Abstract
Pathologies that are causative for neurodegenerative disease (ND) are also frequently present in unimpaired, older individuals. In this retrospective study of 1647 autopsied individuals, we report the incidence of 10 pathologies across ND and normal ageing in attempt to clarify which pathological combinations are disease-associated and which are ageing-related. Eight clinically defined groups were examined including unimpaired individuals and those with clinical Alzheimer’s disease, mixed dementia, amyotrophic lateral sclerosis, frontotemporal degeneration, multiple system atrophy, probable Lewy body disease or probable tauopathies. Up to seven pathologies were observed concurrently resulting in a heterogeneous mix of 161 pathological combinations. The presence of multiple additive pathologies associated with older age, increasing disease duration, APOE e4 allele and presence of dementia across the clinical groups. Fifteen to 67 combinations occurred in each group, with the unimpaired group defined by 35 combinations. Most combinations occurred at a <5% prevalence including 86 that were present in only one or two individuals. To better understand this heterogeneity, we organized the pathological combinations into five broad categories based on their age-related frequency: (i) ‘Ageing only’ for the unimpaired group combinations; (ii) ‘ND only’ if only the expected pathology for that individual’s clinical phenotype was present; (iii) ‘Other ND’ if the expected pathology was not present; (iv) ‘ND + ageing’ if the expected pathology was present together with ageing-related pathologies at a similar prevalence as the unimpaired group; and (v) ‘ND + associated’ if the expected pathology was present together with other pathologies either not observed in the unimpaired group or observed at a greater frequency. ND only cases comprised a minority of cases (19–45%) except in the amyotrophic lateral sclerosis (56%) and multiple system atrophy (65%) groups. The ND + ageing category represented 9–28% of each group, but was rare in Alzheimer’s disease (1%). ND + associated combinations were common in Alzheimer’s disease (58%) and Lewy body disease (37%) and were observed in all groups. The Ageing only and Other ND categories accounted for a minority of individuals in each group. This observed heterogeneity indicates that the total pathological burden in ND is frequently more than a primary expected clinicopathological correlation with a high frequency of additional disease- or age-associated pathologies.
Keywords: Alzheimer’s disease, tauopathy, frontotemporal lobar degeneration, Lewy body disease, neurodegenerative disease
Robinson et al. examine the incidence of 10 pathologies in autopsied individuals with or without neurodegenerative disease. Up to seven pathologies were observed concurrently, resulting in 161 different combinations. The presence of multiple additive pathologies was associated with longer disease duration, clinical dementia, older age and APOE e4 status.
See Younes and Mormino (https://doi.org/10.1093/brain/awad154) for a scientific commentary on this article.
See Younes and Mormino (https://doi.org/10.1093/brain/awad154) for a scientific commentary on this article.
Introduction
Neurodegenerative disease (ND) is frequently associated with multiple neuropathologies.1,2 Even before dementia develops, cognitive impairment related to Alzheimer’s disease pathology is commonly associated with additional comorbid pathologies, including cerebral amyloid angiopathy (CAA) in 50–70% of individuals, transactive response DNA-binding protein-43 (TDP-43) inclusions in 15–35% and Lewy pathology in ∼25%.3 Late-stage Alzheimer’s disease dementia has even higher incidences of comorbid pathology: CAA in 90–95%, TDP-43 inclusions in 50% and Lewy pathology in 50%.3,4 It is now well understood that pathological change also develops in asymptomatic individuals.5,6 By age 80 the estimated prevalence of tau-positive neurofibrillary tangles is 95–99%,7,8 cerebrovascular disease (CVD) lesions is 50–70%,9 amyloid-β plaques is 60–70%,7 TDP-43 inclusions is 30–40%10 and Lewy pathology is 15–30%.11,12 Thus, when an individual is suspected of having an ND, multiple co-pathologies are often present. These can be conceptualized as either comorbid neuropathologies associated with the primary ND or simply incidental pathologies due to increasing age.13 Because co-pathologies are frequently clinically relevant,14-16 understanding whether co-pathologies are ND-associated or age-related may help us elucidate clinically relevant ND processes, which could inform drug trial design and future treatments.
Previous studies have examined the presence of multiple pathologies in ND, primarily in the context of Alzheimer’s disease in comparison to healthy ageing.4,17 One community-based cohort reported that dementia was more likely when four proteinopathies (tau-positive neurofibrillary tangles, amyloid-β plaques, TDP-43 inclusions and α-synuclein-positive Lewy pathology) were present, some of whom also had atherosclerosis, arteriolosclerosis, infarcts, hippocampal sclerosis and/or argyrophilic grain disease.4 One of the larger community-based cohorts reported on the pathological combinations involving nine pathologies (pathological Alzheimer’s disease, CAA, gross and microscopic infarcts, TDP-43 inclusions, atherosclerosis, arteriolosclerosis, cortical Lewy bodies and hippocampal sclerosis). They observed 236 different combinations of neuropathology with many of the combinations only present in a single individual. Their modelling suggested that pathological Alzheimer’s disease only accounted for 50% of the cognitive loss in the cohort, implicating the other eight observed pathologies, in combination with Alzheimer’s disease pathology or in separate pathological combinations, as contributory to clinical Alzheimer’s disease.
In this retrospective study of 1647 individuals, we also report on the heterogeneity in both unimpaired individuals and those with clinical Alzheimer’s disease and extend this analysis to other clinically defined ND conditions. We characterized 10 pathologies across eight clinical groups and observed 161 pathological combinations involving these pathologies. To better understand this heterogeneity, we organized the combinations into five broad categories based on their age-related frequency: (i) ‘Ageing only’ for the unimpaired group combinations; (ii) ‘ND only’ if only the expected pathology for a given clinical group was present; (iii) ‘Other ND’ if the expected pathology was not present; (iv) ‘ND + ageing’ if the expected pathology was present with ageing-related pathologies at a similar prevalence as the unimpaired group; and (v) ‘ND + associated’ if the expected pathology was present together with other pathologies either not observed in the unimpaired group or observed at a greater frequency.
Materials and methods
Participants
As of 1 November 2021, the Center for Neurodegenerative Disease Research brain bank at the University of Pennsylvania consisted of 1889 autopsied adults with a neuropathological diagnosis who were age ≥ 30 years at death. Most individuals with a clinical ND syndrome were referred to the autopsy programme by clinicians in several clinical cores at the University of Pennsylvania, while many of the patients with no impairment were recruited at time of death due to other causes. Cases were excluded due to a clinical diagnosis of schizophrenia (n = 89), other major psychiatric illness (n = 6) or other (n = 13). Of these, 98 were missing pathological measures to diagnosis the level of Alzheimer’s disease neuropathological change according to NIA-AA criteria.18 In addition, 29 were missing arteriolosclerosis or infarct measures to diagnosis cerebrovascular changes and three were missing regional CAA scores to stage CAA. The resulting cohort consisted of 1647 individuals. Informed consent for autopsy was obtained by the next of kin in accordance with local and federal laws and procedures.
Primary clinical groups
Individuals were assigned to one of eight clinically and/or genetically defined groups, each corresponding to different suspected underlying neuropathology: clinical Alzheimer’s disease, Mixed, amyotrophic lateral sclerosis (ALS), frontotemporal degeneration (FTD), clinical Tau, multiple system atrophy (MSA), clinical Lewy body disease (LBD) and unimpaired. The clinical Alzheimer’s disease group included probable Alzheimer’s disease, dementia not otherwise specified, logopenic variant of frontotemporal lobar degeneration, posterior cortical atrophy, Down syndrome and PSEN1 variant cases. The Mixed group included possible Alzheimer’s disease, vascular dementia, Alzheimer’s disease with secondary clinical diagnoses of dementia with Lewy bodies, Parkinson’s disease or FTD and other cognitive impairment cases. The ALS group included definite, probable, possible and suspected ALS cases with and without cognitive impairment, primary muscular atrophy, motor neuron disease not otherwise specified and primary lateral sclerosis cases. The FTD group included behavioural variant FTD, non-logopenic primary progressive aphasia FTD, FTD not otherwise specified and C9orf72 and PGRN variant cases. The Tau group included corticobasal syndrome and progressive supranuclear palsy cases, with and without dementia. The LBD group included Parkinson’s disease, Parkinson’s disease with mild cognitive impairment or dementia and dementia with LBD cases. The MSA group included clinically diagnosed MSA cases. The unimpaired group had no history of clinical neurodegeneration, and were without either cognitive, movement or motor impairments.
Clinical diagnoses were assigned by neurologists according to established clinical criteria, which included clinical and neuropsychological assessments administered by each clinical core. Clinical diagnoses were assigned by ND specialists (neurologists, psychiatrists, gerontologists) according to established clinical criteria, which included clinical and neuropsychological assessments administered by each clinical core. Subjects from the Penn Parkinson’s Disease and Movement Disorder Clinic were diagnosed based on the UK Brain Bank criteria.19 Subjects from the Penn Amyotrophic Lateral Sclerosis Center were diagnosed based on the El Escorial criteria.20,21 Subjects from the Penn Frontotemporal Degeneration Center were designated prospectively based on the current consensus guidelines at weekly multidisciplinary consensus meetings.22,23 Subjects from the Penn Alzheimer’s Disease Research Center were diagnosed clinically using the McKhann criteria24 and then the updated NIA-AA core clinical criteria.25
Dementia status was determined by review of clinical diagnoses. Dementia severity was determined by a Clinical Dementia Rating of 3, which was available for 330 of the 453 Alzheimer’s disease cases and 132 of the 197 FTD cases. Late onset was defined by the start of clinical symptoms at age 65 or older.
Neuropathology
Ten pathologies were determined from neuropathology measures available on each case: A for amyloid-β plaques staged by Thal amyloid phase; B for tangles staged by Braak and Braak neurofibrillary tangle stage; C for neuritic plaques reported by CERAD score; CAA for cerebral amyloid angiopathy stage; TDP for TDP-43 inclusions and neurites; LB for Lewy bodies and neurites; CVD for cerebrovascular disease lesions including infarcts and arteriolosclerosis; Tau for glial and neuronal tau associated with corticobasal degeneration, progressive supranuclear palsy and Pick disease; GCI for α-synuclein-positive glial cytoplasmic inclusions; and Rare (consisting of rare other ND entities). The full description and criteria for each are described below. At least 16 brain regions were examined in the neuropathology evaluations including middle frontal, inferior parietal, superior temporal, occipital striate cortex, hippocampal cornu ammonis, hippocampal dentate gyrus, entorhinal cortex, amygdala, anterior cingulate, putamen, globus pallidus, thalamus, midbrain, substantia nigra, upper pons including basis and locus coeruleus, medulla and cerebellum including dentate nucleus. Immunohistochemistry was performed on each region using the VECTASTAIN® ABC system with ImmPACT DAB (Vector Laboratories) to detect amyloid-β (mouse antibody NAB228 at 1:30 000 dilution with formic acid antigen retrieval, generated in-house), tau (mouse antibody PHF1 at 1:3000 dilution, a gift from Dr Peter Davies), α-synuclein (mouse antibody Syn303 at 1:30 000 dilution with formic acid antigen retrieval, generated in-house) or TDP-43 (rat antibody 1D3 at 1:300 dilution with formic acid antigen retrieval, a gift from Drs Manuela Neumann and Elisabeth Kremmer) pathology. Each region was assigned a semiquantitative score—none, rare, mild, moderate, or severe—for each pathology. Haematoxylin and eosin histology was performed to assess neuron loss, gliosis, arteriolosclerosis, and for the presence of infarcts. Further details are available in Toledo et al.26 All cases were reviewed by a board-certified neuropathologist.
Each of these pathologies was considered present only if intermediate or high levels were reported (Table 1). Braak neurofibrillary tangle stage was assessed as either I/II, III/IV or V/VI corresponding to B1, B2 and B3 scores.27 ,28 While 90% of the cohort had at least a few neurofibrillary tangles (Braak stage I/II, B1), only 53% of the cohort exhibited NIA-AA B2 or B3 scores according to consensus criteria for Alzheimer’s disease and this group was therefore considered B-positive for this study. Other pathologies were not as prevalent as neurofibrillary tangles, but a similar approach to minimal thresholding was applied. The prevalence of each pathology for each clinical group is available before and after thresholding (Supplementary Tables 1 and 2). Thal phases29 were used to assess A positivity but only for cases with NIA-AA scores of A2 or A3. CERAD neuritic plaque scores were available by assessment of at least three cortical areas30 with either C2 or C3 scores deemed C-positive. CAA stages were assigned after screening neocortex for amyloid-β-positive CAA scores. Cases with neocortical CAA were considered CAA-positive if CAA was also present in the amygdala, hippocampus, anterior cingulate, cerebellum, caudate, putamen or thalamus, i.e. CAA stage 2 or 3 as per Thal et al.31 TDP-positive cases included all cases with a frontotemporal lobar degeneration-TDP diagnosis, all ALS cases with TDP-43 inclusions, all cases with limbic-predominant age-related TDP-43 encephalopathy (LATE) neuropathological change pathology was present in the hippocampus or entorhinal cortex10 and all cases with neocortical TDP-43 inclusions.32 LB-positive cases included all cases with a brainstem predominant, transitional or neocortical pattern of Lewy body pathology as per McKeith criteria.33 For cases with an amygdala predominant pattern of pathology, only cases with hippocampal or entorhinal cortex Lewy pathology were included as LB-positive. CVD-positive cases included all cases with two or more of the following pathologies: large, lacunar or micro infarcts; moderate to severe arteriolosclerosis; moderate to severe neocortical CAA.34,35 Tau-positive cases included all cases with coriticobasal degeneration, progressive supranuclear palsy, Pick disease and globular glial tauopathy diagnoses, as well as cases with a diagnosis of tauopathy unclassifiable. GCI-positive cases included all cases with a primary or secondary diagnosis of neuropathological MSA. Rare positive was used for all cases when other pathologies were described in neuropathological reports (n = 25). The prevalence of each individual pathology for each clinical group is reported in Supplementary Table 3.
Table 1.
Examined pathologies and thresholds
| Pathology | Description |
|---|---|
| A | Amyloid-β staged by amyloid phase, A2 or A3a |
| B | Neurofibrillary tangles staged by Braak and Braak, B2 or B3a |
| C | Neuritic plaque severity by CERAD score, C2 or C3a |
| TDP | All ALS cases with TDP-43 pathology and all other cases with neocortical or hippocampal formation TDP-43 pathology |
| LB | All cases with Lewy pathology affecting the substantia nigra, the hippocampal formation and/or neocortical regions |
| CAA | Cerebral amyloid angiopathy in neocortical and amygdala, hippocampus, anterior cingulate, cerebellum, caudate, putamen or thalamus, stage 2 or 3b |
| CVD | Two or more: infarcts (large, lacunar or micro), moderate/severe neocortical CAA or moderate/severe arteriolosclerosisc |
| Tau | Any case with a primary or secondary diagnosis of corticobasal degeneration, progressive supranuclear palsy, Pick disease, globular glial tauopathy or tauopathy unclassifiable |
| GCI | Any case with a primary or secondary diagnosis of MSA |
| Rare | ALS cases with SOD1 mutations, frontotemporal lobar degeneration with fused in sarcoma (FUS)-positive inclusions, frontotemporal lobar degeneration with ubiquitin-positive inclusions, hereditary diffuse leukoenchelopathy with spheroids, adult polyglucosan body disease |
Pathological categories
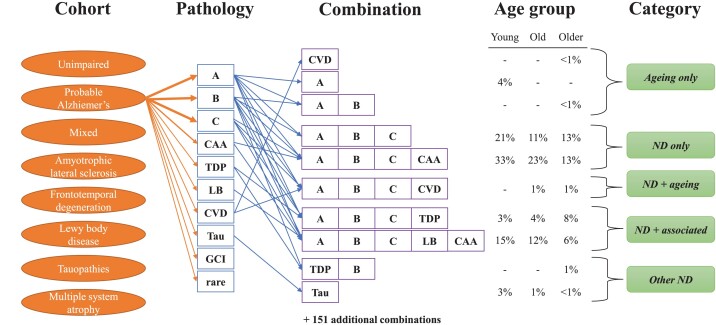
The number of concurrent pathologies determined the pathological combination present in the individual (Fig. 1). One hundred and sixty-one combinations were observed including the combination ‘Limited’, defined as the absence of all the pathologies described above. Pathological combinations were categorized into one of five categories consisting of ND only, Ageing only, ND + ageing, Other ND or ND + associated. More specifically, each clinical cohort had an expected pathology as follows: A-B-C or A-B-C-CAA for the clinical Alzheimer’s disease and Mixed groups; TDP for the ALS group; TDP or Tau for the FTD group; Tau for the clinical Tau group; LB for the clinical LBD group; GCI for the MSA group. ND only is defined when the pathological combination exactly matched the expected pathology. Ageing only combinations are based on the observed frequency of pathological combinations in young (<65), old (65–79) and older (80+) age subgroups in the unimpaired group. Based on these ageing-related combination frequencies, we categorized a case as ND + ageing if the expected pathology is present with additional pathologies matching the Ageing only combinations at a similar age-related frequency (within 5%). For instance, plaques—the combination A—were observed in the young, old and older unimpaired group at an incidence of 8%, 12% and 10%, respectively, and in the LBD group as the combination LB-A at an incidence of 9%, 4% and 0%, respectively. Thus, the pathological combination LB-A was categorized as ND + ageing in the LBD group. Other ND combinations are pathologies not expected or observed in the Ageing only category. For example, cases clinically diagnosed as behavioural variant of FTD in the FTD group but with an A-B-C combination would be classified as Other ND. Finally, ND + associated combinations exhibited their expected ND pathology and additional comorbid pathologies. These comorbid pathologies were either the Ageing only combinations at a higher frequency than seen in the unimpaired group (>5%) or pathologies not observed in the NI group. For example, the LB combination was only observed in the young age subgroup in the unimpaired group at an incidence of 3% (Table 2). So the prevalence of LB in the young, old and older clinical Alzheimer’s disease group at 31%, 20% and 27% as A-B-C-LB or A-B-C-CAA-LB defined these pathological combinations as ND + associated for the AD group. Similarly, the combination Tau was not observed in the unimpaired group so combination A-B-C-Tau would also be categorized as a ND + associated combination in the clinical Alzheimer’s disease group.
Figure 1.
ND pathologies, combinations and categories of combinations. Eight clinically defined cohorts were examined including unimpaired individuals and those with clinical Alzheimer’s disease, mixed dementia, ALS, FTD, MSA, probable LBD, or probable tauopathies. Ten assessed pathologies (see the ‘Materials and methods’ section) resulted in a heterogeneous mix of 161 pathological combinations, including 35 combinations in the unimpaired cohort. To better understand this heterogeneity, we organized the pathological combinations into five broad categories based on their age-related frequency: ‘Ageing only’ for the unimpaired group combinations; ‘ND only’ if only the expected pathology for that individual’s clinical phenotype was present; ‘ND + ageing’ if the expected pathology was present together with ageing-related pathologies at a similar prevalence as the unimpaired group; ‘ND + associated’ if the expected pathology was present together with other pathologies not observed in the unimpaired group or observed at a greater frequency and ‘Other ND’ if the expected pathology was not present. As an example, a subset of observed pathological combinations is shown for clinical Alzheimer’s disease. In this cohort, the combinations A-B-C and A-B-C-CAA are the ND only combinations. A-B-C-CVD was an ND + ageing combination, while A-B-C-TDP and A-B-C-LB-CAA were ND + associated combinations. Only rarely did clinical Alzheimer’s disease involve Other ND combinations such as TDP-B or Tau or Ageing only combinations such as CVD, A or A-B.
Table 2.
Unimpaired pathological combinations
| Combination | Total pathologies | Young <65 | Old 65–79 | Older >80 |
|---|---|---|---|---|
| Limited | 0 | 74% | 54% | 8% |
| A | 1 | 8% | 12% | 10% |
| CAA | 1 | 3% | 9% | 4% |
| CVD | 1 | 5% | 5% | 4% |
| C | 1 | 3% | – | 2% |
| LB | 1 | 3% | – | – |
| TDP | 1 | – | – | 2% |
| A-B | 2 | 3% | – | 2% |
| A-C | 2 | – | 2% | 6% |
| A-CAA | 2 | 3% | – | 4% |
| A-LB | 2 | – | 2% | – |
| A-Rare | 2 | – | – | 2% |
| B-C | 2 | – | – | 2% |
| B-CAA | 2 | – | – | 2% |
| CVD-TDP | 2 | – | 2% | – |
| A-B-CAA | 3 | – | 2% | 4% |
| A-B-CVD | 3 | – | 2% | 2% |
| A-B-LB | 3 | – | 2% | 2% |
| A-C-CAA | 3 | – | 2% | 4% |
| A-C-CVD | 3 | – | 4% | – |
| A-C-TDP | 3 | – | – | 2% |
| A-TDP-CAA | 3 | – | – | 2% |
| B-CAA-CVD | 3 | – | – | 2% |
| C-CAA-CVD | 3 | – | – | 2% |
| A-B-C-TDP | 4 | – | – | 2% |
| A-C-LB-CVD | 4 | – | – | 2% |
| A-LB-CAA-CVD | 4 | – | – | 2% |
| A-B-C | 3 | – | – | 2% |
| A-B-C-CAA | 4 | – | 2% | 4% |
| A-B-C-CVD | 4 | – | – | 2% |
| A-B-C-LB | 4 | – | – | 2% |
| A-B-C-CAA-CVD | 5 | – | 2% | 2% |
| A-B-C-LB-CAA | 5 | – | – | 6% |
| A-B-C-LB-CVD | 5 | – | – | 2% |
| A-B-C-TDP-CVD | 5 | – | – | 2% |
| Number of combinations | – | 8 | 14 | 31 |
Genetics
Genomic DNA was extracted from brain tissues using QIAamp DNA mini kit (Qiagen). C9orf72 hexanucleotide repeat expansions were determined by repeat-primed PCR and capillary electrophoresis or genotyping of surrogate risk allele rs3849942 as previously described.36APOE allele status was defined using two single nucleotide polymorphisms, rs7412 and rs429358, which were genotyped by TaqMan allelic discrimination assays (Thermo Fisher). APOE allele status was available for 1613 individuals. Other genetic variants were primarily determined by single nucleotide polymorphism genotype of top hits from published genome-wide association studies as previously described.26 Data for 203 genetic variants were available including 56 C9orf72, 41 GBA, 25 TREM2, 21 GRN, 16 APP, 10 LRRK2, 10 PSEN1, 9 MAPT, 7 SOD1, 3 CSF1R, 2 GBE1, 2 SNCA and 1 VCP.
Statistical analysis
Two-tailed t-test and χ2 analysis was used to evaluate the association between sum of pathology and age, disease duration, APOE e4 allele status and clinical dementia for each clinical group. Each test was performed independently for each measure with the expected pathology sum as the reference (Table 3) using Microsoft Excel 2016. A comparison of the ND only, ND + associated and ND + ageing categories was done with a multinomial logistic regression analysis using the ‘VGAM’ R package (version 3.4.1). Separate models were fitted for each clinical group with the ND + associated, ND + ageing, and ND only categories serving as the outcome data and the ND only category was selected as the reference category. For each multinomial logistic regression model, covariates in the dataset are included one at a time. Multiple testing adjustment was done using Bonferroni correction for the multinomial logistic regression model. All statistical tests were two-sided.
Table 3.
Sum of pathologies associates with increasing age, disease duration, APOE e4 status and clinical dementia
| Groupa | Sum | n | Onset avg (years) |
t-test | Duration avg (years) |
t-test | Age avg (years) |
t-test | APOE e4 | χ2 | Clinical dementiab | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unimpaired | 0–1 | 97 | – | – | – | – | 68.4 | Ref | 30% | Ref | 0% | – |
| 2–3 | 32 | – | – | – | – | 82.1 | 0.000 | 34% | 0.579 | 0% | – | |
| 4+ | 15 | – | – | – | – | 89.7 | 0.000 | 36% | 0.622 | 0% | – | |
| Clinical AD | 1–2 | 23 | 70.9 | 0.042 | 8.0 | 0.187 | 74.0 | 0.414 | 13% | 0.000 | 38% | 0.243 |
| 3–4 | 206 | 66.4 | Ref | 8.8 | Ref | 74.5 | Ref | 53% | Ref | 50% | Ref | |
| 5+ | 224 | 68.1 | 0.053 | 10.4 | 0.000 | 78.5 | 0.000 | 71% | 0.000 | 56% | 0.047 | |
| Mixed | 1–2 | 13 | 70.9 | 0.190 | 7.5 | 0.387 | 79.8 | 0.405 | 15% | 0.031 | 77% | 0.661 |
| 3–4 | 42 | 74.1 | Ref | 7.1 | Ref | 80.5 | Ref | 45% | Ref | 71% | Ref | |
| 5+ | 50 | 71.7 | 0.399 | 8.7 | 0.194 | 80.7 | 0.375 | 63% | 0.014 | 84% | 0.049 | |
| ALS | 1 | 109 | 55.8 | Ref | 4.4 | Ref | 60.2 | Ref | 20% | Ref | 17% | Ref |
| 2–3 | 42 | 63.3 | 0.000 | 5.3 | 0.140 | 68.6 | 0.000 | 26% | 0.332 | 17% | 0.896 | |
| 4+ | 20 | 71.0 | 0.000 | 4.1 | 0.372 | 75.0 | 0.000 | 47% | 0.002 | 20% | 0.762 | |
| FTD | 1 | 99 | 58.1 | Ref | 7.9 | Ref | 66.1 | Ref | 19% | Ref | 36% | Ref |
| 2–3 | 52 | 63.1 | 0.001 | 8.3 | 0.296 | 71.1 | 0.001 | 25% | 0.198 | 50% | 0.041 | |
| 4+ | 46 | 63.8 | 0.000 | 9.7 | 0.008 | 73.4 | 0.000 | 41% | 0.000 | 66% | 0.000 | |
| Clinical Tau | 1 | 60 | 63.3 | Ref | 5.9 | Ref | 69.6 | Ref | 10% | Ref | 35% | Ref |
| 2–3 | 65 | 66.6 | 0.011 | 7.2 | 0.015 | 73.9 | 0.001 | 25% | 0.000 | 46% | 0.059 | |
| 4+ | 50 | 65.7 | 0.096 | 7.6 | 0.006 | 73.5 | 0.010 | 56% | 0.000 | 70% | 0.000 | |
| Clinical LBD | 1 | 99 | 59.7 | Ref | 14.5 | Ref | 74.1 | Ref | 19% | Ref | 37% | Ref |
| 2–3 | 124 | 61.2 | 0.144 | 15.4 | 0.175 | 76.6 | 0.014 | 36% | 0.000 | 57% | 0.000 | |
| 4+ | 127 | 68.0 | 0.000 | 10.8 | 0.000 | 78.9 | 0.000 | 53% | 0.000 | 76% | 0.000 | |
| MSAc | 1 | 34 | 54.4 | Ref | 7.6 | Ref | 62.6 | Ref | 18% | Ref | 6% | Ref |
| 2–3 | 11 | 60.7 | 0.027 | 8.3 | 0.310 | 69.6 | 0.005 | 27% | 0.434 | 0% | 0.351 | |
| 4+ | 7 | 62.3 | 0.018 | 10.0 | 0.086 | 72.9 | 0.001 | 43% | 0.091 | 60% | 0.000 |
P-values less than 0.05 are given in bold.
The reference subgroup for each statistical test is indicated by Ref for each clinical cohort. For the clinical AD and Mixed groups the reference is three to four pathologies (A-B-C or A-B-C-CAA).
Clinical dementia represents the presence of dementia in all groups except for the AD and FTD groups. In these groups with 100% dementia, we analysed severe dementia as determined by a Clinical Dementia Rating of 3.
Clinical dementia data only available for n = 42 in the MSA group.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of the research participants.
Results
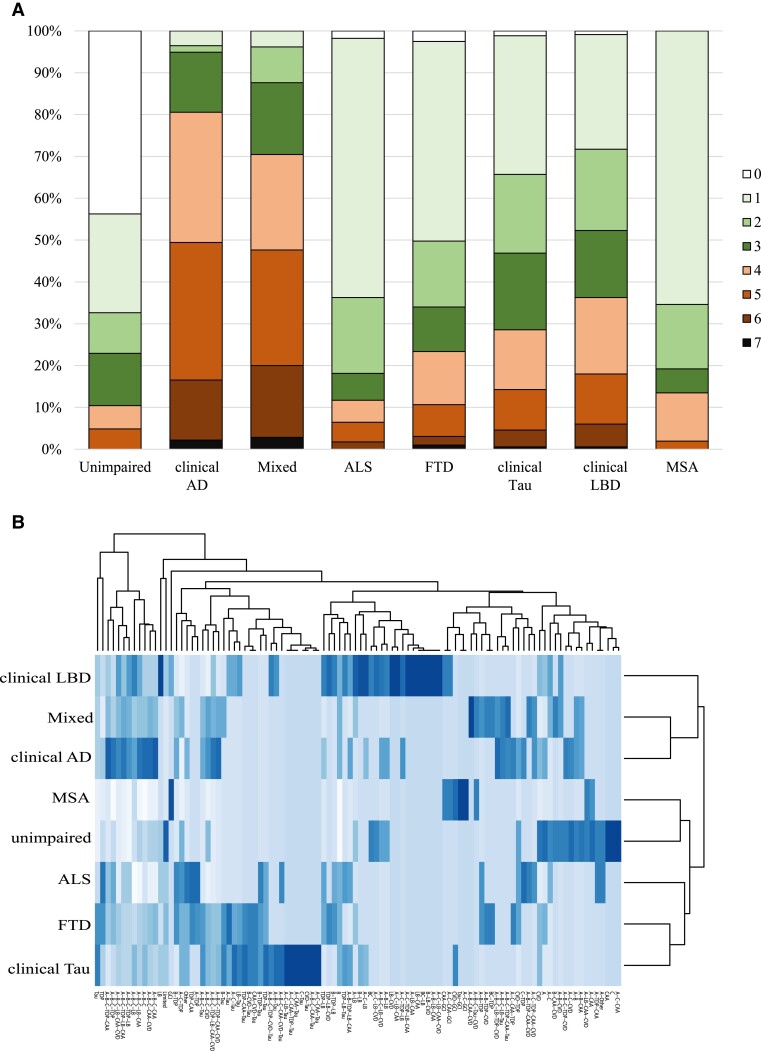
Pathological combinations were assigned to 1647 cases representing a diverse group of ageing-related ND clinical phenotypes including dementias, movement disorders and motor neuron disease. We assessed 10 pathologies (Fig. 1). Only non-rare pathologies were included in our analysis, which we defined by both spread and severity (see Methods). Even neglecting cases with mild burdens of limited pathology such as individuals with a Braak I/II stage of neurofibrillary tangles, the total number of pathologies for each case and the number of pathological combinations observed showed considerable variation across and within each clinical group (Fig. 2). Pathological sums for each case varied from 0 to 7 (Fig. 2A). Four or more pathologies were common in the clinical Alzheimer’s disease and Mixed groups, ≥2 pathologies were common in the clinical Tau and clinical LBD groups, while only one or two pathologies were common in ALS, FTD, MSA and unimpaired groups. Across the entire cohort, 161 combinations of pathologies were observed, including the ‘Limited’ combination which is the absence of any of the 10 defined pathologies. Most groups—excepting MSA—were composed of 30 or more pathological combinations, suggesting that the underlying pathological burden across disease is heterogeneous. Interestingly, the unimpaired group (without any neurological clinical phenotype) also exhibited 35 combinations, indicating that neuropathological heterogeneity exists independent of ND. In addition, this heterogeneity was not uniformly distributed (Fig. 2B). Approximately half of the cohort (n = 875/1647, 53%) exhibited one of 10 combinations (Limited, TDP, Tau, LB, GCI, A-B-C, A-B-C-CAA, A-B-C-LB, A-B-C-CAA-LB, A-B-C-CAA-TDP). Consequently, 151 combinations accounted for the other half of the cohort, with many of the combinations observed in fewer than five individuals. In fact, 61 combinations were uniquely observed in individuals. The remaining 100 combinations are shown in Fig. 2B and the full list of all 161 combinations is available in Supplementary Table 4. In summary, there was considerable heterogeneity across all clinically defined groups in terms of the underlying ND pathological combinations.
Figure 2.
Prevalence of total number of pathologies and specific pathological combinations by clinical group. The total number of pathologies and the specific pathological combinations were variably distributed in each clinical group. (A) The presence of one or more pathologies was not uncommon in the unimpaired group and most cases in the clinical Alzheimer’s disease (AD) group had four or more pathologies. Between these two extremes, the prevalence of multiple pathologies was common in each ND group. (B) One hundred and sixty-one pathological combinations were observed across the cohort with 10 combinations accounting for approximately half of each clinical group. Consequently, the incidence of the remaining 151 combinations ranged from uncommon to rare, with 61 combinations only observed in individuals. The clinical LBD group was the most heterogeneous, the MSA group was the most homogeneous and 35 combinations were observed in the unimpaired group. Shown is a heat map showing the prevalence of the top 100 non-unique combinations after cubed-root transformation, generated using the heatmap function in R Version 4.2.1.
Analysing the total number of pathologies per case in each clinical group is revealing (Table 3). For this analysis, the expected reference pathological sum was a single pathology in most diseases. However, for the clinical Alzheimer’s disease and Mixed cohorts the reference pathological sum was three or four pathologies (A-B-C or A-B-C-CAA). In the unimpaired group, the number of pathologies strongly associated with age at death. Many unimpaired individuals exhibited minimal changes consisting of either no pathologies or a single pathology, but those with two or more pathologies were more likely to be older at death. Across ND, individuals with more pathologies were also older (except for those in the Mixed group). In addition, more pathologies also associated with the presence of the APOE e4 allele. In the clinical Alzheimer’s disease group, for instance, cases with five or more pathologies accounted for approximately half of the group. These cases tended to be older and more likely to have an APOE e4 allele compared to those with only the expected three or four pathologies.
In terms of age of onset, there was a peculiar finding where individuals clinically diagnosed with Alzheimer’s disease but with only one or two pathologies exhibited a later age of onset compared to those with three to four pathologies. However, these individuals with only one or two pathologies were an atypical group representing only 5% of the clinical Alzheimer’s disease group. Indeed, 91% of the cases in this category did not fulfil neuropathological criteria for intermediate to high level of Alzheimer’s disease neuropathological change, indicating that dementia was due to non-Alzheimer’s disease mechanisms.
For many groups including clinical Alzheimer’s disease, FTD, clinical Tau and clinical LBD, increasing disease duration also associated with a greater likelihood of having more pathologies. Finally, an increase in the numbers of pathologies per case correlated with clinical dementia in many groups. Dementia was not always present in the clinical Tau and clinical LBD groups, but was more likely with a higher pathological sum. In the clinical Alzheimer’s disease, FTD and Mixed groups, the cases with more pathologies were not more likely to have dementia, but they were more likely to have severe dementia as assessed by a Clinical Dementia Rating of 3. In summary, an increase in the number of pathologies correlates with APOE e4 status, increasing disease duration, cognitive decline and increasing age across ND.
Age-related pathological combinations
To better understand the pathological heterogeneity that occurs across ND, we first examined the pathological combinations in the unimpaired cohort (Table 2). Arranged by increasing age, either young (<65), old (65–79) or older (≥80), only four combinations—Limited, A, CAA or CVD—were common between all three age groups. While these four combinations represented 89% and 81% of the young and old cohorts, they only represented 27% of the older cohort. In the young group, combinations of three or more pathologies were not observed. However, in the old group, 16% had three or more pathologies and this percentage was 50% in the older group. Similarly, combinations involving A-B-C (i.e. Alzheimer’s disease neuropathological change) were not observed in the young group, but represented 25% of the older group. Of the 35 combinations seen in the unimpaired group, 31 were observed in the older group compared to 14 in the old group and only 8 in the young group. We conclude that much of the pathological heterogeneity in neurologically unimpaired individuals is age-related.
Neurodegenerative disease-related combinations
When comorbid pathologies are present, are they simply an amalgamation of a primary neuropathology with an assortment of ageing combinations? To answer this question, we first have to define the primary neuropathology in each disease. For each clinical cohort, there is an expected pathology which we define as either A-B-C or A-B-C-CAA in the clinical Alzheimer’s disease group, TDP in the ALS group, either TDP or Tau in the FTD group, Tau for the clinical Tau group, LB for the clinical LBD group, and GCI for the MSA group. For the Mixed group, combinations involving A-B-C were the most common so we define A-B-C and A-B-C-CAA as the expected pathologies for this group. By ordering each clinical group into young, old and older subgroups and analysing the observed pathological combinations, five categories of pathological combinations are possible: (i) ‘Ageing only’ for combinations that exactly match and occur at a similar frequency to the unimpaired group; (ii) ‘ND only’ if only the expected pathology was present; (iii) ‘Other ND’ if the expected pathology was not present; (iv) ‘ND + ageing’ if the expected pathology was present with ageing-related pathologies at a similar prevalence as the unimpaired group; and (v) ‘ND + associated’ if the expected pathology was present together with other pathologies either not observed in the unimpaired group or observed at a greater frequency.
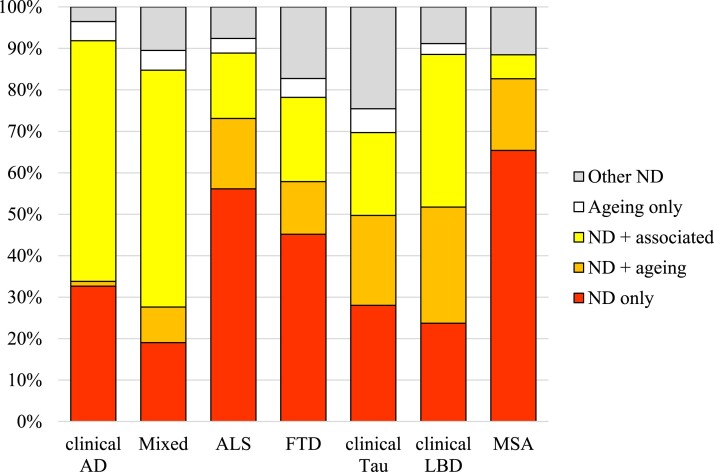
With these categories, we next considered how much of the pathological heterogeneity in ND is due to ageing or other factors (Fig. 3). Several observations were made. First, the expected primary pathology does associate with the clinical disease in 70–92% of cases. The clinical Alzheimer’s disease group had the highest incidence of cases containing the expected A-B-C combination categorized as either ND only, ND + ageing or ND + associated. The other clinical groups also exhibited a high concordance between their clinical phenotype and underlying neuropathological change in the majority of cases. As expected, the FTD and clinical Tau cohorts had the weakest clinicopathological correlations because many cases had another ND as the primary pathology. In the FTD group, both combinations containing TDP or Tau were considered concordant, but many cases were put into the Other ND category as atypical Alzheimer’s disease cases consisting of the A-B-C pathologies. This was also true in the Tau group, consistent with the known fact that corticobasal syndrome can be associated with underlying Alzheimer’s disease neuropathological change. Second, ageing-related pathologies only explain a minority of the pathological burden in ND. In the clinical Alzheimer’s disease group, the ND + ageing category was almost non-existent, implying that comorbid pathologies in Alzheimer’s disease are primarily driven by Alzheimer’s disease processes and not incidental age-related change. In the clinical LBD group, the ND + ageing category was larger, accounting for 28% of the cases, but even in that group, the ND + associated category is the largest category at 37%. Third, the number of ND only cases was relatively low, except in the MSA and ALS groups, where a slim majority were categorized ND only. In the clinical LBD group, for instance, the ND only category accounted for only 24% of cases. Finally, the number of Ageing only cases was infrequent. These were cases where the pathological combination pathologies that were observed occurred at a similar, age-related frequency as in the unimpaired group and did not involve the expected neuropathology or another ND pathology.
Figure 3.
Pathological combination categories. The heterogeneous pathological combinations can be grouped into categories by expected ND including: ‘ND only’ if only the expected pathology or pathologies are present; ‘Other ND’ if the expected pathology is not present; ‘Ageing only’ for combinations also present in age-matched unimpaired cases; ‘ND + ageing’ if the expected pathology was present along with pathologies in age-matched unimpaired cases; and ‘ND + associated’ if the expected pathology was present together with other pathological combinations. We observed that ND only cases are uncommon except in the ALS and MSA groups and that ND + ageing only correlates with a minority of cases, with the implication that additional ND pathologies are frequently observed across disease.
Combinations in pathologically confirmed disease
For the remaining analyses, we focused on pathological confirmed cases, focusing on the ND only, ND + ageing and ND + associated categories, i.e. all cases with a good clinicopathological correlation. We next tested whether the pathological combinations that occur are variably affected by factors such as APOE e4 allele status, increasing age and disease duration (Table 4). Only the significant model parameter estimates after Bonferroni correction are shown for covariates including age of onset, disease duration, age at death, sex, known genetic variants and the presence of an APOE e4 allele. Increasing age significantly affected the risk of being categorized as ND + associated and ND + ageing in most clinical categories relative to the ND only comparison group. In the clinical Tau group and LBD group, the presence of an APOE e4 allele also increased the risk of additional pathologies relative to the ND only comparison group. Interestingly, in the Alzheimer’s disease group disease duration increased the likelihood of ND + associated pathological combinations. Cases with disease-associated genetic variants were just as heterogeneous as sporadic cases. This is consistent with other neuropathology studies of genetic cohorts, where NDs driven by genetic variants exhibit considerable comorbid neuropathological change.37,38Supplementary Table 5 shows the genetic versus sporadic prevalence of ND only, ND + ageing and ND + associated cases for Alzheimer’s disease, ALS and frontotemporal lobar degeneration with TDP-43 inclusions. There were no differences associated with sex.
Table 4.
Factors influencing pathological combinations
| Group | Category | Age at death | Duration | Age of onset | APOE e4 |
|---|---|---|---|---|---|
| Unimpaired | ND ageing | 1.75 (1.43–2.16) | – | – | – |
| Clinical AD | ND associated | 1.22 (1.10–1.36) | 1.56 (1.18–2.07) | – | – |
| ALS | ND associated | 1.71 (1.31–2.23) | – | 1.57 (1.22–2.03) | – |
| ND ageing | 1.78 (1.37–2.31) | – | 1.73 (1.34–2.23) | – | |
| FTD | ND associated | 1.51 (1.19–1.92) | – | 1.47 (1.15–1.88) | – |
| ND ageing | 1.60 (1.21–2.13) | – | 1.56 (1.17–2.09) | – | |
| Clinical LBD | ND associated | – | – | 1.31 (1.13–1.51) | 3.72 (1.90–7.30) |
| ND ageing | – | – | 1.27 (1.09–1.48) | 4.23 (2.10–8.52) | |
| Clinical Tau | ND associated | 1.66 (1.18–2.33) | – | 1.61 (1.14–2.27) | – |
| ND ageing | 1.62 (1.17–2.25) | – | 1.67 (1.19–2.34) | 9.36 (1.93–45.39) |
Odds ratios and 95% confidence intervals shown for P-values <0.01 after accounting for multiple comparison using Bonferroni correction (0.05 divided by number of covariates considered). Odds ratio estimates for age at death and age of onset are for 5-year increments.
Common pathological combinations
As some of the heterogeneity in ND is explained by increasing age, we next focused on the common pathological combinations in late onset and early onset disease (Table 5). Overall, the prevalence of ND only cases is relatively uncommon across the cohorts, especially in late-onset disease. Consequently, there is a concomitant increase in combinations with additional pathologies in either ND + associated or ND + ageing cases. In ALS, as an example, 75% of early-onset disease cases are TDP only cases compared to 36% of late-onset ALS cases. Relatedly, there are three common combinations in late-onset ALS—TDP-B, TDP-A-B-C and TDP-A-B-C-CAA—but TDP-B is the only common combination in early-onset disease. A similar pattern exists for Alzheimer’s disease. In late-onset Alzheimer’s disease, only 31% of cases are described with only A-B-C or A-B-C-CAA pathologies. More common combinations involve the addition of TDP-43 inclusions, with or without LB or CVD pathologies. In early-onset Alzheimer’s disease, in contrast, combinations with TDP are less likely and A-B-C only or A-B-C-CAA only are more likely. In LBD, LB only disease is rarely seen as many cases exhibit Alzheimer’s disease spectrum pathologies, especially in late-onset disease. In early-onset LBD, common combinations include LB-A, LB-B, LB-A-C and LB-A-B-C, while late-onset disease common combinations include LB-A-C-CAA, LB-A-B-C, LB-A-B-C-CAA, LB-A-B-C-CAA-CVD and LB-A-B-C-TDP. Importantly, across disease, rare combinations, which we define as a prevalence of less than 5% often corresponding to combinations seen in only one or very few individuals, account for a substantial portion of each cohort, representing 14–64% of the various clinically defined groups.
Table 5.
Prevalent combinations in early- and late-onset ND
| Age of onset | AD | Mixeda | ALS | FTD | Clinical Tau | LBD | MSAa | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EO | LO | LO | EO | LO | EO | LO | EO | LO | EO | LO | EO | |
| Number of combinations | 13 | 16 | 27 | 14 | 15 | 24 | 24 | 14 | 26 | 35 | 30 | 8 |
| ND only (%)b | 44% | 31% | 18% | 75% | 36% | 66% | 41% | 58% | 28% | 35% | 18% | 81% |
| ND + associated (%) | 53% | 69% | 59% | 13% | 28% | 20% | 39% | 21% | 34% | 37% | 47% | 3% |
| ND + ageing (%) | 1% | 1% | 9% | 11% | 36% | 14% | 20% | 21% | 38% | 29% | 35% | 17% |
| Combinationc | ||||||||||||
| A-B-C | 16% | 13% | 10% | – | – | – | – | – | – | – | – | – |
| A-B-C-CAA | 26% | 19% | 11% | – | – | – | – | – | – | – | – | – |
| A-B-C-CAA-CVD | 9% | 10% | 7% | – | – | – | – | – | – | – | – | – |
| A-B-C-LB | 7% | – | 6% | – | – | – | – | – | – | 5% | 19% | – |
| A-B-C-LB-CAA | 16% | 6% | – | – | – | – | – | – | – | – | 8% | – |
| A-B-C-LB-CAA-CVD | – | 5% | – | – | – | – | – | – | – | – | 6% | – |
| A-B-C-TDP | – | 8% | – | – | 13% | – | – | – | – | – | – | – |
| A-B-C-TDP-CAA | 8% | 14% | 7% | – | 6% | – | – | – | – | – | – | – |
| A-B-C-TDP-CAA-CVD | – | 5% | 6% | – | – | – | – | – | – | – | – | – |
| A-B-C-TDP-LB | – | – | 7% | – | – | – | – | – | – | – | 6% | – |
| A-B-C-TDP-LB-CAA | – | 5% | 6% | – | – | – | – | – | – | – | – | – |
| TDP | – | – | – | 75% | 36% | 42% | 29% | – | – | – | – | – |
| TDP-A | – | – | – | – | 9% | – | – | – | – | – | – | – |
| TDP-B | – | – | – | 7% | 6% | 5% | – | – | – | – | – | – |
| Tau | – | – | – | – | – | 24% | 12% | 31% | 32% | – | – | – |
| Tau-A | – | – | – | – | – | – | 8% | – | – | – | – | – |
| Tau-A-B-C | – | – | – | – | – | – | – | – | 6% | – | – | – |
| Tau-A-B-C-CAA | – | – | – | – | – | – | – | – | 6% | – | – | – |
| Tau-A-C | – | – | – | – | – | – | – | – | 8% | – | – | – |
| Tau-A-C-CAA | – | – | – | – | – | – | – | – | 5% | – | – | – |
| Tau-B | – | – | – | – | – | – | 6% | – | 11% | – | – | – |
| Tau-CVD | – | – | – | – | – | – | – | – | 5% | – | – | – |
| Tau-LB | – | – | – | – | – | – | – | 5% | – | – | – | – |
| Tau-TDP | – | – | – | – | – | – | – | – | 8% | – | – | – |
| LB | – | – | – | – | – | – | – | – | – | 35% | 18% | – |
| LB-A | – | – | – | – | – | – | – | – | – | 10% | – | – |
| LB-A-C | – | – | – | – | – | – | – | – | – | 9% | – | – |
| LB-A-C-CAA | – | – | – | – | – | – | – | – | – | – | 6% | – |
| LB-B | – | – | – | – | – | – | – | – | – | 7% | – | – |
| GCI | – | – | – | – | – | – | – | – | – | – | – | 81% |
| GCI-A-C | – | – | – | – | – | – | – | – | – | – | – | 6% |
| Rare combinations | 17% | 15% | 41% | 18% | 30% | 29% | 45% | 64% | 21% | 35% | 39% | 14% |
EO = early onset; LO = late onset.
Early-onset Mixed and late-onset MSA not shown (n < 20).
A-B-C or A-B-C-CAA are the ND only combinations for the clinical Alzheimer’s disease (AD) and Mixed groups. TDP or Tau are the ND only combinations for FTD group.
Combinations with a prevalence of 5% or higher.
Discussion
The pathological burden for any individual with a suspected ND fits one of five categories: (i) the suspected disease without any additional pathology; (ii) the disease with incidental age-related pathology; (iii) the disease with additional non–age-related pathology; (iv) another ND as the primary pathology; or (v) pathology indistinguishable from incidental age-related pathology. For many cases, the underlying ND is as expected and the clinicopathological correlation is quite high. In fact, diagnostic accuracy is increasing in the age of biomarkers.39 However, the number of patients with additional pathologies appears to be the norm rather than the exception, especially in late-onset disease.40-42 Therefore, the ability to diagnose a disease identifies only the main pathology (or pathologies in the case of Alzheimer’s disease) but still severely underestimates the total pathological burden that is present in the majority of cases.
Our study highlights the neuropathological heterogeneity found across ND. In our cohort, 15–67 pathological combinations were reported for each clinical group (Fig. 2B). This heterogeneity was not uniformly distributed. In ALS, for instance, the TDP only combination accounted for the majority of the ALS cases, with the other 31 combinations distributed across approximately 40% of the cohort. We attempted to explain some of heterogeneity by categorizing some combinations as Other ND—essentially an unexpected neuropathological finding—or as being susceptible to a pathologic burden indistinguishable from that which occurs in individuals without any neurological impairment. However, these instances were uncommon and did not appreciably decrease the heterogeneity observed across clinical groups. We further attempted to explain some of the variability by categorizing some of the combinations as incidental if they were similar in age-adjusted incidence to the 35 combinations observed in the unimpaired group. However, frequently less than a third of the additional pathologies observed in disease were categorized as ND + ageing. This implies that comorbid pathologies are often associated with the primary ND. This has long been recognized in Alzheimer’s disease where both TDP-43 inclusions and Lewy bodies have a high incidence, suggesting that the presence of these comorbid neuropathologies are downstream of the primary disease process.10,43 This is also recognized in LBD, where Alzheimer’s disease pathology is highly prevelant.1,44 Indeed, a CSF analysis of Alzheimer’s disease biomarkers in LBD has already been reported,45 indicating that biomarkers to detect the presence of additional pathologies in LBD is a possibility for patients.
Our study reveals several distinct neuropathological profiles for each disease with implications for clinical trials (Table 5). ND only occurred as the majority outcome only in some of the early-onset diseases. These included ALS, FTD, the clinical Tauopathies and MSA. ND + associated occurred in most cases of both early-onset and late-onset clinical Alzheimer’s disease, and in the late-onset Mixed group. In the other clinical groups no single pathological category accounted for the majority of cases. Clinical Alzheimer’s disease had the most striking neuropathological profile. Because even young unimpaired cases had ageing-related incidental pathology 26% of the time, we would expect some of the pathological combinations in Alzheimer’s disease to match up with the frequency of these pathologies in the unimpaired group. Instead, the ND + ageing profile was almost non-existent in both early- and late-onset Alzheimer’s disease.
Across ND, most patients will exhibit multiple pathologies over their clinical course. These comorbidities include not just those additional pathologies associated with the primary ND, but also the ND + ageing pathologies. The ageing pathologies primarily fall on the Alzheimer’s disease spectrum, including 25% of the older >80 unimpaired group with Alzheimer’s disease neuropathological change. While a patient’s full pathological burden cannot be known ante-mortem, factors such as age, APOE e4 allele status and disease duration can guide a clinician’s understanding of what pathologies may develop (Table 2). Future biomarker studies may be able to examine the time course of multiple pathologies in depth.
Our study highlights the fact that multiple, accumulating pathologies may have detrimental clinical consequences across ND (Table 3). Our analysis is in agreement with similar studies that report concomitant pathologies either lower the threshold for dementia, enhance neurological decline or associate with specific clinical symptoms. These studies have primarily focused on Alzheimer’s disease, where older individuals tend to have a less severe burden of plaques and tangles46 and a higher likelihood of pathologies such as hippocampal sclerosis, CVD and ageing-related tau astrogliopathy, with the implication that the total burden of pathologies in those with dementia is greater than their age-matched peers.2,41,42,47,48 Alternatively, studies have also shown that certain clinical features are associated with the presence of a specific pathology. TDP-43 inclusions, in the context of late-onset dementia, has been implicated in specific episodic memory, semantic memory and perceptual speed deficits, as well as a more rapid cognitive decline.16,42,49
Our analyses of a limited number of simplified and dichotomized pathologies facilitated the comparison of combinations across ND, but in doing so, we oversimplified the neuropathological heterogeneity in the human brain. More specifically, our definitions of the TDP, Tau and CVD pathologies defined several similar, but distinct pathologies as a single pathology. If we consider TDP-43 pathology first, the distribution of TDP-43 inclusions may be present in the human brain as a result of several distinct clinicopathological entities where preferential involvement of the medial temporal lobe corresponds to LATE neuropathological change,50 preferential involvement of motor neurons corresponds to ALS,51 a wider distribution with prominent involvement of the frontal and temporal neocortex corresponding to several subtypes of frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP)52 or threads and astrocytic plaque-like aggregates in cases of corticobasal degeneration.32 Fortunately, there is a clear preponderance of TDP-43 proteinopathy subtype according to clinical diagnosis such that 90.8% of the clinical Alzheimer’s disease cases with TDP-43 proteinopathy exhibited LATE, 99.4% of the clinical ALS cases with TDP-43 proteinopathy exhibited ALS with or without FTLD-TDP, and 84.8% of clinical FTD cases with TDP-43 proteinopathy exhibited FTLD-TDP (Supplementary Table 2). As such, repeating our analysis for clinical Alzheimer’s disease, clinical LBD and clinical FTD cohorts after subdividing TDP-43 proteinopathies into ALS versus FTLD-TDP versus LATE yielded very similar outcomes in terms of the association of age at death, age of onset, and with ND-associated and ND–ageing pathological combinations (cf. Table 4 with Supplementary Table 6). Similar analysis could be done for Tau pathology, which in this study combined the several distinct glial and neuronal inclusions of progressive supranuclear palsy, corticobasal degeneration, Pick’s disease, globular glial tauopathy and other unclassifiable tauopathies (Supplementary Table 2). Finally, we defined CVD pathology by the presence of moderate to severe arteriolosclerosis, CAA and infarcts. While reproducible and clinically relevant,34 modelling these multiple lesions as one disease entity underestimates the heterogeneity of CVD, which has been described by subtypes that differ by their degree of involvement of extra and intracranial vessels, the anatomical location and extent of the lesions and by several pathologies not considered here, such as white matter myelin loss, atherosclerosis, haemorrhages and perivascular space dilation and myelin loss.53
There are several limitations to our study. First, our clinical groups are primarily composed of individuals referred from clinicians practicing at several clinical cores at the University of Pennsylvania. As such, each clinical group may not represent the general population of individuals with an ND and therefore the neuropathological profiles we report may not be generalizable either.10 Second, the ND + ageing category was defined in comparison to the unimpaired group. Again, the referral patterns associated with a given brain bank may influence the composition of their clinically unimpaired group, thereby affecting whether a particular pathological combination is considered ND + ageing versus ND + associated. Third, for the ND + associated category of pathological combinations, while we imply that these combinations are due to either downstream or synergistic processes associated with the primary neuropathology, our results are only observed correlations. Finally, we reported if a pathology was present only if more moderate to severe amount of pathology were present. In late-onset Alzheimer’s disease, for instance, TDP-43 inclusions were present in 54% and Lewy pathology was present in 49% of the cases (Supplementary Table 1), but amygdala-only LATE and Lewy pathology cases were not considered a clinically meaningful comorbidity, resulting in a TDP and LB prevalence of 45% and 29%, respectively (Supplementary Table 3). In fact, it is unclear how much of each pathology contributes to clinical outcomes. Future studies may add additional pathologies and/or directly assess the clinically relevant threshold of each pathology.
In summary, the accumulation of multiple pathologies in ND has significant implications for our understanding of disease processes.54 If concomitant pathologies are a consequence of the primary neuropathology, why do some individuals only have the primary pathology itself? This study highlights that increasing age, APOE allele status and disease duration are significant factors that influence the appearance of additional pathologies, but the heterogeneous distribution of pathological combinations confounds simple explanations. Nonetheless, taking a precision medicine approach to identify the contributory factors that drive these pathologies in patients should be an important component of understanding and treating ND in the future.
Supplementary Material
Acknowledgements
We are grateful to our participants and their families for their commitment to neurodegenerative disease research. We are also grateful for the leadership of the late Dr John Trojanowski in conceiving this study and for his dedication to, and furthering of, our understanding of neurodegenerative disease.
Contributor Information
John L Robinson, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
Sharon X Xie, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA; Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
Daniel R Baer, Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
EunRan Suh, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
Vivianna M Van Deerlin, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
Nicholas J Loh, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
David J Irwin, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA; Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
Corey T McMillan, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA; Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
David A Wolk, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA; Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
Alice Chen-Plotkin, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA; Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
Daniel Weintraub, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA; Department of Psychiatry, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
Theresa Schuck, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
Virginia M Y Lee, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
John Q Trojanowski, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
Edward B Lee, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, Institute on Aging, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA; Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA.
Funding
Funding was provided by US National Institute on Aging (National Institutes of Health U19AG062418 (V.M.Y.L.), P01AG066597 (E.B.L.) and P30AG072979 (E.B.L.).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: A retrospective analysis. Lancet Neurol. 2017;16:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141:2181–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robinson JL, Richardson H, Xie SX, et al. The development and convergence of co-pathologies in Alzheimer’s disease. Brain. 2021;144:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karanth S, Nelson PT, Katsumata Y, et al. Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol. 2020;77:1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wennberg AM, Whitwell JL, Tosakulwong N, et al. The influence of tau, amyloid, alpha-synuclein, TDP-43, and vascular pathology in clinically normal elderly individuals. Neurobiol Aging. 2019;77:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Düzel E, Ziegler G, Berron D, et al. Amyloid pathology but not APOE ε4 status is permissive for tau-related hippocampal dysfunction. Brain. 2022;145:1473–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. [DOI] [PubMed] [Google Scholar]
- 8. Kovacs GG, Milenkovic I, Wöhrer A, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: A community-based autopsy series. Acta Neuropathol (Berl). 2013;126:365–384. [DOI] [PubMed] [Google Scholar]
- 9. Graff-Radford J, Aakre JA, Knopman DS, et al. Prevalence and heterogeneity of cerebrovascular disease imaging lesions. Mayo Clin Proc. 2020;95:1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain. 2019;142:1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wakisaka Y, Furuta A, Tanizaki Y, Kiyohara Y, Iida M, Iwaki T. Age-associated prevalence and risk factors of Lewy body pathology in a general population: The Hisayama study. Acta Neuropathol (Berl). 2003;106:374–382. [DOI] [PubMed] [Google Scholar]
- 12. Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain. 2012;135(Pt 10):3005–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spina S, Farlow MR, Unverzagt FW, et al. The tauopathy associated with mutation +3 in intron 10 of tau: Characterization of the MSTD family. Brain. 2008;131::72–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chung EJ, Babulal GM, Monsell SE, Cairns NJ, Roe CM, Morris JC. Clinical features of Alzheimer disease with and without Lewy bodies. JAMA Neurol. 2015;72:789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA. TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease. Acta Neuropathol Commun. 2018;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyle PA, Yang J, Yu L, et al. Varied effects of age-related neuropathologies on the trajectory of late life cognitive decline. Brain. 2017;140:804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montine TJ, Phelps CH, Beach TG, et al. National institute on aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol (Berl). 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Collaborating Centre for Chronic Conditions (UK) . Parkinson’s disease: National clinical guideline for diagnosis and management in primary and secondary care. Royal College of Physicians, UK; 2006. Accessed 11 November 2022. http://www.ncbi.nlm.nih.gov/books/NBK48513/ [PubMed] [Google Scholar]
- 20. Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on motor neuron diseases/amyotrophic lateral sclerosis of the World Federation of Neurology research group on neuromuscular diseases and the El Escorial “Clinical Limits of Amyotrophic Lateral Sclerosis” workshop contributors. J Neurol Sci. 1994;124(Suppl):96–107. [DOI] [PubMed] [Google Scholar]
- 21. Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Mot Neuron Disord. 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 22. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 25. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Toledo JB, Van Deerlin VM, Lee EB, et al. A platform for discovery: The University of Pennsylvania integrated neurodegenerative disease biobank. Alzheimers Dement J Alzheimers Assoc. 2014;10:477–484.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 28. Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thal DR, Rüb U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. [DOI] [PubMed] [Google Scholar]
- 30. Mirra SS, Heyman A, McKeel Det al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 31. Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: Correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol. 2003;62:1287–1301. [DOI] [PubMed] [Google Scholar]
- 32. Uryu K, Nakashima-Yasuda H, Forman MSet al. Concomitant TAR-DNA-Binding Protein 43 Pathology Is Present in Alzheimer Disease and Corticobasal Degeneration but Not in Other Tauopathies. J Neuropathol Exp Neurol. 2008;67:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McKeith IG, Boeve BF, Dickson DWet al. Diagnosis and management of dementia with Lewy bodies. Neurology. 2017;89:88-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skrobot OA, Attems J, Esiri M, et al. Vascular cognitive impairment neuropathology guidelines (VCING): The contribution of cerebrovascular pathology to cognitive impairment. Brain. 2016;139:2957–2969. [DOI] [PubMed] [Google Scholar]
- 35. Robinson JL, Richardson H, Xie SXet al. Cerebrovascular disease lesions are additive and tied to vascular risk factors and cognitive impairment. FreeNeuropathol. 2022;3:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suh E, Lee EB, Neal D, et al. Semi-automated quantification of C9orf72 expansion size reveals inverse correlation between hexanucleotide repeat number and disease duration in frontotemporal degeneration. Acta Neuropathol (Berl). 2015;130:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cairns NJ, Perrin RJ, Franklin EE, et al. Neuropathologic assessment of participants in two multi-center longitudinal observational studies: The Alzheimer Disease Neuroimaging Initiative (ADNI) and the Dominantly Inherited Alzheimer Network (DIAN). Neuropathol Off J Jpn Soc Neuropathol. 2015;35:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duda JE, Giasson BI, Mabon ME, et al. Concurrence of α-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol (Berl). 2002;104:7–11. [DOI] [PubMed] [Google Scholar]
- 39. Ehrenberg AJ, Khatun A, Coomans E, et al. Relevance of biomarkers across different neurodegenerative diseases. Alzheimers Res Ther. 2020;12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boyle PA, Wang T, Yu L, et al. To what degree is late life cognitive decline driven by age-related neuropathologies? Brain. 2021;144:2166–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol (Berl). 2017;134:171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spina S, La Joie R, Petersen C, et al. Comorbid neuropathological diagnoses in early versus late-onset Alzheimer’s disease. Brain. 2021;144:2186–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lippa CF, Fujiwara H, Mann DM, et al. Lewy Bodies contain altered alpha-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol. 1998;153:1365–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the Consortium on DLB international workshop. Neurology. 1996;47:1113–1124. [DOI] [PubMed] [Google Scholar]
- 45. van Steenoven I, Aarsland D, Weintraub D, et al. Cerebrospinal fluid Alzheimer’s disease biomarkers across the spectrum of Lewy body diseases: Results from a large multicenter cohort. J Alzheimers Dis JAD. 2016;54:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Savva GM, Wharton SB, Ince PG, et al. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. [DOI] [PubMed] [Google Scholar]
- 47. Robinson JL, Corrada MM, Kovacs GG, et al. Non-Alzheimer’s contributions to dementia and cognitive resilience in the 90+ study. Acta Neuropathol (Berl). 2018;136:377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Karanth SD, Schmitt FA, Nelson PT, et al. Four common late-life cognitive trajectories patterns associate with replicable underlying neuropathologies. J Alzheimers Dis. 2021;82:647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilson RS, Yu L, Trojanowski JQ, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol. 2013;70:1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Josephs KA, Murray ME, Tosakulwong Net al. Pathological, imaging and genetic characteristics support the existence of distinct TDP-43 types in non-FTLD brains. Acta Neuropathol. 2019;137:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brettschneider J, Del Tredici K, Toledo JBet al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74:20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee EB, Porta S, Michael Baer Get al. Expansion of the classification of FTLD-TDP: Distinct pathology associated with rapidly progressive frontotemporal degeneration. Acta Neuropathol. 2017;134:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kalaria RN. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol (Berl). 2016;131:659–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bennett DA. Mixed pathologies and neural reserve: Implications of complexity for Alzheimer disease drug discovery. PLoS Med. 2017;14:e1002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of the research participants.