Abstract
Plant height (PH) is closely associated with yield-related traits and environmental adaptation. Seven pairs of near-isogenic lines (NILs) targeting four QTL on 3AL, 4BL, 4AS, and 7AL wheat chromosome arms were assessed for PH and four yield-related traits including yield per plant (Y/P), grain number per spike (G/S), thousand kernel weight (TKW), and biomass per plant (B/P). Significant differences were observed in the NIL pairs for the measured traits. NIL pairs targeting the 3AL QTL differed significantly in PH, G/S, and TKW; NILs targeting the 4BL QTL differed significantly in PH, Y/P, and B/P; NIL pairs targeting the 4AS QTL differed significantly in all the traits; and NIL pairs targeting the 7AL QTL differed significantly in PH. A 90 K SNP genotyping assay of the NILs detected nineteen SNPs associated with fourteen functional genes. Among them, eight candidate genes are related to Rht proteins, four genes are related to hormone pathways and two genes are related to carbohydrate synthesis and transport. By searching the interval marker physical positions, it was found that the four targeted QTL in this study overlapped with eight previously reported QTL for PH, TKW, biomass, and yield. Correlation analysis revealed that PH significantly and positively correlated with B/P and G/S. The SNP and candidate gene information is potentially useful for marker-assisted selection in breeding programs, and the four targeted QTL are proved to be critical genomic regions controlling the investigated agronomic traits, which can be further fine mapped to identify the underlying genes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11032-020-01196-8.
Keywords: Plant height, Yield, Candidate genes, Rht genes, QTL, NIL
Introduction
Green revolution successfully tackled human food demand by cultivating dwarf high-yielding crops (Hazell 2009). In common wheat (Triticum aestivum L.), however, there is still ample room for production improvement (Iizumi and Ramankutty 2016). Wheat production is determined by yield and yield components including grain number per spike (G/S), thousand kernel weight (TKW), and biomass. Plant height (PH), which is the final height at maturity, shows a close relationship to yield and abiotic stress tolerance in wheat (Zhang et al. 2017). Reduced PH minimizes the risk of lodging which can damage canopy structure to decrease photosynthetic capacity and impede water transportation by affecting grain filling (Zhang et al. 2016). As introduction of dwarf or reduced height (Rht) genes has dramatically increased wheat yields during the Green Revolution, exploration of novel Rht genes and revelation of their genetic controls are crucial for breeding high-yield wheat cultivars (Wang et al. 2010).
PH and yield-related traits are determined by many genes and environment factors (Wu et al. 2010; Liu et al. 2020). In wheat, twenty-four genes relating to PH have been reported and designated as Rht genes, distributed on chromosome 2AS, 2BL, 2DS, 3BS, 4BS, 4DS, 5AL, 5DL, 6AS, 7AS, and 7BS (GrainGenes, https://wheat.pw.usda.gov/cgi- bin/GG3/browse.cgi?query=%2ARht%2A;class=gene;begin=1). Rht genes reduce PH by diverse mechanisms that can be divided into two categories: gibberellic acid (GA)-insensitive (GAI) genes and GA-responsive/sensitive genes. GAI-Rht genes, such as Rht-B1 and Rht-D1, encode DELLA proteins to inhibit the sensitivity of GA, which can reduce PH and increase yield in various crops (Peng et al. 1999; Cao et al. 2020). GA-sensitive genes are related to a deficiency in endogenous GA due to blocked biosynthesis and signaling pathway (Milach and Federizzi 2001). Apart from Rht genes, mechanisms controlling PH and yield-related traits are also related to other phytohormone pathways, and protein and carbohydrate metabolism and transport (Cao et al. 2020; Liu et al. 2020).
Various quantitative trait loci (QTL) controlling PH have been identified across all the 21 chromosomes in common wheat. In this study, we used seven pairs of NILs targeting four QTL, namely QPhs.ccsu.3A.1 (Wang et al. 2018), Qphs.ocs-4B.1 (Wang et al. 2019), one unnamed QTL on 4AS (Pinto et al. 2010), and QHtscc.ksu-7A (Talukder et al. 2014; Lu et al. 2020). These genomic regions were chosen because they have overlaps with previously reported QTL for PH and yield-related traits (Huang et al. 2004; Maccaferri et al. 2010; Rebetzke et al. 2008; McCartney et al. 2005; Huang et al. 2004; Araki et al. 1999; Kuchel et al. 2007). In addition, Wang et al. (2018) reported that one NIL pair targeting the pre-harvest sprouting resistance locus QPhs.ccsu.3A.1 had a significant difference in PH. Later, Wang et al. (2019) reported that one NIL pair targeting another pre-harvest sprouting resistance locus Qphs.ocs-4B.1 had significant differences in PH and G/S. Kirigwi et al. (2007) reported an unnamed 4AS QTL controlling drought tolerance which may also play a role in PH. For QHtscc.ksu-7A, Lu et al. (2020) revealed that the isolines of one NIL pair targeting the heat tolerance locus were significantly different in PH, grain numbers, and final yield. The NILs targeting these four loci were, therefore, used to further investigate PH and yield-related traits.
Analysis of near-isogenic lines (NILs) is an essential approach in the characterization of target loci in quantitative genetic studies (Tuinstra et al. 1997). NILs are a pair of plants possessing almost the same genetic background except at one single gene or locus. Hence, any phenotypic differences shown in this pair are supposed to be contributed by the target genes harbored by the locus, which can convert the quantitative QTL to a Mendelian factor with predictable segregation and inheritance. In this study, NILs targeting QTL located on chromosome 3AL, 4AS, 4BL, and 7AL were analyzed by genotype-phenotype association analysis to identify their putative candidate genes responsible for PH and yield-related traits. The study aimed to (1) characterize the NILs targeting the four loci for PH and yield-related traits; (2) identify putative candidate genes controlling the traits through genotype-phenotype association analysis of the contrasting NILs; and (3) test whether any candidate genes identified in this study overlap with previously identified QTL and genes.
Materials and methods
Plant materials
Seven pairs of NILs were developed by a heterogeneous inbred family method with a fast generation cycling system (Yan et al. 2017) with one pair targeting a 3AL QTL Qphs.ccsu-3A.1 derived from Chara/ DM5637B*8 (Wang et al. 2018), one targeting a 4BL QTL Qphs.csc-4B.1 derived from Chara/ DM5637B*8 (Wang et al. 2019), four targeting a 4AS QTL for drought tolerance (Pinto et al. 2010) derived from Babax/Dhwar Dry, and one targeting a 7AL QTL for heat tolerance derived from Cascade/W156 (Talukder et al. 2014; Lu et al. 2020), as listed in Table 1. F8 seeds were grown to characterize the seven pairs of NILs for PH and yield-related traits including Y/P, TKW, G/S, and B/P.
Table 1.
The details of NILs used in this study
| Pair no. | NIL name | Parents | Target QTL name | Chromosome location | Marker interval of target QTL | Physical position of target QTL (Mb) | Screening marker | References |
|---|---|---|---|---|---|---|---|---|
| 1 | NIL-PHS3AL-3R | Chara × DM5637B*8 | Qph.ccsu-3A.1 | 3AL | Xwmc153 and Xgwm155 | 701.7–703.0 | Xgwm155 | Wang et al. 2018 |
| NIL-PHS3AL-3S | ||||||||
| 2 | NIL-PHS4BL-6R | Chara × DM5637B*8 | Qphs.ocs-4B.1 | 4BL | Xgwm495 and Xgwm375 | 482.8–568.6 | Xgwm495 | Wang et al. 2019 |
| NIL-PHS4BL-6S | ||||||||
| 3 | NIL-DT4ABD-1B | Babax × Dharwar Dry | QTL in linkage group 4A-a | 4AS | Xgwm397 and Xwmc491 | 0.14–42.0 | Xwmc491 | Pinto et al. 2010 |
| NIL-DT4ABD-1D | ||||||||
| 4 | NIL-DT4ABD-3B | Babax × Dharwar Dry | QTL in linkage group 4A-a | 4AS | Xgwm397 and Xwmc491 | 0.14–42.0 | Xwmc491 | Pinto et al. 2010 |
| NIL-DT4ABD-3D | ||||||||
| 5 | NIL-DT4ABD-4B | Babax × Dharwar Dry | QTL in linkage group 4A-a | 4AS | Xgwm397 and Xwmc491 | 0.14–42.0 | Xwmc491 | Pinto et al. 2010 |
| NIL-DT4ABD-4D | ||||||||
| 6 | NIL-DT4ABD-5B | Babax × Dharwar Dry | QTL in linkage group 4A-a | 4AS | Xgwm397 and Xwmc491 | 0.14–42.0 | Xwmc491 | Pinto et al. 2010 |
| NIL-DT4ABD-5D | ||||||||
| 7 | NIL-HT3-13R | Cascade × W156 | QHtmd.ksu-7A | 7AL | Xgwm397 and Xwmc491 | 165.1–611.8 | Xbarc49 | Talukder et al. 2014; Lu et al. 2020 |
| NIL-HT3-13S |
Growth conditions
The NILs were grown in a temperature-controlled glasshouse at The University of Western Australia (UWA) (31° 57′, 115° 47′), in which the temperature was set at 15/22 °C (night/day). All the seeds were sown in 20-cm pots to a depth of 2 cm with sterilized soil mixture (fine composted pine barks: coco peat: brown river sand = 5:2:3, pH = 6). Six replicates were used for each line. The pot positions were randomly re-arranged every Friday. Irrigation was applied every 2 days. Plants were fertilized once at 60 days after sowing with a water-soluble NPK fertilizer (poly feed greenhouse grade). The set temperature in the glasshouse was maintained, and all the plants grew in the same well-watered conditions during the whole experiment. All plants were harvested at the physiological mature stage when the loss of green color from the stem was shown in more than 50% of the spikes. After harvesting, all the plants were stored for drying in the temperature-controlled dark room set at a temperature of 35 °C for 1 week before measurements.
Measurements
PH refers to the shortest vertical distance between the upper boundary of the main photosynthetic tissues on a plant and the ground level, which was measured at the maturity stage. A 100 cm ruler was used for measuring PH. Yield-related traits were investigated. Dried plants were put on the balance, accurate to two decimal digitals, for measuring the B/P. All the spikes were gently hand threshed to get the grains, and after threshing, the seeds were counted and weighed using a balance, accurate to three decimal digitals. Yield per plant measured was equivalent to the total weight of grains (g). TKW was calculated by the formula of ×1000 (g).
Statistics
A two-sample t test was used for detecting a significant difference between the isolines. Correlation analysis of the measured traits (PH, B/P, G/S, TKW, and Y/P) was done using RStudio v1.0.153.
90 K SNP genotyping and candidate gene identification
The SNP genotyping was done at UWA. Specifically, Wheat 90K Illumina iSelect Array was used for genotyping genomic DNA samples of the NILs. SNP clustering and genotype calling were done by GenomeStudio 2.0 software (Illumina). SNPs with a call frequency < 0.8 (i.e., missing data points > 20%) or minor allele frequency (MAF) < 0.05 and SNPs with > 0.25 heterozygous calls were removed (Wang et al. 2014).
SNP data analysis was conducted to detect the differences within or close to the QTL intervals between the isolines. Brodie et al. (2016) found that SNPs may be up to 2 Mb away from their associated genes. Therefore, SNPs closely located towards the screening markers, especially those within the targeted QTL region (as they overlapped with QTL for the traits in other studies), were scrutinized. Specifically, the SNPs located on the 3AL, 4AS, 4BL, and 7AL chromosome arms were searched, especially those located closely to the markers used for developing the NILs, namely marker Xgwm155 for Qphs.ccsu.3A.1, Xgwm495 for Qphs.ocs-4B.1, Xwmc491 for the 4AS QTL, and Xbarc49 for QHtscc.ksu-7A. SNPs that differed between the NIL pairs were blasted against the wheat reference genome RefV1.0 (IWGSC et al. 2018). JBrowse (http://www.wheatgenome.org/Tools-and-Resources/Sequences) was used for candidate gene(s) discovery. Genes having key functions related to the investigated traits encoding carbohydrate, hormone, and Rht associated proteins and located within a 2-Mb distance from the SNPs were considered as candidate genes.
Results
Variations of PH and other yield-related traits
The seven NIL pairs showed variations in different traits. PH ranged from 54.1 cm to 80.6 cm, with four pairs including Pair 1, Pair 2, Pair 3, and Pair 7 showed significant differences between the isolines (Table 2). Average PH for the Pair 1 isoline with DM5637B*8 allele was 60.1 cm, significantly taller than the isoline with Chara allele at 54.1 cm; the Pair 2 isoline with DM5637B*8 allele was 59.2 cm, significantly taller than the isoline with Chara allele at 54.2 cm; the Pair 3 isoline with Dhawar Dry allele measured at 80.6 cm, significantly taller than the isoline with Babax allele at 73.6 cm; and the Pair7 isoline with Cascades allele was 70.5 cm, significantly taller than the isolines with W156 allele at 61.7 cm (Table 2; Fig. 1; Fig. 2). No significance in PH was detected for NIL pairs 4, 5, and 6.
Table 2.
The traits where the NIL pairs showing significant differences between their isolines
| Traits | Pair | Mean value of isolines with allele 1 (allele name) | Mean value of isolines with allele 2 (allele name) | p value |
|---|---|---|---|---|
| Plant height | 1 | 60.1 cm (DM5637B*8 allele) | 54.1 cm (Chara allele) | 0.032* |
| 2 | 59.2 cm (DM5637B*8 allele) | 54.2 cm (Chara allele) | 0.049* | |
| 3 | 73.6 cm (Babax allele) | 80.6 cm (Dhwar Dry allele) | 0.024* | |
| 7 | 61.7 cm (W156 allele) | 70.5 cm (Cascades allele) | 0.034* | |
| Biomass per plant | 2 | 12.9 g (DM5637B*8 allele) | 9.5 g (Chara allele) | 0.0017** |
| 4 | 23.2 g (Babax allele) | 10.8 g (Dhwar Dry allele) | 6.84E−12*** | |
| 5 | 11.5 g (Babax allele) | 20.0 g (Dhwar Dry allele) | 8.03E−05*** | |
| 6 | 10.5 g (Babax allele) | 15.9 g (Dhwar Dry allele) | 6.18E−05*** | |
| Yield per plant | 2 | 5.8 g (DM5637B*8 allele) | 4.6 g (Chara allele) | 0.019* |
| 4 | 7.4 g (Babax allele) | 4.6 g (Dhwar Dry allele) | 1.19E−08*** | |
| 5 | 3.3 g (Babax allele) | 6.5 g (Dhwar Dry allele) | 0.0044** | |
| 6 | 5.2 g (Babax allele) | 2.3 g (Dhwar Dry allele) | 5.53E−05*** | |
| Thousand kernel weight | 1 | 38.9 g (DM5637B*8 allele) | 43.5 g (Chara allele) | 0.012* |
| 6 | 40.1 g (Babax allele) | 17.2 g (Dhwar Dry allele) | 5.26E−05*** | |
| Grain number per spike | 1 | 29.3 (DM5637B*8 allele) | 23.4 (Chara allele) | 0.039* |
| 3 | 34.7 (Babax allele) | 54.6 (Dhwar Dry allele) | 0.00097*** | |
| 4 | 48.8 (Babax allele) | 29.3 (Dhwar Dry allele) | 5.2E-06*** | |
| 6 | 53.4 (Babax allele) | 22.7 (Dhwar Dry allele) | 8.76E-06*** |
*Indicates significant difference at p < 0.05, **indicates highly significant difference at p < 0.01, and ***indicates highly significant difference at p < 0.001
Fig. 1.

Average plant height of the NIL isolines at maturity. Pair 1: NIL-PHS3AL-3R and NIL-PHS3AL-3S; Pair 2: NIL-PHS4BL-6R and NIL-PHS4BL-6S; Pair 3: NIL-DT4ABD-1B and NIL-DT4ABD-1D; Pair 4: NIL-DT4ABD-3B and NIL-DT4ABD-3D; Pair 5: NIL-DT4ABD-4B and NIL-DT4ABD-4D; Pair 6: NIL-DT4ABD-5B and NIL-DT4ABD-5D; Pair 7: NIL-HT3-13R and NIL-HT3-13S. AA: allele 1; aa: allele 2 (the allele information is as listed in Table 2). *Denotes significant level at p<0.05
Fig. 2.
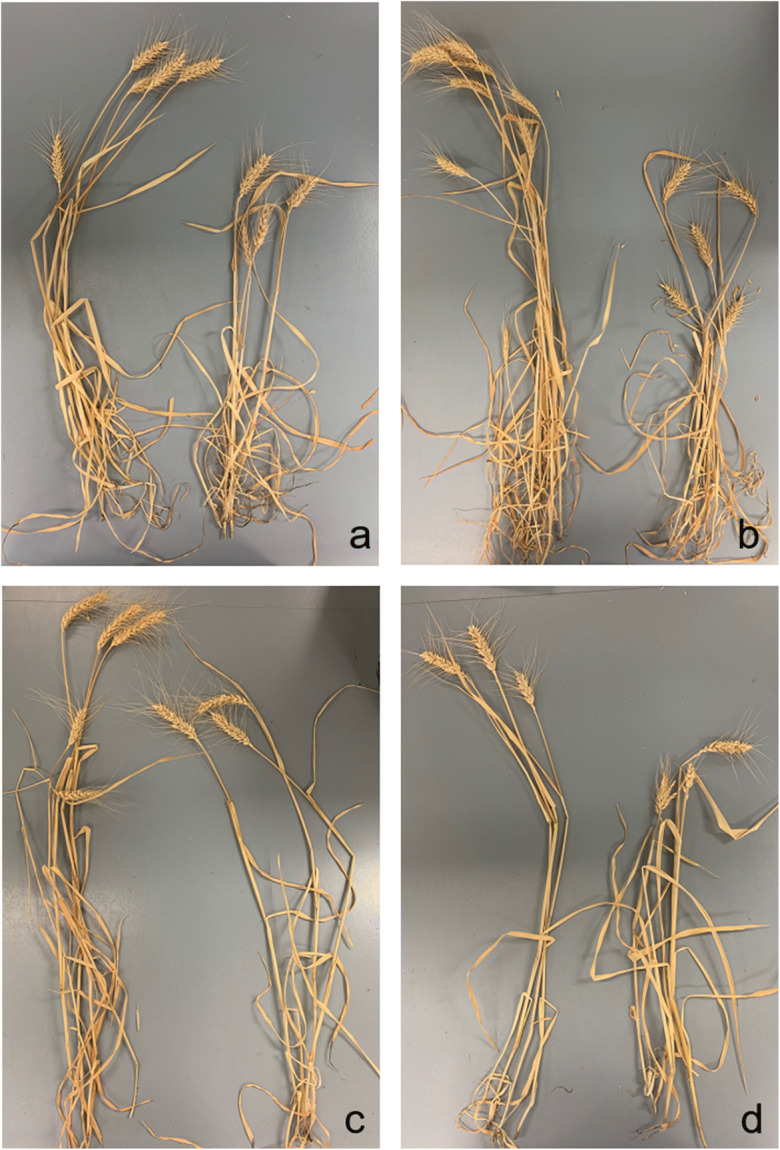
Four pairs of NILs showing significant differences in plant height. a Pair 1: NIL-PHS3AL-3R (left) and NIL-PHS3AL-3S (right) (Wang et al. 2018); b Pair 2: NIL-PHS4BL-6R (left) and NIL-PHS4BL-6S (right) (Wang et al. 2019); c Pair 3: NIL-DT4ABD-1D (left) and NIL-DT4ABD-1B (right) (Pinto et al. 2010); d Pair 7: NIL-HT3-13S (left) and NIL-HT3-13R (right) (Talukder et al. 2014; Lu et al. 2020)
Evaluations of yield-related traits also showed variations. In addition to showing significant difference in PH, Pair 1 showed a significant difference in TKW and G/S, Pair 2 showed a highly significant difference in B/P and a significant difference in Y/P, and Pair 3 showed a highly significant difference in G/S, whereas Pair 7 showed no significant differences in yield-related traits. Although exhibiting no significant differences in PH, Pair 4 showed highly significant differences in B/P, G/S, and Y/P, Pair 5 showed highly significant differences in B/P and Y/P, and Pair 6 showed highly significant differences in all the yield-related traits including Y/P, B/P, G/S and TKW (Table 2; Fig. 3).
Fig. 3.
Four agronomic traits including biomass per plant (a), thousand kernel weight (b), yield per plant (c), and grains per spike (d) measured in the seven NIL pairs. *Denotes significant level at p < 0.05, **denotes significant level at p < 0.01, and ***denotes significant level at p < 0.001. The NIL pair information is as same as in Fig. 1
Correlation of the investigated traits
Correlation analysis revealed the relationship between PH and the four yield-related traits (Y/P, G/S, TKW, and B/P) (Fig. 4). PH showed significantly positive but weak correlation with B/P (p < 0.05, r = 0.26) and highly significantly positive but weak correlation with G/S (p < 0.01, r = 0.28), but no significant correlation with TKW (r = 0.00). With no statistical significance, PH negatively correlated with Y/P (r = − 0.16). Y/P showed highly significantly positive but weak correlation with B/P (p < 0.001, r = 0.37) and G/S (p < 0.001, r = 0.36), and significantly positive but weak correlation with TKW (p < 0.01, r = 0.24). B/P showed significantly positive but weak correlation with G/S (p < 0.05, r = 0.26), but no correlation with TKW (r = 0.00).
Fig. 4.
Correlation between plant height (PH) and four other agronomic traits in the seven NIL pairs. Data are from the results of Pearson’s product-moment correlation. *, **, and ***Denote the significant levels at 0.05, 0.01, and 0.001, respectively. Each plot shows the relationship between the variables in horizontal and vertical axis of the grid. The diagonal shows a histogram of each variable. The numbers represent the ratio of correlation (r) between the variables in horizontal and vertical axis
SNP genotyping analysis
Nineteen SNPs were identified between the isolines and they are associated with fourteen functional genes (Table 3 and Table S1).
Table 3.
SNPs and candidate genes identified in the targeted QTL genomic region
| Targeted QTL | Marker interval | Physical position (Mb) | Traits | SNPs | Genes | Functions |
|---|---|---|---|---|---|---|
| Qph.ccsu.3A.1 | Xwmc153-Xgwm155 | 703 | PH, TKW, G/S | BS00024548_51 | TraesCS3A01G469000 | Hydroxyethylthiazole kinase |
| Qphs.ocs-4B.1 | Xgwm495-Xgwm375 | 482.8 | PH, Y/P, B/P | tplb0043l14_1189 | TraesCS4B01G235400 | Branched-chain-amino-acid aminotransferase |
| Jagger_c1432_289 | TraesCS4B02G230000 | Purple acid phosphatase 3-like | ||||
| Tdurum_contig37811_134 | TraesCS4B02G229900 | Prolyl 3-hydroxylase 2 | ||||
| Unnamed 4A QTL | Xgwm397-Xwmc491 | 0.14 | PH. Y/P, TKW, G/S, B/P | RAC875_c1606_644 | TraesCS4A01G029300LC | Protein FAR1-RELATED SEQUENCE 5 |
| Excalibur_c85853_521 | TraesCS4A01G118900LC | Same to E4 | ||||
| BobWhite_c13975_80 | TraesCS4A01G035700 | Cell division cycle protein 48-like protein | ||||
| BS00066851_51 | TraesCS4A01G049600 | F-box protein | ||||
| D_GBUVHFX01B5RWO_166 | TraesCS4A01G071300 | 26S proteasome non-ATPase regulatory subunit 4 | ||||
| Ku_c10539_2031 | TraesCS4A01G083600 | Protein FAR1-RELATED SEQUENCE 6 | ||||
| GENE-3126_21.2 | TraesCS4A01G001600 | Sn1-specific diacylglycerol lipase alpha | ||||
| QHtscc.ksu-7A | Xbarc121-Xbarc49 | 165.1 | PH | RAC875_c1227_1098 | TraesCS7A01G402900 | Starch synthase 3 |
| RAC875_c1227_1471 | “ | “ | ||||
| wsnp_Ex_c4883_8705816 | “ | “ | ||||
| IAAV3084 | TraesCS7A01G416400 | Auxin response factor | ||||
| BS00022747_51 | TraesCS7A01G419300 | UDP-sugar pyrophosphorylase | ||||
| BS00092631_51 | “ | “ | ||||
| wsnp_CAP11_c78_114341 | “ | “ | ||||
| BobWhite_c17095_237 | TraesCS7A01G597300LC | F-box/LRR-repeat protein 13 | ||||
| BS00075525_51 | TraesCS7A01G412800 | NBS-LRR disease resistance protein-like | ||||
| RAC875_c12665_1272 | TraesCS7A01G408900 | Zinc knuckle family protein | ||||
| wsnp_Ra_c40910_48252997 | “ | “ |
Indicates the value is the same as above. The markers in bold are the screening markers for the NIL development
For NIL Pair 1 targeting the 3AL QTL, 379 polymorphic SNPs out of a total of 53,052 filtered markers were located on the chromosome 3A, and 112 of them were on the long arm of the 3A chromosome. Only one SNP BS00024548_51 was located within the targeted interval and close to the screening markers Xgwm155. The functions of genes within a 2-Mb physical distance from the SNP were examined. A gene TraesCS3A01G469000 near marker BS00024548_51 was detected, which is about 1.1 Mb away from the screening marker, functioning as hydroxyethylthiazole kinase.
For NIL Pair 2 targeting the 4BL QTL, 13 SNPs were identified and assigned to the region of the targeted QTL Qphs.ocs-4B.1. Two SNPs Jagger_c1432_289 and Tdurum_contig37811_134 close to the screening marker Xgwm495 (482.8 Mb) were linked to two functional genes including TraesCS4B01G230000 and TraesCS4B01G22990 that were involved in purple acid phosphatase 3-like and prolyl 3-hydroxylase 2, respectively. In addition, within the QTL Qphs.ocs-4B.1, there was one candidate gene TraesCS4B01G235400 associated with SNP tplb0043l14_1189 having the key function of branched-chain-amino-acid aminotransferase.
For NIL Pair 3 targeting the 4AS QTL, 106 SNPs were detected on the 4A chromosome. For NIL Pair 4 and 5, eighty-three SNPs and 130 SNPs were detected on chromosome 4A respectively. In terms of Pair 6, 145 SNPs were shown on chromosome 4A. Six of these SNPs were located within the QTL interval, which overlapped with the coding sequence (CDS) regions of six candidate genes: gene TraesCS4A01G029300LC with SNP RAC875_c1606_644 and gene TraesCS4A01G118900LC with SNP Excalibur_c85853_521 encode protein FAR1-RELATED SEQUENCE 5; gene TraesCS4A01G035700 with SNP BobWhite_c13975_80 functions as cell division cycle protein 48-like protein; gene TraesCS4A01G049600 with BS00066851_51 encodes F-box protein; gene TraesCS4A01G071300 with SNP D_GBUVHFX01B5RWO_166 functions in production of 26S proteasome non-ATPase regulatory subunit 4; and gene TraesCS4A01G083600 with SNP Ku_c10539_2031 encodes protein FAR1-RELATED SEQUENCE 6. SNP GENE-3126_21.2, located outside of the 4AS QTL but 1.1 Mb away from the screening marker, were overlapping with the CDS of another candidate gene TraesCS4A01G001600, which functions as Sn1-specific diacylglycerol lipase alpha.
For Pair 7 targeting the 7AL QTL, there were a total of 116 SNPs located on chromosome 7A, with eleven of them assigned to the targeted QTL region, which linked to six candidate genes. Three SNPs RAC875_c1227_1098, RAC875_c1227_1471 and wsnp_Ex_c4883_8705816 overlap with gene TraesCS7A01G402900 functioning as starch synthase 3. IAAV3084 is closely located to TraesCS7A01G416400 which functions as an auxin response factor. BS00022747_51, wsnp_CAP11_c78_114341 and BS00092631_51 are located within the CDS region of TraesCS7A01G419300 that functions as UDP-sugar pyrophosphorylase. Gene TraesCS7A01G597300LC is closely linked to BobWhite_c17095_237 functions like NBS-LRR disease resistance protein. RAC875_c12665_1272 and wsnp_Ra_c40910_48252997 overlap the CDS of gene TraesCS7A01G408900 with a function of Zinc knuckle family protein.
Discussion
Candidate genes are identified for the targeted QTL which overlap with previously reported QTL for PH and yield-related traits
`The targeted QTL in this study overlap with eight QTL reported from previous research (Table 4). Specifically, QTgw.igk.3A.1 (Huang et al. 2004) shares the same marker Xgwm155 with QPhs.ccsu.3A.1, and QHt.IDuWUE-4B.2 (Maccaferri et al. 2010) shares the same marker Xgwm495 with Qphs.ocs-4B.1. They both play a role in PH. In addition to PH, QTgw.igk-3A also affects TKW. The 4AS QTL targeted in this study overlapped with two 4AS QTL reported by Rebetzke et al. (2008) that affects plant height, yield, and biomass. Additionally, three QTL Qyld.crc-4A, QGyld.agt-4A.1 and Qyld.ocs-4A.1 overlapping with the targeted 4AS QTL have effects in yield (McCartney et al. 2005; Kuchel et al. 2007; Araki et al. 1999). Meanwhile, QHtscc.ksu-7A is located on chromosome 7AL overlapping with QHt.crc-7A for PH (McCartney et al. 2005).
Table 4.
Summary of previously identified QTL on 3AL, 4BL, 4AS, and 7AL chromosomes for plant height and yield-related traits in wheat
| Trait | Chromosome | Identified QTL | Parents | Genomic region | Phenotypic variance (%) | Physical position (Mb) |
|---|---|---|---|---|---|---|
| Plant height | 3AL | QTgw.igk-3A (Huang et al. 2004) | Flair × XX86 | Xgwm155-Xgwm751 | 8.40 | 701.7–703.0 |
| 4BL | QHt.IDuWUE-4B.2 (Maccaferri et al. 2010) | Cvs. × advanced lines | IWB34975-gwm495 | 482–497 | ||
| 4AS | An unnamed QTL in Rebetzke et al. (2008) | Cranbrook × Hallberd | gwm192 | 10.8–59.7 | ||
| An unnamed QTL in Rebetzke et al. (2008) | Cd87× Katepwa | Abg484 | 17.9–65.9 | |||
| 7AL | QHt.crc-7A (McCartney et al. 2005) | Wmc603-wmc139 | 488.7–530.2 | |||
| TKW | 3AL | QTgw.igk-3A (Huang et al. 2004) | Flair × XX86 | Xgwm155-Xgwm751 | 8.40 | 701.7–703.0 |
| Yield | 4AS | Qyld.crc-4A (McCartney et al. 2005) | RL4452 × AC Domain | Xgwm397-Xwmc680 | 6.30 | 151.7–514.7 |
| An unnamed QTL in Rebetzke et al. (2008) | Cranbrook × Hallberd | gwm192 | 10.8–59.7 | |||
| An unnamed QTL in Rebetzke et al. (2008) | Cd87× Katepwa | Abg484 | 17.9–65.9 | |||
| Qyld.ocs-4A.1(Araki et al.1999) | Chinese Spring × Chinese Spring (Kanto 107 4A) | Xbcd1738 | 27–17 | 90.2 | ||
| QGyld.agt-4A (Kuchel et al. 2007) | Trident × Molineux | Xgwm397 | 151.7 | |||
| Biomass | 4AS | An unnamed QTL in Rebetzke et al. (2008) | Cd87× Katepwa | Abg484 | 17.9–65.9 |
A total of 14 candidate genes responsible for PH and yield-related traits were identified for the four targeted QTL. These genes distributed on chromosome 3AL, 4BL, 4AS and 7AL can be categorized on the basis of distinct mechanisms: two genes related to auxin metabolism pathway, two genes functioning in a similar way as Rht8, one gene related to DELLA protein action, six genes related to ABA pathway, two genes related to starch synthesis, and one gene related to both DELLA protein and ABA.
Mechanisms regulating PH, yield-related traits, and corresponding candidate genes
There are twenty-four Rht genes discovered in previous research (Table 5). The best known Rht genes are Rht-B1 (Rht1) and Rht-D1 (Rht 2) on chromosome arm 4BS and 4DS that are GA-insensitive and inhibit plant growth (Zanke et al. 2014). Rht1 and Rht2 encode DELLA proteins to inhibit the sensitivity of GA, reducing PH, and increasing yield in various crops (Peng et al. 1999). DELLA proteins including GA insensitive (GAI), repressor of GAI (RGA), RGA-like1 (RGL1), RGL2, and RGL3 function in a highly similar fashion. Rht3, Rht10, Rht11, and Rht17 are also GA-insensitive genes (Daba et al. 2020). Rht3 and Rht17 located in the same region of Rht1, affect PH without compromising wheat yield (Navaro et al. 2014; Daba et al. 2020). Rht10 is allelic to Rht2, significantly reducing PH (Ellis et al. 2005). Rht11 is allelic to Rht1, having similar reduction effects in wheat height (Divashuk et al. 2012). Except Rht1, Rht2, Rht3, Rht10, Rht11, and Rht17, other Rht genes are GA-sensitive. GA-sensitive mutants are deficient for GA resulting in blocked biosynthesis pathway (Milach and Federizzi 2001). Among GA-responsive Rht genes, Rht4, Rht5, Rht6, Rht7, and Rht23 affect both wheat height and yield, whereas Rht9, Rht14, Rht16, and Rht18 affect PH but have unknown effects on production (Bachir and Hu 2014; Chen et al. 2013; Worland et al. 1980; Chen et al. 2014; Watanabe 2008). GA-responsive Rht8 is closely linked to Ppd-D1 that is insensitive to photoperiod (Pestsova et al. 2002; Chebotar et al. 2013), causing suppression in production obtained from light promotion. It reduces wheat height by producing the insensitivity of brassinosteroids (BRs) rather than GA, displaying a significant effect on grains per spike but not on yield. Moreover, the combination of Rht4 and Rht8 produces greater TKW and more yield, proposing a useful breeding strategy (Liu et al. 2017a, b; Korzun et al. 1998; Du et al. 2018). Rht12 and Rht13 play a negative role in PH, as well as grain numbers and TKW (Bachir and Hu 2014; Grant et al. 2018). According to Peng et al. (2011), Rht15, Rht16, Rht18, and Rht19 were induced by chemicals and radiation. The identification of Rht21 is debatable (Yang et al. 1995; Börner and Worland 2002). Rht22 affects PH and cell length through a differential mechanism to reduce cell numbers (Peng et al. 2011). Rht24 is reported to be valuable for breeding reduced height wheat with an advantageous effect on Fusarium head blight (FHB) resistance (Herter et al. 2018). Rht25 affects not only PH but also yield-related traits under different conditions (Mo et al. 2018). Although many Rht genes have been reported, the positions of four Rht genes including Rht6, Rht15, Rht19, and Rht20 remain uncertain and need further investigation to locate (Daba et al. 2020). Several candidate genes identified in this study are related to Rht protein functions, as discussed below.
Table 5.
Details of twenty-four Rht genes including names, GA-response mechanism, genetic background, and positions
| Name (Rht genes) | Allelic information | GA response | Genetic background | Position (chromosome) | Traits | Reference |
|---|---|---|---|---|---|---|
| Rht1 (Rht-B1b) | GAI | Norin 10-Brevor 14 | 4BS | PH, Y | Zanke et al. 2014 | |
| Rht2 (Rht-D1b) | GAI | Norin 10-Brevor 14 | 4DS | PH, Y | Zanke et al. 2014 | |
| Rht3 (Rht-B1c) | Allelic to Rht1 | GAI | MINISTER DWARF | 4BS | PH | Navaro et al. 2014; Pearce et al. 2011 |
| Rht4 | GAR | Burt ert 937 | 2BL | PH, Y | Bachir and Hu 2014 | |
| Rht5 | GAR | Marfed ert 1 Mutant 1 | 3BS | PH, Y | Bachir and Hu 2014 | |
| Rht6 | Allelic to Rht2 | GAR | Norin 10-Brevor 14/ BURT | 4D | PH, Y | Bachir and Hu 2014 |
| Rht7 | GAR | Bersee Mutant A/ Bersee Mutant C | 2AS | PH, Y | Worland et al. 1980 | |
| Rht8 | GAR | Akakomugi | 2DS | PH, G/S | Bachir and Hu 2014, Grant et al. 2018 | |
| Rht9 | GAR | Acciao/Akakomugi/FORLANI/MARA | 7BS | PH | Chen et al. 2013 | |
| Rht10 (Rht-D1c) | Allelic to Rht2 | GAI | Aibian | 4DS | PH, Y | Ellis et al. 2005 |
| Rht11(Rht-B1e) | Allelic to Rht1 | GAI | Karlik 1 | 4BS | PH, Y | Divashuk et al. 2012 |
| Rht12 | GAR | Karcagi 522M7K | 5AL | PH, GN, GS, TKW | Grant et al. 2018 | |
| Rht13 | GAR | Matelporignif 41 Mutant 1 | 7BS | PH, GN, TKW | Grant et al. 2018 | |
| Rht14 | GAR | CASTELPORIZANO | 6AS | PH | Watanabe 2008 | |
| Rht15 | GAR | Durox | ? | PH | Daba et al. 2020 | |
| Rht16 | GAR | Edmore Mutant 1 | 6AS | PH | Watanabe 2008 | |
| Rht17(Rht-B1p) | Allelic to Rht1 | GAI | Chris Mutant | 4BS | PH | Daba et al. 2020; Bazhenov et al. 2015 |
| Rht18 | GAR | Icaro | 6AS | PH | Grant et al. 2018, Watanabe 2008 | |
| Rht19 | GAR | Vic Mutant 1 | ? | PH | Daba et al. 2020 | |
| Rht20 | GAR | Burt Mutant 860 | ? | PH | Daba et al. 2020 | |
| Rht21 (debatable) | ? | XN0004 | ? | PH | Yang et al. 1995; Börner and Worland 2002 | |
| Rht22 | GAR | Aiganfanmai | 7AS | PH | Peng et al. 2011 | |
| Rht23 | ? | NAUH164 | 5DL | PH, Y | Chen et al. 2014 | |
| Rht24 | GAR | Aikang 58 | 6AL | PH, FHB resistance | Herter et al. 2018 | |
| Rht25 | GAR | UC1110/PI610750 | 6AS | PH, Y (under different conditions) | Mo et al. 2018 |
GAI: GA insensitive; GAR: GA responsive; PH: plant height, Y: yield, TKW: thousand kernel weight, FHB resistance: Fusarium head blight resistance, GN: grain number per plant, G/S: grain number per spike, GS: grain size
Information provided by Graingenes: https://graingenes.org/cgi-bin/GG3/browse.cgi?query=%2ARht%2A;class=gene;begin=1
TraesCS4A01G049600 on chromosome 4AS encodes F-box protein. SLEEPY1, also belonging to F-box protein family, was involved in the DELLA protein mechanism in Arabidopsis thaliana (Dill et al. 2004). On chromosome 7A, TraesCS7A01G412800 contributes to biosynthesis of LRR family protein, as a receptor of BRs whose mechanism was also reported in Rht8 in wheat (Gasperini et al. 2012). In rice, one NBS-LRR protein Pik-H4 is active in homeodomain-containing protein that is positively reacted to BRs catabolism to reduce PH by ET pathway (Liu et al. 2017a, b). Additionally, TraesCS7A01G412800 has NBS-LRR disease resistance protein-like function that is not only related to disease resistance but also to abiotic stress such as drought stress, which indirectly promotes growth under harsh environment (DeYoung and Innes 2006). TraesCS7A01G408900, encoding Zinc knuckle family protein and belonging to zinc finger family proteins, covers two SNPs on chromosome 7A. Zinc knuckle family protein regulates plant growth through response to light signaling (Loudet et al. 2008). Moreover, the zinc finger family proteins are assigned to synthetic interval of Rht8 locus and function in plant elongation in wheat (Gasperini et al. 2012). TraesCS7A01G597300LC has a function of F-box/LRR-repeat protein 13. One of the F-box protein subfamilies combined with GID2 can interact with DELLA protein to reduce the PH, which works the same as in GAI-Rht genes (Hirsch and Oldroyd 2009).
Although abscisic acid (ABA) levels have no significant difference between crops with Rht genes and those without them, it also plays a role in plant height (King et al. 1983). ABA, as one of the most important hormones in plant, can be regarded as a plant growth inhibitor. A basic zipper transcription factor ABI5 responding to ABA suppresses cell activity and metabolism. Plant growth benefits from the activities of the plasma membrane (PM) H+-ATPase and nutrient transporters combined with electrochemical proton gradients. ABA can inhibit the production of PM H+-ATPase, which corresponds to restrain plant growth (Planes et al. 2014). Several genes are also detected in this study that associated with the ABA pathway.
On chromosome 3AL, the function of TraesCS3A01G469000 is related to hydroxyethylthiazole kinase participating in the biosynthetic of thiamine (vitamin B1) (Yazdani et al. 2013). Accumulation of thiamine is involved in tolerance to various stresses. When plants confront the stressed environment, thiamine is capable of minimizing the negative effects on plant growth by regulating ABA (Fitzpatrick and Chapman 2020). For example, thiamine enhances the adaptability to salinity, oxidative and osmotic stresses, and boosts plant growth and productivity in oil palm (Subki et al. 2018). As Aminifard et al. (2018) reported that thiamin foliar spraying in coriander and fenugreek increased TKW, because thiamin functions as a H+-ATPase pump to intake more nutrients. Thiamin pyrophosphate, as the active form of thiamin, participates in ATP synthesis and photosynthesis, which supplies energy in central metabolism (photosynthesis and respiration) (Rosado-Souza et al. 2020). The balance and coordination of carbon from central metabolism and nitrogen from α-ketoglutarate (α-KG) pathway are related to yield increase (Fitzpatrick and Chapman 2020). On chromosome 4AS, TraesCS4A01G035700 encodes cell division cycle protein 48-like protein. In rice, cell division cycle protein 48-like protein is vital for expression of AAA-ATPase to trigger ATPase activity, which affects PH and yield in rice (Shi et al. 2019). AAA-ATPase would suit stresses of low temperature and high salinity by regulating ABA levels in Arabidopsis (Baek et al. 2011). TraesCS4A01G071300 originating from Arabidopsis thaliana functions in 26S proteasome non-ATPase regulatory subunit 4. The 26S proteasome–related degradation that occurred in the ABA-signaling protein ABI5 or DPBF1 will affect PH in Arabidopsis (Smalle et al. 2003). TraesCS4A01G049600 encodes F-box protein. FOA1, as one of the F-box proteins, reacts to the signaling of ABA in Arabidopsis (Peng et al. 2012). Protein FAR1-RELATED SEQUENCE 6, encoded by gene TraesCS4A01G083600, and protein FAR1-RELATED SEQUENCE 5, encoded by two genes TraesCS4A01G029300LC and TraesCS4A01G118900LC, derive from the multiple FAR1-RELATED SEQUENCE (FRS) families. The FRS family impacts on the growth and development of Arabidopsis via signaling to ABA and light. The decrease transcript abundance of ABI5 happens in FAR1 mutants, suggesting disruption of FAR1 and FHY3 binding to ABI5 can diminish inhibition of plant growth caused by signaling to ABA. Besides, FAR1 shows insensitivity to ABA-triggered stomatal closure, indicating that FAR1 is necessary for responses to drought stress (Ma and Li 2018).
In addition, hormone auxin promotes plant elongation by improving the extensibility of cell walls. The activity of auxin is tightly linked to TRANSPORT INHIBITOR RESISTANT 1/AUXIN SIGNALING F-BOX (TIR1/AFB) nuclear auxin receptor family, the degradation of the transcriptional regulators AUXIN/INDOLE-3-ACETIC ACID (AUX/IAAs) and the AUXIN RESPONSE FACTORs (ARFs). When the auxin levels increase, the reaction between AUX/IAAs and TIR1/AFB will result in AUX/IAAs degrading, further inducing cell expansion (Majda and Robert 2018). The mechanism of auxin regulating plant yield is complex and undefined, but it is commonly known that the auxin signaling factors can modify the grain size and weight, and the interaction between BRs and auxin signaling pathway also plays a role in grain development (Cao et al. 2020). Two candidate genes identified in this study are related to auxin biosynthesis and signaling.
One gene, TraesCS4B01G235400, on chromosome 4BL with the function of producing branched-chain-amino-acid aminotransferase (BCAA) related to l-leucine transaminase activity that can remit the chlorsulfuron-induced growth inhibition. l-leucine transaminase activity is involved in the auxin production through indole-3-pyruvic acid (IPA) pathway as one of most important auxin producing pathways in Neurospora crassa, affecting grain weight and PH (Dastgheib 1993; Sardar and Kempken 2018; Mashiguchi et al. 2011). TraesCS7A01G416400 on chromosome 7AL overlapping with two different SNPs is related to auxin response factors (ARFs), and the regulation of PH by TraesCS7A01G416400 is through auxin signaling in wheat (Qiao et al. 2018).
Moreover, the production and accumulation of starch affect yield. Two main glucose homopolymers, amylose and amylopectin, constitute starch in plant (Dian et al. 2005). Sucrose existing in the cytoplasm of heterotrophic cells can be decomposed to fructose and UDP-glucose (UDPG) recurs to sucrose synthase (SuSy). Then, UDPG was converted to G1P and PPI under the action of UDPG pyrophosphorylase, and G1P is metabolized by cytosolic glucose phosphate mutant enzyme to G6P that can be turned into starch by means of plastidial phosphoglucomutase (pPGM), ADPglucose (AGP), and starch synthase (SS) activities. Two candidate genes located on 7AL are relevant to starch synthesis and transport.
On chromosome 7AL, two genes, TraesCS7A01G402900 and TraesCS7A01G419300, function in the production of carbohydrate, overlapping with two SNPs and having the function of starch synthase 3 (SSIII) and UDP-sugar pyrophosphorylase, respectively. Starch synthase 3 (SSIII) is active in the pathway to starch biosynthesis, and soluble starch synthase (SSS) decreases when wheat plants suffer from heat stress (Mishra et al. 2017). The gene encoding soluble starch synthase can improve heat tolerance in wheat, indirectly boosting wheat yield (Tian et al. 2018). SSIII was proved to participate in the starch synthesis via amylopectin elongation activity in rice (Dian et al. 2005). UDP-glucose (UDPG) pyrophosphorylase, belonging to one of the UDP-sugar pyrophosphorylase family, originates from Arabidopsis thaliana, which is a precursor for biomass producing and plant growing (Decker and Kleczkowski 2019).
By contrast, there are no reports for the functions of 4BL genes TraesCS4B02G230000 and TraesCS4B02G229900 that affect PH and wheat production. Regarding the QTL controlling drought tolerance on chromosome 4AS, one candidate gene TraesCS4A01G001600 was identified for the lipid catabolic process to produce Sn1-specific diacylglycerol lipase alpha. However, there has been no studies showing the relationship between this protein function and yield.
In this study, PH significantly and positively correlated with B/P and G/S. In addition, PH shows a negative correlation with Y/P, but no correlation with TKW. Some of these results are not consistent with previous reports, for instance, Wolde and Eticha (2016) detected the significantly negative correlation between PH and TKW in South Eastern Ethiopia. Sokoto et al. (2012) stated PH in two varieties (Star 11 TR77173/SLM and Kauz/Weaver) significantly and positively correlated with Y/P in the dry conditions. This inconsistency may be caused by differences in plant materials and experimental conditions in individual studies, which may need further investigation.
Conclusion
In this study, seven NIL pairs, originally targeting QTL for pre-harvest sprouting (Qphs.ccsu-3A.1 and Qphs.ocs-4B.1), drought tolerance (the unnamed 4AS QTL), and heat tolerance (QHtscc.ksu-7A), were characterized for PH and yield-related traits. Genotype-phenotype association analysis of the NILs showing contrasting performance between isolines revealed fourteen candidate genes located on 3AL, 4BL, 4AS, 7AL chromosome arms. They have functions related to plant hormone, carbohydrate, and amino-acid metabolism pathways. The four QTL are confirmed to be important in regulating PH and yield-related traits based on findings of this study. The seven pairs of NILs can be used for fine mapping and gene cloning to pinpoint any new genes responsible for PH and productivity, and for the development of useful markers for marker-assisted selection.
Supplementary Information
(XLSX 23 kb)
Acknowledgements
The authors thank Dr. Md Sultan Mia and Dr. Xingyi Wang for providing the NIL seeds for this experiment, Professor Ruihui Wang for technical support, and Professor Jacqueline Batley and Dr. Aneeta Pradhan for assistance in the 90 K SNP array genotyping. The authors also acknowledge the funding body, the Global Innovation Linkage program (GIL53853) from the Australian Department of Industry, Innovation and Science.
Authors’ contributions
YZ, HL and GY conceived and designed the experiments; YZ conducted the experiments; YZ and HL analyzed the data; YZ wrote the manuscript, and HL, GY critically reviewed the manuscript. All authors approved the final version of the manuscript.
Funding
The research was funded by Global Innovation Linkage program (GIL53853) from the Australian Department of Industry, Innovation and Science.
Data availability
The data and materials used in this study from authors are available on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
RStudio v1.0.153, Excel software v16.32.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hui Liu, Email: hui.liu@uwa.edu.au.
Guijun Yan, Email: guijun.yan@uwa.edu.au.
References
- Aminifard MH, Jorkesh A, Fallahi HR, Alipoor K. Foliar application of thiamin stimulates the growth, yield and biochemical compounds production of coriander and fenugreek. J Hortic Res. 2018;26(1):77–85. doi: 10.2478/johr-2018-0009. [DOI] [Google Scholar]
- Araki E, Miura H, Sawada S. Identification of genetic loci affecting amylose content and agronomic traits on chromosome 4A of wheat. Theor Appl Genet. 1999;98(6–7):977–984. doi: 10.1007/s001220051158. [DOI] [Google Scholar]
- Bachir GD, Hu Y. Inheritance of rht5 dwarfing gene in common wheat (Triticum aestivum L.) Int J Innov Res Sci Res. 2014;1(2):75–82. [Google Scholar]
- Baek K, Seo PJ, Park CM. Activation of a mitochondrial ATPase gene induces abnormal seed development in Arabidopsis. Mol Cell. 2011;31(4):361–369. doi: 10.1007/s10059-011-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazhenov MS, Divashuk MG, Amagai Y, Watanabe N, Karloy GI. Isolation of the dwarfing Rht-B1p (Rht17) gene from wheat and the development of an allele-specific PCR marker: new strategies in plant improvement. Mol Breed. 2015;35(11):213–213. doi: 10.1007/s11032-015-0407-1. [DOI] [Google Scholar]
- Börner A, Worland AJ. Does the Chinese dwarf wheat variety ‘XN0004’ carry Rht21? Cereal Res Commun. 2002;30(1):25–29. doi: 10.1007/BF03543385. [DOI] [Google Scholar]
- Brodie A, Azaria JR, Ofran Y. How far from SNP may the causative genes be? Nucieic Acids Res. 2016;44(13):6046–6054. doi: 10.1093/nar/gkw500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Xu D, Hanif M, Xia X, He Z. Genetic architecture underpinning yield component traits in wheat. Theor Appl Genet. 2020;133(6):1811–1823. doi: 10.1007/s00122-020-03562-8. [DOI] [PubMed] [Google Scholar]
- Chebotar GO, Chebotar SV, Motsnyy II, Sivolap YM. Clarification of the Rht8-Ppd-D1 gene linkage on the 2D chromosome of winter bread wheat. Cytol Genet. 2013;47(2):70–74. doi: 10.3103/S0095452713020047. [DOI] [PubMed] [Google Scholar]
- Chen L, Philips AL, Condon AG, Parry M, Hu YG. GA-responsive dwarfing gene Rht12 affects the developmental and agronomic traits in common bread wheat. PLoS One. 2013;8(4):e62285. doi: 10.1371/journal.pone.0062285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Gao R, Wang H, Wen M, Xiao J, Bian N, Zhang R, Hu W, Cheng S, Bie T, Wang X. Characterization of a novel reduced height gene (Rht23) regulating panicle morphology and plant architecture in bread wheat. Euphytica. 2014;203(3):583–594. doi: 10.1007/s10681-014-1275-1. [DOI] [Google Scholar]
- Daba SD, Tyagi P, Brown-Guedira G, Mohammadi M. Genome-wide association study in historical and contemporary U.S. winter wheats identifies height-reducing loci. Crop J. 2020;8(2):243–251. doi: 10.1016/j.cj.2019.09.005. [DOI] [Google Scholar]
- Dastgheib F (1993) Response of wheat cultivars to chlorsulfuron and the effect of nitrogen availability. Dissertation, Lincoln University
- Decker D, Kleczkowski LA (2019) UDP-sugar producing pyrophosphorylases: distinct and essential enzymes with overlapping substrate specificities, providing de novo precursors for glycosylation reactions. Front Plant Sci 9. 10.3389/fpls.2018.01822 [DOI] [PMC free article] [PubMed]
- DeYoung BJ, Innes RW. Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol. 2006;7(12):1243–1249. doi: 10.1038/ni1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dian W, Jiang H, Wu P. Evolution and expression analysis of starch synthase III and IV in rice. J Exp Bot. 2005;56(412):623–632. doi: 10.1093/jxb/eri065. [DOI] [PubMed] [Google Scholar]
- Dill A, Thomas SG, Hu J, Steber CM, Sun TP (2004) The Arabidopsis F-Box protein SLEEPY1 targets gibberellin signaling repressors for gibberellin-induced degradation. The Plant Cell 16(6):1392–1405. 10.1105/tpc.020958 [DOI] [PMC free article] [PubMed]
- Divashuk MG, Vasilyev AV, Bespalova LA, Karlov GI. Identify of the Rht-11 and Rht-B1e reduced plant height genes. Russ J Genet. 2012;48(7):761–763. doi: 10.1134/s1022795412050055. [DOI] [PubMed] [Google Scholar]
- Du Y, Chen L, Wang Y, Yang Z, Saeed I, Daoura BG, Hu YG. The combination of dwarfing genes Rht4 and Rht8 reduced plant height, improved yield traits of rain fed bread wheat (Triticum aestivum L.) Elsevier. 2018;216:149–155. doi: 10.1016/j.fcr.2017.10.015. [DOI] [Google Scholar]
- Ellis MH, Rebetzke GJ, Azanza F, Richards RA, Spielmeyer W. Molecular mapping gibberellin-responsive dwarfing genes in bread wheat. Theor Appl Genet. 2005;111(3):423–430. doi: 10.1007/s00122-005-2008-6. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick TB, Chapman LM. The importance of thiamine (vitamin B1) in plant height: from crop yield to biofortification. J Biol Chem. 2020;295(34):12002–12013. doi: 10.1074/jbc.rev120.010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperini D, Greenland A, Hedden P, Dreos R, Harwood W, Griffiths S. Genetic and physiological analysis of Rht8 in bread wheat: an alternative source of semi-dwarfism with a reduced sensitivity to brassinosteroids. J Exp Bot. 2012;63(12):4419–5536. doi: 10.1093/jxb/ers138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant N, Mohan A, Sandhu D, Gill K. Inheritance and genetic mapping of the reduced height (Rht18) gene in wheat. Plants. 2018;7(3):58. doi: 10.3390/plants7030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell PBR (2009) The Asian green revolution. Res Inst. IFPRI Discussion Paper
- Herter CP, Ebmeyer E, Kollers S, Korzun V, Leiser WL, Wüschum T, Miedaner T. Rht24 reduces height in the winter wheat population ‘Solitär × Bussard’ without adverse effects on Fusarium head blight infection. Theor Appl Genet. 2018;131(6):1263–1272. doi: 10.1007/s00122-018-3076-8. [DOI] [PubMed] [Google Scholar]
- Hirsch S, Oldroyd GED. GRAS-domain transcription factors that regulate plant development. Plant Signal Behav. 2009;4(8):698–700. doi: 10.4161/psb.4.8.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XQ, Kempf H, Ganal MW, Röder MS. Advanced backcross QTL analysis in progenies derived from a cross between a German elite winter wheat variety and a synthetic wheat (Triticum aestivum L.) Theor Appl Genet. 2004;109(5):933–943. doi: 10.1007/s00122-004-1708-7. [DOI] [PubMed] [Google Scholar]
- Iizumi T, Ramankutty N. Changes in yield variability of major crops for 1981–2010 explained by climate change. Environ Res Lett. 2016;11(3):034003. doi: 10.1088/1748-9326/11/3/034003. [DOI] [Google Scholar]
- IWGSC. Appels R, Eversole K, et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. 2018;361(6403):7191. doi: 10.1126/science.aar7191. [DOI] [PubMed] [Google Scholar]
- King RW, Gale MD, Quarrie SA. Effects of NORIN 10 and tom thumb dwarfing genes on morphology, physiology and abscisic acid production in wheat. Ann Bot. 1983;51(2):201–208. doi: 10.1093/oxfordjournals.aob.a086458. [DOI] [Google Scholar]
- Kirigwi FM, Van Ginkel M, Brown-Guedira G, Gill BS, Paulsen GM, Fritz AK. Markers associated with a QTL for grain yield in wheat under drought. Mol Breed. 2007;20(4):401–413. doi: 10.1007/s11032-007-9100-3. [DOI] [Google Scholar]
- Korzun V, Röder MS, Ganal MW, Worland AJ, Law CN. Genetic analysis of the dwarfing gene (Rht8) in wheat. Part I. molecular mapping of Rht8 on the shrot arm of chromosome 2D of bread wheat (Triticum aestivum L.) Theor Appl Genet. 1998;96:1104–1109. doi: 10.1007/s001220050845. [DOI] [Google Scholar]
- Kuchel H, Williams KJ, Langridge P, Langridge P, Eagles HA, Jefferies SP. Genetic dissection of grain yield in bread wheat. I. QTL analysis. Theor Appl Genet. 2007;115(8):1029–1041. doi: 10.1007/s11022-007-0629-7. [DOI] [PubMed] [Google Scholar]
- Liu H, Dong S, Gu F, Liu W, Yang G, Huang M, Xiao W, Liu Y, Guo T, Wang H, Chen Z, Wang J (2017a) NBS-LRR protein Pik-H4 interacts with OsBIHD1 to balance rice blast resistance and growth by coordinating ethylene-brassinosteroid pathway. Front Plant Sci 8. 10.3389/fpls.2017.00127 [DOI] [PMC free article] [PubMed]
- Liu Y, Zhang J, Hu Y, Chen J. Dwarfing genes Rht4 and Rht-B1b affect plant height and key agronomic traits in common wheat under two water regimes. Field Crop Res. 2017;204:242–248. doi: 10.1093/10.1016/j.fcr.2017.01.020. [DOI] [Google Scholar]
- Liu H, Mullan D, Zhang C, Zhao S, Li X, Zhang A, Lu Z, Wang Y, Yan G. Major genomic regions responsible for wheat yield and its components as revealed by meta-QTL and genotype–phenotype association analyses. Planta. 2020;252:65. doi: 10.1007/s00425-020-03466-3. [DOI] [PubMed] [Google Scholar]
- Loudet O, Michael TP, Burger BT, Le Mette C, Mockler TC, Weigel D, Chory J. A zinc knuckle protein that negatively controls morning-specific growth in Arabidopsis thaliana. Proc NatI Acad Sci U S A. 2008;105(44):17193–17198. doi: 10.1073/pnas.0807264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Liu H, Wu Y, Yan G (2020) Development and characterization of near-isogenic lines revealing candidate genes for a major 7AL QTL responsible for heat tolerance in wheat. Front Plant Sci 6. 10.3389/fpls.2020.01316 [DOI] [PMC free article] [PubMed]
- Ma L, Li G (2018) FAR1-RELATED SEQUENCE (FRS) and FRS-RELATED FACTOR (FRF) family proteins in Arabidopsis growth and development. Front Plant Sci 9. 10.3389/fpls.2018.00692 [DOI] [PMC free article] [PubMed]
- Maccaferri M, Sanguineti MC, Demontis A, El-Ahmed A, Moral LG, Maalouf F, Nachit M, Nserallah N, Ouabbou H, Rhouma S, Royo C, Villegas D, Tuberosa R. Association mapping in durum wheat grown across a broad range of water regimes. J Exp Bot. 2010;62(2):409–438. doi: 10.1093/jxb/erq287. [DOI] [PubMed] [Google Scholar]
- Majda M, Robert S. The role of auxin in cell wall expansion. Int J Mol Sci. 2018;19(4):951. doi: 10.3390/ijms19040951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, McSteen P, Zhao Y, Hayashi K, Kamiya Y, Kasahara H. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108(45):18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney CA, Somers DJ, Humphreys DG, Lukow O, Ames N, Noll J, Cloutier S, McCallum BD. Mapping quantitative trait loci controlling agronomic traits in the spring wheat cross RL4452 9 ‘AC Domain’. Genome. 2005;48(5):870–883. doi: 10.1139/g05-055. [DOI] [PubMed] [Google Scholar]
- Milach SC, Federizzi LC. Dwarfing genes in plant improvement. Adv Agron. 2001;73:35–63. doi: 10.1016/S0065-2113(01)73004-0. [DOI] [Google Scholar]
- Mishra BP, Kumar R, Mohan A, Gill KS. Conservation and divergence of starch synthase III genes of monocots and dicots. PLoS One. 2017;12(12):e0189303. doi: 10.1371/journal.pone.0189303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y, Vanzetti L, Hale I, Spagnolo EJ, Guidobaldi F, Al-Oboudi J, Odle N, Pearce S, Helguera M, Dubcovsky J. Identification and characterization of Rht25, a locus on chromosome arm 6AS affecting wheat plant height, heading time, and spike development. Theor Appl Genet. 2018;131(10):2021–2035. doi: 10.1007/s00122-018-3130-6. [DOI] [PubMed] [Google Scholar]
- Navaro CD, Yang Y, Amita M, Grant NP, Gill K, Sandhu D. Microsatellites based genetic linkage map of the Rht3 locus in bread wheat. Mol Plant Breed. 2014;5(8):43–46. doi: 10.5376/mpb.2014.05.0008. [DOI] [Google Scholar]
- Pearce S, Saville R, Vaughan SP, Chandler PM, Wilhelm EP, Sparks CA, Al-Kaff N, Korolev A, Boulton MI, Phillips AL, Hedden P, Nicholson P, Thomas SG. Molecular characterisation of Rht-1 dwarfing genes in wheat. Plant Physiol. 2011;157:1820–1831. doi: 10.1104/pp.111.183657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature. 1999;400(6741):255–261. doi: 10.1038/22307. [DOI] [PubMed] [Google Scholar]
- Peng Z, Li X, Yang Z, Lao M. A new reduced height gene found in the tetraploid semi-dwarf wheat landrace Aiganfanmai. Genet Mol Res. 2011;10(4):2349–2357. doi: 10.4238/2011.october.5.5. [DOI] [PubMed] [Google Scholar]
- Peng J, Yu D, Wang L, Xie M, Yuan C, Wang Y, Tang D, Zhao X, Liu X. Arabidopsis F-box gene FOA1 involved in ABA signaling. Sci China Life Sci. 2012;55(6):497–506. doi: 10.1007/s11427-012-4332-9. [DOI] [PubMed] [Google Scholar]
- Pestsova EG, Korzun V, Röder MS (2002) Pedigree analysis of wheat chromosome 2D, in Abst. 12th Int. EWAC Workshop, July 1–6, 2002, Norwich, UK, pp 122–124
- Pinto RS, Reynolds MP, Mathews KL, Mcintyre CL, Olivares-Villegas JJ, Chapman SC. Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor Appl Genet. 2010;121(6):1001–1021. doi: 10.1007/s00122-010-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planes M, Niñoles R, Rubio L, Bissoli G, Bueso E, Garcia-Sánchez MJ, Alejandro S, Gonzalez-Guzmán M, Hedrich R, Rodriguez PL, Fernández JA, Serrano R. A mechanism of growth inhibition by abscisic acid in germinating seeds of Arabidopsis thaliana based on inhibition of plasma membrane H+-ATPase and decreased cytosolic pH, K+, and anions. J Exp Bot. 2014;66(3):813–825. doi: 10.1093/jxb/eru442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Zhang W, Li X, Zhang L, Zhang X, Li X, Guo H, Ren Y, Zheng J, Chang Z (2018) Characterization and expression patterns of auxin response factors in wheat. Front Plant Sci 9. 10.3389/fpls.2018.013957 [DOI] [PMC free article] [PubMed]
- Rebetzke GJ, Condon AG, Farquhar GD, Appels R, Richards RA. Quantitative trait loci for carbon isotope discrimination are repeatable across environments and wheat mapping populations. Theor Appl Genet. 2008;118(1):123–137. doi: 10.1007/s00122-008-0882-4. [DOI] [PubMed] [Google Scholar]
- Rosado-Souza L, Fernie AR, Aarabi F. Ascorbate and thiamin: metabolic modulators in plant acclimation. Plants. 2020;9(1):101. doi: 10.1007/s00122-008-0882-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardar P, Kempken F (2018) Characterization of indole-3-pyruvic acid pathway-mediated biosynthesis of auxin in Neurospora crassa. 13(2):e0192293. 10.1371/journal.pone.0192293 [DOI] [PMC free article] [PubMed]
- Shi L, Zhang X, Shi Y, Xu X, He Y, Shao G, Huang Q, Wu J (2019) OsCDC48/48E complex is required for plant survival in rice (Oryza sativa L.). Plant Mol. Biol. 100(1–2):163–179. 10.1007/s11103-019-00851-9 [DOI] [PMC free article] [PubMed]
- Smalle J, Kurepa J, Yang P, Emborg TJ, Babiychuk E, Kushnir S, Vierstra RD. The pleiotropic role of the 26S proteasome subunit RPN10 in Arabidopsis growth and development supports a substrate-specific function in abscisic acid signaling. Plant Cell. 2003;15(4):965–980. doi: 10.1105/tpc.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoto MB, Abubakar IU, Dikko AU. Correlation analysis of some growth, yield, yield components and grain quality of wheat (Triticum aestivum L.) Nig J Basic Appl Sci. 2012;20(4):349–356. [Google Scholar]
- Subki A, Abidin AAZ, Yusof ZNB. B group vitamins – current uses and perspectives. London: Intechopen; 2018. The Role of Thiamine in Plants and Current Perspectives in Crop Improvement; pp. 33–44. [Google Scholar]
- Talukder SK, Babar MA, Vijayalakshmi K, Poland J, Prasad PVV, Bowden R, Fritz A (2014) Mapping QTL for the traits associated with heat tolerance in wheat (Triticum aestivum L.). BMC Genet 15(1). 10.1186/s12863-014-0097-4 [DOI] [PMC free article] [PubMed]
- Tian B, Talukder SK, Fu J, Fritz AK, Trick HN (2018) Expression of a rice soluble starch synthase gene in transgenic wheat improves the grain yield under heat stress conditions. In Vitro Cell Dev Biol Plant 54(3):216–227. 10.1007/s11627-018-9893-2 [DOI] [PMC free article] [PubMed]
- Tuinstra MR, Ejeta G, Goldsbrough PB. Heterogeneous inbred family (HIF) analysis: a method for developing near-isogenic lines that differ at quantitative trait loci. Theor Appl Genet. 1997;95(5–6):1005–1011. doi: 10.1007/s001220050654. [DOI] [Google Scholar]
- Wang Z, Wu X, Ren Q, Chang X, Li R, Jing R. QTL mapping for developmental behavior of plant height in wheat (Triticum aestivum L.) Euphytica. 2010;174(3):447–458. doi: 10.1007/s10681-010-0166-3. [DOI] [Google Scholar]
- Wang S, Wong D, Forrest K, Allen A, Chao S, Huang BE, Maccaferri M, Salvi S, Milner SG, Cattivelli L, Mastrangelo AM, Whan A, Stephen S, Barker G, Wieseke R, Plieske JIWGSC, Lillemo M, Mather D, Appels R, Dolferus R, Brown-Guedira G, Korol A, Akhunova AR, Feuillet C, Salse J, Morgante M, Pozniak C, Luo M, Dvorak J, Morell M, Dubcovsky J, Ganal M, Tuberosa R, Lawley C, Mikoulitch I, Cavanagh C, Edwards KJ, Hayden M, Akhunov E. Characterization of polyploid wheat genomic diversity using a high-density 90000 single nucleotide polymorphism array. Plant Biotechnol J. 2014;12(6):787–796. doi: 10.1111/pbi.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu H, Mia MS, Siddique KHM, Yan G. Development of near-isogenic lines targeting a major QTL on 3AL for pre-harvest sprouting resistance in bread wheat. Crop Pasture Sci. 2018;69(9):864. doi: 10.1071/cp17423. [DOI] [Google Scholar]
- Wang X, Liu H, Liu G, Mia MS, Siddique KHM, Yan G (2019) Phenotypic and genotypic characterization of near-isogenic lines targeting a major 4BL QTL responsible for pre-harvest sprouting in wheat. BMC Plant Biol 19(1). 10.1186/s12870-019-1961-1 [DOI] [PMC free article] [PubMed]
- Watanabe N. Genetic collection and development of near-isogenic lines in durum wheat. Vavilov J Genet Breed. 2008;12(4):636–643. [Google Scholar]
- Wolde T, Eticha F (2016) Trait associations in some durum wheat (Triticum durum L.) accessions among yield and yield-related traits at Kulumsa, South Eastern Ethiopia. Adv Crop Sci Tech 4(4). 10.4172/2329-8863.1000234
- Worland AJ, Law CN, Shakoor A. The genetical analysis of an induced height mutant in wheat. Heredity. 1980;45(1):61–71. doi: 10.1038/hdy.1980.50. [DOI] [Google Scholar]
- Wu X, Wang Z, Chang X, Jing R. Genetic dissection of the developmental behaviours of plant height in wheat under diverse water regimes. J Exp Bot. 2010;61(11):2923–2937. doi: 10.1093/jxb/erq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Liu H, Wang H, Lu Z, Wang Y, Mullan D, Hamblin J, Liu C (2017) Accelerated generation of selfed pure line plants for gene identification and crop breeding. Front Plant Sci 8. 10.3389/fpls.2017.01786 [DOI] [PMC free article] [PubMed]
- Yang TZ, Zhang XK, Liu HW, Wang ZH (1995) Chromosomal arm location of a dominant dwarfing gene Rht21 in XN0004 of common wheat. In: Li ZS, Xin ZY (eds) Proc 8th Int Wheat Genet Symp pp 839–842. China Agricultural Scientech Press
- Yazdani M, Zallot R, Tunc-Ozdemir M, de Cré-Lagard V, Shintani DK, Hanson AD. Identification of the thiamine salvage enzyme thiazole kinase in Arabidopsis and maize. Phytochemistry. 2013;94:68–73. doi: 10.1016/j.phytochem.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Zanke CD, Ling J, Plieske J, Kollers S, Ebmeyer E, Korzun V, Argillier O, Stiewe G, Hinze M, Neumann K, Ganal MW, Röder MS. Whole genome association mapping of plant height in winter wheat (Triticum aestivum L.) PLoS One. 2014;9(11):e113287. doi: 10.1371/journal.pone.0113287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu W, Wang H, Fang Y, Dong H, Qi X. Progress in improving stem lodging resistance of Chinese wheat cultivars. Euphytica. 2016;212(2):275–286. doi: 10.1007/s10681-016-1768-1. [DOI] [Google Scholar]
- Zhang N, Fan X, Cui F, Zhao C, Zhang W, Zhao X, Yang L, Pan R, Chen M, Han J, Ji J, Liu D, Zhao Z, Tong Y, Zhang A, Wang T, Li J. Characterization of the temporal and spatial expression of wheat (Triticum aestivum L.) plant height at the QTL level and their influence on yield-related traits. Theor Appl Genet. 2017;130(6):1235–1252. doi: 10.1007/s00122-017-2884-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX 23 kb)
Data Availability Statement
The data and materials used in this study from authors are available on reasonable request.




