Abstract
This scientific commentary refers to ‘Pathological combinations in neurodegenerative disease are heterogeneous and disease-associated’ by Robinson et al. (https://doi.org/10.1093/brain/awad059).
This scientific commentary refers to ‘Pathological combinations in neurodegenerative disease are heterogeneous and disease-associated’ by Robinson et al. (https://doi.org/10.1093/brain/awad059).
Heterogeneity in neurodegenerative diseases is the norm not the exception. Multiple pathological pathways are common among older individuals and these distinct pathologies can often result in similar clinical presentations.1-3 This unwieldy, complex and fascinating reality is at the core of the challenge of establishing accurate pathophysiological understanding and effective therapeutics in neurodegenerative diseases. An individual with cognitive difficulties is unlikely to only have Alzheimer’s disease, Lewy body disease (LBD) or cerebrovascular disease (CVD). Instead, overlapping shades of concurrent pathologies result in a unique signature for each individual that drives their cognitive impairment. This ‘pathotome’ of combinations is what Robinson and colleagues4 elegantly lay out in this issue of Brain.
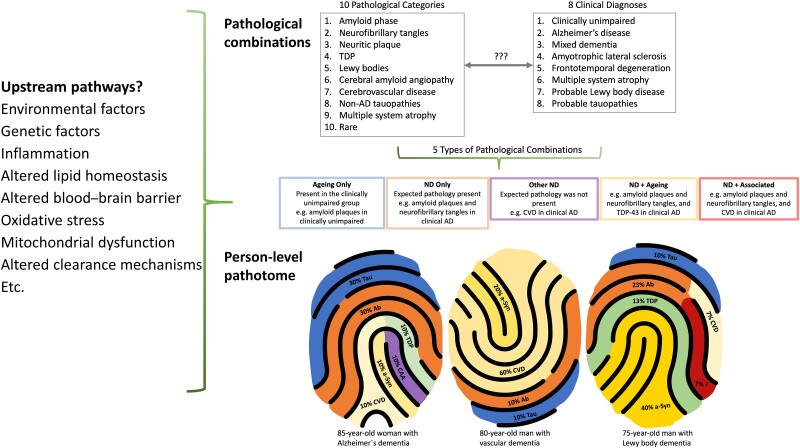
In their work, Robinson et al.4 examine 10 major pathological processes and their possible combinations across eight clinically defined diagnostic groups (Fig. 1). This examination uncovered a heterogeneous mix of 161 pathological combinations present in their sample of 1647 individuals. Concurrent pathologies were common within all clinical groups. Even among the clinically unimpaired group of 144 individuals, 35 different pathological combinations were detected. Although many clinically defined groups included in the study are already known to harbour co-pathology, this work provides additional insights by highlighting the variability in possible combinations of co-pathology. For example, in early-onset clinical Alzheimer’s disease, 16% of patients had only amyloid plaques and tangles (A-B-C), whereas 33% had one additional pathology (A-B-C+1) such as Lewy bodies (LB), TAR DNA-binding protein 43 (TDP), CVD or cerebral amyloid angiopathy (CAA), and 33% had two additional pathologies (A-B-C+2). In late-onset Alzheimer’s disease, 13% had only A-B-C, 27% had A-B-C+1, 30% A-B-C+2 and 15% had A-B-C+3.
Figure 1.
The person-specific pathotome is a representation of the individual-level heterogeneity of neuropathological findings in neurodegenerative disease. Top right: Robinson and colleagues4 considered 10 pathological categories and eight clinical diagnoses. Left: Examples of the potential upstream pathways that may drive the pathological changes observed. Bottom right: Three examples of a pathotome. Similar to a fingerprint, each individual has a particular pattern of pathologies affecting different anatomical brain regions to varying degrees. Ab = amyloid-β; AD = Alzheimer’s disease; a-Syn =α-synuclein; CAA = cerebral amyloid angiopathy; CVD = cerebrovascular disease; TDP = transactive response DNA-binding protein; ND = neurodegenerative disease.
For those with clinical LBD, 35% of the early-onset group and 18% of the late-onset group had Lewy body pathology alone. Of the early-onset group, 31% also had some Alzheimer’s disease co-pathology (LB + AD), compared to 19% of those with late-onset clinical LBD. None of the early-onset clinical LDB group had more than LB + AD pathology, whereas 26% of the late-onset clinical LBD group had 1–2 further pathologies in addition to LB and Alzheimer’s disease. Even among clinical groups known to reflect purer disease processes, three or more pathologies were detected in 18% of cases of amyotrophic lateral sclerosis (ALS), 34% of cases of frontotemporal dementia (FTD), and 19% of cases of multiple system atrophy (MSA).
Importantly, of the 161 combinations observed in the cohort as a whole, there was no single dominant combination, with 1 of 10 combinations (A-B-C, A-B-C-CAA, A-B-C-LB, A-B-C-CAA-LB, A-B-C-CAA-TDP, Limited, TDP, Tau, LB, glial cytoplasmic inclusions) occurring in 53% of individuals and most combinations occurring in less than 5% of the total sample. Older age, longer clinical disease duration, APOE4+ status and worse functional status were associated with a higher burden of total co-pathologies.
One of the challenging conundrums in studying neurodegenerative diseases is the contribution of age. On one hand increasing age is considered a risk factor for dementia syndromes and, on the other hand, the same pathologies present in neurodegenerative diseases are also present in clinically unimpaired older individuals. In order to categorize pathological drivers of clinical decline, Robinson et al.4 determined age-related pathological combinations and their frequencies in the clinically unimpaired group arranged by age (<65 years, 65–79 years and 80+ years). They then conceptualized the 161 observed pathological combinations into a five-category framework: ‘Ageing only’ as defined above, ‘ND (neurodegenerative disease) only’ if underlying pathology was consistent with clinical diagnosis, ‘ND + ageing’ if the expected pathology was present with ageing-related pathologies, ‘ND + associated’ if the expected pathology was present together with other pathologies not observed in the unimpaired group or observed at a greater frequency than in the unimpaired group at that age, and ‘Other ND’ if the expected pathology was not present but other disease-associated pathologies were.
In the clinical Alzheimer’s disease group, the ‘ND + ageing’ profile was almost non-existent, suggesting that co-pathologies in clinical Alzheimer’s disease are driven by the pathophysiology of Alzheimer’s disease and are not simply age-related. The caveat with this conclusion, however, is that low levels of Alzheimer’s disease pathology (Braak I/II) were collapsed into the tangle-negative group and were not considered disease or ageing-related. Thus, the ‘ND + ageing’ profile may be artificially reduced given the exclusion of Braak I/II. Additionally, the authors defined pathological Alzheimer’s disease as only A-B-C or A-B-C-CAA. Nevertheless, in the clinically unimpaired group, A, B, C or CAA were often present in various combinations; albeit not as commonly as only A-B-C or A-B-C-CAA. This could indicate that less developed and early forms of Alzheimer’s disease pathophysiology may not be captured using the approach of Robinson et al.4 In clinical LBD, the largest group was ‘LBD + associated’, and in particular this group was driven by co-Alzheimer’s disease pathology. The more homogenous categories (‘Ageing only’, ‘ND only’ and ‘Other ND’) accounted for a minority of cases, with most cases assigned instead to the ‘’ND + ageing’ and ‘ND + associated’ categories. This finding is consistent with an extensive literature that highlights co-pathology as the largest driver of dementia risk.5
This study has some limitations. For instance, the interpretation of the ‘Ageing only’ pathway is unclear, and it is possible that these combinations reflect early disease as opposed to normal ageing processes. Longitudinal follow-up would be needed to differentiate these possibilities and is not possible in post-mortem studies. The general approach taken in this work was to dichotomize the presence of each major pathological pathway. For instance, B2 and B3 were considered positive for tau tangles, and LB-positive cases included all cases with a brainstem predominant, transitional or neocortical pattern. The impact of lower levels of pathology, especially in the context of co-pathology, is therefore not directly addressed in this work although others have begun to characterize this level of nuance.6 Additionally, ‘ND + Associated’ is a large group that combines both expected pathology with either other pathologies not observed in the unimpaired group or observed at a greater frequency than in the unimpaired group matched for age. It would be helpful to separate this large group into subgroups (e.g. expected pathology + other ND pathology and expected pathology + excessive ageing pathology), as mechanisms underlying these subgroups may differ.
Further, the consideration of A-B-C as three different pathologies contributed to the high number of pathological combinations, for instance A-B-C-LB is considered four pathologies whereas in many other studies it is considered as two pathological processes (Alzheimer's disease and LBD). Finally, subject demographics such as sex and race/ethnicity were not reported, limiting generalizability. Despite these limitations, Robinson and colleagues4 provide convincing evidence that combined pathological processes are common but at the same time do not manifest consistently across individuals. This work underscores the importance of considering disease heterogeneity as opposed to conceptualizing disease progression as a uniform process across individuals.
Although both disease and healthy ageing states encompass a spectrum of diverse pathological combinations varied in their extent and spatial distribution, our current research efforts often focus on one pathological process in isolation. This is especially true for in vivo biofluid and imaging studies, which cannot confidently capture the spectrum of pathological processes that are quantifiable during post-mortem evaluation. The high number of combinations observed by Robinson and colleagues4 emphasizes the need to acknowledge this complex heterogeneity of neurodegenerative diseases as a shortcoming in current in vivo studies. The ability to measure key Alzheimer’s disease pathologies in vivo has accelerated our understanding of the Alzheimer’s disease cascade over the last two decades, and we are entering an era of biomarker research where in vivo markers of other common age-related pathologies such as the aggregation of α-synuclein (LB),7 and TDP are within reach.8 Over the next decade we anticipate that in vivo studies will be better positioned to integrate concepts related to heterogeneity in biomarker cohorts.
As the first anti-amyloid drugs receive accelerated approval for the treatment of clinical Alzheimer’s disease,9 controversy remains regarding whether these drugs produce clinically meaningful improvement. One possibility is that despite clear amyloid removal, focus on a single pathological pathway will not be sufficient if a multitude of underlying pathologies are present. The consistent observation that co-pathology is the norm also highlights the need to consider the upstream pathways that may be driving these pathological processes (Fig. 1). The mechanisms by which different pathologies interact with each other to promote synergistic effects are open avenues of investigation that might still prove fruitful. Within a given clinical diagnosis, individual patients have unique pathotomes of spatiotemporally interacting pathologies of varying severities. It is possible that understanding this heterogeneity in neurodegenerative diseases will enable precision health by leveraging person-specific pathotomes to derive individualized therapeutic approaches.
Contributor Information
Kyan Younes, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, CA, USA.
Elizabeth C Mormino, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, CA, USA; Wu Tsai Neuroscience Institute, Stanford, CA, USA.
Funding
K.Y receives funding from the Iqbal Farrukh and Asad Jamal Fund. E.C.M. receives funding from the National Institutes of Health (R01AG074339, U24AG067418, U24AG074855, P30AG06615).
Competing interests
E.C.M. has received consulting fees from Eli Lilly and Neurotrack. K.Y. reports no competing interests.
References
- 1. Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beach TG, Malek-Ahmadi M. Alzheimer’s disease neuropathological comorbidities are common in the younger-old. J Alzheimers Dis. 2021;79:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141:2181–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robinson JL, Xie SX, Baer DR, et al. Pathological combinations in neurodegenerative disease are heterogeneous and disease-associated. Brain. 2023;146:2557–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. White LR, Edland SD, Hemmy LS, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia aging studies. Neurology. 2016;86:1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ryman SG, Yutsis M, Tian L, et al. Cognition at each stage of Lewy body disease with co-occurring Alzheimer’s disease pathology. J Alzheimers Dis. 2021;80:1243–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shahnawaz M, Mukherjee A, Pritzkow S, et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature. 2020;578:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winston CN, Sukreet S, Lynch H, et al. Evaluation of blood-based exosomes as biomarkers for aging- related TDP-43 pathology. Alzheimers Dement (Amst). 2022;14:e12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9–21. [DOI] [PubMed] [Google Scholar]