Abstract
Chlamydomonas reinhardtii mutants defective in the chloroplast ATP synthase are highly sensitive to light. The ac46 mutant is affected in the MDH1 gene, required for production or stability of the monocistronic atpH mRNA encoding CFO-III. In this and other ATP synthase mutants, we show that short-term exposure to moderate light intensities—a few minutes—induces an inhibition of electron transfer after the primary quinone acceptor of photosystem II (PSII), whereas longer exposure—several hours—leads to a progressive loss of PSII cores. An extensive swelling of thylakoids accompanies the initial inhibition of electron flow. Thylakoids deflate as PSII cores are lost. The slow process of PSII degradation involves the participation of ClpP, a chloroplast-encoded peptidase that is part of a major stromal protease Clp. In the light of the above findings, we discuss the photosensitivity of ATP synthase mutants with respect to the regular photoinhibition process that affects photosynthetic competent strains at much higher light intensities.
The unicellular alga Chlamydomonas reinhardtii has long been used for the isolation of photosynthesis mutants because it can grow in heterotrophic conditions using acetate as an exogenous source of reduced carbon (Levine and Goodenough, 1970). In addition, strains showing various photosynthesis defects can be distinguished based on their fluorescence yield at room temperature (Bennoun and Delepelaire, 1982). This screening criterium is based on the early discovery (Duysens and Sweers, 1963) that the chlorophyll fluorescence yield at room temperature is primarily determined by the redox state of the primary quinone acceptor of photosystem II (QA) that loses its fluorescence quenching properties when reduced. Thus, the identification of photosystem II (PSII)-minus, cytochrome b6f-minus, or photosystem I (PSI)-minus mutants as high fluorescence colonies is easily understood in terms of a block in electron transfer at various steps of the photosynthetic reaction chain. The fluorescence screen used to identify ATP synthase-minus mutants (Bennoun et al., 1978), although very powerful, has not yet been interpreted in molecular terms. Such mutants have a wild-type fluorescence phenotype when analyzed in dim light (i.e. below 6 μE m−2 s−1) but display a fluorescence induction curve similar to that of PSII mutants when transferred for several hours to moderate light (i.e. between 40 and 140 μE m−2 s−1).
Here we report a detailed study of the structural and functional modifications that accompany the changes in fluorescence properties of ATP synthase mutants when transferred from dim light to moderate light (70 μE m−2 s−1). We show that, subsequent to an early and reversible block in photosynthetic electron transfer, the mutants undergo a considerable thylakoid swelling before flattening upon selective photodestruction of PSII. The critical role of the chloroplast protease Clp in PSII degradation in these experimental conditions is demonstrated.
RESULTS
The ac46 Mutant Lacks CFO-III Due to the Absence of the atpH Transcript
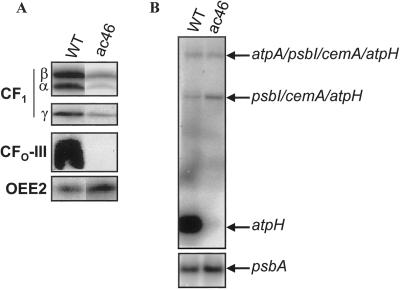
The ac46 mutant lacking chloroplast ATP synthase was chosen for this study because its primary defect is the absence of CFO-III, a major chloroplast-encoded CFO subunit (Lemaire and Wollman, 1989a). The absence of CFO-III in the ac46 mutant is documented on Figure 1A by an immunoblotting experiment using whole-cell protein extracts. Sizeable amounts of CF1 may still assemble in this strain since the α, β, and γ subunits can still be detected by immunoblotting, but they are not associated with the thylakoid membrane (Lemaire and Wollman, 1989a). Thus, this mutant cannot be suspected to undergo any proton leakage accompanying an assembly defect in ATP synthase since it lacks the major subunit of the proton channel.
Figure 1.
Characterization of the ac46 mutant. A, Immunoblots of whole cell protein extracts of the ac46 and WT strains, probed with specific antibodies for ATP synthase subunits. OEE2 was included as a loading control. B, Accumulation of the atpH transcripts in the ac46 and WT strains. The psbA probe was included as a loading control.
Since ac46 is a nuclear mutant, the lack of synthesis of CFO-III, a chloroplast-encoded subunit, points to a mutation in a nuclear gene that could encode a factor specifically controlling the expression of the chloroplast atpH gene at the posttranscriptional level. As previously described (Drapier et al., 1998), the atpH probe reveals three transcripts in the WT strain (Fig. 1B), atpH being the fourth gene in a polycistronic transcription unit containing atpA, psbI, cemA, and atpH. The monocistronic atpH transcript is specifically missing in ac46, whereas the di- and tricistronic transcripts are still present in similar amounts as in the wild type (Fig. 1B). Thus, the mutation defines a novel gene MDH1, whose mutated allele is mdh1-ac46, which is required for production or stability of monocistronic atpH mRNA.
Early Changes in Fluorescence of ATP Synthase Mutants Placed under Moderate Light
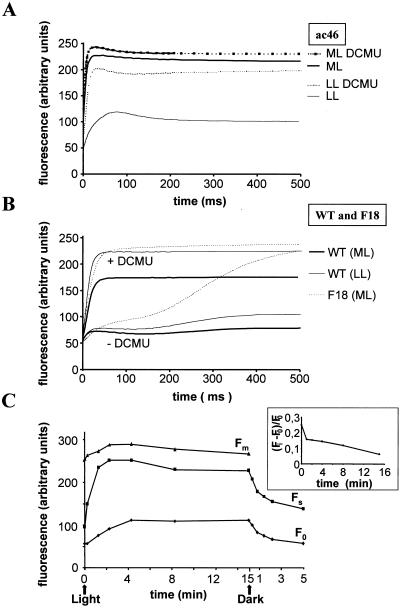
When kept in darkness or under low light, ATP synthase mutants of C. reinhardtii display fluorescence induction kinetics at room temperature that are similar to that of a wild-type strain (Joliot et al., 1998). This is shown on Figure 2A for ac46 grown under 6 μE m−2 s−1 and dark-adapted for 5 s before recording its fluorescence induction. The steady-state level of fluorescence (Fs) attained after a 1.4-s illumination in the apparatus (at 60 μE m−2 s−1) remained well below the maximal fluorescence level (Fm) which can be measured by performing the same experiment in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), a PSII inhibitor that prevents reoxidation of the semiquinone QA− by the secondary quinone acceptor of PSII (QB). This is indicative of an efficient electron transfer along the photosynthetic electron transfer chain insuring the rapid reoxidation of QA− produced by PSII charge separation.
Figure 2.
Early changes in room temperature fluorescence. Fluorescence induction kinetics were recorded with or without DCMU either after incubation in low light (LL = 6 μE m−2 s−1) or after 4-min exposure to moderate light (ML = 70 μE m−2 s−1). A, ac46; B, wild type and cytochrome b6f mutant F18; C, evolution of F0, Fs, and Fm during exposure of ac46 to moderate light. Inset, Evolution of (Fi − F0)/F0.
After as little as 4 min of pre-illumination at moderate light intensities (70 μE m−2 s−1) followed by a 5-s dark adaptation period, ac46 displayed a dramatic change in its fluorescence induction kinetics (Fig. 2A). The fluorescence induction curve measured in the absence of DCMU was now very similar to that obtained in the presence of the inhibitor, with a rapid rise from the initial fluorescence level (F0) to an Fs level very close to Fm. This is reflected in a drop of the parameter (Fm − Fs)/Fv (Table I) and indicates an inhibition of electron transfer beyond QA. However, the persistence of a large variable fluorescence Fv = Fm − F0 demonstrated that PSII charge separation and stable QA reduction still occurred. Similar changes in the fluorescence induction curves were observed with three other ATP synthase mutants: atpC1 (Smart and Selman, 1991), atpA-Fud16;ncc1 (Ketchner et al., 1995), and tbc1-F54 (Drapier et al., 1992) (Table I). In contrast, the wild-type strain retained efficient electron transfer beyond QA after 4 min preillumination at 70 μE m−2 s−1; Fs remained well below Fm (Fig. 2B and Table I). The kinetics of these early changes in fluorescence induction parameters of ATP synthase mutants upon a preillumination at 70 μE m−2 s−1 are shown for ac46 in Figure 2C. The increase in Fs was completed within the first 2 min of preillumination reaching about 85% of the Fm value. The rise at Fs was indicative of a rapid and drastic decrease in the efficiency of QA− reoxidation. In addition to the rise of Fs, we observed a limited but significant increase at Fm (Fig. 2B) that is due in part to an increased state I configuration (see “Discussion”). The inhibition of electron transfer in ac46 was reversible, as shown by the rapid decrease (t1/2 = 1.5 min) of the Fs level back to its initial level when ac46 was placed back into darkness after a 15-min illumination under moderate light.
Table I.
Evolution of the fluorescence parameters in wild type and ATP synthase mutants after various times of illumination at 70 μE m−2 s−1
| Strains | Time in Medium Light
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
5 min
|
24 h
|
48 h
|
|||||||
| Fv/Fm | (Fm − Fs)/Fv | Fm | Fv/Fm | (Fm − Fs)/Fv | Fm | Fv/Fm | Fm | Fv/Fm | Fm | |
| % | % | % | % | |||||||
| ac46 | 0.77 | 0.61 | 100 | 0.52 | 0.13 | 115 | 0.28 | 68 | 0.24 | 25 |
| F54 | 0.68 | 0.70 | 100 | 0.50 | 0.19 | 125 | 0.45 | 63 | 0.22 | 21 |
| atpC1 | 0.67 | 0.70 | 100 | 0.53 | 0.19 | 127 | 0.37 | 63 | 0.30 | 25 |
| atpA-Fud16;ncc1 | 0.64 | 0.74 | 100 | 0.44 | 0.10 | 126 | 0.35 | 100 | 0.19 | 52 |
| Wild type | 0.70 | 0.57 | 100 | 0.72 | 0.75 | 96 | 0.69 | 94 | 0.65 | 102 |
After 5 s dark adaptation, actinic light (60 μE m−2 s−1) is turned on and the F0 and Fs parameters are recorded at time 0 and 1.4 s, respectively. Fi and Fm are measured in the same manner except that DCMU is added during the dark adaptation period. The variable fluorescence Fv is Fm − F0. The normalized parameters Fv/Fm and (Fm − Fs)/Fv reflect the activity of stable PSII charge separation and the activity of electron transfer beyond QA, respectively. Fm at time t is indicated relative to time 0 of illumination at 70 μE m−2 s−1.
The decrease in the rate of QA− reoxidation in ATP synthase mutants is likely to result from the light-induced acidification of the lumen. This acidification should be caused by the release of protons, coupled to photosynthetic electron transfer, that cannot be translocated back to the stroma in the absence of an ATP synthase. We first considered a possible block in the reoxidation of plastoquinol (PQH2) by the cytochrome b6f complex. The latter reaction is coupled to a release of protons in the lumen and is therefore predicted to be considerably slowed down when lumenal pH becomes acidic (Hope, 1993; Finazzi and Rappaport, 1998). We compared the fluorescence induction behavior of ac46 exposed to moderate light with that of the cytochrome b6f mutant F18 (Lemaire et al., 1986) blocked in PQH2 reoxidation. As shown in Figure 2B, this mutant still displays a large difference in the rate of fluorescence rise with and without DCMU. This difference reflects the larger number of electron acceptors after PSII that are available without DCMU, i.e. the plastoquinone pool, whereas only the primary acceptor QA is available when the inhibitor is present. Thus, the similar fluorescence rises with and without DCMU that we observed with ac46 exposed to moderate light point to an impairment of electron transfer to the PQ pool. In order to rule out the possibility that the PQ pool had remained fully reduced in the 5 s of dark adaptation after preillumination of ac46, we added benzoquinone 5 s prior to the measurement of the fluorescence induction. This treatement known to oxidize at least part of the PSII electron acceptors (Lavergne, 1984; Joliot and Joliot, 1985) did not restore electron transfer beyond QA in light-treated ATP synthase mutants (data not shown).
To directly test the hypothesis that electron transfer would be blocked between QA and QB, we compared the initial fluorescence levels observed in the absence (Fo) and presence (Fi) of DCMU. In vivo, Fi is usually higher than the genuine F0 because binding of the inhibitor to the QB pocket requires that it is not occupied by a semiquinone (Wollman, 1978). In the fraction of centers with a semireduced QB− acceptor in the dark, DCMU binding occurs only when the electron is transferred back to QA. Consequently, an interruption of electron transfer between QA and QB should prevent a DCMU-induced rise of the initial fluorescence level. Indeed, when the difference (Fi − F0)/F0 was plotted as a function of time of illumination under 70 μE m−2 s−1 (Fig. 2C, inset), a rapid decrease was observed. The loss of the DCMU-induced rise of the initial level of fluorescence developed together with the inhibition of electron flow. Taken together, our results strongly suggest that electron transfer is blocked between QA and QB in light-treated ATP synthase mutants.
Light-Induced Thylakoid Swelling in ATP Synthase Mutants
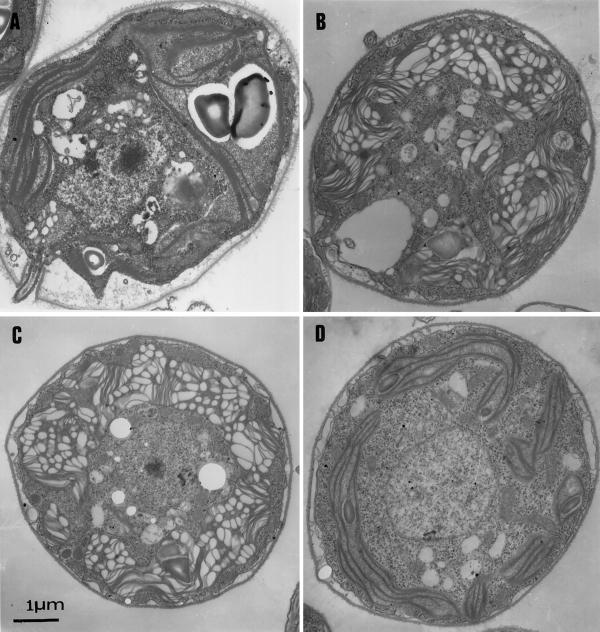
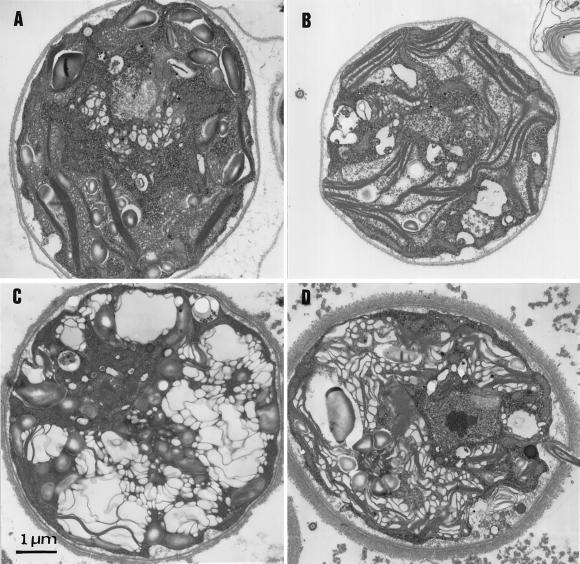
Ultra-thin sections of ac46 were prepared at various time points of illumination at 70 μE m−2 s−1. Prior to exposure to moderate light, low-light-grown ac46 displayed a chloroplast ultrastructural organization very similar to that in a wild-type strain: a dense array of thylakoid membranes extended along a large cup-shaped chloroplast (Fig. 3A). The most conspicuous change that developed upon 70 μE m−2 s−1 illumination was an extensive swelling of the thylakoids, which allowed clear observation of the lumenal space. This change was already apparent after 30 min (Fig. 3B) and increased during the first 3 h of illumination (Fig. 3C). A similar swelling was observed with F54 and other ATP synthase mutants (data not shown), but not with a wild-type strain (Fig. 3D).
Figure 3.
Light-induced thylakoid swelling in ac46. Cells were fixed in low light (A), or after exposure to moderate light for 30 min (B) or 3 h (C). D, Control experiment, wild-type cells exposed for 3 h to moderate light.
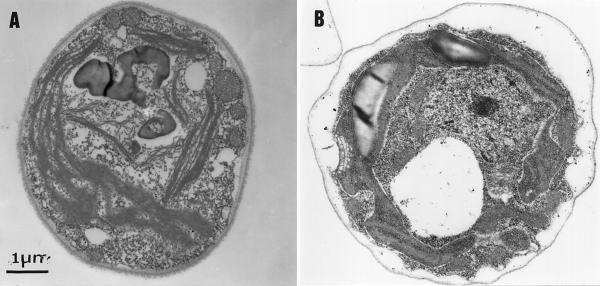
The above observations suggested that swelling resulted from the light-induced electro-chemical proton gradient (Δμ̃H+) that accumulated in the absence of ATP synthase activity. Therefore, we preincubated ac46 with uncouplers [200 μm Crown, 5 μm carbonylcyanide-4-tri(fluoromethoxy)phenylhydrazone]. These high concentrations of uncouplers were necessary to shorten the half time of the light-induced transmembrane electric field to less than 20 ms (F. Rappaport and F.-A. Wollman, unpublished data). Upon illumination, the uncoupler-treated ac46 cells no longer displayed any light-induced swelling (Fig. 4A).
Figure 4.
Light-induced swelling is prevented by addition of uncouplers or the absence of cytochrome b6f. A, ac46 treated with uncouplers [200 μm Crown, 5 μm carbonylcyanide-4-tri(fluoromethoxy)phenylhydrazone], then exposed to moderate light for 3 h. B, F54-F18 exposed to moderate light for 3 h.
Since the light-induced 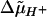 results from proton translocation across the membrane upon photosynthetic electron flow, we also assessed the behavior of ATP synthase mutants that were altered in their electron transfer capacity. The double mutant F54-F18, which lacks the ATP synthase and displays neither linear nor cyclic electron transfer due to the absence of the cytochrome b6f complex, showed no light-induced thylakoid swelling (Fig. 4B). Strangely enough, the double mutant F54-F34, devoid of ATP synthase and PSII, did not undergo thylakoid swelling either (Fig. 5A), despite the fact that it should still be capable of cyclic electron flow between cytochrome b6f and PSI. This unexpected finding was confirmed with ac46 cells when illuminated in the presence of DCMU. No swelling was observed in these conditions (Fig. 5B). Here again, the inhibitor should selectively inhibit linear electron flow from PSII to PSI but not PSI-driven cyclic electron flow. We conclude that the ATP synthase mutants were not able to perform PSI-driven cyclic electron flow at a rate high enough to establish the Δμ̃H+ required for swelling. It has been suggested (Vallon et al., 1991; Finazzi et al., 1999) that cyclic electron flow is active mostly in state II, i.e. when cytochrome b6f moves to unappressed membranes in the vicinity of PSI. Our experimental conditions (illumination of cells with impaired PSII activity) should instead cause a transition to state I, due to a light-induced oxidation of the PQ pool (Keren and Ohad, 1998). Therefore, we repeated our experiments with strains that were preadapted to state II before illumination. This was achieved by adding Glc/Glc oxidase to the resuspension buffer prior to illumination, in order to establish anaerobic conditions (Wollman and Delepelaire, 1984). Remarkably, pretreatment in state II restored a light-induced thylakoid swelling both in the F34-F54 double mutant and in the ac46 mutant treated with DCMU (Fig. 5, C and D). Thus, the Δμ̃H+ responsible for swelling can be generated by linear as well as cyclic electron transfer, the latter only if the cells are placed in State II.
results from proton translocation across the membrane upon photosynthetic electron flow, we also assessed the behavior of ATP synthase mutants that were altered in their electron transfer capacity. The double mutant F54-F18, which lacks the ATP synthase and displays neither linear nor cyclic electron transfer due to the absence of the cytochrome b6f complex, showed no light-induced thylakoid swelling (Fig. 4B). Strangely enough, the double mutant F54-F34, devoid of ATP synthase and PSII, did not undergo thylakoid swelling either (Fig. 5A), despite the fact that it should still be capable of cyclic electron flow between cytochrome b6f and PSI. This unexpected finding was confirmed with ac46 cells when illuminated in the presence of DCMU. No swelling was observed in these conditions (Fig. 5B). Here again, the inhibitor should selectively inhibit linear electron flow from PSII to PSI but not PSI-driven cyclic electron flow. We conclude that the ATP synthase mutants were not able to perform PSI-driven cyclic electron flow at a rate high enough to establish the Δμ̃H+ required for swelling. It has been suggested (Vallon et al., 1991; Finazzi et al., 1999) that cyclic electron flow is active mostly in state II, i.e. when cytochrome b6f moves to unappressed membranes in the vicinity of PSI. Our experimental conditions (illumination of cells with impaired PSII activity) should instead cause a transition to state I, due to a light-induced oxidation of the PQ pool (Keren and Ohad, 1998). Therefore, we repeated our experiments with strains that were preadapted to state II before illumination. This was achieved by adding Glc/Glc oxidase to the resuspension buffer prior to illumination, in order to establish anaerobic conditions (Wollman and Delepelaire, 1984). Remarkably, pretreatment in state II restored a light-induced thylakoid swelling both in the F34-F54 double mutant and in the ac46 mutant treated with DCMU (Fig. 5, C and D). Thus, the Δμ̃H+ responsible for swelling can be generated by linear as well as cyclic electron transfer, the latter only if the cells are placed in State II.
Figure 5.
Effect of state transitions on thylakoid swelling in the absence of PSII-driven electron transfer; F54-F34 double mutant lacking ATP synthase and PSII (A and C) and ac46 incubated with DCMU (B and D). Cells were exposed to moderate light for 30 min, either without Glc/Glc oxidase, i.e. in state I conditions (A and B), or with Glc/Glc oxidase, i.e. in state II conditions (C and D).
Effects of Long-Term Exposure to Moderate Light
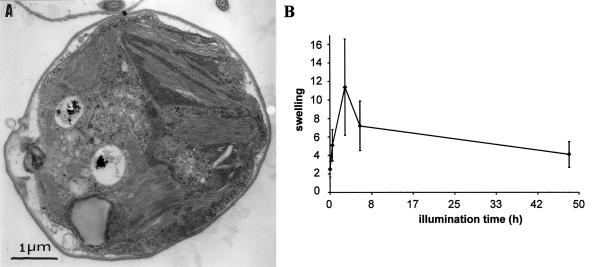
Much to our surprise, ac46 cells showed flattened thylakoids after 48 h at 70 μE m−2 s−1 (Fig. 6A). They appeared only slightly swollen and more densely stacked than in cells that were continuously kept under dim light. Thus, subsequent to their swelling, ac46 thylakoids undergo a deflation process. This deflation starts between 3 and 6 h of exposure to moderate light, as shown on Figure 6B where we have quantified the average width of the thylakoids at various time points of illumination.
Figure 6.
Loss of thylakoid swelling upon long-term exposure to light. A, ac46 cells exposed to 70 μE m−2 s−1 light for 48 h. B, Evolution of the average thylakoid width (arbitrary units) during light treatment.
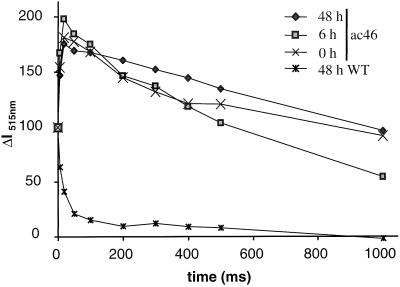
We first considered that prolonged swelling could have caused mechanical damage to the thylakoids, so that membrane leakiness would increase sufficiently to allow flattening. The stability of the light-induced electrochromic shift at 515 nm is a well-established probe of the ionic conductance of the thylakoid membrane in vivo (Junge and Witt, 1968). The 515-nm signal rises with the transmembrane electric field in two steps: in the sub-microsecond time range upon charge separation within the reaction centers and then more slowly, in the millisecond time range, with the electron transfer across the cytochrome b6f complex. In a wild-type strain, the latter phase of rise is most often truncated by a rapid decay phase due to proton extrusion through the ATP synthase that collapses the light-induced electric field while generating ATP (Junge et al., 1970). Typical kinetics of a 515-nm absorbance change after one flash in the wild-type strain are presented on Figure 7 (double crosses), showing a 15-ms half-time of decay of the electric field. As we reported previously (Lemaire and Wollman, 1989a), ATP synthase mutants display a long-lived, light-induced 515-nm absorbance change since the transmembrane electric field is no longer consumed by the ATP synthase. Thus, the slow phase of the electrochromic shift in ac46 adapted to dim light is now well resolved and the half-time of the decay phase is longer than 1 s (Fig. 7, crosses). It is interesting that the decay of the light-induced 515-nm absorbance change was similarly slow after 6 and 48 h of illumination, indicating that the thylakoid membranes retained their impermeability throughout the light treatment. Therefore, restoration of membrane flattening could not be attributed to an increased leakiness of the thylakoids.
Figure 7.
Flash-induced electrochromic signal recorded at 515 nm. PSII activity is inhibited by DCMU + hydroxylamine prior to the measurements. Curves are normalized to the electrochromic shift recorded 100 μs after the actinic flash (phase a, representing PSI charge separation). Times of exposition to moderate light are indicated in hours.
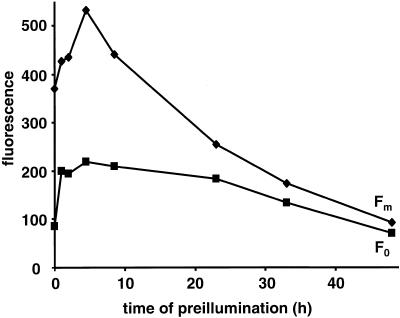
A complete inhibition of photosynthetic electron transport could also end up in membrane flattening since it would extinguish the source of proton accumulation in the thylakoid lumen. Figure 8 shows the changes at Fm and F0 over a 48-h time period of exposure to moderate light. Besides the initial rise at F0 and Fm that was better resolved in Figure 2B, two subsequent phases were observed. A further rise of Fm up to 6 h is probably correlated with changes in thylakoid swelling, since the fluorescence yield in C. reinhardtii varies with the osmotic pressure applied to the cells (F.-A. Wollman and R. Delosme, unpublished data). It is followed by an extensive fluorescence quenching, which is much more pronounced at Fm than at F0. Thus, after 24-h illumination at moderate light, ac46 cells had lost most of their variable fluorescence, which is indicative of a loss of PSII charge separation. A similar evolution of the parameters of fluorescence induction was recorded during light exposure of the atpC1, atpA-Fud16;ncc1, and F54 strains (Table I).
Figure 8.
Changes at Fm and FO upon prolonged exposure of ac46 to moderate light.
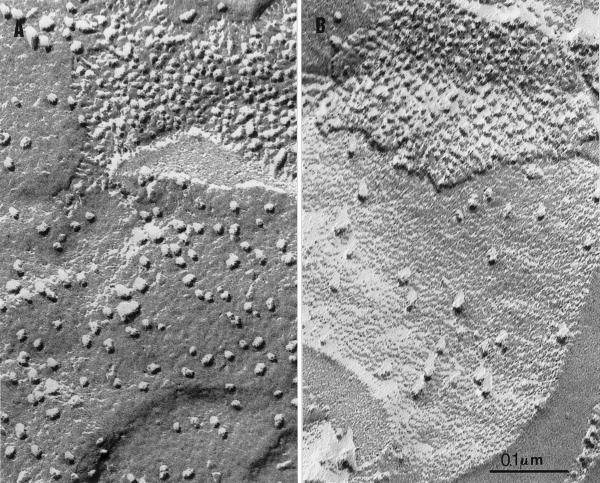
The loss of PSII activity correlated with a major change in PSII content in ac46. Freeze-fracture analysis of C. reinhardtii thylakoid membranes allows visualization of the various transmembrane particles that correspond to the major photosynthetic proteins (for review, see Staehelin, 1986). Figure 9 shows the exoplasmic fracture face (EF) of ac46 grown in dim light and after 48-h illumination at 70 μE m−2 s−1. Most of the intramembrane particles of the EF face correspond to PSII cores (Olive et al., 1979). While ac46 grown under dim light displayed EF faces covered with large particles at a density close to that in the wild type (Table II), their number dropped drastically after 48-h exposure to moderate light. Quantification of the EF particle density at various time points (Table II) showed that the loss started at 6 h and was completed at 24 h, in parallel with the decline of thylakoid swelling and variable florescence. More than 50% of the EF particles were lost during this time period, which suggests that some disassembly or degradation of the PSII core complexes had occurred upon prolonged illumination. The actual loss of PSII subunits upon illumination of ac46 was demonstrated in western blot analysis. A marked decrease in the content of CP43, CP47, and D2 was observed between 6 and 24 h (Fig. 10A). This loss was specific of the PSII complex since neither the cytochrome b6f nor the PSI content varied in the same conditions (Fig. 10A). Taken together, the above data provide the molecular basis for membrane flattening upon prolonged illumination of ac46. Because of the degradation of PSII proteins, ac46 becomes similar to a PSII-ATP synthase double mutant that is unable to perform cyclic electron flow in these state I conditions. Thus, Δμ̃H+ collapses and membranes resume a flat configuration.
Figure 9.
Freeze fracture analysis of ac46 grown in low light (A) or after 48-h exposure to 70 μE m−2 s−1 light (B). Note the loss in most of the large EF particles, whereas protoplasmic faces (top right) remain unaltered.
Table II.
EF particle content in wild type, ac46, and ac46-AUU membranes grown in dim light and after 6, 24, and 48 h illumination at 70 μE m−2 s−1
| Strain | Illumination Time at 70 μE m−2 s−1
|
|||
|---|---|---|---|---|
| 0 | 6 | 24 | 48 | |
| h | ||||
| Wild type | 1,248 | 1,368 | 1,208 | 1,280 |
| 942–1,547 | 896–1,633 | 1,025–1,284 | 1,111–1,711 | |
| ac46 | 1,039 | 948 | 434 | 463 |
| 867–1,584 | 806–1,250 | 290–685 | 260–672 | |
| Ac46-AUU | 1,311 | 1,021 | 1,185 | 1,188 |
| 981–1,710 | 846–1,314 | 883–1,536 | 1,006–1,815 | |
Figure 10.
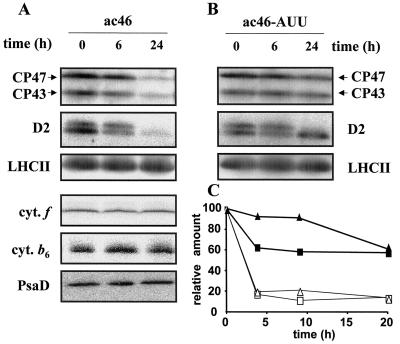
Accumulation of photosynthetic complexes in ATP synthase mutants after exposure to moderate light. A, ac46 was exposed to moderate light for 0, 6, and 24 h. PSII, PSI subunits, and cytochrome b6 were detected by immunoblotting, cytochrome f by TMBZ staining. B, Strain ac46-AUU treated as in A. Only PSII subunits are shown. C, Strains F54 (white symbols) and F54-AUU (black symbols) were exposed to moderate light in the presence of lincomycin, an inhibitor of chloroplast protein synthesis. The levels of CP47 (squares) and CP43 (triangles) were measured by immunoblotting and phosphoimager quantification (ImageQuant software, Molecular Dynamics, Sunnyvale, CA).
PSII Degradation Is Controlled by the Amount of Clp Protease
In a previous study (Majeran et al., 2000), we have shown that ClpP, the chloroplast-encoded peptidase subunit of the stromal protease Clp, contributes to the degradation of a thylakoid membrane protein, the cytochrome b6f complex. In order to examine whether the Clp protease is also involved in the light-induced degradation of PSII in ATP synthase mutants, we constructed double mutants combining a nuclear mutation preventing ATP synthase accumulation (mdh1-ac46) with the chloroplast clpP-AUU mutation that leads to an attenuation of translation initiation for ClpP. In the double mutant ac46-AUU (mdh1-ac46;clpP-AUU), the accumulation of ClpP was reduced, as expected, to about 20% of that in ac46 (data not shown). The double mutant showed the same fluorescence behavior as ac46, which indicated that PSII inactivation still occurred in this strain (data not shown). However, there was only a limited decrease in the content in EF particles during exposure to 70 μE m−2 s−1 (Table I). Accordingly, immunoblotting experiments indicated a moderate loss of integral PSII subunits between 6 and 24 h of illumination (Fig. 10B). Similar results (data not shown) were obtained when comparing the F54 (tda1-F54) ATP synthase mutant to a double mutant F54-AUU (tda1-F54;clpP-AUU).
In order to directly measure the degradation rate of PSII subunits, chase experiments were performed in which lincomycin was added at the beginning of the illumination period. This antibiotic blocks translation of chloroplast proteins shortly after initiation so that the fate of preexisting proteins can be analyzed by immunoblotting without interference from de novo protein synthesis. In these conditions, we observed an increase in the half-life of CP47 and CP43 in the F54-AUU double mutant compared to the F54 parent (Fig. 10C). Similar results were obtained with strains ac46 and ac46-AUU (data not shown). We conclude that the proteolytic disposal of PSII cores that occurred in ATP synthase mutants after PSII inactivation involves, directly or indirectly, the Clp protease.
DISCUSSION
The present analysis of the physiological changes induced by exposure to moderate light in C. reinhardtii mutants lacking the ATP synthase provides the basis for their genetic screening as high fluorescence colonies (Bennoun et al., 1978). The ac46 mutant has been chosen for this analysis because it lacks the CFO ATP synthase subcomplex. This mutant has been described as lacking synthesis of CFO-III, the proteolipid subunit that forms the bulk of the CFO transmembrane channel (Lemaire and Wollman, 1989a). Here we confirm the absence of CFO-III accumulation and present a molecular characterization of the primary defect: the absence of the monocistronic atpH mRNA. This mutation defines a novel gene MDH1 that is required for the production or stability of the monocistronic atpH mRNA. It is interesting that the di- and tricistronic mRNAs were still present in normal amounts. Thus, polycistronic messengers in which atpH is the downstream cistron appear incompetent for translation of subunit III.
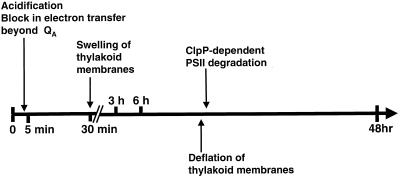
When kept under dim light, ATP synthase mutants behave as wild type, showing a low steady-state level of fluorescence, owing to an efficient electron flow between PSII and PSI. However, after illumination at moderate light intensities (40–140 μE m−2 s−1), they behave as high fluorescent strains. The various changes in functional and supramolecular organization of the photosynthetic apparatus in an ATP synthase mutant over a 48-h illumination period at 70 μE m−2 s−1 are summarized on the scheme of Figure 11.
Figure 11.
Summary of the major events occurring upon illumination of an ATP synthase mutant at 70 μE m−2 s−1.
In wild-type plants and algae, a well-documented effect of a large Δμ̃H+ is to induce qE, the “energetic” component of non-photochemical quenching (for review, see Lavergne and Briantais, 1995; Yamamoto and Bassi, 1996). In this respect, it is paradoxical that ATP synthase mutants that should accumulate a large electrochemical gradient upon illumination do not show rapid fluorescence quenching. qE could be ineffective in our experimental conditions. The sequence of events that transduce the Δμ̃H+ signal into a quenching state for excitons encompasses deepoxidation of violaxanthin to antheraxanthin and zeaxanthin, followed by nonradiative dissipation of light energy through a mechanism that remains poorly understood. It has been reported that the capacity for antheraxanthin and zeaxanthin formation depends on the light intensity used for growing plants (Demmig-Adams et al., 1995) or algae (Niyogi et al., 1997). In particular, the wild-type strain of C. reinhardtii that contains exclusively violaxanthin when grown at 100 μE m−2 s−1 requires exposure to 800 μE m−2 s−1 to display conversion to antheraxanthin and zeaxanthin (Niyogi et al., 1997). Thus, there is a threshold of light intensity, independent of Δμ̃H+, under which nonphotochemical quenching does not develop in C. reinhardtii. The activity of the xanthophyll cycle of the ATP synthase mutants that are grown in dim light and exposed to only 70 μE m−2 s−1 may be too low to elicit transduction of the Δμ̃H+ effect.
The high fluorescence behavior of ATP synthase mutants can be separated kinetically into two phases that develop on widely different time scales. Within the first 5 min of illumination at 70 μE m−2 s−1, most of the ability to reoxidize photoreduced QA is lost. Therefore, the mutant shows a large variable fluorescence nearly reaching Fm even in the absence of DCMU. Then, between 6 and 48 h illumination, the number of active PSII centers decreases steadily and the PSII proteins are degraded. The mutant is initially blocked in a high fluorescence state. After 6 h, a fluorescence quenching develops, leading the mutant cells to lose their high fluorescence properties together with their PSII activity.
The primary defect of a chloroplast ATP synthase mutant resides in its inability to use the proton motive force that develops across the thylakoid membranes during photosynthetic electron transport. During illumination, the lumenal space is then expected to undergo a progressive acidification, limited only by proton leakage and conversion to transmembrane electric potential. Therefore, we suspected that inactivation of photosynthetic electron transport could result from an alteration of a pH-dependent reaction occurring at the inner side of the thylakoid membrane (for review, see Kramer et al., 1999). In particular, lumen acidification can be expected to impair the oxidation of PQH2 at the Qo site of the cytochrome b6f complex, a reaction that requires the release of two protons in the lumenal space (for review, see Hope, 1993). One of the predicted consequences is an increase of Fs, indicative of a more reduced state of the PQ pool under illumination. In this case, however, a substantial difference should remain between the fluorescence induction curves with and without DCMU, reflecting the need to reduce the PQ pool before reaching a high level of fluorescence in the latter case. In our experimental setup, the 5 s of dark adaptation are sufficient to ensure reoxidation of most of PQH2 accumulated during illumination, as documented in Figure 2B with a cytochrome b6f mutant. Furthermore, the rapid fluorescence rise observed in illuminated ATP synthase mutants was also observed in the presence of benzoquinone, an oxidant of the PQ pool (Bulte and Wollman, 1990). Hence, inactivation of the Qo site, even though it may well occur, is not the cause of the fluorescence rise observed in light-treated ATP synthase mutants.
Our results rather suggest a rapid block between QA and QB. The induction curves with and without DCMU become similar. This inhibitor binds to the QB pocket by displacing QB but not QB− (Velthuys, 1981). Because in darkness a fraction of PSII centers are found in the QB− state (Wollman, 1978), these centers will bind the inhibitor only when the electron from the charged semiquinone is visiting QA, an event with low but significant probability, with the equilibrium constant between QA/QB− and QA−/QB being about 20 (Diner, 1977). Thus, addition of DCMU will normally increase the initial fluorescence, by blocking these centers in the QA− state. Our finding (Fig. 2C) that light treatment of ATP synthase mutants decreased the effect of DCMU on the initial fluorescence level is therefore in favor of a block of electron transfer between QA and QB. In essence, PSII centers become similar to the “non QB-reducing” centers (Lavergne and Briantais, 1995), closed after a single turnover, which have been described in plants and algae and can make up between 10% and 35% of PSII centers under certain growth conditions.
The mechanism by which proton accumulation in the lumen causes such dramatic changes at the stromal acceptor side of PSII is still unclear. However, studies by Krieger and coworkers provide interesting clues. They have shown that inactivation of the donor side affects the mid-point redox potential (Em) of QA, raising it from −80 mV to +65 mV (Krieger and Weis, 1992). This probably reflects a physiologically significant conformational change of the PSII center, especially since photoactivation of PSII in dark-grown cells induces a change in the reverse direction, from +110 mV to −80 mV (Johnson et al., 1995). These changes appear to correlate with the Ca2+ binding capacity of the donor side. Interestingly, incubation of PSII membranes at low pH has been shown to cause Ca2+ release, thus inactivating oxygen evolution (Ono and Inoue, 1988). We propose that the rapid acidification of the lumen upon illumination of an ATP synthase mutant could raise the Em of QA to a value too positive to allow downstream electron transfer to QB and the plastoquinone pool, whose Em(pH8) is about +30 mV (Thielen and van Gorkom, 1981). Such a change in the mid-point potential of QA would also explain both the rise of the initial fluorescence level (since QA would now be reducible by PQH2) and its relative insensitivity to DCMU (since single-reduced centers would now have the charge mostly located on QA already before addition of the inhibitor).
Following the rapid block in PSII reoxidation, we observed a loss in variable fluorescence together with a quenching at Fm that slowly developed over the next 48 h of illumination. This behavior is highly reminiscent of the photoinhibition process observed in wild-type algae or in plants, by which excess illumination leads to loss of PSII activity (for review, see Keren and Ohad, 1998). Photoinhibition has been described as a two-step process: an initial reversible stage characterized by a block in electron transfer between QA and QB, and an irreversible stage characterized by a strong fluorescence quenching and a degradation of PSII proteins. Thus, the behavior of ATP synthase mutants in moderate light is strikingly similar to a regular photoinhibition process, the second step of which would be very much delayed. However, the presently described photoinhibition of ATP synthase mutants occurs at light intensities about 30× lower than that required for wild-type cells.
Thylakoid swelling is another feature common to both processes. It has been observed by Topf et al. (1992) using wild-type cells of C. reinhardtii photoinhibited at 625 μE m−2 s−1. Swelling appears as a result of an osmoregulation process driven by lumen acidification and has been suggested to result from amonium uptake into the lumen. In that study, mutants lacking either PSI or plastocyanin or cytochrome b6f failed to show swelling upon photoinhibition, indicating that it is driven by photosynthetic electron flow. Our observation that a double mutant lacking the ATP synthase and cytochrome b6f does not show swelling leads us to the same conclusion. In addition, our experiments with uncoupler-treated ATP synthase mutants clearly point to Δμ̃H+ as the driving force for thylakoid swelling. The exact mechanism for swelling remains unknown, but it is unlikely that protons themselves act as the osmotic species because of the unacceptably low pH that this would entail in the lumen. Linear as well as cyclic electron flow should be capable of generating Δμ̃H+. Our initial observation that the absence of PSII activity prevented swelling was therefore rather intriguing. However, we observed that swelling resumed when PSII-inactive cells were placed in state II prior to illumination. This finding supports the view that, in C. reinhardtii, cyclic electron flow is prevented in state I but is activated in state II, most likely due to the redistribution of cytochrome b6f complexes along the thylakoid membranes (Vallon et al., 1991). It nicely corroborates the recent study by (Finazzi et al., 1999) that shows an interruption of linear electron flow when wild-type cells of C. reinhardtii are placed in state II.
It sounds paradoxical that one of the first effects of light in ATP synthase mutants is an impairment of linear electron transport, because this should in turn counteract the effect of illumination. As outlined by Ohad et al. (1994), the same paradox holds for photoinhibition in the wild type, where both donor and acceptor side models predict that inactivation of PSII will protect the remaining centers. Here, in view of the extreme impermeability of the thylakoid membrane to H+ in the absence of ATP synthase (Lemaire and Wollman, 1989b), we may postulate that even a limited electron transfer activity can maintain a high Δμ̃H+ once the thylakoid ability to extrude H+ is overwhelmed.
The loss of variable fluorescence in ATP synthase mutants was accompanied by a degradation of PSII centers, documented by the loss in the major PSII subunits and in the corresponding EF particles observed on freeze-fractured thylakoid membranes. Here again, the ATP synthase mutant behaved as a wild type upon photoinhibition, but at much lower light intensities and according to a much slower process. We show here that disposal of inactivated PSII centers in ATP synthase mutants depends largely on the action of the Clp protease. This view is supported by our finding that ATP synthase mutants where accumulation of ClpP was reduced by the clpP-AUU mutation display a reduced degradation rate of PSII proteins. This observation is strikingly similar to our previous finding that ClpP controls the rate of degradation of another multisubunit photosynthetic protein, cytochrome b6f (Majeran et al., 2000). Thus, this soluble stromal protease participates in the degradation of large transmembrane proteins. Of possible significance in this regard is the observation that a small fraction of ClpP is consistently found associated with the membrane (W. Majeran and O. Vallon, unpublished observation). Similarly, the cytosolic proteasome is responsible for the degradation of membrane proteins of the endoplasmic reticulum (Wiertz et al., 1996).
However, our results do not imply that Clp is the sole protease carrying out PSII degradation. In fact, PSII degradation in photoinhibitory conditions can readily be observed in vitro, in conditions where ClpP is certainly absent from the preparation (Shipton and Barber, 1992). During photoinhibition, the D1 subunit is the first target of proteolysis, and its degradation in vitro occurs in two steps (De Las Rivas et al., 1992). First, a GTP-stimulated endoproteolytic cleavage generates a 10-kD and a 23-kD fragment. The latter is then degraded by an ATP- and Zn2+-dependant protease, which may be the membrane-bound FtsH protease (Spetea et al., 1999). Hence, photoinactivation may direct the PSII center into two divergent routes of degradation, depending upon the experimental conditions. Indeed, we have found that attenuation of ClpP in a photosynthesis-competent strain does not retard degradation of D1 or the other PSII subunits during photoinhibition (W. Majeran and O. Vallon, unpublished observation). During high light photoinhibition, a conformational change has been implicated in targeting D1 for proteolysis (Zer et al., 1994). The signal that triggers degradation of PSII in ATP synthase mutants is generated under a much lower photon flux and may be of a different nature.
MATERIALS AND METHODS
Strains
All strains were grown under dim light (cool fluorescent white light, 6 μE m−2 s−1) in Tris-acetate medium (Harris, 1989) at 25°C with gentle shaking. The mdh1-ac46 atpC1, atpA-fud16;ncc1, and tda1-F54 nuclear and chloroplastic mutations that prevent formation of the chloroplast ATP synthase have been described before (Lemaire and Wollman, 1989a; Smart and Selman, 1991; Drapier et al., 1992; Ketchner et al., 1995). The tbc1-F34 and ccs3-F18 nuclear mutations preventing the assembly of PSII and cytochrome b6f, respectively, have been previously described (Chua and Bennoun, 1975; Lemaire et al., 1986). They were combined in genetic crosses (Harris, 1989) with the chloroplast clpP-AUU mutation (Majeran et al., 2000), which decreases the accumulation level of ClpP protease. Double mutant segregants were analyzed for the presence of the mutated clpP gene by polymerase chain reaction, and the reduced ClpP protein level was checked by immunoblotting using a homemade anti-ClpP serum (Majeran et al., 2000).
Functional Measurements
Exponentially growing cells were concentrated by centrifugation to a final density of 2 × 107 cells mL−1. After resuspension under dim light for 30 min, cells were placed under moderate light (70 μE m−2 s−1), provided by a tungsten light source. For measurements of fluorescence induction at room temperature, cells were rapidly transferred to a 1-mL cuvette placed in a home-built fluorimeter (Joliot et al., 1998), and fluorescence induction was recorded after 5 s of dark adaptation. When necessary, 10 μm DCMU was added before measurement. The half-life of the light-induced transmembrane electric field was measured by that of the induced electrochromic shift at 515 nm as previously described (Joliot et al., 1998).
Electron Microscopy
For thin-section electron microscopy, cells were fixed by addition of 1% (w/v) glutaraldehyde under stirring in the light, pelleted by centrifugation and further fixed for 1 h on ice, after which they were treated as by Baldan et al. (1991). Freeze fracturing was performed as described (Baldan et al., 1991). Estimation of thylakoid swelling was achieved by measuring the average width of cross-sectioned thylakoids on micrographs of thin sections.
RNA and Protein Analysis
Total RNA was extracted and analyzed as by Drapier et al. (1998). An atpH probe corresponding to the entire reading frame was prepared by photosynthetic carbon reduction using oligonucleotides ATPH1 (5′-ACCCTATCGTAGCTGCAACTTCTGTT-3′) and ATPH2 (5′-AACCAGCGAATGGGTTAGCGAATAG-3′). Plasmid pcp60 (containing the R8 fragment of the chloroplast genome (Rochaix, 1978) was used as a template. The EcoRI fragment R14 of the chloroplast genome was used as a psbA probe.
Whole cell protein extracts were separated in urea/SDS polyacrylamide gels and then transferred to nitrocellulose as by de Vitry et al. (1989). Binding of polyclonal antibodies was detected with 125I-protein A and phosphor-imaging. The monoclonal antibody to CFO subunit III was detected with anti-mouse peroxidase-labeled antibody and enhanced chemiluminescence detection (Amersham, Orsay, France). Heme staining was detected using 3,3′,5,5′-tetramethylbenzidine (Thomas et al., 1976).
ACKNOWLEDGMENTS
The authors thank Michel Recouvreur for his precious help in the preparation of samples for electron microscopy and Fabrice Rappaport for his assistance in flash spectroscopy experiments, for stimulating discussions, and critical reading of this manuscript.
Footnotes
This work was supported by the Centre National de la Recherche Scientifique (Unité Propre de Recherche 1261) and by a fellowship from the Ministère de l'Education Nationale (to W.M.).
LITERATURE CITED
- Baldan B, Girard-Bascou J, Wollman F-A, Olive J. Evidence for thylakoid membrane fusion during zygote formation in Chlamydomonas reinhardtii. J Cell Biol. 1991;114:905–915. doi: 10.1083/jcb.114.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennoun P, Delepelaire P. Isolation of photosynthesis mutants in Chlamydomonas. In: Edelman M, Chua N-H, Hallick RB, editors. Methods in Chloroplast Molecular Biology. Amsterdam: Elsevier Biomedical Press; 1982. pp. 25–38. [Google Scholar]
- Bennoun P, Masson A, Piccioni R, Chua N-H. Uniparental mutants of Chlamydomonas reinhardtii defective in photosynthesis. In: Akoyunoglou G, editor. Chloroplast Development. Amsterdam: Elsevier-North/Holland Biomedical Press; 1978. pp. 721–726. [Google Scholar]
- Bulté L, Wollman F-A. Stabilization of states I and II by p-beazoquinone treatment of intact cells of Chlamydomonas reinhardtii. Biochim Biophys Acta. 1990;1016:253–258. [Google Scholar]
- Chua NH, Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci USA. 1975;72:2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Las Rivas J, Andersson B, Barber J. Two sites of primary degradation of the D1-protein induced by acceptor or donor side photo-inhibition in photosystem II core complexes. FEBS Lett. 1992;301:246–252. doi: 10.1016/0014-5793(92)80250-k. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW, Logan BA, Verhoeven AS. Xanthophyll cycle-dependent energy dissipation and flexible photosystem II efficiency in plants acclimated to light stress. Aust J Plant Physiol. 1995;22:249–260. [Google Scholar]
- Diner BA. Dependence of the deactivation reactions of photosystem II on the redox state of plastoquinone pool varied under anaerobic conditions: equilibria on the acceptor side of photosystem II. Biochim Biophys Acta. 1977;460:247–258. doi: 10.1016/0005-2728(77)90211-0. [DOI] [PubMed] [Google Scholar]
- de Vitry C, Olive J, Drapier D, Recouvreur M, Wollman F-A. Posttranslational events leading to the assembly of photosystem II protein complex: a study using photosynthesis mutants from Chlamydomonas reinhardtii. J Cell Biol. 1989;109:991–1006. doi: 10.1083/jcb.109.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier D, Girard-Bascou J, Wollman F-A. Evidence for nuclear control of the expression of the atpA and atpB chloroplast genes in Chlamydomonas. Plant Cell. 1992;4:283–295. doi: 10.1105/tpc.4.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier D, Suzuki H, Levy H, Rimbault B, Kindle KL, Stern DB, Wollman F-A. The chloroplast atpA gene cluster in Chlamydomonas reinhardtii: functional analysis of a polycistronic transcription unit. Plant Physiol. 1998;117:629–641. doi: 10.1104/pp.117.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysens LNM, Sweers HE. Japanese Society of Plant Physiologists, ed, Studies on Microalgae and Photosynthetic Bacteria. Tokyo: University of Tokyo Press; 1963. Mechanisms of two photochemical reactions in algae as studies by means of fluorescence; pp. 353–372. [Google Scholar]
- Finazzi G, Furia A, Barbagallo RP, Forti G. State transitions, cyclic and linear electron transport and photophosphorylation in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1999;1413:117–129. doi: 10.1016/s0005-2728(99)00089-4. [DOI] [PubMed] [Google Scholar]
- Finazzi G, Rappaport F. In vivo characterization of the electrochemical proton gradient generated in darkness in green algae and its kinetic effects on cytochrome b6f turnover. Biochemistry. 1998;37:9999–10005. doi: 10.1021/bi980320j. [DOI] [PubMed] [Google Scholar]
- Harris EH, editor. The Chlamydomonas Source Book: A Comprehensive Guide to Biology and Laboratory Use. San Diego: Academic Press; 1989. [DOI] [PubMed] [Google Scholar]
- Hope AB. The chloroplast cytochrome bf complex: a critical focus on function. Biochim Biophys Acta. 1993;1143:1–22. doi: 10.1016/0005-2728(93)90210-7. [DOI] [PubMed] [Google Scholar]
- Johnson GN, Rutherford AW, Krieger A. A change in the midpoint potential of the quinone QA in photosystem II associated with photoactivation of oxygen evolution. Biochim Biophys Acta. 1995;1229:202–207. [Google Scholar]
- Joliot P, Beal D, Delosme R. In vivo measurements of photosynthetic activity: methods. In: Rochaix J-D, Goldschmidt-Clermont M, Merchant S, editors. The Molecular Biology of Chloroplast and Mitochondria in Chlamydomonas. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 433–449. [Google Scholar]
- Joliot P, Joliot A. Slow electronic phase and intersystem electron transfer in algae. Biochim Biophys Acta. 1985;806:398–409. [Google Scholar]
- Junge W, Rumberg B, Schroder H. The necessity of an electric potential difference and its use for photophosphorylation in short flash groups. Eur J Biochem. 1970;14:575–581. doi: 10.1111/j.1432-1033.1970.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Junge W, Witt HT. On the ion transport system of photosynthesis: investigations on a molecular level. Z Naturforsch. 1968;23:244–254. doi: 10.1515/znb-1968-0222. [DOI] [PubMed] [Google Scholar]
- Keren N, Ohad I. State transition and photoinhibition. In: Rochaix J-D, Goldschmidt-Clermont M, Merchant S, editors. The Molecular Biology of Chloroplast and Mitochondria in Chlamydomonas. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 569–596. [Google Scholar]
- Ketchner SL, Drapier D, Olive J, Gaudriault S, Girard-Bascou J, Wollman F-A. Chloroplasts can accommodate inclusion bodies: evidence from a mutant of Chlamydomonas reinhardtii defective in the assembly of the chloroplast ATP synthase. J Biol Chem. 1995;270:15299–15306. doi: 10.1074/jbc.270.25.15299. [DOI] [PubMed] [Google Scholar]
- Kramer DM, Sacksteder CA, Cruz JA. How acidic is the lumen? Photosynth Res. 1999;60:151–163. [Google Scholar]
- Krieger A, Weis E. Energy-dependent quenching of chlorophyll-a-fluorescence: the involvement of proton-calcium exchange at photosystem II. Photosynthetica. 1992;27:89–98. [Google Scholar]
- Lavergne J. Absorption changes of Photosystem II donors and acceptors in algal cells. FEBS Lett. 1984;173:9–14. [Google Scholar]
- Lavergne J, Briantais J-M. Photosystem II heterogeneity. In: Ort DR, Yocum CF, editors. Advances in Photosynthesis: Oxygenic Photosynthesis: The Light Reactions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 265–287. [Google Scholar]
- Lemaire C, Girard-Bascou J, Wollman F-A, Bennoun P. Studies on the cytochrome b6f complex: I. Characterization of the complex subunits in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1986;851:229–238. [Google Scholar]
- Lemaire C, Wollman F-A. The chloroplast ATP synthase in Chlamydomonas reinhardtii: I. Characterization of its nine constitutive subunits. J Biol Chem. 1989a;264:10228–10234. [PubMed] [Google Scholar]
- Lemaire C, Wollman F-A. The chloroplast ATP synthase in Chlamydomonas reinhardtii: II. Biochemical studies on its biogenesis using mutants defective in photophosphorylation. J Biol Chem. 1989b;264:10235–10242. [PubMed] [Google Scholar]
- Levine RP, Goodenough UW. The genetics of photosynthesis and of the chloroplast in Chlamydomonas reinhardtii. Annu Rev Genet. 1970;4:397–408. doi: 10.1146/annurev.ge.04.120170.002145. [DOI] [PubMed] [Google Scholar]
- Majeran W, Wollman F-A, Vallon O. Evidence for a role of ClpP in the degradation of the chloroplast cytochrome b6f complex. Plant Cell. 2000;12:137–150. doi: 10.1105/tpc.12.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Bjorkman O, Grossman AR. Chlamydomonas xanthophyll cycle mutants identified by video imaging of chlorophyll fluorescence quenching. Plant Cell. 1997;9:1369–1380. doi: 10.1105/tpc.9.8.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I, Keren N, Zer H, Gong H, Mor TS, Gal A, Tal S, Domovich Y. Light-induced degradation of the photosystem II reaction center D1 protein in vivo: an integrative approach. In: Baker N, editor. Photoinhibition from the Molecule to the Field. Oxford: Bios Scientific Publishers; 1994. pp. 161–177. [Google Scholar]
- Olive J, Wollman F-A, Bennoun P, Recouvreur M. Ultrastructure: function relationship in Chlamydomonas reinhardtii thylakoids, by means of a comparison between the wild type and the F34 mutant which lacks the photosystem II reaction center. Mol Biol Rep. 1979;5:139–143. doi: 10.1007/BF00778412. [DOI] [PubMed] [Google Scholar]
- Ono TA, Inoue Y. Discrete extraction of Ca atom functional for O2 evolution in higher plant photosystem II by a simple low pH treatment. FEBS Lett. 1988;227:147–152. [Google Scholar]
- Rochaix JD. Restriction endonuclease map of the chloroplast DNA of Chlamydomonas reinhardtii. J Mol Biol. 1978;126:597–617. doi: 10.1016/0022-2836(78)90011-6. [DOI] [PubMed] [Google Scholar]
- Shipton CA, Barber J. Characterization of photoinduced breakdown of the D1-polypeptide in isolated reaction centers of photosystem II. Biochim Biophys Acta. 1992;1099:85–90. [PubMed] [Google Scholar]
- Smart EJ, Selman BR. Isolation and characterization of Chlamydomonas reinhardtii mutant lacking the gamma-subunit of chloroplast coupling factor 1 (CF1) Mol Cell Biol. 1991;11:5053–5058. doi: 10.1128/mcb.11.10.5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetea C, Hundal T, Lohmann F, Andersson B. GTP bound to chloroplast thylakoid membranes is required for light-induced, multienzyme degradation of the photosystem II D1 protein. Proc Natl Acad Sci USA. 1999;96:6547–6552. doi: 10.1073/pnas.96.11.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA. Chloroplast structure and supramolecular organization of photosynthetic membranes. In: Staehelin LA, Arntzen CJ, editors. Encyclopedia of Plant Physiology: Photosynthesis III. Berlin: Springer-Verlag; 1986. pp. 1–84. [Google Scholar]
- Thielen AP, van Gorkom HJ. Redox potentials of electron acceptors in Photosystem IIα and IIβ. FEBS Lett. 1981;129:205–209. [Google Scholar]
- Thomas PE, Ryan D, Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976;75:168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Topf J, Gong H, Timberg R, Mets L, Ohad I. Thylakoid membrane energization and swelling in photoinhibited Chlamydomonas cells is prevented in mutants unable to perform cyclic electron flow. Photosynth Res. 1992;32:59–69. doi: 10.1007/BF00028798. [DOI] [PubMed] [Google Scholar]
- Vallon O, Bulté L, Dainese P, Olive J, Bassi R, Wollman F-A. Lateral redistribution of cytochrome b6f complexes along thylakoid membranes upon state transitions. Proc Natl Acad Sci USA. 1991;88:8262–8266. doi: 10.1073/pnas.88.18.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthuys BR. Electron-dependent competition between plastoquinone and inhibitors for binding to photosystem II. FEBS Lett. 1981;126:273–277. [Google Scholar]
- Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- Wollman F-A. Determination and modification of the redox state of the secondary acceptor of photosystem II in the dark. Biochim Biophys Acta. 1978;503:263–273. doi: 10.1016/0005-2728(78)90187-1. [DOI] [PubMed] [Google Scholar]
- Wollman F-A, Delepelaire P. Correlation between changes in light energy distribution and changes in thylakoid membrane polypeptide phosphorylation in Chlamydomonas reinhardtii. J Cell Biol. 1984;98:1–7. doi: 10.1083/jcb.98.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto HY, Bassi R. Carotenoids: localization and function. In: Ort DR, Yocum CF, editors. Oxygenic Photosynthesis. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 539–583. [Google Scholar]
- Zer H, Prasil O, Ohad I. Role of plastoquinol oxidoreduction in regulation of photochemical reaction center II D1 protein turnover in vivo. J Biol Chem. 1994;269:17670–17676. [PubMed] [Google Scholar]