Abstract
Background:
Patients with atopic dermatitis (AD) have systemic biomarker dysregulation that differs by age group; however, the proteomic characteristics of these age-based changes are unknown.
Objective:
To profile blood proteins of patients with AD across different age groups versus age-appropriate controls.
Methods:
Using the Olink high-throughput proteomic platform, we profiled 375 serum proteins of 20 infants (age, 0–5 years), 39 children (age, 6–11 years), 21 adolescents (age, 12–17 years), and 20 adults (age, ≥18 years) with moderate-to-severe AD and 83 age-appropriate controls.
Results:
Each group presented a distinct systemic proteomic signature. Th2-related proteins were increased in infant AD and further intensified with age through adolescence and adulthood (interleukin 4/CCL13/CCL17). In contrast, Th1 axis down-regulation was detected in infants with AD and gradually reversed to increased Th1 products (interferon γ/CXCL9/CXCL10/CCL2) in patients with AD from childhood to adulthood. Despite their short disease duration, infants already had evidence of systemic inflammation, with significant upregulation of innate immunity (interleukin 17C/ interleukin-1RN), T-cell activation/migration (CCL19), Th2 (CCL13/CCL17), and Th17 (PI3) proteins. Adults with AD present unique upregulation of cardiovascular proteins related to coagulation and diabetes.
Limitations:
Cross-sectional observational study with a single time point.
Conclusion:
Systemic immune signatures of AD are age-specific beyond the shared Th2 immune activation. These data advocate for precision medicine approaches based on age-specific AD profiles.
Author Keywords: Olink, atopic dermatitis, pediatric, proteomic
INTRODUCTION
Atopic dermatitis (AD) is the most common inflammatory skin disease, affecting up to 20% of children worldwide.1 Although approximately 60% of patients are diagnosed within the first year of life, chronic persistence of AD into adulthood is reported in up to 50% of school-aged patients.2–6
AD is heterogeneous and changes with age, as was recently shown in the skin and lymphocytes (using flow cytometry) of patients with AD as they mature from infancy through adulthood. All AD age groups present Th2/Th22 skewing. Infants with AD also display strong Th17-related signaling, which decreases during childhood and adolescence, whereas Th1 activation develops during adult AD as a sign of chronicity.7 Flow cytometry blood analysis also established a Th2 axis–related upregulation in AD across all age groups, along with Th17/Th22 skewing from infancy and an increase in interferon γ (Th1) signaling in adults.8 Nevertheless, the evolution of circulating proteins across consecutive AD age groups is still unknown.
AD has been associated with several systemic comorbidities potentially because of the long-standing inflammation characterizing the disease.9,10 However, data on the dysregulation of circulating proteins in AD are largely limited to adults with years of disease activity11–13 and a few reports exploring early-onset infant AD, which show systemic signs of inflammation within months of disease onset.9,13–15 Characterization of the proteomic changes associated with AD at different age groups may shed light on the chronic inflammatory process of AD using a minimally invasive approach. Proteomic analysis may also detect age-specific biomarkers for clinical trials, longitudinal studies, and, in the future, better-tailored clinical management. Moreover, as the treatment of moderate-to-severe pediatric AD is still challenging,16,17 proteomic characterization of AD may ultimately guide age-specific therapeutic development.
Using 375 analytes of the Olink platform, we profiled circulating proteins in patients with AD in different age groups (infants/toddlers, aged 0–5 years; children, aged 5–12 years; adolescents, aged 12–18 years; and adults, aged ≥18 years) in comparison to blood proteins from age-appropriate controls. Our results portray the evolution of the systemic molecular characteristics of AD across ages, revealing unique AD proteomic signatures across different age groups.
METHODS
Patient characteristics
One-hundred patients with AD (20 infants [aged 0–5 years; mean, 2.39 years], 39 children [aged 6–11 years; mean, 8.24 years], 21 adolescents [aged 12–17 years; mean, 15.0 years], and 20 adults [aged ≥18 years; mean, 40.9 years]) with moderate-to-severe AD and 83 healthy age-appropriate controls were enrolled in the study (demographics in Table I). Before study inclusion, parents (and patients if aged ≥12 years) signed institutional review board–approved consent. Immunoglobulin E (IgE) and clinical indices were collected, including the SCORing of Atopic Dermatitis (SCORAD) severity score,18 as well as history of atopic comorbidities (Table I). The exclusion criteria included recent use (<4 weeks) of systemic immunosuppressive treatment before this study. Subjects enrolled in the control groups had a negative personal and family history of atopy and a negative personal history of cardiovascular/immune/neoplastic diseases, hypertension, and hyperlipidaemia.
Table I.
Demographic characteristics
| Infant AD (n = 20) | Infant N (n = 20) | P value | Child AD (n = 39) | Child N (n = 27) | P value | Adolescent AD (n = 21) | Adolescent N (n = 16) | P value | Adult AD (n = 20) | Adult N (n = 20) | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y), mean (SD) | 2.5 (1.8) | 2.5 (1.3) | 0.97 | 8.3 (2) | 8.2 (2) | 0.87 | 15 (2) | 14.5 (1.6) | 0.46 | 42.3 (18) | 37.9 (15.6) | 0.41 |
| Female sex, n (%) | 9 (45) | 8 (40) | 1 | 25 (64.1) | 17 (63) | 1 | 10 (47.6) | 8 (50) | 1 | 10 (50) | 11 (55) | 1 |
| Total serum IgE (IU/mL), mean (SD) | 1072.1 (1838.2) | 26.69 (42.5) | 0.18 | 2026.6 (2401) | 45.99 (49.5) | 1.00E-05 | 3841 (3860) | 236 (533) | 0.002 | 2710 (2334) | 97 (92) | 0.002 |
| SCORAD mean (SD) | 50.5 (18.1) | NA | 54 (15.3) | NA | 57.6 (22.1) | NA | 58.4 (12.1) | NA | ||||
| Ethnicity (%) | ||||||||||||
| Caucasian | 10 (50) | 17 (85) | 0.04 | 26 (66.7) | 21 (77.8) | 0.41 | 14 (66.7) | 13 (81.2) | 0.46 | 15 (75) | 15 (75) | 1 |
| African American | 4 (20) | 1 (5) | 0.34 | 4 (10.3) | 4 (14.8) | 0.71 | 2 (9.5) | 1 (6.3) | 1 | 2 (10) | 2 (10) | 1 |
| Asian | 6 (30) | 2 (10) | 0.23 | 6 (17.9) | 2 (7.4) | 0.45 | 3 (14.3) | 0 (0) | 0.24 | 3 (15) | 3 (15) | 1 |
| Mixed/Unknown | 0 (0) | 0 (0) | NA | 3 (5.1) | 0 (0) | NA | 2 (9.5) | 2(12.5) | NA | 0 (0) | 0 (0) | NA |
| Atopic comorbidities * | 10/20 | None | NA | 37/39 | None | NA | 19/21 | None | NA | 11/20 | None | NA |
AD, Atopic dermatitis; IgE, immunoglobulin E; NA, not applicable; SCORAD, SCORing of Atopic Dermatitis.
Atopic comorbidities included asthma, food allergy, allergic rhinitis, allergic conjunctivitis, and seasonal allergies.
Blood samples
Serum aliquots from subjects with AD and healthy controls were analyzed using a proteomic Olink Proseek multiplex assay. Specifically, we used a comprehensive panel detecting inflammation-related proteins, cardiovascular panels II and III, and the Olink neurology panel. This method quantified protein levels from 10-μL serum for quantification by a real-time polymerase chain reaction platform. IgE was assessed in the sera of all patients with AD.
Statistical analysis
Analyses were performed using the R language (R Core Team and the R Foundation for Statistical Computing; R-project.org). Fold changes (FCHs) for comparisons were estimated with an empirical Bayesian method, and hypothesis testing was conducted using contrasts under the general framework for linear models in the limma package. The results were normalized to healthy controls to differentiate pathologic changes from physiological maturation. A protein with an FCH of >1.2 and a P value of <.05 or a false discovery rate (FDR) of <0.05 was considered differentially expressed. |FCH| of >1.2 was chosen as an arbitrary threshold found appropriate for serum data in which skin-related biomarkers are harder to detect. The sample size was determined on the basis of a power calculation from a previous Olink study in an AD cohort, in which 20 or more AD samples and 9 or more controls had greater than 90% power (P = .05) to detect differences in key Th2 (CCL13 and CCL17) markers.19
The Benjamini-Hochberg procedure was used to adjust P values for multiple hypotheses by controlling the FDR (values in brackets and Supplementary Table I, available via Mendeley at https://data.mendeley.com/datasets/rvybyf8zzd). For correlation studies, unsupervised hierarchical clustering of variables or samples/patients was performed using the correlation coefficient as a distance metric with the average agglomeration algorithm. Pathway analysis was performed with XGR software using canonical/KEGG/Reactome/BioCarta pathways.20
RESULTS
To evaluate circulating AD-related versus growth-related changes across pediatric and adult age groups, we profiled 375 serum proteins from 100 patients with AD and 83 healthy age-appropriate controls using the Olink high-throughput proteomic platform (see demographic characteristics in Table I).
Across the panels, we were able to identify up to 228 differentially expressed proteins (DEPs) using criteria of FCH > |1.2| and P < .05 or FDR < 0.05 as depicted in Supplementary Table I. Because the cohort across age groups was composed primarily of Caucasian patients, we also conducted a sensitivity analysis to confirm that similar results were obtained in subjects of all ethnicities (Supplementary Table II, available via Mendeley at https://data.mendeley.com/datasets/rvybyf8zzd).
Differentially expressed immune-related proteins change across age groups
To characterize the immune phenotype of the different AD age groups, we investigated a panel of immune DEPs that were previously reported as AD-related,13,21–23 as depicted in an unsupervised hierarchical clustering heat map (Fig 1). A common AD signature was shared across all age groups and included key Th2 markers (ie, CCL17/TARC and CCL13; FDR < 0.05) along with the adhesion marker CDH3 (FDR < 0.05).24
Fig 1.

Comparison of differentially expressed immune-related proteins in the blood of patients with atopic dermatitis of different age groups. Heatmap of immune signaling proteins that are differentially expressed in the blood of different AD age groups, using criteria of fold change (FCH) ≥ |1.2| and P < .05. Proteins are ordered by unsupervised hierarchical clustering. The columns of the table on the right show FCH by AD age groups vs age-matched controls, and FCH across normalized groups. +P < .10, ∗P < .05, ∗∗P < .01. AD, atopic dermatitis; Adol, adolescent; N, normal.
In addition to these shared DEPs, each AD age group presented specific trends. The infant AD already presented increased systemic activation compared with that in age-appropriate controls, with significant upregulation of some innate immunity (with interleukin (IL) 17C being uniquely upregulated only in infant AD), cellular activation/migration (CD8A, CD84, CCL19), and Th17 (KYNU, PI3) markers, all presenting the highest expression levels in infants or a unique upregulation in this age group (FDR < 0.05). Moreover, key Th1 markers, such as CXCL10, were uniquely downregulated in the infant AD group (FDR < 0.05; Fig 1 and Supplementary Table II).
In comparison to infant AD and age-appropriate controls, childhood AD presented an increase in innate immunity markers (ie, IL-8), Th2-related markers (CCL7 and transforming growth factor α), and key Th1/Iinterferon γ-associated proteins (CXCL10; FDR < 0.05 for all). On the other hand, children with AD presented a decrease in immune markers related to T-cell activation/recruitment (CD40LG and IL-16) when compared to other age groups (Fig 1 and Supplementary Table I).
Adolescent AD presented intensification of Th2 (CCL13 and CCL17) and Th1/interferon γ (CXCL10/11) markers in comparison to patients of younger age with AD.
Adults with AD displayed a further increase in Th17/Th22-related (S100A12 and IL-20) and Th2-related (CCL13, CCL17, OSM, IL-1RL1/IL-33R, IL-4, and IL-4R) (FDR < 0.05) markers versus younger AD age groups, whereas IL-4R and IL-4 were among the proteins showing upregulation versus controls exclusively in the adult AD age group. Adults also displayed an intensification of Th1 markers as compared with younger age groups (IL-1RN, IL-16, and CCL2; Fig 1). Conversely, LAT, essential for T-cell activation,25 was significantly downregulated in adult AD versus controls as well as versus other AD age groups.
Cardiovascular/atherosclerosis- and neurology-related proteins differ by age
Our analysis also included hallmark proteins of systemic inflammation that may be associated with other conditions or comorbidities, specifically cardiovascular/atherosclerosis,13,26 as depicted in Fig 2, and neurology-related processes27 (Supplementary Table I).
Fig 2.

Comparison of differentially expressed cardiovascular/atherosclerosis-related proteins in the blood of patients with atopic dermatitis of different age groups. Heatmap of cardiovascular/atherosclerosis signaling proteins that are differentially expressed in the blood of different AD age groups, using criteria of fold change (FCH) ≥ |1.2| and P < .05. Proteins are ordered by unsupervised hierarchical clustering. The columns of the table on the right show FCH by AD age groups vs age-matched controls, and FCH across normalized groups. +P < .10, ∗ P < .05, ∗∗ P < .01. AD, atopic dermatitis; Adol, adolescent; N, normal.
Overall, adult patients with AD presented the greatest upregulation of cardiovascular/atherosclerosis-related proteins, as shown in the pink box in Fig 2. Among these, proteins associated with platelet aggregation and thrombus formation (eg, PLAUR, MPO, ADAMTS13, SERPINE1, SELE; FDR < 0.01; Fig 2 and Supplementary Table I).
The infant AD showed a unique and significant upregulation of other circulating proteins linked with vascular growth factors (green box, Fig 2). For example, vascular endothelial growth factor D/FIGF, ADM, tissue inhibitor of metalloproteinase/TIMP4, PLAU, and HB-EGF13,15,19 were all uniquely upregulated in infants, along with proteins associated with increased susceptibility to obesity (ie, LPL and AGRP28,29) (FDR < 0.05 for all). Of note, in comparison to the infant AD, children with AD already displayed upregulation of some atherosclerosis-related markers30,31 (ie, CEACAM8, PTX3, OLR1, AZU1, and MMP9; FDR < 0.05).
Using the Olink neurology panel, we also detected significant upregulation of factors associated with neural development, which were upregulated only in infant AD compared with age-matched controls. These included glial cell line–derived neurotrophic factor receptor α/GFRA3, galectin/4LGALS4, dorsal inhibitory axon guidance protein/DRAXIN, and BCAN (an integral component of the neuro synapses;32 FDR < 0.05 for all; Supplementary Table I).
Proteins related to T-cell activation correlate with AD severity across age groups
The Pearson correlation of protein levels with AD severity scores (assessed by SCORAD), patient’s age, and total serum IgE yielded strong correlations in all AD age groups (Table II). Markers that were correlated with SCORAD across all age groups included the T-cell activation marker IL-16 and the Th17-related marker PI3 (r > 0.4; P < .01). Within specific age groups, infants displayed correlations of AD severity (SCORAD) with proteins implicated in wound recovery and response to infections (TNFRSF13B, ITGB2, SELPG, and FCGR2B)33–36 along with a regulatory protein of keratinocyte proliferation (IGFBP7)37 (r > 0.45, P < .05). MMP12 was correlated with SCORAD in children and adolescents, and children and adults presented the strongest correlations with LGALS9, related to T-regulatory response against Th2. Similar trends were observed for Eczema Area and Severity Index and blood biomarkers, with disease severity correlating with wound healing (PECAM1) in infants, markers of general inflammation in children and adolescents (FCGR2B and TNFRSF10A), and T-cell response markers in adults (IL2R and TNFRSF1B), as depicted in Table II.
Table II.
Spearman correlations of selected serum proteins with Eczema Area and Severity Index, SCORing of Atopic Dermatitis, immunoglobulin E, and age (|r|>0.4, P < .05)
| SCORAD | Age | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Infant | Child | Adolescent | Adult | All age groups | ||||||||||
| Marker | r | P value | Marker | r | P value | Marker | r | P value | Marker | r | P value | Marker | r | P value |
| IL1RL1 | 0.61 | 0.005 | PI3 | 0.54 | <.001 | IL18 | 0.62 | <.001 | IL16 | 0.71 | 0.001 | PRELP | 0.7 | <.001 |
| OSCAR | 0.58 | 0.008 | IL16 | 0.47 | <.001 | XCL1 | 0.6 | <.001 | OSCAR | 0.57 | 0.009 | TIMP4 | 0.64 | <.001 |
| SELE | 0.56 | 0.011 | MMP12 | 0.47 | <.001 | MMP12 | 0.55 | 0.011 | TNFRSF1B | 0.56 | 0.011 | LEP | 0.61 | <.001 |
| TNFRSF13B | 0.55 | 0.014 | ADM | −0.41 | 0.01 | TGM2 | 0.54 | 0.012 | LGALS9 | 0.54 | 0.016 | BMP6 | 0.59 | <.001 |
| ITGB2 | 0.53 | 0.018 | FCGR2B | −0.42 | 0.007 | CHIT1 | 0.54 | 0.013 | CSTB | 0.53 | 0.017 | ADM | 0.58 | <.001 |
| PI3 | 0.53 | 0.018 | MB | −0.43 | 0.006 | CPB1 | 0.5 | 0.021 | PI3 | 0.51 | 0.023 | AMBP | 0.55 | <.001 |
| CCL15-CCL14 | 0.52 | 0.019 | MEPE | −0.52 | <.001 | CTRC | 0.48 | 0.031 | TNFRSF10B | 0.51 | 0.024 | MMP3 | 0.55 | <.001 |
| FABP4 | 0.52 | 0.021 | PI3 | 0.45 | 0.044 | ADM | 0.49 | 0.031 | PLAT | 0.52 | <.001 | |||
| IL2RA | 0.51 | 0.022 | TNFRSF10A | 0.44 | 0.048 | IL2RA | 0.48 | 0.032 | CCL17 | 0.52 | <.001 | |||
| CA5A | 0.48 | 0.034 | MEPE | −0.45 | 0.044 | GDF15 | 0.47 | 0.036 | PAPPA | 0.49 | <.001 | |||
| IGFBP7 | 0.47 | 0.038 | CNTN1 | −0.45 | 0.042 | TNFRSF1A | 0.46 | 0.041 | HBEGF | 0.48 | <.001 | |||
| F3 | 0.46 | 0.041 | MB | −0.46 | 0.036 | SPON1 | 0.46 | 0.044 | CPA1 | 0.48 | <.001 | |||
| IL1RL2 | 0.46 | 0.044 | SFTPD | −0.56 | 0.01 | PDGFB | 0.47 | <.001 | ||||||
| ACP5 | 0.46 | 0.045 | CPB1 | 0.47 | <.001 | |||||||||
| IL1R2 | 0.45 | 0.047 | NPPB | 0.46 | <.001 | |||||||||
| SELPLG | 0.45 | 0.047 | PI3 | 0.46 | <.001 | |||||||||
|
|
||||||||||||||
| EASI | IL16 | 0.43 | <.001 | |||||||||||
|
|
||||||||||||||
| Infant | Child | Adolescent | Adult | FGF21 | 0.42 | <.001 | ||||||||
| Marker | r | P value | Marker | r | P value | Marker | r | P value | Marker | r | P value | IL27 | 0.41 | <.001 |
|
|
||||||||||||||
| IL1RL2 | 0.56 | 0.012 | LGALS9 | 0.49 | <.001 | IL18 | 0.68 | <.001 | PI3 | 0.62 | 0.037 | BLMH | −0.42 | <.001 |
| FCGR2B | 0.5 | 0.028 | AZU1 | 0.46 | <.001 | PI3 | 0.55 | 0.009 | REN | 0.62 | 0.037 | GP6 | −0.43 | <.001 |
| FABP6 | −0.47 | 0.041 | IL6 | 0.43 | 0.006 | TNFRSF10A | 0.53 | 0.013 | TNFRSF1B | 0.6 | 0.043 | SELP | −0.44 | <.001 |
| DECR1 | −0.48 | 0.04 | MPO | 0.42 | 0.007 | CHIT1 | 0.48 | 0.026 | IL2RA | 0.59 | 0.046 | IGFBP2 | −0.44 | <.001 |
| PECAM1 | −0.48 | 0.038 | MMP9 | 0.42 | 0.008 | XCL1 | 0.47 | 0.03 | ACP5 | 0.59 | 0.049 | LTBR | −0.44 | <.001 |
| XCL1 | −0.50 | 0.03 | PRTN3 | 0.41 | 0.01 | SFTPD | −0.45 | 0.038 | CCL3 | −0.78 | 0.005 | TNFRSF14 | −0.45 | <.001 |
| MB | −0.58 | <.001 | CNTN1 | −0.59 | <.001 | IL17RA | −0.45 | <.001 | ||||||
| FCGR2B | −0.66 | <.001 | BOC | −0.46 | <.001 | |||||||||
| PARP1 | −0.49 | <.001 | ||||||||||||
|
IgE
| ||||||||||||||
| Infant | Child | Adolescent | Adult | All age groups | ||||||||||
| Marker | r | P value | Marker | r | P value | Marker | r | P value | Marker | r | P value | Marker | r | P value |
|
| ||||||||||||||
| MMP9 | 0.89 | 0.012 | IL2RA | 0.42 | 0.008 | TGM2 | 0.52 | 0.033 | CCL3 | 0.78 | 0.039 | CCL17 | 0.52 | <.001 |
| COL1A1 | −0.79 | 0.048 | LGALS3 | 0.41 | 0.012 | SLAMF7 | 0.51 | 0.036 | CXCL16 | −0.78 | 0.039 | |||
| CA5A | −0.79 | 0.048 | MMP7 | −0.48 | <.001 | PIGR | −0.85 | 0.015 | ||||||
| TFPI | −0.82 | 0.034 | VWF | −0.85 | 0.015 | |||||||||
| CXCL1 | −0.82 | 0.034 | TNFRSF10C | −0.89 | 0.007 | |||||||||
| HAO1 | −0.86 | 0.024 | ||||||||||||
EASI, Eczema Area and Severity Index; IgE, immunoglobulin E; SCORAD, SCORing of Atopic Dermatitis.S
Multiple correlations were found between age and cardiovascular/hypertension (eg, LEP, ADM, HB-EGF, PDGFB, NPPB). The AD-related biomarker, CCL17/TARC, was also positively correlated with age, as were PI3 and IL-16 (r > 0.43, P < .001). Although all age groups showed correlations between various markers and serum IgE levels, only CCL17/TARC was correlated with IgE across all age groups.
Pathway analysis further distinguishes age-specific proteomic profiles
For an unbiased, broad evaluation of age-specific AD-related changes in proteomic expression, all DEPs in AD versus controls by age groups were analyzed using function-based pathway databases (canonical/KEGG/Reactome/BioCarta pathways)20, as depicted in Fig 3. Top enriched pathways across age groups largely overlapped and included overexpression of cytokine- and inflammation-related signatures along with an ensemble of extracellular matrix-associated proteins (FDR < 0.05). Only adolescents and adults presented enrichment of pathways related to Th1 and Th2 differentiation. JAK-STAT (Janus kinase–signal transducer and activator of transcription) pathway enrichment was detected starting from childhood. The infant AD uniquely presented enrichment of pathways related to TRAIL signaling, β1/2/3 integrin cell surface interactions, and immunoregulatory interactions between lymphoid and nonlymphoid cells. Adult AD uniquely presented significant enrichment of pathways related to complement, coagulation, and diabetes.
Fig 3.
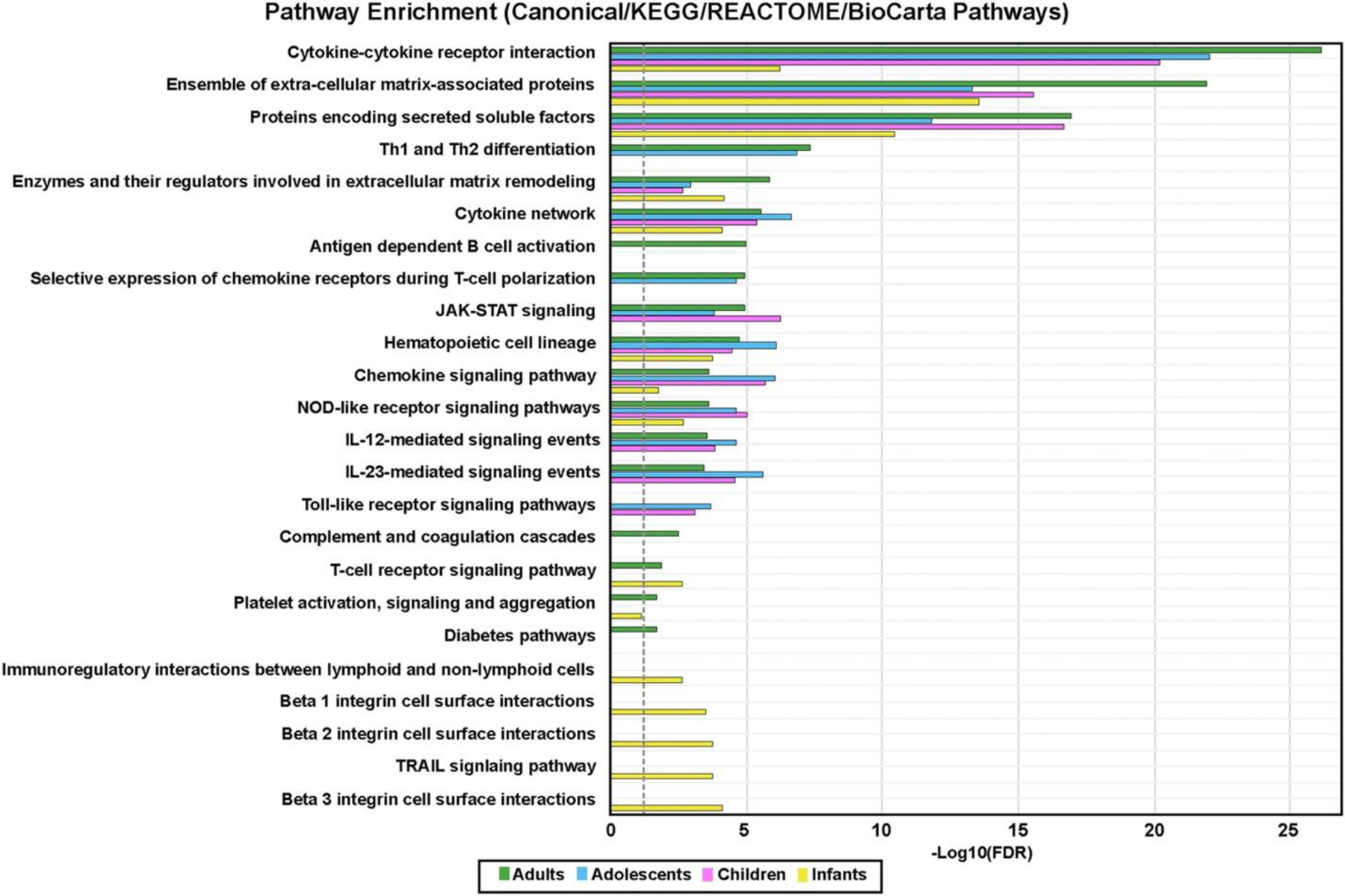
Pathway analysis. Common/most enriched pathways in atopic dermatitis age groups versus age-appropriate controls using differentially expressed proteins in atopic dermatitis blood versus healthy control serum by age groups analyzed with publicly available function-based pathway databases (using canonical/KEGG/Reactome/BioCarta pathways), performed with XGR software. The dashed line denotes the significance threshold (FDR < 0.05).
DISCUSSION
AD often manifests during infancy and persists in adulthood in many patients.38 Recent investigations of the molecular characteristics of AD in adults identified novel therapeutic targets, which transformed the clinical management of AD.22,39,40 Ideally, a better understanding of disease mechanisms will guide age-specific therapeutic development to improve clinical management across all ages, including pediatric populations. Proteomic studies in blood using the Olink proteomic platform, which requires only 10 μL of blood, have been instrumental in identifying biomarkers linked to disease severity and response to various treatments.41–46 We have previously described the blood proteomic profile of early-onset AD in infants.15 However, proteomic studies in the serum of children and adolescents with AD (5–18 years of age) are unavailable. Thus, we performed an analysis of serum from consecutive pediatric AD age groups (infants/toddlers aged 0–5 years, children aged 5–12 years, adolescents aged 12–17 years, and adults with AD aged ≥18years) relative to age-matched healthy controls. This approach allowed us to portray differences in proteomic expressions across age groups and to highlight specific AD signatures throughout life.
A robust Th2 (CCL13 and CCL17) upregulation was detected in all ages and intensified with increasing age. We also found differences in systemic immune polarization across age groups: Th17-related proteins showed the highest increases in infants (KYNU and PI3), while Th1-skewing was most prominent in adults (IL-1RN, CXCL16, and CXCL10), consistent with the fact that overexpression of Th1-related markers is associated with disease chronicity in long-standing AD.47 Correlations between circulating proteins and age revealed that the expression of Th17-related markers significantly decreased with age, potentially reflecting the great burden of infections in AD during early life.48,49
We also evaluated age-related changes that are associated with various biological processes and found a gradual increase in cardiovascular-related proteins. Of note, although adolescents with AD already show upregulation of many cardiovascular-related proteins, such as thrombomodulin/THBD, serine protease 27/PRSS27, CX3CL1, and FABP4, a pathway enrichment analysis shows significant upregulation in cardiovascular/atherosclerosis-associated pathways only in adults. These data suggest an increase in the systemic burden of AD with age and may support the need for early systemic treatment in patients with AD with moderate-to-severe disease. Tracking the cardiovascular burden should be a focus of future longitudinal studies in adolescents and younger children.
Using the Olink neurology/neuroinflammation panel, we also explored the potential changes in proteins associated with neural growth and development because previous studies linked altered neurotrophic factors with AD.50 For example, GFRA3 mediates hypersensitivity to warm sensation, mimicking warmth-provoked pruritus in AD,51 and key AD markers, such as IL-4 and IL-31, can induce the growth of sensory nerves in vitro.52–56 We found that only infants display enrichment of neural development pathways and associated proteins, such as GFRA3 and CADM3, along with unique upregulation of NCAN. Some of these markers have been linked to attention-deficit/hyperactivity disorder and autism,57–60 and further investigation is needed to establish their potential role in the higher risk of developing attention-deficit/hyperactivity disorder in younger populations with AD.61–64 Overall, these findings may be relevant to early disease initiation in AD, particularly in light of recent studies showing a link between sensory neurons, inflammatory pathways, and itch.65–67
This study has a few limitations. Although all age groups from infants to adults were represented in our large cohort, this is not a longitudinal study, and thus, our results reflect a cross-sectional observation at a single time point. In addition, our cohort included a majority of Caucasian participants, potentially limiting the generalizability of our results to other ethnicities.
In summary, our results portray a continuum of the inflammatory blood proteome of AD across ages, filling an important gap in understanding the systemic alterations in AD from infancy to adulthood. Our proteomic data show consistent and progressive Th2 activation across all age groups, as also suggested by the efficacy of Th2-targeting agents, such as dupilumab, in all ages.16,22,68–72 However, because less than 50% of patients achieve disease clearance with Th2-targeting alone across all age groups,73–75 these results potentially suggest the need for additional, age-specific, therapeutic approaches that will improve efficacy and may prevent disease chronicity and the development of associated comorbidities.
Supplementary Material
CAPSULE SUMMARY.
Patients with atopic dermatitis have systemic biomarker dysregulation that differs by age; however, the evolution of circulating proteins across consecutive atopic dermatitis age groups is still unknown.
The results from this study advocate for age-specific therapeutic approaches that can improve clinical outcomes in patients with atopic dermatitis of different ages.
Funding sources
Supported by a research grant from the LEO Foundation.
YRY was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through Rockefeller University, Grant #UL1TR001866. RL received partial salary support from a National Psoriasis Foundation fellowship grant. Tissue and blood collection was supported by Northwestern University Skin Biology and Diseases Resource-based Center, NIAMS #P30AR075049.
IRB approved status: Reviewed and approved by Mount Sinai Hospital, Northwestern University, and Lurie Children’s Hospital IRBs.
Abbreviations used
- AD
atopic dermatitis
- DEP
differentially expressed protein
- FCH
fold change
- FDR
false discovery rate
- IgE
immunoglobulin E
- IL
interleukin
- SCORAD
SCORing of Atopic Dermatitis
Footnotes
Conflicts of interest
Dr Guttman-Yassky is an employee of Mount Sinai; has received research funds (grants paid to the institution) from AbbVie, Celgene, Eli Lilly, Janssen, Medimmune/Astra Zeneca, Novartis, Pfizer, Regeneron, Vitae, Glenmark, Galderma, Asana, Innovaderm, Dermira, and UCB; and is a consultant for Sanofi Aventis, Regeneron, Stiefel/GlaxoSmithKline, MedImmune, Celgene, Anacor, AnaptysBio, Dermira, Galderma, Glenmark, Novartis, Pfizer, Vitae, Leo Pharma, AbbVie, Eli Lilly, Kyowa, Mitsubishi Tanabe, Asana Biosciences, and Promius. Dr Krueger has received research support (grants paid to his institution) and/or personal fees from Pfizer, Amgen, Janssen, Lilly, Merck, Novartis, Kadmon, Dermira, Boehringer, Innovaderm, Kyowa, BMS, Serono, BiogenIdec, Delenex, AbbVie, Sanofi, Baxter, Paraxel, Xenoport, and Kineta. Dr Paller has received research funds (grants paid to the institution) from AbbVie, AnaptysBio, Eli Lilly, Incyte, Janssen, KrystalBio, Regeneron, and UCB and is a consultant for AbbVie, Abeona, Acrotech, Almirall, Amgen, Amryt, Arcutis, Arena, Azitra, BioCryst, BiomX, Bridgebio, Bristol Myers Squibb, Castle Biosciences, Catawba, Eli Lilly, Exicure, Galderma, Kamari, Leo, Novan, Novartis, OM Pharma, Pfizer, Pierre Fabre, RAPT, Regeneron, Sanofi/Genzyme, Seanergy, and UCB. Dr Rangel has received research funds (grants paid to the institution) from AbbVie, DermTech, and Galderma. Drs Del Duca, Renert-Yuval, Pavel, Wu, Lefferdink, Fang, and Facheris and authors Mikhaylov, Sheth, Blumstein, and Estrada have no conflicts of interest to disclose.
REFERENCES
- 1.Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin 2017;35:283–289. [DOI] [PubMed] [Google Scholar]
- 2.Weidinger S, Novak N. Atopic dermatitis. Lancet 2016;387: 1109–1122. [DOI] [PubMed] [Google Scholar]
- 3.Lee HH, Patel KR, Singam V, Rastogi S, Silverberg JI. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J Am Acad Dermatol 2019;80:1526–1532.e7. [DOI] [PubMed] [Google Scholar]
- 4.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol 2011;131:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol 2014;150:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev-Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy 2015;70:836–845. [DOI] [PubMed] [Google Scholar]
- 7.Renert-Yuval Y, Del Duca E, Pavel AB, et al. The molecular features of normal and atopic dermatitis skin in infants, children, adolescents, and adults. J Allergy Clin Immunol 2021;148(1):148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czarnowicki T, He H, Canter T, et al. Evolution of pathologic T-cell subsets in patients with atopic dermatitis from infancy to adulthood. J Allergy Clin Immunol 2020;145:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen YMF, Egeberg A, Skov L, Thyssen JP. Comorbidities of atopic dermatitis: beyond rhinitis and asthma. Curr Dermatol Rep 2017;6:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riis JL, Vestergaard C, Hjuler KF, et al. Hospital-diagnosed atopic dermatitis and long-term risk of myocardial infarction: a population-based follow-up study. BMJ Open 2016;6:e011870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L, Leonard A, Pavel AB, et al. Age-specific changes in the molecular phenotype of patients with moderate-to-severe atopic dermatitis. J Allerg Clin Immunol 2019;144: 144–156. [DOI] [PubMed] [Google Scholar]
- 12.Cole C, Kroboth K, Schurch NJ, et al. Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. J Allerg Clin Immunol 2014;134:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He H, Li R, Choi S, et al. Increased cardiovascular and atherosclerosis markers in blood of older patients with atopic dermatitis. Ann Allergy Asthma Immunol 2020;124:70–78. [DOI] [PubMed] [Google Scholar]
- 14.Buske-Kirschbaum A, Schmitt J, Plessow F, Romanos M, Weidinger S, Roessner V. Psychoendocrine and psychoneuroimmunological mechanisms in the comorbidity of atopic eczema and attention deficit/hyperactivity disorder. Psychoneuroendocrinology 2013;38:12–23. [DOI] [PubMed] [Google Scholar]
- 15.Brunner PM, He H, Pavel AB, et al. The blood proteomic signature of early-onset pediatric atopic dermatitis shows systemic inflammation and is distinct from adult long-standing disease. J Am Acad Dermatol 2019;81:510–519. [DOI] [PubMed] [Google Scholar]
- 16.Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol 2020;83:1282–1293. [DOI] [PubMed] [Google Scholar]
- 17.Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol 2020;156:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carel K, Bratton DL, Miyazawa N, et al. The Atopic Dermatitis Quickscore (ADQ): validation of a new parent-administered atopic dermatitis scoring tool. Ann Allergy Asthma Immunol 2008;101:500–507. [DOI] [PubMed] [Google Scholar]
- 19.Brunner PM, Suárez-Fariñas M, He H, et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep 2017;7: 8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang H, Knezevic B, Burnham KL, Knight JC. XGR software for enhanced interpretation of genomic summary data, illustrated by application to immunological traits. Genome Med 2016;8: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suárez-Fariñas M, Ungar B, Correa da Rosa J, et al. RNA sequencing atopic dermatitis transcriptome profiling provides insights into novel disease mechanisms with potential therapeutic implications. J Allerg Clin Immunol 2015;135:1218–1227. [DOI] [PubMed] [Google Scholar]
- 22.Guttman-Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol 2019; 143:155–172. [DOI] [PubMed] [Google Scholar]
- 23.Sanyal RD, Pavel AB, Glickman J, et al. Atopic dermatitis in African American patients is TH2/TH22-skewed with TH1/TH17 attenuation. Ann Allergy Asthma Immunol 2019;122:99–110.e6. [DOI] [PubMed] [Google Scholar]
- 24.Samuelov L, Sprecher E, Paus R. The role of P-cadherin in skin biology and skin pathology: lessons from the hair follicle. Cell Tissue Res 2015;360:761–771. [DOI] [PubMed] [Google Scholar]
- 25.Bacchelli C, Moretti FA, Carmo M, et al. Mutations in linker for activation of T cells (LAT) lead to a novel form of severe combined immunodeficiency. J. Allergy Clin Immunol 2017; 139:634–642.e5. [DOI] [PubMed] [Google Scholar]
- 26.Pavel AB, Zhou L, Diaz A, et al. The proteomic skin profile of moderate-to-severe atopic dermatitis patients shows an inflammatory signature. J Am Acad Dermatol 2020;82:690–699. [DOI] [PubMed] [Google Scholar]
- 27.Harris SE, Cox SR, Bell S, et al. Neurology-related protein biomarkers are associated with cognitive ability and brain volume in older age. Nat Commun 2020;11:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverberg JI. Atopic disease and cardiovascular risk factors in US children. J Allergy Clin Immunol 2016;137:938–940.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab 2009;297:E271–E288. [DOI] [PubMed] [Google Scholar]
- 30.Wilsgaard T, Mathiesen EB, Patwardhan A, et al. Clinically significant novel biomarkers for prediction of first ever myocardial infarction: the Tromsø Study. Circ Cardiovasc Genet 2015;8:363–371. [DOI] [PubMed] [Google Scholar]
- 31.Eikendal ALM, Bots ML, Gohar A, et al. Circulating levels of P-selectin and E-selectin relate to cardiovascular magnetic resonance-derived aortic characteristics in young adults from the general population, a cross-sectional study. J Cardiovasc Magn Reson 2018;20:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blosa M, Sonntag M, Jäger C, et al. The extracellular matrix molecule brevican is an integral component of the machinery mediating fast synaptic transmission at the calyx of Held. J Physiol 2015;593:4341–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson BN, Hogg N, Svensson LM, et al. A new leukocyte hyperadhesion syndrome of delayed cord separation, skin infection, and nephrosis. Pediatrics 2014;133:e257–e262. [DOI] [PubMed] [Google Scholar]
- 34.Torbica T, Wicks K, Umehara T, et al. Chronic inflammation in response to injury: retention of myeloid cells in injured tissue is driven by myeloid cell intrinsic factors. J Invest Dermatol 2019;139:1583–1592. [DOI] [PubMed] [Google Scholar]
- 35.Kovacs B, Tillmann J, Freund LC, et al. Fcgamma receptor IIB controls skin inflammation in an active model of epidermolysis bullosa acquisita. Front Immunol 2019;10:3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glocker E, Ehl S, Grimbacher B. Common variable immunodeficiency in children. Curr Opin Pediatr 2007;19:685–692. [DOI] [PubMed] [Google Scholar]
- 37.Nousbeck J, Sarig O, Avidan N, et al. Insulin-like growth factor-binding protein 7 regulates keratinocyte proliferation, differentiation and apoptosis. J Invest Dermatol 2010;130:378–387. [DOI] [PubMed] [Google Scholar]
- 38.Lebwohl MG, Del Rosso JQ, Abramovits W, et al. Pathways to managing atopic dermatitis: consensus from the experts. J Clin Aesthet Dermatol 2013;6:S2–S18. [PMC free article] [PubMed] [Google Scholar]
- 39.Frazier W, Bhardwaj N. Atopic dermatitis: diagnosis and treatment. Am Fam Physician 2020;101:590–598. [PubMed] [Google Scholar]
- 40.Zhang L, Du D, Wang L, Guo L, Jiang X. Efficacy and safety of topical Janus kinase and phosphodiesterase inhibitor-4 inhibitors for the treatment of atopic dermatitis: a network meta-analysis. J Dermatol 2021;48:1877–1883. [DOI] [PubMed] [Google Scholar]
- 41.Navrazhina K, Garcet S, Frew JW, et al. The inflammatory proteome of hidradenitis suppurativa skin is more expansive than that of psoriasis vulgaris. J Am Acad Dermatol 2022;86(2): 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubin C, Glickman JW, Del Duca E, et al. Scalp and serum profiling of frontal fibrosing alopecia reveals scalp immune and fibrosis dysregulation with no systemic involvement. J Am Acad Dermatol 2022;86(3):551–562. [DOI] [PubMed] [Google Scholar]
- 43.He H, Olesen CM, Pavel AB, et al. Tape-strip proteomic profiling of atopic dermatitis on dupilumab identifies minimally invasive biomarkers. Front Immunol 2020;11:1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavel AB, Wu J, Renert-Yuval Y, et al. SARS-CoV-2 receptor ACE2 protein expression in serum is significantly associated with age. Allergy 2021;76:875–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glickman JW, Dubin C, Dahabreh D, et al. An integrated scalp and blood biomarker approach suggests the systemic nature of alopecia areata. Allergy 2021;76:3053–3065. [DOI] [PubMed] [Google Scholar]
- 46.He H, Del Duca E, Diaz A, et al. Mild atopic dermatitis lacks systemic inflammation and shows reduced nonlesional skin abnormalities. J Allergy Clin Immunol 2021;147:1369–1380. [DOI] [PubMed] [Google Scholar]
- 47.Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of TH2/TH22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allerg Clin Immunol 2012;130:1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ygberg S, Nilsson A. The developing immune systemdfrom foetus to toddler. Acta Paediatr 2012;101:120–127. [DOI] [PubMed] [Google Scholar]
- 49.Ochs HD, Oukka M, Torgerson TR. TH17 cells and regulatory T cells in primary immunodeficiency diseases. J Allergy Clin Immunol 2009;123:977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raap U, Goltz C, Deneka N, et al. Brain-derived neurotrophic factor is increased in atopic dermatitis and modulates eosinophil functions compared with that seen in nonatopic subjects. J Allergy Clin Immunol 2005;115:1268–1275. [DOI] [PubMed] [Google Scholar]
- 51.Murota H, Izumi M, Abd El-Latif MIA, et al. Artemin causes hypersensitivity to warm sensation, mimicking warmth-provoked pruritus in atopic dermatitis. J Allergy Clin Immunol 2012;130:671–682.e4. [DOI] [PubMed] [Google Scholar]
- 52.Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol 2016;138:500–508.e24. [DOI] [PubMed] [Google Scholar]
- 53.Aebischer I, Stämpfli MR, Miescher S, Horn M, Zürcher AW, Stadler BM. Neuropeptides accentuate interleukin-4 induced human immunoglobuline E synthesis in vitro. Exp Dermatol 1996;5:38–44. [DOI] [PubMed] [Google Scholar]
- 54.Quarcoo D, Fischer TC, Peckenschneider N, Groneberg DA, Welker P. High abundances of neurotrophin 3 in atopic dermatitis mast cell. J Occup Med Toxicol 2009;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salomon J, Baran E. The role of selected neuropeptides in pathogenesis of atopic dermatitis. J Eur Acad Dermatol Venereol 2008;22:223–228. [DOI] [PubMed] [Google Scholar]
- 56.Teresiak-Mikołajczak E, Czarnecka-Operacz M, Jenerowicz D, Silny W. Neurogenic markers of the inflammatory process in atopic dermatitis: relation to the severity and pruritus. Postepy Dermatol Alergol 2013;30:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ribasés M, Hervás A, Ramos-Quiroga JA, et al. Association study of 10 genes encoding neurotrophic factors and their receptors in adult and child attention-deficit/hyperactivity disorder. Biol Psychiatry 2008;63:935–945. [DOI] [PubMed] [Google Scholar]
- 58.Mercati O, Huguet G, Danckaert A, et al. CNTN6 mutations are risk factors for abnormal auditory sensory perception in autism spectrum disorders. Mol Psychiatry 2017;22:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kleijer KT. Neurobiological Properties of Two Autism-Risk Genes: Pten and Cntn5 Utrecht University; 2017. [Google Scholar]
- 60.Cross-Disorder Group of the Psychiatric Genomics Consortium. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 2019;179:1469–1482.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hou A, Silverberg JI. Predictors and age-dependent pattern of psychologic problems in childhood atopic dermatitis. Pediatr Dermatol 2021;38(3):606–612. [DOI] [PubMed] [Google Scholar]
- 62.Lee CY, Chen MH, Jeng MJ, et al. Longitudinal association between early atopic dermatitis and subsequent attention-deficit or autistic disorder: a population-based caseecontrol study. Medicine 2016;95:e5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wan J, Shin DB, Gelfand JM. Association between atopic dermatitis and learning disability in children. J Allergy Clin Immunol Pract 2020;8:2808–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen MH, Su TP, Chen YS, et al. Is atopy in early childhood a risk factor for ADHD and ASD? A longitudinal study. J Psychosom Res 2014;77:316–321. [DOI] [PubMed] [Google Scholar]
- 65.Wang F, Trier AM, Li F, et al. A basophil-neuronal axis promotes itch. Cell 2021;184:422–440.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dehner C, Chen L, Kim B, Rosman IS. Chronic itch of unknown origin is associated with an enhanced Th2 skin immune profile. Am J Dermatopathol 2021;43:773–775. [DOI] [PubMed] [Google Scholar]
- 67.Trier AM, Mack MR, Fredman A, et al. IL-33 signaling in sensory neurons promotes dry skin itch. J Allergy Clin Immunol 2022; 149(4):1473–1480.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol 2020;182:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol 2021; 184(5):857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Licari A, Castagnoli R, Marseglia A, et al. Dupilumab to treat type 2 inflammatory diseases in children and adolescents. Paediatr Drugs 2020;22:295–310. [DOI] [PubMed] [Google Scholar]
- 71.Muzumdar S, Zubkov M, Waldman R, DeWane ME, Wu R, Grant-Kels JM. Characterizing dupilumab facial redness in children and adolescents: a single-institution retrospective chart review. J Am Acad Dermatol 2020;83:1520–1521. [DOI] [PubMed] [Google Scholar]
- 72.Paller AS, Siegfried EC, Simpson EL, et al. A phase 2, open-label study of single-dose dupilumab in children aged 6 months to < 6 years with severe uncontrolled atopic dermatitis: pharmacokinetics, safety and efficacy. J Eur Acad Dermatol Venereol 2021;35(2):464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Faiz S, Giovannelli J, Podevin C, et al. Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life French multicenter adult cohort. J Am Acad Dermatol 2019;81:143–151. [DOI] [PubMed] [Google Scholar]
- 74.Fargnoli MC, Esposito M, Ferrucci S, et al. Real-life experience on effectiveness and safety of dupilumab in adult patients with moderate-to-severe atopic dermatitis. J Dermatolog Treat 2021;32(5):507–513. [DOI] [PubMed] [Google Scholar]
- 75.Wang C, Kraus CN, Patel KG, Ganesan AK, Grando SA. Real-world experience of dupilumab treatment for atopic dermatitis in adults: a retrospective analysis of patients’ records. Int J Dermatol 2020;59:253–256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


