Abstract
Evidence suggests that spironolactone, a nonselective mineralocorticoid receptor (MR) antagonist, modulates alcohol seeking and consumption. Therefore, spironolactone may represent a novel pharmacotherapy for alcohol use disorder (AUD). In this study, we tested the effects of spironolactone in a mouse model of alcohol drinking (drinking-in-the-dark) and in a rat model of alcohol dependence (vapor exposure). We also investigated the association between spironolactone receipt for at least 60 continuous days and change in self-reported alcohol consumption, using the Alcohol Use Disorders Identification Test-Consumption (AUDIT-C), in a pharmacoepidemiologic cohort study in the largest integrated healthcare system in the US. Spironolactone dose-dependently reduced the intake of sweetened or unsweetened alcohol solutions in male and female mice. No effects of spironolactone were observed on drinking of a sweet solution without alcohol, food or water intake, motor coordination, alcohol-induced ataxia, or blood alcohol levels. Spironolactone dose-dependently reduced operant alcohol self-administration in dependent and nondependent male and female rats. In humans, a greater reduction in alcohol consumption was observed among those who received spironolactone, compared to propensity score-matched individuals who did not receive spironolactone. The largest effects were among those who reported hazardous/heavy episodic alcohol consumption at baseline (AUDIT-C ≥ 8) and those exposed to ≥ 50 mg/day of spironolactone. These convergent findings across rodent and human studies demonstrate that spironolactone reduces alcohol use and support the hypothesis that this medication may be further studied as a novel pharmacotherapy for AUD.
INTRODUCTION
Alcohol use disorder (AUD) is a chronic relapsing brain disorder leading to high mortality, morbidity, and economic burden [1]. Compared to other chronic illnesses, currently available medications for AUD are limited. Therefore, there is a critical need to increase the armamentarium of pharmacotherapies to treat individuals with AUD [2]. Neuroendocrine systems involved in alcohol craving and drinking offer promising pharmacologic targets in this regard [3, 4].
The steroid hormone aldosterone and its related mineralocorticoid receptor (MR) regulate fluid and electrolyte homeostasis. In response to decreased blood volume and/or blood pressure, aldosterone is secreted from the cortex of the adrenal gland and binds to MRs located in the principal cells of the kidney. This action facilitates sodium and water reabsorption into the blood and increases blood pressure. MRs are also expressed in brain regions involved in AUD, including the amygdala, prefrontal cortex, and hippocampus [5-8], and modulate processes such as memory formation, fear extinction/recall, and stress responses [9-16]. Preliminary clinical and preclinical studies suggest that aldosterone and the MR play a role in alcohol seeking and consumption [17]. We previously reported that blood aldosterone concentrations are significantly decreased in actively drinking individuals with AUD who maintained alcohol abstinence during a 12-week outpatient follow-up [18]. Aldosterone levels positively correlated with self-reported alcohol craving and anxiety [18]. More recently, we provided evidence supporting the role of this endocrine system in alcohol use across three species [19]. In an alcohol self-administration model in monkeys, blood aldosterone levels increased from baseline to 6 and 12 months, and the MR gene (Nr3c2) expression levels in the central nucleus of the amygdala (CeA) were negatively correlated with average alcohol intake. The study also found a negative correlation between Nr3c2 expression in the CeA and measures of anxiety-like behavior and compulsive-like drinking in alcohol-dependent rats. In a second, 12-week clinical study, participants who remained alcohol abstinent, compared to those not abstinent, had lower blood aldosterone concentrations at the endpoint, and aldosterone levels positively correlated with the amount of drinking, alcohol craving, and anxiety in the non-abstinent group [19]. Collectively, these data suggest that higher MR signaling contributes to increased alcohol consumption, and medications that block MR may represent a novel pharmacotherapeutic approach for AUD.
Spironolactone is an FDA-approved MR antagonist medication used to treat essential hypertension, heart failure, edema, primary hyperaldosteronism, and hypokalemia. Spironolactone has been tested in preclinical studies of alcohol drinking and seeking with inconsistent results. Systemic or intracerebroventricular administration of spironolactone, or the MR antagonist RU28318, did not reduce alcohol drinking in male rats [20, 21] or mice of both sexes [22] tested on a continuous (24 h) two-bottle (water vs. alcohol) choice model nor on a limited (1 h) two-bottle choice model following fluid restriction [23]. Limitations of these studies included low blood alcohol concentration achieved with continuous two-bottle choice models and a “physiological fluid need” (i.e., water restriction) [24]. However, Kashkin et al. reported that seven days of oral spironolactone treatment decreased alcohol drinking (and blood pressure) in high drinking, but not low drinking, male rats given continuous two-bottle choice access [25]. More recently, following the renewed interest in the aldosterone/MR pathway in AUD [19], Makhijani et al. reported that systemic injection of spironolactone reduced operant alcohol self-administration in both male and female rats, and suppressed the persistence of alcohol responding under an extinction condition in female rats [26]. However, spironolactone decreased general locomotion, especially in males, and this may have affected responding for alcohol. In a follow-up study, Makhijani and colleagues found that MR antagonism with eplerenone or MR knockdown in the CeA transiently reduced alcohol self-administration in female rats [27].
Given these promising but inconsistent results, additional studies are needed to better understand the pharmacologic potential of spironolactone in reducing alcohol consumption under different experimental settings, including binge-like and alcohol dependence-associated drinking. Additionally, studies are needed to determine to what extent spironolactone, per se or in combination with alcohol, impairs locomotion and motor coordination, and whether there is an interaction between spironolactone and alcohol pharmacokinetics. Finally, initial bench-to-bedside translation of the potential role of spironolactone in AUD is critically needed. We recently conducted a pharmacoepidemiologic study, using electronic health record data from Kaiser Permanente Northern California. Over 500 individuals treated with spironolactone, for any indication, were propensity score-matched with untreated controls, and the change in weekly alcohol use from baseline to follow-up was compared. Results showed a greater reduction in alcohol drinking among individuals who received spironolactone than those who did not. A significant dose-response relationship was also found, providing clinically relevant data in support of spironolactone for the treatment of AUD [28]. Although this study was promising, independent replication is necessary to confirm these findings, ideally in larger sample sizes and using different methodologies.
The aim of the present study was to address these gaps in knowledge, by testing the effect of spironolactone on alcohol-related behaviors in mice and rats and by conducting a pharmacoepidemiologic study using data from the largest integrated healthcare system in the US. Our hypothesis was that spironolactone would decrease alcohol consumption in rodents, without affecting their general consummatory behavior, causing sedation/motor incoordination, or affecting alcohol-induced ataxia and blood alcohol levels. Further, we hypothesized that patients prescribed spironolactone would display a reduction in their self-reported alcohol consumption, compared to propensity score-matched individuals.
METHODS AND MATERIALS
Spironolactone and alcohol use in rodents: psychopharmacology studies
Full methodological details are described in Appendix 1. Briefly, adult male and female C57BL/6 J mice were used to test the effects of spironolactone (0, 25, 50, 100, 200 mg/kg; injected 30 min before alcohol drinking) on binge-like alcohol consumption (drinking-in-the-dark, DID) [29]. We also assessed food and water intake, blood alcohol levels, motor coordination (rotarod test), and spontaneous locomotion (circular corridor test) in mice. Adult male and female Wistar rats were used to test the effects of spironolactone injections (0, 25, 50, and 75 mg/kg; injected 60 min before alcohol drinking) on operant alcohol self-administration (fixed-ratio 1, FR1) in alcohol-dependent (intermittent alcohol vapor exposure) and nondependent (exposed to air without alcohol) rats [30]. We also assessed blood alcohol levels and motor coordination (rotarod test) in male rats. Doses of spironolactone tested in these rodent experiments were selected based on previous studies and body surface area of species [26, 27, 31].
Spironolactone and alcohol use in humans: a pharmacoepidemiology study
Full methodological details are described in Appendix 2. Briefly, an observational cohort study was conducted, using electronic health record data from the US Department of Veterans Affairs (VA), to examine the association between spironolactone receipt (at least 60 continuous days) and change in self-reported alcohol consumption (Alcohol Use Disorders Identification Test-Consumption, AUDIT-C) [32, 33]. Each spironolactone-exposed patient was propensity score matched [34, 35] to up to five unexposed patients, using a greedy matching algorithm [36]. Multivariable difference-in-difference (Diff-in-Diff) linear regression models [37, 38] estimated the differential change between baseline (pre-index) and follow-up (post-index) AUDIT-C scores among exposed and unexposed patients. Subgroup analyses stratified by baseline AUDIT-C and average daily dose of spironolactone were also performed.
RESULTS
Effects of spironolactone in rodents
Effect of spironolactone on alcohol- and non-alcohol-containing solution drinking in mice.
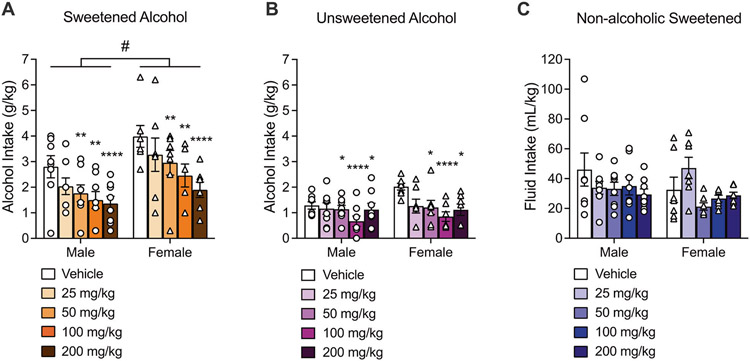
For mice drinking a sweetened alcohol solution (n = 15), two-way repeated measures ANOVA revealed a main effect of dose (F4,52 = 9.09, p < 0.0001) and sex (F1,13 = 6.05, p = 0.02; female > male), but there was no interaction between sex and dose (F4,52 = 0.42, p = 0.78). The Dunnett post hoc comparisons indicated that spironolactone at doses of 50 mg/kg (p = 0.007), 100 mg/kg (p = 0.002), and 200 mg/kg (p < 0.0001) significantly reduced alcohol intake (Fig. 1A). In mice drinking an unsweetened alcohol solution, two-way repeated measures ANOVA revealed a main effect of dose (F4,52 = 5.77, p = 0.0006), but no main effect of sex (F1,13 = 1.41, p = 0.25) and no interaction between sex and dose (F4,52 = 1.26, p = 0.29). Dunnett post hoc analyses revealed that spironolactone significantly reduced alcohol intake at doses of 50 mg/kg (p = 0.04), 100 mg/kg (p < 0.0001), and 200 mg/kg (p = 0.02; Fig. 1B). ANOVA of drinking data in mice receiving a non-alcohol-containing sweet solution showed a main effect of dose (F4,52 = 2.61, p = 0.04). However, the Dunnett post hoc test revealed that none of the tested spironolactone doses significantly reduced non-alcohol-containing sweet solution intake, compared to the vehicle condition. Furthermore, the ANOVA did not show a sex effect (F1,13 = 0.66, p = 0.42) nor an interaction between sex and dose (F4,52 = 2.10, p = 0.09). A female mouse from the non-alcohol-containing sweet group was identified as a significant outlier, so the drinking data for this animal was excluded (Fig. 1C).
Fig. 1. Spironolactone decreased binge-like alcohol drinking in mice.
A Spironolactone dose-dependently reduced alcohol intake (g/kg of body weight) in mice drinking a sweetened alcohol solution [20% alcohol (v/v), 3% glucose (w/v), and 0.1% saccharin (w/v)], and female mice drank significantly more alcohol than male mice. Males: n = 8; Females: n = 7. B Spironolactone dose-dependently reduced alcohol intake (g/kg of body weight) in mice drinking an unsweetened alcohol solution [20% (v/v)]. Males: n = 8; Females: n = 7. C Spironolactone had no effect on the intake (mL/kg of body weight) of a sweet solution without alcohol [0.3% glucose (w/v) and 0.01% saccharin (w/v)] in mice. Males: n = 8; Females: n = 7. Separate cohorts of mice were used for each drinking solution. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, vs. vehicle; #p < 0.05, male vs. female. DID drinking-in−the-dark.
Effect of spironolactone on food and water intake in mice.
Chow and water intake of mice were evaluated at 6 h and 24 h post treatment with vehicle or spironolactone (200 mg/kg). Three-way repeated measures ANOVA on chow intake revealed a main effect of time (F1,11 = 74.98, p < 0.0001) and of sex (F1,11 = 11.70, p = 0.0057), but no main effect of dose (F1,11 = 0.01, p = 0.90) nor an interaction between time and dose (F1,11 = 0.18, p = 0.67) or sex and dose (F1,11 = 0.70, p = 0.41). A significant interaction between time and sex (F1,11 = 6.99, p = 0.02) was detected. The Holm–Sidak post hoc test indicated that females consumed more chow than males 24 h post-treatment, regardless of whether they were treated with vehicle or 200 mg/kg of spironolactone (Table 1A).
Table 1.
Spironolactone did not affect (A) chow or (B) water intake in mice.
| Vehicle-treated | Spironolactone-treated | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | ||||||
| A | Total chow** intake (g) | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM |
| 6 h | 0.95 | 0.172 | 1.28 | 0.235 | 0.43 | 0.197 | 1.22 | 0.397 | |
| 24 h#### | 2.51 | 0.343 | 4.00† | 0.259 | 2.11 | 0.404 | 4.00† | 0.886 | |
| B | Total water* intake (mL) | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM |
| 6 h | 0.80 | 0.096 | 1.06 | 0.225 | 0.80 | 0.177 | 0.98 | 0.328 | |
| 24 h#### | 1.69 | 0.164 | 3.16†† | 0.293 | 1.68 | 0.263 | 2.54 | 0.572 | |
Male: n = 8; female: n = 5.
p < 0.05
p < 0.01, male vs. female (overall sex effect; three-way ANOVA).
p < 0.0001, 6 h vs. 24 h (overall time effect; three-way ANOVA).
p < 0.05, male vehicle-treated 24 h vs. female vehicle-treated 24 h and male spironolactone-treated 24 h vs. female-spironolactone treated 24 h
p < 0.01, male vehicle-treated 24 h vs. female vehicle-treated 24 h (sex vs. time interaction; three-way repeated measures ANOVA followed by Holm–Sidak post hoc test).
Similar results were found for water intake.
Three-way repeated measures ANOVA revealed a main effect of time (F1,11 = 123.30, p < 0.0001) and of sex (F1,11 = 6.10, p = 0.03), but no main effect of dose (F1,11 = 1.24, p = 0.28) and no interaction between time and dose (F1,11 = 0.89, p = 0.36) or sex and dose (F1,11 = 1.15, p = 0.30). A significant interaction between time and sex (F1,11 = 15.10, p < 0.002) was observed. Post hoc analyses indicated that female mice drank significantly more water than male mice at 24 h, regardless of treatment (Table 1B).
Effect of spironolactone on motor coordination and blood alcohol levels in mice.
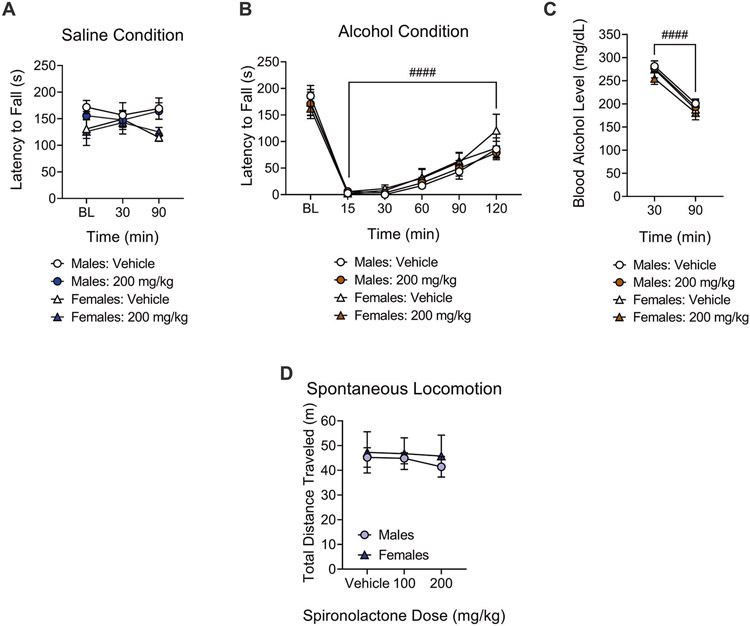
Rotarod performance of spironolactone-treated (200 mg/kg) mice (n = 11) did not significantly differ from vehicle-treated mice at any timepoints. Three-way repeated measures ANOVA did not reveal a main effect of drug (F1,9 = 0.90, p = 0.36), time (F2,18 = 0.17, p = 0.84), or sex (F1,9 = 2.20, p = 0.17) on rotarod performance for the saline condition, i.e., the mice that received a saline injection 30 min before rotarod testing. No interaction was found between dose and time (F2,18 = 0.80, p = 0.46), dose and sex (F1,9 = 0.76, p = 0.40), time and sex (F2,18 = 2.27, p = 0.13), nor between all three of these factors (F2,18 = 0.14, p = 0.86), indicating that spironolactone per se did not disrupt motor coordination (Fig. 2A).
Fig. 2. Spironolactone did not affect motor coordination or spontaneous locomotion in mice.
A Spironolactone treatment had no effect on motor coordination in mice that received a saline injection and were tested 30 min and 90 min later, on the rotarod. Males: n = 6; Females: n = 5. B Systemic administration of alcohol (1.5 g/kg) significantly impaired motor coordination in mice. Males: n = 6; Females: n = 5. Spironolactone treatment had no effect on alcohol-induced ataxia on the rotarod test at any time point. ####p < 0.0001, vs. Baseline. C Spironolactone had no effect on blood alcohol levels 30 min and 90 min after systemic administration of alcohol (1.5 g/kg). ####p < 0.0001, 30 min vs. 90 min. Males: n = 6; Females: n = 5. D Spironolactone had no effect on spontaneous locomotion in the circular corridor. Males: n = 8; Females: n = 6.
A three-way repeated measures ANOVA revealed a main effect of time (F5,45 = 95.97, p < 0.0001) on rotarod performance of spironolactone-treated (200 mg/kg) mice (n = 11) during conditions of alcohol-induced ataxia, i.e., the mice that received an alcohol (1.5 g/kg) injection 30 min before rotarod testing. This observation indicates that alcohol caused motor incoordination in mice and that this effect ameliorated over time. However, the ANOVA did not reveal a main effect of drug (F1,9 = 0.78, p = 0.40) nor an interaction between drug and time (F5,45 = 1.56, p = 0.18), indicating that spironolactone did not affect the ataxic effects of alcohol (Fig. 2B).
Immediately following the rotarod trials at 30 min and 90 min, blood was collected for blood alcohol levels measurement. Three-way repeated measures ANOVA revealed a main effect of time (F1,9 = 223.90, p < 0.0001), indicating that blood alcohol levels decreased over time. The ANOVA did not show a main effect of drug (F1,9 = 0.64, p = 0.44) nor an interaction between drug and time (F1,9 = 0.23, p = 0.63), indicating that spironolactone did not affect alcohol elimination (Fig. 2C). No main effect of sex on alcohol-induced ataxia (F1,9 = 0.31, p = 0.58; Fig. 2B) or blood alcohol levels (F1,9 = 1.33, p = 0.27; Fig. 2C) was found.
Effect of spironolactone on spontaneous locomotion in mice.
Grubb’s test indicated that one female mouse was a significant outlier, and data from this mouse was excluded. Two-way repeated measures ANOVA showed no main effect of dose (F2,24 = 0.80, p = 0.45) or sex (F1,12 = 0.13, p = 0.71) on total distance traveled (m) on the circular corridor, and no dose × sex interaction (F2,24 = 0.18, p = 0.83; Fig. 2D).
Effect of spironolactone on alcohol self-administration, motor coordination, and blood alcohol levels in dependent and nondependent rats.
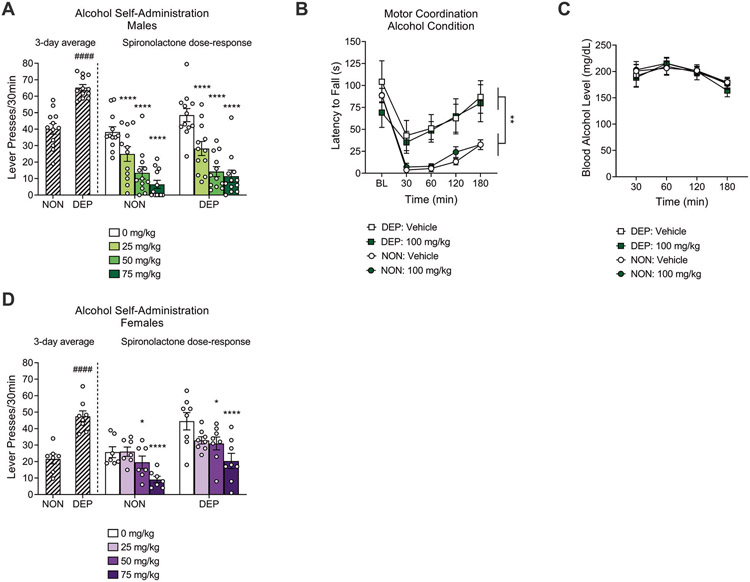
An unpaired Student′s t-test showed a significant difference in the average number of lever presses for alcohol between alcohol-dependent (n = 12) and nondependent (n = 12) male rats over the last three self-administration sessions preceding spironolactone treatment (t22 = 6.7, p = 0.0001). A two-way repeated measures ANOVA showed a significant spironolactone effect (main effect of dose: F3,66 = 43.95, p < 0.0001). The Dunnett post hoc test indicated that spironolactone at 25 mg/kg (p < 0.0001), 50 mg/kg (p < 0.0001), and 75 mg/kg (p < 0.0001) reduced alcohol self-administration in both alcohol-dependent and nondependent male rats (Fig. 3A). The ANOVA did not show a significant effect of alcohol dependence (F1,22 = 2.17, p = 0.53) nor a significant interaction between spironolactone and alcohol dependence (F3,66 = 0.74, p = 0.90).
Fig. 3. Spironolactone decreased operant alcohol self-administration in alcohol-dependent (DEP) and nondependent (NON) rats.
A Spironolactone administration decreased alcohol self-administration in nondependent and alcohol-dependent male rats tested under a fixed-ratio 1 schedule of reinforcement. ****p < 0.0001, vs. vehicle. ####p < 0.0001, vs. NON. Nondependent: n = 12; Dependent: n = 12. B Alcohol-induced ataxia was higher in nondependent than dependent male rats; spironolactone did not affect alcohol-induced ataxia in either group. **p < 0.01, difference between dependent and nondependent male rats. Nondependent: n = 15; Dependent: n = 9. C Spironolactone had no effect on blood alcohol levels 30, 60, 120, and 180 min after systemic administration of alcohol (1.5 g/kg) in male rats. Nondependent: n = 15; dependent: n = 9. D Spironolactone administration decreased alcohol self-administration in nondependent and alcohol-dependent female rats tested under a fixed-ratio 1 schedule of reinforcement. *p < 0.05, ****p < 0.0001, vs. vehicle. ####p < 0.0001, vs. NON. Nondependent: n = 7; Dependent: n = 8.
A three-way repeated measures ANOVA revealed a main effect of time (F4,88 = 40.99, p < 0.0001), a main effect of group (F1,22 = 11.41, p = 0.002), and a time × group interaction (F4,88 = 6.63, p < 0.0001) on rotarod performance of dependent (n = 9) and nondependent (n = 15) male rats during conditions of alcohol-induced ataxia. These results indicate that alcohol caused motor incoordination in both groups of rats, but alcohol-dependent rats showed tolerance to the ataxic effect of alcohol, compared with nondependent rats. However, the ANOVA did not reveal a main effect of spironolactone treatment (F1,22 = 0.38, p = 0.54) nor an interaction between spironolactone and time (F4,88 = 1.42, p = 0.23), indicating that spironolactone had no effect on alcohol-induced ataxia in male rats (Fig. 3B).
Regarding blood alcohol levels, a three-way repeated measures ANOVA revealed a main effect of time (F3,66 = 17.97, p < 0.0001), indicating that blood alcohol levels decreased over time. There was no main effect of group (F1,22 = 0.15, p = 0.70), spironolactone treatment (F1,22 = 0.003, p = 0.95), nor an interaction between spironolactone and time (F3,66 = 0.67, p = 0.56), indicating that alcohol-dependent and nondependent male rats achieved similar blood alcohol levels across timepoints, following a 1.5 g/kg alcohol injection, and that spironolactone did not affect the elimination of alcohol (Fig. 3C).
An unpaired Student′s t-test showed a significant difference in the average number of lever presses for alcohol between alcohol-dependent (n = 8) and nondependent (n = 7) female rats over the last three self-administration sessions preceding spironolactone treatment (t13 = 5.7, p = 0.0001). A two-way repeated measures ANOVA showed a significant effect of alcohol dependence (main effect of group: F1,13 = 15.19, p = 0.002) and a significant spironolactone effect (main effect of dose: F3,39 = 12.06, p < 0.0001). The Dunnett′s post hoc test indicated that spironolactone at 50 mg/kg (p = 0.02) and 75 mg/kg (p < 0.0001) reduced alcohol self-administration in both dependent and nondependent female rats (Fig. 3D). The ANOVA did not show a significant interaction between spironolactone and alcohol dependence (F3,39 = 0.99, p = 0.41).
Effects of spironolactone receipt in humans
Sample.
We identified 30,939 spironolactone-exposed and 2,083,402 unexposed individuals who reported any alcohol consumption in the two years prior to index date. A total 20,382 exposed patients were matched; however, 9,656 (47%) did not have a follow-up AUDIT-C and were unable to be included in analysis. Among those in the final matched cohort, 3016 (28.1%) were matched to five unexposed individuals, 2287 (21.3%) to four, 1541 (14.4%) to three, 1728 (16.1%) to two, and 2154 (20.1%) to one unexposed individual. Thus, the matched cohort consisted of 10,726 exposed and 34,461 unexposed individuals.
Before propensity score matching, the distribution of baseline characteristics differed between exposed and unexposed individuals (Table 2). Consistent with current indications for spironolactone, those who received spironolactone had a higher prevalence of hepatic decompensation (14.6% vs. 0.3%), coronary artery disease (38.0% vs. 13.5%), diabetes (39.6% vs. 21.3%), chest pain (38.8% vs. 22.3%), and chronic medication use (39.6% vs. 7.5% with ≥11 medications), compared to the unexposed group. After propensity score matching, differences were minimized between the two treatment groups (all standardized mean differences ≤0.2, with most ≤0.1) [39]. Thus, matching produced treatment groups that were considered well balanced (Table 2). Among exposed individuals in the matched cohort, 25%, 57%, and 18% were prescribed daily doses of spironolactone <25 mg/day, 25–49 mg/day, and ≥50 mg/day, respectively. Median follow-up time was 542 days (IQR 337–730 days).
Table 2.
Distribution of baseline characteristics in spironolactone-exposed and unexposed individuals before and after propensity score matching.
| Full cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|
| Characteristic | Exposed n = 30,939 |
Unexposed n = 2,083,402 |
SMD | Exposed n = 10,726 |
Unexposed n = 10,726a |
SMD |
| Age (years) | ||||||
| <55 | 5389 (17.4) | 549,459 (26.4) | 0.19 | 1683 (15.7) | 1716 (16.0) | 0.03 |
| 55–59 | 6747 (21.8) | 425,267 (20.4) | 2059 (19.2) | 2171 (20.2) | ||
| 60–64 | 12,016 (38.8) | 682,783 (32.8) | 4227 (39.4) | 4198 (39.1) | ||
| ≥65 | 6787 (21.9) | 425,893 (20.4) | 2757 (25.7) | 2641 (24.6) | ||
| Race/ethnicity | ||||||
| White | 20,573 (66.5) | 1,413,221 (67.8) | 0.13 | 7317 (68.2) | 7195 (67.1) | 0.06 |
| Black | 7136 (23.1) | 391,367 (18.8) | 2401 (22.4) | 2459 (22.9) | ||
| Hispanic | 1,315 (4.3) | 108,479 (5.2) | 357 (3.3) | 480 (4.5) | ||
| Other | 704 (2.3) | 61,807 (3.0) | 253 (2.4) | 231 (2.2) | ||
| Missing | 1211 (3.9) | 108,528 (5.2) | 398 (3.7) | 361 (3.4) | ||
| Male sex | 29,540 (95.5) | 1,958,068 (94.0) | 0.06 | 10,229 (95.4) | 10,177 (94.9) | 0.03 |
| HCV+ | 4822 (15.6) | 119,706 (5.8) | 0.33 | 979 (9.1) | 1290 (12.0) | 0.06 |
| AUD | ||||||
| Never | 20,398 (65.9) | 1,676,893 (80.5) | 0.32 | 7503 (70.0) | 7417 (69.2) | 0.07 |
| Lifetime | 3162 (10.2) | 187,489 (9.0) | 1202 (11.2) | 1389 (13.0) | ||
| Current | 7379 (23.9) | 219,020 (10.5) | 2021 (18.8) | 1920 (17.9) | ||
| Substance use treatment program visit | 9108 (29.4) | 323,974 (15.6) | 0.31 | 2095 (19.5) | 1912 (17.8) | 0.05 |
| Any hospitalization | 9976 (32.2) | 169,306 (8.1) | 0.64 | 2873 (26.8) | 2280 (21.3) | 0.18 |
| Coronary artery disease | 11,743 (38.0) | 281,078 (13.5) | 0.55 | 4673 (43.6) | 3983 (37.1) | 0.16 |
| Diabetes | 12,255 (39.6) | 444,048 (21.3) | 0.38 | 4793 (44.7) | 4631 (43.2) | 0.04 |
| Hepatic decompensation | 4515 (14.6) | 5492 (0.3) | 0.57 | 201 (1.9) | 209 (1.9) | 0.06 |
| Hyperlipidemia | 19,366 (62.6) | 1,139,384 (54.7) | 0.01 | 7676 (71.6) | 7181 (67.0) | 0.08 |
| Abdominal pain | 11,201 (36.2) | 512,747 (24.6) | 0.19 | 3693 (34.4) | 3588 (33.5) | 0.04 |
| Chest pain | 12,017 (38.8) | 464,716 (22.3) | 0.30 | 4498 (41.9) | 4015 (37.4) | 0.11 |
| Any chronic pain | 26,863 (86.8) | 1,722,836 (82.7) | 0.01 | 9466 (88.3) | 9590 (89.4) | 0.03 |
| Number of medications | ||||||
| ≤5 | 6163 (19.9) | 1,553,268 (74.6) | 1.29 | 1573 (14.7) | 1640 (15.3) | 0.13 |
| 6–10 | 12,531 (40.5) | 374,143 (18.0) | 4483 (41.8) | 4804 (44.8) | ||
| ≥11 | 12,245 (39.6) | 155,991 (7.5) | 4670 (43.5) | 4283 (39.9) | ||
| VACS Index score | ||||||
| <20 | 117 (0.4) | 38,767 (1.9) | 0.80 | 39 (0.4) | 35 (0.3) | 0.03 |
| 20–34 | 7748 (25.0) | 1,035,820 (49.7) | 3151 (29.4) | 3033 (28.3) | ||
| 35–54 | 14,501 (46.9) | 701,034 (33.7) | 5734 (53.5) | 5702 (53.2) | ||
| ≥55 | 6393 (20.7) | 55,826 (2.7) | 961 (9.0) | 1224 (11.4) | ||
| Missing | 2180 (7.1) | 251,955 (12.1) | 841 (7.8) | 732 (6.8) | ||
All statistics reported as n (%); up to five unexposed individuals were matched to each exposed individual.
SMD standardized mean difference, HCV hepatitis C virus, AUD alcohol use disorder, VACS Veterans Aging Cohort Study.
Unexposed matches were weighted according to the number of matches.
Changes in alcohol consumption.
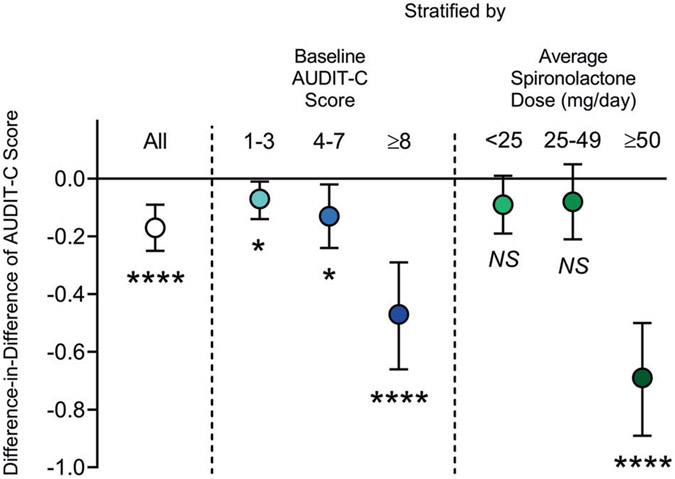
Overall, AUDIT-C scores decreased during the study period in both treatment groups. Average AUDIT-C scores decreased from 3.07 (standard deviation [SD] 0.02) to 2.16 (SD 0.02) among exposed individuals and from 2.96 (SD 0.01) to 2.22 (SD 0.01) among unexposed individuals (Table 3). Therefore, on average, AUDIT-C scores decreased 0.17 points more among spironolactone-exposed individuals, compared to the unexposed individuals (Diff-in-Diff: −0.17 points, 95% CI: −0.09, −0.25; p < 0.0001). In the analysis stratified by baseline AUDIT-C, average scores decreased 0.07 points (95% CI: −0.01, −0.14; p = 0.02), 0.13 points (95% CI: −0.02, −0.24; p = 0.02), and 0.47 points (95% CI: −0.29, −0.66; p < 0.0001) more among exposed, compared to unexposed individuals, in those with baseline AUDIT-C scores of 1–3, 4–7, and ≥8, respectively, indicating that the largest effect was observed in the group with highest severity of alcohol use at baseline. Similarly, the analysis stratified by average spironolactone dosage found the largest Diff-in-Diff estimate among individuals exposed to ≥50 mg/day of spironolactone (Diff-in-Diff: −0.69 points, 95% CI: −0.50, −0.89; p < 0.0001) (Fig. 4).
Table 3.
Estimated average pre- and post-index date AUDIT-C scores and Diff-in-Diff, overall, by baseline AUDIT-C score, and by average daily dose of spironolactone.
| Exposed | Unexposed | ||
|---|---|---|---|
| n = 10,726 | n = 34,461 | ||
| All patients | Pre | 3.07 (0.02) | 2.96 (0.01) |
| Post | 2.16 (0.02) | 2.22 (0.01) | |
| Dn | −0.91 (0.03) | −0.75 (0.02) | |
| Diff-in-Diff (95% CI) | −0.17 (−0.09, −0.25), p < 0.0001 | ||
| By baseline AUDIT-C score | |||
| 1–3 | n = 7362 | n = 24,098 | |
| Pre | 1.64 (0.02) | 1.62 (0.01) | |
| Post | 1.46 (0.02) | 1.52 (0.01) | |
| Dn | −0.18 (0.03) | −0.11 (0.02) | |
| Diff-in-Diff (95% CI) | −0.07 (−0.01, −0.14), p = 0.0231 | ||
| 4–7 | n = 2439 | n = 7701 | |
| Pre | 4.85 (0.04) | 4.83 (0.02) | |
| Post | 3.29 (0.04) | 3.39 (0.02) | |
| Dn | −1.56 (0.05) | −1.43 (0.03) | |
| Diff-in-Diff (95% CI) | −0.13 (−0.02, −0.24), p = 0.0221 | ||
| ≥8 | n = 925 | n = 2662 | |
| Pre | 9.72 (0.06) | 9.70 (0.03) | |
| Post | 4.68 (0.06) | 5.13 (0.03) | |
| Dn | −5.04 (0.08) | −4.57 (0.05) | |
| Diff-in-Diff (95% CI) | −0.47 (−0.29, −0.66), p < 0.0001 | ||
| By average dose of spironolactone (mg/day) | |||
| <25 | n = 2640 | n = 34,461 | |
| Pre | 3.00 (0.03) | 2.96 (0.01) | |
| Post | 2.16 (0.03) | 2.22 (0.01) | |
| Dn | −0.84 (0.05) | −0.75 (0.02) | |
| Diff-in-Diff (95% CI) | −0.09 (0.01, −0.19), p = 0.0658 | ||
| 25–49 | n = 6110 | n = 34,461 | |
| Pre | 2.98 (0.04) | 2.96 (0.01) | |
| Post | 2.15 (0.04) | 2.22 (0.01) | |
| Dn | −0.83 (0.06) | −0.75 (0.02) | |
| Diff-in-Diff (95% CI) | −0.08 (0.05, −0.21), p = 0.2140 | ||
| ≥50 | n = 1976 | n = 34,461 | |
| Pre | 3.61 (0.07) | 2.96 (0.01) | |
| Post | 2.17 (0.07) | 2.22 (0.01) | |
| Dn | −1.44 (0.10) | −0.75 (0.02) | |
| Diff-in-Diff (95% CI) | −0.69 (−0.50, −0.89), p < 0.0001 | ||
Statistics reported as mean (standard error).
AUDIT-C Alcohol Use Disorders Identification Test-Consumption, Pre pre-index AUDIT-C score, Post post-index AUDIT-C score, Dn change in AUDIT-C score, Diff-in-Diff difference-in-difference, CI confidence interval.
Fig. 4. Difference-in-difference estimates and 95% confidence intervals of self-reported changes in Alcohol Use Disorders Identification Test-Consumption-C (AUDIT-C) scores associated with spironolactone exposure, overall, by baseline AUDIT-C score, and by average daily dose of spironolactone.
Difference-in-differences = reported AUDIT-C decrease among spironolactone-exposed individuals minus reported AUDIT-C decrease among propensity score-matched unexposed controls during the study period. *p < 0.05, ****p < 0.0001, NS not significant.
DISCUSSION
The present findings provide translational evidence across three species (mice, rats, and humans) supporting the hypothesis that spironolactone represents a promising pharmacological treatment for AUD. Using a mouse model of alcohol drinking [29], we observed that spironolactone dose-dependently decreased the consumption of sweetened and unsweetened alcohol solutions in male and female mice. Although female mice drank significantly more sweetened alcohol than male mice, spironolactone was equally effective in both sexes. Of note, spironolactone had no effect on consumption of a non-alcohol-containing sweet solution and on food or water intake. Spironolactone per se did not affect spontaneous locomotion (circular corridor) or motor coordination (rotarod) and did not interfere with alcohol-induced ataxia (rotarod) or blood alcohol levels in mice. These findings suggest that spironolactone did not change motor and consummatory behaviors in general or alcohol pharmacokinetics. Using a rat model of alcohol dependence [30], we observed that spironolactone decreased operant alcohol self-administration (fixed-ratio 1 schedule of reinforcement) in dependent and nondependent male and female rats. Spironolactone did not affect motor coordination in alcohol-dependent and nondependent male rats and did not reverse the already established tolerance to the alcohol-induced ataxia in dependent male rats. More specifically, compared with nondependent rats, alcohol vapor-exposed dependent rats exhibited less motor impairment under alcohol intoxication, and this was not influenced by spironolactone treatment. Consistent with these preclinical results, our pharmacoepidemiologic study indicated that individuals who received spironolactone for any indication reported greater reduction in alcohol drinking than matched controls who did not receive spironolactone.
We used the DID test as a model of alcohol binge-like drinking in mice and used both unsweetened alcohol and sweetened alcohol solutions, which lead to different levels of alcohol drinking. When mice were given the sweetened alcohol solution, they consumed on average approximately 3–4 g/kg of alcohol in 4 h, which is reported to produce blood alcohol levels approaching 0.08 g/dL [31]. This drinking level is considered by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) as alcohol ‘binge drinking’, which increases risky behaviors, the development of chronic illnesses, and the risk of developing AUD [40]. The use of a sweetened alcohol solution also resembles the types of alcohol-containing beverages commonly consumed by humans. To test the specificity of the spironolactone effect to alcohol, we also tested the effects of spironolactone on the consumption of an unsweetened alcohol solution and a sweetened solution without alcohol.
Our results of decreased alcohol drinking in mice given spironolactone are consistent with previous studies in rats that operantly self-administrated a sweetened alcohol (15–20% alcohol + 2% sucrose) solution [26, 27] or rats that were given two-bottle choice between unsweetened alcohol (9%) and water [25]. However, spironolactone did not affect drinking of a non-alcohol-containing sweetened solution or spontaneous locomotion in mice of both sexes herein. In contrast, spironolactone decreased self-administration of a non-alcohol-containing sucrose solution in male rats and locomotion in male and female rats in previous work [26]. We also did not observe an effect of spironolactone on motor performance on the rotarod, indicating that spironolactone per se did not cause ataxia. A previous study reported that MR inactivation in the forebrain of male and female mice had no impact on rotarod performance [41]. Also important for our data interpretation is that spironolactone did not influence alcohol-induced ataxia on the rotarod nor blood alcohol levels in mice of both sexes. A previous study showed that repeated spironolactone treatment did not affect alcohol (1.5 g/kg)-induced motor impairment (hanging on a rod with front paws) in male rats or blood alcohol levels [42]. We also provide novel evidence that spironolactone decreased alcohol intake in male and female rats that were made dependent on alcohol via chronic, intermittent alcohol vapor exposure [43] and allowed to operantly self-administer unsweetened alcohol. In this model of alcohol dependence, the rats exhibit motivational and somatic signs of withdrawal [44], analogous to those observed in humans with AUD. However, spironolactone did not alter already established alcohol tolerance in male rats suggesting that the effect of spironolactone on alcohol seeking is not via a reversal of tolerance in general [45]. Together, the present results and prior studies provide complimentary evidence that spironolactone reduces nondependent drinking, binge-like drinking, and dependent drinking, without affecting alcohol tolerance or motor and consummatory behaviors in general.
In parallel, we conducted a pharmacoepidemiologic cohort study to translate our rodent findings to humans and observed consistent results, i.e., a significant association between spironolactone treatment and reduction in self-reported alcohol consumption. As is expected in a middle-aged cohort of patients, AUDIT-C scores decreased over time in both spironolactone-exposed and unexposed individuals; however, there was a significantly greater decrease in the scores of individuals exposed to spironolactone. Consistent with our rodent studies, we found a dose-dependent effect of spironolactone in humans, which suggests a potential causal relationship between spironolactone dose and change in alcohol drinking. Specifically, individuals exposed to ≥50 mg/day of spironolactone had a significantly greater decrease in AUDIT-C scores than those exposed to <50 mg/day. Biological gradient, i.e., dose-response relationship, is one of the Hill’s criteria for causation in traditional epidemiology [46]. When an incremental change of the exposure leads to a respective incremental change of the outcome, like the relationship observed in this study between spironolactone dose and change in AUDIT-C score, a causal relationship may be assumed, although other potentially confounding factors should be considered [47]. These findings are consistent with, and substantially extend, a recent retrospective cohort study comparing 523 spironolactone-treated adults and 2,305 untreated adults using electronic health record data from Kaiser Permanente Northern California (KPNC) [28]. First, the present data are derived from a much larger patient population, and different in terms of geography (national vs. regional) and demographics (younger and predominantly male), compared to the KPNC cohort. Of note, US Veterans have an increased likelihood of developing AUD [48]; therefore, the present human results are particularly relevant as they were generated in a cohort at higher risk of AUD. Second, we analyzed a different alcohol-related outcome (AUDIT-C), which is important and clinically relevant given that AUDIT-C scores have been associated with alcohol-related medical consequences, including alcohol dependence [49] and mortality [50]. AUDIT-C scores have been routinely collected in the VA since 2008, providing the longitudinal data necessary for our analysis. Third, herein we analyzed a sample size that was ~20 times larger for the spironolactone-treated group and ~15 times larger for the untreated group. Finally, the present study had a longer follow-up period (~2 years), compared to our previous study (~6 months). Thus, the present human data, together with those in Palzes et al. [28], provide strong pharmacoepidemiology-based evidence supporting a role of spironolactone in AUD.
The mechanism(s) of action by which spironolactone reduces alcohol consumption is an area of current investigation. We hypothesize that increased levels of circulating aldosterone may contribute to alcohol drinking by increasing anxiety, facilitating brain stress system activation, and/or inducing neuroinflammation. Alterations in the levels of hormones that regulate fluid and electrolyte homeostasis, including aldosterone, have been proposed to accompany and potentially contribute to AUD [3]. Aldosterone levels significantly correlated with alcohol withdrawal [51], anxiety, obsessive craving [18, 19], and alcohol drinking [19] in patients with AUD. Primary aldosteronism in humans was associated with increased anxiety [52, 53], and chronic treatment with aldosterone increased anxiety-like behavior in rats [54]. Many drugs that have shown promise in treating AUD (e.g., baclofen, gabapentin, pregabalin) also have anxiolytic effects [2, 55], and it is conceivable that at least part of spironolactone’s effect on alcohol use may be driven by anxiety reduction [56-59]. Moreover, in adrenalectomized rats, aldosterone increased corticotropin-releasing factor (CRF) mRNA levels in the paraventricular nucleus of the hypothalamus and in the CeA [60]. Increased CRF activity in limbic areas, particularly the amygdala, drives negative emotional states and drinking associated with alcohol dependence in rodent models [44, 45, 61, 62].
The exact role of MR in stress and alcohol drinking is intriguingly multifaceted. In the brain, MR expression is enriched in limbic regions, such as the prefrontal cortex, hippocampus, and extended amygdala, as well as the nucleus of the solitary tract [63]. Brain MRs are involved in various cognitive processes, regulate basal hypothalamic-pituitary-adrenal (HPA) axis activity, and mediate the autonomic and HPA axis response to stress, which are dysregulated in alcohol dependence [62]. Although cortisol/corticosterone (CORT) binds MR with high affinity and its concentration exceeds that of aldosterone, aldosterone activity in the brain in the presence of corticosteroid has been reported [64]. Because MRs in the brain are almost completely occupied at basal circadian CORT levels, it is hypothesized that receptor turnover plays an important role in MR function, as well as the existence of a membrane-localized MR, and the formation of steroid receptor heterodimers, such as MR-glucocorticoid receptor (GR) heterodimers, during gene transcription that may all facilitate different outcomes of MR activation in various physiological states. Thus, increased blood aldosterone may be expected to increase brain MR signaling, and perhaps GR signaling, the latter of which has been shown to be involved in alcohol dependence in rodents and humans [65].
There is also evidence that enhanced MR activity in brain regions mediating emotional responses is beneficial for a healthy state and may be protective in the face of stress and possibly psychiatric illness. High expression of MRs is associated with enhanced cognitive function, usage of active coping strategies, and resistance to chronic stress-induced cognitive dysfunction [66]. Consistent with the role of MR activity in cognition [9], both preclinical and clinical studies indicate that MR blockade may negatively impact certain cognitive domains, such as selective attention, visuospatial memory, reversal learning, and decision making [41, 67-72]. Such effects are more likely with repeated and continuous MR blockade, as opposed to acute spironolactone doses that we used in our rodent experiments. Future preclinical and human studies should incorporate cognitive assessments over time to examine the extent to which spironolactone, or other MR antagonists, combined with alcohol, may produce cognitive deficits. Decreased MR expression was observed in post-mortem brains of individuals with depression [73], and MR expression was increased by antidepressant administration in rats [74]. MR agonism with fludrocortisone enhanced cognitive function and antidepressant efficacy in humans [75, 76]. Similarly, we identified a relationship between decreased MR expression and alcohol dependence. Decreased MR in the CeA was correlated with compulsive-like alcohol drinking in dependent rats and with increased alcohol drinking in monkeys [19].
Altogether, the present study and previous findings indicate dysregulations in the aldosterone/MR pathway in alcohol dependence, but whether the effects of spironolactone are peripheral, central, or both is unknown. Acute MR antagonism with eplerenone or downregulation of MR in the amygdala decreased alcohol drinking in rats [27], supporting a central effect. Spontaneously hypertensive rats, which are typically high alcohol drinkers [77], exhibited increased MR binding capacity and expression in the brain and peripheral organs (for review, see: [78]). In normotensive high drinking rats, chronic treatment with spironolactone decreased both alcohol drinking and blood pressure [25], potentially suggesting a parallel central and peripheral effect.
The extent to which spironolactone effects are specifically MR-mediated remains unclear. Spironolactone and its metabolites are nonselective MR antagonists, such that they also bind GRs, progesterone, and androgen receptors [79, 80]. Chronic treatment with aldosterone increased proinflammatory cytokine expression in the mouse brain and this effect was blocked by spironolactone [81]. Another target of spironolactone is pannexin 1 channels, which regulate adenosine triphosphate and, thereby, contribute to many physiological processes, particularly cardiovascular function [82]. Spironolactone, via pannexin 1 channel inhibition, rapidly lowers blood pressure and inhibits α1-adrenergic activity in mice [83]. We have recently reported that the pannexin 1 channel inhibitor probenecid reduced alcohol drinking in nondependent and dependent rats, as well as binge-like drinking in mice [84]. Overall, future studies are needed to elucidate the exact mechanism(s) of action of spironolactone on alcohol-related outcomes.
Spironolactone is a widely used medication for a variety of indications, mostly related to cardiovascular diseases and hemodynamic disturbances in patients with chronic disorders (e.g., patients with liver cirrhosis or nephrotic syndrome). As such, a strength of this work is that we are testing a medication with known tolerability, safety, and side effects, even in individuals with severe chronic diseases. AUD leads to several chronic diseases, such as alcohol-related liver and cardiovascular diseases [85, 86], and there is certainly a need to identify effective and safe medications to treat patients with AUD and comorbid disease [87, 88]. Future prospective human studies are needed not only to test the putative efficacy of spironolactone in AUD via double-blind, placebo-controlled, randomized, clinical trials, but also to confirm its safety and tolerability in individuals with AUD. Potential drug-alcohol interactions will also need to be examined in humans. Based on the observation that spironolactone reduced alcohol self-administration in alcohol-dependent and nondependent rats, in mice exhibiting a binge-like level of alcohol drinking, and in mice that consumed a relatively lower amount of alcohol, we suggest that spironolactone may play a general role in alcohol reinforcement, although our pharmacoepidemiologic data suggested a higher effect of spironolactone in those with higher baseline drinking. Additional preclinical and clinical studies on alcohol consumption, as well as other aspects associated with AUD such as craving/relapse, and reward and stress function [89-91], will help with the understanding of spironolactone’s effects observed in the present study. Furthermore, due to the heterogeneity of AUD, which likely influences the effect sizes of medications in clinical studies [2], it will be important for future work to identify potential biomarkers of spironolactone efficacy in sub-populations with AUD.
In conclusion, the present study provides converging evidence, across psychopharmacologic experiments in mice and rats and pharmacoepidemiologic observations in humans, supporting that spironolactone represents a promising pharmacological treatment for AUD. These findings collectively support future prospective randomized, controlled studies testing spironolactone in patients with AUD, as well as additional work to understand the mechanisms related to the role of the MR in AUD and how spironolactone reduces alcohol drinking.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank all staff involved in data collection, management, and support at the National Institutes of Health Intramural Research Program (NIH IRP) and the following joint post-baccalaureate fellows in the NIDA/NIAAA Clinical Psychoneuroendocrinology and Neuropsychopharmacology and NIDA Neurobiology of Addiction Sections: Brandon Blank, Adriana Gregory-Flores, Claire Pince, and Lia Zallar. The authors would also like to thank Dr. Kendall Bryant (NIAAA) for providing administrative support and guidance during the initial development of the human pharmacoepidemiology study. Finally, the authors would like to thank Dr. Gail Seabold (NIDA) for professional proofreading and editing the manuscript.
FUNDING
This work was supported by the NIH intramural funding ZIA-DA-000635 and ZIA-AA000218 (Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section – PI: LL), ZIA-DA000602–06 (Neurobiology of Addiction Section – PI: GFK), and NIAAA extramural funding (R01-AA023733, U24-AA020794, U01-AA020790, and U10-AA013566). The content of this article is solely the responsibility of the authors and does not necessarily represent the official view of the funding agencies.
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41380-022-01736-y.
REFERENCES
- 1.Heilig M, MacKillop J, Martinez D, Rehm J, Leggio L, Vanderschuren L. Addiction as a brain disease revised: why it still matters, and the need for consilience. Neuropsychopharmacology 2021;46:1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witkiewitz K, Litten RZ, Leggio L. Advances in the science and treatment of alcohol use disorder. Sci Adv. 2019;5:eaax4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Addolorato G, Leggio L, Hillemacher T, Kraus T, Jerlhag E, Bleich S. Hormones and drinking behaviour: new findings on ghrelin, insulin, leptin and volume-regulating hormones. An ESBRA Symposium report. Drug Alcohol Rev. 2009;28:160–165. [DOI] [PubMed] [Google Scholar]
- 4.Kenna GA, Swift RM, Hillemacher T, Leggio L. The relationship of appetitive, reproductive and posterior pituitary hormones to alcoholism and craving in humans. Neuropsychol Rev. 2012;22:211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funder JW. Mineralocorticoid receptors: distribution and activation. Heart Fail Rev. 2005;10:15–22. [DOI] [PubMed] [Google Scholar]
- 6.Molinari AM, Machado-Rada MY, Mazaira GI, Erlejman AG, Galigniana MD. Molecular basis of mineralocorticoid receptor action in the nervous system. CNS Neurol Disord Drug Targets. 2013;12:1163–1174. [PubMed] [Google Scholar]
- 7.Kellner M, Wiedemann K. Mineralocorticoid receptors in brain, in health and disease: possibilities for new pharmacotherapy. Eur J Pharmacol. 2008;583:372–378. [DOI] [PubMed] [Google Scholar]
- 8.Reul JM, Gesing A, Droste S, Stec IS, Weber A, Bachmann C, et al. The brain mineralocorticoid receptor: greedy for ligand, mysterious in function. Eur J Pharmacol. 2000;405:235–249. [DOI] [PubMed] [Google Scholar]
- 9.Wingenfeld K, Otte C. Mineralocorticoid receptor function and cognition in health and disease. Psychoneuroendocrinology 2019;105:25–35. [DOI] [PubMed] [Google Scholar]
- 10.de Kloet ER, Rots NY, van den Berg DT, Oitzl MS. Brain mineralocorticoid receptor function. Ann N Y Acad Sci. 1994;746:8–20. [PubMed] [Google Scholar]
- 11.Dorey R, Piérard C, Shinkaruk S, Tronche C, Chauveau F, Baudonnat M, et al. Membrane mineralocorticoid but not glucocorticoid receptors of the dorsal hippocampus mediate the rapid effects of corticosterone on memory retrieval. Neuropsychopharmacology 2011;36:2639–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou M, Kindt M, Joëls M, Krugers HJ. Blocking mineralocorticoid receptors prior to retrieval reduces contextual fear memory in mice. PLoS ONE. 2011;6:e26220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol. 2014;4:965–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitra R, Ferguson D, Sapolsky RM. Mineralocorticoid receptor overexpression in basolateral amygdala reduces corticosterone secretion and anxiety. Biol Psychiatry. 2009;66:686–690. [DOI] [PubMed] [Google Scholar]
- 15.de Kloet ER, Otte C, Kumsta R, Kok L, Hillegers MH, Hasselmann H, et al. Stress and depression: a crucial role of the mineralocorticoid receptor. J Neuroendocrinol. 2016;28:1–12. [DOI] [PubMed] [Google Scholar]
- 16.Vogel S, Fernández G, Joëls M, Schwabe L. Cognitive adaptation under stress: a case for the mineralocorticoid receptor. Trends Cogn Sci. 2016;20:192–203. [DOI] [PubMed] [Google Scholar]
- 17.Cannavo A, Bencivenga L, Liccardo D, Elia A, Marzano F, Gambino G, et al. Aldosterone and mineralocorticoid receptor system in cardiovascular physiology and pathophysiology. Oxid Med Cell Longev. 2018;2018:1204598–1204598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leggio L, Ferrulli A, Cardone S, Miceli A, Kenna GA, Gasbarrini G, et al. Renin and aldosterone but not the natriuretic peptide correlate with obsessive craving in medium-term abstinent alcohol-dependent patients: a longitudinal study. Alcohol. 2008;42:375–381. [DOI] [PubMed] [Google Scholar]
- 19.Aoun EG, Jimenez VA, Vendruscolo LF, Walter NAR, Barbier E, Ferrulli A, et al. A relationship between the aldosterone–mineralocorticoid receptor pathway and alcohol drinking: preliminary translational findings across rats, monkeys and humans. Mol Psychiatry. 2018;23:1466–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fahlke C, Hård E, Eriksson CJ, Engel JA, Hansen S. Consequence of long-term exposure to corticosterone or dexamethasone on ethanol consumption in the adrenalectomized rat, and the effect of type I and type II corticosteroid receptor antagonists. Psychopharmacology 1995;117:216–224. [DOI] [PubMed] [Google Scholar]
- 21.Fahlke C, Hård E, Hansen S. Facilitation of ethanol consumption by intracerebroventricular infusions of corticosterone. Psychopharmacology 1996;127:133–139. [DOI] [PubMed] [Google Scholar]
- 22.O’Callaghan MJ, Croft AP, Jacquot C, Little HJ. The hypothalamopituitary-adrenal axis and alcohol preference. Brain Res Bull. 2005;68:171–178. [DOI] [PubMed] [Google Scholar]
- 23.Koenig HN, Olive MF. The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrinology. 2004;29:999–1003. [DOI] [PubMed] [Google Scholar]
- 24.Tunstall BJ, Vendruscolo LF, Allen–Worthington K. Chapter 26 - Rat models of alcohol use disorder. In: Suckow MA, Hankenson FC, Wilson RP, Foley PL, editors. The laboratory rat (3rd Edition). Cambridge, MA, USA: Academic Press; 2020. p. 967–986. [Google Scholar]
- 25.Kashkin VA, Shekunova EV, Egorov AY, Bagrov AY. Marinobufagenin in urine: a potential marker of predisposition to ethanol and a target for spironolactone. Curr Hypertens Rev. 2018;14:35–38. [DOI] [PubMed] [Google Scholar]
- 26.Makhijani VH, Van Voorhies K, Besheer J. The mineralocorticoid receptor antagonist spironolactone reduces alcohol self-administration in female and male rats. Pharmacol Biochem Behav. 2018;175:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makhijani VH, Irukulapati P, Van Voorhies K, Fortino B, Besheer J. Central amygdala mineralocorticoid receptors modulate alcohol self-administration. Neuropharmacology 2020;181:108337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palzes VA, Farokhnia M, Kline-Simon AH, Elson J, Sterling S, Leggio L, et al. Effectiveness of spironolactone dispensation in reducing weekly alcohol use: a retrospective high-dimensional propensity score-matched cohort study. Neuropsychopharmacology. 2021;46:2140–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. [DOI] [PubMed] [Google Scholar]
- 30.McGinn MA, Tunstall BJ, Schlosburg JE, Gregory-Flores A, George O, de Guglielmo G, et al. Glucocorticoid receptor modulators decrease alcohol self-administration in male rats. Neuropharmacology 2021;188:108510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. [DOI] [PubMed] [Google Scholar]
- 33.Fiellin DA, Reid MC, O’Connor PG. Screening for alcohol problems in primary care: a systematic review. Arch Intern Med. 2000;160:1977–1989. [DOI] [PubMed] [Google Scholar]
- 34.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearl J. Invited commentary: understanding bias amplification. Am J Epidemiol. 2011;174:1223–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cormen TH. Introduction to algorithms. Cambridge, MA: MIT Press; 2009. [Google Scholar]
- 37.Donald SG, Lang K. Inference with difference-in-differences and other panel data. Rev Econ Stat. 2007;89:221–233. [Google Scholar]
- 38.Lechner M. The estimation of causal effects by difference-in-difference methods. Found Trends® Econ. 2011;4:165–224. [Google Scholar]
- 39.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Understanding binge drinking. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/binge-drinking.
- 41.Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM, et al. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci USA. 2006;103:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grupp LA, Elias J, Perlanski E, Stewart RB. Modification of ethanol-induced motor impairment by diet, diuretic, mineralocorticoid, or prostaglandin synthetase inhibitor. Psychopharmacology 1985;87:20–24. [DOI] [PubMed] [Google Scholar]
- 43.Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: focus on the vapor model Alcohol. 2014;48:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol. 2014;35:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberto M, Spierling SR, Kirson D, Zorrilla EP. Corticotropin-releasing factor (CRF) and addictive behaviors. Int Rev Neurobiol. 2017;136:5–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimonovich M, Pearce A, Thomson H, Keyes K, Katikireddi SV. Assessing causality in epidemiology: revisiting Bradford Hill to incorporate developments in causal thinking. Eur J Epidemiol. 2021;36:873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meeks TW, Bekman NM, Lanouette NM, Yung KA, Vienna RP. Alcohol and alcohol use disorder. In: Ritchie EC, Llorente MD, editors. Veteran psychiatry in the US: optimizing clinical outcomes. Cham: Springer International Publishing; 2019. p. 135–56. [Google Scholar]
- 49.Rubinsky AD, Kivlahan DR, Volk RJ, Maynard C, Bradley KA. Estimating risk of alcohol dependence using alcohol screening scores. Drug Alcohol Depend. 2010;108:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris AHS, Bradley KA, Bowe T, Henderson P, Moos R. Associations between AUDIT-C and mortality vary by age and sex. Popul Health Manag. 2010;13:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovács GL. The role of atrial natriuretic peptide in alcohol withdrawal: a peripheral indicator and central modulator? Eur J Pharmacol. 2000;405:103–112. [DOI] [PubMed] [Google Scholar]
- 52.Sonino N, Fallo F, Fava GA. Psychological aspects of primary aldosteronism. Psychother Psychosom. 2006;75:327–330. [DOI] [PubMed] [Google Scholar]
- 53.Sonino N, Tomba E, Genesia ML, Bertello C, Mulatero P, Veglio F, et al. Psychological assessment of primary aldosteronism: a controlled study. J Clin Endocrinol Metab. 2011;96:E878–883. [DOI] [PubMed] [Google Scholar]
- 54.Hlavacova N, Jezova D. Chronic treatment with the mineralocorticoid hormone aldosterone results in increased anxiety-like behavior. Horm Behav. 2008;54:90–97. [DOI] [PubMed] [Google Scholar]
- 55.Farokhnia M, Browning BD, Leggio L. Prospects for pharmacotherapies to treat alcohol use disorder: an update on recent human studies. Curr Opin Psychiatry. 2019;32:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smythe JW, Murphy D, Timothy C, Costall B. Hippocampal mineralocorticoid, but not glucocorticoid, receptors modulate anxiety-like behavior in rats. Pharm Biochem Behav. 1997;56:507–513. [DOI] [PubMed] [Google Scholar]
- 57.Korte SM, de Boer SF, de Kloet ER, Bohus B. Anxiolytic-like effects of selective mineralocorticoid and glucocorticoid antagonists on fear-enhanced behavior in the elevated plus-maze. Psychoneuroendocrinology 1995;20:385–394. [DOI] [PubMed] [Google Scholar]
- 58.Calvo N, Volosin M. Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinology 2001;73:261–271. [DOI] [PubMed] [Google Scholar]
- 59.Ding H, Cui SY, Cui XY, Liu YT, Hu X, Zhao HL, et al. Anti-stress effects of combined block of glucocorticoid and mineralocorticoid receptors in the paraventricular nucleus of the hypothalamus. Br J Pharmacol. 2021;178:3696–3707. [DOI] [PubMed] [Google Scholar]
- 60.Watts AG, Sanchez-Watts G. Region-specific regulation of neuropeptide mRNAs in rat limbic forebrain neurones by aldosterone and corticosterone. J Physiol. 1995;484:721–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Guglielmo G, Kallupi M, Pomrenze MB, Crawford E, Simpson S, Schweitzer P, et al. Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat Commun. 2019;10:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vendruscolo LF, Koob GF. Alcohol dependence conceptualized as a stress disorder. In: Harkness KL, Hayden EP, editors. The Oxford handbook of stress and mental health. Oxford, United Kingdom: Oxford University Press; 2020. [Google Scholar]
- 63.van Eekelen JA, Bohn MC, de Kloet ER. Postnatal ontogeny of mineralocorticoid and glucocorticoid receptor gene expression in regions of the rat tel- and diencephalon. Brain Res Dev Brain Res. 1991;61:33–43. [DOI] [PubMed] [Google Scholar]
- 64.Yongue BG, Roy EJ. Endogenous aldosterone and corticosterone in brain cell nuclei of adrenal-intact rats: regional distribution and effects of physiological variations in serum steroids. Brain Res. 1987;436:49–61. [DOI] [PubMed] [Google Scholar]
- 65.Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Investig. 2015;125:3193–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joëls M, de Kloet ER. 30 Years of the mineralocorticoid receptor: the brain mineralocorticoid receptor: a saga in three episodes. J Endocrinol. 2017;234:T49–t66. [DOI] [PubMed] [Google Scholar]
- 67.Yau JL, Noble J, Seckl JR. Continuous blockade of brain mineralocorticoid receptors impairs spatial learning in rats. Neurosci Lett. 1999;277:45–48. [DOI] [PubMed] [Google Scholar]
- 68.Douma BR, Korte SM, Buwalda B, la Fleur SE, Bohus B, Luiten PG. Repeated blockade of mineralocorticoid receptors, but not of glucocorticoid receptors impairs food rewarded spatial learning. Psychoneuroendocrinology 1998;23:33–44. [DOI] [PubMed] [Google Scholar]
- 69.Thai CA, Zhang Y, Howland JG. Effects of acute restraint stress on set-shifting and reversal learning in male rats. Cogn Affect Behav Neurosci. 2013;13:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Otte C, Moritz S, Yassouridis A, Koop M, Madrischewski AM, Wiedemann K, et al. Blockade of the mineralocorticoid receptor in healthy men: effects on experimentally induced panic symptoms, stress hormones, and cognition. Neuropsychopharmacology 2007;32:232–238. [DOI] [PubMed] [Google Scholar]
- 71.Young KD, Preskorn SH, Victor T, Misaki M, Bodurka J, Drevets WC. The effect of mineralocorticoid and glucocorticoid receptor antagonism on autobiographical memory recall and amygdala response to implicit emotional stimuli. Int J Neuropsychopharmacol 2016;19:pyw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wingenfeld K, Kuehl LK, Dziobek I, Roepke S, Otte C, Hinkelmann K. Effects of mineralocorticoid receptor blockade on empathy in patients with major depressive disorder. Cogn Affect Behav Neurosci. 2016;16:902–910. [DOI] [PubMed] [Google Scholar]
- 73.Klok MD, Alt SR, Irurzun Lafitte AJ, Turner JD, Lakke EA, Huitinga I, et al. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in post-mortem brain regions of patients with major depressive disorder. J Psychiatr Res. 2011;45:871–878. [DOI] [PubMed] [Google Scholar]
- 74.Seckl JR, Fink G. Antidepressants increase glucocorticoid and mineralocorticoid receptor mRNA expression in rat hippocampus in vivo. Neuroendocrinology 1992;55:621–626. [DOI] [PubMed] [Google Scholar]
- 75.Otte C, Hinkelmann K, Moritz S, Yassouridis A, Jahn H, Wiedemann K, et al. Modulation of the mineralocorticoid receptor as add-on treatment in depression: a randomized, double-blind, placebo-controlled proof-of-concept study. J Psychiatr Res. 2010;44:339–346. [DOI] [PubMed] [Google Scholar]
- 76.Otte C, Wingenfeld K, Kuehl LK, Kaczmarczyk M, Richter S, Quante A, et al. Mineralocorticoid receptor stimulation improves cognitive function and decreases cortisol secretion in depressed patients and healthy individuals. Neuropsychopharmacology 2015;40:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vendruscolo LF, Terenina-Rigaldie E, Raba F, Ramos A, Takahashi RN, Mormède P. Evidence for a female-specific effect of a chromosome 4 locus on anxiety-related behaviors and ethanol drinking in rats. Genes Brain Behav. 2006;5:441–450. [DOI] [PubMed] [Google Scholar]
- 78.de Kloet ER, Meijer OC, de Nicola AF, de Rijk RH, Joëls M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front Neuroendocrinol. 2018;49:124–145. [DOI] [PubMed] [Google Scholar]
- 79.de Gasparo M, Joss U, Ramjoué HP, Whitebread SE, Haenni H, Schenkel L, et al. Three new epoxy-spirolactone derivatives: characterization in vivo and in vitro. J Pharmacol Exp Ther. 1987;240:650–656. [PubMed] [Google Scholar]
- 80.Kolkhof P, Bärfacker L. 30 Years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol. 2017;234:T125–T140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dinh QN, Young MJ, Evans MA, Drummond GR, Sobey CG, Chrissobolis S. Aldosterone-induced oxidative stress and inflammation in the brain are mediated by the endothelial cell mineralocorticoid receptor. Brain Res. 2016;1637:146–153. [DOI] [PubMed] [Google Scholar]
- 82.Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta. 2013;1828:15–22. [DOI] [PubMed] [Google Scholar]
- 83.Good ME, Chiu Y-H, Poon IKH, Medina CB, Butcher JT, Mendu SK, et al. Pannexin 1 channels as an unexpected new target of the anti-hypertensive drug spironolactone. Circ Res. 2018;122:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tunstall BJ, Lorrai I, McConnell SA, Gazo KL, Zallar LJ, de Guglielmo G, et al. Probenecid reduces alcohol drinking in rodents. is pannexin1 a novel therapeutic target for alcohol use disorder? Alcohol Alcohol. 2019;54:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Farinelli LA, Piacentino D, Browning BD, Brewer BB, Leggio L. Cardiovascular consequences of excessive alcohol drinking via electrocardiogram: a systematic review. J Addict Nurs. 2021;32:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vuittonet CL, Halse M, Leggio L, Fricchione SB, Brickley M, Haass-Koffler CL, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71:1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mirijello A, Tarli C, Vassallo GA, Sestito L, Antonelli M, d’Angelo C, et al. Alcoholic cardiomyopathy: what is known and what is not known. Eur J Intern Med. 2017;43:1–5. [DOI] [PubMed] [Google Scholar]
- 89.Martinotti G, Di Nicola M, Tedeschi D, Callea A, Di Giannantonio M, Janiri L. Craving Typology Questionnaire (CTQ): a scale for alcohol craving in normal controls and alcoholics. Compr Psychiatry. 2013;54:925–932. [DOI] [PubMed] [Google Scholar]
- 90.Addolorato G, Leggio L, Abenavoli L, Gasbarrini G. Neurobiochemical and clinical aspects of craving in alcohol addiction: a review. Addict Behav. 2005;30:1209–1224. [DOI] [PubMed] [Google Scholar]
- 91.Leggio L, Kenna GA, Fenton M, Bonenfant E, Swift RM. Typologies of alcohol dependence. From Jellinek to genetics and beyond. Neuropsychol Rev. 2009;19:115–129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.