Abstract
For many years it was believed that the aging process is inevitable and age-related diseases could not be prevented or reversed. The geroscience hypothesis, however, posits that aging is in fact malleable and, by targeting the hallmarks of biological aging, it is indeed possible to alleviate age-related diseases and dysfunction and longevity. This field of geroscience thus aims to prevent the development of multiple disorders with age, thereby extending health span, with contraction of morbidity toward the end of life. Experts in the field have made remarkable advancements in understanding the mechanisms underlying biological aging and identified ways to target aging pathways using both novel agents and repurposed therapies. While geroscience researchers currently face significant barriers to bringing therapies through clinical development, proof-of-concept studies, as well as early-stage clinical trials, are underway to assess the feasibility of drug evaluation and lay a regulatory foundation for future FDA approvals in the future.
Keywords: biological aging, health span, hallmarks of aging, geroscience, longevity
Graphical Abstract
For many years it was believed that the aging process is inevitable and age-related diseases could not be prevented or reversed. The geroscience hypothesis, however, posits that aging is in fact malleable and, by targeting the hallmarks of biological aging, it is indeed possible to increase median, if not maximum, longevity.
Introduction
With the advent of modern medicine, life expectancy has more than doubled since 1900.1 However, increased longevity comes with a price—the longer we live, the greater the risk of developing age-related diseases. In fact, aging is the strongest risk factor of multiple morbidities, ranging from cardiovascular disease to neurological conditions.2,3
The COVID-19 pandemic, which poses a greater risk for older people and resulted in disproportionate mortality in this population, has highlighted the importance of understanding health risks related to aging.4 Insights garnered from centenarians have shown that these individuals appear to be protected from declines in health related to normal aging.5 Thus, research into the mechanisms underlying normal aging, such as genetics and environment, can provide important insights into how to optimize physiological health.6
The goal of geroscience is to extend health span by delaying the onset of age-related diseases, or by extending health span.7 The field posits that biological aging is in fact modifiable, rather than a fixed process immune to intervention. Geroscience research has therefore focused on determining if biological processes can be targeted to delay aging and perhaps stop and even reverse its effects on health span (Fig. 1).
Figure 1.
Key geroscience targets. Adapted from Kennedy et al. https://doi.org/10.1016/j.cell.2014.10.039.
Several molecular pathways associated with aging are being investigated, including autophagy (proteostasis component), cellular senescence, the growth hormone/insulin-like growth factor (GH/IGF) axis, epigenetics, metabolomics, proteomics, DNA damage, and mitochondrial dysfunction. Novel agents targeting various mechanisms, such as mitochondrial function and epigenetics, are being evaluated for clinical use. In addition, a large body of work has indicated that several approved therapies such as metformin may be repurposed to treat specific age-related diseases and positively impact biological aging as a whole.8,9
This line of research, however, is not without challenges, as it is not widely accepted that biological aging can in fact be modified. The FDA currently does not recognize aging as an indication for drug approval, which poses a significant roadblock in getting compounds through the clinical development process.10 Proof-of concept studies are now underway to evaluate the feasibility of conducting interventional trials to assess novel or repurposed interventions. While preliminary findings are indeed promising, researchers must identify comprehensive clinical outcomes, including both biological and functional measures, and biomarkers, and go beyond standard disease assessments to determine if these agents have a significant impact on the hallmarks of aging.
On May 19, 2021, experts in geroscience met virtually at the New York Academy of Sciences symposium “Extending Human Health Span and Longevity,” organized by Stephanie Lederman, Glenda Greenwald, Orla Smith, Nir Barzilai, James Kirkland, and Judith Campisi, to discuss the molecular mechanisms that contribute to longevity and how those insights show that disease emergence can be prevented or reversed by repurposing or developing novel therapies that target these processes. This report summarizes the speakers’ presentations at the one-day symposium.
Age later: translational geroscience
Nir Barzilai of Albert Einstein College of Medicine opened the symposium with a keynote presentation that provided an overview of the geroscience field, as well as work from his laboratory. Barzilai discussed the importance of targeting the key hallmarks of biological aging to extend health span.
Genetic insights into aging have stemmed from data on centenarians who stay healthy 20–30 years longer than their counterparts, with only 30% experiencing disease at age 100.5,11
In collaboration with Zhengdong Zhang, Barzilai and his team used exome sequencing to identify links between single nucleotide polymorphisms (SNPs) and longevity. This network approach confirmed findings from animal studies that have identified pathways such as MAPK, insulin/IGF-1 signaling, and MTOR.12 [place holder for Barzilai Nature Aging, in press]
A study performed in collaboration with Sanish Sathyan used SOMAmer technology to identify proteins related to longevity expressed at older age.13 The network analysis revealed that potential biomarkers are associated with the breakdown of tissues including the extracellular matrix and platelet degradation.
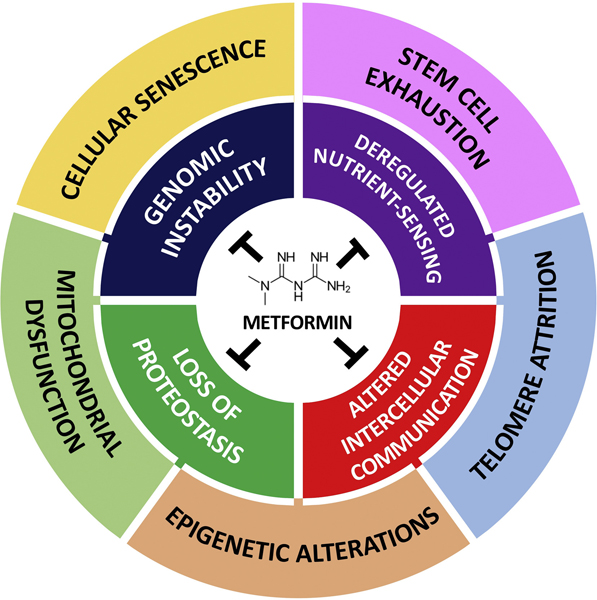
Clinical trials are currently evaluating agents that target multiple pathways. Metformin has been used for 70 years to treat type 2 diabetes mellitus (T2DM), and both interventional and association studies have shown that metformin attenuates aspects of of aging.9 (Fig. 2)
Figure 2.
Primary and secondary targets of metformin across the hallmarks of aging. From Kulkarni et al. https://doi.org/10.1016/j.cmet.2020.04.001.
Metformin has been associated with a lower mortality rate in T2DM patients, and non-diabetics taking metformin may experience a delay in cognitive decline.8 The TAME (Targeting Aging with Metformin) trial is designed to evaluate 3,000 people between the age of 65 and 80 years to determine the effect of metformin on the time to any major age-related disease incidence and evaluate its correlation with biomarkers. As metformin is very safe and affordable, results from this trial will reveal utility as a possible therapeutic.
Barzilai concluded by sharing a matrix developed by his lab ranking therapies for their repurposing potential. This analysis revealed several candidates, of which Barzilai highlighted canagliflozin and dapagliflozin as the ones he believes should be evaluated next.8,14,15
Targetable aging processes
Targeting selective autophagy in aging and age-related diseases
For the first session of the day, Ana Maria Cuervo of Albert Einstein College of Medicine discussed work that her laboratory has been pursuing on the role of selective autophagy in aging. Cuervo and her team employ several different models to study proteostasis to better understand protein quality control.
Cuervo’s research focuses on selective types of autophagy, such as chaperone-mediated autophagy (CMA), and how proteins are targeted and then degraded. The lysosome-associated membrane protein type 2A (LAMP-2A) plays a key role in allowing proteins to cross the lysosomal membrane for degradation. This pathway is a very favorable target, as its activity decreases with age.16
Using rodent models with a fluorescent reporter allowed visualization of CMA in multiple organs including the brain.17 This study, in collaboration with J.J. Bravo-Cordero (Mt. Sinai), revealed spatial-temporal changes on CMA under physiological conditions. Furthermore, by crossing the fluorescent reporter mouse with mouse models of age-related diseases, they recently demonstrated a cell-type specific decline in neuronal CMA in the brain of Alzheimer’s disease mice models.18 To understand the consequences of this decline of CMA in disease and aging, they experimentally blocked CMA in neurons and found that it leads to neurodegeneration.18 Neuronal CMA is required to maintain a metastable proteome, since blocking this pathway results in an increase in protein aggregation.18
Taking a network approach to create an index to measure autophagy, Cuervo and her team found that CMA decreases in neurons in brains from Alzheimer’s disease patients, and that the decrease in neuronal CMA activity levels correlate with disease state and changes in brain pathology in tissue samples from these patients.
Targeting autophagy, and specifically the CMA pathway, could thus lead to development of pharmaceuticals to reduce protein aggregation and preserve cellular function in neurological conditions and other age-related conditions. Cuervo’s team has initiated this line of work in collaboration with E. Gavathiotis (Albert Einstein) and found that chemical activation of CMA in a mouse model of Alzheimer’s preserves memory and reduces tau and β-amyloid protein accumulation and microglia activation.18
Also, on the same line, they have demonstrated that genetic preservation of CMA activity until late in life in mice prevents a decline in the regenerative capacity of hematopoietic stem cells that occurs with age, in this case, by restoring proper cellular energetics.19 Chemical activation of CMA in hematopoietic stem cells proved effective not only to prevent but also to restore repopulating capabilities of the cells both in mouse and human donors.19 Overall, these findings support the potential value of targeting selective autophagy to improve several of the cellular pathways associated with aging, such as proteostasis, metabolism, or stem cell function.
Senescent cells as a therapeutic target
Laura Niedernhofer of the University of Minnesota Medical School discussed the role of cellular senescence in driving the aging process. Niedernhofer and her team are particularly interested in studying endogenous DNA damage as a potent trigger of senescence.
Senescent cells are irreversibly arrested, very resistant to apoptosis, and secrete pro-inflammatory molecules.20 Immune system function declines with age, as does its ability to tag and remove senescent cells from the body. Selectively targeting p16Ink4a-positive senescent cells for clearance can improve median lifespan and overall health, demonstrating that senescence not only occurs with age but also drives aging.21–23
The extreme heterogeneity of secretory senescent cells has made characterization a significant challenge and thus has hindered development of senotherapeutics. However, existing drugs with senotherapeutic properties have been identified, including kinase inhibitors like dasatinib and other natural products with promiscuous mechanisms of action, which could affect multiple molecular systems.24
In collaboration with researchers at the University of Minnesota and the Mayo Clinic, Niedernhofer and her team applied findings from the COVID-19 pandemic in their study of community-acquired microbes. Exposing older pathogen-free lab mice to pet store mice results in full mortality within fourteen days, while young mice survive the exposure. Death of the old mice is driven by the mouse hepatitis virus (MHV), a betacoronavirus related to SARS-CoV-2, because inoculation of the mice with a sublethal dose of the virus prior to exposure to pet store mice ablated mortality. Treating aged mice with fisetin, a natural product with senolytic activity,25 reduced the mortality rate of old mice by half.26 Mortality rates were also partially rescued in transgenic models that clear p16-positive senescent cells or in old mice treated with the senolytic cocktail dasatinib plus quercetin, indicating a role for senescent cells in contributing to mortality of old mice upon infection with a viral pathogen. Evidence was provided that senescent cells over-react to pathogen-associated molecular patterns, leading to increased inflammation, cytokine storm, and multiorgan failure. This study strongly supports the geroscience hypothesis, revealing that aging biology contributes to the loss of resilience and vulnerability to disease in old organisms and can be therapeutically targeted to restore resilience.
Niedernhofer’s team recently utilized a genetic model of a tissue-specific knockout of a DNA repair molecule to increase senescence selectively in immune cells. This resulted in increased systemic spread of senescent cells, organ damage, and reduced lifespan of mice.27 This line of work demonstrates that senescent immune cells play a key role in driving age-related accumulation of senescent cells, suggesting that senescent immune cells could be a favorable target of senotherapeutics.
Biomarkers and omics for therapies
Translational geroscience: role of IGF-1 in human health span and lifespan
For the second session of the day, Sofiya Milman of Albert Einstein College of Medicine presented findings from recent work from her laboratory on the GH/IGF-1 pathway and its contribution to health span. Reduced GH/IGF-1 action is associated with extension in health span and lifespan across multiple species, including humans, suggesting a conserved mechanism for longevity.28–32
Growth hormone, which is secreted by the pituitary gland, binds the GH receptor, and stimulates transcription of IGFs, which promote growth and development via action at the IGF-1 receptor; its highly regulated activity requires cleavage of its binding proteins by proteases.33 IGF-1 peaks during the second decade of life but falls quickly and declines with aging; its age-related downward trajectory raises the possibility that it may be an aging-promoting candidate. Yet, inhibiting this pathway can extend lifespan, even if the pathway is blocked later in life.34 This time course effect is very favorable since IGF-1 is important for growth early in life.
Using serum IGF-1 levels as a surrogate for growth hormone, Milman and her team found a two-fold increase in the survival of females with low IGF-1 levels.32 This population was also approximately 50% less likely to be cognitively impaired compared to those with higher levels.35 Centenarians that carry an IGF-1 mutation have reduced receptor levels and lower signaling activity, indicating a genetic contribution to the effect of IGF-1 on lifespan.36 Recent work by Milman’s team revealed that low IGF-1 levels delay cognitive impairment, multi-morbidities, and all-cause mortality in older adults.37
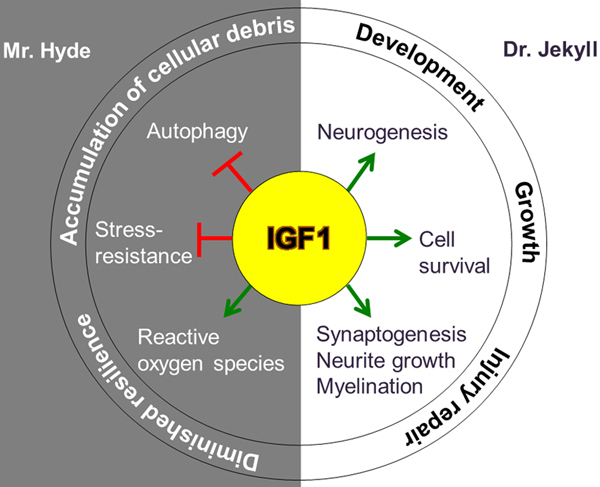
While there are inconsistencies in the literature regarding IGF-1 and its link to aging, Milman pointed out that the NHANES study, which did not show significant effects, included people over 18 years old and did not separate results by age.37–40 Using samples from the UK Biobank of nearly 450,000 people, Milman’s group found that lower IGF-1 levels in younger people were associated with an increased risk of mortality and age-related diseases compared to older adults.41 These data suggest an interaction between age and IGF-1, where higher levels of IGF-1 appear to be protective for younger adults but not for older adults. Milman contributes these effects to antagonistic pleiotropy of IGF-1, which promotes development and growth in youth but antagonizes proteostasis and other cell maintenance mechanisms at older age.42 (Fig. 3)
Figure 3.
Antagonistic pleiotropy of IGF-1. From Gubbi et al. https://doi.org/10.1530/JME-18-0093.
Epigenetic biomarker of aging for lifespan and health span
Morgan Levine of Yale School of Medicine shared research that her laboratory is pursuing to better understand epigenetic changes that occur across aging. Levine takes a biological systems approach to look at how aging at higher levels of biological organization emerge from changes at lower levels.
Epigenetic aging reflects the operating system of the cell, and epigenetic signatures affects cell identity across aging. Her lab has developed epigenetic clocks to compare biological to chronological age using DNA methylation and supervised machine learning.43 Analysis of data from the landmark Framingham heart study showed that higher epigenetic age in the blood was associated with increased mortality risk and other age-related outcomes after adjusting for chronological age.44 In collaboration with colleagues at Yale, Levine also demonstrated that age patterns were accelerated in normal tissue of breast cancer patients relative to healthy controls, which may also be the case in the liver of nonalcoholic fatty liver disease (NAFLD) patients compared to controls.45,46
Highly proliferative tissue shows greater biological aging, which affects timing of aging in each organ. Levine’s team established a method to statistically improve reliability in a new epigenetic clock model, as original clocks developed to measure biological age are not reliable. Her team found that accelerated epigenetic aging affects cellular and molecular hallmarks of aging, including senescence and mitochondrial decline.47
Levine has also demonstrated that caloric restriction in rats slows epigenetic aging over time.43 In vitro studies demonstrated the ability to reverse epigenetic aging in mouse fibroblasts using epigenetic reprogramming, demonstrating that this clock is modifiable.48 In collaboration with Yuancheng Lu and David Sinclair, her team confirmed these data in vivo using an injury and aging model.
Metabolomics in the search for biomarkers and mechanisms of aging
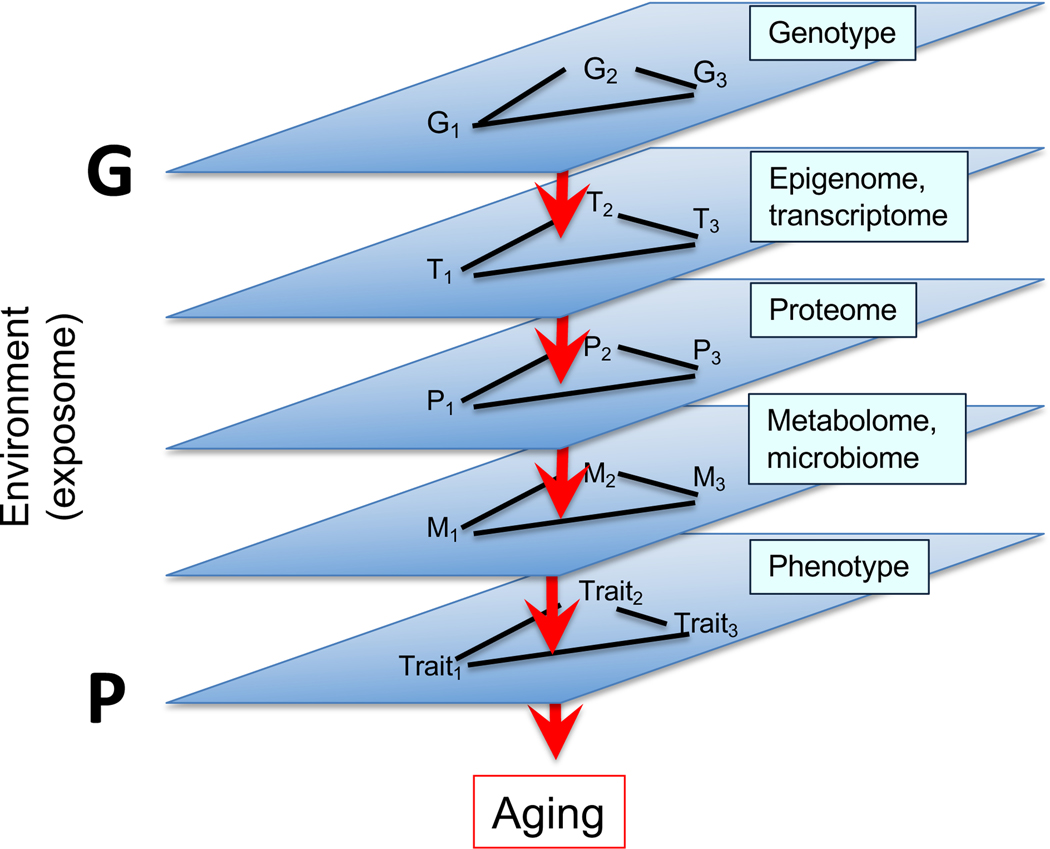
Daniel Promislow of the University of Washington presented research from his laboratory on the role of genetic variation in lifespan. It is challenging to identify the link between genetic polymorphisms and longevity, as most polymorphisms explain less than 0.5% in variation.49,50 While complex traits are typically influenced by large numbers of genetic variants, the path is extremely complex leading from genotype, through multiple domains of complex networks, to downstream phenotype (Fig. 4). In this light, the goal of Promislow’s research is to bridge the gap between genotype and phenotype by focusing on the metabolome.
Figure 4.
Distance between genotypic and phenotypic relationships.
The metabolome comprises small molecules that make up the building blocks of all organismal features, from cell membranes to metabolic cycles, to genes and proteins. Studies in Drosophila have shown that the metabolome is a predictive biomarker of genotypes under stressful situations.51 In a study of diet restriction in Drosophila, Promislow and colleagues showed that the treatment not only made flies live longer, but also reversed age-related changes in the metabolome.52 In a follow-up study of the effects of dietary restriction across almost 200 inbred strains of flies, Promislow and colleagues used metabolomic profiles to successfully predict whether diet restriction would increase or decrease lifespan.53 Moreover, while a direct search for genetic variants associated with dietary restriction did not identify significant genes, a network model that included metabolites linking genes to longevity highlighted several genes that modify the diet restriction response.53
Research in marmosets has suggested that the metabolome may be a gender-specific biomarker of mortality risk.54 Analysis of metabolic profiles of over 44,000 people identified several metabolites that are highly predictive of 5-year survival, underscoring the power of metabolome profiles as a powerful biomarker of aging, with the potential to point to specific causal pathways.55
Promislow noted the importance of conducting longitudinal studies to identify biomarkers of biological aging, as recent theoretical models suggest that biomarkers from cross-sectional studies will be biased toward the discovery of biomarkers that are not causal with respect to aging.56 His team is now pursuing a nationwide, open-data study of aging in companion dogs to identify the biological and environmental determinants of healthy versus nonhealthy aging.57
Translational potential of the biology of aging
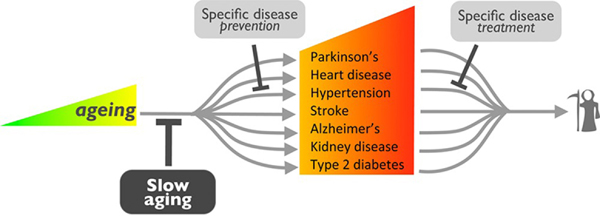
Luigi Ferrucci of the National Institute on Aging at the National Institutes of Health discussed different mechanisms that may be therapeutically targeted to slow biological aging. While common practice is to treat each age-related disease one-by-one, Ferrucci discussed an alternative strategy to target the hallmarks of biological aging to prevent these diseases from developing.58,59 (Fig. 5) Data from animal models support this approach, as existing therapies like rapamycin can increase longevity, as can many other commonly used drugs like aspirin.60–62
Figure 5.
Preventing multimorbidities across aging.
The rate of biological aging correlates with the speed of damage accumulation at the level of macromolecular, organelle, and cellular levesl, and the capacity of the body to repair this damage. Ferrucci focuses on ATP, which is tremendously affected by this damage, as these mechanisms all require an enormous amount of ATP to sustain mitochondrial function, which declines dramatically with aging.63
A skeletal muscle proteomics analysis identified a decline in mitochondrial function in muscle across the age spectrum; however, there were differential effects in protein regulation in older adults based on activity levels, which are related to declines in VEGFRa-156a/VEGFa-156b, a pathway that regulates blood vessel growth.64,65
Ferrucci closed by summarizing the aims of geroscience translational research, which include minimizing damage and enhancing resilience, tracking effectiveness, and evaluating both middle- and long-term outcomes.
Translational research at the forefront of health and lifespans
Targeting immunity: what is it about immune aging that makes older adults so vulnerable to COVID-19?
For the second session of the day, George Kuchel of the University of Connecticut presented work from his group and others on the role of immunity in the aging process and, specifically, how immune aging makes older adults vulnerable to COVID-19. His team takes a geroscience-guided approach in their research that encompasses the complexity and heterogeneity of aging, integrating both reductionist and system-based perspectives.
Older adults aged 65–74 years have a 90-fold greater risk of dying from COVID-19 compared to younger adults (18–29 years), and older adults aged 85 years or more have a 630-fold higher risk. Analysis of 2,000 COVID-19 patients in Sao Paolo revealed that higher frailty levels also correlate with lower survival probability.66
Relatively high mortality rates due to infection with pneumonia, flu, or COVID-19 have illustrated the vulnerability of the aging population to pathogens. Men over 65 years old have higher innate immune activity and inflammation but less adaptive T and B cell activity compared to women, suggesting that primary response and immune memory are diminished in older adults.67,68 In addition, CD4+ and CD8+ cells exhibit less coordination in older compared to younger adults, reflecting immune dysregulation that confers a higher risk from COVID-19–related mortality.69
The ability to clear infection with SARS-CoV-2 in nasal and airway epithelium tissues declines with age, and data in macaques showed that older animals, but not younger animals, lose up to 10% of their weight following infection, and younger but not older animals could clear CD11b+ and CD8+ cells, reflecting what is seen in aged mice infected with influenza virus.70,71 Younger animals also experience viral-induced fever while older animals do not, mirroring symptoms experienced by older COVID-19 patients living in nursing homes. Kuchel therefore recommended that a lower temperature threshold should be set when evaluating COVID-19 immune response in older adults.
Kuchel proposed that vaccines and interventions targeting biological aging could be used to reduce the risk of age-related chronic diseases. He summarized by emphasizing that the specific nature of immune and biological deficits must still be defined to better understand immune resilience – the capacity of the immune system to maintain or rapidly return to normal function when confronted by stressors such as a novel pathogen (e.g., SARS-CoV-2).72
Developing novel therapies targeting the biology of aging
Joan Mannick of Life Biosciences discussed three platforms that the company is pursuing to target conditions linked to biological aging. The first platform is focused on mitochondrial uncouplers, which allow hydrogen ions to leak into the cellular matrix independently of ATP production. These agents enable the body to burn more calories to generate same amount of ATP, resulting in increased metabolism and longer lifespan.
Life Biosciences is developing a mitochondrial uncoupler compound called BAM15 that reduces fat mass accumulation in mice fed a high-fat Western diet but does not affect muscle mass.73 BAM15 was also shown to improve cellular metabolic parameters, including glucose tolerance and lipid profiles. Uncoupler therapies like BAM15 could be particularly advantageous to address the global obesity epidemic and related diseases, such as nonalcoholic steatohepatitis (NASH).
The second platform she discussed is chaperone-mediated autophagy, which is aimed at removing cellular waste accumulation to treat neurodegenerative diseases. Ana Maria Cuervo and her team evaluated their CMA activator compound in a mutant mouse model (PS19) that mimics frontotemporal dementia.18,74 They found that the typical accumulation of insoluble tau protein across different brain regions, including the hippocampus, ventral amygdala, and piriform cortex, was markedly reduced upon treatment with the CMA activator; treatment also reduced tau aggregates and improved visual memory in the PS19 mice, suggesting potential benefits for Alzheimer’s disease.18
Life Biosciences is also targeting CMA in hematopoietic stem cells building on the work mentioned in the previous section from Ana Maria Cuervo’s group, where they found that treatment with a CMA activator restored the function of aged stem cells and increased blood cell production by hematopoietic stem cells in older mice.17 They are now investigating whether this agent can be used to improve stem cell function in older adults.
Mannick also discussed research that Life Biosciences is conducting in collaboration with David Sinclair. Their proprietary gene therapy, OSK, which expresses 3 Yamanaka factors, reprograms the epigenome to a younger state; initial findings have shown that this approach is safe after 16 months of treatment in mice. Sinclair also demonstrated that intravitreal OSK administration could reverse aging and injury-associated changes in ganglion cells. This therapy also accelerated regeneration following injury to the optic nerve and improved visual function in a mouse model of glaucoma.75
Development of clinical trials to extend healthy lifespan
Jamie Justice of Wake Forest School of Medicine discussed the challenges facing the field of geroscience with regard to getting new compounds or repurposed therapies through the clinical trial pipeline. Geroscientists like Justice are working on designing trials tailored to assess senotherapeutics, as many have shown promise for treating specific conditions like diabetic kidney disease and Alzheimer’s disease.76–78
Cellular senescence may contribute to the development of idiopathic pulmonary fibrosis (IPF), a disease usually diagnosed in older adults. Based on pre-clinical research suggesting that senolytics might be effective in treating lung injury, Justice and Nambiar and their colleagues conducted a proof-of-concept single-arm, open-label, three-week study to determine the feasibility and tolerability of dasatinib and quercetin in IPF patients.79 They found improvements in mobility but no effect on other disease-specific outcomes, possibly due to the short-term treatment.79
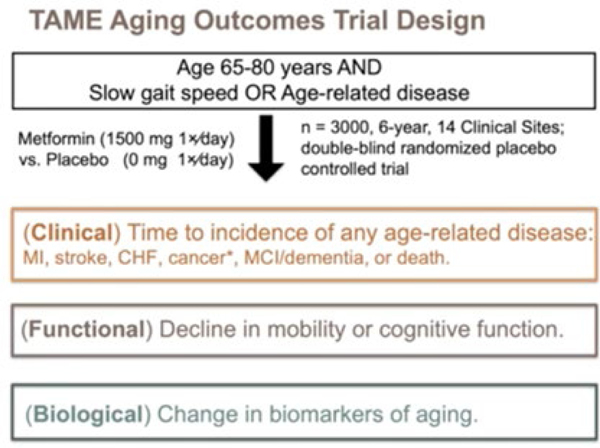
Justice is also working on the TAME trial, a six-year double blind, randomized, placebo-controlled, multicenter study that will evaluate the effects of metformin on 3,000 nondiabetic older adults aged 65–80 years. The outcomes of the TAME trial will include clinical measures related to age-related diseases, and will assess function, biomarkers, and patient-reported outcomes. This trial is the first to study aging outcomes, and the goal is to create a regulatory framework that future therapies can follow to achieve FDA approval (Fig. 6). Estimated annual event rates from the LIFE and Health ABC studies were used to calculate the annual rates they might expect to see in trials with aging outcomes, like the incidence of new major chronic disease or multimorbidity. These event rate data provide support that aging outcomes trials may be feasible to conduct within 4–6 years when simple trial eligibility criteria such as older age and slow gait speed are used.
Figure 6.
TAME trial design.
Justice emphasized the importance of identifying and adding health span–relevant outcome measures that encompass additional factors that affect biological aging rather than focusing on disease-specific outcomes alone. She also noted the importance of identifying biomarkers that reflect the interaction between the underlying biology of aging and the change in clinical disease and functional endpoints, but that successful trials with clinical “aging” indicators are needed to validate and qualify biomarkers for use as surrogate endpoints.80
Justice summarized by reiterating that trials testing geroscience-guided interventions are needed to evaluate therapeutic effects in specific age-related diseases and broadly on aging outcomes, and that because aging is multifactorial, trial endpoints should reflect this by including aggregate endpoints to measure change in a collection of diseases, conditions, functional measures, and biomarkers.
Senolytics and Alzheimer’s disease
Mitzi M. Gonzales of the University of Texas Health Sciences Center San Antonio discussed work in her laboratory aimed at evaluating the potential of senolytics to treat Alzheimer’s disease (NCT04063124; NCT04685590).77 Although numerous small nucleotide polymorphisms have been associated with increased risk of developing Alzheimer’s disease, they each contribute to only a small percentage of this risk.81 Therefore, the mechanisms underlying Alzheimer’s appear to be multifactorial.82
Cellular senescence is a key mechanism that underlies normal aging and can contribute to the development Alzheimer’s disease, as senescence affects multiple cell types in the brain.83 Senescent cells develop a sensenscence-associated secretory phenotype (SASP), which can be toxic to neighboring cells and allow senescence to propagate within tissues.84,85 Gonzales’ colleagues and other investigators have focused on senescence as a way to target neuroinflammation.86,87
Analyses of post-mortem brain tissue of individuals with Alzheimer’s disease revealed elevated senescence markers in neurofibrillary tangles; these results were confirmed in tau transgenic mice.88 Twelve weeks of intermittent treatment with dasatinib and quercetin in this animal model led to a 35% reduction in tau expression and increased tau clearance. MRI data confirmed these results, showing that senolytic treatment reduced atrophy and white matter pathology and improved cerebral blood flow.
Gonzales and her team are currently evaluating this treatment in the STOMP-AD trial (NCT04063124).77 The open-label, proof-of-concept study will assess 12-week intermittent treatment in individuals with early-stage Alzheimer’s disease. All participants will undergo safety evaluations every two weeks at the clinic. Preliminary data from five patients enrolled in the study indicate a favorable tolerability profile.
The primary goal of the study is to determine the degree of CNS drug penetration in the CSF. Additional outcomes include assessing senescence markers in the blood, evaluating cognitive changes, and using neuroimaging and biomarkers to determine if the treatment affects the pathogenesis of Alzheimer’s.
Senolytics: the path to translation
James Kirkland of the Mayo Clinic discussed work that he and his team are pursuing regarding the effects of different senolytics on network pathways, as the fundamental mechanisms of aging appear to be highly interlinked. Kirkland is also interested in whether combining senolytic therapies with other drugs may provide synergistic effects.
Since some senescent cells mirror some types of cancer cells, pro-survival networks likely support their resistance to apoptosis. To pursue this hypothesis, Kirkland utilized a bioinformatics-based approach to identify several anti-apoptotic regulator pathways in senescent cells, many of which are redundant.89
Anti-apoptotic regulators reduce viability in senescent preadipocytes and endothelial cells through different pathways.90 Dasatinib specifically affected senescent preadipocytes while quercetin affected endothelial cells. Although some other senescent cell types were not responsive to one of these drugs, they did respond to the combination of both. Oral dosing of this combination in mice with transplanted senescent cells successfully killed cells with SASP.91 Senolytic treatment in human adipose tissue from obese diabetic individuals also killed cells with a SASP within 18 hours of exposure.
Dasatinib and quercetin treatment over the course of seven mouths reversed impairments in mobility caused by leg radiation.90 Similar effects were seen in younger mice in which senescent cells had been transplanted; these results were also confirmed in older adult mice treated with senolytics.91
Kirkland summarized by discussing the Translational Geroscience Network, composed of several scientists from various institutions, which is aimed at clinically evaluating multiple senolytics to target serious conditions lacking sufficient interventions. Early data show that dasatinib and quercetin cleared senescent cells and reduced macrophage infiltration and fibrosis in adipose tissue from patients with diabetes. However, Kirkland cautioned that people should not take any of these therapies to extend lifespan or treat certain conditions, as their full safety profiles have not yet been elucidated.
Mitochondrial-derived peptides (MDPs) and the regulation of aging processes
Pinchas Cohen of the USC Leonard Davis School of Gerontology closed the day’s discussion with a final keynote presentation. Cohen’s research focuses on mitochondrial dysfunction, one of the hallmarks of aging that contributes to the pathogenesis of age-related diseases such as Alzheimer’s disease.
Microproteins are bioactive peptides within an open reading frame in the mitochondrial genome of under 100 codons, of which eight have been published so far.92,93 These peptides are part of growing class of microproteins identified from nuclear small ORFs.94,95 Cohen and his colleagues have demonstrated that humanin, one of these microproteins, exerts protective effects on the heart, brain, and liver.96,97
Humanin is transcribed from mitochondrial DNA and is expressed after translation on cytoplasmic ribosomes when its mRNA is carried out of mitochondria to the cytoplasm to be translated by mRNA binding proteins. Humanin exerts its biological effects via the JAK–STAT and ERK3 pathways.98
Using mitochondrial-derived peptide (MDP) assays against humanin, Cohen and his colleagues found that mitochondrial proteins are age-dependent and suppressed by growth hormone and IGF-1.99 They also found that humanin levels are correlated with endothelial function and AD status, indicating that humanin could potentially serve as a biomarker of mitochondrial function.100–102
Data from animal studies have shown that sustained humanin levels are positively linked to longevity;101 these findings are mirrored in data from centenarians and their offspring, who have higher levels of humanin.103 Cohen and his colleague have identified an MDP called SHLP2, which predicts prostate cancer risk.104–108
Their group also takes a population genetic approach using Mitochondrial Wide Association Studies, or MiWAS, to look at genetic relationships in single ethnic groups. The advantage of using MiWAS is that it allows for more comprehensive analysis using a small sample size rather than looking at the whole genome. Cohen’s team revealed that a D-loop SNP results in a five-fold greater risk of cataracts in Hispanic people.109,110 In addition, they identified a SNP in the humanin gene, which lowers humanin levels, that is associated with a higher risk of cognitive decline in African Americans.92
Based on data from his lab and others, Cohen has proposed that mitochondria might be a site of action that mimics the effects of diet and exercise on aging via production of peptides like MOTS-c, which promotes metabolic homeostasis and reduces obesity and insulin resistance.111–113 MOTS-c markedly reduced fat accumulation in mice after given a high-fat diet. These findings led to initiation of a phase I trials of MOTS-c for diabetes, obesity, and NASH.
A novel SNP in MOTS-c called K14Q, leading to the expression of a bioinactive form of MOTS-c and resulting in increased MOTS-c levels, has been linked to a higher risk of diabetes in Japanese males.114 Although this risk is nearly doubled in sedentary men, it can be overcome through daily exercise. Since SNP carriers have higher levels of abdominal fat, a therapy targeting MOTS-c could benefit those with this mutation, as MOTS-c treatment also leads to improved exercise capacity, increased muscle mass, and extended lifespan in mice.115,116
Cohen summarized by highlighting the promise of MDPs as a novel class of genes that may represent significant health span regulators, as well as potential therapeutics to address age-related diseases in specific ethnic populations.
Conclusions
The remarkable progress made recently in aging research has been born from the realization that aging is likely a malleable process, and that it affects virtually every tissue and organ. This realization is the basis for the geroscience hypothesis. Geroscience has, in turn, spawned a plethora of academic and industrial endeavors to develop interventions that can ameliorate, postpone, or even reverse age-related phenotypes and pathologies. These interventions currently hold significant promise for extending the health span (years of healthy life) of human populations.
In-depth study of the interactions among underlying mechanisms of aging are needed to answer the following:
Is there a hierarchical relation among these mechanisms?
Are there organ/cell-type differences in the interaction among these mechanisms?
Would it be possible to reach a synergistic effect through combinatorial interventions targeting several of the process that drive aging?
Additional research is also required to understand the status of these drivers of aging in the human population to determine the mechanistic “signatures” for successful aging, as well as to improve experimental models to include aging (in single disease models) and co-morbidities to understand how they affect the drivers of aging.
Acknowledgments
Work in the Cuervo laboratory is supported by NIH/NIA Grants AG021904, AG038072, and AG031782, and the generous support of the JPB Foundation, and the Rainwater Charitable Foundation. Laura J. Niedernhofer is supported by R01 AG063543, R01 AG063543-03S1, P01 AG043376, and U19 AG056278. Sofiya Milman is supported by NIH/NIA K23AG051148, R01 AG061165, and the American Federation of Aging Research. Pinchas Cohen is supported by NIH Grants R01 AG069698, RF1 AG061834, R01 AG068405, P30 AG068345, and P01 AG055369. Nir Barzilai is supported by the Nathan Shock Centers of Excellence in the Basic Biology of Aging (P30 AG038072) and the Paul G. Glenn Center for the Biology of Human Aging.
Footnotes
Competing interests
A.M.C. is co-funder of Selphagy, a program now under Life Biosciences LLC (MA), and consults for Generian Pharmaceuticals, Inc. and Cognition Therapeutics, Inc.
L.J.N. is a founder of NRTK Biosciences.
J.C. is a scientific founder and shareholder of Unity Biotechnology, which is developing senolytics to treat age-related diseases.
Patents on senolytic drugs and their uses are held by Mayo Clinic and the University of Minnesota; this research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
References
- 1.Crimmins EM. Lifespan and health span: past, present, and promise. Gerontologist. 2015;55(6):901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller RA. Extending life: Scientific prospects and political obstacles. Milbank Q. 2002;80(1):155–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitty CJM, Watt FM. Map clusters of diseases to tackle multimorbidity. Nature. 2020;579(7800):494–496. [DOI] [PubMed] [Google Scholar]
- 4.Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging (Albany NY). 2020;12(10):9959–9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ismail K, et al. Compression of morbidity is observed across cohorts with exceptional longevity. J Am Geriatr Soc. 2016;64(8):1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sierra F. The emergence of geroscience as an interdisciplinary approach to the enhancement of health span and life span. Cold Spring Harb Perspect Med. 2016;6(4):a025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23(6):1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulkarni AS, Gubbi S, Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020;32(1):15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall SS. “Feature: The man who wants to beat back aging.” Science Magazine. 2015. Available at: https://www.sciencemag.org/news/2015/09/feature-man-who-wants-beat-back-aging [Google Scholar]
- 11.Atzmon G, Rincon M, Schechter CB, et al. Lipoprotein genotype and conserved pathway for exceptional longevity in humans. PLoS Biol. 2006;4(4):e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.[place holder for Barzilai Nature Aging, in press; ] [Google Scholar]
- 13.Sathyan S, Ayers E, Gao T, et al. Plasma proteomic profile of age, health span, and all-cause mortality in older adults. Aging Cell. 2020;19(11):e13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller RA, Harrison DE, Allison DB, et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight. 2020;5(21):e140019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. [DOI] [PubMed] [Google Scholar]
- 16.Kaushik S, Cuervo AM. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 2018;19(6):365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong S, Aguirre-Hernandez C, Scrivo A, et al. Monitoring spatiotemporal changes in chaperone-mediated autophagy in vivo. Nat Commun. 2020;11(1):645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourdenx M, Martín-Segura A, Scrivo A, et al. Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome. Cell. 2021;184(10):2696–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong S, Wang Q, Kao YR, et al. Chaperone-mediated autophagy sustains haematopoietic stem-cell function. Nature. 2021;591(7848):117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbins PD, Jurk D, Khosla S, et al. Senolytic drugs: reducing senescent cell viability to extend health span. Annu Rev Pharmacol Toxicol. 2021;61:779–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burd CE, Sorrentino JA, Clark KS, et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell. 2013;152(1–2):340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker DJ, Childs BG, Durik M, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasanna PG, Citrin DE, Hildesheim J, et al. Therapy-induced senescence: opportunities to improve anti-cancer therapy. J Natl Cancer Inst. 2021:djab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camell CD, Yousefzadeh MJ, Zhu Y, et al. Senolytics reduce coronavirus-related mortality in old mice. Science. 2021:eabe4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yousefzadeh MJ, Flores RR, Zhu Y, et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594(7861):100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366(6454):461–4. [DOI] [PubMed] [Google Scholar]
- 29.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997; 277(5328):942–6. [DOI] [PubMed] [Google Scholar]
- 30.Clancy DJ, Gems D, Harshman LG, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 200;292(5514):104–6. [DOI] [PubMed] [Google Scholar]
- 31.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. [DOI] [PubMed] [Google Scholar]
- 32.Milman S, Atzmon G, Huffman DM, et al. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. 2014;13(4):769–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Argente J, Chowen JA, Pérez-Jurado LA, Frystyk J, Oxvig C. One level up: abnormal proteolytic regulation of IGF activity plays a role in human pathophysiology. EMBO Mol Med. 2017;9(10):1338–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao K, Quipildor GF, Tabrizian T, et al. Late-life targeting of the IGF-1 receptor improves health span and lifespan in female mice. Nat Commun. 2018;9(1):2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perice L, Barzilai N, Verghese J, et al. Lower circulating insulin-like growth factor-I is associated with better cognition in females with exceptional longevity without compromise to muscle mass and function. Aging (Albany NY). 2016;8(10):2414–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suh Y, Atzmon G, Cho MO, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA. 2008;105(9):3438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang WB, Aleksic S, Gao T, et al. Insulin-like growth factor-1 and IGF binding proteins predict all-cause mortality and morbidity in older adults. Cells. 2020;9(6):1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saydah S, Graubard B, Ballard-Barbash R, Berrigan D. Insulin-like growth factors and subsequent risk of mortality in the United States. Am J Epidemiol. 2007;166(5):518–26. [DOI] [PubMed] [Google Scholar]
- 39.Burgers AM, Biermasz NR, Schoones JW, et al. Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab. 2011;96(9):2912–20. [DOI] [PubMed] [Google Scholar]
- 40.Svensson J, Carlzon D, Petzold M, et al. Both low and high serum IGF-I levels associate with cancer mortality in older men. J Clin Endocrinol Metab. 2012;97(12):4623–30. [DOI] [PubMed] [Google Scholar]
- 41.Zhang WB, Ye K, Barzilai N, Milman S. The antagonistic pleiotropy of insulin-like growth factor 1. Aging Cell. 2021, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 43.Levine M, McDevitt RA, Meer M, et al. A rat epigenetic clock recapitulates phenotypic aging and co-localizes with heterochromatin. Elife. 2020;9:e59201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and health span. Aging (Albany NY). 2018;10(4):573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hofstatter E, Levine M, Liu Z, O’Meara T, Dalela D, Pusztai L. Abstract P2–09-02: Evidence of accelerated epigenetic aging of breast tissues in patients with breast cancer compared to women without cancer. Cancer Res. 2020;80:4 Supplement. P2–09-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyun J, Jung Y. DNA Methylation in nonalcoholic fatty liver disease. Int J Mol Sci. 2020;21(21):8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z, Leung D, Thrush K, et al. Underlying features of epigenetic aging clocks in vivo and in vitro. Aging Cell. 2020;19(10):e13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levine ME, Leung D, Minteer C, Gonzalez J. A DNA methylation fingerprint of cellular senescence. bioRxiv. 2019:674580. [Google Scholar]
- 49.Park JH, Wacholder S, Gail MH, et al. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet. 2010;42(7):570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deelen J, Beekman M, Uh HW, et al. Genome-wide association meta-analysis of human longevity identifies a novel locus conferring survival beyond 90 years of age. Hum Mol Genet. 2014;23(16):4420–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison BR, Wang L, Gajda E, et al. The metabolome as a link in the genotype-phenotype map for peroxide resistance in the fruit fly, Drosophila melanogaster. BMC Genomics. 2020;21(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laye MJ, Tran V, Jones DP, Kapahi P, Promislow DE. The effects of age and dietary restriction on the tissue-specific metabolome of Drosophila. Aging Cell. 2015;14(5):797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin K, Wilson KA, Beck JN, et al. Genetic and metabolomic architecture of variation in diet restriction-mediated lifespan extension in Drosophila. PLoS Genet. 2020;16(7):e1008835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffman JM, Ross C, Tran V, Promislow DEL, Tardif S, Jones DP. The metabolome as a biomarker of mortality risk in the common marmoset. Am J Primatol. 2019;81(2):e22944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deelen J, Kettunen J, Fischer K, et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat Commun. 2019;10(1):3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson PG, Promislow DEL, Masel J. Biomarkers for aging identified in cross-sectional studies tend to be non-causative. J Gerontol A Biol Sci Med Sci. 2020;75(3):466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaeberlein M, Creevy KE, Promislow DE. The dog aging project: translational geroscience in companion animals. Mamm Genome. 2016;27(7–8):279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase health span and achieve optimal longevity. J Physiol. 2016;594(8):2001–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fabbri E, An Y, Zoli M, et al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci. 2015;70(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strong R, Miller RA, Astle CM, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7(5):641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrison DE, Strong R, Allison DB, et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrison D, Strong R, Sharp Z, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi S, Reiter DA, Shardell M, et al. 31P Magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2016;71(12):1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ubaida-Mohien C, Gonzalez-Freire M, Lyashkov A, et al. Physical activity associated proteomics of skeletal muscle: being physically active in daily life may protect skeletal muscle from aging. Front Physiol. 2019;10:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ubaida-Mohien C, Lyashkov A, Gonzalez-Freire M, et al. Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. Elife. 2019;8:e49874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aliberti MJR, Szlejf C, Avelino-Silva VI, et al. COVID-19 is not over and age is not enough: Using frailty for prognostication in hospitalized patients. J Am Geriatr Soc. 2021;69(5):1116–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Márquez EJ, Chung CH, Marches R, et al. Sexual-dimorphism in human immune system aging. Nat Commun. 2020;11(1):751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moderbacher CR, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song TZ, Zheng HY, Han JB, et al. Delayed severe cytokine storm and immune cell infiltration in SARS-CoV-2-infected aged Chinese rhesus macaques. Zool Res. 2020;41(5):503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bartley JM, Pan SJ, Keilich SR, et al L. Aging augments the impact of influenza respiratory tract infection on mobility impairments, muscle-localized inflammation, and muscle atrophy. Aging (Albany NY). 2016;8(4):620–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Justice JN, Gubbi S, Kulkarni AS, Bartley JM, Kuchel GA, Barzilai N. A geroscience perspective on immune resilience and infectious diseases: a potential case for metformin. Geroscience. 2021;43(3):1093–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alexopoulos SJ, Chen SY, Brandon AE, et al. Mitochondrial uncoupler BAM15 reverses diet-induced obesity and insulin resistance in mice. Nat Commun. 2020;11(1):2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anguiano J, Garner TP, Mahalingam M, Das BC, Gavathiotis E, Cuervo AM. Chemical modulation of chaperone-mediated autophagy by retinoic acid derivatives. Nat Chem Biol. 2013;9(6):374–82. Erratum in: Nat Chem Biol. 2013;9(11):746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu Y, Brommer B, Tian X, et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature. 2020;588(7836):124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clinicaltrials.gov. Senescence in chronic kidney disease. NCT02848131. [Google Scholar]
- 77.Clinicaltrials.gov. Senolytic therapy to modulate progression of Alzheimer’s disease (SToMP-AD). NCT04063124, NCT04685590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hickson LJ, Langhi Prata LGP, Bobart SA, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. Erratum in: EBioMedicine. 2020;52:102595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kritchevsky SB, Justice JN. Testing the geroscience hypothesis: early days. J Gerontol A Biol Sci Med Sci. 2020;75(1):99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma Y, Vardaraja BN, Bennett DA, et al. Alzheimer’s disease GWAS weighted by multi-omics and endophenotypes identifies novel risk loci: genetics/genetic factors of Alzheimer’s disease. Alzheimer’s & Dementia. 2020;16(S2):e043977. [Google Scholar]
- 82.Delgado-Morales R, Agís-Balboa RC, Esteller M, Berdasco M. Epigenetic mechanisms during ageing and neurogenesis as novel therapeutic avenues in human brain disorders. Clin Epigenetics. 2017;9:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baker DJ, Petersen RC. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest. 2018;128(4):1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yun MH. Cellular senescence in tissue repair: every cloud has a silver lining. Int J Dev Biol. 2018;62(6–7-8):591–604. [DOI] [PubMed] [Google Scholar]
- 85.Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015;64(7):2289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hara Y, McKeehan N, Fillit HM. Translating the biology of aging into novel therapeutics for Alzheimer disease. Neurology. 2019;92(2):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ogrodnik M, Evans SA, Fielder E, et al. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell. 2021;20(2):e13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Musi N, Valentine JM, Sickora KR, et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 2018;17(6):e12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fuhrmann-Stroissnigg H, Ling YY, Zhao J, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. 2017;8(422):1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yen K, Wan J, Mehta HH, et al. Humanin prevents age-related cognitive decline in mice and is associated with improved cognitive age in humans. Sci Rep. 2018;8(1):14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee C, Zeng J, Drew BG, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21(3):443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mackowiak SD, Zauber H, Bielow C, et al. Extensive identification and analysis of conserved small ORFs in animals. Genome Biol. 2015;16:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saghatelian A, Couso JP. Discovery and characterization of smORF-encoded bioactive polypeptides. Nat Chem Biol. 2015;11(12):909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ikonen M, Liu B, Hashimoto Y, et al. Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci USA. 2003;100(22):13042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lue Y, Swerdloff R, Wan J, et al. The potent humanin analogue (HNG) protects germ cells and leucocytes while enhancing chemotherapy-induced suppression of cancer metastases in male mice. Endocrinology. 2015;156(12):4511–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim SJ, Xiao J, Wan J, Cohen P, Yen K. Mitochondrially derived peptides as novel regulators of metabolism. J Physiol. 2017;595(21):6613–6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee C, Wan J, Miyazaki B, et al. IGF-I regulates the age-dependent signaling peptide humanin. Aging Cell. 2014;13(5):958–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muzumdar RH, Huffman DM, Atzmon G, et al. Humanin: a novel central regulator of peripheral insulin action. PLoS One. 2009;4(7):e6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Widmer RJ, Flammer AJ, Herrmann J, et al. Circulating humanin levels are associated with preserved coronary endothelial function. Am J Physiol Heart Circ Physiol. 2013;304(3):H393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oh YK, Bachar AR, Zacharias DG, et al. Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice. Atherosclerosis. 2011;219(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yen K, Mehta HH, Kim SJ, et al. The mitochondrial derived peptide humanin is a regulator of lifespan and health span. Aging (Albany NY). 2020;12(12):11185–11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mehta HH, Xiao J, Ramirez R, et al. Metabolomic profile of diet-induced obesity mice in response to humanin and small humanin-like peptide 2 treatment. Metabolomics. 2019;15(6):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cobb LJ, Lee C, Xiao J, et al. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY). 2016;8(4):796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiao J, Howard L, Wan J, et al. Low circulating levels of the mitochondrial-peptide hormone SHLP2: novel biomarker for prostate cancer risk. Oncotarget. 2017;8(55):94900–94909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nashine S, Cohen P, Nesburn AB, et al. Characterizing the protective effects of SHLP2, a mitochondrial-derived peptide, in macular degeneration. Sci Rep. 2018;8:15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Okada AK, Teranishi K, Lobo F, et al. The mitochondrial-derived peptides, HumaninS14G and small humanin-like peptide 2, exhibit chaperone-like activity. Sci Rep. 2017;7(1):7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miller B, Arpawong TE, Jiao H, et al. Comparing the utility of mitochondrial and nuclear DNA to adjust for genetic ancestry in association studies. Cells. 2019;8(4):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miller B, Torres M, Jiang X, et al. A mitochondrial genome-wide association study of cataract in a Latino population. Transl Vis Sci Technol. 2020;9(6):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miller B, Kim SJ, Kumagai H, et al. Peptides derived from small mitochondrial open reading frames: Genomic, biological, and therapeutic implications. Exp Cell Res. 2020;393(2):112056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim KH, Son JM, Benayoun BA, Lee C. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab. 2018;28(3):516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee C, Kim KH, Cohen P. MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free Radic Biol Med. 2016;100:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zempo H, Kim SJ, Fuku N, et al. A pro-diabetogenic mtDNA polymorphism in the mitochondrial-derived peptide, MOTS-c. Aging (Albany NY). 2021;13(2):1692–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kumagai H, Coelho AR, Wan J, et al. MOTS-c reduces myostatin and muscle atrophy signaling. Am J Physiol Endocrinol Metab. 2021;320(4):E680–E690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reynolds JC, Lai RW, Woodhead JST, et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat Commun. 2021;12(1):470. [DOI] [PMC free article] [PubMed] [Google Scholar]