Abstract
Background and Aims
Vitamin D has a regulatory role in innate and adaptive immune processes. Previous studies have reported that low pretreatment vitamin D concentrations are associated with primary non-response (PNR) and non-remission to anti-TNF therapy. This study aimed to assess whether pretreatment 25-hydroxyvitamin D concentrations predicted PNR and non-remission to infliximab and adalimumab in patients with active luminal Crohn’s disease.
Methods
25-Hydroxyvitamin D concentrations were measured in stored baseline samples from 659 infliximab- and 448 adalimumab-treated patients in the Personalised Anti-TNF Therapy in Crohn’s disease (PANTS) study. Cut-offs for vitamin D were deficiency <25 nmol/L, insufficiency 25–50 nmol/L, and adequacy/sufficiency >50 nmol/L.
Results
About 17.1% (189/1107; 95% CI, 15.0–19.4) and 47.7% (528/1107; 95% CI, 44.8–50.6) of patients had vitamin D deficiency and insufficiency, respectively. 22.2% (246/1107) of patients were receiving vitamin D supplementation. Multivariable analysis confirmed that sampling during non-summer months, South Asian ethnicity, lower serum albumin concentrations, and non-treatment with vitamin D supplementation were independently associated with lower vitamin D concentrations. Pretreatment vitamin D status did not predict response or remission to anti-TNF therapy at week 14 (infliximab Ppnr = .89, adalimumab Ppnr = .18) or non-remission at week 54 (infliximab P = .13, adalimumab P = .58). Vitamin D deficiency was, however, associated with a longer time to immunogenicity in patients treated with infliximab, but not adalimumab.
Conclusions
Vitamin D deficiency is common in patients with active Crohn’s disease. Unlike previous studies, pretreatment vitamin D concentration did not predict PNR to anti-TNF treatment at week 14 or nonremission at week 54.
Keywords: vitamin D, IBD, Crohn’s disease, PANTS
Background
By binding to the vitamin D receptor expressed on most immune cells, vitamin D has a key regulatory role in innate and adaptive immune processes. Relevant to the pathogenesis of inflammatory bowel disease (IBD), in animal and in vitro experimental models, vitamin D modulates tight junctions, maintaining intestinal epithelial integrity and regulating host–microbiota interactions.1
Patients with IBD have multiple risk factors for vitamin D deficiency including chronic diarrhea, bile salt malabsorption, dietary restrictions, and reduced sunlight exposure. Consequently, vitamin D deficiency is more common than in the general population,2–4 and while it does not cause IBD,5–8 because of the link with active disease,9–12 there is considerable interest in the role of vitamin D as an adjunct to IBD therapies.13
Over the last 3 decades, the anti-TNF monoclonal antibodies, infliximab and adalimumab, have become the most frequently prescribed biologics for immune-mediated inflammatory diseases. Unfortunately, anti-TNF treatment failure in patients with Crohn’s disease is common: One-quarter of patients experience primary nonresponse, one-third of responders lose response, and only 40% of patients are in remission at the end of a year.14,15 Anti-TNF monotherapy, obesity, smoking, disease severity, and the development of antidrug antibodies are associated with low drug concentrations and subsequent anti-TNF treatment failure.15,16 Carriage of the HLA-DQA1*05 allele confers a 2-fold risk of developing antibodies to anti-TNF treatment.17,18
In small retrospective studies, vitamin D deficiency has been associated with primary non-response, non-remission, and durability of anti-TNF therapy.19–21 We sought to assess whether pretreatment 25-hydroxyvitamin D concentrations predicted primary non-response and non-remission to infliximab and adalimumab in patients with Crohn’s disease.
Methods
Study Design
The Personalised Anti-TNF Therapy in Crohn’s Disease study (PANTS) is a UK-wide, multicentre, prospective observational cohort reporting the treatment failure rates of the anti-TNF drugs infliximab (originator, Remicade [Merck Sharp & Dohme, UK] and biosimilar, CT-P13 [Celltrion, South Korea]) and adalimumab (Humira [Abbvie, USA]) in anti-TNF-naïve patients with active luminal Crohn’s disease.15
Patients were recruited between February 2013 and June 2016 at the time of first anti-TNF exposure and studied for 12 months or until drug withdrawal. After 12 months, patients were invited to continue follow-up for a further 2 years. Eligible patients were aged ≥6 years with objective evidence of active luminal Crohn’s disease involving the colon and/or small intestine. Exclusion criteria included prior exposure to, or contraindications for the use of, anti-TNF therapy. The choice of anti-TNF was at the discretion of the treating physician and prescribed according to the licensed dosing schedule. Study visits were scheduled at the first dose, week 14, and at weeks 30 and 54. Additional visits were planned for infliximab-treated patients at each infusion and for both groups at treatment failure or exit.
For this analysis, we included all patients who had stored serum available from baseline visits and effectiveness outcomes. Patients were excluded from our effectiveness analysis if they had a stoma as Harvey Bradshaw Index (HBI) and short pediatric Crohn’s disease activity index (sPCDAI) scores have not been validated in these patient groups. Patients who were recruited into the study with normal prescreening visit 1 fecal calprotectin and C-reactive protein (CRP) levels, and where the only indication for anti-TNF was perianal disease, were also excluded.
Outcomes
Treatment failure endpoints were primary non-response at week 14, non-remission at week 54, and adverse events leading to drug withdrawal.
We used composite endpoints using the HBI in adults and the sPCDAI in children, corticosteroid use, and CRP to define primary non-response (Figure S1). Remission was defined as CRP of ≤3 mg/L and HBI of ≤4 points (sPCDAI ≤15 in children), without corticosteroid therapy or exit for treatment failure.
Secondary outcomes included anti-TNF drug concentration measured at weeks 14 and 54 and the time to development of anti-TNF antibodies. Drug persistence was defined as the duration of time from initiation of anti-TNF therapy to exit from the study due to treatment failure.
Patients exited the study when they stopped anti-TNF therapy or had an intestinal resection regardless of surgical outcome. They were deemed to be in non-remission for subsequent time points. Patients who declined to participate in the 2-year extension or who exited the study for loss to follow-up, withdrawal of consent, or elective withdrawal of drug, including for pregnancy, were censored at the time of study exit and excluded from the denominator for subsequent analyses.
Clinical Variables and Laboratory Analyses
Variables recorded at baseline by sites were demographics (age, sex, ethnicity, comorbidities, height and weight, and smoking status) and IBD phenotype and its treatments (age at diagnosis, disease duration, Montreal classification, prior medical and drug history, and previous Crohn’s disease-related surgeries). At every visit, disease activity score, weight, current therapy, and adverse events were recorded.
Blood and stool samples were processed through the central laboratory at the Royal Devon University Healthcare NHS Foundation Trust (https://www.exeterlaboratory.com/) for hemoglobin, white cell count, platelets, serum albumin, CRP, anti-TNF drug and antidrug antibody concentrations, and fecal calprotectin, respectively. Genotyping methods and the genetic analysis have been reported previously.17
25-Hydoxyvitamin D concentrations
Serum 25-hydoxyvitamin D was measured in baseline samples between January 22, 2020, and March 20, 2020, using the Elecsys 25-hydoxyvitamin electrochemiluminescence immunoassay (Roche) using the Cobas 801 module on the Cobas 8000 analyzer.22 This competitive electrochemiluminescence assay uses a ruthenium-complexed vitamin D binding protein to capture vitamin D3 (25-OH) and vitamin D2 (25-OH). The local reference range defines vitamin D deficiency as <25 nmol/L, insufficiency as 25–50 nmol/L, and adequacy/sufficiency >50 nmol/L. Preanalytical stability of serum 25-hydroxyvitamin D following long-term sample storage at up to −40 °C has been demonstrated previously.23,24
TNF drug-level assays
The IDKmonitor free infliximab (K9655) and adalimumab (K9657) drug-level assays permit quantitative measurement of free therapeutic drug in serum.15 The assays follow a standard ELISA format using a specific monoclonal antidrug antibody fragment as a capture antibody and peroxidase-labeled anti-human IgG antibody as a detection antibody. The measuring range for both assays is 0.8–45 mg/L, with the absence of the drug being defined using a cutoff of <0.8 mg/L.
Drug-tolerant anti-TNF antibody assays
Total antidrug antibody concentrations were measured with IDKmonitor® ELISA assays (Immundiagnostik AG, Bensheim, Germany) performed on the Dynex DS2 ELISA robot (Dynex technologies, Worthing, UK). The Immundiagnostik (IDK) AG (Bensheim, Germany) IDKmonitor infliximab (K9654) and adalimumab (K9651) total antidrug antibody assays allow semi-quantitative measurement of both free and bound antidrug antibodies.15 A pretreatment acid dissociation step is used to separate antidrug antibodies from the therapeutic antibody. The assay then follows a standard ELISA format using a recombinant therapeutic antibody as a capture and detection antibody. The positivity thresholds for the infliximab and adalimumab assays are 9 and 6 AU/mL, respectively.25
Study Size and Statistical Methods
The sample size calculation for the PANTS study has been reported previously.15 Here we included all patients who had sufficient stored serum from their baseline visit and had outcome data at week 14.
Statistical analyses were undertaken in R 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria). All tests were 2-tailed and P-values of <0.05 were considered significant. We included patients with missing clinical variables in analyses for which they had data and have specified the denominator for each variable. Continuous data are reported as median and interquartile range (IQR), and discrete data as numbers and percentages. We performed univariable analyses using Fisher’s exact, Mann–Whitney U, and Spearman’s rank tests to identify differences in baseline characteristics between infliximab- and adalimumab-treated patients, and to determine categorical and continuous factors associated with vitamin D levels and the predefined clinical outcomes above. Multivariable logistic regression analyses were used to confirm factors independently associated with vitamin D deficiency. Rates of immunogenicity and drug persistence were estimated using the Kaplan–Meier method, and comparative analyses were performed using univariable and multivariable Cox proportional hazards regression. Further sensitivity analyses using fecal calprotectin at week 54 as an outcome and stratifying the cohort by vitamin D supplementation and/or corticosteroid treatments at baseline were undertaken.
Results
Participants
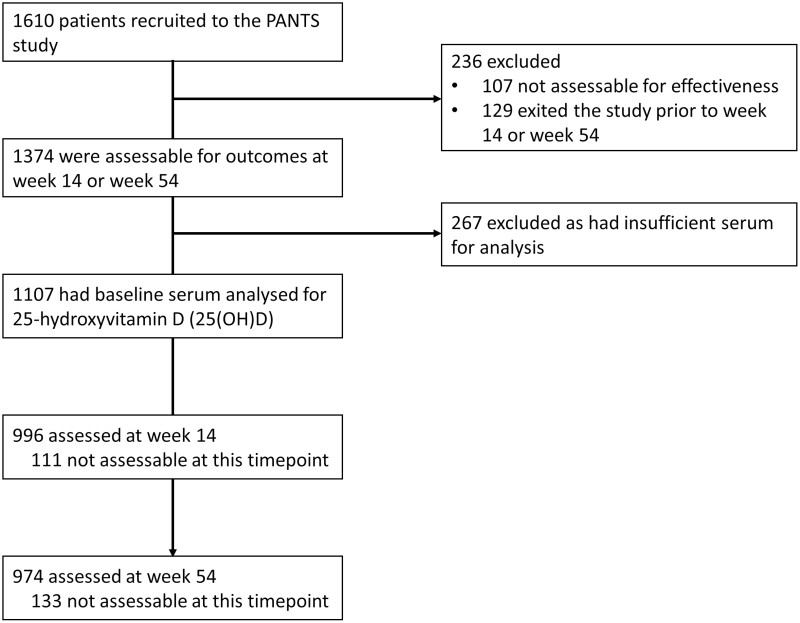
Overall, 80.6% (1107/1374) of patients who participated in the PANTS study who were assessable for effectiveness were included: 659 (59.5%) were treated with infliximab (526 [47.5%] with originator infliximab, and 133 [12.0%] with biosimilar CT-P13) and 448 (40.5%) were treated with adalimumab (Figure 1). At baseline, 22.2% (246/1107) patients were receiving a form of vitamin D supplementation, of whom 52.8% (130/246) were prescribed corticosteroids. Differences between demographic and clinical characteristics of infliximab- and adalimumab-treated patients are shown in Table 1.
Figure 1.
Study profile. Patients were not assessable when 1 or more of the key data items were missing.
Table 1.
Baseline demographic and clinical characteristics, stratified by anti-TNF.
| Variable | Level | Infliximab | Adalimumab | Overall | P |
|---|---|---|---|---|---|
| n | 659 | 448 | |||
| Sex | Female | 50.68% (334/659) | 53.57% (240/448) | 51.85% (574/1107) | .358 |
| Male | 49.32% (325/659) | 46.43% (208/448) | 48.15% (533/1107) | ||
| Ethnicity | White | 89.53% (590/659) | 96.65% (433/448) | 92.41% (1023/1107) | <.001 |
| South Asian | 5.01% (33/659) | 1.79% (8/448) | 3.70% (41/1107) | ||
| Other | 5.46% (36/659) | 1.56% (7/448) | 3.88% (43/1107) | ||
| Anti-TNF | Adalimumab | 0.00% (0/659) | 100.00% (448/448) | 40.47% (448/1107) | <.001 |
| CT-P13 | 20.18% (133/659) | 0.00% (0/448) | 12.01% (133/1107) | ||
| Remicade | 79.82% (526/659) | 0.00% (0/448) | 47.52% (526/1107) | ||
| Age at first dose of anti-TNF | 30.03 (18.95–44.69) | 38.60 (28.53–50.49) | 33.26 (22.76–47.29) | <.001 | |
| Age at first dose of anti-TNF < 18 | 22.91% (151/659) | 3.12% (14/448) | 14.91% (165/1107) | <.001 | |
| Disease duration | 2.08 (0.61–7.40) | 2.80 (0.74–10.52) | 2.26 (0.66–8.83) | .009 | |
| Montreal disease location | L1 | 28.09% (184/655) | 33.26% (147/442) | 30.17% (331/1097) | .306 |
| L2 | 24.12% (158/655) | 21.72% (96/442) | 23.15% (254/1097) | ||
| L3 | 46.72% (306/655) | 44.34% (196/442) | 45.76% (502/1097) | ||
| L4 | 1.07% (7/655) | 0.68% (3/442) | 0.91% (10/1097) | ||
| Montreal L4 modifier | 13.28% (87/655) | 4.52% (20/442) | 9.75% (107/1097) | <.001 | |
| Montreal disease behavior | B1 | 63.57% (417/656) | 57.79% (256/443) | 61.24% (673/1099) | <.001 |
| B2 | 25.91% (170/656) | 36.79% (163/443) | 30.30% (333/1099) | ||
| B3 | 10.52% (69/656) | 5.42% (24/443) | 8.46% (93/1099) | ||
| Perianal disease | 14.42% (95/659) | 7.81% (35/448) | 11.74% (130/1107) | <.001 | |
| Smoking history | Current | 14.18% (92/649) | 21.40% (95/444) | 17.11% (187/1093) | <.001 |
| Ex | 25.73% (167/649) | 35.59% (158/444) | 29.73% (325/1093) | ||
| Never | 60.09% (390/649) | 43.02% (191/444) | 53.16% (581/1093) | ||
| Body mass index (kg/m2) | 22.49 (19.55–27.06) | 24.28 (21.48–28.30) | 23.29 (20.31–27.68) | <.001 | |
| Baseline immunomodulator use | TRUE | 61.91% (408/659) | 51.79% (232/448) | 57.81% (640/1107) | .001 |
| Baseline steroid use | TRUE | 29.29% (193/659) | 27.01% (121/448) | 28.36% (314/1107) | .416 |
| C-reactive protein (mg/L) | 9.00 (3.00–23.00) | 7.00 (2.00–14.00) | 8.00 (3.00–19.00) | <.001 | |
| Fecal calprotectin (μg/g) | 458.00 (186.50–898.25) | 317.50 (141.50–629.00) | 372.50 (163.50–761.50) | <.001 | |
| Hemoglobin (g/L) | 125.00 (114.00–136.00) | 131.00 (120.00–142.00) | 127.00 (117.00–138.50) | <.001 | |
| Albumin (g/L) | 39.00 (34.00–42.00) | 40.00 (36.00–43.00) | 39.00 (34.00–42.00) | .003 | |
| Harvey Bradshaw Index | 6.00 (3.00–9.00) | 5.00 (3.00–8.00) | 5.00 (3.00–9.00) | .418 | |
| Short pediatric Crohn’s disease activity index | 25.0 (15.0–50.0) | NA | 25.0 (15.0–50.0) | NA | |
Similar to the whole cohort, there were significant differences at baseline between the infliximab- and adalimumab-treated patients, including in age, ethnicity, smoking, body mass index, disease duration, and disease behavior. Patients treated with infliximab had more active disease at baseline than patients treated with adalimumab, as evidenced by higher serum CRP and fecal calprotectin concentrations. At the initiation of anti-TNF treatment, immunomodulator use was higher in patients treated with infliximab compared to those treated with adalimumab, but there was no difference in the proportion of patients treated with corticosteroids.
Baseline Factors Associated With Vitamin D Concentrations
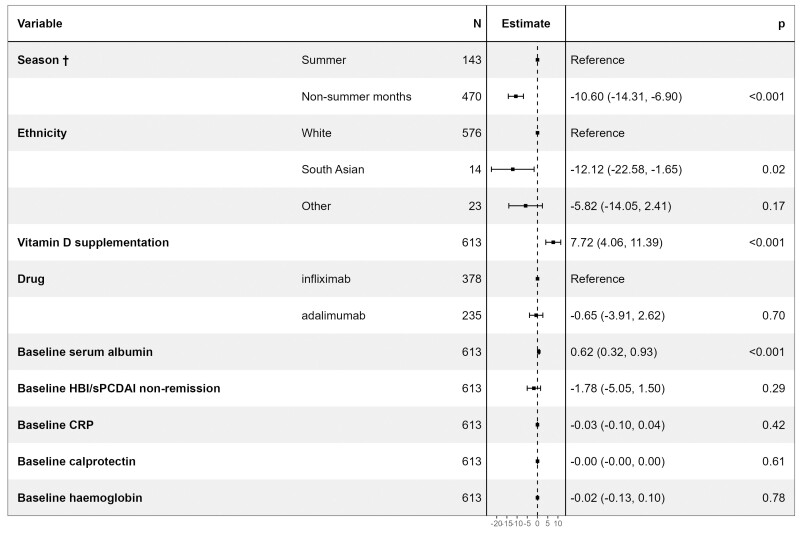
Median [IQR] vitamin D concentrations were lower in patients subsequently treated with infliximab than adalimumab (39.0 nmol/L [29.0–56.0] versus 44.0 nmol/L [31.0–59.0], P = .02). The other univariable factors associated with vitamin D concentrations are shown in Table 2 and Table S2. Multivariable linear regression analysis confirmed that baseline sampling during non-summer months (Figure S2), South Asian ethnicity, lower serum albumin concentrations, and nontreatment with vitamin D supplementation were independently associated with lower vitamin D concentrations (Figure 2).
Table 2.
Baseline demographic and clinical characteristics associated with vitamin D concentrations.
| Categorical variables | ||||
|---|---|---|---|---|
| Variable | Level | n | Vitamin D (nmol/L) | P |
| Month of sampling | Non-summer | 849 | 38.0 (27.0–54.0) | <.001 |
| Summera | 258 | 51.0 (39.0–65.0) | ||
| Ethnicity | South Asian | 41 | 30.0 (22.0–44.0) | .001 |
| White/Others | 1066 | 42.0 (30.0–58.0) | ||
| Pretreatment vitamin D supplementation | No | 861 | 39.0 (28.0–55.0) | <.001 |
| Yes | 246 | 50.0 (36.0–64.0) | ||
| Drug | Infliximab | 659 | 39.0 (29.0–56.0) | .021 |
| Adalimumab | 448 | 44.0 (31.0–59.0) | ||
| Continuous variables | ||||
|---|---|---|---|---|
| Variable | Spearman’s rho (R) | P | ||
| Serum albumin | 0.18 | <.001 | ||
| Harvey Bradshaw Index | −0.07 | .040 | ||
| Short pediatric Crohn’s disease activity index | −0.33 | .003 | ||
| CRPb | −0.12 | <.001 | ||
| Fecal calprotectinb | −0.11 | .003 | ||
| Hemoglobin | 0.10 | .002 | ||
aSampling during the summer was defined as a blood sample obtained for vitamin D analysis in the months of June, July, and August.
bVariables were log-transformed for analysis.
Abbreviation: CRP, C-reactive protein.
Figure 2.
Forest plot showing the coefficients from a multivariable linear regression model of associations with pretreatment vitamin D concentrations. The resultant values represent the change in vitamin D concentrations associated with each variable. CRP, C-reactive protein; HBI, Harvey Bradshaw Index; sPCDAI, short pediatric Crohn’s disease activity index. †Sampling during the summer was defined as a blood sample obtained in the months of June, July, and August.
Baseline Vitamin D Status and Clinical Outcomes
Overall, 17.0% (189/1107; 95% CI, 15.0–19.4) and 47.7% (528/1107; 95% CI, 44.8–50.6) patients had vitamin D deficiency and insufficiency, respectively. Primary non-response at week 14 and non-remission at week 54 occurred in 19.3% (116/600; 95% CI, 16.4–22.7) and 58.8% (351/597; 95% CI, 54.8–62.7) patients treated with infliximab and 25.3% (100/396; 95% CI, 21.2–29.8) and 65.3% (246/377; 95% CI, 60.3–69.9) of patients treated with adalimumab, respectively.
Pretreatment vitamin D status did not predict response or remission status to anti-TNF therapy at week 14 (primary non-response: infliximab P = .89, adalimumab P = .18; remission: infliximab P = .19, adalimumab P = .38) or non-remission at week 54 (infliximab P = .13, adalimumab P = .58; Figure 3). Overall, there were no differences in median (IQR) vitamin D levels at baseline according to response or remission status at weeks 14 and 54, respectively (Figure S3).
Figure 3:
Proportion of patients stratified by their pretreatment vitamin D status and outcomes to anti-TNF at (A) week 14 and (B) week 54. Infliximab-treated patients on the left panel, and adalimumab-treated patients on the right panel. The number of patients experiencing each outcome is annotated in the plot, with the proportion in brackets (%). PNR, primary non-response.
In patients who continued in the study beyond week 14, there was no difference in drug persistence between patients with vitamin D deficiency (hazard ratio [HR] 0.98 [95% CI, 0.71–1.34], P = 0.89) or insufficiency (HR 1.08 [95% CI, 0.85–1.36], P = .54) at baseline compared to those with adequate concentrations.
Sensitivity Analyses
In a subset of 47.1% (520/1107) of patients who had week 54 fecal calprotectin data, we found a weak negative correlation between vitamin D concentrations at baseline and fecal calprotectin concentrations at week 54 (Rho = −0.09, P = .04).
Of the 28.4% (314/1107) patients treated with corticosteroids at baseline, 41.4% (130/314) were receiving concurrent vitamin D supplementation. Vitamin D concentrations were higher in those receiving vitamin D supplementation compared to those who were not (50.0 nmol/L [36.3–64.8] vs 36.0 [25.8–48.0], P < .001); however, there was no difference in primary non-response rates at week 14 (35.3% vs 32.7%, P = .70) or non-remission at week 54 (65.5% vs 63.1%, P = .71).
We then excluded from the whole cohort, all patients who were receiving vitamin D supplementation at baseline. Of 773 patients remaining, pretreatment vitamin D status was not associated with primary non-response at week 14 (P = .15) or non-remission at week 54 (P = .26).
Anti-TNF Drug Concentrations and Time to Immunogenicity
We observed a weak positive correlation between pretreatment vitamin D concentration and anti-TNF drug concentrations at week 14 (infliximab: Rho = 0.10, P = .03; adalimumab: Rho = 0.20, P < .001); however, when we included the factors previously associated with week 14 drug level, vitamin D concentrations were not independently associated in our multivariable models (Figure S4). We did not demonstrate associations with infliximab or adalimumab drug concentrations at week 54.
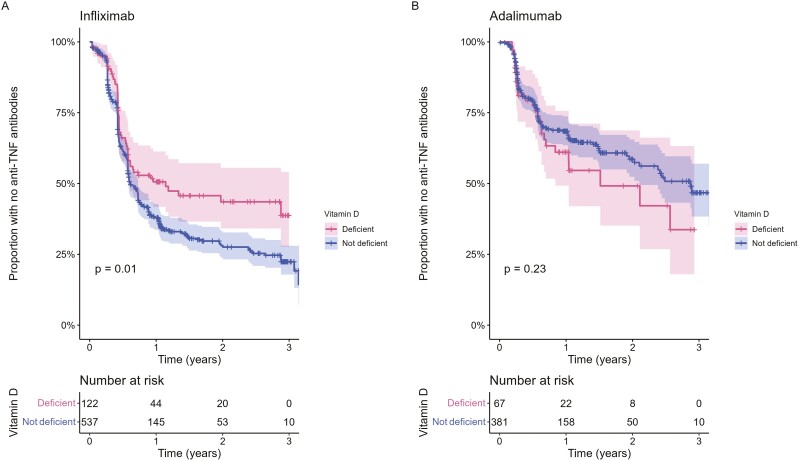
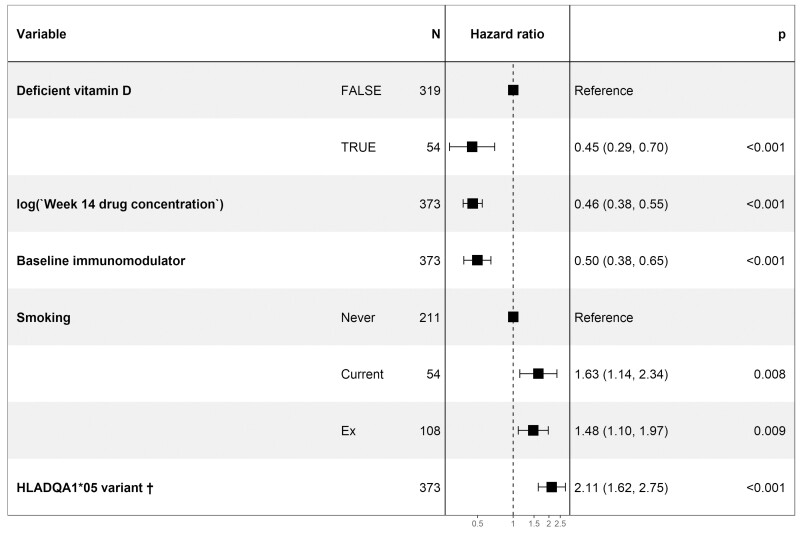
The estimated proportion of patients who developed antidrug antibodies for the first, second, and third years was 64.4% (95% CI, 60.0–68.4), 69.6% (95% CI, 64.9–73.6), and 78.4% (95% CI, 69.1–84.9) in infliximab-treated patients; and 36.9% (95% CI, 31.5–41.8), 45.8% (95% CI, 38.7–52.1), and 55.1% (95% CI, 45.6–62.9) in adalimumab-treated patients, respectively. Time to immunogenicity was longer in patients with vitamin D deficiency in infliximab-treated (HR 0.69 [95% CI, 0.52–0.91], P = .01]), but not adalimumab-treated (HR 1.29 [95% CI, 0.85–1.95], P = .23) patients (Figure 4). Multivariable analysis, including drug concentration at week 14, immunomodulator use, smoking, and carriage of the HLA-D1A1*05 variant that we have previously reported to be associated with time to immunogenicity in this cohort, confirmed that vitamin D deficiency was independently associated with a longer time to immunogenicity in infliximab-treated patients (Figure 5).
Figure 4.
Kaplan–Meier estimates of time to the development of anti-TNF antibodies in patients stratified by pretreatment vitamin D status. Infliximab-treated patients are shown in (A) and adalimumab-treated patients in (B). P-values calculated using the log-rank test. Shaded regions represent the 95% CI.
Figure 5.
Forest plot showing the hazard ratio of the factors associated with time to the development of anti-TNF antibodies in infliximab-treated patients. †Patients with either 1 or 2 copies of the allele were considered to have carriage of the HLA-DQA1*05 variant.
Discussion
Key Results
Vitamin D deficiency is common in patients with active Crohn’s disease. Unlike previous studies, pretreatment serum 25-hydroxyvitamin D concentrations did not predict primary non-response, non-remission, or anti-TNF drug persistence. Vitamin D deficiency was, however, associated with a longer time to immunogenicity in patients treated with infliximab.
Interpretation
Our observation that 17% and 48% of patients had vitamin deficiency and insufficiency, respectively, is consistent with previous estimates in patients with active IBD and almost double that compared of healthy controls.3–5 Moreover, our model of 25-hydroxyvitamin D concentration confirmed independent associations with baseline sampling during non-summer months, South Asian ethnicity, lower serum albumin concentrations, and nontreatment with vitamin D supplementation further validates our findings against clinical outcome. Unlike Winter et al.,19 Zator et al.,20 and Xia et al.,21 in their small mixed cohorts, we did not see associations with primary non-response, durability of IBD therapy or remission at week 54, respectively. The major criticisms of these studies are measurement bias (to be included patients needed to have had vitamin D measured); the retrospective assessment of remission; how disease severity was controlled for; and the lack of data relating to concomitant corticosteroid therapy or vitamin D supplementation.26 This is the first prospective study with a large enough sample size to adjust for potential confounders to examine the association between pretreatment vitamin D status/concentration and clinical outcomes in patients with Crohn’s disease treated with anti-TNF therapy. Our negative findings are consistent with the findings from a small (56 Crohn’s disease and 12 ulcerative colitis) prospective study reported by Santos-Antunes et al.27 In our sensitivity analyses, we did not observe any association between pretreatment vitamin D concentrations and clinical outcomes when we stratified our data by vitamin D supplementation in patients who were treated with corticosteroids at baseline. Our weak association with fecal calprotectin suggests that vitamin D supplementation might have, at best, a modest immunoregulatory role in anti-TNF therapy. Overall, however, and unlike previous reports, our data provide no additional justification for the use of vitamin D supplementation in anti-TNF treatment over current indications.
The finding that vitamin D deficiency was lower in infliximab-treated patients is likely to be explained by more active disease at baseline evidenced by a higher serum CRP and fecal calprotectin observed in infliximab- compared to adalimumab-treated patients in this real-world study.
Our observation that the time to immunogenicity was longer in patients with vitamin D deficiency is of interest. Vitamin D has a central role in antigen presentation and T cell function, with effects on immune tolerance in adaptive immune responses.28 While vitamin D deficiency did not predict primary non-response to anti-TNF treatment, whether low vitamin D levels protect against the development of antidrug antibodies requires further study.
Limitations and Generalizability
We acknowledge the following limitations. We accept that our data would have been strengthened by endoscopic outcomes or cross-sectional imaging. However, in PANTS,15 we observed a significant association between clinical outcomes at weeks 14 and 54 with fecal calprotectin, which correlates closely with endoscopic findings. In our sensitivity analysis, we did not observe a clinically useful correlation (Rho = −0.09, P = .04) between pretreatment vitamin D concentrations and calprotectin. The addition of cross-sectional imaging would have strengthened our data in those with a disease affecting the small bowel, but less so in those with disease affecting the large bowel only. Because our stored samples are slowly being exhausted, in particular in children,16 we accept there was some missingness in our cohort. We may have been underpowered to detect associations between vitamin D concentrations and time to the development of anti-adalimumab antibodies, because immunogenicity events were less common and fewer in adalimumab- than in infliximab-treated patients who were vitamin D deficient at baseline.
Our findings are likely to be generalizable to other patients with Crohn’s disease, at least at latitudes similar to those in the United Kingdom. It is possible that vitamin D deficiency in patients with IBD at different latitudes will be less prevalent and whether our findings are generalizable in these populations remain unknown. It is perhaps less likely that vitamin D deficiency influences anti-TNF treatment responses in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis, hidradenitis suppurativa, and uveitis because these conditions are not associated with intestinal malabsorption of dietary vitamin D. Whether our findings are generalizable to other anti-TNF drugs, including certolizumab, golimumab, etanercept, and other biologicals, is unknown.
Conclusions
Vitamin D deficiency is common in patients with active Crohn’s disease. Unlike previous studies, pretreatment serum 25-hydroxyvitamin D concentration did not predict primary non-response to anti-TNF treatment at week 14 or non-remission at week 54.
Supplementary Material
Acknowledgments
Laboratory tests were undertaken by the Exeter Blood Sciences Laboratory at the Royal Devon University Hospital NHS Trust (https://www.exeterlaboratory.com/). The Exeter NIHR Clinical Research Facility coordinated sample storage and management. The sponsor of the study was the Royal Devon University Healthcare NHS Foundation Trust. The South West Research Ethics committee approved the study (REC Reference: 12/SW/0323) in January 2013. N.C. acknowledges support from Crohn’s & Colitis UK. S.L. is supported by a Wellcome GW4-CAT fellowship (222850/Z/21/Z). . C.B. is supported by the National Institute for Health and Care Research Exeter Biomedical Research Centre. We thank the patients who participated in the PANTS study, the Inflammatory Bowel Disease Pharmacogenetic Study Group, and research nurses who collected clinical data and biological samples at each study visit. We acknowledge the study coordinators Marian Parkinson and Helen Gardner-Thorpe for their ongoing administrative support to the study. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Contributor Information
Neil Chanchlani, Gastroenterology, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK; Exeter Inflammatory Bowel Disease and Pharmacogenetics Research Group, University of Exeter, Exeter, UK.
Simeng Lin, Gastroenterology, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK; Exeter Inflammatory Bowel Disease and Pharmacogenetics Research Group, University of Exeter, Exeter, UK.
Rebecca Smith, Gastroenterology, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK; Exeter Inflammatory Bowel Disease and Pharmacogenetics Research Group, University of Exeter, Exeter, UK.
Christopher Roberts, Gastroenterology, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK; Exeter Inflammatory Bowel Disease and Pharmacogenetics Research Group, University of Exeter, Exeter, UK.
Rachel Nice, Biochemistry, Exeter Clinical Laboratory International, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK.
Timothy J McDonald, Biochemistry, Exeter Clinical Laboratory International, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK.
Benjamin Hamilton, Gastroenterology, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK; Exeter Inflammatory Bowel Disease and Pharmacogenetics Research Group, University of Exeter, Exeter, UK.
Maria Bishara, Gastroenterology, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK; Exeter Inflammatory Bowel Disease and Pharmacogenetics Research Group, University of Exeter, Exeter, UK.
Claire Bewshea, Exeter Inflammatory Bowel Disease and Pharmacogenetics Research Group, University of Exeter, Exeter, UK.
Nicholas A Kennedy, Gastroenterology, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK; Exeter Inflammatory Bowel Disease and Pharmacogenetics Research Group, University of Exeter, Exeter, UK.
James R Goodhand, Gastroenterology, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK; Exeter Inflammatory Bowel Disease and Pharmacogenetics Research Group, University of Exeter, Exeter, UK.
Tariq Ahmad, Gastroenterology, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK; Exeter Inflammatory Bowel Disease and Pharmacogenetics Research Group, University of Exeter, Exeter, UK.
Author Contributions
N.C., S.L., N.A.K., J.R.G., and T.A. participated in the conception and design of the work. C.B. was the project manager and coordinated recruitment. R.N. and T.J.M. coordinated all biochemical analyses and central laboratory aspects of the project. N.C., S.L., R.S., C.R., R.N., T.J.M., C.B., B.H., M.B., N.A.K., J.R.G., and T.A. and were involved in the acquisition, analysis, or interpretation of data. The data analysis was performed by S.L. and N.A.K. Drafting of the manuscript was conducted by N.C., S.L., R.S., C.R., N.A.K., J.R.G., and T.A. All the authors contributed to the critical review and final approval of the manuscript. N.C. and T.A. obtained the funding for the study. T.A. is the guarantor of the article.
Ethics and Funding
The sponsor of the study was the Royal Devon University Healthcare NHS Foundation Trust. The South West Research Ethics committee approved the study (REC Reference: 12/SW/0323) in January 2013. PANTS is an investigator-led study, and was supported by the National Institute for Health and Care Research (NIHR) Exeter Biomedical Research Centre. It was funded by the research charities CORE, Crohn's & Colitis UK, and C3, and by unrestricted educational grants from Abbvie (USA), Merck Sharp & Dohme (UK), NAPP Pharmaceuticals (UK), Pfizer (USA), and Celltrion Healthcare (South Korea). Our vitamin D study was funded by grants from the European Crohn’s Colitis Organisation (ECCO) and the European Society of Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). No funding bodies had any role in study design, data collection or analysis, writing, or decision to submit for publication.
Conflict of Interest
S.L. reports nonfinancial support from Pfizer outside the submitted work. N.A.K. reports grants from F. Hoffmann-La Roche AG, grants from Biogen Inc, grants from Celltrion Healthcare, grants from Galapagos NV, nonfinancial support from Immundiagnostik, grants and nonfinancial support from AbbVie, grants and personal fees from Celltrion, personal fees and nonfinancial support from Janssen, personal fees from Takeda, personal fees and nonfinancial support from Dr Falk, outside the submitted work. T.A. reports grants and nonfinancial support from F. Hoffmann-La Roche AG, grants from Biogen Inc, grants from Celltrion Healthcare, grants from Galapagos NV, nonfinancial support from Immundiagnostik, personal fees from Biogen inc, grants and personal fees from Celltrion Healthcare, personal fees and nonfinancial support from Immundiagnostik, personal fees from Takeda, personal fees from ARENA, personal fees from Gilead, personal fees from Adcock Ingram Healthcare, personal fees from Pfizer, personal fees from Genentech, nonfinancial support from Tillotts, outside the submitted work. J.R.G. reports grants from F. Hoffmann-La Roche AG, grants from Biogen Inc, grants from Celltrion Healthcare, grants from Galapagos NV, nonfinancial support from Immundiagnostik, outside the submitted work. The following authors have nothing to declare: N.C., R.S., C.R., R.N., T.J.M., C.B., B.H., and M.B.
Data Availability
Individual participant deidentified data that underlie the results reported in this article will be available immediately after publication for a period of 5 years. The data will be made available to investigators whose proposed use of the data has been approved by an independent review committee. Analyses will be restricted to the aims in the approved proposal. Proposals should be directed to tariq.ahmad1@nhs.net. To gain access, data requestors will need to sign a data access agreement.
References
- 1. Cantorna MT, McDaniel K, Bora S, Chen J, James J.. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med (Maywood). 2014;239(11):1524–1530. doi: 10.1177/1535370214523890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nic Suibhne T, Cox G, Healy M, O’Morain C, O’Sullivan M.. Vitamin D deficiency in Crohn’s disease: prevalence, risk factors and supplement use in an outpatient setting. J Crohns Colitis. 2012;6(2):182–188. doi: 10.1016/J.CROHNS.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 3. McCarthy D, Duggan P, O’Brien M, et al. . Seasonality of vitamin D status and bone turnover in patients with Crohn’s disease. Aliment Pharmacol Ther. 2005;21(9):1073–1083. doi: 10.1111/J.1365-2036.2005.02446.X [DOI] [PubMed] [Google Scholar]
- 4. Kabbani TA, Koutroubakis IE, Schoen RE, et al. . Association of vitamin D level with clinical status in inflammatory bowel disease: a 5-year longitudinal study. Am J Gastroenterol. 2016;111(5):712–719. doi: 10.1038/AJG.2016.53 [DOI] [PubMed] [Google Scholar]
- 5. Ananthakrishnan AN, Khalili H, Higuchi LM, et al. . Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142(3):482–489. doi: 10.1053/J.GASTRO.2011.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Limketkai BN, Singla MB, Rodriguez B, et al. . Levels of vitamin D are low after Crohn’s disease is established but not before. Clin Gastroenterol Hepatol. 2020;18(8):1769–1776.e1. doi: 10.1016/J.CGH.2019.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lund-Nielsen J, Vedel-Krogh S, Kobylecki CJ, Brynskov J, Afzal S, Nordestgaard BG.. Vitamin D and inflammatory bowel disease: Mendelian randomization analyses in the Copenhagen studies and UK Biobank. J Clin Endocrinol Metab. 2018;103(9):3267–3277. doi: 10.1210/JC.2018-00250 [DOI] [PubMed] [Google Scholar]
- 8. Opstelten JL, Chan SSM, Hart AR, et al. . Prediagnostic serum vitamin D levels and the risk of Crohn’s disease and ulcerative colitis in European populations: a nested case–control study. Inflamm Bowel Dis. 2018;24(3):633–640. doi: 10.1093/IBD/IZX050 [DOI] [PubMed] [Google Scholar]
- 9. Jørgensen SP, Hvas CL, Agnholt J, Christensen LA, Heickendorff L, Dahlerup JF.. Active Crohn’s disease is associated with low vitamin D levels. J Crohns Colitis. 2013;7(10):e407-e413. doi: 10.1016/J.CROHNS.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 10. Garg M, Rosella O, Lubel JS, Gibson PR.. Association of circulating vitamin D concentrations with intestinal but not systemic inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(12):2634–2643. doi: 10.1097/01.MIB.0000436957.77533.B2 [DOI] [PubMed] [Google Scholar]
- 11. Gilman J, Shanahan F, Cashman KD.. Determinants of vitamin D status in adult Crohn’s disease patients, with particular emphasis on supplemental vitamin D use. Eur J Clin Nutr 2006 607. 2006;60(7):889–896. doi: 10.1038/sj.ejcn.1602395 [DOI] [PubMed] [Google Scholar]
- 12. Frigstad SO, Høivik M, Jahnsen J, et al. . Vitamin D deficiency in inflammatory bowel disease: prevalence and predictors in a Norwegian outpatient population. Scand J Gastroenterol. 2016;52(1):100–106. doi: 10.1080/00365521.2016.1233577 [DOI] [PubMed] [Google Scholar]
- 13. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. doi: 10.1136/GUTJNL-2019-318484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papamichael K, Afif W, Drobne D, et al. Therapeutic drug monitoring of biologics in inflammatory bowel disease: unmet needs and future perspectives. Lancet Gastroenterol Hepatol. 2022;7(2):171–185. doi: 10.1016/S2468-1253(21)00223-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4(5):341–353. doi: 10.1016/S2468-1253(19)30012-3 [DOI] [PubMed] [Google Scholar]
- 16. Lin S, Chanchlani N, Carbery I, et al. Understanding anti-TNF treatment failure: does serum triiodothyronine-to-thyroxine (T3/T4) ratio predict therapeutic outcome to anti-TNF therapies in biologic-naïve patients with active luminal Crohn’s disease? Aliment Pharmacol Ther. 2022;56(5):783–793. doi: 10.1111/APT.17089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sazonovs A, Kennedy NA, Moutsianas L, et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn’s disease. Gastroenterology. 2020;158(1):189–199. doi: 10.1053/j.gastro.2019.09.041 [DOI] [PubMed] [Google Scholar]
- 18. Wilson A, Peel C, Wang Q, Pananos AD, Kim RB.. HLADQA1*05 genotype predicts anti-drug antibody formation and loss of response during infliximab therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51(3):356–363. doi: 10.1111/APT.15563 [DOI] [PubMed] [Google Scholar]
- 19. Winter RW, Collins E, Cao B, Carrellas M, Crowell AM, Korzenik JR.. Higher 25-hydroxyvitamin D levels are associated with greater odds of remission with anti-tumour necrosis factor-α medications among patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45(5):653–659. doi: 10.1111/APT.13936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zator ZA, Cantu SM, Konijeti GG, et al. . Pretreatment 25-hydroxyvitamin D levels and durability of anti-tumor necrosis factor-α therapy in inflammatory bowel diseases. JPEN J Parenter Enteral Nutr. 2014;38(3):385–391. doi: 10.1177/0148607113504002 [DOI] [PubMed] [Google Scholar]
- 21. Xia SL, Min QJ, Shao XX, et al. . Influence of Vitamin D3 supplementation on infliximab effectiveness in Chinese patients with Crohn’s disease: a retrospective cohort study. Front Nutr. 2021;8:789. doi: 10.3389/FNUT.2021.739285/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roche Diagnostics. Elecsys® Vitamin D total II. Published online. 2018.
- 23. Borai A, Khalil H, Alghamdi B, et al. . The pre-analytical stability of 25-hydroxyvitamin D: Storage and mixing effects. J Clin Lab Anal. 2020;34(2):e23037. doi: 10.1002/JCLA.23037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bozkurt B. Pre-analytical stability of 25 hydroxy vitamin D in human serum. Int J Med Biochem. 2018;1(1):0–2. doi: 10.14744/ijmb.2017.08208 [DOI] [Google Scholar]
- 25. Nice R, Chanchlani N, Green H, et al. . Validating the positivity thresholds of drug-tolerant anti-infliximab and anti-adalimumab antibody assays. Aliment Pharmacol Ther. 2021;53(1):128–137. doi: 10.1111/apt.16135 [DOI] [PubMed] [Google Scholar]
- 26. Hawthorne AB. Editorial: clinical benefits of vitamin D therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45(10):1365–1366. doi: 10.1111/APT.13997 [DOI] [PubMed] [Google Scholar]
- 27. Santos-Antunes J, Nunes ACR, Lopes S, Macedo G.. The relevance of vitamin D and antinuclear antibodies in patients with inflammatory bowel disease under anti-TNF treatment: a prospective study. Inflamm Bowel Dis. 2016;22(5):1101–1106. doi: 10.1097/MIB.0000000000000697 [DOI] [PubMed] [Google Scholar]
- 28. Chun RF, Liu PT, Modlin RL, Adams JS, Hewison M.. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol. 2014;5:151. doi: 10.3389/FPHYS.2014.00151/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant deidentified data that underlie the results reported in this article will be available immediately after publication for a period of 5 years. The data will be made available to investigators whose proposed use of the data has been approved by an independent review committee. Analyses will be restricted to the aims in the approved proposal. Proposals should be directed to tariq.ahmad1@nhs.net. To gain access, data requestors will need to sign a data access agreement.