Abstract
Background
One in six people with cancer will develop depression at some point in their care. Untreated depression affects quality of life, cancer care satisfaction and healthcare expenditure. Treatments for this vulnerable heterogenous population should be evidence based and specific. A common sentiment is that psychiatric research does not reflect the prevalence of patients with cancer and comorbid depression and is biased towards certain cancers, but this has not been empirically shown.
Study selection and analysis
A systematic review of studies on psychological and pharmacological treatments for depression in people with cancer was conducted. Of 4621 papers identified from a search of PubMed and PsycINFO up to 27 June 2020, 84 met inclusion criteria (eg, adults with cancer; depression diagnosis; treatment study) and comprised 6048 participants with depression with cancer.
Findings
Cancer types are not proportionally represented in depression research in accordance with their incidence. Breast cancer is over-represented (relative frequency in research 49.3%, but 11.7% of global cancer). Cancers of the head and neck and bone and soft tissue were close to parity. All other cancers are under-represented. Representativeness varied 40-fold across different cancers.
Conclusions
The evidence base for depression treatments is dominated by a single cancer. Given heterogeneity in cancer populations (eg, stage of illness; psychological impact; cancer treatments), it is possible that depression treatments may not have the same benefits and harms across all cancers, impeding the ability to offer people with different cancers the best depression treatment. While the dominant opinion within this research field is that a cancer bias exists, this is the first study to demonstrate as such.
Keywords: depression & mood disorders
Background
In 2018, there was an estimated 18.1 million new cases of cancer worldwide and 9.6 million cancer deaths.1 The frequencies of cancers vary across regions, populations and sexes.1 Fortunately, more than half of people diagnosed with cancer now survive the disease or live with the disease for many years after diagnosis. Many people diagnosed with cancer experience distress because of the physical and physiological consequences of the disease and its treatments, and develop psychological, spiritual, employment and financial forms of distress.2 Distress in cancer can oftentimes go on to become severe and sustained, leading to the development of psychiatric conditions such as depression. Depression is more prevalent in people affected by cancer compared with the general population.3 4 Depression is associated with lower quality of life and may increase mortality in people with cancer5 and patients with depression are less likely to accept and commence systemic cancer therapies.4 One trial of depression treatment in people with cancer showed improved quality of life, but no effect on overall survival.6 Treating depression in people with cancer has been shown to reduce annual healthcare costs by 30% per patient.7 However, almost three-quarters of patients with depression with cancer do not receive appropriate treatment and only 5% have contact with a mental health professional.8
The general prevalence of comorbid depression in cancer is between 10.8%9 and 15%,4 but substantial variation exists depending on diagnostic methods10 (eg, interview, self-report measures), clinical settings (eg, inpatient, outpatient, palliative) and types of cancer.11 To highlight some examples, a meta-analysis found higher rates of depression when using self-report measures in brain cancer (pooled mean 33%), breast cancer (20%) and respiratory tract cancers (21%) compared with diagnostic interviews (brain cancer 28%; breast cancer 11%; respiratory tract cancer 3%).10 The prevalence of any depressive disorder (eg, major, minor and dysthymia) is higher among palliative settings (24.6%) than oncological and haematological settings (20.7%).4
Once a diagnosis of depression is confirmed, the clinician should be able to access evidence-based treatments for depression in that particular cancer.6 12 Growth of psycho-oncology depression research has been rapid, but the generalisability and application of findings and treatments may be limited because of several factors. First, cancer is a single term for a heterogenous collection of diseases involving different organ systems, treatments, side effects and psychological impacts. Second, research across the entire cancer continuum is dominated by particular cancers (eg, breast cancer) and this may limit generalisability.13 14
Objective
The aim of this project was to determine whether there is alignment between depression treatment research in people with cancer and epidemiological patterns for cancer and comorbid depression. Within the field of cancer with comorbid depression, several meta-analyses and systematic reviews have been done on the diagnosis, prevalence and treatment of depression,9–11 15 16 but none have attempted to quantify and describe the research base according to cancer type.
Study selection and analysis
A two-part strategy was undertaken. First, a new systematic review of depression treatment studies in people with cancer was conducted in order to describe research effort in terms of participants’ cancer types. Second, a comparison was made with cancer types included in depression treatment research with overall prevalence of cancer and comorbid depression.
Systematic review search strategy
A search framework according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses17 was created and the systematic review protocol was registered with PROSPERO (number CRD42018089928). The original protocol included searches related to depression and anxiety, but this was later refined to depression because of the volume of studies. PubMed and PsycINFO were searched with the aid of an independent librarian on 27 June 2020 for human studies up to that date, on adults and authored in English. The search was done using the following MeSH terms and constructs in PubMed (and equivalent in PsycINFO): “neoplasm” AND “depression” AND (“therap* OR “intervention” OR “treatment”).
Inclusion criteria
Studies were eligible if they were original works published in peer-reviewed journals. Specific inclusion criteria were as follows:
Participants: participants aged over 18 years with cancer who met criteria for a likely diagnosis of major depressive disorder according to clinical cut-off scores on a validated self-report measure, or a current diagnosis of major depressive disorder established using a formal diagnostic interview. Studies on carers, partners and family members were excluded.
Interventions: antidepressant pharmacological treatment or specified/manualised psychological interventions for depression were included. Psychological therapy modalities (eg, cognitive–behavioural therapy; mindfulness) were included regardless of mode of delivery (eg, self-guided; clinician-assisted; computerised). Studies that evaluated non-specific psychosocial interventions (eg, art therapy; music therapy; massage; exercise; support groups; expressive writing) or that did not specifically target depressive symptoms (eg, menopausal symptoms; anxiety; fear of cancer recurrence; grief; well-being; stress reduction) or that involved electroconvulsive therapy were excluded.
Outcomes: reported outcomes for depressive symptoms using a validated self-report or clinician-administered measure.
Study design: randomised controlled trials, uncontrolled trials, observational studies, case series and case reports were included. Conference proceedings, abstracts only, unpublished reports and theses were excluded.
Data extraction
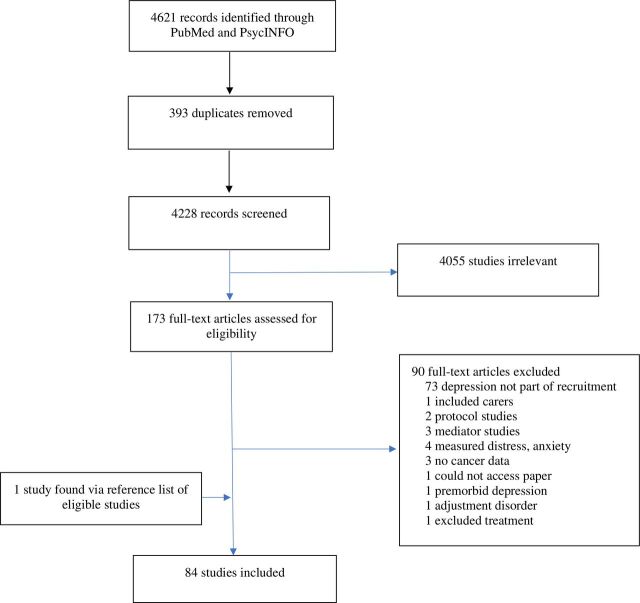
Studies were identified and exported to data management software (www.covidence.org) from individual electronic databases. After results were collated and duplicates removed, two investigators (BB and SL) independently screened titles and abstracts (figure 1). Disagreements were settled by a third investigator (MM). All eligible papers were retrieved for full-text screening and independently reviewed (BB and SL). Of those that met inclusion criteria, study sample size and participant characteristics, including sex and cancer types, were extracted to a Microsoft Excel spreadsheet for analysis.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses processes for study selection.
Cancer types in depression research
Terminology used to denote cancer type was extracted verbatim from each eligible study to aid subsequent grouping and analysis. Cancer types were then organised according to the ICD-11 topography (www.who.int/classifications/icd/en/) based on the highest level classifiers within the ‘neoplasm’ branch as major groups.
Comparison with global cancer burden
Global cancer data were obtained from the Global Cancer Observatory at the International Agency for Research on Cancer using incidence data for 2018 for 185 countries and the 36 most common cancers.1 The Cancer Today visualisation tool (gco.iarc.fr/today) was used to extract the total number of new cancer cases globally for adults aged over 20 years old, including non-melanoma skin cancer and separating colon, rectum and anus cancer. This age and geographical range most closely matched the inclusion criteria of our systematic review and therefore allowed for the most comprehensive comparison.
Comparison with comorbid depression population
Comparing cancer characteristics of participants with depression with cancer incidence is useful, but a stronger method is to compare cancer types of participants with depression with all people with cancer and comorbid depression. To control for variation in the rates of depression, participant data were compared with an estimate of the population of people with cancer and comorbid depression. There are no global data available for the incidence of people with cancer and comorbid depression. To estimate the global cancer population with depression, the literature was searched for level one evidence (systematic reviews or meta-analyses) for rates of depression across different cancer types. When a review was not available, single cross-sectional studies were used (see references in footnote to table 1). The average rate of depression (as a mean of the proportions if more than one study was available) for each cancer type was then multiplied by global cancer incidence to estimate the number of people with comorbid depression across cancer types.
Table 1.
Cancer types represented in depression treatment research and the global burden of those cancers and cancers in comorbid depression populations
| Cancer type* | Research participants, n (%) |
Global cancer incidence† | Cancer cases (%) | Depression prevalence‡ | Comorbid depression incidence§ | Comorbid cases (%)¶ | Research participation per 100 000 comorbid cases |
| Bone, soft tissue | 53 (0.9) | 68 245 | 0.4 | 0.33 | 22 521 | 0.6 | 235 |
| Brain | 12 (0.2) | 266 745 | 1.5 | 0.28 | 74 689 | 2.1 | 16 |
| Breast | 2983 (49.5) | 2 088 035 | 11.7 | 0.20 | 417 607 | 11.8 | 714 |
| Digestive | 680 (11.2) | 4 965 321 | 27.9 | 0.27 | 1 340 637 | 37.7 | 51 |
| Endocrine | 18 (0.3) | 557 307 | 3.1 | 0.17 | 94 742 | 2.7 | 19 |
| Female genital | 375 (6.2) | 1 303 419 | 7.3 | 0.26 | 338 889 | 9.5 | 111 |
| Haematological | 295 (4.9) | 1 071 531 | 6.0 | 0.25 | 267 883 | 7.5 | 110 |
| Head and neck** | 343 (5.7) | 880 469 | 4.9 | 0.20 | 176 094 | 5.0 | 195 |
| Male genital | 89 (1.5) | 1 374 153 | 7.7 | 0.10 | 137 415 | 3.9 | 65 |
| Respiratory | 550 (9.1) | 2 092 686 | 11.8 | 0.21 | 439 464 | 12.4 | 125 |
| Skin | 40 (0.7) | 1 325 473 | 7.4 | 0.07 | 92 783 | 2.6 | 43 |
| Urinary tract | 189 (3.1) | 938 930 | 5.3 | 0.16 | 150 229 | 4.2 | 126 |
| Total†† | 5627 | 16 932 314 | 3 512 928 |
*Cancer types categorised according to ICD-11 where possible.
†GLOBOCAN global incidence data.
‡Estimated depression prevalence rates from cross-sectional study or meta-analysis.3 8–10
§Estimated from cancer incidence and depression prevalence within cancer types.
¶Estimated percentage of all cases of cancer comorbid with depression.
**Not an ICD-11 category, grouped according to clinical usage in depression meta-analyses and includes ICD group ‘Lip, oral cavity and pharynx’ plus laryngeal cancer from ICD group ‘Middle ear, respiratory, intrathoracic organs’.
††Total number of participants does not equal all participants in review as depression prevalence is not known for some cancers and these were not included in comparison.
To estimate the contribution of each cancer to the comorbid depression population, the estimate of depressed people with each cancer type was divided by the total number of depressed people across all cancers. Then, participation in studies compared with the incidence of people with cancer and comorbid depression was expressed as a participation rate per 100 000 cases of comorbid depression.
Findings
Figure 1 outlines the main results. Four thousand six hundred and twenty-one potential papers were screened, and 173 studies were eligible for inclusion based on title and abstract. After full-text review, 84 studies were included in the final analysis (see online supplementary appendix A). Table 2 shows the main findings of the 84 selected studies. Overall, 6048 depressed participants were included in this review. Controlled trials were the most common study design (45%), and the majority of studies examined psychological treatments for depression (51%).
Table 2.
Characteristics of studies evaluating depression treatments in a cancer population (N=84)
| N (%) | |
| Study type | |
| Randomised controlled trial | 38 (45) |
| Non-controlled trial | 26 (31) |
| Feasibility or pilot | 1 (1) |
| Case series | 4 (5) |
| Case report | 15 (18) |
| Method of depression diagnosis | |
| Interview | 44 (52) |
| Self-report instrument | 26 (31) |
| Both | 14 (17) |
| Intervention | |
| Pharmacotherapy | 39 (46) |
| Psychological | 43 (51) |
| Both | 2 (3) |
| Cancer type* | |
| Brain, central nervous system | 12 (0.2) |
| Haematopoietic, lymphoid | 295 (4.9) |
| Mesenchymal | 53 (0.9) |
| Lip, oral cavity, pharynx | 183 (3) |
| Digestive organs | 680 (11.2) |
| Middle ear, respiratory, intrathoracic organs | 701 (11.6) |
| Skin | 40 (0.7) |
| Peripheral nerve, autonomic nervous system | 4 (0.1) |
| Retroperitoneum, peritoneum, omentum | 4 (0.1) |
| Breast | 2983 (49.3) |
| Female genital organs | 375 (6.2) |
| Male genital organs | 89 (1.5) |
| Urinary tract | 189 (3.1) |
| Eye, ocular adnexa | 0 (0) |
| Endocrine glands | 18 (0.3) |
| Ill-defined sites (eg, neuroendocrine), unknown primary | 14 (0.2) |
| ‘Other’ | 408 (6.7) |
*Classified according to ICD-11 topographic clusters.
ebmental-2020-300145supp001.pdf (62.5KB, pdf)
Cancer and tumour types were denoted inconsistently across studies, frequently clustered following unclear methodologies and bespoke cancer terms (eg, ‘aerodigestive’), or not fully detailed and grouped as ‘other’ cancers. Across the 6048 participants, we extracted 54 different labels for types of cancer, some of which overlapped (eg, ‘digestive’ and ‘upper GIT’). These were initially merged into 17 subgroups and later organised according to ICD-11 to make appropriate comparisons. Almost half of all people (49.3%) participating in depression research were identified as having breast cancer (table 1).
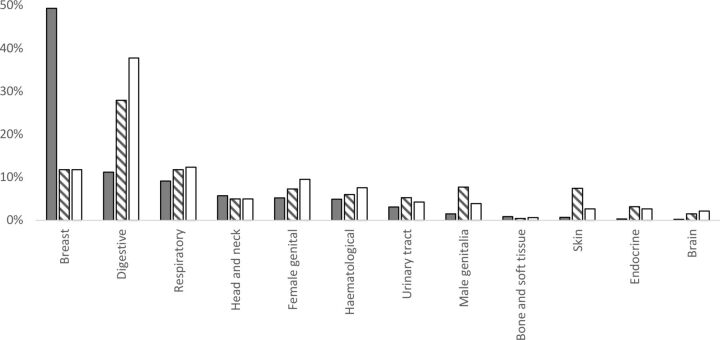
According to global estimates, 17 806 354 new cases of cancer were diagnosed in adults aged 20 years and older in 2018. The available individual incidence data for the 36 cancers listed in the GLOBOCAN database accounts for 16 932 314 or 95% of all new cancers in 2018. Table 1 outlines the respective rates of cancer incidence. Figure 2 shows a graphical representation to enable easier appreciation of the relative frequencies. For example, breast cancer research represents a higher relative percentage of total research (49.3% of participants) than its corresponding cancer incidence percentage rate (11.7%) and its corresponding depression in cancer percentage rate (11.8%). Bone and soft-tissue cancers and head and neck cancers were also over-represented in depression research, but only marginally. All other cancers were under-represented when compared with their corresponding cancer incidence percentage rate and comorbid depression percentage rate (figure 2).
Figure 2.
The relative frequency of cancer types represented in depression research (shaded bars) compared with the relative global frequency of these cancers (striped bars) and the relative global frequency of people with these cancers and comorbid depression (white bars). Cancers are ordered from highest to lowest relative frequency in research according to the present systematic review.
Expressing each cancer’s inclusion in depression research as a function of the incidence of cancer-specific depression shows that for every 100 000 people with breast cancer and depression, 714 appear in a study (table 1). This is followed by bone and soft-tissue cancers at 235 participants for every 100 000 cases, and head and neck cancers at 195 participants per 100 000 cases. There was over a 40-fold difference in inclusion rates across cancers. For example, brain cancer had the lowest measured relative representation in depression studies at just 16 participants per 100 000 cases (compared with 714 participants per 100 000 cases of breast cancer with depression).
Conclusions and clinical implications
This paper presents a systematic review of treatment for depression in people with cancer, specifically examining whether certain cancer types dominate this research. It then used data to see whether the pattern within research is reflective of the actual cancer incidence and the relative rate of patients with comorbid depression. We identified 84 relevant studies representing over 6000 participants and looked across many cancer types, revealing several discrepancies. The disparity between cancers is large.
So why are cancers not included in depression research at a level commensurate with the relative frequency of each cancer nor their individual contributions to the population of depressed people with cancer? A systematic review of psychosocial oncology and quality of life research (including depression) found that study participation was not affected by cancer type, patient age and patient sex.18 The same review found that participation was higher in longitudinal studies compared with controlled trials,18 but our review includes an array of study designs and small differences in participation are unlikely to explain the pattern measured here. Factors such as stage at diagnosis or treatment morbidity may be responsible for disparities, but respiratory cancers tend to be late stage with high mortality and are represented in research at a rate nearly commensurate to their overall incidence.
Breast cancer currently dominates this field of psycho-oncology research (both pharmacological and psychological treatments of comorbid depression in cancer). Such is the dominance of breast cancer in this area, no other cancer reaches representativeness and inclusion commensurate with incidence (except perhaps bone and soft-tissue cancer). Factors such as research funding models, advocacy models, public engagement, philanthropic giving and clinician and patient bias, plus the ‘success’ of the breast cancer movement are a likely driver of bias in research on cancer and comorbid depression towards breast cancer. Identifying causal factors for biased research effort detected here is beyond the primary research question of this study and requires a more complex approach.
Generally, in cancer survivorship research, projects on breast cancer dominate the field and in 2011 in the USA there were seven times more projects on breast cancer being conducted than the next best studied cancer, prostate.14 The dominance of breast cancer models and narratives in survivorship research has been referred to as the ‘breast cancerisation’ of the understanding of life with and beyond cancer.13 For those with breast cancer, this bias means that guidelines for the management of depression in cancer are likely based on patients with their cancer and represents an appropriate evidence base for this patient group.
Only two cancers approach relevant parity: head and neck cancer and female genital cancer. Cancers least represented by depression research include digestive cancers, male genital cancers and skin cancer. The most substantial under-representation occurred in digestive cancers, which form 37.7% of people with cancer comorbid with depression but only 11.2% of people in depression studies. The disproportionate attention afforded certain cancers whereby particular cancers receive smaller funding shares and less consideration is well documented.19–21 Only when under-represented patient groups are routinely included and studied in depression research will the evidence base reflect the full distribution of patients in need of treatment.
Last, many cancers were inconsistently reported in psycho-oncology literature. There was limited use of consensus classification systems of neoplasms. As this can lead to difficulties in undertaking systematic reviews in this area, future research should report findings using a recognised system (for example ICD-11). In addition to the correct classification, future research should comment on whether patient and/or cancer factors affected enrolment in studies on treating depression.
This review quantifies the current evidence base for depression treatments in specific cancer populations. Breast cancer accounts for half of all participants with depression. In addition to the impact on psychiatric treatments, the dominance of a particular cancer has the potential to negatively impact the psychological experiences of people with other cancers if their experience does not align.13 Further research is required to investigate the efficacy of depression treatments in other specific cancer populations, particularly digestive (eg, stomach, pancreatic, liver and colorectal) and respiratory cancers (eg, lung), which represent the highest global burden of comorbid disease. This would allow targeted, evidence-based treatments for depression in specific cancer populations.
This study has several limitations. First, our search strategy was limited to MeSH terms which may not have selected relevant papers published in the month prior to the search. However, a search of the grey literature was conducted. Second, because cancer types are inconsistently reported in depression research, assumptions were made when classifying cancer types of participants. For example, when several cancer types were associated with a single number of participants, we divided the participants equally among clustered cancers, such as ‘aerodigestive’ or ‘genitourinary’, which are terms not recognised in the ICD. Finally, level 1 evidence for the prevalence of depression within cancer types was not available for all cancers extracted during our systematic review. For this reason, we limited the comparison between participation in depression research with the incidence of depression to cancer types for which level 1 evidence was available. This is a limitation that will have primarily affected under-represented cancer types. Our estimate of the incidence of depression within each cancer was conducted using the fewest assumptions possible. Ideally, this analysis would have been itemised for each cancer type and used specific prevalence and incidence data. Many reviews of depression in cancer have noted an inability to analyse depression by cancer type because of too few studies and study heterogeneity. This is something that will improve in the future as the number of studies continues to expand.
Acknowledgments
The authors would like to thank Julie Williams at the St Vincent’s Hospital library for assistance with the search protocol and tracking down articles. This work was completed as part of BB’s medical degree honours project and did not receive any funding.
Footnotes
Contributors: MM and BB designed the research strategy and review protocol. BB and SL conducted the review with supervision from MM. All authors have contributed to and approved the final version of the manuscript for publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Data available from benbravery@yahoo.com.au.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Yeh M-L, Chung Y-C, Hsu M-YF, et al. Quantifying psychological distress among cancer patients in interventions and scales: a systematic review. Curr Pain Headache Rep 2014;18:399. 10.1007/s11916-013-0399-7 [DOI] [PubMed] [Google Scholar]
- 3. Linden W, Vodermaier A, Mackenzie R, et al. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord 2012;141:343–51. 10.1016/j.jad.2012.03.025 [DOI] [PubMed] [Google Scholar]
- 4. Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 Interview-Based studies. Lancet Oncol 2011;12:160–74. 10.1016/S1470-2045(11)70002-X [DOI] [PubMed] [Google Scholar]
- 5. Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med 2010;40:1797–810. 10.1017/S0033291709992285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mulick A, Walker J, Puntis S, et al. Does depression treatment improve the survival of depressed patients with cancer? a long-term follow-up of participants in the smart Oncology-2 and 3 trials. Lancet Psychiatry 2018;5:321–6. 10.1016/S2215-0366(18)30061-0 [DOI] [PubMed] [Google Scholar]
- 7. Mausbach BT, Bos T, Irwin SA. Mental health treatment dose and annual healthcare costs in patients with cancer and major depressive disorder. Health Psychol 2018;37:1035–40. 10.1037/hea0000670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walker J, Hansen CH, Martin P, et al. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry 2014;1:343–50. 10.1016/S2215-0366(14)70313-X [DOI] [PubMed] [Google Scholar]
- 9. Ng CG, Boks MPM, Zainal NZ, et al. The prevalence and pharmacotherapy of depression in cancer patients. J Affect Disord 2011;131:1–7. 10.1016/j.jad.2010.07.034 [DOI] [PubMed] [Google Scholar]
- 10. Krebber AMH, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology 2014;23:121–30. 10.1002/pon.3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caruso R, Nanni MG, Riba M, et al. Depressive spectrum disorders in cancer: prevalence, risk factors and screening for depression: a critical review. Acta Oncol 2017;56:146–55. 10.1080/0284186X.2016.1266090 [DOI] [PubMed] [Google Scholar]
- 12. Karageorge A, Murphy MJ, Newby JM, et al. Acceptability of an Internet cognitive behavioural therapy program for people with early-stage cancer and cancer survivors with depression and/or anxiety: thematic findings from focus groups. Support Care Cancer 2017;25:2129–36. 10.1007/s00520-017-3617-8 [DOI] [PubMed] [Google Scholar]
- 13. Bell K. The breast-cancer-ization of cancer survivorship: implications for experiences of the disease. Soc Sci Med 2014;110:56–63. 10.1016/j.socscimed.2014.03.031 [DOI] [PubMed] [Google Scholar]
- 14. Harrop JP, Dean JA, Paskett ED. Cancer survivorship research: a review of the literature and summary of current NCI-designated cancer center projects. Cancer Epidemiol Biomarkers Prev 2011;20:2042–7. 10.1158/1055-9965.EPI-11-0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith HR. Depression in cancer patients: pathogenesis, implications and treatment (review). Oncol Lett 2015;9:1509–14. 10.3892/ol.2015.2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walker J, Holm Hansen C, Martin P, et al. Prevalence of depression in adults with cancer: a systematic review. Ann Oncol 2013;24:895–900. 10.1093/annonc/mds575 [DOI] [PubMed] [Google Scholar]
- 17. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wakefield CE, Fardell JE, Doolan EL, et al. Participation in psychosocial oncology and quality-of-life research: a systematic review. Lancet Oncol 2017;18:e153–65. 10.1016/S1470-2045(17)30100-6 [DOI] [PubMed] [Google Scholar]
- 19. Plage S, Gibson A, Burge M, et al. Cancer on the margins: experiences of living with neuroendocrine tumours. Health Sociology Review 2018;27:153–67. 10.1080/14461242.2017.1387068 [DOI] [Google Scholar]
- 20. Griffiths J, Willard C, Burgess A, et al. Meeting the ongoing needs of survivors of rarer cancer. Eur J Oncol Nurs 2007;11:434–41. 10.1016/j.ejon.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 21. Carter AJR, Nguyen CN. A comparison of cancer burden and research spending reveals discrepancies in the distribution of research funding. BMC Public Health 2012;12:526. 10.1186/1471-2458-12-526 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ebmental-2020-300145supp001.pdf (62.5KB, pdf)