Abstract
Cross-frequency coupling (CFC) reflects (nonlinear) interactions between signals of different frequencies. Evidence from both patient and healthy participant studies suggests that CFC plays an essential role in neuronal computation, interregional interaction, and disease pathophysiology. The present review discusses methodological advances and challenges in the computation of CFC with particular emphasis on potential solutions to spurious coupling, inferring intrinsic rhythms in a targeted frequency band, and causal interferences. We specifically focus on the literature exploring CFC in the context of cognition/memory tasks, sleep, and neurological disorders, such as Alzheimer's disease, epilepsy, and Parkinson's disease. Furthermore, we highlight the implication of CFC in the context and for the optimization of invasive and noninvasive neuromodulation and rehabilitation. Mainly, CFC could support advancing the understanding of the neurophysiology of cognition and motor control, serve as a biomarker for disease symptoms, and leverage the optimization of therapeutic interventions, e.g., closed-loop brain stimulation. Despite the evident advantages of CFC as an investigative and translational tool in neuroscience, further methodological improvements are required to facilitate practical and correct use in cyborg and bionic systems in the field.
Introduction
Cyborg and bionic systems (CBS) focus on the integration of organic and biomechatronic components, with the aim of either restoring lost function or normalizing disease symptoms. Examples of such techniques may include brain–computer interfaces or neuromodulation technologies [e.g., deep brain stimulation (DBS)] [1,2].
Targeting a reliable set of biomarkers is crucial for the development of a useful CBS [3]. Electrophysiological systems such as the brain or heart generate oscillatory activity over a spectrum of frequencies. System outputs such as movement or cognitive process reflect a complex and nonlinear integration of oscillatory neural population activity [4]. This can be accessed using a range of approaches including invasive local field potential or electrocorticogram recordings, or non-invasive measures with either electroencephalography (EEG) or magnetoencephalography.
Multiple neural oscillations across temporal and spatial scales participate in neural information processing [5,6]. In general, low-frequency oscillations are thought to control long-range synchronization, while high-frequency oscillations (HFOs) are believed to be linked to local computation [7]. The question of how these neural oscillations contribute to top-down neural transmission has raised great interest [8,9]. Oscillatory neural activities in multiple frequencies are modulated during a range of tasks (e.g., cognitive tasks) [10–12]. Furthermore, brain stimulation techniques that entrain (or alter) oscillatory activity are in turn known to impact task performance [13,14]. This has led to the belief that oscillatory neural population activity has a causal impact on behavior [15]. In keeping with this, it is also becoming increasingly apparent that neurophysiological oscillations may serve as a biomarker for pathophysiological states such as Parkinson's disease [16].
One particular type of oscillatory coupling, known as cross-frequency coupling (CFC), has gained great interest in medicine and neuroscience. CFC characterizes interactions across different frequency rhythms and is modulated during both physiological processing and pathological states, such as spasticity [17–19]. CFC denotes the statistical association between the phase, amplitude, or frequency of 2 rhythms [17]. CFC applied on simultaneous recordings from different cortical areas reveals a coordinated information exchange in cognitive, sensory, and motor events from long distance to local computation [17]. There are 4 commonly studied types of CFC: phase–amplitude coupling (PAC), amplitude–amplitude coupling (AAC), phase–frequency coupling, and phase–phase coupling [7].
PAC [20–22] and AAC [23,24] attracted much attention for their association with physiological processing and pathological states. These 2 types of CFCs are depicted in Fig. 1. Figure 1F shows a simulation of PAC, where the activity at 13 Hz is modulated by the phase of a 2.5-Hz wave, forming a nested structure, i.e., XPAC(t) = (xp(t)+1)·sin(2π×13t), where xp(t) = sin(2π×2.5t). On the other hand, AAC refers to when the amplitude of the 13-Hz activity is modulated by the envelope of a 2.5-Hz wave (Fig. 1G). In this case, the form of the resulting signal is XAAC(t) = |H[xa(t)]|·sin(2π×13t), where H[·] represents the Hilbert transform (HT) of a signal xa(t) = V·sin(2π×2.5t), and V is a time-varying variable (Fig. 1B). Both XPAC(t) and XAAC(t) formulated here refer to 13-Hz activities, and both the instantaneous phase (Fig. 1D) and amplitude (Fig. 1E) are derived from the HT of the inputs.
Fig. 1.

Concepts of phase–amplitude coupling (PAC) and amplitude–amplitude coupling (AAC). (A) High-frequency oscillation (13 Hz). (B) Low-frequency oscillation (2.5 Hz). (C) Low-frequency oscillation with varying amplitude modulations. (D) Phase of a 2.5-Hz oscillation. (E) Envelope of xa(t) oscillation. (F) Oscillatory coupling formations of PAC with 2.5-Hz phase shown in (D) modulating 13-Hz amplitude shown in (A). (G) Oscillatory coupling formations of AAC with 2.5-Hz amplitude shown in (C) modulating 13-Hz amplitude shown in (A).
This review seeks to offer a comprehensive overview of the latest developments in CFC research, with a special focus on methodologies, neural mechanisms, and potential applications in CBS, clinical interventions in particular. Firstly, the review will commence by defining CFC and summarizing the current state of knowledge regarding its methodological advances. Next, we will summarize the latest studies on CFC in cognitive processes, and various neurological disorders, including but not limited to Alzheimer's disease, epilepsy, and Parkinson's disease, plus discussions over the potential neuromodulation techniques for clinical interventions. Lastly, the review will consider the challenges and opportunities for the integration of CFC technology into CBS, with future trends in this field being highlighted.
Methodological Considerations in CFC
CFC provides an approach to encode multiple bodies of CBS. Specifically, the slow wave encodes temporal information via phase coding, while the fast oscillation reflects rhythmic spiking activity [7]. Many methods had been introduced for the computation of CFC. Traditionally, linear approaches were applied to quantify CFC [25–27]. Considering findings suggesting that CFC is prone to dynamic fluctuations [20,28,29], methods for computing time-varying CFC are required.
Aru et al. [30] reported risks of bias in measuring CFC along with several recommendations to evaluate the reliability of different CFC methods. Indicators to evaluate CFC methodologies are summarized as follows: (a) The bandwidth of the extracted decomposition should adequately cover its riding wave (i.e., amplitude modulation). (b) The effect of oscillatory nonlinearity on coupling strength, authentic waveform characteristics, and possible harmonics needs to be carefully validated. (c) The accuracy of the quantitative approach in calculating the instantaneous phase/amplitude modulations also matters. (d) Preserving input-related nonstationarity is important. (e) Either healthy control or surrogate data are needed. (f) Sustaining temporal structure and transient coupling is a necessity.
Extracting a broad range of phase and amplitude modulations from electrophysiological oscillations is crucial for assessing CFC [30,31]. The traditional Fourier transform may result in harmonic artifacts with a loss of information [23,32–34]. Empirical mode decomposition (EMD)—a method of adaptive decomposition [35]—has been proven to capture nonlinear/nonstationary features of irregular patterns more effectively than the Fourier transform. EMD decomposes data into intrinsic mode functions (IMFs) at different frequencies. IMFs are considered promising for calculating CFC [23,36]. However, the sifting process of EMD may result in intermittent patterns at different frequency ranges being mixed within the same IMF (i.e., mode mixing). Therefore, several advanced methods have been proposed for the calculation of CFC. Ensemble EMD-based PAC eliminates the mode mixing phenomenon by iteratively adding Gaussian white noise to ensure refined scale in phase/amplitude-given components [37]. This leads to increased computational complexity. The proposal of masking PAC is computationally efficient and resolves the trade-off between nonlinearity and frequency specificity [38,39]. Recently, variational mode decomposition-derived PAC estimation techniques have been proposed. To avoid spurious couplings caused by dyadic filter banks or harmonics, PACs between irregular oscillators around preferred center frequencies are measured [40].
Traditionally, the HT has been used to calculate phase and amplitude modulations. PAC can subsequently be computed by using metrics such as the modulation index (MI). One well-known method to quantify MI is to measure the nonuniformity of the distribution of the averaged high-frequency amplitude over the low-frequency phase bin. Precisely, a probability distribution, P of the high-frequency amplitudes at each low-frequency phase can be constructed. This observed distribution can be compared to a uniform distribution (which would imply no relationship between phase and amplitude) using information theoretic measures such as the Kullback–Leibler (KL) distance. The KL distance can then be normalized by considering the maximum possible entropy, resulting in MI values ranging from 0 to 1.
Tort et al. [41] compared 8 different PAC indicators and concluded that MI had the best performance. PAC can also be estimated through the phase-locking value (PLV) [42] or synchronization index (SI) [43]. Some studies used mean vector length (MVL) as a measure to assess the dependence between phase and amplitude time series by clustering complex vectors [44,45]. Penny et al. compared PLV [46], MVL [44], and envelope-to-signal correlation (ESC) [47] with the general linear model and concluded that all methods comparably performed with suitable conditions (e.g., long epoch with less noise contamination). Moreover, a growing number of toolboxes are devised to calculate CFC, wherein some have relied on Matlab such as Fieldtrip [48] and Brainstorm [49], and others are Python-based toolboxes such as pactools and Tensorpac [50]. These tools support multiple CFC measures and statistical analyses to obtain a corrected CFC. However, the common use of linear analyses could more or less result in spurious couplings.
After the decomposition procedure, the standard process to calculate coupling strength is as follows. Illustrated by the case of PAC, either cycle-by-cycle frequencies or instantaneous ones are applied to eliminate the effects of intra-wave variation per decomposition. To obtain the cycle-based frequencies of the ith decomposition of the jth channel recording, the phase series is unwound, then the expansion phase series at points in time spanning 2π integer increments is identified to generate a cycle-by-cycle conversion. The cycle-based frequency can be approximated by a secant to the instantaneous frequency [36,37]. Here, i = 1, 2, …, N, where the value of N represents the total number of IMFs. Meanwhile, the phase/amplitude modulations derived from the HT of all selected decompositions across montages of interest are used to assess the cross-channel and/or cross-decomposition coupling intensity [11,41]. The statistical significance of the MI can be tested by generating surrogate data from individual MI-contributed decompositions using a bootstrap strategy in a cycle base [51,52]. The average and standard deviation of the permuted MIs are used to determine the z-score of the original MI, and a significance threshold (α = 0.05) can then be applied. A cross-frequency comodulogram can then be used to visualize coupling strengths. Figure 2 shows the methodology of CFC exemplified by a signal with a 6-Hz phase modulating a 65-Hz amplitude. Table 1 lists the bibliography of “methodological advances of CFC”.
Fig. 2.
Demonstrating methodology of CFC between 6-Hz phase and 65-Hz amplitude modulations. The left panels show the 5 steps to calculate CFC. The middle panels summarize the indicators of each step to guarantee a reliable CFC. The right panels present schematic diagrams of each step. Firstly, a raw signal with a 65-Hz amplitude modulated by a 6-Hz phase is illustrated. Secondly, all phase-given Sp and amplitude-given SA IMFs are calculated, wherein all IMFs are extracted by IMF-based decompositions. Next, the instantaneous phases φp and envelopes Aa of the corresponding IMF are obtained by HT with MI serving as a measure of coupling strength. After that, surrogate data are created to access the significance of MI. Lastly, a cross-frequency comodulogram is adopted to display coupling strength across multiple frequencies. The white block denotes the desired coupling between the 6-Hz phase and 65-Hz amplitude, while the blue block represents a spurious coupling.
Table 1.
Bibliography of methodological advances of CFC.
| Steps | Ref. | Key points |
|---|---|---|
| Accessing phase and amplitude modulations | Aru et al. [30] | Some potential risks of bias in measuring CFC are summarized. |
| Kramer et al. [32] | Fourier analysis may result in harmonic artifacts. | |
| Colgin et al. [26] | The wavelet technique was applied to quantify CFC. | |
| Pittman-Polletta et al. [36] | The integration of EMD enables extracting nonstationary and nonlinear broadband rhythms in calculating PAC. | |
| Shi et al. [37] | Ensemble EMD-based PAC eliminates the mode mixing phenomenon. | |
| Yeh and Shi [38] | Masking PAC is computationally efficient and resolves the trade-off between nonlinearity and frequency specificity. | |
| Zhang et al. [40] | Variational PAC avoids occurrence of spurious couplings due to dyadic filter banks or harmonics. | |
| Estimating coupling intensity | Tort et al. [41] | MI exhibited superior performance among 8 different PAC indicators. |
| Penny et al. [42] | PLV was originally developed to quantify phase synchronization between trials. | |
| Cohen [43] | SI was presented to test CFC between upper θ and γ. | |
| Canolty et al. [44] | Coupling strengths during cognitive processes were assessed by MVL. | |
| Bruns and Eckhorn [47] | ESC was proposed to measure correlations between different bands. | |
| Statistical analysis | Pittman-Polletta et al. [36] | To sustain the temporal structures, surrogate data with cycle-shuffled amplitude and phase were generated. |
| Visualization | Yeh et al. [23] | Redistributing CFC across a frequency scatter plot based on the cycle-by-cycle frequencies corresponding to each IMF pair. |
Progress of Electrophysiological Couplings in Physiology and Neuroscience
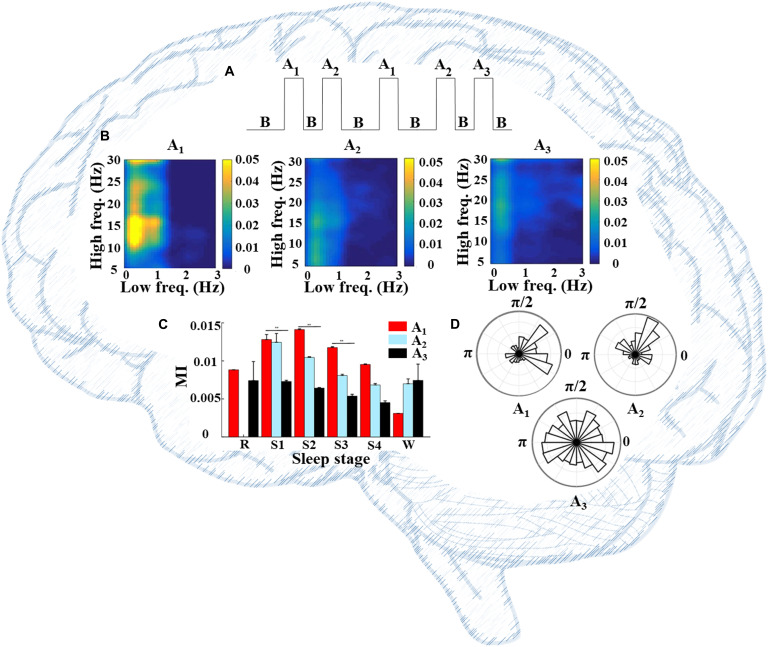
Information flow typically involves multiple sites of specialized processing [53]. CFC can provide a framework for both local and distributed information processing within neural networks, thereby serving the coordination of neural oscillations over multiple spatial scales [17,54–61]. Owing to this, disturbances of information processing in certain neurological disease states may be inferred through the observation of changes in CFC relative to healthy control populations [62,63]. In general, past findings have shown that stronger coupling tends to occur with higher neural computational needs. For example, during sleep, PAC coordinates various brain rhythms and varies across cyclic alternating patterns (CAPs) (Fig. 3). CAPs are the periodic pattern of sleep comprising A and B phases, in which A contains 3 subtypes including A1, A2, and A3 (Fig. 3A). Past findings suggest that δ-α/low β PAC is stronger in subtype A1 (Fig. 3B) than in the 2 other subtypes. This strong coupling may regulate sleep structure and preserve working memory [38]. The relatively disperse distribution of phase differences between δ phase and α/low β amplitude in A3 supports a weaker δ-α/low β PAC (Fig. 3D). Table 2 shows the bibliography of the “progress of CFC in neuroscience and physiology”.
Fig. 3.
PAC associating various brain rhythms and varying by physiological states. (A) CAP consists of A and B phases, in which A contains 3 subtypes including A1, A2, and A3. (B) PAC comodulograms differ by phase-A subtypes, of which A1 shows stronger α/low β-amplitude-related PACs. (C) Significant differences (P < 0.0001) of δ-α/low β PACs among phase-A subtypes in all sleep stages except S4 were shown. (D) The distribution of phase difference between δ phase and α/low β amplitude is displayed in the polar histogram chart. Subtype A3 showed a relatively disperse distribution compared to the 2 other subtypes.
Table 2.
Bibliography of the progress of CFC in neuroscience and physiology.
| Ref. | Disease | Subtype of CFC | Key points |
|---|---|---|---|
| Axmacher et al. [10] | Pharmacoresistant temporal lobe epilepsy | θ–γ | θ–γ PAC exhibited greater prominence during cognitive tasks. |
| Rajji et al. [71] | Healthy | θ–γ | PAS increases θ–γ PAC in the dorsolateral prefrontal cortex. |
| Mondragón-Rodríguez et al. [75] | Alzheimer's disease | θ–γ | A reduction in θ–γ PAC between the hippocampus and prefrontal cortex indicates early cognitive impairments. |
| Etter et al. [81] | Alzheimer's disease | θ–γ | Optogenetic stimulation can restore memory performance and hippocampal θ–γ PAC. |
| Turi et al. [83] | Healthy | θ–γ | Stimulations at the θ–γ frequency over the trough impaired cognitive control. |
| Amiri et al. [97] | Mesiotemporal lobe epilepsy | δ/θ-HFO | Interictal HFOs in the SOZ were modulated by δ/θ phase. |
| Ma et al. [102] | Frontal lobe epilepsy | δ–β/γ | Strong δ–β/γ PAC emerges around the SOZ during pre-seizure periods. |
| de Hemptinne et al. [128] | Parkinson's disease | β–γ | β phase waveform in both the primary M1 and STN modulate broadband-γ amplitude in M1. |
| de Hemptinne et al. [129] | Parkinson's disease | β–γ | STN DBS can reduce β–γ coupling. |
| He et al. [136] | Parkinson's disease | Gait phase-α/β | Gait phases associated modulations of α/β band activity in PPN. |
| Jin et al. [122] | Parkinson's disease | δ/θ-gait-related β | Gait-related β amplitude is driven by lower-frequency components modulated by auditory stimuli. |
| Yin et al. [137] | Parkinson's disease | β–γ | Increased PAC in MI indicates higher probabilities of gait problems. |
| Muthuraman et al. [143] | Parkinson's disease | γ-stimulation frequency | The presence of CFC suggests that DBS utilizes clinically effective frequencies to induce intrinsic FTG oscillations through a mechanism of entrainment. |
The role and potential of CFC as a biomarker for cognitive and memory tasks
CFC is believed to play a special role in regulating performance in cognitive and memory tasks. Oscillations in both the θ (5 to 8 Hz) and γ (30 to 150 Hz) bands display modulations in such tasks. Studies in rodents have reported that a strong coupling between θ and γ activities emerges when decision-making or learning tasks are performed [64,65]. Strong θ–γ PAC also emerges within the hippocampus during the performance of context-learning tasks [51]. Similarly, θ–γ CFC is considered to play a crucial role in cognition and memory in humans [10,46,55,66,67]. A recent study revealed that the extent of θ–γ CFC negatively correlated with the development of mild cognitive impairment [68], hence suggesting that reductions in θ–γ coupling may relate to degenerative pathologies.
One reported transcranial magnetic stimulation (TMS) paradigm for inducing neuroplasticity is paired associative stimulation (PAS). One recent study showed that PAS can increase θ–γ PAC within the dorsolateral prefrontal cortex (DLPFC; an area of the frontal lobe that is crucially involved in executive functions such as working memory [69]), suggesting that PAC could be a potential indicator of neuroplasticity [70]. θ–γ PAC was reported by Rajji et al. [71] to support the organization of information in N-back working memory tasks. Soto and Jerbi [72] observed a clear decrease in θ–γ PAC during N-back trials that did not involve information ordering. Closed-loop auditory stimulation was reported to locally modulate δ-α/low β coupling in the frontal area. This modulation could potentially be utilized to influence neuroplasticity that occurs during sleep in the targeted brain network [73].
The diagnosis of common neurodegenerative disorders such as Parkinson's disease (PD) or Alzheimer's disease (AD) can only be confidently made once suggestive clinical signs and symptoms are present. It is well recognized, however, that these conditions often have a prodrome [74], which can be many years long. PAC may serve as a biomarker of prodromal states of neurodegeneration. For instance, in AD, an early sign of neuronal dysfunction leading to cognitive impairments could be a reduction in θ–γ coupling between the hippocampus and prefrontal cortex [75,76]. This could be relevant in terms of identifying at-risk populations and trialing both pharmacological and non-pharmacological neuroprotective therapies [77,78]. Interestingly, θ–γ coupling has been reported to be enhanced by the use of CBS [79,80]. For example, Etter et al. [81] used optogenetic stimulation to restore memory performance and hippocampal θ–γ PAC in a mouse model of AD. Transcranial alternating current stimulation (tACS) has been recently shown to modulate top-down control and functional connectivity (θ–γ coupling) across the frontal-occipital regions, leading to enhanced performance in working memory tasks [82]. Of note, stimulations at the θ–γ frequency over the trough have been found to impair cognitive control [83]. Hence, it could theoretically be possible to develop a closed-loop cognitive rehabilitation training set, integrated with techniques/paradigms such as PAS and cognitive exercise. Such a system is designed to increase θ–γ PAC feedback in the hippocampal/cortical regions to favor memory consolidation.
Coupling to facilitate the diagnosis and treatment of epilepsy: Biomarkers for seizure onset and non-pharmacological treatment options
HFOs may serve as a biomarker of the epileptogenic zone (EZ) or seizure onset zones (SOZs) [84,85]. Although HFOs occur more commonly within the SOZ/EZ than in other brain areas [86–89], they can also be generated by the nonepileptic somatosensory or motor cortices at rest or during movement [90–93]. Therefore, the application of HFO recordings for guiding epilepsy surgery resection margins has been limited [94–96].
Many studies have reported that interictal HFOs in the SOZ are modulated by the slow-wave phase [97–100]. Ibrahim et al. [101] showed that PAC between HFO amplitude and θ/α phase was significantly higher in the SOZ than in other cortical regions. Also, several studies exploring CFC in epilepsy found that δ–γ PAC could be a promising biomarker for locating the SOZ/EZ [98,102]. For frontal lobe epilepsy, it has been observed that a prominent δ–β/γ PAC occurs around the SOZ during pre-seizure periods [102]. This would support a role for PAC in regulating seizure onset [103]. Interestingly, Guirgis et al. [104] observed that the presence of δ-modulated HFOs provided a satisfactory indicator of the resection margin of an EZ.
For some patients with drug-resistant epilepsy, the removal of brain tissue is not advisable due to the presence of multiple seizure foci or fears of postoperative functional deficits [105]. Developing sophisticated neural regulatory techniques, for instance, vagus nerve stimulation (VNS ®, LivaNova, Inc.) or responsive neurostimulation (RNS ®, NeuroPace, Inc.), has been a recent focus [106,107]. VNS and RNS were approved as non-pharmacological treatments for focal epilepsy by the U.S. Food and Drug Administration in 1997 and 2014, respectively. These neurostimulation devices deliver electrical stimulation with adjustable parameters to reduce the frequency of seizures [108]. The RNS system is designed as a closed-loop device that delivers electrical stimulation immediately upon recognizing possible electrocorticogram seizure activity [109,110]. Meanwhile, the VNS with the recently released generator model 106 AspireSR® can trigger simulation automatically based on the increased heart rate, which may indicate seizures [111]. Randomized trials using VNS and RNS have demonstrated the effectiveness of neuromodulation—with a responder rate of up to 50% [112–114]. However, the mechanisms of neurostimulation remain unclear, and this hinders the development of further improvements in this technology.
Movement-related neurophysiological basis and neuromodulation techniques for Parkinson's disease rehabilitation
Motor impairment in PD is characterized by excessive synchronization in the β band within the basal ganglia (BG) [115,116] (Fig. 4B). Additionally, β power can be suppressed with both dopaminergic medication and stimulation and the extent of treatment-related suppression correlates with treatment-related clinical improvements in bradykinesia and rigidity [117–122], hence suggesting an important pathophysiological role of β oscillations. γ oscillation, in contrast, synchronizes strongly in the BG and thalamus at the initiation of contralateral movements [123,124]. Movement-related broadband γ synchrony (30 to 100 Hz) increases excitability between periods of inhibition, supporting interregional interaction/communication [125,126]. Note that the occurrence of narrowband γ activity (60 to 90 Hz) induced either by levodopa or DBS is linked to dyskinesia [127]. Studies have suggested that cross-frequency interactions may have a marked impact on BG information processing in PD [119,120]. The phase of the β waveform within both the primary motor cortex (M1) and the subthalamic nucleus (STN) has been shown to modulate broadband-γ amplitude in M1 in PD [128–133]. This cortical β–γ coupling may also be suppressed by STN DBS.
Fig. 4.
An example of applying CFC with multiple neuromodulations to rehabilitation interventions for individuals with Parkinson's disease. (A) A 3-dimensional gait signal and gait-related EEG of a PD patient with motor impairment. (B) After signal decomposition, excessive β power emerged in basal ganglia. (C) Excessive PAC between Lβ/Hβ and HFO in the STN was observed in a patient with PD, as shown in the left panel, which was suppressed by stimulation (middle panel), with a subsequent rebound after the stimulation was removed (right panel). (D) Neuromodulations, such as DBS, TMS, and sensory stimulation. (E) The varying PAC with stimulation provides feedback to the neuromodulation paradigm. (F) PAC results reflect the interregional interaction/communication in the brain.
Studies exploring the relationship between oscillatory activity and gait have revealed that low-γ frequency oscillations in the motor cortex are modulated by the gait phase [134] and central midline sites [135]. In PD, gait phases are known to associate with modulations of α/β band activity within the pedunculopontine nucleus (PPN) [136]. Our past work has also shown that β band modulation within the STN is time-locked to contralateral steps in PD [121]. High-β synchronization is suppressed when the contralateral foot is raised and displays a rebound following a heel strike. Recently, we observed that these gait-related β frequency amplitude modulations could themselves be driven by lower-frequency components that can be modulated by auditory stimuli [122], hence providing a neurophysiological substrate for a link between auditory and motor processing. This can be partially explained by the fact that elevated PAC in PD may result in greater (cortical) processing demands, which, in turn, could contribute to gait problems like freezing episodes [137]. Studies such as this provide insights into the cortical circuits that need to be modulated to target specific disease symptoms. The inhibitory circuit formation in the cortex of PD patients is more strongly inhibited in response to stimulation than normal individuals, indicating that the occurrence of cortical disinhibition could be an early, and possibly prodromal, characteristic of PD [138]. Pezzopane et al. [139] reported targeting θ–γ tACS to short intracortical inhibition through stimulation resulted in a decrease in inhibition following the stimulation.
Neuromodulation techniques for PD can either be invasive or non-invasive. Noninvasive tools include TMS, transcranial direct current stimulation, and tACS, while the most frequently employed invasive technique is DBS [140]. High-frequency DBS (at frequencies of around 130 Hz) can suppress increased BG β activity and ameliorate symptoms such as bradykinesia or rigidity [141]. While conventional continuous DBS is already established, a promising next-generation DBS technique is closed-loop DBS, in which the delivery of stimulation is titrated based on a neurophysiological biomarker [142]. For example, the CFC observed between γ oscillations and the volume of tissue-activated power at stimulation frequency in Muthuraman et al.'s report [143] indicates that DBS as per clinically effective frequencies may induce intrinsic FTG oscillations through an entrainment mechanism. Closed-loop DBS has attracted a growing amount of attention for the treatment of PD [144]. Gilron et al. reported the effectiveness of adaptive DBS with the Summit RC+S (Medtronic) device. This adjusts stimulation parameters based on the detection of neurophysiological activity that may relate to particular symptoms such as dyskinesia [142]. A recent study showed that within a gait cycle, significant positive correlations were observed between low β power and gait muscle activities that can be used to forecast the gait events and freezing episodes [145]. This also supports the use of closed-loop neuromodulation therapies that can be controlled through specific commands.
Thinking about rehabilitation approaches, robot-assisted devices such as the Tymo system (Tyromotion, Austria) have advantages over traditional physiotherapy (e.g., stretching and muscle strengthening) for impacting gait. These devices enable the task-oriented design of exercises and adjustment of the intensity of exercises [49,146–148].
Figure 4 illustrates how CFC biomarkers can be applied to several neuromodulation or rehabilitation techniques. As per the 3-dimensional gait signal, the gait-related EEGs from a PD patient with motor impairment are extracted (Fig. 4A). Excessive β power is observed in BG after signal decomposition (Fig. 4B). Obvious Lβ/Hβ-HFO PAC emerges in the STN (left panel in Fig. 4C), while stimulation techniques, e.g., DBS, TMS, and sensory stimulation (Fig. 4D), can suppress the abnormal PAC (middle panel in Fig. 4C), with a subsequent rebound after the stimulation is removed (right panel in Fig. 4C). The varying PAC under stimulation provides feedback to the neuromodulation paradigm (Fig. 4E). The PAC dynamics support the interregional interaction/communication in the brain (Fig. 4F), which facilitates the understanding of the pathological neural network.
Conclusion and Future Outlook
This review focuses on the methodologies, mechanisms, and applications (neuro-control and rehabilitation treatment) of CFC in neuroscience and medicine. Reliable CFC enables characterizing multi-frequency interactions and reflects how these coupled oscillations contribute to top-down neural transmission. CFC, PAC in particular, provides relatively precise metrics of entangling temporal structure in neural circuits and is, hence, linked to both motor and cognitive function in healthy and diseased states, which manifests that it holds great promise in serving as an electrophysiological feature to inform real-time neuromodulation. In addition to their use in measuring cognitive and motor states, CFC metrics may also allow for the monitoring of disease progression and therapeutic responses, supporting clinicians and scientists to allocate brain regions and temporal periods with deteriorated neural functions.
Referenced to other biomarkers, such as band power [149], evoked compound action potential [150], and abnormal synchrony [151], CFC may offer a deeper understanding of the underlying entangling oscillatory mechanisms of neurological disorders. By identifying the interested CFC patterns that are disrupted by changing physiological or pathophysiological status, researchers can gain insight into the affected neural circuits, which could facilitate the development of more targeted and effective interventions.
We have discussed some common pitfalls to consider when computing CFC metrics, including techniques to avoid the detection of spurious CFC. The criteria proposed by Aru et al. can serve as quality control to avoid methodological confounds. The cycle-based permuted nonlinear approaches introduced in this review suggest a feasible path toward a more reliable CFC estimation.
Although CFC has been greatly developed, several challenges to implementing CFC in cyborg and bionic systems need to be addressed to facilitate translating CFC findings into these systems. One crucial challenge is the development of reliable and effective CFC measurement techniques to integrate into a CBS, relying on sensors and devices to real-time access brain activities, followed by translating them into control signals for prosthetic limbs, etc. Thus, developing promising algorithms to evaluate CFC intensities without introducing clear delays or bias is highlighted next. Successful translation of CFC findings into a CBS requires precise decoding of electrophysiological recordings and then translating CFC biomarkers into controls of CBS devices. Such algorithms and controls require minimizing time lags and false alarms in real use. Lastly, these translation uses require rigorous testing and validation to ensure their safety, effectiveness, and reliability in real-world applications. These involve extensive testing and validation in preclinical and clinical settings, along with continued monitoring and optimization of CFC-based CBS over time.
Although many challenges remain, including the handling of brain stimulation artifacts and real-time deployment, robust algorithms and controls, and rigorous testing and validation of these systems in real uses, there is much to be optimistic about regarding the therapeutic deployment of CFC-based CBS.
Acknowledgments
Funding: This work was partially supported by the National Natural Science Foundation of China (Nos. 62171028 and 62001026), the BIT National High-level Fellow Research Fund Program (No. 3050012222022), the MRC Clinician Scientist Fellowship (No. MR/W024810/1), the Swiss National Science Foundation (No. PZ00P3_202166), and the National Science and Technology Council (Nos. 110-2221-E-008-093, 111-2221-E-008-015-MY3, 111-2221-E008-103-MY3, and 111-2218-E-008-009). Author contributions: C.-H.Y. and C.Z. analyzed the data and wrote the manuscript. C.-H.Y. and W.S. were responsible for the conceptualization of the study. M.-T.L., G.T., and A.O. collected the references and surveyed the development. All authors read and approved the final manuscript. Competing interests: The authors declare that they have no competing interests.
Data Availability
Data of this paper are available by emailing chien-hung.yeh@bit.edu.cn.
References
- 1.Eusebio A, Thevathasan W, Doyle Gaynor L, Pogosyan A, Bye E, Foltynie T, Zrinzo L, Ashkan K, Aziz T, Brown P. Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatry. 2011;82(5):569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley C, Nydam AS, Dux PE, Mattingley JB. State-dependent effects of neural stimulation on brain function and cognition. Nat Rev Neurosci. 2022;23(8):459–475. [DOI] [PubMed] [Google Scholar]
- 3.Scangos KW, Khambhati AN, Daly PM, Makhoul GS, Sugrue LP, Zamanian H, Liu TX, Rao VR, Sellers KK, Dawes HE, et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat Med. 2021;27(10):1696–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormick DA, McGinley MJ, Salkoff DB. Brain state dependent activity in the cortex and thalamus. Curr Opin Neurobiol. 2015;31:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94(3):1904–1911. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Shi W, Yeh C-H. A novel measure of cardiopulmonary coupling during sleep based on the synchrosqueezing transform algorithm. IEEE J Biomed Health Inform. 2023;27(4):1790–1800. [DOI] [PubMed] [Google Scholar]
- 7.Hyafil A, Giraud AL, Fontolan L, Gutkin B. Neural cross-frequency coupling: Connecting architectures, mechanisms, and functions. Trends Neurosci. 2015;38(11):725–740. [DOI] [PubMed] [Google Scholar]
- 8.Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–480. [DOI] [PubMed] [Google Scholar]
- 9.Amzica F, Steriade M. Short- and long-range neuronal synchronization of the slow (<1 Hz) cortical oscillation. J Neurophysiol. 1995;73(1):20–38. [DOI] [PubMed] [Google Scholar]
- 10.Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc Natl Acad Sci USA. 2010;107(7):3228–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsáki G. Cross-frequency phase-phase coupling between θ and γ oscillations in the hippocampus. J Neurosci. 2012;32(2):423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheffer-Teixeira R, Tort ABL. On cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. eLife. 2016;5: e20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pogosyan A, Gaynor LD, Eusebio A, Brown P. Boosting cortical activity at beta-band frequencies slows movement in humans. Curr Biol. 2009;19(19):1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joundi RA, Jenkinson N, Brittain JS, Aziz TZ, Brown P. Driving oscillatory activity in the human cortex enhances motor performance. Curr Biol. 2012;22(5):403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinogradov S, Herman A. Psychiatric illnesses as oscillatory connectomopathies. Neuropsychopharmacology. 2016;41(1):387–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oswal A, Brown P, Litvak V. Synchronized neural oscillations and the pathophysiology of Parkinson's disease. Curr Opin Neurol. 2013;26(6):662–670. [DOI] [PubMed] [Google Scholar]
- 17.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14(11):506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riddle J, Alexander ML, Schiller CE, Rubinow DR, Frohlich F. Reward-based decision-making engages distinct modes of cross-frequency coupling. Cereb Cortex. 2022;32(10):2079–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh CH, Young HWV, Wang CY, Wang YH, Lee PL, Kang JH, Lo MT. Quantifying spasticity with limited swinging cycles using pendulum test based on phase amplitude coupling. IEEE Trans Neural Syst Rehabil Eng. 2016;24(10):1081–1088. [DOI] [PubMed] [Google Scholar]
- 20.Voytek B, Canolty RT, Shestyuk A, Crone NE, Parvizi J, Knight RT. Shifts in gamma phase-amplitude coupling frequency from theta to alpha over posterior cortex during visual tasks. Front Hum Neurosci. 2010;4:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wingerden M, Meij R, Kalenscher T, Maris E, Pennartz CMA. Phase-amplitude coupling in rat orbitofrontal cortex discriminates between correct and incorrect decisions during associative learning. J Neurosci. 2014;34(2):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen KH, Tang AM, Gilbert ZD, Martin del Campo-Vera R, Sebastian R, Gogia AS, Sundaram S, Tabarsi E, Lee Y, Lee R, et al. Theta low-gamma phase amplitude coupling in the human orbitofrontal cortex increases during a conflict-processing task. J Neural Eng. 2022;19(1):016026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh CH, Lo MT, Hu K. Spurious cross-frequency amplitude-amplitude coupling in nonstationary, nonlinear signals. Physica A. 2016;454:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazaheri A, Nieuwenhuis ILC, Van Dijk H, Jensen O. Prestimulus alpha and mu activity predicts failure to inhibit motor responses. Hum Brain Mapp. 2009;30(6):1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel M, Warden MR, Miller EK. Phase-dependent neuronal coding of objects in short-term memory. Proc Natl Acad Sci USA. 2009;106(50):21341–21346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462(7271):353–357. [DOI] [PubMed] [Google Scholar]
- 27.Igarashi KM, Lu L, Colgin LL, Moser MB, Moser EI. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature. 2014;510(7503):143–147. [DOI] [PubMed] [Google Scholar]
- 28.Kikuchi Y, Attaheri A, Wilson B, Rhone AE, Nourski KV, Gander PE, Kovach CK, Kawasaki H, Griffiths TD, Howard MA, et al. Sequence learning modulates neural responses and oscillatory coupling in human and monkey auditory cortex. PLOS Biol. 2017;15(4): e2000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amemiya S, Redish AD. Hippocampal theta-gamma coupling reflects state-dependent information processing in decision making. Cell Rep. 2018;22(12):3328–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aru J, Aru J, Priesemann V, Wibral M, Lana L, Pipa G, Singer W, Vicente R. Untangling cross-frequency coupling in neuroscience. Curr Opin Neurobiol. 2015;31:51–61. [DOI] [PubMed] [Google Scholar]
- 31.Yeh CH, al-Fatly B, Kühn AA, Meidahl AC, Tinkhauser G, Tan H, Brown P. Waveform changes with the evolution of beta bursts in the human subthalamic nucleus. Clin Neurophysiol. 2020;131(9):2086–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramer MA, Tort ABL, Kopell NJ. Sharp edge artifacts and spurious coupling in EEG frequency comodulation measures. J Neurosci Methods. 2008;170(2):352–357. [DOI] [PubMed] [Google Scholar]
- 33.Lo MT, Novak V, Peng CK, Liu Y, Hu K. Nonlinear phase interaction between nonstationary signals: A comparison study of methods based on Hilbert-Huang and Fourier transforms. Phys Rev E Stat Nonlinear Soft Matter Phys. 2009;79(6): 061924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lozano-Soldevilla D, Huurne N, Oostenveld R. Neuronal oscillations with non-sinusoidal morphology produce spurious phase-to-amplitude coupling and directionality. Front Comput Neurosci. 2016;10:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang NE, Shen Z, Long SR, Wu MC, Shih HH, Zheng Q, Yen NC, Tung CC, Liu HH. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc R Soc A. 1998;454:903–995. [Google Scholar]
- 36.Pittman-Polletta B, Hsieh WH, Kaur S, Lo MT, Hu K. Detecting phase-amplitude coupling with high frequency resolution using adaptive decompositions. J Neurosci Methods. 2014;226:15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi W, Yeh CH, Hong Y. Cross-frequency transfer entropy characterize coupling of interacting nonlinear oscillators in complex systems. IEEE Trans Biomed Eng. 2019;66(2):521–529. [DOI] [PubMed] [Google Scholar]
- 38.Yeh CH, Shi W. Identifying phase-amplitude coupling in cyclic alternating pattern using masking signals. Sci Rep. 2018;8(1):2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Y, Shi W, Yeh C-H. A novel nonlinear bispectrum analysis for dynamical complex oscillations. Cogn Neurodyn. 2023;1–21. [Google Scholar]
- 40.Zhang C, Yeh C-H, Shi W. Variational phase-amplitude coupling characterizes signatures of anterior cortex under emotional processing. IEEE J Biomed Health Inform. 2023;27(4):1935–1945. [DOI] [PubMed] [Google Scholar]
- 41.Tort ABL, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104(2):1195–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penny WD, Duzel E, Miller KJ, Ojemann JG. Testing for nested oscillation. J Neurosci Methods. 2008;174(1):50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen MX. Assessing transient cross-frequency coupling in EEG data. J Neurosci Methods. 2008;168(2):494–499. [DOI] [PubMed] [Google Scholar]
- 44.Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313(5793):1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Özkurt TE, Schnitzler A. A critical note on the definition of phase-amplitude cross-frequency coupling. J Neurosci Methods. 2011;201(2):438–443. [DOI] [PubMed] [Google Scholar]
- 46.Mormann F, Fell J, Axmacher N, Weber B, Lehnertz K, Elger CE, Fernández G. Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus. 2005;15(7):890–900. [DOI] [PubMed] [Google Scholar]
- 47.Bruns A, Eckhorn R. Task-related coupling from high- to low-frequency signals among visual cortical areas in human subdural recordings. Int J Psychophysiol. 2004;51(2):97–116. [DOI] [PubMed] [Google Scholar]
- 48.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011: 156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput Intell Neurosci. 2011;2011: 879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Combrisson E, Nest T, Brovelli A, Ince RAA, Soto JLP, Guillot A, Jerbi K. Tensorpac: An open-source Python toolbox for tensor-based phase-amplitude coupling measurement in electrophysiological brain signals. PLOS Comput Biol. 2020;16(10): e1008302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tort ABL, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci USA. 2009;106(49):20942–20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu CC, Chien JH, Kim JH, Chuang YF, Cheng DT, Anderson WS, Lenz FA. Cross-frequency coupling in deep brain structures upon processing the painful sensory inputs. Neuroscience. 2015;303:412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jirsa V, Müller V. Cross-frequency coupling in real and virtual brain networks. Front Comput Neurosci. 2013;7:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klimesch W, Freunberger R, Sauseng P, Gruber W. A short review of slow phase synchronization and memory: Evidence for control processes in different memory systems? Brain Res. 2008;1235:31–44. [DOI] [PubMed] [Google Scholar]
- 55.Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, Hummel FC. Brain oscillatory substrates of visual short-term memory capacity. Curr Biol. 2009;19(21):1846–1852. [DOI] [PubMed] [Google Scholar]
- 56.Bonnefond M, Kastner S, Jensen O. Communication between brain areas based on nested oscillations. eNeuro. 2017;4(2):ENEURO.0153-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38(3):301–313. [DOI] [PubMed] [Google Scholar]
- 58.Lopes-dos-Santos V, Ven GM, Morley A, Trouche S, Campo-Urriza N, Dupret D. Parsing hippocampal theta oscillations by nested spectral components during spatial exploration and memory-guided behavior. Neuron. 2018;100(4):940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salimpour Y, Anderson WS. Cross-frequency coupling based neuromodulation for treating neurological disorders. Front Neurosci. 2019;13:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zielinski MC, Shin JD, Jadhav SP. Coherent coding of spatial position mediated by theta oscillations in the hippocampus and prefrontal cortex. J Neurosci. 2019;39(23):4550–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maris E, Vugt M, Kahana M. Spatially distributed patterns of oscillatory coupling between high-frequency amplitudes and low-frequency phases in human iEEG. NeuroImage. 2011;54(2):836–850. [DOI] [PubMed] [Google Scholar]
- 62.Lega B, Burke J, Jacobs J, Kahana MJ. Slow-theta-to-gamma phase-amplitude coupling in human hippocampus supports the formation of new episodic memories. Cereb Cortex. 2016;26(1):268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tok S, Maurin H, Delay C, Crauwels D, Manyakov NV, van der Elst W, Moechars D, Drinkenburg WHIM. Pathological and neurophysiological outcomes of seeding human-derived tau pathology in the APP-KI NL-G-F and NL-NL mouse models of Alzheimer's disease. Acta Neuropathol Commun. 2022;10(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tort ABL, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci USA. 2008;105(51):20517–20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shirvalkar PR, Rapp PR, Shapiro ML. Bidirectional changes to hippocampal theta-gamma comodulation predict memory for recent spatial episodes. Proc Natl Acad Sci USA. 2010;107(15):7054–7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bandarabadi M, Boyce R, Gutierrez Herrera C, Bassetti CL, Williams S, Schindler K, Adamantidis A. Dynamic modulation of theta-gamma coupling during rapid eye movement sleep. Sleep. 2019;42(12):zsz182. [DOI] [PubMed] [Google Scholar]
- 67.Daume J, Gruber T, Engel AK, Friese U. Phase-amplitude coupling and long-range phase synchronization reveal frontotemporal interactions during visual working memory. J Neurosci. 2017;37(2):313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Musaeus CS, Nielsen MS, Musaeus JS, Høgh P. Electroencephalographic cross-frequency coupling as a sign of disease progression in patients with mild cognitive impairment: A pilot study. Front Neurosci. 2020;14:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rajji TK, Zomorrodi R, Barr MS, Blumberger DM, Mulsant BH, Daskalakis ZJ. Ordering information in working memory and modulation of gamma by theta oscillations in humans. Cereb Cortex. 2017;27(2):1482–1490. [DOI] [PubMed] [Google Scholar]
- 70.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. [DOI] [PubMed] [Google Scholar]
- 71.Rajji TK, Sun Y, Zomorrodi-Moghaddam R, Farzan F, Blumberger DM, Mulsant BH, Fitzgerald PB, Daskalakis ZJ. PAS-induced potentiation of cortical-evoked activity in the dorsolateral prefrontal cortex. Neuropsychopharmacology. 2013;38(12):2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soto JLP, Jerbi K. Investigation of cross-frequency phase-amplitude coupling in visuomotor networks using magnetoencephalography. Annu Int Conf IEEE Eng Med Biol Soc. 2012;1550–1553. [DOI] [PubMed] [Google Scholar]
- 73.Krugliakova E, Volk C, Jaramillo V, Sousouri G, Huber R. Changes in cross-frequency coupling following closed-loop auditory stimulation in non-rapid eye movement sleep. Sci Rep. 2020;10(1):10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Babiloni C, Lizio R, Marzano N, Capotosto P, Soricelli A, Triggiani AI, Cordone S, Gesualdo L, del Percio C. Brain neural synchronization and functional coupling in Alzheimer's disease as revealed by resting state EEG rhythms. Int J Psychophysiol. 2016;103:88–102. [DOI] [PubMed] [Google Scholar]
- 75.Mondragón-Rodríguez S, Gu N, Manseau F, Williams S. Alzheimer's transgenic model is characterized by very early brain network alterations and β-CTF fragment accumulation: Reversal by β-secretase inhibition. Front Cell Neurosci. 2018;12:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ries ML, McLaren DG, Bendlin BB, GuofanXu, Rowley HA, Birn R, Kastman EK, Sager MA, Asthana S, Johnson SC. Medial prefrontal functional connectivity—Relation to memory self-appraisal accuracy in older adults with and without memory disorders. Neuropsychologia. 2012;50(5):603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brueggen K, Kasper E, Ochmann S, Pfaff H, Webel S, Schneider W, Teipel S. Cognitive rehabilitation in Alzheimer's disease: A controlled intervention trial. J Alzheimers Dis. 2017;57(4):1315–1324. [DOI] [PubMed] [Google Scholar]
- 78.Buschert VC, Friese U, Teipel SJ, Schneider P, Merensky W, Rujescu D, Möller HJ, Hampel H, Buerger K. Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild Alzheimer's disease: A pilot study. J Alzheimers Dis. 2011;25(4):679–694. [DOI] [PubMed] [Google Scholar]
- 79.Guo Y, Dang G, Hordacre B, Su X, Yan N, Chen S, Ren H, Shi X, Cai M, Zhang S, et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex modulates electroencephalographic functional connectivity in Alzheimer's disease. Front Aging Neurosci. 2021;13: 679585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hötting K, Röder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev. 2013;37(9):2243–2257. [DOI] [PubMed] [Google Scholar]
- 81.Etter G, Veldt S, Manseau F, Zarrinkoub I, Trillaud-Doppia E, Williams S. Optogenetic gamma stimulation rescues memory impairments in an Alzheimer's disease mouse model. Nat Commun. 2019;10(1):5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim S-E, Kim H-S, Kwak Y, Ahn M-H, Choi KM, Min B-K. Neurodynamic correlates for the cross-frequency coupled transcranial alternating current stimulation during working memory performance. Front Neurosci. 2022;16:1013691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turi Z, Mittner M, Lehr A, Bürger H, Antal A, Paulus W. θ-γ cross-frequency transcranial alternating current stimulation over the trough impairs cognitive control. eNeuro. 2020;7(5):ENEURO.0126-20.2020–0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bragin A, Engel J, Staba RJ. High-frequency oscillations in epileptic brain. Curr Opin Neurol. 2010;23(2):151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thomschewski A, Hincapié AS, Frauscher B. Localization of the epileptogenic zone using high frequency oscillations. Front Neurol. 2019;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andrade-Valenca LP, Dubeau F, Mari F, Zelmann R, Gotman J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology. 2011;77(6):524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacobs J, Kobayashi K, Gotman J. High-frequency changes during interictal spikes detected by time-frequency analysis. Clin Neurophysiol. 2011;122(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jacobs J, Levan P, Chtillon CD, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132(4):1022–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sakuraba R, Iwasaki M, Okumura E, Jin K, Kakisaka Y, Kato K, Tominaga T, Nakasato N. High frequency oscillations are less frequent but more specific to epileptogenicity during rapid eye movement sleep. Clin Neurophysiol. 2016;127(1):179–186. [DOI] [PubMed] [Google Scholar]
- 90.Nagasawa T, Juhász C, Rothermel R, Hoechstetter K, Sood S, Asano E. Spontaneous and visually driven high-frequency oscillations in the occipital cortex: Intracranial recording in epileptic patients. Hum Brain Mapp. 2012;33(3):569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang S, Wang IZ, Bulacio JC, Mosher JC, Gonzalez-Martinez J, Alexopoulos AV, Najm IM, So NK. Ripple classification helps to localize the seizure-onset zone in neocortical epilepsy. Epilepsia. 2013;54(2):370–376. [DOI] [PubMed] [Google Scholar]
- 92.Schnitzler S, Hartmann CJ, Groiss SJ, Wojtecki L, Schnitzler A, Vesper J, Hirschmann J. Occurrence of thalamic high frequency oscillations in patients with different tremor syndromes. Clin Neurophysiol. 2018;129(5):959–966. [DOI] [PubMed] [Google Scholar]
- 93.Hirschmann J, Schoffelen JM, Schnitzler A, Gerven MAJ. Parkinsonian rest tremor can be detected accurately based on neuronal oscillations recorded from the subthalamic nucleus. Clin Neurophysiol. 2017;128(10):2029–2036. [DOI] [PubMed] [Google Scholar]
- 94.Engel J, Bragin A, Staba R, Mody I. High-frequency oscillations: What is normal and what is not? Epilepsia. 2009;50(4):598–604. [DOI] [PubMed] [Google Scholar]
- 95.Gliske SV, Irwin ZT, Chestek C, Hegeman GL, Brinkmann B, Sagher O, Garton HJL, Worrell GA, Stacey WC. Variability in the location of high frequency oscillations during prolonged intracranial EEG recordings. Nat Commun. 2018;9(1):2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zweiphenning W, Klooster MA', van Klink NEC, Leijten FSS, Ferrier CH, Gebbink T, Huiskamp G, van Zandvoort MJE, van Schooneveld MMJ, Bourez M, et al. Intraoperative electrocorticography using high-frequency oscillations or spikes to tailor epilepsy surgery in the Netherlands (the HFO trial): A randomised, single-blind, adaptive non-inferiority trial. Lancet Neurol. 2022;21(11):982–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amiri M, Frauscher B, Gotman J. Interictal coupling of HFOs and slow oscillations predicts the seizure-onset pattern in mesiotemporal lobe epilepsy. Epilepsia. 2019;60(6):1160–1170. [DOI] [PubMed] [Google Scholar]
- 98.Motoi H, Miyakoshi M, Abel TJ, Jeong JW, Nakai Y, Sugiura A, Luat AF, Agarwal R, Sood S, Asano E. Phase-amplitude coupling between interictal high-frequency activity and slow waves in epilepsy surgery. Epilepsia. 2018;59(10):1954–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nonoda Y, Miyakoshi M, Ojeda A, Makeig S, Juhász C, Sood S, Asano E. Interictal high-frequency oscillations generated by seizure onset and eloquent areas may be differentially coupled with different slow waves. Clin Neurophysiol. 2016;127(6):2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ren G, Yan J, Sun Y, Ren J, Dai J, Mei S, Li Y, Wang X, Yang X, Wang Q. Association between interictal high-frequency oscillations and slow wave in refractory focal epilepsy with good surgical outcome. Front Hum Neurosci. 2020;14:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ibrahim GM, Wong SM, Anderson RA, Singh-Cadieux G, Akiyama T, Ochi A, Otsubo H, Okanishi T, Valiante TA, Donner E, et al. Dynamic modulation of epileptic high frequency oscillations by the phase of slower cortical rhythms. Exp Neurol. 2014;251:30–38. [DOI] [PubMed] [Google Scholar]
- 102.Ma H, Wang Z, Li C, Chen J, Wang Y. Phase–amplitude coupling and epileptogenic zone localization of frontal epilepsy based on intracranial EEG. Front Neurol. 2021;12: 718683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yeh C-H, Hsin Y-L. Frequency-frequency coupling of brain oscillations in studying ictal EEG activity. Brain Stimul. 2015;8(2):345. [Google Scholar]
- 104.Guirgis M, Chinvarun Y, Del Campo M, Carlen PL, Bardakjian BL. Defining regions of interest using cross-frequency coupling in extratemporal lobe epilepsy patients. J Neural Eng. 2015;12(2): 026011. [DOI] [PubMed] [Google Scholar]
- 105.Gooneratne IK, Green AL, Dugan P, Sen A, Franzini A, Aziz T, Cheeran B. Comparing neurostimulation technologies in refractory focal-onset epilepsy. J Neurol Neurosurg Psychiatry. 2016;87(11):1174–1182. [DOI] [PubMed] [Google Scholar]
- 106.Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, Srinivasan S, Jobst B, Gross RE, Shields DC, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84(8):810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toffa DH, Touma L, El Meskine T, Bouthillier A, Nguyen DK. Learnings from 30 years of reported efficacy and safety of vagus nerve stimulation (VNS) for epilepsy treatment: A critical review. Seizure. 2020;83:104–123. [DOI] [PubMed] [Google Scholar]
- 108.Ryvlin P, Rheims S, Hirsch LJ, Sokolov A, Jehi L. Neuromodulation in epilepsy: State-of-the-art approved therapies. Lancet Neurol. 2021;20(12):1038–1047. [DOI] [PubMed] [Google Scholar]
- 109.Kokkinos V, Sisterson ND, Wozny TA, Richardson RM. Association of closed-loop brain stimulation neurophysiological features with seizure control among patients with focal epilepsy. JAMA Neurol. 2019;76(7):800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Skarpaas TL, Jarosiewicz B, Morrell MJ. Brain-responsive neurostimulation for epilepsy (RNS® System). Epilepsy Res. 2019;153:68–70. [DOI] [PubMed] [Google Scholar]
- 111.Fisher B, DesMarteau JA, Koontz EH, Wilks SJ, Melamed SE. Responsive vagus nerve stimulation for drug resistant epilepsy: A review of new features and practical guidance for advanced practice providers. Front Neurol. 2021;11: 610379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Englot DJ, Rolston JD, Wright CW, Hassnain KH, Chang EF. Rates and predictors of seizure freedom with vagus nerve stimulation for intractable epilepsy. Neurosurgery. 2016;79(3):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Batson S, Shankar R, Conry J, Boggs J, Radtke R, Mitchell S, Barion F, Murphy J, Danielson V. Efficacy and safety of VNS therapy or continued medication management for treatment of adults with drug-resistant epilepsy: Systematic review and meta-analysis. J Neurol. 2022;269(6):2874–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nair DR, Laxer KD, Weber PB, Murro AM, Park YD, Barkley GL, Smith BJ, Gwinn RP, Doherty MJ, Noe KH, et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology. 2020;95(9):e1244–e1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith Y, Wichmann T, Factor SA, Delong MR. Parkinson's disease therapeutics: New developments and challenges since the introduction of levodopa. Neuropsychopharmacology. 2012;37(1):213–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pötter-Nerger M, Volkmann J. Deep brain stimulation for gait and postural symptoms in Parkinson's disease. Mov Disord. 2013;28(11):1609–1615. [DOI] [PubMed] [Google Scholar]
- 117.Kühn AA, Kempf F, Brücke C, Doyle LG, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider G-H, Hariz MI, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson's disease in parallel with improvement in motor performance. J Neurosci. 2008;28(24):6165–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Little S, Brown P. The functional role of beta oscillations in Parkinson's disease. Parkinsonism Relat Disord. 2014;20(Suppl. 1):S44–S48. [DOI] [PubMed] [Google Scholar]
- 119.López-Azcárate J, Tainta M, Rodríguez-Oroz MC, Valencia M, González R, Guridi J, Iriarte J, Obeso JA, Artieda J, Alegre M. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson's disease. J Neurosci. 2010;30(19):6667–6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.AuYong N, Malekmohammadi M, Ricks-Oddie J, Pouratian N. Movement-modulation of local power and phase amplitude coupling in bilateral globus pallidus interna in Parkinson disease. Front Hum Neurosci. 2018;12:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fischer P, Chen CC, Chang YJ, Yeh CH, Pogosyan A, Herz DM, Cheeran B, Green AL, Aziz TZ, Hyam J, et al. Alternating modulation of subthalamic nucleus beta oscillations during stepping. J Neurosci. 2018;38(22):5111–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jin L, Shi W, Zhang C, Yeh C-H. Frequency nesting interactions in the subthalamic nucleus correlate with the step phases for Parkinson's disease. Front Physiol. 2022;13: 890753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brücke C, Huebl J, Schönecker T, Neumann WJ, Yarrow K, Kupsch A, Blahak C, Lütjens G, Brown P, Krauss JK, et al. Scaling of movement is related to pallidal γ oscillations in patients with dystonia. J Neurosci. 2012;32(3):1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jenkinson N, Kühn AA, Brown P. Gamma oscillations in the human basal ganglia. Exp Neurol. 2013;245:72–76. [DOI] [PubMed] [Google Scholar]
- 125.Buzśaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fries P, Nikolić D, Singer W. The gamma cycle. Trends Neurosci. 2007;30(7):309–316. [DOI] [PubMed] [Google Scholar]
- 127.Wiest C, Torrecillos F, Tinkhauser G, Pogosyan A, Morgante F, Pereira EA, Tan H. Finely-tuned gamma oscillations: Spectral characteristics and links to dyskinesia. Exp Neurol. 2022;351: 113999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, Ostrem JL, Galifianakis NB, Starr PA. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci USA. 2013;110(12):4780–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, Starr PA. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson's disease. Nat Neurosci. 2015;18(5):779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Damborská A, Lamoš M, Brunet D, Vulliemoz S, Bočková M, Deutschová B, Baláž M, Rektor I. Resting-state phase-amplitude coupling between the human subthalamic nucleus and cortical activity: A simultaneous intracranial and scalp EEG study. Brain Topogr. 2021;34(3):272–282. [DOI] [PubMed] [Google Scholar]
- 131.Wang DD, de Hemptinne C, Miocinovic S, Qasim SE, Miller AM, Ostrem JL, Galifianakis NB, San Luciano M, Starr PA. Subthalamic local field potentials in Parkinson's disease and isolated dystonia: An evaluation of potential biomarkers. Neurobiol Dis. 2016;89:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wijk BCM, Beudel M, Jha A, Oswal A, Foltynie T, Hariz MI, Limousin P, Zrinzo L, Aziz TZ, Green AL, et al. Subthalamic nucleus phase-amplitude coupling correlates with motor impairment in Parkinson's disease. Clin Neurophysiol. 2016;127(4):2010–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang AI, Vanegas N, Lungu C, Zaghloul KA. Beta-coupled high-frequency activity and beta-locked neuronal spiking in the subthalamic nucleus of Parkinson's disease. J Neurosci. 2014;34(38):12816–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Seeber M, Scherer R, Wagner J, Solis-Escalante T, Müller-Putz GR. EEG beta suppression and low gamma modulation are different elements of human upright walking. Front Hum Neurosci. 2014;8:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wagner J, Solis-Escalante T, Grieshofer P, Neuper C, Müller-Putz G, Scherer R. Level of participation in robotic-assisted treadmill walking modulates midline sensorimotor EEG rhythms in able-bodied subjects. NeuroImage. 2012;63(3):1203–1211. [DOI] [PubMed] [Google Scholar]
- 136.He S, Deli A, Fischer P, Wiest C, Huang Y, Martin S, Khawaldeh S, Aziz TZ, Green AL, Brown P, et al. Gait-phase modulates alpha and beta oscillations in the pedunculopontine nucleus. J Neurosci. 2021;41(41):8390–8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yin Z, Zhu G, Liu Y, Zhao B, Liu D, Bai Y, Zhang Q, Shi L, Feng T, Yang A, et al. Cortical phase–amplitude coupling is key to the occurrence and treatment of freezing of gait. Brain. 2022;145(7):2407–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ammann C, Dileone M, Pagge C, Catanzaro V, Mata-Marín D, Hernández-Fernández F, Monje MHG, Sánchez-Ferro Á, Fernández-Rodríguez B, Gasca-Salas C, et al. Cortical disinhibition in Parkinson's disease. Brain. 2020;143(11):3408–3421. [DOI] [PubMed] [Google Scholar]
- 139.Pezzopane V, D'Acunto A, Casula EP, Spampinato D, Koch G, Fadiga L. Neuromodulation using cross-frequency coupling tACS: Preliminary data. Brain Stimul. 2023;16(1):329. [Google Scholar]
- 140.Chen KHS, Chen R. Invasive and noninvasive brain stimulation in Parkinson's disease: Clinical effects and future perspectives. Clin Pharmacol Ther. 2019;106(4):763–775. [DOI] [PubMed] [Google Scholar]
- 141.Farokhniaee A, Lowery MM. Cortical network effects of subthalamic deep brain stimulation in a thalamo-cortical microcircuit model. J Neural Eng. 2021;18(5). [DOI] [PubMed] [Google Scholar]
- 142.Gilron R, Little S, Perrone R, Wilt R, de Hemptinne C, Yaroshinsky MS, Racine CA, Wang SS, Ostrem JL, Larson PS, et al. Long-term wireless streaming of neural recordings for circuit discovery and adaptive stimulation in individuals with Parkinson's disease. Nat Biotechnol. 2021;39(9):1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Muthuraman M, Bange M, Koirala N, Ciolac D, Pintea B, Glaser M, Tinkhauser G, Brown P, Deuschl G, Groppa S. Cross-frequency coupling between gamma oscillations and deep brain stimulation frequency in Parkinson's disease. Brain. 2021;143(11):3393–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Potel SR, Marceglia S, Meoni S, Kalia SK, Cury RG, Moro E. Advances in DBS technology and novel applications: Focus on movement disorders. Curr Neurol Neurosci Rep. 2022;22(9):577–588. [DOI] [PubMed] [Google Scholar]
- 145.Thenaisie Y, Lee K, Moerman C, Scafa S, Gálvez A, Pirondini E, Burri M, Ravier J, Puiatti A, Accolla E, et al. Principles of gait encoding in the subthalamic nucleus of people with Parkinson's disease. Sci Transl Med. 2022;14(661):eabo1800. [DOI] [PubMed] [Google Scholar]
- 146.Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson's disease: Current outlook and future challenges. Parkinsonism Relat Disord. 2016;22(Suppl. 1):S60–S64. [DOI] [PubMed] [Google Scholar]
- 147.Bevilacqua R, Maranesi E, di Rosa M, Luzi R, Casoni E, Rinaldi N, Baldoni R, Lattanzio F, di Donna V, Pelliccioni G, et al. Rehabilitation of older people with Parkinson's disease: An innovative protocol for RCT study to evaluate the potential of robotic-based technologies. BMC Neurol. 2020;20(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alhasan HS, Wheeler PC, Fong DTP. Application of interactive video games as rehabilitation tools to improve postural control and risk of falls in prefrail older adults. Cyborg Bionic Syst. 2021;2021:9841342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bouthour W, Mégevand P, Donoghue J, Lüscher C, Birbaumer N, Krack P. Biomarkers for closed-loop deep brain stimulation in Parkinson disease and beyond. Nat Rev Neurol. 2019;15(6):343–352. [DOI] [PubMed] [Google Scholar]
- 150.Kent AR, Swan BD, Brocker DT, Turner DA, Gross RE, Grill WM. Measurement of evoked potentials during thalamic deep brain stimulation. Brain Stimul. 2015;8(1):42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hoang KB, Cassar IR, Grill WM, Turner DA. Biomarkers and stimulation algorithms for adaptive brain stimulation. Front Neurosci. 2017;11:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data of this paper are available by emailing chien-hung.yeh@bit.edu.cn.