Graphical Abstract
Graphical Abstract.
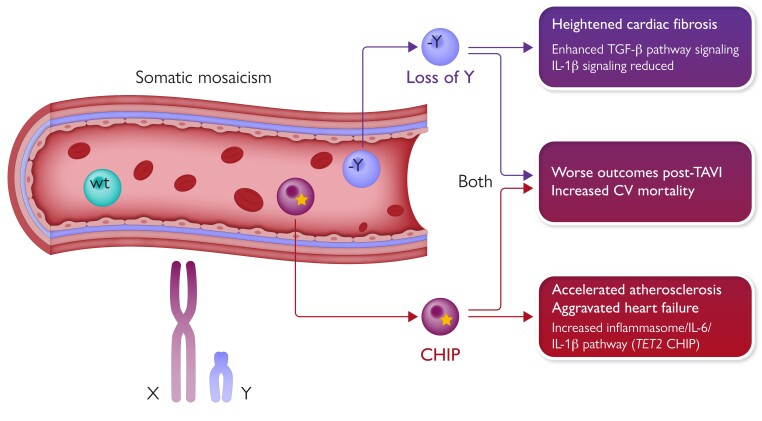
Somatic mosaicism and cardiovascular risk. Somatic mosaicism refers to genetic heterogeneity in cells due to acquired mutations. Recent work has implicated two types of somatic mosaicism in enhancing cardiovascular disease. The representation of the vessel shown on the upper right depicts wild-type leucocytes (wt), leucocytes with loss of Y (–Y), and myeloid cells with mutations in known leukaemia driver genes (indicated by an *) linked to clonal haematopoiesis of indeterminate potential. Both types of somatic mosaicism convey worse outcomes post-transcatheter aortic valve insertion (TAVI). The molecular pathways linked to cardiac fibrosis in mice with loss of Y involve enhanced signalling through the transforming growth factor-β pathway, while interleukin (IL)-1β pathway signalling decreases. In contrast, in certain forms of clonal haematopoiesis of indeterminate potential (CHIP), the inflammasome–IL-6–IL-1β pathway appears to participate in increased atherothrombotic and heart failure risk.
This editorial refers to ‘Mosaic loss of Y chromosome in monocytes is associated with lower survival after transcatheter aortic valve replacement’, by S. Mas-Peiro et al., https://doi.org/10.1093/eurheartj/ehad093.
The puny Y chromosome has engendered considerable concern of late. In men, the partner to the X chromosome measures up poorly. If size matters, the Y chromosome comes up short. Only one-third the expanse of its X counterpart, it encodes a mere 55 genes, while its sister X boasts some 900 genes (Graphical Abstract). The Y chromosome may even be shrinking, raising concerns about its eventual erasure.1 In another blow to the status of this runty chromosome, much of its length, such as it is, contains repetitive sequences of DNA of dubious genetic utility. The claim to fame of this otherwise laggard chromosome is its SRY gene that signals development of the male genitalia. To add insult to injury, with age, many men accumulate blood cells which have cast away this challenged chromosome, making mosaic males with an acquired condition called ‘loss of Y’ or LOY.
Knowledge of the cardiovascular consequences of LOY has recently burgeoned. Men with LOY have a reduced life span compared with those without this mosaic condition. Much of the excess mortality appears to be due to cardiovascular disease, although cancer rates also increase in men with LOY. Sano and colleagues recently provided experimental insight into the mechanisms by which LOY can worsen cardiac outcomes.2 They created mice engineered to mimic LOY using CRISPR gene editing technology to generate LOY cells which were then transplanted into a recipient mouse’s bone marrow. These manipulations did not alter the haemogram of the mice. However, those with the engineered LOY had shortened survival compared with controls. Echocardiographic assessment showed reduced left ventricular fractional shortening with age and accentuated adverse remodelling when the mice underwent thoracic aortic constriction to produce experimental pressure overload. These mice also displayed greater fibrosis as determined histologically. The engineered LOY mice had greater fibroblast content than controls as well.
Transforming growth factor-β (TGF-β) augments the expression of many extracellular matrix genes implicated in the formation of fibrous tissue. The mice with engineered LOY showed increased expression of TGF-β-related genes in macrophages and, curiously, a decrease in those regulated by interleukin-1β. Building on prior observational studies, interrogation of mortality due to cardiovascular disease in men with LOY in the UK Biobank revealed an increased in proportion to the number of leucocytes with LOY.
LOY, like clonal haematopoiesis of indeterminate potential (CHIP),3 which is another acquired somatic mosaic condition, increases in prevalence with age. Aortic stenosis, a common cardiovascular condition, also associates with older age. Mas-Peiro and associates previously studied outcomes following transcatheter aortic valve replacement (TAVR) in patients with CHIP, hypothesizing that the presence of CHIP might portend worse survival. Accordingly, they presented evidence for worsened outcomes including increased mortality in individuals undergoing TAVR who had CHIP. As experimental pressure overload in mice is associated with accelerated fibrosis in the mice with engineered LOY described above, they reasoned that outcomes post-TAVR, usually conducted in individuals with adverse left ventricular remodelling, might likewise display worsened outcomes. Indeed, as reported in this issue of the European Heart Journal, they found a striking increase in mortality in men with LOY undergoing TAVR compared with controls.4
The study authors further performed single-cell RNA sequencing to ask if cells that did not express Y chromosome genes had altered gene expression. In concordance with the mouse observations of Sano et al., they found that fibrosis-related genes, including those related to TGF-β, increased in the patients undergoing TAVR who had LOY. These new results affirm the clinical relevance of the experimental observations by Sano et al. Mas-Peiro and colleagues opened up a new perspective on factors that may alter outcomes after undergoing TAVR. Given their prior interest in CHIP and TAVR outcomes, they tested whether there was an interaction between CHIP and LOY; however, low numbers prevented a conclusive exploration of an interaction. Not commented upon was the increased expression of the mRNA that encodes the calgranulin family member S100A8 in men with LOY. We have previously implicated the product of this message, myeloid-related protein 8 (MRP8), in a variety of experimental cardiovascular conditions and as a biomarker of cardiovascular outcomes in humans.5,6
These novel findings of the Frankfurt group raise several interesting questions ripe for future investigation. Do individuals with calcific aortic stenosis have a higher prevalence of LOY than age- and risk factor-matched men? Could LOY lead to more rapid progression of calcific aortic stenosis than in those without LOY? Or even, in the presence of aortic stenosis, could LOY lead to more rapid progression of ventricular remodelling before valve intervention than in those without LOY?
Chronic mitral regurgitation causes a different sort of ventricular remodelling compared with the pressure overload of aortic stenosis. Could LOY correlate with outcomes in patients undergoing intervention for mitral regurgitation? Are smokers with LOY at even greater risk? It would be of interest to assess the effect of LOY on other cardiovascular outcomes, particularly heart failure, especially in the subset of patients with heart failure with preserved ejection fraction who have signs of impaired diastolic function. Could LOY alter outcome in cardiac amyloidosis?
Mechanistically, how does LOY alter gene expression? Mas-Peiro and colleagues profiled gene expression in peripheral blood. Do the alterations to gene expression that they documented in the peripheral blood reflect processes also at work in cardiac muscle, the arterial system, or other relevant tissues? Do measurements of arterial stiffness correlate with LOY? Would non-invasive measurement of blood biomarkers of collagen turnover inform whether those with LOY have evidence for altered extracellular matrix metabolism, a prediction of accentuated TGF-β-related gene expression? These and many other questions provide a fertile field for future research.
Many have questioned the utility of the Y chromosome. Perhaps the recent plethora of findings regarding heightened cardiovascular risk may cast a new light on this prodigal son of our genetic material. The Y chromosome cannot save us from cardiovascular disease, but its absence may aggravate certain cardiovascular conditions. At long last, we can point to a post-natal use for the shrinking violet of our chromosomal complement.
Contributor Information
Aeron M Small, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School Boston, 77 Avenue Louis Pasteur, Boston, MA 02115, USA.
Peter Libby, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School Boston, 77 Avenue Louis Pasteur, Boston, MA 02115, USA.
Funding
A.S. is funded by a precision and genomic medicine T32 co-led by Dr Jordan Smoller and Dr Heidi Rehm. P.L. receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892 and 1R01HL163099-01), the American Heart Association (18CSA34080399), the RRM Charitable Fund, and the Simard Fund.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Griffin DK. Is the Y chromosome disappearing?—Both sides of the argument. Chromosome Res 2012;20:35–45. 10.1007/s10577-011-9252-1 [DOI] [PubMed] [Google Scholar]
- 2. Sano S, Horitani K, Ogawa H, Halvardson J, Chavkin NW, Wang Y, et al. . Hematopoietic loss of Y chromosome leads to cardiac fibrosis and heart failure mortality. Science 2022;377:292–297. 10.1126/science.abn3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mas-Peiro S, Hoffmann J, Fichtlscherer S, Dorsheimer L, Rieger MA, Dimmeler S, et al. . Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. Eur Heart J 2019;41:933–939. 10.1093/eurheartj/ehz591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mas-Piero S, Abplanalp WT, Rasper T, Berkowitsch A, Leistner DM, Dimmeler S, et al. . Mosaic loss of Y chromosome in monocytes is associated with lower survival after transcatheter aortic valve replacement. Eur Heart J 2023;44:1943–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Healy AM, Pickard MD, Pradhan AD, Wang Y, Chen Z, Croce K, et al. . Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation 2006;113:2278–2284. 10.1161/CIRCULATIONAHA.105.607333 [DOI] [PubMed] [Google Scholar]
- 6. Croce K, Gao H, Wang Y, Mooroka T, Sakuma M, Shi C, et al. . Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation 2009;120:427–436. 10.1161/CIRCULATIONAHA.108.814582 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.