More than 30,000 monkeypox (mpox) cases have been diagnosed in the United States since May 2022, primarily among gay, bisexual, and other men who have sex with men (MSM) (1,2). In recent months, diagnoses have declined to one case per day on average. However, mpox vaccination coverage varies regionally, suggesting variable potential risk for mpox outbreak recurrence (3). CDC simulated dynamic network models representing sexual behavior among MSM to estimate the risk for and potential size of recurrent mpox outbreaks at the jurisdiction level for 2023 and to evaluate the benefits of vaccination for preparedness against mpox reintroduction. The risk for outbreak recurrence after mpox reintroduction is linearly (inversely) related to the proportion of MSM who have some form of protective immunity: the higher the population prevalence of immunity (from vaccination or natural infection), the lower the likelihood of recurrence in that jurisdiction across all immunity levels modeled. In contrast, the size of a potential recurrent outbreak might have thresholds: very small recurrences are predicted for jurisdictions with mpox immunity of 50%–100%; exponentially increasing sizes of recurrences are predicted for jurisdictions with 25%–50% immunity; and linearly increasing sizes of recurrences are predicted for jurisdictions with <25% immunity. Among the 50 jurisdictions examined, 15 are predicted to be at minimal risk for recurrence because of their high levels of population immunity. This analysis underscores the ongoing need for accessible and sustained mpox vaccination to decrease the risk for and potential size of future mpox recurrences.
CDC adapted models of mpox transmission to estimate the risk for and size of potential mpox recurrences at varying levels of population-level mpox immunity (4,5). Immunity varied from 0%–99% in increments of approximately 4%. Immunity levels included persons who had received 1 or 2 vaccine doses or had a history of infection, which conveyed 37%, 67%, and 100% reduction in susceptibility to infection, respectively (6). At each immunity level modeled, 29%, 67%, and 4% of those with some immunity were assumed to have 1-dose, 2-dose, or infection-acquired immunity, respectively, with immunity concentrated among MSM with higher levels of sexual activity (3,4). Sensitivity analyses considered 65% and 83% reductions in susceptibility associated with receipt of 1 or 2 doses, respectively (6).
To model mpox reintroduction, five MSM with infectious mpox and high levels of sexual activity were introduced to the sexual network. Depending on the level of immunity and chance, introduced cases either initiated an outbreak (of variable size) or failed to sustain transmission. An outbreak was defined as a simulation with sustained mpox incidence 3 months after reintroduction. For each immunity level, model simulations were conducted until 50 simulated recurrent outbreaks occurred. This analysis assumed no additional vaccination after April 28, 2023, no behavioral adaptation (such as decreasing partner acquisition rates) among MSM in response to new mpox cases, and no loss of immunity due to demographic turnover during the 2-year period modeled.
Risk was assessed at the jurisdiction level for the 50 nonstate, Ending the HIV Epidemic (EHE) Initiative jurisdictions based on these results and each jurisdiction’s case and vaccine administration data, through April 2023 (4,5). These jurisdictions, many of which contain urban centers with large MSM populations, account for more than one half of all new HIV diagnoses.* The numerator for jurisdiction immunity level was the sum of persons who had received 1 and 2 doses of JYNNEOS vaccine and those who had already been infected, accounting for potential incomplete reporting (2,3,5). The denominator was based on the population at increased risk for Monkeypox virus exposure, estimated as the number of MSM aged ≥16 years who were recommended to receive HIV preexposure prophylaxis and the number of MSM aged ≥13 years living with HIV in each jurisdiction, using publicly available data (7,8). The estimated population was then increased by 25% for each jurisdiction to account for additional persons eligible for vaccination (e.g., MSM with lower levels of sexual activity than their already-eligible partners). Statistical models were fit to the simulated outbreak results to summarize the relationship between immunity level and both risk for and size of outbreak recurrence; each jurisdiction’s risk for and size of outbreak recurrence was then then inferred based on jurisdiction-specific immunity using these fitted curves. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.†
Conditional on mpox reintroduction into a jurisdiction, the risk for a recurrent mpox outbreak is linearly related to immunity: each percentage point increase in population immunity reduces outbreak risk by 0.62 percentage points across all immunity levels modeled (Figure 1). For example, Suffolk County, Massachusetts, with an estimated at-risk population immunity of 64%, has a 21% risk for a recurrent outbreak, whereas Harris County, Texas, with 17% immunity has a 50% risk, conditional on reintroduction (Table). No critical threshold to avert a recurrent mpox outbreak was identified; higher vaccination coverage among MSM at risk provides continued decreased recurrence risk across all immunity levels modeled. Sensitivity analyses using higher efficacy estimates of 65% and 83%, respectively, decreased the recurrence risk by 6 percentage points when population immunity was >20% (Supplementary Figure, https://stacks.cdc.gov/view/cdc/128422).
FIGURE 1.
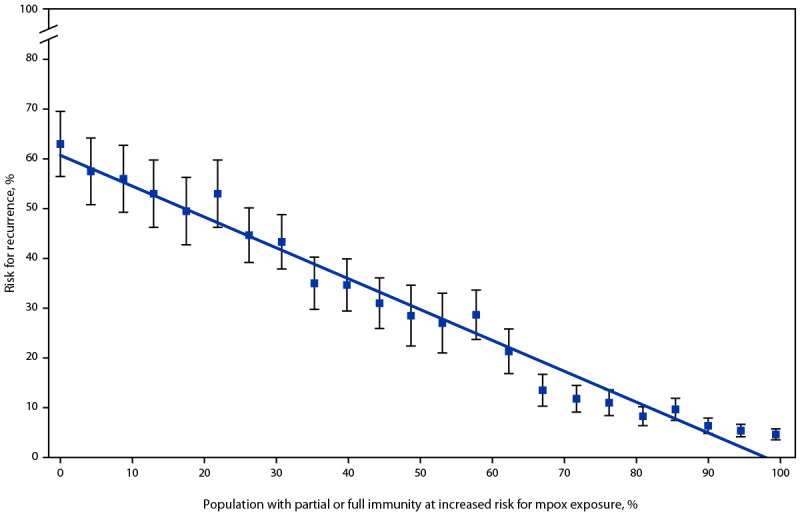
Risk* for recurrent mpox outbreak lasting >3 months, by immunity level† — United States, 2023
Abbreviations: mpox = monkeypox; MSM = gay, bisexual, and other men who have sex with men.
* Data points reflect simulated risk for a specific immunity level; line reflects predictions from linear model using immunity level as the independent variable and risk for recurrence as the dependent variable; error bars indicate 95% CIs assuming a binomial distribution based on the number of simulations required to produce 50 outbreaks.
† Immunity was varied from 0% to 99% in increments of approximately 4%; at each level of immunity, 29%, 67%, and 4% of MSM with some immunity are assumed to have 1-dose, 2-dose, or infection-acquired immunity, conveying 37%, 67%, and 100% protection, respectively.
TABLE. Jurisdiction-specific estimates of immunity and inferred* risk and size of mpox recurrence — United States, 2023.
| Jurisdiction | Estimated immunity level, %† | Inferred* risk for recurrence, % | Inferred* cumulative Monkeypox virus infections vs. 2022§ | Jurisdictional immunity grouping¶ | MSM at increased risk for Monkeypox virus exposure** |
|---|---|---|---|---|---|
| Duval County, Florida |
6 |
57 |
4.08 |
Low |
12,425 |
| Shelby County, Tennessee |
10 |
55 |
3.77 |
Low |
10,626 |
| Hamilton County, Ohio |
10 |
55 |
3.79 |
Low |
9,970 |
| Bexar County, Texas |
11 |
54 |
3.67 |
Low |
17,916 |
| Dallas County, Texas |
12 |
53 |
3.62 |
Low |
45,264 |
| Tarrant County, Texas |
15 |
51 |
3.32 |
Low |
15,909 |
| Palm Beach County, Florida |
15 |
52 |
3.36 |
Low |
12,824 |
| Hillsborough County, Florida |
15 |
52 |
3.39 |
Low |
17,802 |
| Wayne County, Michigan |
16 |
51 |
3.29 |
Low |
14,705 |
| Harris County, Texas |
17 |
50 |
3.16 |
Low |
60,769 |
| San Bernardino County, California |
18 |
49 |
3.07 |
Low |
15,829 |
| East Baton Rouge Parish, Louisiana |
18 |
50 |
3.14 |
Low |
3,735 |
| Baltimore City, Maryland |
19 |
49 |
3.04 |
Low |
10,800 |
| Pinellas County, Florida |
20 |
48 |
2.96 |
Low |
13,430 |
| Gwinnett County, Georgia |
21 |
48 |
2.89 |
Low |
5,672 |
| Marion County, Indiana |
24 |
46 |
2.60 |
Low |
12,681 |
| Fulton County, Georgia |
25 |
45 |
2.58 |
Low |
27,831 |
| Prince George's County, Maryland |
26 |
44 |
2.03 |
Medium |
9,007 |
| Orange County, Florida |
26 |
45 |
2.09 |
Medium |
21,838 |
| Dekalb County, Georgia |
26 |
45 |
2.12 |
Medium |
14,053 |
| Cuyahoga County, Ohio |
27 |
44 |
1.90 |
Medium |
11,470 |
| Cobb County, Georgia |
27 |
44 |
1.96 |
Medium |
5,980 |
| Essex County, New Jersey |
29 |
43 |
1.66 |
Medium |
7,806 |
| Franklin County, Ohio |
31 |
42 |
1.41 |
Medium |
15,752 |
| Travis County, Texas |
32 |
41 |
1.30 |
Medium |
16,218 |
| San Juan Municipio, Puerto Rico |
32 |
41 |
1.30 |
Medium |
3,773 |
| Maricopa County, Arizona |
32 |
41 |
1.33 |
Medium |
33,513 |
| Mecklenburg County, North Carolina |
33 |
40 |
1.18 |
Medium |
12,947 |
| Montgomery County, Maryland |
34 |
40 |
1.10 |
Medium |
7,515 |
| Clark County, Nevada |
36 |
39 |
0.97 |
Medium |
20,231 |
| Bronx County, New York |
36 |
39 |
0.98 |
Medium |
19,723 |
| Hudson County, New Jersey |
37 |
38 |
0.86 |
Medium |
8,009 |
| Miami-Dade County, Florida |
40 |
36 |
0.72 |
Medium |
40,489 |
| Orange County, California |
45 |
33 |
0.48 |
Medium |
17,090 |
| Philadelphia County, Pennsylvania |
47 |
32 |
0.42 |
Medium |
18,771 |
| Sacramento County, California |
52 |
28 |
0.20 |
High |
9,723 |
| San Diego County, California |
54 |
27 |
0.19 |
High |
27,536 |
| Riverside County, California |
58 |
25 |
0.18 |
High |
21,314 |
| Broward County, Florida |
59 |
24 |
0.18 |
High |
33,886 |
| Orleans Parish, Louisiana |
61 |
23 |
0.18 |
High |
8,057 |
| Cook County, Illinois |
63 |
22 |
0.17 |
High |
60,444 |
| Los Angeles County, California |
63 |
22 |
0.17 |
High |
117,361 |
| Suffolk County, Massachusetts |
64 |
21 |
0.17 |
High |
10,356 |
| King County, Washington |
65 |
20 |
0.16 |
High |
24,308 |
| Alameda County, California |
75 |
14 |
0.14 |
High |
14,167 |
| Queens County, New York |
78 |
12 |
0.13 |
High |
20,057 |
| District of Columbia |
98 |
<1 |
0.08 |
High |
22,348 |
| Kings County, New York |
99 |
<1 |
0.07 |
High |
30,540 |
| New York County, New York |
100 |
<1 |
0.07 |
High |
37,900 |
| San Francisco County, California | 100 | <1 | 0.07 | High | 23,577 |
Abbreviations: mpox = monkeypox; MSM = gay, bisexual, and other men who have sex with men.
* Risk and size of potential recurrence inferred from each jurisdiction's estimated immunity level based on primary simulated results.
† At each level of immunity, 29%, 67%, and 4% of MSM with some immunity are assumed to have 1-dose, 2-dose, or infection-acquired immunity, conveying 37%, 67%, and 100% protection, respectively.
§ Median cumulative infections from simulations, relative to the size of the CDC-modeled 2022 mpox outbreak in the absence of additional vaccination or behavioral adaptation.
¶ Grouping based on thresholds identified in primary analysis. Threshold values are as follows: high >50%, medium = 25%–50%, and low <25%.
** Estimated as the number of MSM aged ≥16 years who were recommended to receive HIV preexposure prophylaxis and the number of MSM aged ≥13 years living with HIV in each jurisdiction, using publicly available data. The estimated population was then increased by 25% for each jurisdiction to account for additional persons eligible for vaccination (e.g., MSM with lower levels of sexual activity than their already-eligible partners).
In contrast to the linear relationship between increasing levels of immunity in the population at risk and the risk for a recurrent outbreak, immunity does have a threshold effect on the size of a recurrent outbreak (Figure 2). Three distinct jurisdictional groupings were identified: those with high, medium, and low immunity, defined as 50%–100%, 25%–49%, and <25% vaccine- or infection-induced immunity, respectively. High-immunity jurisdictions were predicted to experience small recurrences that resolved within 1 year. Medium-immunity jurisdictions were transitional, with more uncertainty: recurrence size increased exponentially and lasted 12–17 months. Finally, low-immunity jurisdictions were predicted to experience recurrences lasting 18–20 months that increased linearly in size with decreasing levels of immunity.
FIGURE 2.
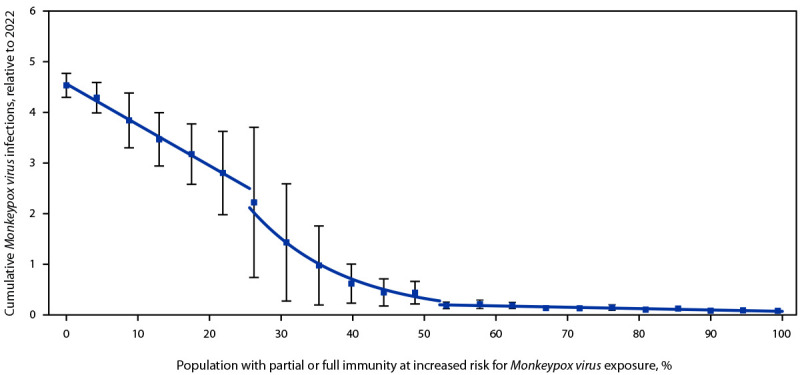
Cumulative Monkeypox virus infections* relative to 2022, by immunity level† — United States, 2023
Abbreviations: mpox = monkeypox; MSM = gay, bisexual, and other men who have sex with men.
* Median cumulative infections from simulations, measured among simulations in which an outbreak occurred, relative to the size of the CDC-modeled 2022 mpox outbreak; data points reflect simulated cumulative infection magnitude for a specific immunity level; lines reflect three separate linear model fits using immunity level as the independent variable and median cumulative incidence as the dependent variable among each threshold; first and last threshold were fit using estimates directly, and middle threshold was fit using log-transformed incidence estimates to reflect the rapid change in outbreak magnitude during this period; error bars indicate 25th–75th quartile observed across outbreaks.
† Immunity was varied from 0% to 99% in increments of approximately 4%; at each level of immunity, 29%, 67%, and 4% of MSM with some immunity are assumed to have 1-dose, 2-dose, or infection-acquired immunity, conveying 37%, 67%, and 100% protection, respectively.
Overall, 44% of MSM at increased risk for Monkeypox virus exposure who live in EHE jurisdictions live in high-immunity jurisdictions that are likely to have minimal risk for recurrence, including Los Angeles, New York City, San Francisco, and Washington, DC (Table). However, 56% of MSM at increased risk for exposure who live in EHE jurisdictions live in low- or medium-immunity jurisdictions that are potentially at risk for mpox recurrences capable of sustained transmission should reintroduction occur (Table).
Discussion
This analysis, which highlights the association between population mpox immunity and the risk for outbreak recurrence, underscores the need for accessible and sustained mpox vaccination services, particularly in communities with low vaccination coverage and among MSM at highest risk. Jurisdictions that achieved high vaccination coverage among populations at risk are not expected to experience large recurrences in the immediate future. Success in achieving high coverage in these jurisdictions was partially driven by early prioritization for vaccine distribution because of high early case counts when there was high vaccination demand. In other jurisdictions, vaccines became available after the outbreak had peaked and when demand had declined because of reduced perception of risk (5).
The findings in this report are subject to at least four limitations. First, to consider the downstream effects of mpox reintroduction and guide preparedness, mpox reintroduction was assumed to be certain. However, actual reintroduction risk will be influenced by global and local mpox infection dynamics. Although the intensity of the 2022 multinational outbreak has diminished substantially, areas with ongoing transmission remain. Attendance at large social engagements that attract domestic and international MSM travelers could result in reintroduction of mpox into local sexual networks. Assuming reintroduction is independent of subsequent outbreak recurrence risk, reintroduction has a proportional effect on recurrence: for example, if there were a 50% probability of reintroduction, the risk for recurrence would be 50% lower than that presented in the current analysis. In the absence of additional vaccination or mpox cases, immunity will decline in future years because of demographic turnover, thereby increasing recurrence risk. In addition, small outbreaks might occur even when the estimated risk for a large outbreak is relatively low. As an example, based on this model, Cook County, Illinois, has an estimated 22% risk for a sustained mpox recurrence; however, the city of Chicago, which is part of Cook County, has reported a new cluster of mpox cases that emerged in April 2023 (9). Second, inferred estimates of vaccine efficacy from Israel and the United States show a range of effectiveness (6). Conservative estimates for 1- and 2-dose efficacy of 37% and 67%, respectively, were used in the primary analysis, although sensitivity analysis examining higher efficacy did not appreciably change the results. Third, many of the sexual behavior data were derived from surveys conducted in 2012, and it is possible that patterns have changed over time and in response to recent disruptive public health events, including this mpox outbreak (10). The actual risk for and size of recurrent outbreaks might differ among the actual social and sexual engagements of MSM, which might also vary between communities. Finally, this analysis assumed no interventions took place in response to mpox reintroduction. However, this assumption highlights the benefits of vaccination for preparedness against mpox reintroduction. Delays between detection of reintroduction, mobilization of additional vaccination sites, vaccine administration, and immunologic protection are difficult to shorten, potentially leaving vulnerable communities at risk.
This analysis highlights the importance of public health programs identifying opportunities to promote vaccination before Pride-related and other events when vaccination interest might be higher, rather than vaccinating after reintroduction is identified. Focusing these vaccination efforts in low-coverage areas, and even in high-coverage areas, among MSM who are younger and newly sexually active and among groups with disproportionally low vaccination coverage, can help protect both individual persons and the entire community against a resurgence of mpox. CDC continues to recommend a full 2-dose course of the JYNNEOS vaccine for MSM and others at risk for Monkeypox virus exposure.
Summary.
What is already known about this topic?
Monkeypox (mpox) has disproportionately affected gay, bisexual, and other men who have sex with men (MSM); the percentage of MSM with immunity due to vaccination or infection varies among jurisdictions.
What is added by this report?
Mathematical modeling suggests that the risk for future outbreaks depends linearly on the level of immunity in the population at risk; cumulative incidence, on the other hand, has multiple thresholds. More than 592,000 MSM live in jurisdictions with risk for mpox recurrences capable of sustained transmission if a cluster of infectious cases were reintroduced.
What are the implications for public health practice?
Increasing vaccination coverage among MSM at risk and in jurisdictions with low immunity has the potential to reduce the risk for and potential size of future mpox outbreaks.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Footnotes
45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
References
- 1.Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of monkeypox cases—United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1018–22. 10.15585/mmwr.mm7132e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Mpox: 2022 U.S. map & case count. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. Accessed March 14, 2023. https://www.cdc.gov/poxvirus/mpox/response/2022/us-map.html
- 3.Owens LE, Currie DW, Kramarow EA, et al. JYNNEOS vaccination coverage among persons at risk for mpox—United States, May 22, 2022–January 31, 2023. MMWR Morb Mortal Wkly Rep 2023;72:342–7. 10.15585/mmwr.mm7213a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spicknall IH, Pollock ED, Clay PA, et al. Modeling the impact of sexual networks in the transmission of Monkeypox virus among gay, bisexual, and other men who have sex with men—United States, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1131–5. 10.15585/mmwr.mm7135e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clay PA, Asher JM, Carnes N, et al. Modelling the impact of vaccination and sexual behavior change on reported cases of mpox in Washington, DC. medRxiv [Preprint posted online February 14, 2023]. 10.1101/2023.02.10.23285772 [DOI]
- 6.Chard A. JYNNEOS vaccine effectiveness. Presented at the Advisory Committee on Immunization Practices meeting; February 23, 2023, Atlanta, Georgia. https://stacks.cdc.gov/view/cdc/124951
- 7.CDC. Mpox science brief: detection and transmission of mpox (formerly Monkeypox) virus during the 2022 Clade IIb outbreak. Atlanta, GA: US Department of Health and Human Services, CDC; 2023. Accessed February 21, 2023. https://www.cdc.gov/poxvirus/mpox/about/science-behind-transmission.html
- 8.CDC. AtlasPlus. Atlanta, GA: US Department of Health and Human Services, CDC; 2023. Accessed March 14, 2023. https://www.cdc.gov/nchhstp/atlas/index.htm
- 9.Chicago Department of Public Health. Mpox (Monkeypox) data dashboard. Chicago, IL: Chicago Department of Public Health; 2023. Accessed May 10, 2023. https://www.chicago.gov/city/en/sites/monkeypox/home/data.html
- 10.Hernández-Romieu AC, Sullivan PS, Rothenberg R, et al. Heterogeneity of HIV prevalence among the sexual networks of black and white men who have sex with men in Atlanta: illuminating a mechanism for increased HIV risk for young black men who have sex with men. Sex Transm Dis 2015;42:505–12. 10.1097/OLQ.0000000000000332 [DOI] [PMC free article] [PubMed] [Google Scholar]


