Abstract
Case series
Patients: —
Final Diagnosis: Vulvar leiomyosarcoma
Symptoms: Vulvar mass • vulvar pain • vulvar swelling
Clinical Procedure: —
Specialty: Obstetrics and Gynecology • Oncology
Objective:
Rare disease
Background:
Leiomyosarcomas of the vulva (VLMS) are very rare among gynecological malignancies, with a lack of knowledge on clinical presentation, prognosis, and therapeutic management.
Case Reports:
The database of the German Clinical Center of Competence for Genital Sarcomas and Mixed Tumors in Greifswald (DKSM) was reviewed between the years 2010 and 2020. A total of 8 cases of VLMS were retrieved and analyzed retrospectively.
One exemplary case of VLMS was outlined in detail: A 45-year-old premenopausal woman presented with increasing vulvar swelling and discomfort. Given the suspicion of a Bartholin’s gland abscess, the mass was excised. Final pathology revealed a solid tumor consistent with a moderately differentiated leiomyosarcoma of the vulva. A wide local excision was subsequently performed followed by adjuvant external beam radiation. The clinical features of these 8 cases of VLMS were compared to 26 cases of VLMS found in a review of the literature and to a total of 276 cases of uterine leiomyosarcoma (ULMS) from the same database (DKSM).
Conclusions:
In addition to rapid growth, observed in both tumor entities, VLMS most commonly presented as Bartholin’s gland abscess or cyst and ULMS as leiomyoma. In this cohort, the prognosis of VLMS was much better than that of ULMS, most probably due to the significantly smaller tumor size of VLMS at diagnosis. Further data and larger studies on VLMS are needed to calculate recurrence and survival rates more accurately and define the role of adjuvant radiotherapy.
Keywords: Bartholin’s Glands, Leiomyosarcoma, Uterine Neoplasms, Vulvar Neoplasms
Background
Soft tissue sarcomas are an extremely rare malignant tumor of the female genital tract (<1% of all female genital tract malignancies) [1,2]. Vulvar sarcomas account for approximately 1–3% of all vulvar malignancies and include leiomyosarcomas (LMS), malignant fibrous histiocytomas, dermatofibrosarcomas, malignant rhabdoid tumors, angiosarcomas, angiomyxomas, liposarcomas, chondrosarcomas, rhabdomyosarcomas, and epithelioid sarcomas [1,3–5]. Vulvar LMS (VLMS) is one of the types most frequently found among vulvar sarcomas, accounting for 30–55% of all vulvar sarcomas [1,4–7]. The mean age at diagnosis reported in the literature is 49.8 years (range, 15–84) [4].
VLMS are nonspecifically localized in the subcutaneous tissue of the vulva and are classified as a soft tissue sarcoma (STS) of the extremities and the trunk wall rather than an independent gynecological malignancy or independent tumor entity [5]. Classification and staging of VLMS is performed according to STS. Therapeutic management recommendations are defined in the ESMO and NCCN guidelines [8,9].
In the case of localized disease, the goal of STS surgery is to achieve a complete resection (R0) with an adequate margin of normal tissue [8–10]. The definition of an adequate minimal surgical margin for STS is difficult, as these tumors often involve critical structures and functional considerations have to be made; therefore, in certain cases “just” clear margins are acceptable [8,9,11]. In case of R1 or R2 resections, additional surgery is recommended whenever possible, with the goal to achieve negative margins [8–10]. However, also in the case of positive margins, there is no uniform definition for STS [12]. The most important prognostic factors for local recurrence are tumor grade, margins of excision, and application of radiotherapy [10]. Local recurrence rates for STS of the extremities and the trunk wall range from 5% to 10% [13]. Regarding the influence of surgical margins on the outcome of STS, it has been shown that for critical structure-positive margins, the 5-year local recurrence rate is 14.6%, whereas for negative margins it is 6.5% [11]. The 5-year cause-specific survival rates of STS are 80.3% for negative margins, 59.4% for critical structure-positive margins, 84.7% for tumor bed re-excision-positive margins, and 59.2% for unexpected positive margins during primary resection [11].
For vulvar sarcomas, the 5-year overall survival (OS) rate is approximately 70%. High-grade tumors display a lower 5-year OS rate, tumor size however does not affect the prognosis [7]. Median time to local recurrence has been reported to be 37 months for vulvar sarcomas [6]. For vulvar LMS, the local recurrence rate and rate of metastasis are reported to be 68% and 32%, respectively [4]. The tumor size of VLMS does not seem to affect the risk of local recurrence [4].
In general, diagnosis of LMS can be made if at least 2 of the histological criteria “tumor cell necrosis”, “mitotic index (MI) ≥10 mitoses (M) per 10 high-power fields (HPF)”, and “significant diffuse or multifocal moderate-to-severe atypia” are present. The median MI is approximately 20 M/10 HPF [14]. Immunohistochemical analysis of LMS is typically positive for the markers representing smooth-muscle differentiation, such as smooth-muscle actin (SMA), desmin, and h-caldesmon [5,15]. The marker S-100 is usually negative, but weak positivity has been reported in a few cases [15].
Regarding hormone receptor expression, data on vulvar LMS are scarce. For uterine LMS, findings in the literature are contradictory. While some report absent or only weak expression of estrogen and progesterone receptors (ER, PR), others have observed ER expression in 63% [16] to 100% [17] and PR expression in up to 50% [16] of ULMS.
About 40% of all LMS arise from the uterine tissue [18] and ULMS is the most common subtype of uterine sarcomas, constituting approximately 25–30% [5].
The mean age at disease onset for ULMS is 53.9 years, with a range from 31 to 90 years [5]. In a recent multicenter cohort study [19], the main symptoms of ULMS have been described in detail: additional uterine bleeding (19.5%), postmenopausal bleeding (28.2%), hypermenorrhea (10%), dysmenorrhea (1.8%), rapid growth (53.4%), and other symptoms without bleeding disorders (41.6%). Eleven to fourteen percent of ULMS are diagnosed incidentally during a diagnostic workup for other reasons [5,20]. The mean tumor size of ULMS is 9.7 cm (range, 0.7–30 cm) [5], and in 67–87.9% the tumor has already reached a diameter of 5 cm at the time of diagnosis [5,21].
Tumor staging of ULMS is reported according to FIGO stage [22] and therapeutic management of ULMS in Europe is summarized in the guidelines of the DGGG and OEGGG [22]. The gold standard for surgical treatment of ULMS confined to the uterus is a total hysterectomy without morcellation and with or without bilateral salpingo-oophorectomy, depending on the patient’s menopausal status [22–24]. Systematic pelvic and paraaortic lymph node dissection (LND) is not generally recommended due to the low incidence of lymph node metastasis of around 6.6% in ULMS [22,23,25,26].
Adjuvant therapeutic regimens are also not generally recommended, as there is a lack of evidence regarding their benefit on OS [22,27]. However, adjuvant chemotherapy (CHT) could be discussed in individual cases, in which additional risk factors, such as higher tumor stage, are present [28]. Adjuvant radiation therapy (RT) might be considered in cases with positive surgical margins (R1/R2 resections) [22].
The prognosis of ULMS in general is poor. Five-year OS rates vary across studies and are highly dependent on the tumor stage. For stage I ULMS, 5-year OS rates have been shown to range from 38% [29] to 55.2% [30]. Other studies have reported 5-year OS rates of 57% for FIGO stages I and II [31]. Across all tumor stages (FIGO I–IV), 5-year OS has been shown to be approximately 40% [14,31]. The recurrence rate for stage I disease was found to be up to 76%, and median time to recurrence, calculated across all stages, 19.7 months [29]. The most important prognostic factors identified for ULMS are age, FIGO stage, tumor size, resection margin, and mitotic index [5,31].
Because of their rarity, there is a scarcity of data on VLMS with regards to clinical presentation, prognostic factors, and prognosis in general. Therefore, we retrospectively analyzed 8 cases of VLMS from the DKSM and gave detailed insight into an exemplary case of VLMS to provide additional data on this tumor entity, help identify the most common clinical symptoms and possible prognostic factors, and shed light on their overall prognosis. The current findings are compared with the existing body of literature on VLMS as well as available data from the DKSM on ULMS.
Case Reports
Report of an Exemplary Case of the 8 VLMS Cases
In December 2020, a 45-year-old premenopausal woman (gravida V, para IV) of Arabic origin presented to the Department of Gynecology and Obstetrics with a 6-week history of a vulvar mass with an increase in size over the past 2 days. The patient reported vulvar discomfort and no other symptoms. Her medical history was solely significant for 4 spontaneous vaginal deliveries.
The clinical examination revealed a firm, plum-sized (6–7 cm) swelling of the vulva on the left side, located at the caudal end of the labium minus, in the expected region of the Bartholin’s gland. It was tender to palpation and blocked the introitus. The findings were considered most consistent with an abscess or cyst of the Bartholin’s gland. No further examinations were performed due to the pain, and a local excision was recommended.
The surgery was performed under general anesthesia. Intraoperatively, the mass was located in the region of the Bartholin’s gland on the left side. The vaginal walls were smooth and the cervix was unsuspicious. On palpation, the uterus was normal in size and the adnexa, parametria, and rectum were free. Incision of the skin covering the mass revealed a solid, greenish tumor located directly subcutaneously. There was no evidence of an abscess. The tumor was macroscopically excised entirely and sent for histological analysis.
The final pathology revealed a moderately differentiated LMS of the vulva, measuring 6.5 cm with positive margins (not further specified), resulting in a tumor stage pT1b L0 V0 Pn0 R1. The case was presented at the interdisciplinary sarcoma board, and a pelvic MRI performed 2 weeks after the resection revealed no evidence of a residual mass or inguino-femoral lymph node enlargement. A CT scan of the thorax, abdomen, and pelvis did not show distant metastases.
Given the evidence of microscopical disease on the resection margin, a left hemi-vulvectomy was performed. Residual microscopic disease was detected in the re-excision specimen, resulting in an R0-resection with a minimum of 5-mm tumor-free resection margins towards the medial margin and >10 mm towards the remaining circumference. The final tumor stage was pT2 pNX L0 V0 R0 G2. Tumor cells were positive for SMA, desmin, and caldesmon, the proliferation index Ki-67 was 5%, and the MI was 16 M/10 HPF.
Based on the report of the external counseling by the DKSM and the interdisciplinary sarcoma board at the Technical University Munich, adjuvant external beam radiation therapy (EBRT) was recommended along with an intensive follow-up consisting of clinical examinations, pelvic MRIs, and CT imaging of the thorax every 3 months.
The patient was discharged on postoperative day 5 on oral pain medication. She underwent the recommended adjuvant RT from 03/10/21 until 04/23/21, consisting of 25×2 Gy for a total of 50 Gy with a boost to the former tumor bed of 2 Gy for a total of 60 Gy in 5 fractions. Aside from some moist epitheliolysis on the mons pubis and the bilateral groins, the RT was well tolerated. The first follow-up appointment and the proposed imaging showed no evidence of residual or recurrent local or distant disease. After 16 months, the patient reports a good control of her anal sphincter and has resumed sexual activity.
Patients
Data were taken from the prospectively designed DKSM registry and retrospectively analyzed. The DKSM Greifswald is the referral center for second opinions on gynecological sarcomas in Germany. From 2010 to 2020, 8 cases of VLMS that received a second opinion were identified in the database. Additionally, a search for ULMS within the database of the DKSM revealed 335 counseling cases. Of these, 276 cases with stage pT1 and pT2 were extracted for further analysis.
Of the symptoms analyzed, the definition of rapid growth is controversial. For practical reasons, we refer, for the purposes of this analysis, to the approach of a recent multicenter cohort study [19], and consider VLMS and ULMS as exhibiting rapid or conspicuous growth if the attending medical physician clinically or sonographically described this condition per se.
All patients had given written informed consent for the anonymized scientific utilization of their data.
Statistical Analysis
Using SPSS 27, OS rates of VLMS and ULSM were determined by Kaplan-Meier survival analyses concomitant with the log-rank test. Differences in categorial variables were compared using the chi-square test or Fisher’s exact test and in continuous variables by means of the t test. A P value of <0.05 was considered to be statistically significant.
Method of Literature Search
A literature search was performed to provide an overview of the cases and case characteristics of VLMS described in the past 20 years. We conducted the search in the PubMed® database from the years 2002 to 2022 for the terms “vulva AND leiomyosarcoma”. This yielded a total of 52 citations. Based on the titles, the abstracts, or the full text articles, we excluded 36 of them, mostly due to insufficient information on the tumor characteristics of the cases with VLMS or because of the lack of full text availability. In the end, 16 papers on LMS of the vulva were selected from this search. Four additional articles on LMS of the vulva were selected due to their citation in several of the papers. Thus, a total of 20 original publications on LMS of the vulva were taken into account to use for the discussion and review of literature, including the table of literature (Figure 1) [32–51].
Figure 1.
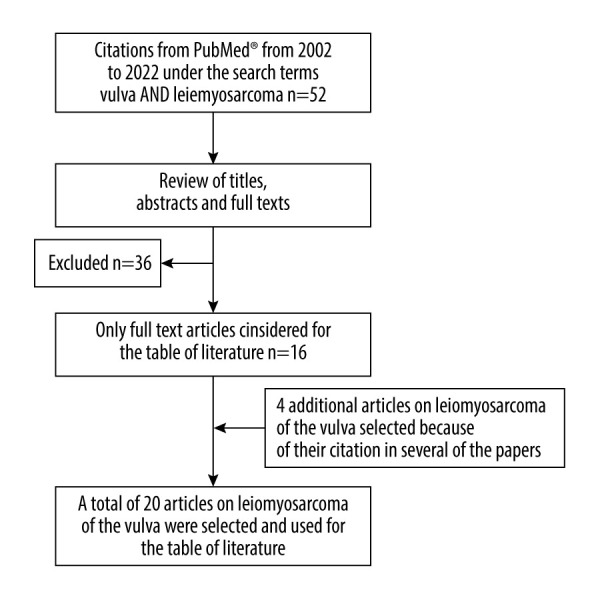
Flowchart of the literature search and selection process for the literature used in the review and discussion.
Results of the Data of the 8 VLMS Cases and ULMS Cases of the DKSM
The clinical data and characteristics of the VLMS and ULMS of the DKSM database are summarized in Table 1. Additionally, to provide a comprehensive overview, the case characteristics of the 8 VLMS of the DKSM are laid out in Table 2. Of the 8 VLMS, 50% were found to be stage pT1 and 50% were stage pT2. Of the 276 ULMS, 85.9% were at stage pT1 and 14.1% at stage pT2. The mean and median age of the patients with VLMS was 59.8 (range, 45–81 years) and 54 years, respectively. Three of the 8 women (37.5%) were postmenopausal at the time of diagnosis. The mean and median age of patients with an ULSM was 53.0 (range, 25–80 years) and 51 years, respectively. Of the 276 women, 148 (53.6%) were postmenopausal. The differences in age and postmenopausal state at the time of diagnosis in VLMS compared to ULMS were not statistically significant (t test, P=0.08 and Fisher’s exact test, P=0.48).
Table 1.
Clinical data of vulvar and uterine leiomyosarcomas of the DKSM database.
| VLMS | ULMS | Significance (P value) | |
|---|---|---|---|
| n | 8 | 276 | – |
| Stage 1/2 | 50/50 | 85.9/14.1 | – |
| Age (years) median/mean, range | 59.8/54, 45–81 | 53.0/51, 25–80 | 0.08 |
| Postmenopause (%) | 37.5 | 53.6 | 0.48 |
| Rapid growth (%) | 100 | 75 | – |
| Misdiagnosis (%) | 66.7 (Bartholin’s gland abscess/cyst) | 75 (leiomyoma) | – |
| Tumor diameter (cm) | 4.9, 2.5–7.6 | 10.3, 1.5–40 | <0.01 |
| Lymphadenectomy (%) | 0 | 14 | – |
| External beam radiation (%) | 37.5 | 3.4 | – |
| Chemotherapy (%) | 0 | 8 | – |
| Follow-up (months) median, range | 73, 16–106 | 49, 2–220 | – |
| Overall survival (%) | 100 | 59 | 0.043 |
VLMS – vulvar leiomyosarcoma; ULMS – uterine leiomyosarcoma.
Table 2.
Summary of the case characteristics of the 8 VLMS of the DKSM.
| Pt Nr | Age (years) | Menopausal state | Presenting symptom | Initial diagnosis | Tumor size | Initial surgery | 2° surgery | R-Status |
|---|---|---|---|---|---|---|---|---|
| 1 | 45 | Premenopausal | Painful growing swelling | Bartholin‘s gland cyst or abscess | 76 mm | TE; not completely removed (R1) | Partial vulvectomy with partial negative margins after resection of the M. sphincter ani | R0 (positive margins after tumor excision, 2° operation) |
| 2 | 52 | Premenopausal | Occlusion of the introitus | chronic Bartholin‘s gland cyst | 47 mm | TE | Re-excision recommended | R1 |
| 3 | 81 | Postmenopausal | n.s. | n.s. | 64 mm | radical vulvectomy with intraoperative frozen section to ensure complete resection | None | R0 |
| 4 | 53 | Premenopausal | n.s. | vulvar tumor | 25 mm | TE | Left hemi-vulvectomy | R0 |
| 5 | 73 | Postmenopausal | n.s. | n.s. | 35 mm | TE; not completely removed (R1) | Re-excision | R0 |
| 6 | 52 | Peri-menopausal | Foreign body sensation, growing tumor | Bartholin‘s gland abscess | 50 mm | marsupialization; tumor not completely removed (R1) | Right hemi-vulvectomy and negative margins after reconstruction by VY-flap plastic | R0 (positive margins after tumor excision, 2° operation) |
| 7 | 68 | Postmenopausal | Growing mass | tumor increasing in size | 25 mm | TE; not completely removed (R1) | Radical right vulvectomy | R0 |
| 8 | 54 | Peri-menopausal | n.s. | Bartholin‘s gland abscess | 33 mm | TE; completely removed | None | R0 |
| Pt Nr | Margins | Tumor stage | Grading | Caldesmon | Desmin | SMA | S-100 | Ki-67 |
|---|---|---|---|---|---|---|---|---|
| 1 | Resection margin ≥5 mm | pT2 pNx L0 V0 Pn0 cM0 | G2 | + | + | + | n.s. | 5% |
| 2 | Margins partially infiltrated | pT1a pNx Lx Vx Pnx cM0 | G2 | n.s. | n.s. | + | n.s. | 30–70% |
| 3 | n.s. | pT2a pNx L0 V0 Pnx cM0 | G1 | + | n.s. | + | Negative | Slightly increased |
| 4 | Resection margin >10 mm | pT1a pNx Lx Vx Pnx cM0 | G2 | n.s. | + | + | n.s. | 60% |
| 5 | Resection margin 3 mm | pT1a pNx Lx Vx Pnx cM0 | G1 | n.s. | + | + | n.s. | n.s. |
| 6 | n.s. | pT2a pNx Lx Vx Pnx cM0 | G2 | Focally + | + | + | Negative | 30% |
| 7 | Resection margin ≥10 mm | pT1a pNx Lx Vx Pnx cM0 | G3 | Negative | Negative | + | Nuclear + in 10% | 80% |
| 8 | n.s. | pT1a pNx L0 V0 Pnx cM0 | G2 | n.s. | + | (+) | Negative | 50% |
| Pt Nr | Mitosis rate | ER | PR | Recommendation for adjuvant therapy | RT | CHT | Endocrine therapy | Follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | 16 MF/10 HPF | n.s. | n.s. | RT | EBRT with boost; 25×2 Gy with a boost to the former tumor bed of 5×2 Gy (total of 60 Gy) | None | none | 16 months |
| 2 | Frequent MF | n.s. | n.s. | Could not be made due to missing information on re-excision | none | None | none | 106 months |
| 3 | 5 MF/10 HPF | + | + | RT | n.s. | n.s. | n.s. | 24 months |
| 4 | >10 MF/10 HPF | (+) | (+) | None | None | None | None | 76 months |
| 5 | n.s. | n.s. | n.s. | RT | n.s. | n.s. | n.s. | 48 months |
| 6 | n.s. | n.s. | n.s. | RT | EBRT, total dose of 60 Gy (single dose 2 Gy) to tumor region and regional lymph nodes | None | None | 80 months |
| 7 | 33 MF/10 HPF | (+), 5% | Negative | RT | applied (no further information) | None | None | 70 months |
| 8 | 13 MF/10 HPF | n.s. | n.s. | None | None | None | None | 95 months |
n.s. – not specified; TE – tumor excision; R1 – tumor cells found in the resection margin/tumor not completely removed; R0 – negative resection margins/tumor completely removed; pT – pathological tumor size; pNx – unknown pathological lymph node status; L0 – no lymphatic vessel invasion; Lx – unknown lymphatic vessel invasion; V0 – no blood vessel invasion; Vx – unknown blood vessel invasion; Pn0 – no perineural invasion; Pnx – unknown perineural invasion; cM0 – clinically no distant metastasis; MF – mitotic figure; HPF – high-power field; G1/G2/G3 – low/intermediate/high-grade; “+” – positive; (+) – weak positive; SMA – smooth muscle actin; ER – estrogen receptor; PR – progesterone receptor; S-100 – “soluble”-100; Ki-67 – (Kiel)-67 proliferation marker; RT – radiation therapy; EBRT – external beam radiation therapy; Gy – Gray; CHT – chemotherapy.
At presentation, the main symptom of VLMS was a rapidly growing vulvar mass or space-occupying process in 100% (in 4 of the 4 cases with information on initial symptoms). Rapid growth was also the most common symptom in ULMS, accounting for 58%. Further symptoms in ULMS not comparable to VLMS were postmenopausal bleeding (52.7%) and additional uterine bleeding (48.4%).
In 66.7% of the VLMS cases, the initial diagnosis was a Bartholin’s gland abscess or cyst (n=4 of 6 cases with available data). The 2 remaining patients were suspected to have a solid process of the vulva. Of the ULMS 75.6% had surgery under the diagnosis “leiomyoma”, resulting in a high rate of misdiagnosis in both entities.
The mean tumor diameter of VLMS was 4.9 cm (range, 2.5–7.6 cm), whereas the mean tumor diameter of ULMS was 10.3 cm (range, 1.5–40 cm). Despite the small sample size of VLMS, this difference was significant (t test, P<0.01, Cohens d 1.0).
All patients with VLMS were initially treated surgically. Seven underwent tumor resection and 1 had a primary radical vulvectomy (an 81-year-old woman with a 6.4-cm vulvar mass). Five of the patients with initial tumor resection underwent a re-resection (1/5), a partial vulvectomy (1/5), or a hemi-vulvectomy (3/5). None of the VLMS patients and only 14% of the ULMS patients underwent an LND. Margins were tumor-free (R0) in 7 of the 8 VLMS. One specimen was found to have “partially involved” margins. In 5 of the cases of VLMS, the tumor was completely resected with residual tumor found in re-resection specimen (4/5). Of 276 ULMS, 37% were initially treated by total hysterectomy without injuring the uterine surface and exposing the tumor. In 45% of the cases total hysterectomy was performed, but injury to the uterine surface or exposure of the tumor occurred intraoperatively. Other surgical procedures were performed in 18%, resulting in an injury to the uterine surface or exposure of the tumor in all cases.
Histology revealed a LMS of the vulva in all 8 cases. Information on grading was available for each of the 8 patients with VLMS: 2 patients had a well-differentiated (G1) LMS, 5 had a moderately differentiated (G2) LMS, and 1 had a poorly differentiated (G3) LMS.
Immunohistochemical analysis of the tumor cells in the VLMS showed positivity for SMA in all 8 (100.0%) cases. Desminpositivity was detected in 5 of the 6 (83.3%) cases in which the marker was evaluated. Staining for ER and PR was done in 3 of the 8 cases of VLMS. In all 3 cases, ER was at least partially positive, and 2 of 3 cases displayed a weak or strong positivity for PR. The tumor cells in 3 of 4 cases of the VLMS did not show staining for the S-100 protein. A high variability was observed for the proliferation marker Ki-67; it ranged from 5% to 80%. Accordingly, the MI also showed a wide range from 5 to 33 mitoses/10 HPF.
Adjuvant RT was recommended for 5 of the 8 patients with VLMS and was actually performed in 3 of these patients. Information on the radiation dose was available for 2 patients, both of whom were treated by EBRT for a total of 60 Gy. None of the women with VLMS received adjuvant CHT or antihormonal treatment. Of the patients with ULMS, 3.4% received EBRT and 8% received CHT.
Data on follow-up were available for all 8 patients with VLMS. The median duration of follow-up was 73 months (range, 16–106 months). All patients were recurrence-free at the time of their last reported consultation. The median duration of follow-up for ULMS patients was 49 months (range, 2–220 month).
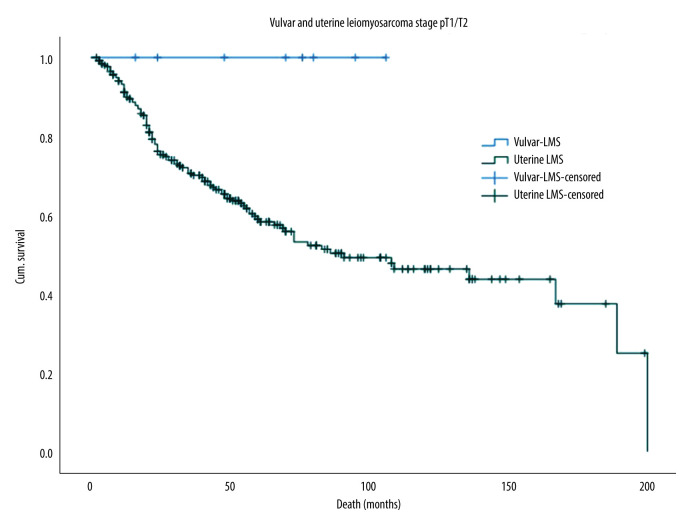
The 5-year OS rate for VLMS and ULMS was 100.0% and 59.0%, respectively, as illustrated in the Kaplan-Meier survival curves in Figure 2. This difference was statistically significant despite the small sample size (log-rank test, P=0.043).
Figure 2.
Kaplan-Meier survival curves for the OS of VLMS and ULMS. OS – overall survival; LMS – leiomyosarcoma; ULMS – uterine leiomyosarcoma; VLMS – vulvar leiomyosarcoma.
Discussion
VLMS and Review of the Literature
Vulvar sarcomas represent an uncommon malignant tumor of the female genital tract and account for 1–3% of all vulvar malignancies. VLMS are the most common type of vulvar sarcomas, constituting approximately 30–55%, but they are still extremely rare. The current body of literature consists of case reports or small case series, reporting only tumor characteristics and follow-up, which makes it difficult to predict prognosis, recurrence risk, and a potential efficacy of adjuvant treatment modalities. In order to increase the knowledge of and the insight into VLMS, we compared our case series (summarized in Table 2) to the available cases of the past 20 years (summarized in Table 3) found in PubMed® [32–51].
Table 3.
Summary of the published cases of leiomyosarcoma of the vulva and their characteristics over the past 20 years [32–51].
| Author, yr | No of Pts | Age(yrs) | Localization | Symptoms | Initial diagnosis | Diagnosis | Tumor size | Staging |
|---|---|---|---|---|---|---|---|---|
| Yordanov et al, 2020 | 1 | 73 | Symphysis | Pain syndrome, present for years, rapid growth in the last 3 months | n.s. | Leiomyosarcoma | 70 mm, satellite nodules | cM0 |
| Saquib et al, 2020 | 1 | 63 | Bartholin‘s gland, left | Painless swelling, >1y | Chronic Bartholin‘s gland cyst | Leiomyosarcoma | 28 mm | cM0, cN0 |
| Aljehani et al, 2021 | 1 | 38, pregnant | Bartholin‘s gland, left | Small vulvar mass since 1y, rapid growth in pregnancy, painless | Benign, Bartholin‘s cyst | Leiomyosarcoma | 100 mm | cM0 |
| Korkmaz et al, 2016 | 1 | 65 | Bartholin‘s gland, left | Vulvar lump with progressive enlargement over 6 months | Angiofibroma (after biopsy) | Leiomyosarcoma | 60 mm | cM0 |
| Alnafisah et al, 2016 | 1 | 37 | Vulva, left | Vulvar lump with progressive enlargement | n.s. | Leiomyosarcoma | 50 mm | Initially cM0 cN+ |
| Akrivi et al, 2021 | 1 | 42 | Area of the left Bartholin‘s gland | 6 months, progressive swelling | Chronic Bartholin‘s gland abscess | Leiomyosarcoma | 65 mm | cM0 |
| Mowers et al, 2014 | 1 | 48 | Bartholin‘s gland, left | Induration for 5 years, sudden rapid growth, pain, difficulty urinating | Bartholin‘s Gland cyst | Myxoid leiomyosarcoma | 46 mm | cM0 |
| Sameeta et al, 2019 | 1 | 63 | Right posterior fourchette | 6 months, slow growing | n.s. | Leiomyosarcoma | 21 mm | cM0 |
| Sayeed et al, 2017 | 3 | 72 | n.s. | vulvar mass | n.s. | Leiomyosarcoma | 110 mm | n.s. |
| 56 | n.s. | vulvar mass | n.s. | Leiomyosarcoma | 135 mm | n.s. | ||
| 68 | n.s. | vulvar mass | n.s. | Leiomyosarcoma | 55 mm | n.s. | ||
| Swanson et al, 2020 | 3 | 80 | n.s. | n.s. | n.s. | Leiomyosarcoma | 97 mm | n.s. |
| 31 | n.s. | n.s. | n.s. | Leiomyosarcoma | 25 mm | n.s. | ||
| 80 | n.s. | n.s. | n.s. | Leiomyosarcoma | 52 mm | cM1 (liver) | ||
| Nath et al, 2019 | 1 | 38 | Right upper vulva | Pain, ulceration, gradually growing mass over 8 months | n.s. | Leiomyosarcoma | 80 mm | cM0 |
| Smith et al, 2020 | 1 | 46 | Left labia majora | Painless mass | n.s. | Leiomyosarcoma | 30 mm | cM0 |
| Shankar, 2006 | 1 | 58 | Bartholin‘s gland, right | 4-months history, enlarging lump, painless | Not typical features for Bartholin‘s cyst | Leiomyosarcoma | n.s. | n.s. |
| González-Bugatto et al, 2009 | 1 | 52 | Bartholin‘s gland, left | 1–2 cm nodule for 4 years, rapid growth over past 6 months | Left Bartholin‘s gland cyst | Leiomyosarcoma | 60 mm | cM0 |
| Levy et al, 2014 | 1 | 57 | Perineal region of left labia | Tender and uncomfortable mass, increasing in size over past 4–6 weeks | Left Bartholin‘s gland cyst | Leiomyosarcoma | 25 mm | n.s. |
| Teramae et al, 2014 | 1 | 51 | Right side of vulva | Palpable mass for 20 years, rapid growth over 1 year | Aggressive angiomyxoma of the vulva | Leiomyosarcoma | 135 mm | cM0 |
| Tjalma et al, 2005 | 1 | 85 | Right side of vulva | Vulvar discomfort | Soft tissue tumor | Myxoid leiomyosarcoma | 60 mm | n.s. |
| Di Gilio el al, 2004 | 1 | 36, pregnant | Left labia majora | Palpable mass, rapid growth | Left Bartholin‘s gland cyst | Myxoid leiomyosarcoma | 60 mm | cM0 |
| Rawal et al, 2005 | 1 | 81 | Right side of vulva | Exophytic lesion | n.s. | Leiomyosarcoma | 50 mm | cM0 |
| Ulutin et al, 2003 | 3 | 39 | Labia majora | n.s. | n.s. | Leiomyosarcoma | 70 mm | n.s. |
| 37 | Labia majora | n.s. | n.s. | Leiomyosarcoma | 35 mm | n.s. | ||
| 18 | Labia majora | n.s. | n.s. | Leiomyosarcoma | 30 mm | n.s. |
| Author, yr | Surgical therapy | Margins | Microscopy | Grading | Immunohistochemistry | Adjuvant therapy | Follow-up | Ref. No. |
|---|---|---|---|---|---|---|---|---|
| Yordanov et al, 2020 | Wide local excision | n.s. | n.s. | n.s. | SMA +, S-100 –, MyoD – | RT | 3 months, recurrence-free | [32] |
| Saquib et al, 2020 | Excision, 2° left hemi-vulvectomy and left inguinal LNE | Negative margins, pN0 (0/8) | 22 MF/ 10 HPF | G2 | SMA +, Caldesmon +, Desmin +, Ki-67 30%, Vimentin –, S-100 – | None | n.s. | [33] |
| Aljehani et al, 2021 | Tumor resection | n.s. | 9 MF/ 10 HPF | n.s. | SMA +, Caldesmon +, Desmin + | None | 12 months, recurrence-free | [34] |
| Korkmaz et al, 2016 | Local excision | n.s. | 20 MF/ 10 HPF | n.s. | Calponin +, S-100 –, Ki-67 5% | None | 6 months, recurrence-free | [35] |
| Alnafisah et al, 2016 | Wide local excision; 2° left radical vulvectomy plus left inguinal LNE (2° operation after chemotherapy) | Negative margins at wide excision, 2° negative margins but 3.5 mm tumor rest, pN0 (0/3) | n.s. | G3 | SMA +, ER/PR + | CHT, 3 cycles gemcitabine/ docetaxel; again, CHT after 2° operation, 3 cycles gemcitabine/ docetaxel | 15 months: local recurrence, resection and RT; 18 months: lung metastasis, resection | [36] |
| Akrivi et al, 2021 | Wide local excision | Positive margins | 8 MF/ 10 HPF | n.s. | SMA +, Desmin +, Caldesmon +, Vimentin +, ER/ PR +, S-100 –, Myoglobulin – | None | 53 months, recurrence-free | [37] |
| Mowers et al, 2014 | Left radical hemi-vulvectomy; 2° re-excision | Positive margins; negative margins after re-excision | n.s. | G3 | SMA +, Desmin +, ER/PR + | CHT, 6 cycles doxorubicin/ ifosfamide | 18 months, recurrence-free | [38] |
| Sameeta et al, 2019 | Wide local excision | Positive margins | 25 MF/ 10 HPF | G3 | SMA +, Desmin +, ER +, S-100 – | RT | n.s. | [39] |
| Sayeed et al, 2017 | Wide local excision | Positive margins | 8 MF/ 10 HPF | n.s. | n.s. | n.s. | Died of disease | [40] |
| Wide local excision | Positive margins | 34 MF/ 10 HPF | n.s. | n.s. | n.s. | Died of disease | [40] | |
| Wide local excision | Negative margins | 23 MF/ 10 HPF | n.s. | n.s. | n.s. | Died of disease | [40] | |
| Swanson et al, 2020 | Tumor resection | Positive margins | 36 MF/10 HPF | n.s. | SMA +, Caldesmon +, Desmin – | n.s. | 1 month | [41] |
| Tumor resection | Positive margins | 12 MF/ 10 HPF | n.s. | SMA +, Caldesmon +, Desmin +, S-100 – | n.s. | 1 month | [41] | |
| Tumor resection | Negative margins | 12 MF/ 10 HPF | n.s. | n.s. | CHT | 4 months | [41] | |
| Nath et al, 2019 | Wide local excision | n.s. | n.s. | n.s. | SMA +, Vimentin +, Desmin –, S-100 –, ER/PR – | Chemoradiation | 21 months, recurrence-free | [42] |
| Smith et al, 2020 | Tumor resection, 2° left partial radical vulvectomy, 3° posterior radical vulvectomy plus bilateral inguino-femoral LNE | Positive margins, 2° positive margins, 3° negative margins, pN0 | n.s. | n.s. | SMA +, Desmin +, S-100 –, Caldesmon –, MyoD1 – | n.s. | n.s. | [43] |
| Shankar, 2006 | Wide local excision | n.s. | n.s. | n.s. | SMA +, Desmin + | RT intended but not carried out | 42 months, recurrence-free | [44] |
| González- Bugatto et al, 2009 | Tumor resection, 2° hemi-vulvectomy with ipsilateral inguinal LNE | Positive margins, 2° negative margins, pN0 | 21 MF/ 10 HPF | G3 | SMA +, Vimentin +, Desmin +, ER/PR +, S-100 – | RT, 66.6 Gy; CHT with mesna, epirubicin, ifosfamide and cisplatin | 12 months: local recurrence, wide excision, negative margins; 48 months: disease free | [45] |
| Levy et al, 2014 | Tumor resection, 2° radical excision | Positive margins, 2° negative margins | 16 MF/ 10 HPF | n.s. | SMA +, Desmin + | n.s. | n.s. | [46] |
| Teramae et al, 2014 | Tumor resection | Negative margins | 7 MF/ 10 HPF | n.s. | Desmin + | Adjuvant treatment (CHT or RT) was recommended but not wanted | 32 months, recurrence-free | [47] |
| Tjalma et al, 2005 | Wide local excision | Negative margins | 34 MF/ 10 HPF | n.s. | SMA +, Desmin +, ER/PR partially +, S-100 –, Ki-67 20% | None | 25 months, recurrence-free | [48] |
| Di Gilio el al, 2004 | Tumor resection, 2° wide local excision with ipsilateral superficial inguinal node sampling | Positive margins, 2° negative margins, pN0 | 2 MF/ 10 HPF | n.s. | SMA +, Vimentin +, S-100 – | n.s. | 30 months, recurrence-free | [49] |
| Rawal et al, 2005 | Wide local excision | n.s. | 35–40 MF/10 HPF | n.s. | SMA + | none | 9 months: suspicion of local recurrence, died of unrelated cause | [50] |
| Ulutin et al, 2003 | Radical vulvectomy with groin LNE | Close surgical margin | n.s. | G2 | n.s. | RT, 52.2 Gy | 73 months, recurrence-free | [51] |
| Simple vulvectomy | n.s. | n.s. | G2 | n.s. | None | 150 months, recurrence-free | [51] | |
| Radical vulvectomy | n.s. | n.s. | G1 | n.s. | None | 172 months, recurrence-free | [51] |
n.s. – not specified; cM0 – clinically no distant metastasis; cM1 – clinically distant metastasis; cN0 – clinically no lymph node metastasis; pN0 – pathologically no lymph node metastasis; LNE – lymphadenectomy; MF – mitotic figure; HPF – high-power field; G1/G2/G3 – low/intermediate/high-grade; SMA – smooth muscle actin; ER – estrogen receptor; PR – progesterone receptor; S-100 – “soluble”-100; Myo-D1 – myoblast determination protein 1; Ki-67 – (Kiel)-67 proliferation marker; “+” – positive; “–“ – negative; Gy – Gray; RT – radiation therapy; CHT – chemotherapy.
Regarding the age at diagnosis, the 26 cases reported on in Table 3 showed a median age of 54 years (range, 18–85), which was identical with the 54 years (range, 45–81 years) in our case series of 8 patients. Interestingly, the cases retrieved from the literature included 11 patients (42.3%) under the age of 50 (Table 3), but in our series there was only 1 patient (12.5%) younger than 50 years.
Reviewing the selected literature on patients ≥50 years, an adverse event rate of 41.7% (5/12) was observed. Three patients died of disease [40], 1 experienced local recurrence [45], and 1 was suspected to have a local recurrence but died of an unrelated cause [50] (information available on 12 cases). In patients <50 years, 1 adverse event, in the sense of local recurrence, occurred (information available on 10 cases=10%) [36]. These results seem to point towards age (<50 years and ≥50 years) being a possible independent prognostic factor. It is possible, however, that the presumably larger proportion of premenopausal women among the VLMS also has an influence on the prognosis, at least in this cohort.
All in all, there was very inconsistent information on the menopausal state in the cases in the literature, so that no statement about the menopausal state as prognostic factor could be made. However, as already stated above, with an adverse event rate of 41.7% in the age group ≥50 years and 10% in the age group <50 years, an age ≥50 years appears to be an unfavorable prognostic factor. A potential influence of the post-menopause on the OS could not be determined, as the 5-year survival rate for the VLMS of our case series was 100% and the size of the cohort was small.
The main presenting symptoms of VLMS reported in the literature are a vulvar mass or lump (80%; n=16), a rapidly growing vulvar mass (50%; n=10), and pain (25%; n=5) (Table 3). In 20% (n=4) of the cases, the mass or swelling was reported to be painless. In 5 of the 20 cases (25%), a swelling had been present at the same location for at least 1 year, often (n=4) even several years, before rapid growth occurred (information available on 20/26 cases). The main presenting symptom in our case series was a rapid growing vulvar mass in 100% and pain in 25% of the cases with available information.
In our case series, 66.7% of VLMS were initially misdiagnosed as a Bartholin’s gland abscess or cyst. When compared to the existing body of literature on VLMS, 7 of 11 cases were initially diagnosed as a Bartholin’s gland cyst or abscess (63.6%) (Table 3) [33,34,37,38,45,46,49]. There was no information on the initial diagnosis in 15 of the 26 cases.
Given the frequency of misdiagnosis as a benign process and the fact that the anatomical location of some VLMS may be suggestive of a Bartholin’s gland abscess or cyst, the solid nature of the mass and the lack of inflammatory symptoms should encourage clinicians to broaden the differential diagnosis and include more rare, potentially malignant vulvar processes as well.
According to the guidelines of the European Society of Medical Oncology (ESMO) and the National Comprehensive Cancer Network (NCCN) on soft tissue sarcoma, the surgical resection with negative margins is the standard primary treatment [8,9]. However, the extent of the resection margins must be adapted to critical anatomic structures in/close to the area of resection. The potential benefit of wide negative margins has to be weighed against the adverse effects of injuring critical structures. Consequently, neoadjuvant RT or chemoradiation therapy is often used to downstage the tumor and possibly preserve critical structures [9].
Of the 26 cases of VLMS reported in the literature, 100% were treated by primary surgical resection (Table 3). A wide local excision was the most common form of surgical treatment (n=12; 46.2%) of the VLMS, followed by a vulvectomy (hemi or radical) in 30.8% (n=8) and a tumor resection in 23.1% (n=6). A secondary operation due to positive margins was performed in 7 of the 26 cases (re-operation rate 26.9%) and a third operation was done in 1 case (3.8%). In our case series, the re-operation rate was 62.5%. The most common form of definite surgical treatment was a vulvectomy in 50% (radical or partial).
Of the 26 cases summarized in Table 3, 11 patients had negative margins after the definitive operation, 6 had positive margins, 1 had close surgical margins, and in 8 cases no data were available. Five patients with negative margins (45.5%) were reported to be recurrence-free (follow-up time 4–32 months), 2 patients (18.2%) developed a local recurrence after 12 and 15 months, respectively [36,45], 1 patient (9.1%) was diagnosed with pulmonary metastases after 18 months [36], and 1 patient (9.1%) died of disease [40].
Of the 5 patients with negative margins who were reported to be recurrence-free, 2 had received no adjuvant treatment [47,48] and 2 had completed an adjuvant CHT [38,41]. The 2 patients who developed local recurrence [36,45] and metastasis in 1 of these 2 cases [36], had received adjuvant RT plus CHT [45] and adjuvant CHT [36], respectively.
Of the 6 patients with positive margins, 2 died of disease (33.3%) [40], 1 (16.7%) was reported to be recurrence-free after 53 months of follow-up without adjuvant treatment [37], and for 2 (33.3%) the follow-up time was only 1 month [41]. The 1 patient with close surgical margins was found to have a recurrence-free follow-up period of 73 months, following adjuvant RT [51].
Although there is a relatively limited number of cases (n=26) and a lack of data on margins, use of adjuvant treatments, and follow-up, the data from the literature suggests that the margin status is an independent prognostic factor regarding the recurrence rate (Table 3). These findings are in accordance with previous studies [4].
In our case series, 1 patient had a R1-resection and did not undergo the recommended re-resection or adjuvant RT. She remains recurrence-free after a follow-up time of 106 months. Follow-up data are available on all of the 5 patients with an initial incomplete resection: They all remain recurrence-free (follow-up time 16–106 months). In 3 of these cases, adjuvant RT was administered. Despite the small sample size, our data suggest that an R1-resection does not have an influence on the OS rate.
A systematic LND is not considered part of the recommended first-line surgical treatment of soft tissue sarcomas (STS) [8,9]. Of the 26 cases of VLMS reported in the literature, 6 patients underwent a LND, although imaging suggested possible lymph node metastases in the bilateral external iliac lymph nodes in only 1 case (Table 3) [36]. In 5 of these 6 cases, an inguinal LND was performed, and in 1 case an inguino-femoral LND was performed [43]. The inguino-femoral LND was done bilaterally, and 4 of the 5 inguinal LND were done ipsilaterally. Information on the side of the LND was missing in 1 case [51]. No lymph node metastases were detected in any of these 6 cases [33,36,43,45,49,51]. Information on follow-up was provided for 4 of the 6 patients with LND [36,45,49,51]. Two remained recurrence-free after 30 [49] and 73 months [51], respectively, and 2 developed local recurrence after 12 [45] and 15 months [36], respectively. The patient with a local recurrence after 15 months also developed pulmonary metastases 3 months later [36]. Of note, the patient with a local recurrence after 12 months had negative resection margins and had completed adjuvant RT [45]. The patient with a local recurrence and distant metastases had received 3 cycles of adjuvant CHT [36].
None of the patients in our case series underwent an inguinal or inguino-femoral LND. There was no evidence of metastases in imaging in any of the 8 cases, and all patients were reported to be recurrence-free at the time of the last follow-up. These findings suggest that a systematic LND does not reduce the risk of recurrence or improve OS in VLMS and can therefore most likely be omitted, provided staging exams display no evidence of lymph node involvement.
The mean tumor size of VLMS reported in the literature at the time of diagnosis was 6.2 cm (range, 2.1–13.5 cm) (Table 3). In 68% (17 of 25 cases with information on tumor size), the tumor was ≥5.0 cm. Of these patients, 11.8% (n=2) had a local recurrence (at 12 and 15 months, respectively), 5.9% (n=1) had suspicion of local recurrence (but died of non-related disease), and 17.6% (n=3) died of disease. Thus, adverse events occurred in 35.5% of the cases with a VLMS ≥5.0 cm. No recurrences were reported for the group with VLMS <5.0 cm; however, follow-up information was often not available. Based on the cases of the literature, tumor size seems to be an independent prognostic factor (Table 3). These findings are contradictory to the ones reported by Aartsen et al, who found that the risk of local recurrence was unrelated to the tumor size [4].
The mean tumor size in our case series was 4.9 cm (range, 2.5–7.6 cm); 37.5% (n=3) had a tumor that was ≥5.0 cm and 62.5% (n=5) had a tumor that was <5.0 cm. Because no recurrences occurred, a statement on tumor size as a prognostic factor cannot be made.
Information on tumor grading of the VLMS extracted from the literature was provided in 8 of the 26 cases (Table 3). One patient was found to have a G1 tumor [51], 3 had a G2 tumor [33,51], and 4 had a G3 tumor [36,38,39,45].
The patient with the G1 tumor was reported to be recurrence-free after 172 months [51]. Two of the patients with a G2 tumor were recurrence-free after 73 and 150 months, respectively [51]. Information on follow-up was missing on 1 patient with a G2 tumor [33]. Of the 4 patients with a G3 tumor, 50% (n=2) developed a local recurrence after 12 [45] and 15 months [36], respectively, and 25% (n=1) were diagnosed with distant metastases (lung) 18 months following the initial therapy [36]. One patient (25%) with a G3 tumor was recurrence-free after 18 months [38], and follow-up data was missing on 1 patient [39]. Although information regarding the grading of VLMS was scarce, these findings suggest that tumor grade may also be a prognostic factor, with a higher risk for local recurrence and distant metastases in poorly differentiated VLMS.
In our case series, information on tumor grade was available on all of the 8 cases; 2 patients were diagnosed with a G1 tumor, 5 with a G2 tumor, and 1 with a G3 tumor. The median duration of follow-up was 73 months (range, 16–106 months) for all tumors. For the 2 patients with the G1 tumor, follow-up after 24 and 48 months, respectively, showed no signs of relapse. The 5 patients with a G2 tumor were reported to be recurrence-free after 16, 76, 80, 95, and 106 months, respectively, and the 1 patient with a G3 tumor remained recurrence-free after 70 months.
VLMS appear to metastasize very rarely. In our case series, none of the 8 patients had distant metastases at the time of diagnosis or at the time of their last follow-up. In the literature, distant metastases at the time of initial diagnosis were reported in 1 of 26 cases (3.8%, hepatic) [41]. Lymph node metastasis was not described in any of the patients included in our case series or extracted from the literature (Table 3). An imaging-based suspicion of lymph node metastases in 1 patient was not confirmed histologically [36].
The histological and immunohistochemical characteristics of VLMS reported in the literature are summarized in Table 3. Positivity for SMA was found in 100% (17/17 cases with staining information available), positivity for desmin in 85.7% (12/14 cases), positivity for caldesmon in 83.3% (5/6 cases), and positivity for vimentin in 80% (4/5 cases) of the cases. Analysis for S-100 was documented in 11 cases, all of which were negative. ER was positive in 85.7% (6/7 patients) and PR was positive in 83.3% (5/6 patients).
The patients in our case series displayed a similar histological and immunohistochemical pattern. SMA was positive in 100% of the cases (8/8) and desmin in 83.3% (5/6). The staining for S-100 was negative in 75% (3/4) of the VLMS. ER and PR were assessed in a total of 3 of the 8 cases. ER was at least partially positive in all 3 cases (100%), whereas PR was positive in 66.7% (2/3).
One of the histological criteria for LMS is a MI ≥10 M/10 HPF, with a median MI of approximately 20 M/10 HPF [14]. Information on the MI was available in 17 of the 26 cases from the literature (Table 3). The median MI was calculated to be 20 M/10 HPF, ranging from 2 to 35–40 M/10 HPF. Our case series demonstrated a median MI of 13 M/10 HPF with a range from 5 to 33 M/10 HPF.
According to the ESMO and the NCCN clinical practice guidelines on soft tissue sarcoma, RT can be administered in the neoadjuvant or adjuvant setting [8,9]. Adjuvant RT is recommended for high-grade, deep tumors (located beneath the superficial fascia, on both sides of the fascia or growing through the superficial fascia) of >5 cm, as well as in the case of positive or close resection margins [8,9]. Postoperative RT has been shown to improve local control in patients with positive surgical margins, with the risk of added radiation-related toxicity [9]. The total recommended radiation dose is 50 Gy in fractions of 1.8–2 Gy, with a possible boost up to 66 Gy [8]. The goal of preoperative RT is to reduce the surgery-related morbidity and improve the functional outcome and quality of life [8]. At present, there is no consensus on the role of adjuvant CHT in STS. While the effect on OS is not clear, postoperative CHT may improve relapse-free survival [9]. CHT regimens that have been used for STS include doxorubicin mono, doxorubicin and ifosfamide, epirubicin and ifosfamide, cyclophosphamide/ vincristine/doxorubicin±dacarbazine, and ifosfamide/dacarbazine plus doxorubicin [9]. Although it is not part of the standard treatment in adult-type STS, adjuvant CHT can be proposed for high-risk situations, such as high-grade, deep tumor, and those >5 cm [8]. Neoadjuvant CHT has not been shown to improve survival, but may downstage the disease [9]. In patients with high-grade STS of the extremity and body wall, preoperative chemoradiation followed by surgery and postoperative doxorubicin-based CHT seems to improve local control, disease-free survival, and OS in long-term follow-up [9].
In our review of the literature, 10 of the 26 patients with VLMS underwent adjuvant therapy post-operatively, while 10 cases received no additional therapeutic measures (information missing on 6 patients, Table 3). Of the 10 patients treated by surgery alone, 9 were recurrence-free (mean of 55.7 months, range, 6–172 months, data missing on 1 patient).
Three of the 10 cases with adjuvant therapy (33.3%) underwent adjuvant RT. Of these 3, 2 patients were reported to be recurrence-free after 3 months [32] (tumor size 7.0 cm, margins and grade not reported) and 73 months [51] (tumor size 7.0 cm, close surgical margins, G2), respectively. Follow-up data were missing on 1 patient (tumor size 2.1 cm, R1, G3) [39]. In the case reported by Ulutin et al [51], the patient received a total dose of 52.2 Gy.
Two of 10 patients with adjuvant treatment (20%) underwent adjuvant combined chemoradiation. The patient reported by González-Bugatto et al received a total radiation dose of 66.6 Gy and CHT consisting of epirubicin, ifosfamide, and cisplatin. This patient’s tumor size was 6.0 cm, surgical margins were negative, and final histology showed a high-grade LMS. She developed local recurrence after 12 months [45]. Details on the radiation dose or CHT regimen of the other patient were not provided [43]. The tumor size, however, was 8.0cm, and the patient remained recurrence-free after a follow-up period of 21 months [43].
Three of the 10 cases with adjuvant treatment (33.3%) received adjuvant CHT alone; 2 patients were recurrence-free after 4 and 18 months, respectively [38,41]. The patient who remained recurrence-free for 18 months presented with a poorly differentiated tumor of 4.6 cm in size with negative surgical margins. She was treated with 6 cycles of adjuvant doxorubicin and ifosfamide [38]. One other patient treated with adjuvant CHT, consisting of 3 cycles of gemcitabine and docetaxel, developed a local recurrence after 15 months [36]. Repeat surgery was performed at the time of local recurrence, and another 3 cycles of gemcitabine and docetaxel were administered. However, the patient developed pulmonary metastases only 3 months later. The initial tumor size was 5.0 cm, margins were negative, and the histology showed a high-grade LMS [36].
In our case series, adjuvant EBRT was applied in 3 of the 8 patients; 2 patients received a total of 60 Gy (25×2 Gy with a boost of 5×2 Gy to the former tumor bed). None of the patients received pre- or postoperative CHT or chemoradiation.
The mean follow-up duration described in the literature was 37.8 months (range, 1 [41] to 172 months [51], information available on 22/26 patients, Table 3). Three patients (13.6%) died of disease [40], local recurrence occurred in 2 patients (9.1%) [36,45], and there was suspicion of local recurrence in 1 patient who then died of an unrelated cause [50]. The reported rate of local recurrence is therefore approximately 13.6%, occurring after a mean of 12 months after the initial diagnosis. One patient (4.5%) developed pulmonary metastases 18 months after primary treatment [36]. Sixteen patients were reported to be recurrence-free (72.7%).
For the 8 patients in our case series, the median duration of follow-up was 73 months (range, 16–106 months) and 100% were reported to be recurrence-free at the end of the observation period.
Comparison of VLMS and ULMS
The VLMS cases extracted from the DKSM database including the case presented above were compared to the data on ULMS from the same database. Although the mean age of the patients diagnosed with VLMS (59.8 years) was slightly higher compared to the mean age of patients with ULMS (53.0 years), this difference did not reach statistical significance. Contrary to the younger age of women diagnosed with ULMS, the proportion of postmenopausal women was higher in ULMS (53.6%) compared to VLMS (37.5%).
The mean tumor diameter of the VLMS was 4.9 cm (range, 2.5–7.6 cm). Despite the small sample size, this was significantly smaller (P<0.01) than the mean tumor diameter of ULMS of 10.3 cm (range, 1.5–40.0 cm).
Typical for both tumor entities was a primary misdiagnosis; as Bartholin’s gland abscess or cyst in the case of VLMS in 66.7% and as a leiomyoma in the case of ULMS in 75.6%. In VLMS, caution should be exercised when a suspected Bartholin’s gland cyst or abscess presents as a solid mass on palpation and ultrasound. In ULMS, the preoperative LMS risk score [19] can be applied to significantly reduce the rate of leiomyoma misdiagnosis.
The 5-year OS rate for VLMS and ULMS was 100.0% and 59.0%, respectively. Although the cohort of VLMS patients was considerably smaller than that of ULMS, the difference in survival between VLMS and ULMS was statistically significant (P=0.043) and clinically striking. This may be attributable to the fact that VMLS were diagnosed at a much smaller tumor size and the vast majority (7/8; 87.5%) underwent a complete surgical re-section with negative surgical margins, either during initial surgery (2/8) or at the latest by the time of re-resection (5/8). In the 1 case with final positive margins, the patient had declined the recommended repeat resection.
Unlike ULMS, an incomplete initial surgery in the case of VLMS does not lead to the intraabdominal dissemination of tumor cells. Thus, the anatomical barriers between the vulva and the abdominal cavity could also play a role in the recurrence rates of VLMS.
Hypothetically, the significantly smaller tumor size of VLMS arises from the more superficial and clinically accessible localization of this tumor, resulting in the development of earlier symptoms compared to ULMS. Furthermore, VLMS are easily accessible for biopsy procedures, with minimal surgical risk. This may reduce the threshold for immediate histological clarification, but also for an inadequate surgical approach. In contrast, in ULMS the situation must be thoroughly evaluated by a preoperative LMS risk score [19] and weighed against potential risks in the case of abdominal surgery. Thus, in asymptomatic, and in particular premenopausal women, with a uterine mass, initial expectant management could potentially lead to a delay in diagnosis and treatment.
Conclusions
Given the rarity of VLMS, it is crucial to understand the presenting symptoms and provide the appropriate surgical management without delay. The main presenting symptom is unilateral swelling in combination with a fast-growing tumor, sometimes with pain. Despite mostly absent signs of inflammation, the lesion is often interpreted as a Bartholin’s gland abscess or cyst. Caution should be exercised when a suspected Bartholin’s gland cyst or abscess presents as a solid mass on palpation and ultrasound. In those cases, adequate surgical procedure should be planned.
Because of their superficial localization, patients develop symptoms earlier than with ULMS and are diagnosed at a significantly smaller tumor size. The significantly better prognosis of the VLMS compared to the ULMS is likely a result of smaller tumor size at diagnosis.
The benefit of LND and postoperative CHT is not supported by any data. Regarding postoperative radiotherapy, the data are inconsistent. An age of ≥50 years, resection margins, tumor size ≥5 cm, and a poor differentiation (G3) seemed to be independent prognostic factors regarding recurrence in VLMS. The impact on the OS is still unclear.
Clearly, the significance of the statements made by this publication are limited by the small sample size of VLMS.
We recommend that all cases of VLMS be treated at a specialized referral center and presented at an interdisciplinary tumor board to provide patients with the greatest possible expertise in therapeutic management and to enable data collection on such rare tumors.
Abbreviations
- CHT
chemotherapy;
- DKSM
German Clinical Center of Competence for Genital Sarcomas and Mixed Tumors in Greifswald;
- EBRT
external beam radiation therapy;
- ER
estrogen receptor;
- ESMO
European Society of Medical Oncology;
- HPF
high-power fields;
- LMS
leiomyosarcoma;
- LND
lymph node dissection;
- MI
mitotic index;
- NCCN
National Comprehensive Cancer Network;
- OS
overall survival;
- PR
progesterone receptor;
- RT
radiation therapy;
- SMA
smooth muscle actin;
- STS
soft tissue sarcoma;
- ULMS
uterine leiomyosarcoma;
- VLMS
vulvar leiomyosarcoma.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Department and Institution Where Work Was Done
The work was done at the Department of Obstetrics and Gynecology, Lucerne Cantonal Hospital and at the University Medicine Greifswald, German Clinical Center of Competence for Genital Sarcomas and Mixed Tumors (DKSM), Department of Gynecology and Obstetrics, Greifswald, Germany.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Jones ISC, Crandon A, Sanday K. Vulvar sarcomas: A 25 plus-year experience from Queensland. Open J Obstet Gynecol. 2013;3(0):37–40. [Google Scholar]
- 2.Sleijfer S, Seynaeve C, Verweij J. Gynaecological sarcomas. Curr Opin Oncol. 2007;19(5):492–96. doi: 10.1097/CCO.0b013e3282748eaa. [DOI] [PubMed] [Google Scholar]
- 3.Iavazzo C, Gkegkes ID, Vrachnis N. Dilemmas in the management of patients with vulval epithelioid sarcoma: A literature review. Eur J Obstet Gynecol Reprod Biol. 2014;176:1–4. doi: 10.1016/j.ejogrb.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Aartsen EJ, Albus-Lutter ChE. Vulvar sarcoma: Clinical implications. Eur J Obstet Gynecol Reprod Biol. 1994;56(3):181–89. doi: 10.1016/0028-2243(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 5.Köhler G, Evert M, Evert K, Zygmunt M. Leiomyosarcoma. In: Köhler G, Evert M, Evert K, Zygmunt M, editors. Sarcoma of the female genitalia. Vol 1: Smooth muscle and stromal tumors and prevention of inadequate surgery [Internet] Vol. 1. Berlin/Boston: De Gruyter; 2016. [Google Scholar]
- 6.Behranwala KA, Latifaj B, Blake P, et al. Vulvar soft tissue tumors. Int J Gynecol Cancer. 2004;14(1):94–99. doi: 10.1111/j.1048-891x.2004.14946.x. [DOI] [PubMed] [Google Scholar]
- 7.Curtin J. Soft-tissue sarcoma of the vagina and vulva: A clinicopathologic study. Obstet Gynecol. 1995;86(2):269–72. doi: 10.1016/0029-7844(95)00160-s. [DOI] [PubMed] [Google Scholar]
- 8.Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii102–12. doi: 10.1093/annonc/mdu254. [DOI] [PubMed] [Google Scholar]
- 9.von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(6):758–86. doi: 10.6004/jnccn.2016.0078. [DOI] [PubMed] [Google Scholar]
- 10.Grimer R, Judson I, Peake D, Seddon B. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:1–15. doi: 10.1155/2010/506182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimer RJ. On the effect of setting of a positive surgical margin in soft tis-sue sarcoma: Editorial. Cancer. 2014;120(18):2803–5. doi: 10.1002/cncr.28781. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell PW, Griffin AM, Eward WC, et al. The effect of the setting of a positive surgical margin in soft tissue sarcoma: Positive Margins in Soft Tissue Sarcoma. Cancer. 2014;120(18):2866–75. doi: 10.1002/cncr.28793. [DOI] [PubMed] [Google Scholar]
- 13.Gronchi A, Colombo C, Raut CP. Surgical management of localized soft tis-sue tumors: Surgery in Soft Tissue Tumors. Cancer. 2014;120(17):2638–48. doi: 10.1002/cncr.28715. [DOI] [PubMed] [Google Scholar]
- 14.Iasonos A, Keung EZ, Zivanovic O, et al. External validation of a prognostic nomogram for overall survival in women with uterine leiomyosarcoma: External Validation of Nomogram for ULMS. Cancer. 2013;119(10):1816–22. doi: 10.1002/cncr.27971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abeler VM, Nenodovic M. Diagnostic immunohistochemistry in uterine sarcomas: A study of 397 cases. Int J Gynecol Pathol. 2011;33(3):236–43. doi: 10.1097/PGP.0b013e318200caff. [DOI] [PubMed] [Google Scholar]
- 16.Hensley ML, Wathen JK, Maki RG, et al. Adjuvant therapy for high-grade, uterus-limited leiomyosarcoma: Results of a phase 2 trial (SARC 005) Cancer. 2013;119(8):1555–61. doi: 10.1002/cncr.27942. [DOI] [PubMed] [Google Scholar]
- 17.Ioffe YJ, Li AJ, Walsh CS, et al. Hormone receptor expression in uterine sarcomas: Prognostic and therapeutic roles. Gynecol Oncol. 2009;115(3):466–71. doi: 10.1016/j.ygyno.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int J Cancer. 2006;119(12):2922–30. doi: 10.1002/ijc.22239. [DOI] [PubMed] [Google Scholar]
- 19.Köhler G, Vollmer M, Nath N, et al. Benign uterine mass – discrimination from leiomyosarcoma by a preoperative risk score: A multicenter cohort study. Arch Gynecol Obstet. 2019;300(6):1719–27. doi: 10.1007/s00404-019-05344-0. [DOI] [PubMed] [Google Scholar]
- 20.Leitao MM, Tornos C, Wolfson A, O’Cearbhaill R. Corpus: Mesenchymaltumors. In: Barakat RR, Berchuk A, Markman M, Randall ME, editors. Principles and practice of gynecologic oncology. 6th edn. Lippincott Williams & Wilkins; Philadelphia: 2013. online ed. pos. 47172–74393. In: Principles and Practice of Gynecologic Oncology Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 21.Abeler VM, Røyne O, Thoresen S, et al. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54(3):355–64. doi: 10.1111/j.1365-2559.2009.03231.x. [DOI] [PubMed] [Google Scholar]
- 22.Denschlag D, Ackermann S, Battista MJ, et al. Sarcoma of the uterus. Guideline of the DGGG and OEGGG (S2k Level, AWMF Register Number 015/074, February 2019). Geburtshilfe Frauenheilkd. 2019;79(10):1043–60. doi: 10.1055/a-0882-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: Emphasis on impact of lymphadenectomy and oophorectomy. Cancer. 2008;112(4):820–30. doi: 10.1002/cncr.23245. [DOI] [PubMed] [Google Scholar]
- 24.Nasioudis D, Chapman-Davis E, Frey M, Holcomb K. Safety of ovarian preservation in premenopausal women with stage I uterine sarcoma. J Gynecol Oncol. 2017;28(4):e46. doi: 10.3802/jgo.2017.28.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leitao MM, Sonoda Y, Brennan MF, et al. Incidence of lymph node and ovarian metastases in leiomyosarcoma of the uterus. Gynecol Oncol. 2003;91(1):209–12. doi: 10.1016/s0090-8258(03)00478-5. [DOI] [PubMed] [Google Scholar]
- 26.Seagle B-LL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: treatment of uterine leiomyosarcoma: A National Cancer Database study. Gynecol Oncol. 2017;145(1):61–70. doi: 10.1016/j.ygyno.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Bogani G, Fucà G, Maltese G, et al. Efficacy of adjuvant chemotherapy in early stage uterine leiomyosarcoma: A systematic review and meta-analysis. Gynecol Oncol. 2016;143(2):443–47. doi: 10.1016/j.ygyno.2016.07.110. [DOI] [PubMed] [Google Scholar]
- 28.Denschlag D, Ackermann S, Battista MJ, et al. Sarcoma of the uterus. Guideline of the DGGG, OEGGG and SGGG (S2k-Level, AWMF Registry No. 015/074, April 2021). Geburtshilfe Frauenheilkd. 2022;82(12):1337–67. doi: 10.1055/a-1897-5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia C, Kubat JS, Fulton RS, et al. Clinical outcomes and prognostic mark-ers in uterine leiomyosarcoma: prognostic markers in uterine leiomyosarcoma: A population-based cohort. Int J Gynecol Cancer. 2015;25(4):622–28. doi: 10.1097/IGC.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 30.Zivanovic O, Jacks LM, Iasonos A, et al. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer. 2012;118(3):660–69. doi: 10.1002/cncr.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelmus M, Penault-Llorca F, Guillou L, et al. Prognostic factors in early-stage leiomyosarcoma of the uterus. Int J Gynecol Cancer. 2009;19(3):385–90. doi: 10.1111/IGC.0b013e3181a1bfbc. [DOI] [PubMed] [Google Scholar]
- 32.Yordanov A, Slavchev S, Kostov S, et al. Leiomyosarcoma of the vulva: A case report. Menopausal Rev. 2020;19(4):184–87. doi: 10.5114/pm.2020.101949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saquib S, Cherawala M, Rahman OA, Keloth TE. Leiomyosarcoma of the vulva mimicking as chronic bartholin cyst: A case report. Oman Med J. 2020;35(4):e153. doi: 10.5001/omj.2020.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aljehani AM, Quatei A, Qattea L, et al. Vulvar leiomyosarcoma in pregnancy. Cureus. 2021 Oct 14;13(10):e18772. doi: 10.7759/cureus.18772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korkmaz V, Kurdoğlu Z, Karadag B, et al. A rare case of leiomyosarcoma localized in the Bartholin’s gland area and review of the literature: Leiomyosarcoma in Bartholin’s gland area. J Obstet Gynaecol Res. 2016;42(5):589–92. doi: 10.1111/jog.12943. [DOI] [PubMed] [Google Scholar]
- 36.Alnafisah F, Alfieri J. Lung metastasis in a case of recurrent poorly differentiated leiomyosarcoma of the Bartholin gland: A case report and review of the literature. Cureus. 2016;8(3):e550. doi: 10.7759/cureus.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akrivi S, Varras M, Anastasiadi Z, et al. Primary vulvar leiomyosarcoma localized in the Bartholin’s gland area: A case report and review. Mol Clin Oncol. 2021;14(4):69. doi: 10.3892/mco.2021.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mowers EL, Shank JJ, Frisch N, Reynolds RK. Myxoid leiomyosarcoma of the bartholin gland. Obstet Gynecol. 2014;124(2):433–35. doi: 10.1097/AOG.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 39.Sameeta F, Haque M, Akbar S, et al. Leiomyosarcoma: A rare tumor of the vulva. Am J Clin Pathol. 2019;152(Suppl. 1):S46. [Google Scholar]
- 40.Sayeed S, Xing D, Jenkins SM, et al. Criteria for risk stratification of vulvar and vaginal smooth muscle tumors: An evaluation of 71 cases comparing proposed classification systems. Am J Surg Pathol. 2018;42(1):84–94. doi: 10.1097/PAS.0000000000000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanson AA, Howitt BE, Schoolmeester JK. Criteria for risk stratification of vulvar and vaginal smooth muscle tumors: A follow-up study with application to leiomyoma variants, smooth muscle tumors of uncertain malignant potential, and leiomyosarcomas. Hum Pathol. 2020;103:83–94. doi: 10.1016/j.humpath.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Nath B, Gaikwad HS, Rajamani N, et al. Vulvar smooth muscle tumours: Case series and review of literature. J Clin Diagn Res. 2019;13(6):1–4. [Google Scholar]
- 43.Smith SA, Bou Zgheib N, Vallejos AM, Cuda JD. Case report on leiomyosarcoma of the vulva: A rare pathology. Marshall J Med. 2020;6(3):20. [Google Scholar]
- 44.Shankar S, Todd P, Rytina E, Crawford R. Leiomyosarcoma of the vulva. J Eur Acad Dermatol Venereol. 2006;20(1):116–17. doi: 10.1111/j.1468-3083.2006.01341.x. [DOI] [PubMed] [Google Scholar]
- 45.González-Bugatto F, Añón-Requena MJ, López-Guerrero MA, et al. Vulvar leiomyosarcoma in Bartholin’s gland area: A case report and literature review. Arch Gynecol Obstet. 2009;279(2):171–74. doi: 10.1007/s00404-008-0652-1. [DOI] [PubMed] [Google Scholar]
- 46.Levy RA, Winham WM, Bryant CS, Quick CM. Smooth muscle neoplasms of the vulva masquerading as bartholin gland duct cysts. Bayl Univ Med Cent Proc. 2014;27(1):25–27. doi: 10.1080/08998280.2014.11929043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teramae M, Fukuda T, Imai K, et al. Leiomyosarcoma of the vulva: A case report. Int J Reprod Contracept Obstet Gynecol. 2014;3(1):225–28. [Google Scholar]
- 48.Tjalma WAA, Colpaert CGA. Myxoid leiomyosarcoma of the vulva. Gynecol Oncol. 2005;96(2):548–51. doi: 10.1016/j.ygyno.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Di Gilio AR, Cormio G, Resta L, et al. Rapid growth of myxoid leiomyosarcoma of the vulva during pregnancy: A case report. Int J Gynecol Cancer. 2004;14(1):172–75. doi: 10.1111/j.1048-891x.2004.14152.x. [DOI] [PubMed] [Google Scholar]
- 50.Rawal N, Saridogan E, Khan N, Weekes A. Leiomyosarcoma of the vulva in association with lichen sclerosus. J Obstet Gynaecol. 2005;25(1):87–88. doi: 10.1080/01443610400025549. [DOI] [PubMed] [Google Scholar]
- 51.Ulutin HC, Zellars RC, Frassica D. Soft tissue sarcoma of the vulva: A clinical study. Int J Gynecol Cancer. 2003;13(4):528–31. doi: 10.1046/j.1525-1438.2003.13305.x. [DOI] [PubMed] [Google Scholar]