Abstract
The accumulation of calcium in atherosclerotic plaques is a prominent feature of advanced atherosclerosis, and it has a strong positive correlation with the total burden of atherosclerosis. Atherosclerotic calcification usually appears first at the necrotic core, indicating that cell death and inflammatory processes are involved in calcification. During atherosclerotic inflammation, various cell types, such as vascular smooth muscle cells, nascent resident pericytes, circulating stem cells, or adventitial cells, have been assumed to differentiate into osteoblastic cells, which lead to vascular calcification. Among these cell types, vascular smooth muscle cells are considered a major contributor to osteochondrogenic cells in the atherosclerotic milieu. In this review, we summarize the molecular mechanisms underlying the osteochondrogenic switch of vascular smooth muscle cells in atherosclerotic plaques.
Keywords: Vascular smooth muscle cells, Atherosclerosis, Calcification, Osteochondrogenic
INTRODUCTION
Atherosclerosis is a major pathological process underlying most cardiovascular diseases (such as heart disease, stroke, angina pectoris, and aneurysms), which combined are the leading cause of global death.1 Once largely limited to Western nations, atherosclerosis now affects people worldwide, including those living in developing countries, mainly due to population growth and increased life expectancy.2 Atherosclerosis reproducibly affects multifocal sites of the arterial tree. It typically impacts the inner curvatures and branch points, such as the coronary arteries, iliofemoral arteries, abdominal arteries, and carotid bifurcations, which are areas with low or oscillatory endothelial shear stress.3 Atherosclerosis is primarily driven by native or aggregated low-density lipoproteins (LDLs) in combination with other risk factors, including diabetes mellitus, smoking, hypertension, and male sex. Once accumulated in the arterial intima, LDLs are modified by oxidation and aggregation and act as chronic stimulators of innate and adaptive immune responses. In turn, endothelial cells and vascular smooth muscle cells (VSMCs) express adhesion molecules, chemoattractants, and variable cytokines that attract monocytes, which then differentiate into macrophages and monocyte-derived dendritic cells. Lipid-phagocytized macrophages are involved in various pathological events, including apoptosis, necrosis, VSMC proliferation, matrix synthesis, and calcification.3
Based on their progression, atherosclerotic lesions can be classified into several histological subtypes, including adaptive intimal thickening, intimal xanthoma, pathological intimal thickening, fibroatheroma, and fibrocalcific plaque.4 Adaptive intimal thickening—the earliest type of lesion to appear—is characterized by the intimal accumulation of VSMCs, while intimal xanthoma involves the intimal accumulation of foam cells. The lesion then progresses to pathological intimal thickening, characterized by the extracellular accumulation of lipid pools without apparent necrosis. Fibroatheroma indicates an advanced stage of atherosclerosis, characterized by the formation of a fibrous cap and necrotic core, as well as the accumulation of a collagenous extracellular matrix (ECM). The lesion eventually accumulates calcified areas in the necrotic core and surrounding tissues and becomes a fibrocalcific plaque.
Atherosclerosis causes clinical complications primarily via thrombosis or the limitation of blood flow. Depending on the affected vascular beds, various clinical sequelae can occur, such as angina and myocardial infarction in the coronary arteries or stroke in the carotid arteries.5 In the past, the rupture of vulnerable plaques, histologically characterized by a large lipid- and macrophage-rich necrotic core and a thin fibrous cap, was considered a major mechanism of atherosclerotic thrombosis.3 However, recent clinical studies have suggested that other mechanisms—such as plaque erosion, which is histologically characterized by thrombosis formation on an ECM-rich, less inflamed, and lipid-laden plaque without a thin fibrous cap—can be principal causes of atherosclerosis-induced thrombosis.6 In addition to plaque rupture and erosion, calcified nodules, which are formed by atherosclerotic calcification and have distinct morphology, reportedly contribute to a small proportion (up to 5%) of atherosclerosis-induced thrombosis cases.4,7
In this review, we discuss calcification in atherosclerosis, which is a characteristic feature of advanced atherosclerotic plaques. We detail the cell types and underlying mechanisms responsible for atherosclerotic calcification, particularly VSMCs, which are considered a major contributor to atherosclerotic calcification through the osteochondrogenic transition.
CALCIFICATION IN ATHEROSCLEROSIS
Atherosclerotic lesions contain various noncellular components, such as collagen, elastin, proteoglycans, glycosaminoglycan, and calcium. As lesions progress, these noncellular components, especially fibrous tissue and calcium, tend to accumulate gradually and often become the primary plaque components.3 Coronary artery calcification can be readily measured and quantified through computed tomography. The resulting coronary artery calcium (CAC) score is strongly positively associated with the total burden of atherosclerosis, indicating that calcification is a characteristic phenomenon of atherosclerosis.8 The CAC score can be used as a sensitive and specific predictor of clinically significant coronary artery disease and identify patients at risk for adverse cardiac events. Currently, it is generally agreed that high CAC scores predict high risk at the patient level rather than a vulnerable plaque or vessel. Potential roles of atherosclerotic calcification in plaque stability have been proposed based on the size, location, and shape of the calcification. For instance, microcalcifications and spotty calcifications are regarded as pro-inflammatory processes and negatively impact plaque stability. In contrast, macrocalcifications formed in the deep intima or necrotic core are considered to stabilize plaques.9 However, substantial debate persists regarding whether atherosclerotic calcification can serve as a marker of plaque stability or instability.8
Atherosclerotic calcification can give rise to a distinct type of lesion termed a calcified nodule, which is histologically characterized by erupted and fractured calcified plates on a disrupted fibrous cap, accompanied by a luminal thrombus.10 Although calcified nodules account for a minority (up to 5%) of the causes of atherosclerosis-related thrombosis, their clinical implications have been highlighted in a retrospective study. Calcified nodules were found to be associated with higher incidence rates of hypertension, chronic kidney disease (CKD), and maintenance hemodialysis. A follow-up study revealed that calcified nodules are highly correlated with major adverse cardiac events, as defined by a combined outcome of death related to cardiac failure, recurrence of acute coronary syndrome, and target lesion revascularization. In addition, when target lesion revascularization was performed after the installment of drug-eluting stents in patients with calcified nodules, the recurrence rate of the calcified nodules within the stents was 82.4%.7 These observations indicate that calcified nodules can hinder the neointimal suppression capacity of drug-eluting stents, suggesting that different treatment approaches may be required for atherosclerotic lesions that harbor calcified nodules. To date, drugs that prevent or treat atherosclerotic calcification—including statins, a major treatment option for atherosclerosis—are not available.11
Atherosclerotic calcification usually appears first in the necrotic core, implying that cell death (necrosis and apoptosis) and inflammation are involved in the calcification process. Previous findings indicate that apoptotic bodies and matrix vesicles from intralesional cells (e.g., macrophages/foam cells and VSMCs) can act as nucleating agents to promote calcification. Various environmental cues such as inflammatory cytokines (including tumor necrosis factor alpha and interleukin 6), oxidative stress, bone morphogenetic proteins (BMPs) 2 and 4, changes in pyrophosphate levels, and osteocalcin can accelerate atherosclerotic calcification.12 Conversely, various cytokines and molecules such as osteopontin, matrix Gla protein, osteoprotegerin, sclerostin, fibroblast growth factor 23, fetuin, and BMP7 can inhibit atherosclerotic calcification. Systemic factors, including CKD, parathyroid hormone, vitamin D, glucocorticoids, diabetes, menopause, and osteoporosis, are also known to affect vascular calcification.11,12
In the past, vascular calcification was regarded as a passive, degenerative, and unregulated process. However, calcifications of both the intima (atherosclerotic calcification) and the media (medial calcification) are now considered to be tightly regulated active processes that recapitulate bone morphogenesis.11 Studies have indicated the existence of osteoblast- and chondrocyte-like cells that express bone- and cartilage-related transcription factors such as MSX2, RUNX2, and SP7 during vascular calcification. Studies have suggested that various cell types, such as VSMCs, nascent resident pericytes, circulating stem cells, or adventitial cells, can differentiate into osteoblastic cells in vascular calcification.12 Among these cell types, VSMCs are considered the major contributor of the osteochondrogenic cells in the atherosclerotic milieu that produce calcification.11 VSMCs integrate various atherogenic signals and differentiate into osteochondrogenic cells primarily through the BMP signaling pathway (Fig. 1), a process that we will discuss in further detail below.
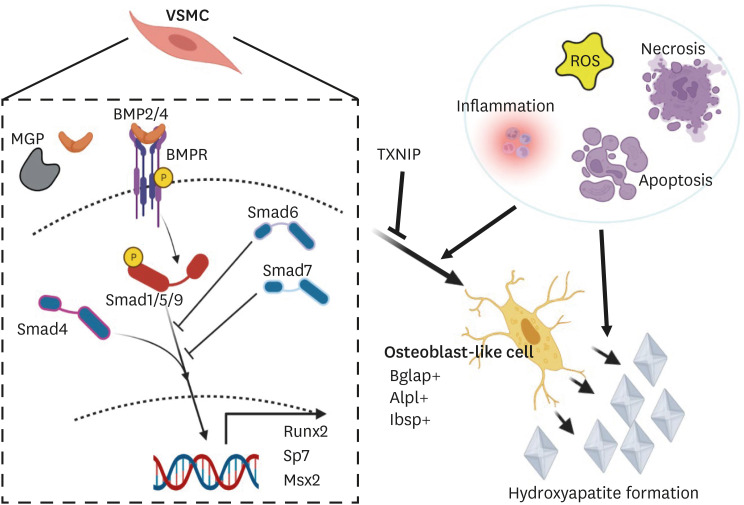
Fig. 1. Graphical abstract summarizing the osteochondrogenic switch of VSMCs and underlying mechanism of atherosclerotic calcification. BMP signaling is known to play a pivotal role in vascular calcification. During atherogenesis, VSMCs can undergo various phenotypic switches, including to synthetic, foam cell, macrophage-like, adipocyte-like, myofibroblast-like, MSC-like, endothelial cell-like, and osteochondrogenic cells. The osteochondrogenic switch of VSMCs appears to be a key step in atherosclerotic calcification and is mediated by canonical and non-canonical BMP signaling. TXNIP can suppress the osteochondrogenic switch of VSMCs during atherosclerosis by inhibiting BMP signaling.
VSMCs, vascular smooth muscle cells; BMP, bone morphogenetic protein; MSC, mesenchymal stem cell; TXNIP, thioredoxin-interacting protein; ROS, reactive oxygen species.
VSMCs IN ATHEROSCLEROSIS
VSMCs occupy the largest cellular portion of the aorta, constituting the tightly woven medial layer along with elastic fibers. Under steady-state conditions, VSMCs are densely packed, exhibit spindle-shaped morphology, and express typical contractile markers, such as ACTA2, TAGLN, and MYH11; thus, they can exert contractile properties to fulfill their role of controlling vascular tone and blood pressure. During vascular injury or atherosclerosis, VSMCs undergo phenotypic switching, which is initially characterized by the downregulation of contractile genes.13 In early VSMC research, the loss of such typical contractile markers hampered the accurate assessment of VSMC-derived cells in atherosclerotic lesions. However, the development of a lineage tracing reporter system method (a mouse model harboring the Myh11-CreERT2 transgene along with the Cre-inducible fluorescent reporter transgene) has enabled accurate tracking of VSMC-derived cells, even when VSMC-specific contractile marker expression is lost. Lineage tracing studies have provided solid evidence that VSMC-derived cells constitute a substantial proportion (30%–70%) of all plaque cells; they have also shown that VSMCs can yield multiple phenotypes, such as macrophage-like, myofibroblast-like, or osteochondrogenic cells.14,15,16,17 In addition, mouse studies involving lineage tracing with a multicolor reporter system, such as a confetti or rainbow system, have revealed an interesting phenomenon: only a few VSMCs from the medial layer give rise to the VSMC-derived cells of the entire lesion through clonal expansion.15,18 The underlying mechanisms of this phenomenon are under investigation.
Various in vivo and in vitro studies have indicated that VSMCs can adopt multiple phenotypes, including synthetic, foam cell, macrophage-like, adipocyte-like, myofibroblast-like, mesenchymal stem cell (MSC)-like, endothelial cell-like, and osteochondrogenic cells.13 Recently, mouse studies using a combination of single-cell RNA sequencing (scRNA-seq) and a VSMC-specific lineage tracing system unbiasedly characterized several atherosclerotic VSMC-derived cell types at the transcriptomic level. Based on the scRNA-seq data, VSMCs produce transcriptomically distinct clusters, namely modulated VSMCs, osteochondrogenic cells, and foam cells/macrophage-like cells.16,19,20,21
Modulated VSMCs, or named differently depending on the groups albeit showing similar transcriptomic profiles (such as the intermediate cell state,16 fibromyocytes,21 and Lgals3+ VSMCs19) despite showing similar transcriptomic profiles, express the stem cell markers Ly6a and Lgals3 with decreased contractile gene expression. The modulated VSMC cluster appears to act as a pioneer cell population that eventually transdifferentiates into the osteochondrogenic cluster. Through reference-based integration of mouse and human scRNA-seq data, Pan et al.16 showed that modulated VSMC and osteochondrogenic clusters also exist in human atheromas.
The regulation of dedifferentiation and phenotypic switching of VSMCs is primarily achieved at the transcriptional level.13 The contractile properties of VSMCs are transcriptionally controlled by serum response factor (SRF) and its coactivator myocardin (MYOCD).22,23 SRF binds to the DNA consensus sequence CC(A/T)6GG, termed the CArG box, within the promoters of contractile genes. SRF is ubiquitously expressed, whereas MYOCD is specifically expressed in smooth muscle cells (SMCs) and cardiomyocytes. Because SRF must be bound to MYOCD for contractile genes to be expressed, the combination of SRF and MYOCD enables the SMC-specific expression of contractile genes. MYOCD serves as a primary regulator of VSMC differentiation, as many factors related to the phenotypic change of VSMCs directly or indirectly alter the expression or activity of MYOCD.13 Other relatively well-studied factors that regulate the phenotypic transition of VSMCs include stem cell pluripotency factor Krüppel-like factor 4 (KLF4), octamer-binding transcription factor 4 (OCT4), and transcription factor 21 (TCF21). Using SMC-specific deletion of KLF4 in an atherosclerotic mice model, the research group led by Gary K. Owens has shown that KLF4 promotes transdifferentiation of VSMCs into macrophage-like and Ly6a+ MSC-like phenotypes17 as well as Lagls3+ osteogenic cells.19 Several mechanisms by which KLF4 suppresses the contractile VSMC phenotype have been proposed.24 Specifically, KLF4 binds the G/C repressor element of contractile gene promoters or recruits histone deacetylase (HDAC) 2/5 to suppress contractile gene expression. In addition, KLF4 antagonizes the binding of SRF to the CArG box through direct interaction with SRF; alternatively, it forms a complex with p-ELK1 and SRF to hinder the binding of MYOCD to SRF. In addition, research indicates that KLF4 can bind to RUNX2 to enhance its activity.25 Another stem cell pluripotency factor, OCT4, also regulates VSMC phenotypic modulation, but has an effect opposite to that of KLF4. The atherosclerotic phenotypes of VSMC-specific KLF4 and OCT4 deletions are inverses.17,26 The activation of OCT4 in VSMCs is related to the hydroxymethylation of the OCT4 promoter and is KLF4- and hypoxia-inducible factor-1α–dependent.26 TCF21, which is known to be a causal gene of the coronary artery disease-associated locus at 6q23.2, also promotes VSMC differentiation by decreasing both SRF and MYOCD gene expression and interfering with SRF-MYOCD complex formation.27
MicroRNAs (miRNAs), epigenetic modifications, and various environmental stimuli can post-transcriptionally regulate the VSMC phenotype.13 Among miRNAs, miRNA143 and miRNA145 are known to be involved in the regulation of the VSMC contractile phenotype. SRF and MYOCD induce the expression of miRNA 143/145, which supports the VSMC contractile phenotype and suppresses proliferation. miRNA 143/145 expression has been reported to be reduced in injured or atherosclerotic aortas, while cholesterol loading promotes VSMC transdifferentiation via the miRNA 143/145-MYOCD axis, collectively suggesting that the downregulation of miRNA 143/145 may promote VSMC dedifferentiation.28,29 Other miRNAs involved in the phenotypic switching of VSMCs are miRNA221, miRNA222, and miRNA124.13 Of note, miRNA 221/222 has been reported to promote the osteochondrogenic transition of VSMCs, leading to calcification.30
Epigenetic regulation is another important mechanism governing the contractile phenotype of VSMCs. Histone modifications (such as acetylation or methylation) modify chromatin structure, thereby regulating its accessibility for transcription factors.31 Studies have indicated that during VSMC differentiation, histone acetylation of ACTA2 and MYH11 enables their activation by allowing the binding of the SRF/MYOCD complex.32 The transcriptional activity of MYOCD can be enhanced by histone acetylation through P300 histone acetyltransferase (HAT) or inhibited by HDACs.33 In addition, HATs and HDACs have been reported to regulate VSMC characteristics, such as matrix production, proliferation, and migration.13 Lastly, histone methylation is also involved in the binding of MYOCD/SRF to the CArG box loci.34 During VSMC differentiation, the H3K4me2 histone modification at the contractile gene promotor is enriched, facilitating MYOCD binding. Interestingly, H3K4me2 methylation persists even when VSMCs lose contractile marker expression during phenotypic modulation. Researchers have exploited this phenomenon to track VSMC-derived cells in human atherosclerotic lesions for which lineage-tracing techniques are unavailable.35
Within the atherosclerotic milieu, VSMCs are acted upon by various cytokines in response to environmental cues that can promote or inhibit the phenotypic modulation of VSMCs. Platelet-derived growth factor (PDGF)-BB promotes the differentiation and proliferation of VSMCs.36 PDGF-BB can stimulate the phosphorylation of ELK1 through the PDGFRβ receptor, which in turn competitively inhibits the interaction between MYOCD and SRF.37 KLF4 and miRNA221 can partially mediate the effect of PDGF-BB on phenotypic modulation.38,39 Wnt/β-catenin signaling has been reported to promote the proliferation of VSMCs, leading to intimal hyperplasia,40 and it is also involved in the osteodifferentiation of VSMCs through the activation of RUNX2.41 Transforming growth factor beta (TGF-β) is involved in various VSMC properties, such as the contractile phenotype, proliferation, hypertrophy, matrix synthesis, and proteolytic activities.13 TGF-β is one of the few growth factors to promote the VSMC contractile phenotype. This is achieved via binding of the TGF-β signaling molecule SMAD2/3 to the contractile gene promoter or through CArG-dependent interaction with SRF.42 In addition, TGF-β can downregulate KLF4 via miRNA 143/145 to suppress VSMC phenotypic switching.43 Integrin beta 3 has been reported to be involved in the transdifferentiation, proliferation, and migration of VSMCs in the atherosclerotic milieu through cell-autonomous and paracrine effects.44
ROLE OF VSMCs IN ATHEROSCLEROTIC CALCIFICATION
Numerous studies have shown that VSMCs are the major cell type responsible for vascular calcification through their phenotypic transition to osteogenic cells. VSMC-specific deletion of the osteogenic transcription factors Msx1 and Msx2 has been shown to attenuate arteriosclerotic calcification in LDLR−/− mice with a diabetogenic background.45 Transgenic mice that are forced to express the pro-osteogenic factors BMP2 or S100A12 in VSMCs display increased osteoblastic differentiation, leading to atherosclerotic calcification.46,47 VSMC-specific deletion of the osteogenic inhibitors peroxisome proliferator-activated receptor γ or lipoprotein-receptor-related protein 6 increases the osteochondrogenic differentiation of VSMCs by augmenting Wnt signaling.48,49 However, the most convincing evidence comes from a lineage tracing study using VSMC-specific reporter mice, which demonstrated that approximately 98% of osteochondrogenic cells expressing Runx2 in atherosclerotic plaques were derived from VSMCs.50 Notably, Kramann et al.51 showed that Gli1+ adventitial MSC-like progenitor cells can substantially contribute to the osteoblast-like cells of atherosclerotic lesions under CKD conditions. In contrast, Wang et al.52 demonstrated that Ly6a+ adventitial stem cells contribute little to the VSMC population of atherosclerotic lesions in the absence of other systemic diseases (e.g., CKD). Although the Gli1+ and Ly6a+ cells of the adventitia may not be completely identical, multiple lineage-tracing studies collectively suggest that VSMCs are the principal cell type responsible for the osteochondrogenic cells in atherosclerosis.
The development of the scRNA-seq technique has enabled unbiased characterization of multiple heterogeneous cell populations in tissues or organs of interest. In addition, combining scRNA-seq with a lineage tracing system enables unambiguous tracking of the transition of specific cells, even when the typical marker expression of the cells has been lost. Recent scRNA-seq studies of mouse atherosclerotic lesions using a combination of fluorescence reporters (e.g., ZsGreen1 or tdTomato) with the inducible Cre recombinase on SMC-specific contractile gene promoter (Myh-CreERT2) have revealed generally consistent findings regarding the process of VSMC transition toward osteochondrogenic clusters.16,19,20 VSMCs first give rise to the pioneer cell population (cells with similar transcriptomic profiles but categorized into separate subgroups, such as the intermediate cell state, fibromyocytes, or Lgals3+ VSMCs) and then transform into the osteochondrogenic population (fibrochondrocytes, chondromyocytes, or osteogenic cells). The osteochondrogenic population is enriched with multiple collagen-producing and osteochondrogenic genes, representing the cell population responsible for calcification and chondroid metaplasia in atherosclerotic lesions. Two of these studies, published by the research groups led by Thomas Quertermous and Gary K. Owens, additionally showed that the VSMC-derived osteochondrogenic population can be manipulated via SMC-specific ablation of a specific gene, Ahr or Klf4.19,20 Notably, SMC-specific ablation of Ahr or Klf4 also altered the lesion size, fibrous cap, and intermediate VSMC clusters, suggesting that the regulatory function of these genes in the SMC transition is not confined to the osteochondrogenic phenotype. To date, the key regulatory factors and underlying mechanisms that govern the osteochondrogenic transition of VSMCs have yet to be elucidated.
ROLES OF THE BMP SIGNALING PATHWAY IN OSTEOBLAST DIFFERENTIATION AND THIOREDOXIN-INTERACTING PROTEIN AS A CRITICAL REGULATOR OF BMP SIGNALING IN ATHEROSCLEROSIS
The TGF-β superfamily comprises TGF-β, BMPs, activin, and other related proteins.53 BMP signaling plays a central role in skeletal system development and homeostasis. BMP signaling is initiated by type I and type II BMP receptors. The binding of BMP ligands produces a tetrameric complex composed of homomeric dimers of type I and type II receptors, which then affects the transphosphorylation of type I receptors. This phosphorylation results in the transduction of signals through either canonical Smad-related or noncanonical p38 mitogen-activated protein kinase (MAPK)-related pathways, which consequently stimulates the osteogenic transcription factors that lead to osteoblast differentiation.53 Among the types of BMPs (14 in total), BMP2, BMP4, BMP5, BMP6, BMP7, and BMP9 are known to have osteogenic activity.54,55 BMP2 and BMP7 have been widely studied, and recombinant proteins are currently being tested in human clinical trials for various bone-related defects.56,57 Short-term expression of BMP2 is necessary and sufficient to induce bone formation,58 and BMP7 induces the expression of osteoblastic markers, such as alkaline phosphatase, and accelerates mineralization.59,60,61 BMP3 is a noncanonical BMP ligand that transduces its signaling through the type IIB activin receptor and Smad2/3-related pathway to oppose the osteogenic function of other BMPs.62
Smad molecules constitute the canonical arm of TGF-β/BMP signaling. Vertebrates have eight Smad proteins, which can be divided into three subtypes: the receptor-regulated Smads (R-Smads; Smad1, Smad2, Smad3, Smad5, and Smad8), the co-mediator Smad (Smad4), and the inhibitory Smads (I-Smads; Smad6 and Smad7).63 Upon BMP ligand binding, the heteromeric BMP receptor complex phosphorylates Smad1/5/8. The phosphorylated Smad1/5/8 forms heteromeric complexes with Smad4 and eventually translocates to the nucleus, where it acts as a transcription factor or repressor.53 During osteoblast differentiation, the Smad1/5/8-Smad4 complex forms a transcription complex with RUNX2 and other molecules to initiate the expression of multiple osteoblast-related genes.53
p38 MAPKs constitute noncanonical arms of the TGF-β/BMP signaling pathway. MAPKs are a family of enzymes that respond to various extracellular stimuli such as environmental stress, growth factors, and cytokines. Conventional MAPKs consist of the extracellular signal-regulated kinase (ERK) 1/2 and ERK 5, c-Jun amino (N)-terminal kinase 1/2/3, and p38 isoforms (p38α, p38β, p38γ, and p38δ). MAPK signaling constitutes a series of phosphorylation events. Once stimuli reach the cell, MAPK kinase kinase (MAP3K) is activated and phosphorylates MAPK kinase (MAP2K), which in turn phosphorylates and activates MAPKs. In the TGF-β/BMP signaling pathway, p38α/β exerts its osteogenic potential by inducing expression or increasing activity through the phosphorylation of key osteogenic transcription factors such as DLX5, RUNX2, and OSX.64
TGF-β/BMP signaling can be fine-tuned through multiple mechanisms. Various ECM proteins, such as noggin, chordin, gremlin, and follistatin, can competitively bind BMPs to prevent receptor activation.65,66,67 I-Smads inhibit TGF-β/BMP signaling in multiple steps. Of the 2 I-Smads, Smad6 preferentially inhibits BMP signaling, whereas Smad7 inhibits both BMP and TGF-β signaling. I-Smads can prevent the phosphorylation and nuclear translocation of R-Smads, promote R-Smad degradation, and promote receptor degradation via the ubiquitin-proteasome degradation pathway.63 The latter is achieved by the E3 ubiquitin ligase Smuf1/2. Smuf1 can widely target components of BMP signaling machinery, such as BMP type I receptors, Smad1/5, RUNX2, and MAPK kinase 2, leading to ubiquitin-proteasome degradation.53 Another E3 ubiquitin ligase, Arkadia, can degrade the suppressors of TGF-β/BMP signaling, such as Smad6/7 and c-Ski/SnoN, which in turn promotes the osteoblastic phenotype (Arkadia). Small ubiquitin-related modifier proteins and ubiquitin-conjugating enzyme 9 target Smad4 for degradation and counteract BMP2-induced osteoblast differentiation.68,69 Other factors and mechanisms, such as transcriptional repressors (Ski/SnoN, Tob), miRNAs (mi133, mi30, mi141, mi542-3p, mi20a, mi140, mi199a), and epigenetic regulation, are also involved in the regulation of TGF-β/BMP signaling.53
Within the atherosclerotic milieu, BMPs are expressed in various cells, including endothelial cells, foam cells, and VSMCs. Several causes of endothelial dysfunction, such as oxidative stress, turbulent blood flow, and hypoxia, have been reported to increase BMP expression in endothelial cells.12 Among BMPs, BMP2 and BMP4 have been reported to accelerate the osteogenic differentiation of VSMCs, whereas BMP7 inhibits the induction of p21 and the upregulation of Smad6/7.11 Recently, we defined the osteochondrogenic populations derived from VSMCs, consistent with previous reports, and newly reported that thioredoxin-interacting protein (TXNIP) is a critical regulator of osteochondrogenic switch of VSMCs in atherosclerotic plaque.70 In our study, using three mouse models (whole-body, smooth muscle cell-specific, and hematopoietic TXNIP ablation) combined with scRNA-seq analysis and primary VSMC culture experiments, we demonstrated that TXNIP deficiency in hyperlipidemic mice enhances the phenotypic transition of modulated VSMCs toward the osteochondrogenic population by upregulating canonical and non-canonical BMP signaling, leading to plaque calcification (Fig. 1). In a human transcriptome database, TXNIP was also downregulated in modulated VSMC and osteochondrogenic clusters of calcified atherosclerotic lesions. Together, these data indicate that TXNIP is a critical checkpoint for the osteochondrogenic switch of VSMCs in the atherosclerotic milieu and highlight its importance in plaque calcification.
CONCLUSION
Calcification, a characteristic feature of advanced atherosclerotic plaques, is highly correlated with total plaque burden and may lead to clinical complications through thrombosis or compromise anti-atherosclerotic treatment. Once thought to just contribute to plaque stabilization through fibrous cap formation, VSMCs are now highlighted as a crucial contributor to plaque formation, including the intimal lipid-rich areas. Studies combining single-cell transcriptomics and fate mapping have clearly shown that VSMCs have a highly plastic nature and can differentiate into various cell types, including the macrophage-like, MSC-like, fibroblastic, and osteochondrogenic cells responsible for atherosclerotic calcification. Previous studies have revealed that pathological cues such as inflammation, cell death, and reactive oxygen species, as well as osteogenic pathways such as BMP signaling, are involved in the osteochondrogenic transition of VSMCs. However, the exact molecular and cellular mechanisms responsible for the phenotypic switch of VSMCs into each cell type, including osteochondrogenic cells, remain to be elucidated. Importantly, the identification of key regulators of VSMC phenotypic change is a precondition for the development of novel therapeutic modalities for atherosclerosis.
Footnotes
Funding: This work was supported by the National Research Foundation of Korea, funded by the Korean government (grant number NRF-2016M3A9D5A01952416, 2016M3A9D5A01952413, 2020R1A6A1A06046728, and 2021R1A2C3004586).
Conflict of Interest: The authors have no conflicts of interest to declare.
Data Availability Statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
- Conceptualization: Woo SH, Kim DY, Choi JH.
- Funding acquisition: Kim DY, Choi JH.
- Investigation: Woo SH, Choi JH.
- Supervision: Kim DY, Choi JH.
- Writing - original draft: Woo SH.
- Writing - review & editing: Kim DY, Choi JH.
References
- 1.Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 3.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 5.Basatemur GL, Jørgensen HF, Clarke MC, Bennett MR, Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16:727–744. doi: 10.1038/s41569-019-0227-9. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Pasterkamp G. Requiem for the ‘vulnerable plaque’. Eur Heart J. 2015;36:2984–2987. doi: 10.1093/eurheartj/ehv349. [DOI] [PubMed] [Google Scholar]
- 7.Sugane H, Kataoka Y, Otsuka F, Nakaoku Y, Nishimura K, Nakano H, et al. Cardiac outcomes in patients with acute coronary syndrome attributable to calcified nodule. Atherosclerosis. 2021;318:70–75. doi: 10.1016/j.atherosclerosis.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos N, Raggi P. Calcification in atherosclerosis. Nat Rev Cardiol. 2009;6:681–688. doi: 10.1038/nrcardio.2009.165. [DOI] [PubMed] [Google Scholar]
- 9.Shi X, Gao J, Lv Q, Cai H, Wang F, Ye R, et al. Calcification in atherosclerotic plaque vulnerability: friend or foe? Front Physiol. 2020;11:56. doi: 10.3389/fphys.2020.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato Y, Finn AV, Virmani R. Calcified nodule: a rare but important cause of acute coronary syndrome with worse clinical outcomes. Atherosclerosis. 2021;318:40–42. doi: 10.1016/j.atherosclerosis.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018;114:590–600. doi: 10.1093/cvr/cvy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima Y, Wight TN, Sueishi K. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc Res. 2008;79:14–23. doi: 10.1093/cvr/cvn099. [DOI] [PubMed] [Google Scholar]
- 14.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, et al. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen K, Lund MB, Shim J, Gunnersen S, Füchtbauer EM, Kjolby M, et al. Diverse cellular architecture of atherosclerotic plaque derives from clonal expansion of a few medial SMCs. JCI Insight. 2017;2:e95890. doi: 10.1172/jci.insight.95890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan H, Xue C, Auerbach BJ, Fan J, Bashore AC, Cui J, et al. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation. 2020;142:2060–2075. doi: 10.1161/CIRCULATIONAHA.120.048378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, et al. Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contributes to neointimal formation in mouse injury and atherosclerosis models. Circ Res. 2016;119:1313–1323. doi: 10.1161/CIRCRESAHA.116.309799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alencar GF, Owsiany KM, Karnewar S, Sukhavasi K, Mocci G, Nguyen AT, et al. Stem cell pluripotency genes Klf4 and Oct4 regulate complex SMC phenotypic changes critical in late-stage atherosclerotic lesion pathogenesis. Circulation. 2020;142:2045–2059. doi: 10.1161/CIRCULATIONAHA.120.046672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JB, Zhao Q, Nguyen T, Pjanic M, Cheng P, Wirka R, et al. Environment-sensing aryl hydrocarbon receptor inhibits the chondrogenic fate of modulated smooth muscle cells in atherosclerotic lesions. Circulation. 2020;142:575–590. doi: 10.1161/CIRCULATIONAHA.120.045981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wirka RC, Wagh D, Paik DT, Pjanic M, Nguyen T, Miller CL, et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat Med. 2019;25:1280–1289. doi: 10.1038/s41591-019-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2003;100:9366–9370. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida T, Sinha S, Dandré F, Wamhoff BR, Hoofnagle MH, Kremer BE, et al. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res. 2003;92:856–864. doi: 10.1161/01.RES.0000068405.49081.09. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida T, Hayashi M. Role of Krüppel-like factor 4 and its binding proteins in vascular disease. J Atheroscler Thromb. 2014;21:402–413. doi: 10.5551/jat.23044. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida T, Yamashita M, Hayashi M. Krüppel-like factor 4 contributes to high phosphate-induced phenotypic switching of vascular smooth muscle cells into osteogenic cells. J Biol Chem. 2012;287:25706–25714. doi: 10.1074/jbc.M112.361360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherepanova OA, Gomez D, Shankman LS, Swiatlowska P, Williams J, Sarmento OF, et al. Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective. Nat Med. 2016;22:657–665. doi: 10.1038/nm.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagao M, Lyu Q, Zhao Q, Wirka RC, Bagga J, Nguyen T, et al. Coronary disease-associated gene TCF21 inhibits smooth muscle cell differentiation by blocking the myocardin-serum response factor pathway. Circ Res. 2020;126:517–529. doi: 10.1161/CIRCRESAHA.119.315968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boettger T, Beetz N, Kostin S, Schneider J, Krüger M, Hein L, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackenzie NC, Staines KA, Zhu D, Genever P, Macrae VE. miRNA-221 and miRNA-222 synergistically function to promote vascular calcification. Cell Biochem Funct. 2014;32:209–216. doi: 10.1002/cbf.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez D, Swiatlowska P, Owens GK. Epigenetic control of smooth muscle cell identity and lineage memory. Arterioscler Thromb Vasc Biol. 2015;35:2508–2516. doi: 10.1161/ATVBAHA.115.305044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manabe I, Owens GK. Recruitment of serum response factor and hyperacetylation of histones at smooth muscle-specific regulatory regions during differentiation of a novel P19-derived in vitro smooth muscle differentiation system. Circ Res. 2001;88:1127–1134. doi: 10.1161/hh1101.091339. [DOI] [PubMed] [Google Scholar]
- 33.Cao D, Wang Z, Zhang CL, Oh J, Xing W, Li S, et al. Modulation of smooth muscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol Cell Biol. 2005;25:364–376. doi: 10.1128/MCB.25.1.364-376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo . J Clin Invest. 2006;116:36–48. doi: 10.1172/JCI26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez D, Shankman LS, Nguyen AT, Owens GK. Detection of histone modifications at specific gene loci in single cells in histological sections. Nat Methods. 2013;10:171–177. doi: 10.1038/nmeth.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31:1495–1505. doi: 10.1161/ATVBAHA.110.221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428:185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- 38.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284:3728–3738. doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deaton RA, Gan Q, Owens GK. Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am J Physiol Heart Circ Physiol. 2009;296:H1027–H1037. doi: 10.1152/ajpheart.01230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsaousi A, Mill C, George SJ. The Wnt pathways in vascular disease: lessons from vascular development. Curr Opin Lipidol. 2011;22:350–357. doi: 10.1097/MOL.0b013e32834aa701. [DOI] [PubMed] [Google Scholar]
- 41.Cai T, Sun D, Duan Y, Wen P, Dai C, Yang J, et al. WNT/β-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp Cell Res. 2016;345:206–217. doi: 10.1016/j.yexcr.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Qiu P, Feng XH, Li L. Interaction of Smad3 and SRF-associated complex mediates TGF-β1 signals to regulate SM22 transcription during myofibroblast differentiation. J Mol Cell Cardiol. 2003;35:1407–1420. doi: 10.1016/j.yjmcc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, et al. down-regulation of Krüppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-β and bone morphogenetic protein 4. J Biol Chem. 2011;286:28097–28110. doi: 10.1074/jbc.M111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Misra A, Feng Z, Chandran RR, Kabir I, Rotllan N, Aryal B, et al. Integrin beta3 regulates clonality and fate of smooth muscle-derived atherosclerotic plaque cells. Nat Commun. 2018;9:2073. doi: 10.1038/s41467-018-04447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng SL, Behrmann A, Shao JS, Ramachandran B, Krchma K, Bello Arredondo Y, et al. Targeted reduction of vascular Msx1 and Msx2 mitigates arteriosclerotic calcification and aortic stiffness in LDLR-deficient mice fed diabetogenic diets. Diabetes. 2014;63:4326–4337. doi: 10.2337/db14-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hofmann Bowman MA, Gawdzik J, Bukhari U, Husain AN, Toth PT, Kim G, et al. S100A12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein E-null mice by activating an osteogenic gene regulatory program. Arterioscler Thromb Vasc Biol. 2011;31:337–344. doi: 10.1161/ATVBAHA.110.217745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakagawa Y, Ikeda K, Akakabe Y, Koide M, Uraoka M, Yutaka KT, et al. Paracrine osteogenic signals via bone morphogenetic protein-2 accelerate the atherosclerotic intimal calcification in vivo . Arterioscler Thromb Vasc Biol. 2010;30:1908–1915. doi: 10.1161/ATVBAHA.110.206185. [DOI] [PubMed] [Google Scholar]
- 48.Cheng SL, Ramachandran B, Behrmann A, Shao JS, Mead M, Smith C, et al. Vascular smooth muscle LRP6 limits arteriosclerotic calcification in diabetic LDLR−/− mice by restraining noncanonical Wnt signals. Circ Res. 2015;117:142–156. doi: 10.1161/CIRCRESAHA.117.306712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woldt E, Terrand J, Mlih M, Matz RL, Bruban V, Coudane F, et al. The nuclear hormone receptor PPARγ counteracts vascular calcification by inhibiting Wnt5a signalling in vascular smooth muscle cells. Nat Commun. 2012;3:1077. doi: 10.1038/ncomms2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naik V, Leaf EM, Hu JH, Yang HY, Nguyen NB, Giachelli CM, et al. Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study. Cardiovasc Res. 2012;94:545–554. doi: 10.1093/cvr/cvs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, et al. Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell. 2016;19:628–642. doi: 10.1016/j.stem.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Zhao H, Zhu H, Li Y, Tang J, Li Y, et al. Sca1+ cells minimally contribute to smooth muscle cells in atherosclerosis. Circ Res. 2021;128:133–135. doi: 10.1161/CIRCRESAHA.120.317972. [DOI] [PubMed] [Google Scholar]
- 53.Wu M, Chen G, Li YP. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abula K, Muneta T, Miyatake K, Yamada J, Matsukura Y, Inoue M, et al. Elimination of BMP7 from the developing limb mesenchyme leads to articular cartilage degeneration and synovial inflammation with increased age. FEBS Lett. 2015;589:1240–1248. doi: 10.1016/j.febslet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25:665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 56.Kanakaris NK, Giannoudis PV. Clinical applications of bone morphogenetic proteins: current evidence. J Surg Orthop Adv. 2008;17:133–146. [PubMed] [Google Scholar]
- 57.Razzouk S, Sarkis R. BMP-2: biological challenges to its clinical use. N Y State Dent J. 2012;78:37–39. [PubMed] [Google Scholar]
- 58.Noël D, Gazit D, Bouquet C, Apparailly F, Bony C, Plence P, et al. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells. 2004;22:74–85. doi: 10.1634/stemcells.22-1-74. [DOI] [PubMed] [Google Scholar]
- 59.Gu K, Zhang L, Jin T, Rutherford RB. Identification of potential modifiers of Runx2/Cbfa1 activity in C2C12 cells in response to bone morphogenetic protein-7. Cells Tissues Organs. 2004;176:28–40. doi: 10.1159/000075025. [DOI] [PubMed] [Google Scholar]
- 60.Shen B, Wei A, Whittaker S, Williams LA, Tao H, Ma DD, et al. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro . J Cell Biochem. 2010;109:406–416. doi: 10.1002/jcb.22412. [DOI] [PubMed] [Google Scholar]
- 61.Tsuji K, Cox K, Gamer L, Graf D, Economides A, Rosen V. Conditional deletion of BMP7 from the limb skeleton does not affect bone formation or fracture repair. J Orthop Res. 2010;28:384–389. doi: 10.1002/jor.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kokabu S, Gamer L, Cox K, Lowery J, Tsuji K, Raz R, et al. BMP3 suppresses osteoblast differentiation of bone marrow stromal cells via interaction with Acvr2b. Mol Endocrinol. 2012;26:87–94. doi: 10.1210/me.2011-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyazawa K, Miyazono K. Regulation of TGF-β family signaling by inhibitory Smads. Cold Spring Harb Perspect Biol. 2017;9:a022095. doi: 10.1101/cshperspect.a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodríguez-Carballo E, Gámez B, Ventura F. p38 MAPK signaling in osteoblast differentiation. Front Cell Dev Biol. 2016;4:40. doi: 10.3389/fcell.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O’Brien CA, Economides AN, et al. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res. 2000;15:663–673. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- 66.Gazzerro E, Gangji V, Canalis E. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J Clin Invest. 1998;102:2106–2114. doi: 10.1172/JCI3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wan DC, Pomerantz JH, Brunet LJ, Kim JB, Chou YF, Wu BM, et al. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem. 2007;282:26450–26459. doi: 10.1074/jbc.M703282200. [DOI] [PubMed] [Google Scholar]
- 68.Shimada K, Suzuki N, Ono Y, Tanaka K, Maeno M, Ito K. Ubc9 promotes the stability of Smad4 and the nuclear accumulation of Smad1 in osteoblast-like Saos-2 cells. Bone. 2008;42:886–893. doi: 10.1016/j.bone.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 69.Yukita A, Hosoya A, Ito Y, Katagiri T, Asashima M, Nakamura H. Ubc9 negatively regulates BMP-mediated osteoblastic differentiation in cultured cells. Bone. 2012;50:1092–1099. doi: 10.1016/j.bone.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Woo SH, Kyung D, Lee SH, Park KS, Kim M, Kim K, et al. TXNIP suppresses the osteochondrogenic switch of vascular smooth muscle cells in atherosclerosis. Circ Res. 2023;132:52–71. doi: 10.1161/CIRCRESAHA.122.321538. [DOI] [PMC free article] [PubMed] [Google Scholar]