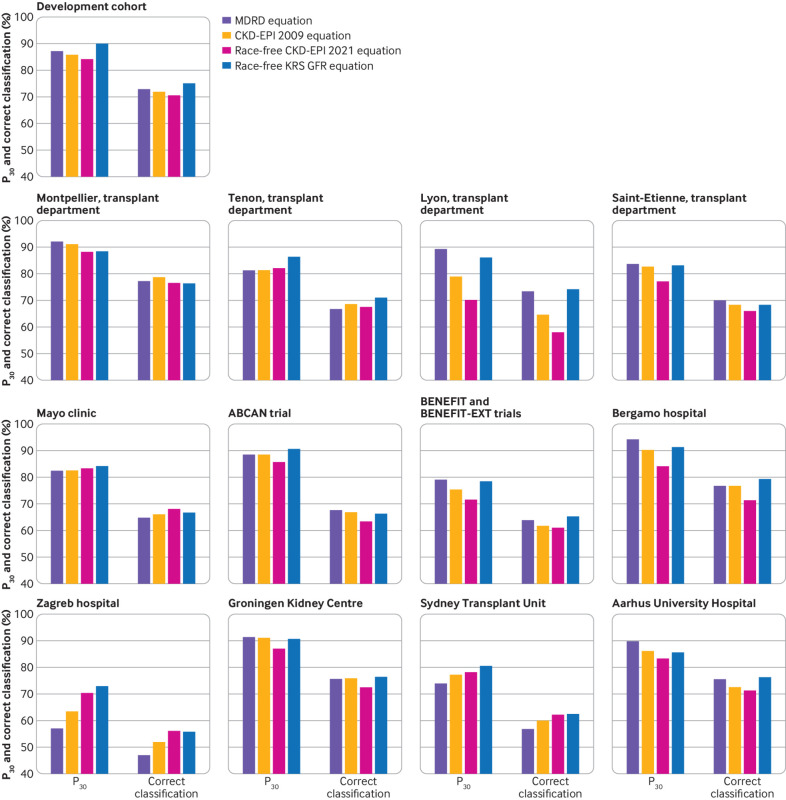
In the external validation centres, the P30 for the Modification of Diet in Renal Disease equation was 92.3% in Montpellier, 81.1% in Tenon, 89.5% in Lyon, 83.9% in Saint-Etienne, 82.4% in Mayo Clinic, 88.5% in the ABCAN trial, 79.1% in BENEFIT, 94.3% in Bergamo, 57.1% in Zagreb, 91.2% in Groningen, 74.0% in Sydney, and 89.9% in Aarhus (fig 1). The corresponding P30 values for the CKD-EPI 2009 equation were 91.3%, 81.3%, 79.0%, 82.8%, 82.4%, 88.5%, 75.3%, 90.3%, 63.6%, 91.1%, 77.3% and 86.3%, and for the race-free CKD-EPI 2021 equation were 88.4%, 82.1%, 70.2%, 77.2%, 83.4%, 85.6%, 71.6%, 84.2%, 70.6%, 87.0%, 78.3% and 83.5%. Figure 1 presents the correct classification values. Overall, the race-free CKD-EPI 2021 equation showed the lowest performance in estimating mGFR (see supplementary table 13). Supplementary table 14 shows the confidence intervals for the P30 and correct classification values, and supplementary table 15 shows the confidence intervals for the P10 values.
Fig 1.
P30 (proportion of eGFR within 30% of mGFR) and correct classification (agreement between eGFR and mGFR according to GFR stages) metrics for four GFR equations in development cohort and external validation cohorts. eGFR was calculated on the basis of recipient creatinine level, age, sex, and race (if required by the equation). ABCAN=Angiotensin II Blockade for Chronic Allograft Nephropathy; BENEFIT=Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial; BENEFIT-EXT=Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial-EXTended criteria donors; CKD-EPI=Chronic Kidney Disease Epidemiology Collaboration; GFR=glomerular filtration rate; eGFR=estimated glomerular filtration rate; KRS=kidney recipient specific; mGFR=measured glomerular filtration rate; MDRD=Modification of Diet in Renal Disease
Development of a kidney recipient specific GFR equation
Based on the metrics used to evaluate the model fit and equation prediction performances, we developed a new race-free kidney recipient specific GFR equation. When we investigated the impact of using race in the equation, performances remained unchanged (see supplementary figures 3-6). The best model was based on an additive model (see equation). This equation showed a good adjusted R2 of 0.73 and a good root mean square error of 0.18. Supplementary table 8 presents the different metrics used for evaluating the model’s fit, and supplementary table 7 presents the steps for developing the equation. Supplementary table 16 shows the final model.

The use of Lasso regressions for model selection was not associated with an increase in the performances of the model (P30 of 89.8%). Instead, using Lasso regressions resulted in more a complex and thus less clinically implementable and usable model (see supplementary table 17).
Performances of the race-free kidney recipient specific GFR equation
Comparison with current GFR equations
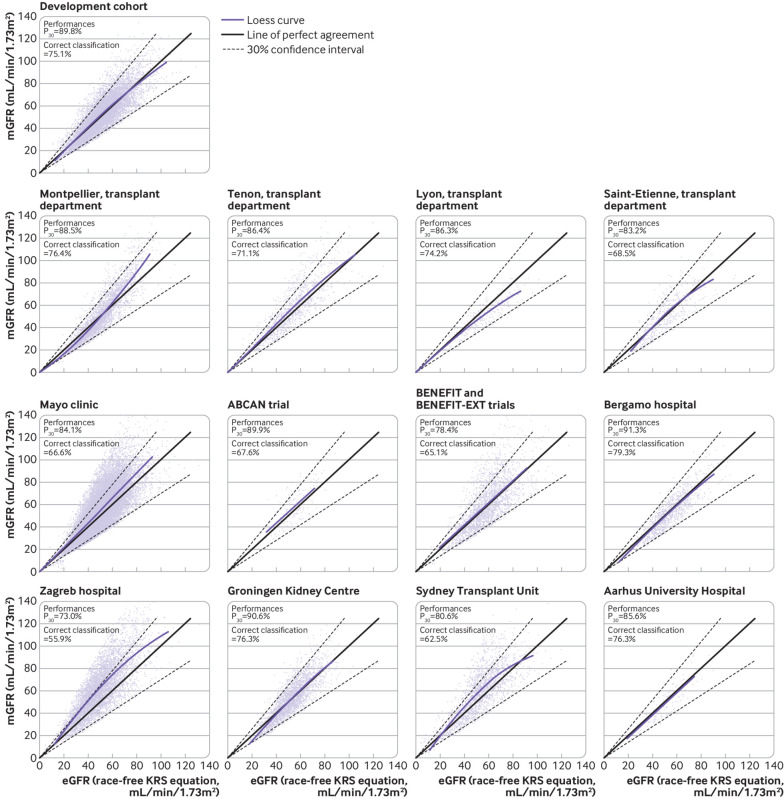
The P30 and correct classification values for the race-free kidney recipient specific GFR equation in the development cohort were 89.8% and 75.1%, respectively (fig 1 and fig 2). The P30 of the race-free kidney recipient specific GFR equation in the external validation centres was 88.5% in Montpellier, 86.4% in Tenon, 86.3% in Lyon, 83.2% in Saint-Etienne, 84.1% in the Mayo Clinic, 90.6% in the ABCAN trial, 78.4% in BENEFIT, 91.3% in Bergamo, 73.0% in Zagreb, 90.6% in Groningen, 80.6% in Sydney, and 85.6% in Aarhus. Figure 1 shows the correct classification values.
Fig 2.
Distribution of eGFR according to mGFR in development cohort and external validation cohorts. eGFR was calculated with the kidney recipient specific GFR equation on the basis of recipient creatinine level, age, and sex. P30 is the proportion of eGFR within 30% of mGFR. Correct classification is the agreement between eGFR and mGFR according to GFR stages. ABCAN=Angiotensin II Blockade for Chronic Allograft Nephropathy; BENEFIT=Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial; BENEFIT-EXT=Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial-EXTended criteria donors; CKD-EPI=Chronic Kidney Disease Epidemiology Collaboration; GFR=glomerular filtration rate; eGFR=estimated glomerular filtration rate; KRS=kidney recipient specific; mGFR=measured glomerular filtration rate
Overall, the performances of the race-free kidney recipient specific GFR equation were similar to those of the Modification of Diet in Renal Disease equation, which uses a correction factor for race (P=0.85), but showed better performance than the CKD-EPI 2009 (P=0.04) and race-free CKD-EPI 2021 (P=0.003) equations (see supplementary table 13). On the basis of these findings we developed an online application that estimates GFR based on recipient’s creatinine level, age, and sex (https://transplant-prediction-system.shinyapps.io/eGFR_equation_KTX/).
Performances in black and non-black patients
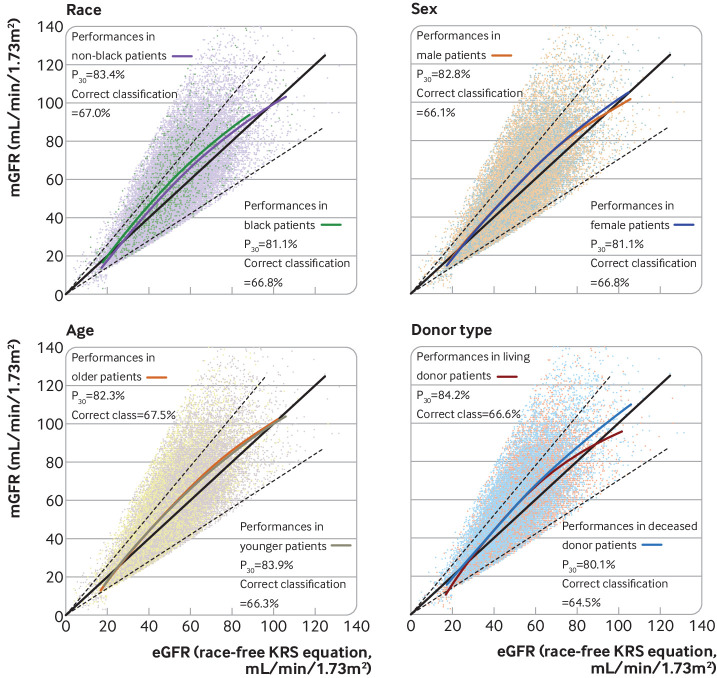
Overall, 598 black patients were included in the external validation cohorts. When we focused on centres with black patients, the P30 of the race-free kidney recipient specific GFR equation for non-black patients was 88.5% for Montpellier, 87.5% for Tenon, 78.6% for BENEFIT, and 84.4% for the Mayo Clinic, whereas for black patients the corresponding values were 88.7%, 83.6%, 76.6%, and 74.1%. Supplementary figures 5 and 6 show the correct classification values. Overall, the race-free kidney recipient specific GFR equation performed better than the current race-free CKD-EPI 2021 equation (see supplementary figures 5 and 6). The race-free kidney recipient specific GFR equation was found to slightly underestimate mGFR in both black and non-black patients (fig 3). We also found that the race-free kidney recipient specific GFR equation performed well in people from other ethnicities, such as Arab, Indian, people of other Asian origin, and Hispanic patients from the French cohorts and the Mayo clinic centres (see supplementary figure 7). To have a better sense of the performance of the race-free kidney recipient specific GFR equation, see supplementary figure 8 for Bland-Altman plots for each cohort.
Fig 3.
P30 (proportion of eGFR within 30% of mGFR) and correct classification (agreement between eGFR and mGFR according to GFR stages) metrics for four GFR equations in external validation cohorts when stratified by race, sex, age (threshold set at median), and donor type. eGFR was calculated with race-free kidney recipient specific GFR equation on basis of recipient creatinine level, age, and sex. Supplementary figures 5-35 show comparisons with Modification of Diet in Renal Disease, CKD-EPI 2009, and race-free CKD-EPI 2021 equations. eGFR=estimated glomerular filtration rate; KRS=kidney recipient specific; mGFR=measured glomerular filtration rate
Subpopulations and treatment
We investigated the prediction performance of the race-free kidney recipient specific GFR equation in a series of key subpopulations. Overall, the equation showed good prediction performances in male patients (supplementary figure 9), female patients (supplementary figure 10), older patients (supplementary figure 11), younger patients (supplementary figure 12), underweight patients (supplementary figure 13), patients with normal weight (supplementary figure 14), overweight patients (supplementary figure 15), obese patients (supplementary figure 16), patients with creatinine values measured using the enzymatic method (supplementary figure 17), patients with creatinine values measured using the colorimetric method (supplementary figure 18), patients with GFR values measured using 99Tc-DTPA clearance (supplementary figure 19), patients with GFR values measured using 51Cr-EDTA clearance (supplementary figure 20), patients with GFR values measured using inulin clearance (supplementary figure 21), patients with GFR values measured using iohexol clearance (supplementary figure 22), patients with GFR values measured using iothalamate clearance (supplementary figure 23), patients with an allograft from a living donor (supplementary figure 24), patients with an allograft from a deceased donor (supplementary figure 25), patients with an allograft from a younger donor (supplementary figure 26), patients with an allograft from an older donor (supplementary figure 27), patients whose age discrepancy with the donor was >10 years (supplementary figure 28), patients whose age discrepancy with the donor was <10 years (supplementary figure 29), patients treated with calcineurin inhibitor based regimens (supplementary figures 30 and 32), patients treated with mammalian target of rapamycin inhibitor based regimens (supplementary figures 31 and 32), patients treated with belatacept (supplementary figure 33), patients with GFR values measured before one year post-transplantation (supplementary figure 34), patients with GFR values measured after one year post-transplantation (supplementary figure 35), patients with pre-emptive transplantation (supplementary figure 36), and patients who received a transplant after initiation of dialysis (supplementary figure 36). Overall, for all subpopulations, the race-free kidney recipient specific GFR equation performed better than the race-free CKD-EPI 2021 equation.
Discussion
In this development and validation study comprising 15 489 kidney transplant recipients and 50 464 GFR measurements, we developed a new race-free kidney recipient specific GFR equation to estimate GFR. We compared the performance of this equation with those of the three current main GFR equations developed in individuals with native kidneys and concluded that the race-free kidney recipient specific GFR equation performed better in kidney transplant recipients than the current GFR equations. The newly developed equation meets a specific requirement for this population and avoids the need to include race as a variable compared with historical models.
Differences between native and transplanted kidneys
In 2021, the KDIGO consortium launched a series of conferences to improve the management of kidney allografts.13 One of the recognised key issues of this initiative was the fundamental differences between individuals with native kidneys and those with transplanted kidneys regarding creatinine metabolism and secretion and therefore the effect on GFR estimation. The frequent use of corticosteroids in kidney transplant recipients causes a direct catabolic effect43 leading to a lower skeletal muscle mass and thereby altering muscle mass to total body weight ratio.44 Abnormal muscle mass also results from other catabolic events common in kidney transplant recipients, including recurrent infections and acute rejection episodes.16 In addition, creatinine secretion in kidney transplant recipients is blocked by commonly used drugs such as trimethoprim-sulfamethoxazole,45 and through chronic rejection and acute tubular necrosis episodes.17 Kidney transplant recipients therefore represent a complex, distinct population in terms of comorbidities46 and determinants of kidney failure and death.4 Overall, this multidimensional complexity justified the need to develop an equation to estimate GFR in kidney transplant recipients and might explain our findings of lower performances of the GFR equations developed in individuals with native kidneys.
Use of race
The use of race for estimating kidney function has been questioned recently.47 It has been suggested that including a race coefficient might delay referral to a nephrologist.21 48 Studies have also shown that not including race can lead to systematic misclassification23 and overestimation of CKD diagnosis in black people, and thus overtreatment and increased healthcare costs.24 49 Another study also found that in patients with cancer the removal of race from the GFR equation led to undertreatment of black patients and negatively affected their outcomes.50 Therefore we investigated the impact of race on the kidney recipient specific GFR equation. Interestingly, the inclusion of race in our equation was not associated with a statistically significant increase in performance. Based on this finding, our equation is race-free, which is consistent with our finding that the race-free kidney recipient specific GFR equation performs well in black patients. Furthermore, the equation performed better than the race-free CKD-EPI 2021 equation in European black patients but had similar performances in North American black patients, suggesting that these two populations may differ.
Bias in the bias metric
One of the reference metrics for assessing the performance of GFR equations is the median bias, which is the raw median difference between the mGFR and eGFR. The median bias is, however, prone to bias itself as it is unequal between low and high mGFR values. This bias in the median bias was highlighted in a seminal study in 2020,24 in which the CKD-EPI equations were found to underestimate mGFR at values lower than 60 mL/min/1.73m2 and overestimate mGFR at values higher than 60 mL/min/1.73m2. Since there are more values higher than 60 mL/min/1.73m2, the bias metric indicates that the CKD-EPI equations overall overestimate mGFR, which is not the case at lower values. This bias is also visible in a recent study with new race-free equations.22 For these reasons, we chose not to use the median bias and focused on the P30 and correct classification, which are more reliable metrics. We acknowledge that in several centres the race-free kidney recipient specific GFR equation underestimated mGFR, especially at higher levels.
Variable selection
To avoid producing a biased model from use of stepwise procedures only, or univariate evaluation of associations,51 52 we adopted an alternative variable selection approach comprising stepwise procedures, univariate evaluation of associations, the investigation of many combinations of parameters based on the literature search performed in the present study, and the experience of the numerous nephrologists and researchers involved in the study. Besides, the Lasso penalised regressions were not used to generate the final GFR equation because the corresponding performances were similar, with a higher number of parameters identified in the final model, which would be more difficult to implement and use in clinical practice.
Performances of GFR equation and Americentrism
We showed that the performance of the race-free kidney recipient specific GFR equation, developed on the basis of a great number of GFR measurements, was good regardless of recipient race, sex, age, body mass index, donor type, donor age, age discrepancy between donor and recipient, treatments, whether transplantation was pre-emptive or after the initiation of dialysis, and method used to measure creatinine level and GFR. In addition, our equation was externally validated in France, Europe, Australia, and the US. This last point is crucial as “Americentrism” in GFR equations—the current GFR equations were mainly developed in North American patients—may hamper generalisability.10
Clinical and policy implication
Based on these results, we think that that the race-free kidney recipient specific GFR equation could contribute to more accurate clinical decisions for kidney transplant recipients. In particular, our equation may be of interest in clinical trials, as the US Food and Drug Administration and European Medicines Agency are currently willing to consider a decrease in GFR as a surrogate endpoint for kidney failure for clinical trials.53
Limitations of this study
This study has limitations. Firstly, we could not develop equations based on cystatin C, or use the new race-free cystatin C based equations, because none of the included centres performed routine cystatin C assessments. Nevertheless, most transplant centres worldwide do not perform cystatin C assessments, making our equation well adapted to current clinical practice. Secondly, we did not have data on genetic ancestry markers, which are not available for most cohorts worldwide. We therefore used the binary approach of race (black versus non-black), as in previous studies developing GFR equations. However, we showed that our race-free equation performed well in black and non-black patients. We also showed in sensitivity analysis that our equation performed well in additional ethnicities, comprising people of Arab, Indian, and other Asian origin, and Hispanic people. Thirdly, we acknowledge the low number of black patients included in the study. In addition, we validated the race-free kidney recipient specific GFR equation in European and American patients. Further validating the equation in other transplant centres, such as South American or Asian transplant centres, is important. Moreover, although we presented the performances of the GFR equations according to immunosuppressive treatment, we could not retrieve these data for most of the external validation cohorts. Further investigations are needed to determine the impact of immunosuppressive treatment on the race-free kidney recipient specific GFR equation. Lastly, although patients were not involved in the study design and conduct, we plan to involve patient associations such as RENALOO and FNAIR for the dissemination of the study findings, given the important impact the race-free kidney recipient specific GFR equation will have on transplant healthcare.
Conclusion
We developed and validated a new race-free kidney recipient specific GFR equation, which showed good performance. In particular, the race-free kidney recipient specific GFR equation performs better than the race-free CKD-EPI 2021 equation developed in individuals with native kidneys and was validated in various countries and subpopulations. Our equation is based on creatinine measurement, as most transplant centres do not assess cystatin C in routine clinical practice. Further studies using cystatin C might help improve the performance of the equation. Based on these results, we think that the race-free kidney recipient specific GFR equation may contribute to more accurate and confident clinical decision making in the daily care of kidney transplant recipients.
What is already known on this topic
Accurate prediction of glomerular filtration rate (GFR) is crucial for the management and monitoring of kidney transplant recipients; however, the current GFR equations were developed in patients with native kidneys, and “Americentrism” in estimation of GFR equations also may be a concern
The Kidney Disease: Improving Global Outcomes consortium has advocated for new race-free equations in kidney transplant recipients
In the previous studies that developed kidney transplant specific GFR equations, validation of the equations was hampered by methodological shortcomings such as single centre design, small cohort size, and lack of external validation sets
What this study adds
A newly developed race-free kidney recipient specific GFR equation in well phenotyped cohorts across France, Europe, Oceania, and the US, performed better than the current race-free Chronic Kidney Disease Epidemiology Collaboration 2021 equation
The race-free kidney recipient specific GFR equation should help in the adoption of race-free GFR equations without impacting the prediction of GFR in black patients
Overall, because of the high performances in various countries and subpopulations, the race-free kidney recipient specific GFR equation may help improve monitoring and patient risk stratification worldwide
Acknowledgments
We thank Andrew S Levey and Lesley A Inker for their feedback on the methods and findings of the manuscript and Radu Vadanici for providing data from BENEFIT and BENEFIT-EXT.
Web extra.
Extra material supplied by authors
Supplementary information: Additional methods, tables 1-17, and figures 1-36
Contributors: MR and SAA contributed equally to the article as co-first authors. AJB and AL contributed to the article as co-last authors. MR and AL designed and supervised the study. IJ, GD, YL, NBJ, LD, IM, CM, DP, VP, MLQ, TL, MS, BB, PR, LM, HI, MBN, AM, BN, SB, RP, SBa, CL, NK, BS, HW, AD, FV, GR, CLeg, AB, and AL collected the data. MR, SAA, and AL analysed the data and wrote the original draft of the manuscript. MR, SAA, IJ, GD, YL, NBJ, LD, IM, CM, DP, VP, MLQ, TL, MS, BB, PR, LM, HI, MBN, AM, BN, SB, RP, SBa, XJ, CL, NK, BS, HW, AD, FV, GR, CLeg, AB, and AL reviewed and edited the manuscript. MR and AL are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study received financial support from the French government through the National Research Agency under the programme “Investissements d’avenir” KTD-Innov (grant ANR-17-RHUS-0010) and the European Union’s Horizon Europe 2020 research and innovation programme EU-TRAIN (grant No 754995). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the French government through the National Research Agency and the European Union’s Horizon Europe programme; AL holds shares in Cibiltech, a company that develops software; No other relationships or activities that could appear to have influenced the submitted work.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The use of deidentified data precludes direct dissemination to participants. We plan to share the work with public communities through a press release, social media, scientific conferences, and medical and nephrology clinical presentations. Results will also be disseminated by all co-authors through their home institutions.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Each patient from the Paris Transplant Group cohort provided written informed consent to be included in the Paris Transplant Group database. This database has been approved by the National French Commission for Bioinformatics, Data, and Patient Liberty (CNIL registration No 363505, validated 3 April 1996). The institutional review boards of the Paris Transplant Group participating centres approved the study.
Data availability statement
Data are available from the corresponding author at alexandreloupy@gmail.com upon reasonable request for research purpose only. Deidentified participant level data from the development cohort will be made available upon reasonable request. Requests will be assessed by the members of the Paris Transplant Group. For validation cohorts, data access is not covered by our data transfer agreements.
References
- 1. Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Measured and estimated glomerular filtration rate: current status and future directions. Nat Rev Nephrol 2020;16:51-64. 10.1038/s41581-019-0191-y [DOI] [PubMed] [Google Scholar]
- 2. Nitsch D, Grams M, Sang Y, et al. Chronic Kidney Disease Prognosis Consortium . Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ 2013;346:f324. 10.1136/bmj.f324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coemans M, Süsal C, Döhler B, et al. Analyses of the short- and long-term graft survival after kidney transplantation in Europe between 1986 and 2015. Kidney Int 2018;94:964-73. 10.1016/j.kint.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 4. Loupy A, Aubert O, Orandi BJ, et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: international derivation and validation study. BMJ 2019;366:l4923. 10.1136/bmj.l4923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clayton PA, Lim WH, Wong G, Chadban SJ. Relationship between eGFR Decline and Hard Outcomes after Kidney Transplants. J Am Soc Nephrol 2016;27:3440-6. 10.1681/ASN.2015050524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Josephson MA. Monitoring and managing graft health in the kidney transplant recipient. Clin J Am Soc Nephrol 2011;6:1774-80. 10.2215/CJN.01230211 [DOI] [PubMed] [Google Scholar]
- 7. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461-70. 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 8. Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inker LA, Schmid CH, Tighiouart H, et al. CKD-EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20-9. 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delanaye P, Pottel H, Glassock RJ. Americentrism in estimation of glomerular filtration rate equations. Kidney Int 2022;101:856-8. 10.1016/j.kint.2022.02.022 [DOI] [PubMed] [Google Scholar]
- 11. Flamant M, Vidal-Petiot E, Metzger M, et al. NephroTest Study Group . Performance of GFR estimating equations in African Europeans: basis for a lower race-ethnicity factor than in African Americans. Am J Kidney Dis 2013;62:182-4. 10.1053/j.ajkd.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 12. Masson I, Flamant M, Maillard N, et al. MDRD versus CKD-EPI equation to estimate glomerular filtration rate in kidney transplant recipients. Transplantation 2013;95:1211-7. 10.1097/TP.0b013e318288caa6 [DOI] [PubMed] [Google Scholar]
- 13.KDIGO Controversies Conference on Challenges in Management of the Kidney Allograft: From Decline to Failure. https://kdigo.org/wp-content/uploads/2021/05/KDIGO-Challenging-Allograft-Conference_Scope-for-Public-Review.pdf. [DOI] [PubMed]
- 14. Murata K, Baumann NA, Saenger AK, Larson TS, Rule AD, Lieske JC. Relative performance of the MDRD and CKD-EPI equations for estimating glomerular filtration rate among patients with varied clinical presentations. Clin J Am Soc Nephrol 2011;6:1963-72. 10.2215/CJN.02300311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raynaud M, Aubert O, Reese PP, et al. Trajectories of glomerular filtration rate and progression to end stage kidney disease after kidney transplantation. Kidney Int 2021;99:186-97. 10.1016/j.kint.2020.07.025 [DOI] [PubMed] [Google Scholar]
- 16. Nankivell BJ, Gruenewald SM, Allen RD, Chapman JR. Predicting glomerular filtration rate after kidney transplantation. Transplantation 1995;59:1683-9. 10.1097/00007890-199506270-00007 [DOI] [PubMed] [Google Scholar]
- 17. Santos J, Martins LS. Estimating glomerular filtration rate in kidney transplantation: Still searching for the best marker. World J Nephrol 2015;4:345-53. 10.5527/wjn.v4.i3.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. White CA, Huang D, Akbari A, Garland J, Knoll GA. Performance of creatinine-based estimates of GFR in kidney transplant recipients: a systematic review. Am J Kidney Dis 2008;51:1005-15. 10.1053/j.ajkd.2008.02.308 [DOI] [PubMed] [Google Scholar]
- 19. Salvador CL, Hartmann A, Åsberg A, Bergan S, Rowe AD, Mørkrid L. Estimating Glomerular Filtration Rate in Kidney Transplant Recipients: Comparing a Novel Equation With Commonly Used Equations in this Population. Transplant Direct 2017;3:e332. 10.1097/TXD.0000000000000742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int 2006;69:399-405. 10.1038/sj.ki.5000073 [DOI] [PubMed] [Google Scholar]
- 21. Diao JA, Inker LA, Levey AS, Tighiouart H, Powe NR, Manrai AK. In Search of a Better Equation - Performance and Equity in Estimates of Kidney Function. N Engl J Med 2021;384:396-9. 10.1056/NEJMp2028243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inker LA, Eneanya ND, Coresh J, et al. Chronic Kidney Disease Epidemiology Collaboration . New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med 2021;385:1737-49. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsu CY, Yang W, Parikh RV, et al. CRIC Study Investigators . Race, Genetic Ancestry, and Estimating Kidney Function in CKD. N Engl J Med 2021;385:1750-60. 10.1056/NEJMoa2103753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levey AS, Tighiouart H, Titan SM, Inker LA. Estimation of Glomerular Filtration Rate With vs Without Including Patient Race. JAMA Intern Med 2020;180:793-5. 10.1001/jamainternmed.2020.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raynaud M, Aubert O, Loupy A. New Equations for Estimating the GFR without Race. N Engl J Med 2022;386:1669-70. 10.1056/NEJMc2119761 [DOI] [PubMed] [Google Scholar]
- 26. Inker LA, Eneanya ND, Coresh J, et al. Chronic Kidney Disease Epidemiology Collaboration . New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med 2021;385:1737-49. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010;10:535-46. 10.1111/j.1600-6143.2009.03005.x [DOI] [PubMed] [Google Scholar]
- 28. Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant 2010;10:547-57. 10.1111/j.1600-6143.2010.03016.x [DOI] [PubMed] [Google Scholar]
- 29. Ibrahim HN, Jackson S, Connaire J, et al. Angiotensin II blockade in kidney transplant recipients. J Am Soc Nephrol 2013;24:320-7. 10.1681/ASN.2012080777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 2009;20:2305-13. 10.1681/ASN.2009020171 [DOI] [PubMed] [Google Scholar]
- 31. Speeckaert MM, Seegmiller J, Glorieux G, et al. Measured Glomerular Filtration Rate: The Query for a Workable Golden Standard Technique. J Pers Med 2021;11:949. 10.3390/jpm11100949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Medeiros FS, Sapienza MT, Prado ES, et al. Validation of plasma clearance of 51Cr-EDTA in adult renal transplant recipients: comparison with inulin renal clearance. Transpl Int 2009;22:323-31. 10.1111/j.1432-2277.2008.00799.x [DOI] [PubMed] [Google Scholar]
- 33. Soveri I, Berg UB, Björk J, et al. SBU GFR Review Group . Measuring GFR: a systematic review. Am J Kidney Dis 2014;64:411-24. 10.1053/j.ajkd.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 34. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5:303-11, discussion 312-3. [PubMed] [Google Scholar]
- 35. Delgado C, Baweja M, Crews DC, et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis 2022;79:268-288.e1. 10.1053/j.ajkd.2021.08.003 [DOI] [PubMed] [Google Scholar]
- 36.Guidelines CP. For Chronic Kidney Disease: Evaluation, Classification and Stratification. https://www.kidney.org/sites/default/files/docs/ckd_evaluation_classification_stratification.pdf.
- 37. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015;350:g7594. 10.1136/bmj.g7594 [DOI] [PubMed] [Google Scholar]
- 38. Schneider A, Hommel G, Blettner M. Linear regression analysis: part 14 of a series on evaluation of scientific publications. Dtsch Arztebl Int 2010;107:776-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michael H. Kutner CJN, John Neter, William Li. Applied Linear Statistical Models. https://users.stat.ufl.edu/~winner/sta4211/ALSM_5Ed_Kutner.pdf.
- 40. Pottel H, Björk J, Courbebaisse M, et al. Development and Validation of a Modified Full Age Spectrum Creatinine-Based Equation to Estimate Glomerular Filtration Rate : A Cross-sectional Analysis of Pooled Data. Ann Intern Med 2021;174:183-91. 10.7326/M20-4366 [DOI] [PubMed] [Google Scholar]
- 41. Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089-100. 10.1111/j.1523-1755.2005.00365.x [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization - Body mass index. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
- 43. Horber FF, Scheidegger J, Frey FJ. Overestimation of renal function in glucocorticosteroid treated patients. Eur J Clin Pharmacol 1985;28:537-41. 10.1007/BF00544064 [DOI] [PubMed] [Google Scholar]
- 44. El Haggan W, Hurault de Ligny B, Partiu A, et al. The evolution of weight and body composition in renal transplant recipients: Two-year longitudinal study. Transplant Proc 2006;38:3517-9. 10.1016/j.transproceed.2006.10.121 [DOI] [PubMed] [Google Scholar]
- 45. Berglund F, Killander J, Pompeius R. Effect of trimethoprim-sulfamethoxazole on the renal excretion of creatinine in man. J Urol 1975;114:802-8. 10.1016/S0022-5347(17)67149-0 [DOI] [PubMed] [Google Scholar]
- 46. Wu C, Evans I, Joseph R, et al. Comorbid conditions in kidney transplantation: association with graft and patient survival. J Am Soc Nephrol 2005;16:3437-44. 10.1681/ASN.2005040439 [DOI] [PubMed] [Google Scholar]
- 47. Ioannidis JPA, Powe NR, Yancy C. Recalibrating the Use of Race in Medical Research. JAMA 2021;325:623-4. 10.1001/jama.2021.0003 [DOI] [PubMed] [Google Scholar]
- 48. Eneanya ND, Yang W, Reese PP. Reconsidering the Consequences of Using Race to Estimate Kidney Function. JAMA 2019;322:113-4. 10.1001/jama.2019.5774 [DOI] [PubMed] [Google Scholar]
- 49. Vilson FL, Schmidt B, White L, et al. Removing Race from eGFR calculations: Implications for Urologic Care. Urology 2022;162:42-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Casal MA, Ivy SP, Beumer JH, Nolin TD. Effect of removing race from glomerular filtration rate-estimating equations on anticancer drug dosing and eligibility: a retrospective analysis of National Cancer Institute phase 1 clinical trial participants. Lancet Oncol 2021;22:1333-40. 10.1016/S1470-2045(21)00377-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walter S, Tiemeier H. Variable selection: current practice in epidemiological studies. Eur J Epidemiol 2009;24:733-6. 10.1007/s10654-009-9411-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Talbot D, Massamba VK. A descriptive review of variable selection methods in four epidemiologic journals: there is still room for improvement. Eur J Epidemiol 2019;34:725-30. 10.1007/s10654-019-00529-y [DOI] [PubMed] [Google Scholar]
- 53. Levey AS, Gansevoort RT, Coresh J, et al. Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration With the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 2020;75:84-104. 10.1053/j.ajkd.2019.06.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Additional methods, tables 1-17, and figures 1-36
Data Availability Statement
Data are available from the corresponding author at alexandreloupy@gmail.com upon reasonable request for research purpose only. Deidentified participant level data from the development cohort will be made available upon reasonable request. Requests will be assessed by the members of the Paris Transplant Group. For validation cohorts, data access is not covered by our data transfer agreements.