Abstract
Background
The Mini-Mental State Examination (MMSE), the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and the Severe Impairment Battery (SIB) are widely used rating scales to assess cognition in Alzheimer’s disease.
Objective
To understand the correspondence between these rating scales, we aimed to examine the linkage of MMSE with the ADAS-Cog and SIB total and change scores.
Methods
We used individual-level data on participants with Alzheimer’s disease (n=2925) from five pivotal clinical trials of donepezil. Data were collected at baseline and scheduled visits for up to 6 months. We used equipercentile linking to identify the correspondence between simultaneous measurements of MMSE with ADAS-Cog, and SIB total and change ratings.
Findings
Spearman’s correlation coefficients were of strong magnitude between the MMSE total score and the ADAS-Cog (rs from −0.82 to −0.87; p<0.05) and SIB total scores (rs from 0.70 to 0.75; p<0.05). Weaker correlations between the change scores were observed between the MMSE change score and the ADAS-Cog (week 1: r=−0.11, p=0.18; rs thereafter: −0.28 to −0.45; p<0.05) and SIB change scores (rs from 0.31 to 0.44; p<0.05). Linking suggested that the MMSE total scores were sensitive to moderate and severe cognitive impairment levels. Despite weak to moderate correlations for the change scores, moderate change levels linked well, indicating ceiling and floor effects.
Conclusions
The current results can be used in meta-analyses, data harmonisation and may contribute to increasing statistical power when pooling data from multiple sources.
Clinical implications
The current study results help clinicians to understand these cognitive rating scale scores.
Keywords: adult psychiatry, delirium & cognitive disorders
Background
Alzheimer’s disease is a chronic neurodegenerative disease characterised by the progressive deterioration of neurons and atrophy of brain tissue.1 Onset is insidious and gradual, with initial cognitive impairment in short-term memory and with disease progression spreads to multiple cognitive domains (e.g., executive functions, attention, language) become impaired.2 To assess the extent of cognitive impairment, various rating scales have been developed to evaluate cognitive functioning in research and clinical settings at different stages of the disease.
The most widely used cognitive rating scale is the Mini-Mental State Examination (MMSE), which was designed to screen for cognitive impairment in under 10 minutes. However, the MMSE lacks sensitivity to distinguish severe levels of impairment. Subsequently, the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) was developed as a comprehensive assessment of the extent of cognitive dysfunction in Alzheimer’s disease.3 Despite an administration time of approximately 40 minutes, it has become the gold standard to assess the efficacy of antidementia treatments in clinical trials.4 For instance, a 4-point difference between the treatment and the placebo groups is considered clinically relevant,5 at least in most Alzheimer’s disease trials where symptomatology is moderate.6 For populations in the advanced stages of the illness, the Severe Impairment Battery (SIB) was developed to address the cognitive and behavioural characteristics of severe dementia.7 It has an administration time of around 30 minutes. There are design trade-offs between these scales, including administration time, and ceiling and floor effects (i.e., scales that measure the most severe cases lack sensitivity to differentiate moderate cases and vice versa).
Nonetheless, being able to convert between scale scores is often desirable, even a requirement. Research has demonstrated conversions between rating scales in schizophrenia,8 depression,9 panic disorder10 and Alzheimer’s disease.11 12 Prior studies linking rating scales in Alzheimer’s disease have been informative but are restricted to cross-sectional information, do not link change scores, do not link MMSE and SIB scores and do not consider learning effects from the first measure administered to the second. Nonetheless, there are benefits to converting measures.12 First, most clinicians are probably used to the MMSE, therefore an explanation as to what scores the other rating scales (e.g., the SIB mean will be useful. Second, in meta-analyses two different rating scales often need to be transformed into a single common metric. Third, healthcare maintenance organisations frequently have information on different rating scales that could be harmonised for a single statistical analysis. Fourth, datasets with different scale ratings can be pooled to increase statistical power.
Objective
In the current study, we aim to link the MMSE to the SIB and the ADAS-Cog based on five clinical trials of Alzheimer’s disease.
Study selection and analysis
We obtained access to the individual-level participant data of all randomised controlled double-blinded trials of donepezil conducted by Eisai Co in which two or more of the cognitive scales of interest were administered simultaneously. Data access was provided following the submission of an a priori analytic plan and analysed via a secure internet cloud-based platform (http://www.clinicalstudydatarequest.com). We included trials in which patients with Alzheimer’s disease were assessed with at least two of MMSE,13 the SIB14 15 and/or the ADAS-Cog.3
Measures
The following three measures were administered in the trials.
Mini-Mental State Examination
The MMSE is comprised of 20 items and takes between 5 and 10 min to administer. It consists of components of cognitive functioning (e.g., time-space orientation, short-term memory, attention, language and construction) that constitute a single underlying entity of cognitive functioning.13 MMSE total scores range from 0 to 30, with lower scores representing a worse cognitive deficit.
Alzheimer’s Disease Assessment Scale–Cognitive Subscale
The ADAS-Cog is a neuropsychological index of the severity of the cognitive symptoms of dementia.4 It is comprised of 11 tasks (word recall, word recognition, constructional praxis, orientation, naming objects and fingers, commands, ideational praxis, remembering test instruction, spoken language, word-finding, comprehension) that include both participant-completed and observer-based assessments. ADAS-Cog total scores range from 0 to 70, with higher scores representing a greater cognitive deficit.
The Severe Impairment Battery
The SIB consists of 40 one-step questions and commands, with a scale score of 100 points and nine aspects of cognitive functioning (social interaction, memory, orientation, language, attention, praxis, visuospatial ability, construction, orienting to name). SIB total scores may range from 0 to 100, with higher scores representing a lesser cognitive deficit.14
The MMSE was administered in every trial. In contrast, the ADAS-Cog and SIB assessments did not coincide in the same trial or week (table 1). Across the trials, the MMSE was administered before the ADAS-Cog and SIB, except at week 24 in one trial.16
Table 1.
Trial characteristics of the analytic dataset
| Authors | N | Sex | Mean age (SD) | Assessments available for linkage by visit | Trial inclusion criteria |
| Rogers and Friedhoff29 | 156 | Male: 63 Female: 93 |
71.9 (7.4) | ADAS-Cog, MMSE 0, 1, 3, 6, 9, 12 |
MMSE: 10–26 CDR: 1 or 2 |
| Rogers et al 23 | 481 | Male: 176 Female: 305 |
74.0 (7.6) | ADAS-Cog, MMSE 0, 3, 6, 9, 12 |
MMSE: 10–26 CDR: 1 or 2 |
| Rogers et al 30 | 473 | Male: 180 Female: 293 |
73.5 (7.2) | ADAS-Cog, MMSE 0, 6, 12, 18, 24 |
MMSE: 10–26 CDR: 1 or 2 |
| Black et al 16 | 342 | Male: 101 Female: 241 |
78.1 (7.6) | MMSE: 0 to 24 SIB: −1, 8, 16, 24 |
MMSE: 1–12 Modified Hachinski Ischemic Score: 6 or less Functional Assessment Staging: 6 or more |
| Farlow et al 24 | 1444 | Male: 539 Female: 905 |
74.2 (7.9) | MMSE, SIB 0, 6, 12, 18, 24 |
MMSE: 0–20 SIB: 90 or less Cornell Scale for Depression in Dementia score: under 12 |
Week −1 refers to the screening assessment that was 28±7 before the drug administration (mean 23.67 days).
ADAS-Cog, Alzheimer’s Disease Assessment Scale–Cognitive Subscale; CDR, Clinical Dementia Rating; MMSE, Mini-mental State Examination; SIB, Severe Impairment Battery.
Statistical analysis
At step one of the analysis, we presented the trial characteristics and demographics. At step two, we conducted linking of each available pair of ratings assessed at the same week (between the MMSE with the ADAS-Cog and then the MMSE with the SIB) with one exception. One trial16 had screening rather than baseline SIB assessments (conducted within the 28±7 days before drug administration, median 22 days). Therefore, it so was used as a baseline appraisal to maximise the sample size and because there was no alternative treatment for Alzheimer’s disease when the trials were conducted.
We computed Spearman’s correlation coefficients and equipercentile linking. Although there are no agreed thresholds to interpret Spearman’s correlation coefficient values, we follow prior guidelines and interpret the magnitude as weak (0.10–0.39), moderate (0.40–0.69) and strong (0.70–0.89).17 We computed equipercentile linking to examine the extent to which it was possible to convert between the study measures at each time point. Prior studies of schizophrenia18 and depression9 19 have used equipercentile linking. Equipercentile linking is a statistical method to equate scores between two test scales. In short, the method ranks percentile scores between tests, and there is no independent or dependent variable. In this way, transformations between test scores are ascertained.20 We analysed all trials collectively as a unique population rather than by trial to maximise the sample size and so attain robust linkage estimates. Finally, we took the median value from the equipercentile linking values across different measurement points to define the corresponding scores between the scales. We report cut-offs that link to mild (21-25), moderate (11-20), and severe (0-10) dementia on the MMSE.21 In this way, different scores become interchangeable and comparable. Equipercentile linking was computed using the equate library20 in R 3.6.2.22
Sensitivity analysis
We conducted sensitivity analysis precisely as above, but without the trial by Rogers et al 23 owing to 13 participants at one site, which violated the trial protocol.
Findings
Trial characteristics
We identified four randomised placebo-controlled clinical trials and one trial24 comparing random dose levels (of 10mg or 23mg of donepezil) cumulating in 2925 trial participants. Four participants were removed owing to missing information following the screening phase in one trial,24 leaving 2921 trial participants with information in the dataset. Of the 2921 participants, 1110 participants had MMSE and ADAS-Cog scores and 1786 had MMSE and SIB scores. Hence, the analytic dataset consisted of 2896 trial participants (table 1). In addition, eight participants who contributed to the linkage between ADAS-Cog and MMSE total and one participant who contributed to the linkage between MMSE and SIB total did not contribute to change scores due to missing baseline assessments. The trial characteristics of the analytic dataset are summarised in table 1. There were 1059 (36.6%) males and 1837 (63.4%) females, with a mean age (SD) of 74.4 (7.8) years. Total and change rating scale score descriptive statistics are shown in table 2.
Table 2.
Sample characteristics
| Visit | MMSE-ADAS-Cog total scores | MMSE-SIB total scores | ||||||
| Week | N | MMSE | ADAS-Cog | r | N | MMSE | SIB | r |
| Baseline | 1102 | 19.33 (4.73) | 26.69 (11.16) | −0.82 | 1785 | 12.01 (5.17) | 72.68 (19.19) | 0.70 |
| Week 1 | 152 | 19.64 (5.34) | 26.29 (10.88) | −0.86 | ||||
| Week 3 | 597 | 20.06 (5.23) | 24.57 (11.32) | −0.84 | ||||
| Week 6 | 996 | 20.10 (5.39) | 25.04 (11.38) | −0.85 | 1225 | 13.53 (5.46) | 76.41 (18.78) | 0.72 |
| Week 9 | 562 | 20.35 (5.62) | 24.87 (11.17) | −0.86 | ||||
| Week 12 | 961 | 20.17 (5.62) | 25.40 (11.88) | −0.86 | 1156 | 13.83 (5.72) | 77.05 (18.78) | 0.72 |
| Week 18 | 381 | 19.45 (5.82) | 26.31 (12.30) | −0.86 | 1095 | 13.82 (5.76) | 77.47 (19.22) | 0.72 |
| Week 24 | 380 | 19.09 (6.05) | 26.73 (12.98) | −0.87 | 1549 | 12.74 (6.16) | 74.61 (21.60) | 0.75 |
ADAS-Cog, Alzheimer’s Disease Assessment Scale–Cognitive Subscale; MMSE, Mini-Mental State Examination; r, Spearman’s correlation coefficient; SIB, Severe Impairment Battery.
| Visit | MMSE-ADAS-Cog total change scores | MMSE-SIB total change scores | ||||||
| Week | N | MMSE | ADAS-Cog | r | N | MMSE | SIB | r |
| Week 1 | 149 | 0.69 (2.34) | −0.92 (3.68) | −0.11 | ||||
| Week 3 | 594 | 0.76 (2.61) | −2.04 (4.31) | −0.28 | ||||
| Week 6 | 991 | 0.74 (2.82) | −1.64 (4.65) | −0.28 | 1225 | 0.52 (2.35) | 2.05 (7.07) | 0.31 |
| Week 9 | 560 | 0.98 (3.05) | −1.57 (4.70) | −0.32 | ||||
| Week 12 | 957 | 0.76 (3.05) | −1.17 (5.11) | −0.36 | 1156 | 0.76 (2.76) | 2.36 (7.72) | 0.37 |
| Week 18 | 380 | 0.06 (3.16) | −0.36 (5.31) | −0.39 | 1095 | 0.71 (2.94) | 2.47 (8.74) | 0.43 |
| Week 24 | 379 | −0.31 (3.35) | 0.03 (5.76) | −0.45 | 1548 | 0.53 (3.08) | 1.02 (10.42) | 0.44 |
All r values were statistically significant (p<0.05), except the correlation at week 1 between the MMSE and ADAS-Cog where p=0.18. Spearman’s correlation coefficient values may be interpreted as weak (0.10–0.39), moderate (0.40–0.69) and strong (0.70–0.89).
ADAS-Cog, Alzheimer’s Disease Assessment Scale–Cognitive Subscale; MMSE, Mini-Mental State Examination; N, the sample size that varies owing to different visit schedules across trials; r, Spearman’s correlation coefficient; SIB, Severe Impairment Battery.
Equipercentile linking
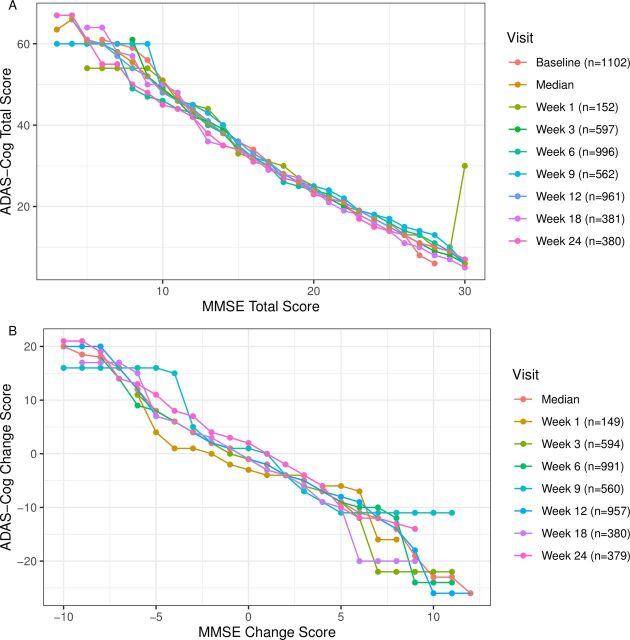
Spearman’s correlation coefficients between the MMSE and the ADAS-Cog total were statistically significant (p<0.05) and of large magnitude at all time points (r=−0.82 to −0.87; table 2). Equipercentile linking of the MMSE total scores with ADAS-Cog total scores was calculated. Across different time points, each 10 point increase on the MMSE total score corresponded to a decrease of approximately 20 on the ADAS-Cog total score (see figure 1A and the conversion table: online supplemental eTable 1). In general, one MMSE point was equivalent to two ADAS-Cog points. Spearman’s correlation coefficient between the MMSE and the ADAS-Cog change scores was null at baseline (r=−0.11), but was statistically significant (p<0.05) for the remaining weeks with weak to moderate magnitude (range from r=−0.28 to r=−0.45; p<0.05; table 2). These lower correlations may partly reflect the inability of the MMSE change to capture severe cases of ADAS-Cog change. Equipercentile linking between the MMSE and the ADAS-Cog change scores showed slight fluctuations over time from −2 to 5 on the MMSE with floor and ceiling effects where change was under −3 and over 5 (see figure 1B and the conversion table: online supplemental eTable 2).
Figure 1.
Linking the MMSE and ADAS-Cog scores. Fig. 1A shows the MMSE total score linked to the ADAS-Cog total score from baseline to week 24. Figure 1B shows the MMSE change score linked to the ADAS-Cog change score from week one to 24. ADAS-Cog, Alzheimer’s Disease Assessment Scale–Cognitive Subscale; MMSE, Mini-Mental State Examination.
ebmental-2020-300184supp001.pdf (160.2KB, pdf)
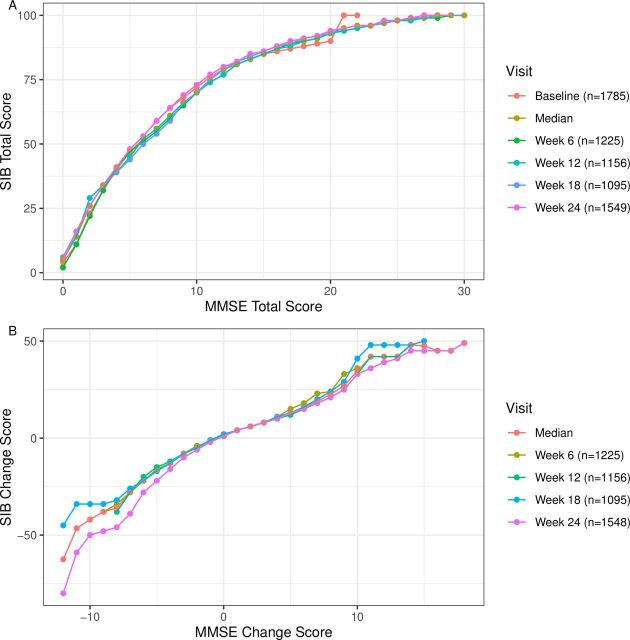
Spearman’s correlation coefficients between the MMSE and the SIB scores were of strong magnitude for the total scores (range r=0.70–0.75; p values <0.05; table 2) and a moderate magnitude for the change scores (range r=0.31–0.45; p values <0.05; table 2). Each point increase in the range 0–6 on the MMSE total score corresponded to an increment of approximately 8 on the SIB total score; for MMSE total scores ranging from 7 to 11 each point increase was linked to 4–5 point increases on the SIB score; and for MMSE total scores ranging from 12 to 30, each point increase linked to 1–2 point increments on the SIB total (figure 2A, and conversion online supplemental eTable 3). Examination of the linkage between the MMSE and SIB total change scores showed consistent linkage over time for MMSE change scores ranging from −2 to 8 (and vice versa). MMSE total change scores under −3 to −9 generally linked to a point reduction of −4 on the SIB change score, with lower values linking at −11 MMSE scores (see figure 2B, and conversion online supplemental eTable 4).
Figure 2.
Linking the MMSE and SIB scores. Figure 2A shows the MMSE total score linked to the SIB total score from baseline to week 24. Figure 2B shows the MMSE change score linked to the SIB change score to week six to 24. MMSE, Mini-Mental State Examination; SIB, Severe Impairment Battery.
Table 3 summarises the corresponding scores across MMSE, ADAS-Cog and SIB. To interpret table 3, we used established guidelines to classify the MMSE scores into mild (21-25), moderate (11-20), and severe (0-10) dementia on the MMSE.21 MMSE scores of mild dementia (i.e., 21–25) linked to ADAS-Cog scores from 15 to 22 and SIB scores from 95 to 98; moderate dementia (i.e., 11–20) linked to ADAS-Cog scores from 24 to 46 and SIB scores from 75 to 93 and severe dementia (i.e., 0–10) linked to ADAS-Cog scores over 48 and SIB scores under 70.
Table 3.
Conversion table between the study measures
| Observed | Change | ||||
| MMSE | ADAS-Cog | SIB | MMSE | ADAS-Cog | SIB |
| 0 | 5 | −12 | −62 | ||
| 1 | 14 | −11 | −46 | ||
| 2 | 26 | −10 | 20 | −42 | |
| 3 | 64 | 34 | −9 | 18 | −38 |
| 4 | 66 | 40 | −8 | 18 | −36 |
| 5 | 60 | 47 | −7 | 16 | −28 |
| 6 | 60 | 52 | −6 | 12 | −22 |
| 7 | 58 | 56 | −5 | 8 | −16 |
| 8 | 56 | 61 | −4 | 6 | −13 |
| 9 | 52 | 66 | −3 | 4 | −8 |
| 10 | 48 | 70 | −2 | 2 | −5 |
| 11 | 46 | 75 | −1 | 1 | −2 |
| 12 | 44 | 79 | 0 | −1 | 1 |
| 13 | 40 | 81 | 1 | −2 | 4 |
| 14 | 38 | 83 | 2 | −4 | 6 |
| 15 | 34 | 85 | 3 | −5 | 8 |
| 16 | 32 | 87 | 4 | −7 | 10 |
| 17 | 31 | 89 | 5 | −9 | 13 |
| 18 | 28 | 90 | 6 | −11 | 16 |
| 19 | 26 | 91 | 7 | −12 | 19 |
| 20 | 24 | 93 | 8 | −14 | 22 |
| 21 | 22 | 95 | 9 | −19 | 27 |
| 22 | 21 | 96 | 10 | −23 | 34 |
| 23 | 19 | 96 | 11 | −23 | 42 |
| 24 | 17 | 97 | 12 | −26 | 42 |
| 25 | 15 | 98 | 13 | 42 | |
| 26 | 13 | 98 | 14 | 48 | |
| 27 | 11 | 99 | 15 | 48 | |
| 28 | 10 | 100 | 16 | 45 | |
| 29 | 9 | 100 | 17 | 45 | |
| 30 | 6 | 100 | 18 | 49 | |
ADAS-Cog and SIB scores are based on median value across different measurement points to define the corresponding scores between the scales.
ADAS-Cog, Alzheimer’s Disease Assessment Scale–Cognitive Subscale; MMSE, Mini-Mental State Examination; SIB, Severe Impairment Battery.
Sensitivity analysis by removing the trial by Rogers et al 23 owing to 13 participants at one site that violated the trial protocol did not manifestly impact the results (conversion online supplemental eTables 5 and 6).
Conclusions and clinical implications
The current study is the first linkage between the MMSE with ADAS-Cog and SIB total and change from baseline scores. The results show a generally consistent linking pattern over time and are easy to translate into practice. For instance, at baseline, an MMSE score of 10 was linked to an ADAS-Cog score of 48 and a SIB score of 70, and across time each 10 point increase on the MMSE total score linked to a decrease of approximately 20 on the ADAS-Cog total score.
There are several potential reasons that the correlations between the total scores increased over time (see table 2). One explanation is that as the trial progressed, the variability of the scale scores increased and so did the correlation coefficients between them. Another possibility is that the natural progression of the disease induced an increase in generalised cognitive impairment. Furthermore, possibly repeated testing induced learning effects. Notably, the magnitudes of the change score correlations were less than that of the total scores, suggesting that conversion of change scores may be less reliable.25
Interestingly, the plots between the MMSE and the ADAS-Cog and SIB totals differ in form. The graph of the MMSE-SIB total linkage resembles an asymptotic function (i.e., SIB scores seem to level off at high MMSE scores). This likely reflects ceiling effects, namely, that the SIB is sensitive to severe stages but is insensitive to more normal range scores represented by MMSE scores of 25–30. The presence of ceiling effects was less evident on the linkage between the MMSE and the ADAS-Cog total scales.
There are several limitations to our study. First, as the results are based on clinical trial data with inclusion criteria, they may have restricted generalisability. Second, in one trial16 ratings of the MMSE at screening were used because there were no baseline scores. Considering there were approximately 3 weeks between screening and baseline and the rate of deterioration in Alzheimer’s disease, this artefact is unlikely to impact the study results. Third, the change scores did not correlate highly owing to outlying extreme scores. Fourth, we lacked item-level MMSE scores for a fine-level analysis. One might expect equivalence in well-defined domains of the same aspect of cognitive functioning. Fifth, the combined sample size and distribution did not enable us to analyse the placebo and donepezil groups separately. Sixth, we lacked information on the Montreal Cognitive Assessment (MoCA), which is a widely used measure to index cognitive impairment. Hence, the lack of information on the MoCA restricts the generalisability of the current results.26 Seventh, MMSE was administered before the other rating scales, except for one trial where the SIB was administered before the MMSE.16 This may raise concerns about the temporal order of each rating measure pair and learning cross-over effects between tests. However, at each visit, we consider learning effects are unlikely to adversely impact the result since the questions on the MMSE capture less severe impairment than the other rating scales.
A notable advantage of the current study was the large number of participants making the results robust. Moreover, all rating scale total scores were generally strongly correlated with each other, with little variability over time. This feature reinforces our faith in the robustness of linking functions.
In conclusion, our results provide linkages of the MMSE with the ADAS-Cog and SIB total and change scores often used in clinical trials of Alzheimer’s disease.27 The study results have the potential to contribute to clinical cross-walks when different clinicians use different scales and to meta-analytic calculations when researchers want to pool studies using different scales. Furthermore, our results can assist in data harmonisation and thus increase the statistical power of analyses that combine data from multiple sources. To facilitate the uptake of our results in practice, we have provided detailed conversion tables to directly link between rating scores of cognitive impairment in Alzheimer’s disease. From a clinical point of view, individual priorities and goals of care will vary significantly depending on the stage of dementia, and discussions need to be tailored to the stage of illness. It is important, however, that complex decisions are discussed at a relatively early stage, when a person with dementia may be actively involved in care planning.28
Acknowledgments
We acknowledge Eisai Co for providing us with the study data. Eisai Co did not provide study design, critical input or manuscript review for the study.
Footnotes
Twitter: @szlevine, @And_Cipriani, @Toshi_FRKW
Contributors: SZL contributed to manuscript drafting, statistical analysis, data management and study conceptualisation. KY contributed to critical manuscript feedback, data management and statistical analysis. YG contributed to critical manuscript feedback and statistical analysis. MS and AC contributed to study conceptualisation, interpretation and critical manuscript feedback. OE contributed to critical manuscript feedback and statistical interpretation. TI and SL contributed to study conceptualisation, interpretation and critical manuscript feedback. SL contributed to study conceptualisation, interpretation and critical manuscript feedback. TAF contributed to critical manuscript feedback, statistical review, study conceptualisation and mentorship.
Funding: AC is supported by the National Institute for Health Research (NIHR) Oxford Cognitive Health Clinical Research Facility, by an NIHR Research Professorship (grant RP-2017-08-ST2-006), by the NIHR Oxford and Thames Valley Applied Research Collaboration and by the NIHR Oxford Health Biomedical Research Centre (grant BRC-1215-20005). OE is supported by project grant No 180 083 from the Swiss National Science Foundation (SNSF).
Competing interests: TI has served as a consultant of Eisai, Roche and Biogen in the last 3 years. AC has received research and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation and Angelini Pharma. In the past 3 years, SL has received honoraria as a consultant or for lectures for LB Pharma, Otsuka, Lundbeck, Boehringer Ingelheim, LTS Lohmann, Janssen, Johnson&Johnson, TEVA, MSD, Sandoz, SanofiAventis, Angelini, Sunovion, Recordati and Geodon Richter. TAF reports personal fees from Mitsubishi-Tanabe, MSD and Shionogi, and a grant from Mitsubishi-Tanabe, outside the submitted work; TAF has a patent 2018-177688 pending.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Data requests can be submitted via http://www.clinicalstudydatarequest.com.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Institutional review boards approved each study, and all patients had given their written informed consent.
References
- 1. Whitehouse PJ, Price DL, Struble RG, et al. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science 1982;215:1237–9. 10.1126/science.7058341 [DOI] [PubMed] [Google Scholar]
- 2. Alzheimer's Association . 2020 Alzheimer's disease facts and figures. Alzheimers Dement 2020;15:321–87. 10.1016/j.jalz.2019.01.010 [DOI] [Google Scholar]
- 3. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–64. 10.1176/ajp.141.11.1356 [DOI] [PubMed] [Google Scholar]
- 4. Kueper JK, Speechley M, Montero-Odasso M. The Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog): Modifications and Responsiveness in Pre-Dementia Populations. A Narrative Review. J Alzheimers Dis 2018;63:423–44. 10.3233/JAD-170991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rockwood K, Fay S, Gorman M, et al. The clinical meaningfulness of ADAS-Cog changes in Alzheimer's disease patients treated with donepezil in an open-label trial. BMC Neurol 2007;7:26. 10.1186/1471-2377-7-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knopman DS. Clinical trial design issues in mild to moderate Alzheimer disease. Cogn Behav Neurol 2008;21:197–201. 10.1097/WNN.0b013e318190cf75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saxton J, McGonigle-Gibson KL, Swihart AA, et al. Assessment of the severely impaired patient: description and validation of a new neuropsychological test battery. Psychol Assess 1990;2:298–303. 10.1037/1040-3590.2.3.298 [DOI] [Google Scholar]
- 8. Levine SZ, Leucht S. Identifying clinically meaningful symptom response cut-off values on the SANS in predominant negative symptoms. Schizophr Res 2013;145:125–7. 10.1016/j.schres.2012.12.032 [DOI] [PubMed] [Google Scholar]
- 9. Furukawa TA, Reijnders M, Kishimoto S, et al. Translating the BDI and BDI-II into the HAMD and vice versa with equipercentile linking. Epidemiol Psychiatr Sci 2019;29:e24. 10.1017/S2045796019000088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Furukawa TA, Katherine Shear M, Barlow DH, et al. Evidence-based guidelines for interpretation of the panic disorder severity scale. Depress Anxiety 2009;26:922–9. 10.1002/da.20532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roalf DR, Moore TM, Mechanic-Hamilton D, et al. Bridging cognitive screening tests in neurologic disorders: a crosswalk between the short Montreal cognitive assessment and Mini-Mental state examination. Alzheimers Dement 2017;13:947–52. 10.1016/j.jalz.2017.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balsis S, Benge JF, Lowe DA, et al. How do scores on the ADAS-Cog, MMSE, and CDR-SOB correspond? Clin Neuropsychol 2015;29:1002–9. 10.1080/13854046.2015.1119312 [DOI] [PubMed] [Google Scholar]
- 13. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 14. Panisset M, Roudier M, Saxton J, et al. Severe impairment battery. A neuropsychological test for severely demented patients. Arch Neurol 1994;51:41–5. 10.1001/archneur.1994.00540130067012 [DOI] [PubMed] [Google Scholar]
- 15. Schmitt FA, Ashford W, Ernesto C, et al. The severe impairment battery: concurrent validity and the assessment of longitudinal change in Alzheimer's disease. the Alzheimer's disease Cooperative study. Alzheimer Dis Assoc Disord 1997;11(Suppl 2):S51–6. [PubMed] [Google Scholar]
- 16. Black SE, Doody R, Li H, et al. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology 2007;69:459–69. 10.1212/01.wnl.0000266627.96040.5a [DOI] [PubMed] [Google Scholar]
- 17. Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg 2018;126:1763–8. 10.1213/ANE.0000000000002864 [DOI] [PubMed] [Google Scholar]
- 18. Levine SZ, Rabinowitz J, Engel R, et al. Extrapolation between measures of symptom severity and change: an examination of the PANSS and CGI. Schizophr Res 2008;98:318–22. 10.1016/j.schres.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 19. Leucht S, Fennema H, Engel RR, et al. Translating the HAM-D into the MADRS and vice versa with equipercentile linking. J Affect Disord 2018;226:326–31. 10.1016/j.jad.2017.09.042 [DOI] [PubMed] [Google Scholar]
- 20. Albano AD. equate : An R Package for Observed-Score Linking and Equating. J Stat Softw 2016;74. 10.18637/jss.v074.i08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perneczky R, Wagenpfeil S, Komossa K, et al. Mapping scores onto stages: Mini-Mental state examination and clinical dementia rating. Am J Geriatr Psychiatry 2006;14:139–44. 10.1097/01.JGP.0000192478.82189.a8 [DOI] [PubMed] [Google Scholar]
- 22. R Core Team . R: a language and environment for statistical computing R foundation for statistical computing, 2013. [Google Scholar]
- 23. Rogers SL, Doody RS, Mohs RC, et al. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med 1998;158:1021–31. 10.1001/archinte.158.9.1021 [DOI] [PubMed] [Google Scholar]
- 24. Farlow MR, Salloway S, Tariot PN, et al. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer's disease: a 24-week, randomized, double-blind study. Clin Ther 2010;32:1234–51. 10.1016/j.clinthera.2010.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cronbach LJ, Furby L. How we should measure "change": Or should we? Psychol Bull 1970;74:68–80. 10.1037/h0029382 [DOI] [Google Scholar]
- 26. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 27. Takeshima N, Ishiwata K, Sozu T, et al. Primary endpoints in current phase II/III trials for Alzheimer disease: a systematic survey of trials registered at ClinicalTrials.gov. Alzheimer Dis Assoc Disord 2020;34:97–100. 10.1097/WAD.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 28. Fetherston AA, Rowley G, Allan CL. Challenges in end-of-life dementia care. Evid Based Ment Health 2018;21:107–11. 10.1136/eb-2018-102889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rogers SL, Friedhoff LT. The efficacy and safety of donepezil in patients with Alzheimer's disease: results of a US multicentre, randomized, double-blind, placebo-controlled trial. The donepezil Study Group. Dementia 1996;7:293–303. 10.1159/000106895 [DOI] [PubMed] [Google Scholar]
- 30. Rogers SL, Farlow MR, Doody RS, et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group. Neurology 1998;50:136–45. 10.1212/WNL.50.1.136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ebmental-2020-300184supp001.pdf (160.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data requests can be submitted via http://www.clinicalstudydatarequest.com.