Abstract
Background
The use of intravenous antibiotics at anaesthetic induction in colorectal surgery is the standard of care. However, the role of mechanical bowel preparation, enemas, and oral antibiotics in surgical site infection, anastomotic leak, and other perioperative outcomes remains controversial. The aim of this study was to determine the optimal preoperative bowel preparation strategy in elective colorectal surgery.
Methods
A systematic review and network meta-analysis of RCTs was performed with searches from PubMed/MEDLINE, Scopus, Embase, and the Cochrane Central Register of Controlled Trials from inception to December 2022. Primary outcomes included surgical site infection and anastomotic leak. Secondary outcomes included 30-day mortality rate, ileus, length of stay, return to theatre, other infections, and side effects of antibiotic therapy or bowel preparation.
Results
Sixty RCTs involving 16 314 patients were included in the final analysis: 3465 (21.2 per cent) had intravenous antibiotics alone, 5268 (32.3 per cent) had intravenous antibiotics + mechanical bowel preparation, 1710 (10.5 per cent) had intravenous antibiotics + oral antibiotics, 4183 (25.6 per cent) had intravenous antibiotics + oral antibiotics + mechanical bowel preparation, 262 (1.6 per cent) had intravenous antibiotics + enemas, and 1426 (8.7 per cent) had oral antibiotics + mechanical bowel preparation. With intravenous antibiotics as a baseline comparator, network meta-analysis demonstrated a significant reduction in total surgical site infection risk with intravenous antibiotics + oral antibiotics (OR 0.47 (95 per cent c.i. 0.32 to 0.68)) and intravenous antibiotics + oral antibiotics + mechanical bowel preparation (OR 0.55 (95 per cent c.i. 0.40 to 0.76)), whereas oral antibiotics + mechanical bowel preparation resulted in a higher surgical site infection rate compared with intravenous antibiotics alone (OR 1.84 (95 per cent c.i. 1.20 to 2.81)). Anastomotic leak rates were lower with intravenous antibiotics + oral antibiotics (OR 0.63 (95 per cent c.i. 0.44 to 0.90)) and intravenous antibiotics + oral antibiotics + mechanical bowel preparation (OR 0.62 (95 per cent c.i. 0.41 to 0.94)) compared with intravenous antibiotics alone. There was no significant difference in outcomes with mechanical bowel preparation in the absence of intravenous antibiotics and oral antibiotics in the main analysis.
Conclusion
A bowel preparation strategy with intravenous antibiotics + oral antibiotics, with or without mechanical bowel preparation, should represent the standard of care for patients undergoing elective colorectal surgery.
A systematic review and network meta-analysis of RCTs was performed to determine the optimal preoperative bowel preparation strategy in elective colorectal surgery. Sixty RCTs involving 16 314 patients were included; network meta-analysis demonstrated a significant reduction in surgical site infection and anastomotic leak risk with strategies consisting of combined intravenous and oral antibiotics. Bowel preparation with intravenous and oral antibiotics, with or without mechanical bowel preparation, should represent the standard of care for patients undergoing elective colorectal surgery.
Introduction
Infectious complications are common after colorectal surgery. The incidence of surgical site infection (SSI) is between 5.4 and 23.2 per cent1, comprising superficial and deep incisional SSIs as well as intra-abdominal abscess (IAA)/organ space infections, which occur in 7.9–11.5 per cent of cases2,3. The most feared complication is anastomotic leak (AL), which occurs in 2–10 per cent of cases, and more frequently in the setting of low rectal, complex inflammatory, and cancer resections4,5. Superficial SSIs incur a large burden on the healthcare system with increased utilization and costs6,7. IAA and AL represent significant morbidity that often requires further intervention, with an associated mortality rate as high as 16 per cent in the setting of AL8,9.
Routine prophylactic intravenous antibiotics (IAB) at induction of anaesthesia is the standard of care in elective colorectal surgery10,11, due to the observed reduction in SSI and other infectious complications. In contrast, ongoing debate exists regarding the role of mechanical bowel preparation (MBP) and oral antibiotics (OAB) in elective colorectal surgery. MBP theoretically allows for improved bowel handling, reduced faecal contamination and spillage, and reduced luminal pressure and bacterial load within the intestinal lumen. Moreover, OAB may further target the gastrointestinal bacterial flora, with direct exposure and further elimination of the colonic mucosa-related microflora12,13.
Early RCTs demonstrated evidence in support of the use of combined OAB + MBP for major colorectal surgery, with an observed reduction in SSI and AL rates14–16. However, concerns surrounding the effectiveness and morbidity of bowel preparation (including prolonged hospital admission, dehydration, electrolyte imbalances, Clostridium difficile infection (CDI), and patient discomfort) were subsequently raised17–19. Multiple RCTs in the early 2000s–2010s reported a marginal advantage for the use of preoperative MBP20–27, which was further supported in a Cochrane review performed by Güenaga et al.28 in 2011 and a subsequent meta-analysis performed by Cao et al.29 in 2012. Unfortunately, most of these studies failed to include OAB20,21,23,25,26,30, and MBP and OAB subsequently fell out of favour.
More recently, several large retrospective cohort studies of the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) colectomy database demonstrated significant reductions in the rates of SSI, AL, and post-operative ileus with OAB + MBP31–33. The ACS and Surgical Infection Society (2017)34, the American Society of Colon and Rectal Surgeons (2019)35, and the WHO10 guidelines recommend the use of OAB + MBP in elective colorectal surgery. However, there remains a large evidence–practice gap, with a recent international European multicentre audit in 2017 demonstrating low rates of utilization of this bowel preparation strategy, with only 16.8 per cent of patients receiving OAB + MBP versus 52.9 per cent receiving MBP36.
Given recent publication of further RCTs, the aim of this study was to review the prospective literature to evaluate the optimal bowel preparation to be used for elective colorectal surgery.
Methods
A systematic review was performed according to the guidelines and recommendations of PRISMA37. Institutional review board approval was not required. The protocol for this meta-analysis was registered with the PROSPERO database (CRD42021287956).
Search strategy
An electronic search for relevant publications was performed using the following sources: PubMed/MEDLINE, Scopus, Embase, and the Cochrane Central Register of Controlled Trials. The search headings and medical subject headings (MeSH) can be seen in Fig. S1. The search of the Cochrane Central Register of Controlled Trials, Embase, and Scopus databases was performed by combining the following search terms using the Boolean AND/OR operators: ‘colorectal surgery’, ‘surgery’, ‘colorectal’, ‘antibiotic’, and ‘bowel prep*’.
Publications were limited to those published in the English language. All titles were initially screened, and appropriate abstracts were reviewed. The reference section of each relevant publication and Google Scholar were also screened for other applicable publications. The function ‘related article’ in PubMed was also used to identify articles. In addition, clinicaltrials.gov was searched for proposed or ongoing trials. The last date of the search was 31 December 2022.
Inclusion criteria
To be included in the analysis, the studies had to meet the following criteria: report on patients undergoing elective colorectal resection; compare the use of oral or systemic antibiotic use or MBP administration or both; report on surgical and clinical outcome measures mentioned below; have a clear research methodology and prospective randomization of patients; have the longest follow-up or the largest sample size when two or more studies were reported by the same institution; and be published in full-text format and in the English language.
Exclusion criteria
Studies were excluded from the analysis if they failed to meet the above inclusion criteria.
Outcomes of interest
The primary outcomes were total SSI and AL rates. SSI rates were subdivided into superficial SSI, deep incisional SSI, and IAA/organ space infection rates. Secondary outcomes included ileus, return to theatre, length of stay, urinary tract infection, respiratory tract infection, CDI, 30-day mortality rate, and side effects of antibiotic therapy or bowel preparation.
Data extraction
Two authors (J.T. and F.T.M.) independently reviewed the literature according to the above predefined strategy and criteria. Each of these authors extracted the following data variables: titles and reference details (first author, journal, year, and country), study population characteristics (number in study, number treated by each approach, sex, and age), disease characteristics, type and approach of surgical intervention, and outcome data. All data were extracted independently into separate databases and compared at the end of the reviewing process to limit selection bias. Duplicates were removed, and any disparities were clarified. A third author (É.J.R.) independently reviewed the database and resolved any discrepancies.
Statistical analysis
Descriptive statistics were used to report the characteristics of eligible trials. Binary data were compared using ORs. ORs were calculated using crude event data from the original articles to compare the efficacy of the various reconstructive strategies. Weighted-mean differences were calculated for continuous variables. If means and standard deviations were not available, estimates were derived from study data using the methods described by Hozo et al.38 and Luo et al.39.
Network meta-analysis (NMA) was conducted using the netmeta40 and Shiny41 packages for R. Effect sizes for the NMA are described with a 95 per cent c.i. Study heterogeneity was assessed via Cochrane Q score, I2 scores, and deviance information criterion between random- and fixed-effects models. A deviance information criterion difference of greater than three was deemed to indicate significant study heterogeneity and a random-effects model was used for all study arms42. Where appropriate, estimates of group means and standard deviations were calculated if the required data were available38,39,43. The authors plotted rank probabilities against the possible ranks for all competing treatments. The confidence in estimates of the outcome was assessed using Confidence in Network Meta-Analysis (CINeMA)44. Methodological assessment of included studies was undertaken by J.T. and F.T.M. using the Cochrane risk-of-bias assessment tool45 and the Jadad scale46, with papers achieving a score of less than 3 being considered low quality. Sensitivity analysis was performed based on study quality, chronology, type of resection, and whether antibiotic regimens had anaerobic and aerobic coverage consistent with contemporary guidelines.
Results
Literature search and clinical characteristics
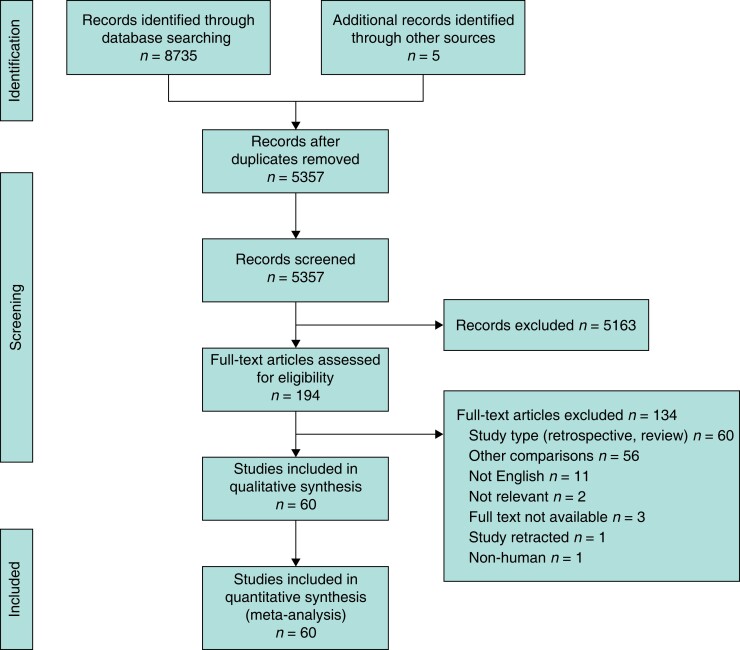
From the 8735 studies identified, 60 RCTs including 16 314 patients were included (Fig. 1)20–24,26,27,30,47–99. A summary of the details of all included RCTs is included in Table S1. Study publication dates ranged from 1979 to 2022. Of the 60 RCTs included in this analysis, 17 studies (28 per cent) were specific to cancer resections, seven studies (12 per cent) were specific to left-sided/rectal anastomoses, and three studies (5 per cent) were specific to a laparoscopic approach. Of the 16 314 patients, 3465 (21.2 per cent) had IAB alone, 5268 (32.3 per cent) had IAB + MBP, 1710 (10.5 per cent) had IAB + OAB, 4183 (25.6 per cent) had IAB + OAB + MBP, 262 (1.6 per cent) had IAB + enemas (EN), and 1426 (8.7 per cent) had OAB + MBP.
Fig. 1.
PRISMA flow diagram outlining the systematic search
Primary outcomes
Total SSI
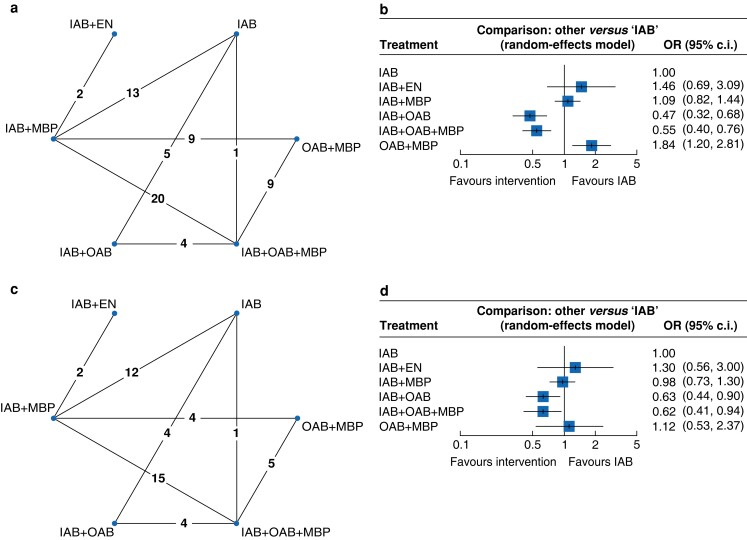
All studies assessed SSI (Fig. 2a), with an overall SSI rate of 10.9 per cent. SSI rates were lowest in the IAB + OAB + MBP (7.5 per cent) and IAB + OAB (7.6 per cent) groups, compared with IAB (12.2 per cent), IAB + MBP (12.8 per cent), IAB + EN (14.1 per cent), and OAB + MBP (14.6 per cent). Using the IAB group as a baseline comparator, NMA demonstrated a statistically significant reduction in risk of total SSI with IAB + OAB (OR 0.47 (95 per cent c.i. 0.32 to 0.68)) and IAB + OAB + MBP (OR 0.55 (95 per cent c.i. 0.40 to 0.76)) (Fig. 2b). The SSI rate was higher in the OAB + MBP group (OR 1.84 (95 per cent c.i. 1.20 to 2.81)) compared with the IAB group. There was no significant difference with regards to total SSI rates between the IAB group and the other compared groups. League ranking tables showed IAB + OAB to be the best ranked treatment in terms of reducing the total SSI rate (Fig. S2a).
Fig. 2.
Network plots (n = number of studies) and forest plots for total surgical site infection and anastomotic leak
a Network plot for total surgical site infection. b Forest plot for total surgical site infection. c Network plot for anastomotic leak. d Forest plot for anastomotic leak. Forest plots compare different bowel preparation methods against intravenous antibiotics. IAB, intravenous antibiotics; EN, enemas; MBP, mechanical bowel preparation; OAB, oral antibiotics.
Anastomotic leak
A total of 47 studies reported AL20–24,26,27,30,47–58,60–64,66,67,69,71,73–76,78,80,81,83–87,90–92,94,97–99, including 13 912 patients, with an overall leak rate of 3.6 per cent (Figure 2c). AL rates were 1.9 per cent with IAB + OAB + MBP, 2.6 per cent with OAB + MBP, 3.5 per cent with IAB + OAB, 4.1 per cent with IAB + MBP, 5.0 per cent with IAB, and 6.1 per cent with IAB + EN. NMA showed a reduction in AL with IAB + OAB + MBP (OR 0.62 (95 per cent c.i. 0.41 to 0.94)) and IAB + OAB (OR 0.63 (95 per cent c.i. 0.44 to 0.90)) compared with IAB alone (Fig. 2d). There were no significant differences between the IAB group and the other compared groups. League ranking tables showed IAB + OAB + MBP to be the best treatment option, followed by IAB + OAB (Fig. S2b).
Secondary outcomes
Superficial SSI
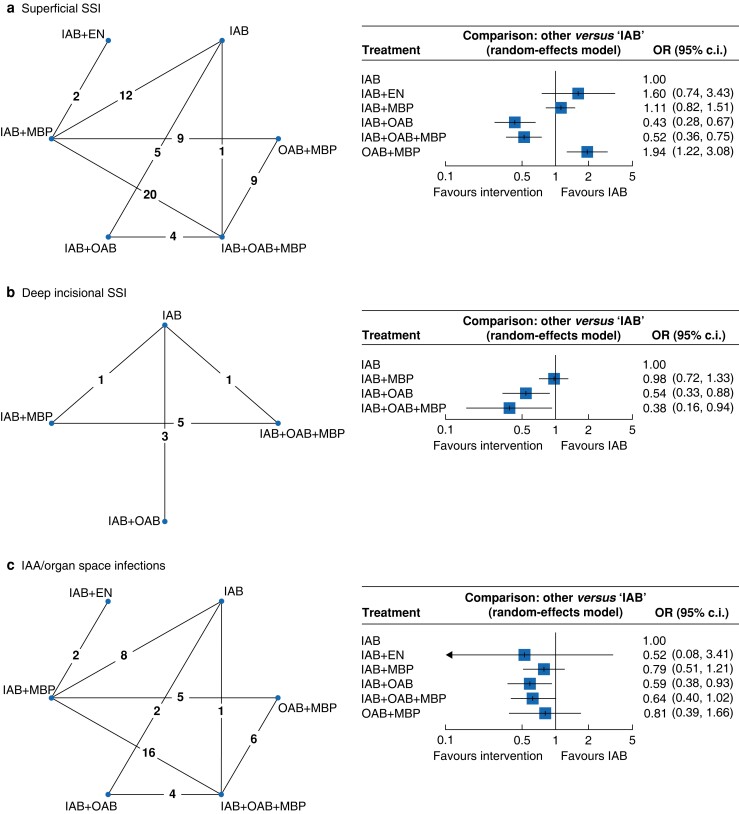
All studies assessed superficial SSI (16 289 patients). The overall superficial SSI rate was 8.0 per cent and rates were lowest in the IAB + OAB (4.0 per cent) and IAB + OAB + MBP (5.2 per cent) groups, followed by IAB (7.7 per cent), IAB + MBP (10.2 per cent), OAB + MBP (12.8 per cent), and IAB + EN (13.4 per cent). NMA demonstrated a significant reduction in superficial SSI risk with IAB + OAB (OR 0.43 (95 per cent c.i. 0.28 to 0.67)) and IAB + OAB + MBP (OR 0.52 (95 per cent c.i. 0.36 to 0.75)) when compared with IAB alone, with an increased superficial SSI risk with OAB + MBP (OR 1.94 (95 per cent c.i. 1.22 to 3.08)) (Fig. 3a).
Fig. 3.
Network plots (n = number of studies) and forest plots for superficial surgical site infection, deep incisional surgical site infection, and intra-abdominal abscess/organ space infection
a Network plot and forest plot for superficial surgical site infection. b Network plot and forest plot for deep incisional surgical site infection. c Network plot and forest plot for intra-abdominal abscess/organ space infection. Forest plots compare different bowel preparation methods against intravenous antibiotics. SSI, surgical site infection; IAB, intravenous antibiotics; EN, enemas; MBP, mechanical bowel preparation; OAB, oral antibiotics; IAA, intra-abdominal abscess.
Deep incisional SSI
Ten RCTs20,47,49–52,54,58,61,65 assessed for deep incisional SSI (4893 patients), with an overall rate of 6.0 per cent. The rate was lowest in the IAB + OAB + MBP group (0.7 per cent), followed by IAB + OAB (3.6 per cent), IAB + MBP (7.2 per cent), and IAB alone (9.1 per cent). In comparison with IAB alone, NMA demonstrated a reduction in deep incisional SSI risk with IAB + OAB + MBP (OR 0.38 (95 per cent c.i. 0.16 to 0.94)) and IAB + OAB (OR 0.54 (95 per cent c.i. 0.33 to 0.88)) (Fig. 3b).
IAA/organ space infection
IAA/organ space infection was assessed in 47 RCTs (14 327 patients)20,22–24,26,27,30,47,49,50,52–54,56,58,59,61–67,69–76,79,80,83–91,93–97, with an overall rate of 2.6 per cent. IAA/organ space infection rates were lower across all treatment arms in comparison with IAB alone, with a statistically significant reduction in the IAB + OAB group (OR 0.59 (95 per cent c.i. 0.38 to 0.93)) (Fig. 3c). The rate was lowest in the IAB + EN group (0.8 per cent), followed by OAB + MBP (2.1 per cent), IAB + OAB + MBP (2.2 per cent), IAB + OAB (2.5 per cent), IAB + MBP (2.5 per cent), and IAB alone (3.7 per cent). IAB + OAB ranked as the best treatment option, followed by IAB + OAB + MBP, IAB + EN, IAB + MBP, OAB + MBP, and IAB alone (Fig. S2e).
There were no statistically significant differences between treatment options for ileus (5535 patients, 16 RCTs), return to theatre (6757 patients, 17 RCTs), respiratory tract infection (9218 patients, 22 RCTs), urinary tract infection (8884 patients, 23 RCTs), 30-day mortality rate (12350 patients, 38 RCTs), CDI (3854 patients, 13 RCTs), length of stay (8484 patients, 26 RCTs), or side effects of antibiotic therapy or bowel preparation (4461 patients, 12 RCTs) (Fig. S3).
Risk-of-bias assessment
Risk-of-bias assessment demonstrated an overall low–moderate risk of bias (Figs S4, S5). RCT methodological quality assessment using the Jadad scale (Table S2) deemed 36 studies (60 per cent) to be of good quality. Participant blinding was not possible during studies that assessed MBP, contributing to a higher risk of performance bias, particularly in these trials.
Sensitivity analysis
Study quality—Jadad score greater than or equal to 3
Analysis of RCTs with a Jadad score greater than or equal to 3 included 36 RCTs and 11 382 patients (Fig. S6a). Observed significant differences remained, with significant reductions in SSI and AL rates with IAB + OAB (OR 0.43 (95 per cent c.i. 0.30 to 0.63) and OR 0.62 (95 per cent c.i. 0.43 to 0.90) respectively) and IAB + OAB + MBP (OR 0.58 (95 per cent c.i. 0.41 to 0.82) and OR 0.56 (95 per cent c.i. 0.34 to 0.92) respectively).
Chronology—RCTs published after 2000
Sensitivity analysis was performed for all RCTs after 2000 (12 455 patients and 38 RCTs). This demonstrated similar results to the overall primary outcome analysis, with significant reductions in SSI and AL rates in the IAB + OAB (OR 0.46 (95 per cent c.i. 0.33 to 0.64) and OR 0.62 (95 per cent c.i. 0.43 to 0.89) respectively) and IAB + OAB + MBP (OR 0.52 (95 per cent c.i. 0.38 to 0.70) and OR 0.56 (95 per cent c.i. 0.36 to 0.89) respectively) treatment arms (Fig. S6b).
Left-sided/rectal anastomoses
Analysis of RCTs specific to left-sided/rectal anastomoses included eight RCTs and 1304 patients. There was a trend toward lower AL rates in the IAB + OAB and IAB + OAB + MBP groups (OR 0.20 (95 per cent c.i. 0.02 to 2.21) and OR 0.37 (95 per cent c.i. 0.10 to 1.40) respectively), with a statistically significant reduction in total SSI rate in the IAB + OAB + MBP group (OR 0.39 (95 per cent c.i. 0.16 to 0.95)) (Fig. S6c).
Adequacy of antibiotic coverage
A total of 28 RCTs (8229 patients) were found to utilize IAB with both aerobic and anaerobic coverage consistent with latest guidance. Results for total SSI were similar to the primary analysis, with lower SSI rates in the IAB + OAB and IAB + OAB + MBP groups (OR 0.40 (95 per cent c.i. 0.29 to 0.57) and OR 0.45 (95 per cent c.i. 0.31 to 0.66) respectively). A significant reduction in AL rate was noted in the IAB + OAB group (OR 0.63 (95 per cent c.i. 0.44 to 0.91)) (Fig. S6d).
Discussion
Controversy exists over the optimal bowel preparation strategy in elective colorectal surgery, with previous European studies demonstrating low utilization of OAB (10–16.8 per cent)36,100. This NMA comparing different bowel preparation strategies consists solely of RCT data, including 60 RCTs and 16 289 patients. The advantage of employing NMA methodology in this analysis is that it facilitates simultaneous direct and indirect comparisons of greater than two strategies, providing more insightful results101,102. The most important findings were a significant reduction in risk of overall SSI and AL with strategies consisting of a combined IAB and OAB bowel preparation (that is IAB + OAB and IAB + OAB + MBP). Interestingly, the addition of MBP did not confer a statistically significant advantage over IAB + OAB alone in the overall analysis. These findings support the importance of the intestinal microbiome on anastomotic wound healing and provide strong evidence that selective antibiotic decontamination of the gastrointestinal tract with combined IAB + OAB should represent the standard of care for patients undergoing elective colorectal resection.
This NMA demonstrated a statistically significant reduction in overall SSI rates with OAB use. These findings correspond with two recently published meta-analyses103,104, with both showing a reduction in SSI rates with the use of preoperative OAB, and one study reporting a reduction in AL rates with OAB104. These data suggest that while prophylactic IAB at induction reduce the microbial burden at surgical sites through bactericidal effects, OAB may play a role in further reducing the SSI risk by eliminating the colonic mucosa-related microflora12,13.
The most significant finding of this NMA is a significant reduction in AL rate with the addition of OAB to IAB. The results of this NMA add to a growing body of evidence that the addition of OAB therapy reduces AL rates compared with IAB alone, and further supports the argument for preoperative OAB in elective colorectal surgery.
Historically, mechanical cleansing was thought to be among the most important factors influencing outcomes in colorectal surgery105. Today, the use of preoperative MBP is still commonly practiced100,106,107 and widely recommended35. The current study found no clear advantage from the addition of MBP, which contrasts with results of several previous studies, including previous large retrospective cohort studies of the ACS NSQIP colectomy database31–33,108, which favoured combined MBP + OAB. These findings support recent National Institute for Health and Care Excellence (NICE) and WHO guidelines, which advise that MBP alone (in the absence of OAB) should not be used to reduce SSI risk10,109. This brings into question the true efficacy of and necessity for MBP, which has been shown to cause alterations in the colonic mucosa and inflammatory changes in the lamina propria110–112, which may result in increased risk of bacterial translocation113–115. Furthermore, attempted but ineffective bowel preparation may lead to difficulties controlling large volumes of liquid stool, increasing the risk of faecal spillage and contamination21. Nevertheless, ranking tables suggest that MBP may still confer a potential advantage over IAB + OAB, with regards to AL risk, and sensitivity analysis suggests that MBP may play a role in left-sided/rectal resections. Overall, the proportion of benefit attributable to each of MBP and OAB remains uncertain, and ongoing large-volume RCTs116, including the ORALEV2 (NCT04161599)117, MOBILE2 (NCT04281667)118, and SELDDEC119 studies, may shed more light on this long-standing debate.
Some authors have advocated against the use of OAB, due to a theoretical higher risk of CDI17, yet this NMA did not identify a significant difference in CDI risk with OAB. Currently, there is limited evidence to suggest a significant causal relationship between OAB and CDI in the setting of colorectal surgery64,120, with some studies even suggesting a lower rate of C. difficile colitis with OAB33,121,122. Overall, the benefits of OAB outweigh the theoretical low risk of post-colectomy C. difficile colitis, and OAB should not be omitted over concerns regarding potential CDI.
While the strength of a NMA methodology is its ability to allow for simultaneous comparisons between several treatment options both directly and indirectly, there are pertinent limitations of such techniques101. As with any meta-analysis, clinical and methodological heterogeneity will exist given the differences in patient, antibiotic, and bowel preparation selection within the individual included studies. The results should also be interpreted with caution given the differences between sizes of each treatment arm—the majority of patients had IAB, IAB + MBP, or IAB + OAB + MBP; IAB + OAB represented 10.5 per cent of patients, with fewer studies reporting outcomes for this antimicrobial strategy. The methods of bowel preparation and types of OAB selection were also not distinguished in the analysis. OAB and MBP strategies varied, with noted heterogeneity between studies. Where OAB were utilized, most included non-absorbable aminoglycosides (for example neomycin and kanamycin). Many contemporary studies also utilized polyethylene glycol, sodium picosulfate, or sodium phosphate solutions for MBP. The current methodology did not differentiate between resections for benign and malignant disease or those in receipt of preoperative chemoradiation, laparoscopic versus open procedures, operative time, stapled versus handsewn anastomoses, and the presence or absence of a diverting stoma—all of which may be important confounding factors that may ultimately influence outcomes.123–125
These data demonstrate that OAB produce a significant reduction in SSI and AL, and provide high-level evidence to recommend the implementation of preoperative OAB as the standard of care in elective colorectal resection. There is less clarity surrounding the role of MBP, and the authors eagerly anticipate the results of the large ongoing, multicentre RCTs that may provide a more conclusive answer to this question.117,118
Supplementary Material
Acknowledgements
The authors would like to thank Dr Amirhossein Jalali (Department of Mathematics & Statistics, Faculty of Science & Engineering, University of Limerick, Limerick, Ireland) for his expertise and assistance with the study.
Contributor Information
Jonavan Tan, Department of Colorectal Surgery, Tallaght University Hospital, Tallaght, Dublin, Ireland.
Éanna J Ryan, Department of Colorectal Surgery, Tallaght University Hospital, Tallaght, Dublin, Ireland.
Matthew G Davey, Department of Surgery, Royal College of Surgeons in Ireland, Dublin, Ireland.
Fiachra T McHugh, Department of Colorectal Surgery, Tallaght University Hospital, Tallaght, Dublin, Ireland.
Ben Creavin, Department of Colorectal Surgery, Tallaght University Hospital, Tallaght, Dublin, Ireland.
Maria C Whelan, Department of Colorectal Surgery, Tallaght University Hospital, Tallaght, Dublin, Ireland.
Michael E Kelly, Department of Colorectal Surgery, Tallaght University Hospital, Tallaght, Dublin, Ireland.
Paul C Neary, Department of Colorectal Surgery, Tallaght University Hospital, Tallaght, Dublin, Ireland; School of Medicine, Trinity College Dublin, College Green, Dublin, Ireland.
Dara O Kavanagh, Department of Colorectal Surgery, Tallaght University Hospital, Tallaght, Dublin, Ireland; Department of Surgical Affairs, Royal College of Surgeons in Ireland, Dublin, Ireland.
James M O’Riordan, Department of Colorectal Surgery, Tallaght University Hospital, Tallaght, Dublin, Ireland; School of Medicine, Trinity College Dublin, College Green, Dublin, Ireland.
Funding
No funding was received for this study.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
All data are included in the manuscript, referenced articles, or the supplementary material.
References
- 1. Young H, Knepper B, Moore EE, Johnson JL, Mehler P, Price CS. Surgical site infection after colon surgery: national healthcare safety network risk factors and modeled rates compared with published risk factors and rates. J Am Coll Surg 2012;214:852–859 [DOI] [PubMed] [Google Scholar]
- 2. Gomila A, Carratalà J, Camprubí D, Shaw E, Badia JM, Cruz Aet al. Risk factors and outcomes of organ-space surgical site infections after elective colon and rectal surgery. Antimicrob Resist Infect Control 2017;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biondo S, Kreisler E, Fraccalvieri D, Basany EE, Codina-Cazador A, Ortiz H. Risk factors for surgical site infection after elective resection for rectal cancer. A multivariate analysis on 2131 patients. Colorectal Dis 2012;14:e95–e102 [DOI] [PubMed] [Google Scholar]
- 4. Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 2009;208:269–278 [DOI] [PubMed] [Google Scholar]
- 5. Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KYet al. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg 2013;257:665–671 [DOI] [PubMed] [Google Scholar]
- 6. Gantz O, Zagadailov P, Merchant AM. The cost of surgical site infections after colorectal surgery in the United States from 2001 to 2012: a longitudinal analysis. Am Surg 2019;85:142–149 [PubMed] [Google Scholar]
- 7. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009;37:387–397 [DOI] [PubMed] [Google Scholar]
- 8. Bakker IS, Grossmann I, Henneman D, Havenga K, Wiggers T. Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br J Surg 2014;101:424–432 [DOI] [PubMed] [Google Scholar]
- 9. Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier-Konrad B, Morel P. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis 2008;23:265–270 [DOI] [PubMed] [Google Scholar]
- 10. WHO . Global Guidelines for the Prevention of Surgical Site Infection (2nd edn). 2018 [PubMed] [Google Scholar]
- 11. Royal College of Surgeons in Ireland/Royal College of Physicians of Ireland . Preventing Surgical Site Infections, Key Recommendations for Practice. 2012
- 12. Smith MB, Goradia VK, Holmes JW, McCluggage SG, Smith JW, Nichols RL. Suppression of the human mucosal-related colonic microflora with prophylactic parenteral and/or oral antibiotics. World J Surg 1990;14:636–641 [DOI] [PubMed] [Google Scholar]
- 13. Arabi Y, Dimock F, Burdon DW, Alexander-Williams J, Keighley MR. Influence of bowel preparation and antimicrobials on colonic microflora. Br J Surg 1978;65:555–558 [DOI] [PubMed] [Google Scholar]
- 14. Rosenberg IL, Graham NG, De Dombal FT, Goligher JC. Preparation of the intestine in patients undergoing major large-bowel surgery, mainly for neoplasms of the colon and rectum. Br J Surg 1971;58:266–269 [DOI] [PubMed] [Google Scholar]
- 15. Nichols RL, Broido P, Condon RE, Gorbach SL, Nyhus LM. Effect of preoperative neomycin-erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Ann Surg 1973;178:453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clarke JS, Condon RE, Bartlett JG, Gorbach SL, Nichols RL, Ochi S. Preoperative oral antibiotics reduce septic complications of colon operations: results of prospective, randomized, double-blind clinical study. Ann Surg 1977;186:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wren SM, Ahmed N, Jamal A, Safadi BY. Preoperative oral antibiotics in colorectal surgery increase the rate of Clostridium difficile colitis. Arch Surg 2005;140:752–756 [DOI] [PubMed] [Google Scholar]
- 18. Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis Net al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 2013;37:259–284 [DOI] [PubMed] [Google Scholar]
- 19. Holte K, Nielsen KG, Madsen JL, Kehlet H. Physiologic effects of bowel preparation. Dis Colon Rectum 2004;47:1397–1402 [DOI] [PubMed] [Google Scholar]
- 20. Contant CM, Hop WC, van't Sant HP, Oostvogel HJ, Smeets HJ, Stassen LPet al. Mechanical bowel preparation for elective colorectal surgery: a multicentre randomised trial. Lancet 2007;370:2112–2117 [DOI] [PubMed] [Google Scholar]
- 21. Fa-Si-Oen P, Roumen R, Buitenweg J, van de Velde C, van Geldere D, Putter Het al. Mechanical bowel preparation or not? Outcome of a multicenter, randomized trial in elective open colon surgery. Dis Colon Rectum 2005;48:1509–1516 [DOI] [PubMed] [Google Scholar]
- 22. Jung B, Påhlman L, Nyström PO, Nilsson E. Multicentre randomized clinical trial of mechanical bowel preparation in elective colonic resection. Br J Surg 2007;94:689–695 [DOI] [PubMed] [Google Scholar]
- 23. Miettinen RP, Laitinen ST, Mäkelä JT, Pääkkönen ME. Bowel preparation with oral polyethylene glycol electrolyte solution vs. no preparation in elective open colorectal surgery: prospective, randomized study. Dis Colon Rectum 2000;43:669–675; discussion 675–677 [DOI] [PubMed] [Google Scholar]
- 24. Ram E, Sherman Y, Weil R, Vishne T, Kravarusic D, Dreznik Z. Is mechanical bowel preparation mandatory for elective colon surgery? A prospective randomized study. Arch Surg 2005;140:285–288 [DOI] [PubMed] [Google Scholar]
- 25. Van't Sant HP, Weidema WF, Hop WC, Oostvogel HJ, Contant CM. The influence of mechanical bowel preparation in elective lower colorectal surgery. Ann Surg 2010;251:59–63 [DOI] [PubMed] [Google Scholar]
- 26. Bucher P, Gervaz P, Soravia C, Mermillod B, Erne M, Morel P. Randomized clinical trial of mechanical bowel preparation versus no preparation before elective left-sided colorectal surgery. Br J Surg 2005;92:409–414 [DOI] [PubMed] [Google Scholar]
- 27. Zmora O, Mahajna A, Bar-Zakai B, Hershko D, Shabtai M, Krausz MMet al. Is mechanical bowel preparation mandatory for left-sided colonic anastomosis? Results of a prospective randomized trial. Tech Coloproctol 2006;10:131–135 [DOI] [PubMed] [Google Scholar]
- 28. Güenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2011;2011:CD001544. [DOI] [PubMed] [Google Scholar]
- 29. Cao F, Li J, Li F. Mechanical bowel preparation for elective colorectal surgery: updated systematic review and meta-analysis. Int J Colorectal Dis 2012;27:803–810 [DOI] [PubMed] [Google Scholar]
- 30. Bretagnol F, Panis Y, Rullier E, Rouanet P, Berdah S, Dousset Bet al. Rectal cancer surgery with or without bowel preparation: the French GRECCAR III multicenter single-blinded randomized trial. Ann Surg 2010;252:863–868 [DOI] [PubMed] [Google Scholar]
- 31. Kiran RP, Murray AC, Chiuzan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg 2015;262:416–425; discussion 423–425 [DOI] [PubMed] [Google Scholar]
- 32. Midura EF, Jung AD, Hanseman DJ, Dhar V, Shah SA, Rafferty JFet al. Combination oral and mechanical bowel preparations decreases complications in both right and left colectomy. Surgery 2018;163:528–534 [DOI] [PubMed] [Google Scholar]
- 33. Klinger AL, Green H, Monlezun DJ, Beck D, Kann B, Vargas HDet al. The role of bowel preparation in colorectal surgery: results of the 2012–2015 ACS-NSQIP data. Ann Surg 2019;269:671–677 [DOI] [PubMed] [Google Scholar]
- 34. Ban KA, Minei JP, Laronga C, Harbrecht BG, Jensen EH, Fry DEet al. American College of Surgeons and Surgical Infection Society: surgical site infection guidelines, 2016 update. J Am Coll Surg 2017;224:59–74 [DOI] [PubMed] [Google Scholar]
- 35. Migaly J, Bafford A, Francone T, Gaertner W, Eskicioglu C, Bordeianou Let al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the use of bowel preparation in elective colon and rectal surgery. Dis Colon Rectum 2019;62:3–8 [DOI] [PubMed] [Google Scholar]
- 36. The 2017 European Society of Coloproctology (ESCP) Collaborating Group . Association of mechanical bowel preparation with oral antibiotics and anastomotic leak following left sided colorectal resection: an international, multi-centre, prospective audit. Colorectal Dis 2018; 20(Suppl 6): 15–32 [DOI] [PubMed] [Google Scholar]
- 37. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron Cet al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–784 [DOI] [PubMed] [Google Scholar]
- 38. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785–1805 [DOI] [PubMed] [Google Scholar]
- 40. Rücker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods 2012;3:312–324 [DOI] [PubMed] [Google Scholar]
- 41. Chang W, Cheng J, Allaire J, Xie Y, McPherson J. Shiny: Web Application Framework for R. R Package Version. 2017 [Google Scholar]
- 42. Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Ser B (Statist Methodol) 2002;64:583–639 [Google Scholar]
- 43. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the quality of evidence from a network meta-analysis. PLoS One 2014;9:e99682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman ADet al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJet al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 47. Futier E, Jaber S, Garot M, Vignaud M, Panis Y, Slim Ket al. Effect of oral antimicrobial prophylaxis on surgical site infection after elective colorectal surgery: multicentre, randomised, double blind, placebo controlled trial. BMJ 2022;379:e071476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arezzo A, Mistrangelo M, Bonino MA, Salusso P, Forcignanò E, Vettoretto Net al. Oral neomycin and bacitracin are effective in preventing surgical site infections in elective colorectal surgery: a multicentre, randomized, parallel, single-blinded trial (COLORAL-1). Updates Surg 2021;73:1775–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Papp G, Saftics G, Szabó BE, Baracs J, Vereczkei A, Kollár Det al. Systemic versus Oral and Systemic Antibiotic Prophylaxis (SOAP) study in colorectal surgery: prospective randomized multicentre trial. Br J Surg 2021;108:271–276 [DOI] [PubMed] [Google Scholar]
- 50. Espin Basany E, Solís-Peña A, Pellino G, Kreisler E, Fraccalvieri D, Muinelo-Lorenzo Met al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol 2020;5:729–738 [DOI] [PubMed] [Google Scholar]
- 51. Mulder T, Kluytmans-van den Bergh M, Vlaminckx B, Roos D, de Smet AM, de Vos tot Nederveen Cappel Ret al. Prevention of severe infectious complications after colorectal surgery using oral non-absorbable antimicrobial prophylaxis: results of a multicenter randomized placebo-controlled clinical trial. Antimicrob Resist Infect Control 2020;9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rybakov E, Nagudov M, Sukhina M, Shelygin Y. Impact of oral antibiotic prophylaxis on surgical site infection after rectal surgery: results of randomized trial. Int J Colorectal Dis 2021;36:323–330 [DOI] [PubMed] [Google Scholar]
- 53. Schardey HM, Wirth U, Strauss T, Kasparek MS, Schneider D, Jauch KW. Prevention of anastomotic leak in rectal cancer surgery with local antibiotic decontamination: a prospective, randomized, double-blind, placebo-controlled single center trial. Int J Colorectal Dis 2020;35:847–857 [DOI] [PubMed] [Google Scholar]
- 54. Koskenvuo L, Lehtonen T, Koskensalo S, Rasilainen S, Klintrup K, Ehrlich Aet al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. Lancet 2019;394:840–848 [DOI] [PubMed] [Google Scholar]
- 55. Mai-Phan AT, Nguyen H, Nguyen TT, Nguyen DA, Thai TT. Randomized controlled trial of mechanical bowel preparation for laparoscopy-assisted colectomy. Asian J Endosc Surg 2019;12:408–411 [DOI] [PubMed] [Google Scholar]
- 56. Suzuki T, Sadahiro S, Tanaka A, Okada K, Saito G, Miyakita Het al. Usefulness of preoperative mechanical bowel preparation in patients with colon cancer who undergo elective surgery: a prospective randomized trial using oral antibiotics. Dig Surg 2020;37:192–198 [DOI] [PubMed] [Google Scholar]
- 57. Abis GSA, Stockmann HBAC, Bonjer HJ, van Veenendaal N, van Doorn-Schepens MLM, Budding AEet al. Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). Br J Surg 2019;106:355–363 [DOI] [PubMed] [Google Scholar]
- 58. Anjum N, Ren J, Wang G, Li G, Wu X, Dong Het al. A randomized control trial of preoperative oral antibiotics as adjunct therapy to systemic antibiotics for preventing surgical site infection in clean contaminated, contaminated, and dirty type of colorectal surgeries. Dis Colon Rectum 2017;60:1291–1298 [DOI] [PubMed] [Google Scholar]
- 59. Uchino M, Ikeuchi H, Bando T, Chohno T, Sasaki H, Horio Yet al. Efficacy of preoperative oral antibiotic prophylaxis for the prevention of surgical site infections in patients with Crohn disease: a randomized controlled trial. Ann Surg 2017;269:420–426 [DOI] [PubMed] [Google Scholar]
- 60. Bhat AH, Parray FQ, Chowdri NA, Wani RA, Thakur N, Nazki Set al. Mechanical bowel preparation versus no preparation in elective colorectal surgery: a prospective randomized study. Int J Surg Open 2016;2:26–30 [Google Scholar]
- 61. Hata H, Yamaguchi T, Hasegawa S, Nomura A, Hida K, Nishitai Ret al. Oral and parenteral versus parenteral antibiotic prophylaxis in elective laparoscopic colorectal surgery (JMTO PREV 07-01): a phase 3, multicenter, open-label, randomized trial. Ann Surg 2016;263:1085–1091 [DOI] [PubMed] [Google Scholar]
- 62. Ikeda A, Konishi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Yet al. Randomized clinical trial of oral and intravenous versus intravenous antibiotic prophylaxis for laparoscopic colorectal resection. Br J Surg 2016;103:1608–1615 [DOI] [PubMed] [Google Scholar]
- 63. Bhattacharjee PK, Chakraborty S. An open-label prospective randomized controlled trial of mechanical bowel preparation vs nonmechanical bowel preparation in elective colorectal surgery: personal experience. Indian J Surg 2015;77:1233–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sadahiro S, Suzuki T, Tanaka A, Okada K, Kamata H, Ozaki Tet al. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: prospective randomized trial. Surgery 2014;155:493–503 [DOI] [PubMed] [Google Scholar]
- 65. Oshima T, Takesue Y, Ikeuchi H, Matsuoka H, Nakajima K, Uchino Met al. Preoperative oral antibiotics and intravenous antimicrobial prophylaxis reduce the incidence of surgical site infections in patients with ulcerative colitis undergoing IPAA. Dis Colon Rectum 2013;56:1149–1155 [DOI] [PubMed] [Google Scholar]
- 66. Sasaki J, Matsumoto S, Kan H, Yamada T, Koizumi M, Mizuguchi Yet al. Objective assessment of postoperative gastrointestinal motility in elective colonic resection using a radiopaque marker provides an evidence for the abandonment of preoperative mechanical bowel preparation. J Nippon Med Sch 2012;79:259–266 [DOI] [PubMed] [Google Scholar]
- 67. Bertani E, Chiappa A, Biffi R, Bianchi PP, Radice D, Branchi Vet al. Comparison of oral polyethylene glycol plus a large volume glycerine enema with a large volume glycerine enema alone in patients undergoing colorectal surgery for malignancy: a randomized clinical trial. Colorectal Dis 2011;13:e327–e334 [DOI] [PubMed] [Google Scholar]
- 68. Watanabe M, Murakami M, Nakao K, Asahara T, Nomoto K, Tsunoda A. Randomized clinical trial of the influence of mechanical bowel preparation on faecal microflora in patients undergoing colonic cancer resection. Br J Surg 2010;97:1791–1797 [DOI] [PubMed] [Google Scholar]
- 69. Pena-Soria MJ, Mayol JM, Anula R, Arbeo-Escolar A, Fernandez-Represa JA. Single-blinded randomized trial of mechanical bowel preparation for colon surgery with primary intraperitoneal anastomosis. J Gastrointest Surg 2008;12:2103–2108; discussion 2108–2109 [DOI] [PubMed] [Google Scholar]
- 70. Kobayashi M, Mohri Y, Tonouchi H, Miki C, Nakai K, Kusunoki Met al. Randomized clinical trial comparing intravenous antimicrobial prophylaxis alone with oral and intravenous antimicrobial prophylaxis for the prevention of a surgical site infection in colorectal cancer surgery. Surg Today 2007;37:383–388 [DOI] [PubMed] [Google Scholar]
- 71. Platell C, Barwood N, Makin G. Randomized clinical trial of bowel preparation with a single phosphate enema or polyethylene glycol before elective colorectal surgery. Br J Surg 2006;93:427–433 [DOI] [PubMed] [Google Scholar]
- 72. Espin-Basany E, Sanchez-Garcia JL, Lopez-Cano M, Lozoya-Trujillo R, Medarde-Ferrer M, Armadans-Gil Let al. Prospective, randomised study on antibiotic prophylaxis in colorectal surgery. Is it really necessary to use oral antibiotics? Int J Colorectal Dis 2005;20:542–546 [DOI] [PubMed] [Google Scholar]
- 73. Zmora O, Mahajna A, Bar-Zakai B, Rosin D, Hershko D, Shabtai Met al. Colon and rectal surgery without mechanical bowel preparation: a randomized prospective trial. Ann Surg 2003;237:363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lewis RT. Oral versus systemic antibiotic prophylaxis in elective colon surgery: a randomized study and meta-analysis send a message from the 1990s. Can J Surg 2002;45:173–180 [PMC free article] [PubMed] [Google Scholar]
- 75. Ishida H, Yokoyama M, Nakada H, Inokuma S, Hashimoto D. Impact of oral antimicrobial prophylaxis on surgical site infection and methicillin-resistant Staphylococcus aureus infection after elective colorectal surgery. Results of a prospective randomized trial. Surg Today 2001;31:979–983 [DOI] [PubMed] [Google Scholar]
- 76. Takesue Y, Yokoyama T, Akagi S, Ohge H, Murakami Y, Sakashita Yet al. A brief course of colon preparation with oral antibiotics. Surg Today 2000;30:112–116 [DOI] [PubMed] [Google Scholar]
- 77. Yabata E, Okabe S, Endo M. A prospective, randomized clinical trial of preoperative bowel preparation for elective colorectal surgery–comparison among oral, systemic, and intraoperative luminal antibacterial preparations. J Med Dent Sci 1997;44:75–80 [PubMed] [Google Scholar]
- 78. Burke P, Mealy K, Gillen P, Joyce W, Traynor O, Hyland J. Requirement for bowel preparation in colorectal surgery. Br J Surg 1994;81:907–910 [DOI] [PubMed] [Google Scholar]
- 79. Taylor EW, Lindsay G. Selective decontamination of the colon before elective colorectal surgery. West of Scotland surgical infection study group. World J Surg 1994;18:926–931; discussion 931–932 [DOI] [PubMed] [Google Scholar]
- 80. Schoetz DJ Jr, Roberts PL, Murray JJ, Coller JA, Veidenheimer MC. Addition of parenteral cefoxitin to regimen of oral antibiotics for elective colorectal operations. A randomized prospective study. Ann Surg 1990;212:209–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Khubchandani IT, Karamchandani MC, Sheets JA, Stasik JJ, Rosen L, Riether RD. Metronidazole vs. erythromycin, neomycin, and cefazolin in prophylaxis for colonic surgery. Dis Colon Rectum 1989;32:17–20 [DOI] [PubMed] [Google Scholar]
- 82. Kling PA, Dahlgren S. Oral prophylaxis with neomycin and erythromycin in colorectal surgery. More proof for efficacy than failure. Arch Surg 1989;124:705–707 [DOI] [PubMed] [Google Scholar]
- 83. Lau WY, Chu KW, Poon GP, Ho KK. Prophylactic antibiotics in elective colorectal surgery. Br J Surg 1988;75:782–785 [DOI] [PubMed] [Google Scholar]
- 84. Playforth MJ, Smith GM, Evans M, Pollock AV. Antimicrobial bowel preparation. Oral, parenteral, or both? Dis Colon Rectum 1988;31:90–93 [DOI] [PubMed] [Google Scholar]
- 85. Petrelli NJ, Conte CC, Herrera L, Stulc J, O'Neill P. A prospective, randomized trial of perioperative prophylactic cefamandole in elective colorectal surgery for malignancy. Dis Colon Rectum 1988;31:427–429 [DOI] [PubMed] [Google Scholar]
- 86. Raahave D, Hesselfeldt P, Pedersen TB. Cefotaxime i.v. versus oral neomycin-erythromycin for prophylaxis of infections after colorectal operations. World J Surg 1988;12:369–372 [DOI] [PubMed] [Google Scholar]
- 87. University of Melbourne Colorectal Group . Systemic Timentin is superior to oral tinidazole for antibiotic prophylaxis in elective colorectal surgery. Dis Colon Rectum 1987;30:786–789 [PubMed] [Google Scholar]
- 88. University of Melbourne Colorectal Group . Clinical trial of prophylaxis of wound sepsis in elective colorectal surgery comparing ticarcillin with tinidazole. Aust N Z J Surg 1986;56:209–213 [PubMed] [Google Scholar]
- 89. Weaver M, Burdon DW, Youngs DJ, Keighley MR. Oral neomycin and erythromycin compared with single-dose systemic metronidazole and ceftriaxone prophylaxis in elective colorectal surgery. Am J Surg 1986;151:437–442 [DOI] [PubMed] [Google Scholar]
- 90. Coppa GF, Eng K, Gouge TH, Ranson JH, Localio SA. Parenteral and oral antibiotics in elective colon and rectal surgery. A prospective, randomized trial. Am J Surg 1983;145:62–65 [DOI] [PubMed] [Google Scholar]
- 91. Condon RE, Bartlett JG, Greenlee H, Schulte WJ, Ochi S, Abbe Ret al. Efficacy of oral and systemic antibiotic prophylaxis in colorectal operations. Arch Surg 1983;118:496–502 [DOI] [PubMed] [Google Scholar]
- 92. Kaiser AB, Herrington JL Jr, Jacobs JK, Mulherin JL Jr, Roach AC, Sawyers JL. Cefoxitin versus erythromycin, neomycin, and cefazolin in colorectal operations. Importance of the duration of the surgical procedure. Ann Surg 1983;198:525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lazorthes F, Legrand G, Monrozies X, Fretigny E, Pugnet G, Cordova JAet al. Comparison between oral and systemic antibiotics and their combined use for the prevention of complications in colorectal surgery. Dis Colon Rectum 1982;25:309–311 [DOI] [PubMed] [Google Scholar]
- 94. Beggs FD, Jobanputra RS, Holmes JT. A comparison of intravenous and oral metronidazole as prophylactic in colorectal surgery. Br J Surg 1982;69:226–227 [DOI] [PubMed] [Google Scholar]
- 95. Hoffmann CE, McDonald PJ, Watts JM. Use of peroperative cefoxitin to prevent infection after colonic and rectal surgery. Ann Surg 1981;193:353–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dion YM, Richards GK, Prentis JJ, Hinchey EJ. The influence of oral versus parenteral preoperative metronidazole on sepsis following colon surgery. Ann Surg 1980;192:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hanel KC, King DW, McAllister ET, Reiss-Levy E. Single-dose parenteral antibiotics as prophylaxis against wound infections in colonic operations. Dis Colon Rectum 1980;23:98–101 [DOI] [PubMed] [Google Scholar]
- 98. Keighley MR, Arabi Y, Alexander-Williams J, Youngs D, Burdon DW. Comparison between systemic and oral antimicrobial prophylaxis in colorectal surgery. Lancet 1979;1:894–897 [DOI] [PubMed] [Google Scholar]
- 99. Koskenvuo L, Lehtonen T, Koskensalo S, Rasilainen S, Klintrup K, Ehrlich Aet al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation in right and left colectomy: subgroup analysis of MOBILE trial. BJS Open 2021;5:zrab011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Devane LA, Proud D, O'Connell PR, Panis Y. A European survey of bowel preparation in colorectal surgery. Colorectal Dis 2017;19:O402–O406 [DOI] [PubMed] [Google Scholar]
- 101. Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ 2013;346:f2914 [DOI] [PubMed] [Google Scholar]
- 102. Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med 2013;11:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Woodfield JC, Clifford K, Schmidt B, Turner GA, Amer MA, McCall JL. Strategies for antibiotic administration for bowel preparation among patients undergoing elective colorectal surgery: a network meta-analysis. JAMA Surg 2022;157:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rollins KE, Javanmard-Emamghissi H, Acheson AG, Lobo DN. The role of oral antibiotic preparation in elective colorectal surgery: a meta-analysis. Ann Surg 2019;270:43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chung RS, Gurll NJ, Berglund EM. A controlled clinical trial of whole gut lavage as a method of bowel preparation for colonic operations. Am J Surg 1979;137:75–81 [DOI] [PubMed] [Google Scholar]
- 106. Badia JM, Casey AL, Rubio-Pérez I, Arroyo-García N, Espin E, Biondo Set al. Awareness of practice and comparison with best evidence in surgical site infection prevention in colorectal surgery. Surg Infect (Larchmt) 2019;21:218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Markell KW, Hunt BM, Charron PD, Kratz RJ, Nelson J, Isler JTet al. Prophylaxis and management of wound infections after elective colorectal surgery: a survey of the American Society of Colon and Rectal Surgeons membership. J Gastrointest Surg 2010;14:1090–1098 [DOI] [PubMed] [Google Scholar]
- 108. Scarborough JE, Mantyh CR, Sun Z, Migaly J. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: an analysis of colectomy-targeted ACS NSQIP. Ann Surg 2015;262:331–337 [DOI] [PubMed] [Google Scholar]
- 109. National Institute for Health and Care Excellence . Surveillance of Surgical Site Infections: Prevention and Treatment (NICE Guideline NG125). 19 August 2019 [PubMed]
- 110. Bucher P, Gervaz P, Egger J-F, Soravia C, Morel P. Morphologic alterations associated with mechanical bowel preparation before elective colorectal surgery: a randomized trial. Dis Colon Rectum 2006;49:109–112 [DOI] [PubMed] [Google Scholar]
- 111. Fa-Si-Oen PR, Penninckx F. The effect of mechanical bowel preparation on human colonic tissue in elective open colon surgery. Dis Colon Rectum 2004;47:948–949 [DOI] [PubMed] [Google Scholar]
- 112. Rejchrt S, Bures J, Siroký M, Kopácová M, Slezák L, Langr F. A prospective, observational study of colonic mucosal abnormalities associated with orally administered sodium phosphate for colon cleansing before colonoscopy. Gastrointest Endosc 2004;59:651–654 [DOI] [PubMed] [Google Scholar]
- 113. Kale TI, Kuzu MA, Tekeli A, Tanik A, Aksoy M, Cete M. Aggressive bowel preparation does not enhance bacterial translocation, provided the mucosal barrier is not disrupted: a prospective, randomized study. Dis Colon Rectum 1998;41:636–641 [DOI] [PubMed] [Google Scholar]
- 114. Poole GV. Spontaneous bacterial peritonitis during bowel preparation: an example of clinical translocation. South Med J 1991;84:1412–1413 [DOI] [PubMed] [Google Scholar]
- 115. Berg RD. Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol 1999;473:11–30 [DOI] [PubMed] [Google Scholar]
- 116. Gao RQ, Wang WD, Yu PF, Mo ZC, Dong DH, Yang XSet al. Are preoperative oral antibiotics effective in reducing the incidence of anastomotic leakage after colorectal cancer surgery? Study protocol for a prospective, multicentre, randomized controlled study. Trials 2022;23:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pellino G, Solís-Peña A, Kraft M, Huguet BM, Espín-Basany E. Preoperative oral antibiotics with versus without mechanical bowel preparation to reduce surgical site infections following colonic resection: protocol for an international randomized controlled trial (ORALEV2). Colorectal Dis 2021;23:2173–2181 [DOI] [PubMed] [Google Scholar]
- 118. Koskenvuo L, Lunkka P, Varpe P, Hyöty M, Satokari R, Haapamäki Cet al. Mechanical bowel preparation and oral antibiotics versus mechanical bowel preparation only prior rectal surgery (MOBILE2): a multicentre, double-blinded, randomised controlled trial-study protocol. BMJ Open 2021;11:e051269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Balasubramanian I, Sehgal R, McNamara E, Condon E, Waldron D, Coffey JCet al. The role of selective decontamination of the digestive tract in preventing surgical site infections in elective colorectal resections: a randomized controlled trial (SELDDEC trial). Colorectal Dis 2018;20:14130182470 [Google Scholar]
- 120. Yeom CH, Cho MM, Baek SK, Bae OS. Risk factors for the development of Clostridium difficile-associated colitis after colorectal cancer surgery. J Korean Soc Coloproctol 2010;26:329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Krapohl GL, Morris AM, Cai S, Englesbe MJ, Aronoff DM, Campbell DA Jret al. Preoperative risk factors for postoperative Clostridium difficile infection in colectomy patients. Am J Surg 2013;205:343–347; discussion 347–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Al-Mazrou AM, Hyde LZ, Suradkar K, Kiran RP. Effect of inclusion of oral antibiotics with mechanical bowel preparation on the risk of Clostridium difficile infection after colectomy. J Gastrointest Surg 2018;22:1968–1975 [DOI] [PubMed] [Google Scholar]
- 123. Veyrie N, Ata T, Muscari F, Couchard AC, Msika S, Hay JMet al. Anastomotic leakage after elective right versus left colectomy for cancer: prevalence and independent risk factors. J Am Coll Surg 2007;205:785–793 [DOI] [PubMed] [Google Scholar]
- 124. Choy PY, Bissett IP, Docherty JG, Parry BR, Merrie AE. Stapled versus handsewn methods for ileocolic anastomoses. Cochrane Database Syst Rev 2007; (3)CD004320 [DOI] [PubMed] [Google Scholar]
- 125. Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 2007;246:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript, referenced articles, or the supplementary material.
References
- 1. Young H, Knepper B, Moore EE, Johnson JL, Mehler P, Price CS. Surgical site infection after colon surgery: national healthcare safety network risk factors and modeled rates compared with published risk factors and rates. J Am Coll Surg 2012;214:852–859 [DOI] [PubMed] [Google Scholar]
- 2. Gomila A, Carratalà J, Camprubí D, Shaw E, Badia JM, Cruz Aet al. Risk factors and outcomes of organ-space surgical site infections after elective colon and rectal surgery. Antimicrob Resist Infect Control 2017;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biondo S, Kreisler E, Fraccalvieri D, Basany EE, Codina-Cazador A, Ortiz H. Risk factors for surgical site infection after elective resection for rectal cancer. A multivariate analysis on 2131 patients. Colorectal Dis 2012;14:e95–e102 [DOI] [PubMed] [Google Scholar]
- 4. Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 2009;208:269–278 [DOI] [PubMed] [Google Scholar]
- 5. Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KYet al. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg 2013;257:665–671 [DOI] [PubMed] [Google Scholar]
- 6. Gantz O, Zagadailov P, Merchant AM. The cost of surgical site infections after colorectal surgery in the United States from 2001 to 2012: a longitudinal analysis. Am Surg 2019;85:142–149 [PubMed] [Google Scholar]
- 7. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009;37:387–397 [DOI] [PubMed] [Google Scholar]
- 8. Bakker IS, Grossmann I, Henneman D, Havenga K, Wiggers T. Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br J Surg 2014;101:424–432 [DOI] [PubMed] [Google Scholar]
- 9. Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier-Konrad B, Morel P. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis 2008;23:265–270 [DOI] [PubMed] [Google Scholar]
- 10. WHO . Global Guidelines for the Prevention of Surgical Site Infection (2nd edn). 2018 [PubMed] [Google Scholar]
- 11. Royal College of Surgeons in Ireland/Royal College of Physicians of Ireland . Preventing Surgical Site Infections, Key Recommendations for Practice. 2012
- 12. Smith MB, Goradia VK, Holmes JW, McCluggage SG, Smith JW, Nichols RL. Suppression of the human mucosal-related colonic microflora with prophylactic parenteral and/or oral antibiotics. World J Surg 1990;14:636–641 [DOI] [PubMed] [Google Scholar]
- 13. Arabi Y, Dimock F, Burdon DW, Alexander-Williams J, Keighley MR. Influence of bowel preparation and antimicrobials on colonic microflora. Br J Surg 1978;65:555–558 [DOI] [PubMed] [Google Scholar]
- 14. Rosenberg IL, Graham NG, De Dombal FT, Goligher JC. Preparation of the intestine in patients undergoing major large-bowel surgery, mainly for neoplasms of the colon and rectum. Br J Surg 1971;58:266–269 [DOI] [PubMed] [Google Scholar]
- 15. Nichols RL, Broido P, Condon RE, Gorbach SL, Nyhus LM. Effect of preoperative neomycin-erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Ann Surg 1973;178:453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clarke JS, Condon RE, Bartlett JG, Gorbach SL, Nichols RL, Ochi S. Preoperative oral antibiotics reduce septic complications of colon operations: results of prospective, randomized, double-blind clinical study. Ann Surg 1977;186:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wren SM, Ahmed N, Jamal A, Safadi BY. Preoperative oral antibiotics in colorectal surgery increase the rate of Clostridium difficile colitis. Arch Surg 2005;140:752–756 [DOI] [PubMed] [Google Scholar]
- 18. Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis Net al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 2013;37:259–284 [DOI] [PubMed] [Google Scholar]
- 19. Holte K, Nielsen KG, Madsen JL, Kehlet H. Physiologic effects of bowel preparation. Dis Colon Rectum 2004;47:1397–1402 [DOI] [PubMed] [Google Scholar]
- 20. Contant CM, Hop WC, van't Sant HP, Oostvogel HJ, Smeets HJ, Stassen LPet al. Mechanical bowel preparation for elective colorectal surgery: a multicentre randomised trial. Lancet 2007;370:2112–2117 [DOI] [PubMed] [Google Scholar]
- 21. Fa-Si-Oen P, Roumen R, Buitenweg J, van de Velde C, van Geldere D, Putter Het al. Mechanical bowel preparation or not? Outcome of a multicenter, randomized trial in elective open colon surgery. Dis Colon Rectum 2005;48:1509–1516 [DOI] [PubMed] [Google Scholar]
- 22. Jung B, Påhlman L, Nyström PO, Nilsson E. Multicentre randomized clinical trial of mechanical bowel preparation in elective colonic resection. Br J Surg 2007;94:689–695 [DOI] [PubMed] [Google Scholar]
- 23. Miettinen RP, Laitinen ST, Mäkelä JT, Pääkkönen ME. Bowel preparation with oral polyethylene glycol electrolyte solution vs. no preparation in elective open colorectal surgery: prospective, randomized study. Dis Colon Rectum 2000;43:669–675; discussion 675–677 [DOI] [PubMed] [Google Scholar]
- 24. Ram E, Sherman Y, Weil R, Vishne T, Kravarusic D, Dreznik Z. Is mechanical bowel preparation mandatory for elective colon surgery? A prospective randomized study. Arch Surg 2005;140:285–288 [DOI] [PubMed] [Google Scholar]
- 25. Van't Sant HP, Weidema WF, Hop WC, Oostvogel HJ, Contant CM. The influence of mechanical bowel preparation in elective lower colorectal surgery. Ann Surg 2010;251:59–63 [DOI] [PubMed] [Google Scholar]
- 26. Bucher P, Gervaz P, Soravia C, Mermillod B, Erne M, Morel P. Randomized clinical trial of mechanical bowel preparation versus no preparation before elective left-sided colorectal surgery. Br J Surg 2005;92:409–414 [DOI] [PubMed] [Google Scholar]
- 27. Zmora O, Mahajna A, Bar-Zakai B, Hershko D, Shabtai M, Krausz MMet al. Is mechanical bowel preparation mandatory for left-sided colonic anastomosis? Results of a prospective randomized trial. Tech Coloproctol 2006;10:131–135 [DOI] [PubMed] [Google Scholar]
- 28. Güenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2011;2011:CD001544. [DOI] [PubMed] [Google Scholar]
- 29. Cao F, Li J, Li F. Mechanical bowel preparation for elective colorectal surgery: updated systematic review and meta-analysis. Int J Colorectal Dis 2012;27:803–810 [DOI] [PubMed] [Google Scholar]
- 30. Bretagnol F, Panis Y, Rullier E, Rouanet P, Berdah S, Dousset Bet al. Rectal cancer surgery with or without bowel preparation: the French GRECCAR III multicenter single-blinded randomized trial. Ann Surg 2010;252:863–868 [DOI] [PubMed] [Google Scholar]
- 31. Kiran RP, Murray AC, Chiuzan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg 2015;262:416–425; discussion 423–425 [DOI] [PubMed] [Google Scholar]
- 32. Midura EF, Jung AD, Hanseman DJ, Dhar V, Shah SA, Rafferty JFet al. Combination oral and mechanical bowel preparations decreases complications in both right and left colectomy. Surgery 2018;163:528–534 [DOI] [PubMed] [Google Scholar]
- 33. Klinger AL, Green H, Monlezun DJ, Beck D, Kann B, Vargas HDet al. The role of bowel preparation in colorectal surgery: results of the 2012–2015 ACS-NSQIP data. Ann Surg 2019;269:671–677 [DOI] [PubMed] [Google Scholar]
- 34. Ban KA, Minei JP, Laronga C, Harbrecht BG, Jensen EH, Fry DEet al. American College of Surgeons and Surgical Infection Society: surgical site infection guidelines, 2016 update. J Am Coll Surg 2017;224:59–74 [DOI] [PubMed] [Google Scholar]
- 35. Migaly J, Bafford A, Francone T, Gaertner W, Eskicioglu C, Bordeianou Let al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the use of bowel preparation in elective colon and rectal surgery. Dis Colon Rectum 2019;62:3–8 [DOI] [PubMed] [Google Scholar]
- 36. The 2017 European Society of Coloproctology (ESCP) Collaborating Group . Association of mechanical bowel preparation with oral antibiotics and anastomotic leak following left sided colorectal resection: an international, multi-centre, prospective audit. Colorectal Dis 2018; 20(Suppl 6): 15–32 [DOI] [PubMed] [Google Scholar]
- 37. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron Cet al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–784 [DOI] [PubMed] [Google Scholar]
- 38. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785–1805 [DOI] [PubMed] [Google Scholar]
- 40. Rücker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods 2012;3:312–324 [DOI] [PubMed] [Google Scholar]
- 41. Chang W, Cheng J, Allaire J, Xie Y, McPherson J. Shiny: Web Application Framework for R. R Package Version. 2017 [Google Scholar]
- 42. Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Ser B (Statist Methodol) 2002;64:583–639 [Google Scholar]
- 43. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the quality of evidence from a network meta-analysis. PLoS One 2014;9:e99682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman ADet al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJet al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 47. Futier E, Jaber S, Garot M, Vignaud M, Panis Y, Slim Ket al. Effect of oral antimicrobial prophylaxis on surgical site infection after elective colorectal surgery: multicentre, randomised, double blind, placebo controlled trial. BMJ 2022;379:e071476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arezzo A, Mistrangelo M, Bonino MA, Salusso P, Forcignanò E, Vettoretto Net al. Oral neomycin and bacitracin are effective in preventing surgical site infections in elective colorectal surgery: a multicentre, randomized, parallel, single-blinded trial (COLORAL-1). Updates Surg 2021;73:1775–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Papp G, Saftics G, Szabó BE, Baracs J, Vereczkei A, Kollár Det al. Systemic versus Oral and Systemic Antibiotic Prophylaxis (SOAP) study in colorectal surgery: prospective randomized multicentre trial. Br J Surg 2021;108:271–276 [DOI] [PubMed] [Google Scholar]
- 50. Espin Basany E, Solís-Peña A, Pellino G, Kreisler E, Fraccalvieri D, Muinelo-Lorenzo Met al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol 2020;5:729–738 [DOI] [PubMed] [Google Scholar]
- 51. Mulder T, Kluytmans-van den Bergh M, Vlaminckx B, Roos D, de Smet AM, de Vos tot Nederveen Cappel Ret al. Prevention of severe infectious complications after colorectal surgery using oral non-absorbable antimicrobial prophylaxis: results of a multicenter randomized placebo-controlled clinical trial. Antimicrob Resist Infect Control 2020;9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rybakov E, Nagudov M, Sukhina M, Shelygin Y. Impact of oral antibiotic prophylaxis on surgical site infection after rectal surgery: results of randomized trial. Int J Colorectal Dis 2021;36:323–330 [DOI] [PubMed] [Google Scholar]
- 53. Schardey HM, Wirth U, Strauss T, Kasparek MS, Schneider D, Jauch KW. Prevention of anastomotic leak in rectal cancer surgery with local antibiotic decontamination: a prospective, randomized, double-blind, placebo-controlled single center trial. Int J Colorectal Dis 2020;35:847–857 [DOI] [PubMed] [Google Scholar]
- 54. Koskenvuo L, Lehtonen T, Koskensalo S, Rasilainen S, Klintrup K, Ehrlich Aet al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. Lancet 2019;394:840–848 [DOI] [PubMed] [Google Scholar]
- 55. Mai-Phan AT, Nguyen H, Nguyen TT, Nguyen DA, Thai TT. Randomized controlled trial of mechanical bowel preparation for laparoscopy-assisted colectomy. Asian J Endosc Surg 2019;12:408–411 [DOI] [PubMed] [Google Scholar]
- 56. Suzuki T, Sadahiro S, Tanaka A, Okada K, Saito G, Miyakita Het al. Usefulness of preoperative mechanical bowel preparation in patients with colon cancer who undergo elective surgery: a prospective randomized trial using oral antibiotics. Dig Surg 2020;37:192–198 [DOI] [PubMed] [Google Scholar]
- 57. Abis GSA, Stockmann HBAC, Bonjer HJ, van Veenendaal N, van Doorn-Schepens MLM, Budding AEet al. Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). Br J Surg 2019;106:355–363 [DOI] [PubMed] [Google Scholar]
- 58. Anjum N, Ren J, Wang G, Li G, Wu X, Dong Het al. A randomized control trial of preoperative oral antibiotics as adjunct therapy to systemic antibiotics for preventing surgical site infection in clean contaminated, contaminated, and dirty type of colorectal surgeries. Dis Colon Rectum 2017;60:1291–1298 [DOI] [PubMed] [Google Scholar]
- 59. Uchino M, Ikeuchi H, Bando T, Chohno T, Sasaki H, Horio Yet al. Efficacy of preoperative oral antibiotic prophylaxis for the prevention of surgical site infections in patients with Crohn disease: a randomized controlled trial. Ann Surg 2017;269:420–426 [DOI] [PubMed] [Google Scholar]
- 60. Bhat AH, Parray FQ, Chowdri NA, Wani RA, Thakur N, Nazki Set al. Mechanical bowel preparation versus no preparation in elective colorectal surgery: a prospective randomized study. Int J Surg Open 2016;2:26–30 [Google Scholar]
- 61. Hata H, Yamaguchi T, Hasegawa S, Nomura A, Hida K, Nishitai Ret al. Oral and parenteral versus parenteral antibiotic prophylaxis in elective laparoscopic colorectal surgery (JMTO PREV 07-01): a phase 3, multicenter, open-label, randomized trial. Ann Surg 2016;263:1085–1091 [DOI] [PubMed] [Google Scholar]
- 62. Ikeda A, Konishi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Yet al. Randomized clinical trial of oral and intravenous versus intravenous antibiotic prophylaxis for laparoscopic colorectal resection. Br J Surg 2016;103:1608–1615 [DOI] [PubMed] [Google Scholar]
- 63. Bhattacharjee PK, Chakraborty S. An open-label prospective randomized controlled trial of mechanical bowel preparation vs nonmechanical bowel preparation in elective colorectal surgery: personal experience. Indian J Surg 2015;77:1233–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sadahiro S, Suzuki T, Tanaka A, Okada K, Kamata H, Ozaki Tet al. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: prospective randomized trial. Surgery 2014;155:493–503 [DOI] [PubMed] [Google Scholar]
- 65. Oshima T, Takesue Y, Ikeuchi H, Matsuoka H, Nakajima K, Uchino Met al. Preoperative oral antibiotics and intravenous antimicrobial prophylaxis reduce the incidence of surgical site infections in patients with ulcerative colitis undergoing IPAA. Dis Colon Rectum 2013;56:1149–1155 [DOI] [PubMed] [Google Scholar]
- 66. Sasaki J, Matsumoto S, Kan H, Yamada T, Koizumi M, Mizuguchi Yet al. Objective assessment of postoperative gastrointestinal motility in elective colonic resection using a radiopaque marker provides an evidence for the abandonment of preoperative mechanical bowel preparation. J Nippon Med Sch 2012;79:259–266 [DOI] [PubMed] [Google Scholar]
- 67. Bertani E, Chiappa A, Biffi R, Bianchi PP, Radice D, Branchi Vet al. Comparison of oral polyethylene glycol plus a large volume glycerine enema with a large volume glycerine enema alone in patients undergoing colorectal surgery for malignancy: a randomized clinical trial. Colorectal Dis 2011;13:e327–e334 [DOI] [PubMed] [Google Scholar]
- 68. Watanabe M, Murakami M, Nakao K, Asahara T, Nomoto K, Tsunoda A. Randomized clinical trial of the influence of mechanical bowel preparation on faecal microflora in patients undergoing colonic cancer resection. Br J Surg 2010;97:1791–1797 [DOI] [PubMed] [Google Scholar]
- 69. Pena-Soria MJ, Mayol JM, Anula R, Arbeo-Escolar A, Fernandez-Represa JA. Single-blinded randomized trial of mechanical bowel preparation for colon surgery with primary intraperitoneal anastomosis. J Gastrointest Surg 2008;12:2103–2108; discussion 2108–2109 [DOI] [PubMed] [Google Scholar]
- 70. Kobayashi M, Mohri Y, Tonouchi H, Miki C, Nakai K, Kusunoki Met al. Randomized clinical trial comparing intravenous antimicrobial prophylaxis alone with oral and intravenous antimicrobial prophylaxis for the prevention of a surgical site infection in colorectal cancer surgery. Surg Today 2007;37:383–388 [DOI] [PubMed] [Google Scholar]
- 71. Platell C, Barwood N, Makin G. Randomized clinical trial of bowel preparation with a single phosphate enema or polyethylene glycol before elective colorectal surgery. Br J Surg 2006;93:427–433 [DOI] [PubMed] [Google Scholar]
- 72. Espin-Basany E, Sanchez-Garcia JL, Lopez-Cano M, Lozoya-Trujillo R, Medarde-Ferrer M, Armadans-Gil Let al. Prospective, randomised study on antibiotic prophylaxis in colorectal surgery. Is it really necessary to use oral antibiotics? Int J Colorectal Dis 2005;20:542–546 [DOI] [PubMed] [Google Scholar]
- 73. Zmora O, Mahajna A, Bar-Zakai B, Rosin D, Hershko D, Shabtai Met al. Colon and rectal surgery without mechanical bowel preparation: a randomized prospective trial. Ann Surg 2003;237:363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lewis RT. Oral versus systemic antibiotic prophylaxis in elective colon surgery: a randomized study and meta-analysis send a message from the 1990s. Can J Surg 2002;45:173–180 [PMC free article] [PubMed] [Google Scholar]
- 75. Ishida H, Yokoyama M, Nakada H, Inokuma S, Hashimoto D. Impact of oral antimicrobial prophylaxis on surgical site infection and methicillin-resistant Staphylococcus aureus infection after elective colorectal surgery. Results of a prospective randomized trial. Surg Today 2001;31:979–983 [DOI] [PubMed] [Google Scholar]
- 76. Takesue Y, Yokoyama T, Akagi S, Ohge H, Murakami Y, Sakashita Yet al. A brief course of colon preparation with oral antibiotics. Surg Today 2000;30:112–116 [DOI] [PubMed] [Google Scholar]
- 77. Yabata E, Okabe S, Endo M. A prospective, randomized clinical trial of preoperative bowel preparation for elective colorectal surgery–comparison among oral, systemic, and intraoperative luminal antibacterial preparations. J Med Dent Sci 1997;44:75–80 [PubMed] [Google Scholar]
- 78. Burke P, Mealy K, Gillen P, Joyce W, Traynor O, Hyland J. Requirement for bowel preparation in colorectal surgery. Br J Surg 1994;81:907–910 [DOI] [PubMed] [Google Scholar]
- 79. Taylor EW, Lindsay G. Selective decontamination of the colon before elective colorectal surgery. West of Scotland surgical infection study group. World J Surg 1994;18:926–931; discussion 931–932 [DOI] [PubMed] [Google Scholar]
- 80. Schoetz DJ Jr, Roberts PL, Murray JJ, Coller JA, Veidenheimer MC. Addition of parenteral cefoxitin to regimen of oral antibiotics for elective colorectal operations. A randomized prospective study. Ann Surg 1990;212:209–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Khubchandani IT, Karamchandani MC, Sheets JA, Stasik JJ, Rosen L, Riether RD. Metronidazole vs. erythromycin, neomycin, and cefazolin in prophylaxis for colonic surgery. Dis Colon Rectum 1989;32:17–20 [DOI] [PubMed] [Google Scholar]
- 82. Kling PA, Dahlgren S. Oral prophylaxis with neomycin and erythromycin in colorectal surgery. More proof for efficacy than failure. Arch Surg 1989;124:705–707 [DOI] [PubMed] [Google Scholar]
- 83. Lau WY, Chu KW, Poon GP, Ho KK. Prophylactic antibiotics in elective colorectal surgery. Br J Surg 1988;75:782–785 [DOI] [PubMed] [Google Scholar]
- 84. Playforth MJ, Smith GM, Evans M, Pollock AV. Antimicrobial bowel preparation. Oral, parenteral, or both? Dis Colon Rectum 1988;31:90–93 [DOI] [PubMed] [Google Scholar]
- 85. Petrelli NJ, Conte CC, Herrera L, Stulc J, O'Neill P. A prospective, randomized trial of perioperative prophylactic cefamandole in elective colorectal surgery for malignancy. Dis Colon Rectum 1988;31:427–429 [DOI] [PubMed] [Google Scholar]
- 86. Raahave D, Hesselfeldt P, Pedersen TB. Cefotaxime i.v. versus oral neomycin-erythromycin for prophylaxis of infections after colorectal operations. World J Surg 1988;12:369–372 [DOI] [PubMed] [Google Scholar]
- 87. University of Melbourne Colorectal Group . Systemic Timentin is superior to oral tinidazole for antibiotic prophylaxis in elective colorectal surgery. Dis Colon Rectum 1987;30:786–789 [PubMed] [Google Scholar]
- 88. University of Melbourne Colorectal Group . Clinical trial of prophylaxis of wound sepsis in elective colorectal surgery comparing ticarcillin with tinidazole. Aust N Z J Surg 1986;56:209–213 [PubMed] [Google Scholar]
- 89. Weaver M, Burdon DW, Youngs DJ, Keighley MR. Oral neomycin and erythromycin compared with single-dose systemic metronidazole and ceftriaxone prophylaxis in elective colorectal surgery. Am J Surg 1986;151:437–442 [DOI] [PubMed] [Google Scholar]
- 90. Coppa GF, Eng K, Gouge TH, Ranson JH, Localio SA. Parenteral and oral antibiotics in elective colon and rectal surgery. A prospective, randomized trial. Am J Surg 1983;145:62–65 [DOI] [PubMed] [Google Scholar]
- 91. Condon RE, Bartlett JG, Greenlee H, Schulte WJ, Ochi S, Abbe Ret al. Efficacy of oral and systemic antibiotic prophylaxis in colorectal operations. Arch Surg 1983;118:496–502 [DOI] [PubMed] [Google Scholar]
- 92. Kaiser AB, Herrington JL Jr, Jacobs JK, Mulherin JL Jr, Roach AC, Sawyers JL. Cefoxitin versus erythromycin, neomycin, and cefazolin in colorectal operations. Importance of the duration of the surgical procedure. Ann Surg 1983;198:525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lazorthes F, Legrand G, Monrozies X, Fretigny E, Pugnet G, Cordova JAet al. Comparison between oral and systemic antibiotics and their combined use for the prevention of complications in colorectal surgery. Dis Colon Rectum 1982;25:309–311 [DOI] [PubMed] [Google Scholar]
- 94. Beggs FD, Jobanputra RS, Holmes JT. A comparison of intravenous and oral metronidazole as prophylactic in colorectal surgery. Br J Surg 1982;69:226–227 [DOI] [PubMed] [Google Scholar]
- 95. Hoffmann CE, McDonald PJ, Watts JM. Use of peroperative cefoxitin to prevent infection after colonic and rectal surgery. Ann Surg 1981;193:353–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dion YM, Richards GK, Prentis JJ, Hinchey EJ. The influence of oral versus parenteral preoperative metronidazole on sepsis following colon surgery. Ann Surg 1980;192:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hanel KC, King DW, McAllister ET, Reiss-Levy E. Single-dose parenteral antibiotics as prophylaxis against wound infections in colonic operations. Dis Colon Rectum 1980;23:98–101 [DOI] [PubMed] [Google Scholar]
- 98. Keighley MR, Arabi Y, Alexander-Williams J, Youngs D, Burdon DW. Comparison between systemic and oral antimicrobial prophylaxis in colorectal surgery. Lancet 1979;1:894–897 [DOI] [PubMed] [Google Scholar]
- 99. Koskenvuo L, Lehtonen T, Koskensalo S, Rasilainen S, Klintrup K, Ehrlich Aet al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation in right and left colectomy: subgroup analysis of MOBILE trial. BJS Open 2021;5:zrab011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Devane LA, Proud D, O'Connell PR, Panis Y. A European survey of bowel preparation in colorectal surgery. Colorectal Dis 2017;19:O402–O406 [DOI] [PubMed] [Google Scholar]
- 101. Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ 2013;346:f2914 [DOI] [PubMed] [Google Scholar]
- 102. Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med 2013;11:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Woodfield JC, Clifford K, Schmidt B, Turner GA, Amer MA, McCall JL. Strategies for antibiotic administration for bowel preparation among patients undergoing elective colorectal surgery: a network meta-analysis. JAMA Surg 2022;157:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rollins KE, Javanmard-Emamghissi H, Acheson AG, Lobo DN. The role of oral antibiotic preparation in elective colorectal surgery: a meta-analysis. Ann Surg 2019;270:43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chung RS, Gurll NJ, Berglund EM. A controlled clinical trial of whole gut lavage as a method of bowel preparation for colonic operations. Am J Surg 1979;137:75–81 [DOI] [PubMed] [Google Scholar]
- 106. Badia JM, Casey AL, Rubio-Pérez I, Arroyo-García N, Espin E, Biondo Set al. Awareness of practice and comparison with best evidence in surgical site infection prevention in colorectal surgery. Surg Infect (Larchmt) 2019;21:218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Markell KW, Hunt BM, Charron PD, Kratz RJ, Nelson J, Isler JTet al. Prophylaxis and management of wound infections after elective colorectal surgery: a survey of the American Society of Colon and Rectal Surgeons membership. J Gastrointest Surg 2010;14:1090–1098 [DOI] [PubMed] [Google Scholar]
- 108. Scarborough JE, Mantyh CR, Sun Z, Migaly J. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: an analysis of colectomy-targeted ACS NSQIP. Ann Surg 2015;262:331–337 [DOI] [PubMed] [Google Scholar]
- 109. National Institute for Health and Care Excellence . Surveillance of Surgical Site Infections: Prevention and Treatment (NICE Guideline NG125). 19 August 2019 [PubMed]
- 110. Bucher P, Gervaz P, Egger J-F, Soravia C, Morel P. Morphologic alterations associated with mechanical bowel preparation before elective colorectal surgery: a randomized trial. Dis Colon Rectum 2006;49:109–112 [DOI] [PubMed] [Google Scholar]
- 111. Fa-Si-Oen PR, Penninckx F. The effect of mechanical bowel preparation on human colonic tissue in elective open colon surgery. Dis Colon Rectum 2004;47:948–949 [DOI] [PubMed] [Google Scholar]
- 112. Rejchrt S, Bures J, Siroký M, Kopácová M, Slezák L, Langr F. A prospective, observational study of colonic mucosal abnormalities associated with orally administered sodium phosphate for colon cleansing before colonoscopy. Gastrointest Endosc 2004;59:651–654 [DOI] [PubMed] [Google Scholar]
- 113. Kale TI, Kuzu MA, Tekeli A, Tanik A, Aksoy M, Cete M. Aggressive bowel preparation does not enhance bacterial translocation, provided the mucosal barrier is not disrupted: a prospective, randomized study. Dis Colon Rectum 1998;41:636–641 [DOI] [PubMed] [Google Scholar]
- 114. Poole GV. Spontaneous bacterial peritonitis during bowel preparation: an example of clinical translocation. South Med J 1991;84:1412–1413 [DOI] [PubMed] [Google Scholar]
- 115. Berg RD. Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol 1999;473:11–30 [DOI] [PubMed] [Google Scholar]
- 116. Gao RQ, Wang WD, Yu PF, Mo ZC, Dong DH, Yang XSet al. Are preoperative oral antibiotics effective in reducing the incidence of anastomotic leakage after colorectal cancer surgery? Study protocol for a prospective, multicentre, randomized controlled study. Trials 2022;23:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pellino G, Solís-Peña A, Kraft M, Huguet BM, Espín-Basany E. Preoperative oral antibiotics with versus without mechanical bowel preparation to reduce surgical site infections following colonic resection: protocol for an international randomized controlled trial (ORALEV2). Colorectal Dis 2021;23:2173–2181 [DOI] [PubMed] [Google Scholar]
- 118. Koskenvuo L, Lunkka P, Varpe P, Hyöty M, Satokari R, Haapamäki Cet al. Mechanical bowel preparation and oral antibiotics versus mechanical bowel preparation only prior rectal surgery (MOBILE2): a multicentre, double-blinded, randomised controlled trial-study protocol. BMJ Open 2021;11:e051269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Balasubramanian I, Sehgal R, McNamara E, Condon E, Waldron D, Coffey JCet al. The role of selective decontamination of the digestive tract in preventing surgical site infections in elective colorectal resections: a randomized controlled trial (SELDDEC trial). Colorectal Dis 2018;20:14130182470 [Google Scholar]
- 120. Yeom CH, Cho MM, Baek SK, Bae OS. Risk factors for the development of Clostridium difficile-associated colitis after colorectal cancer surgery. J Korean Soc Coloproctol 2010;26:329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Krapohl GL, Morris AM, Cai S, Englesbe MJ, Aronoff DM, Campbell DA Jret al. Preoperative risk factors for postoperative Clostridium difficile infection in colectomy patients. Am J Surg 2013;205:343–347; discussion 347–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Al-Mazrou AM, Hyde LZ, Suradkar K, Kiran RP. Effect of inclusion of oral antibiotics with mechanical bowel preparation on the risk of Clostridium difficile infection after colectomy. J Gastrointest Surg 2018;22:1968–1975 [DOI] [PubMed] [Google Scholar]
- 123. Veyrie N, Ata T, Muscari F, Couchard AC, Msika S, Hay JMet al. Anastomotic leakage after elective right versus left colectomy for cancer: prevalence and independent risk factors. J Am Coll Surg 2007;205:785–793 [DOI] [PubMed] [Google Scholar]
- 124. Choy PY, Bissett IP, Docherty JG, Parry BR, Merrie AE. Stapled versus handsewn methods for ileocolic anastomoses. Cochrane Database Syst Rev 2007; (3)CD004320 [DOI] [PubMed] [Google Scholar]
- 125. Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 2007;246:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]