Abstract
Background
Detailed information is needed on the dynamic pattern of antimicrobial resistance (AMR) in Neisseria gonorrhoeae in Latin America and the Caribbean (LAC).
Objectives
To conduct a systematic review of AMR in N. gonorrhoeae in LAC.
Methods
Electronic searches without language restrictions were conducted in PubMed, Embase, Cochrane Library, EconLIT, Cumulative Index of Nursing and Allied Health Literature, Centre for Reviews and Dissemination, and Latin American and Caribbean Literature in Health Sciences. Studies were eligible if published between 1 January 2011 and 13 February 2021, conducted in any LAC country (regardless of age, sex and population) and measured frequency and/or patterns of AMR to any antimicrobial in N. gonorrhoeae. The WHO Global Gonococcal Antimicrobial Surveillance Programme (WHO-GASP) for LAC countries and Latin American AMR Surveillance
Network databases were searched. AMR study quality was evaluated according to WHO recommendations.
Results
AMR data for 38, 417 isolates collected in 1990–2018 were included from 31 publications, reporting data from Argentina, Brazil, Colombia, Peru, Uruguay, Venezuela and WHO-GASP. Resistance to extended-spectrum cephalosporins was infrequent (0.09%–8.5%). Resistance to azithromycin was up to 32% in the published studies and up to 61% in WHO-GASP. Resistance to penicillin, tetracycline and ciprofloxacin was high (17.6%–98%, 20.7%–90% and 5.9%–89%, respectively). Resistance to gentamicin was not reported, and resistance to spectinomycin was reported in one study.
Conclusions
This review provides data on resistance to azithromycin, potentially important given its use as first-line empirical treatment, and indicates the need for improved surveillance of gonococcal AMR in LAC.
Trial registration: Registered in PROSPERO, CRD42021253342.
Introduction
Gonorrhoea is a common sexually transmitted infection (STI) caused by the bacterium Neisseria gonorrhoeae, with an estimated 86.9 million new gonorrhoea cases worldwide in 2016 in people aged 15–49 years.1 Along with Africa and Western Pacific, prevalence and incidence were highest in the WHO Region of the Americas (in 2016, prevalence in women 0.9%, 95% confidence interval [CI] 0.6–1.5; prevalence in men 0.8%, 95% CI 0.4–1.3).1 It may present as urethritis or cervicitis, and may also affect sites outside the genital tract such as the pharynx, rectum or eyes.2 Gonorrhoea may result in serious complications such as pelvic inflammatory disease and infertility,3 increases the risk of transmission of HIV4 and is a major public health challenge globally.5
Confirmed diagnosis requires laboratory techniques such as bacterial culture, nucleic acid amplification tests or Gram stain. Microbiological diagnosis of gonorrhoea can be difficult, especially in resource-limited countries, as many regions do not have a laboratory-based diagnostic capability,5 and in these settings diagnosis is often made clinically6 and treatment relies on syndromic management to guide empirical antimicrobial treatment. Treatment of gonorrhoea is complicated by rapidly changing patterns of antimicrobial resistance (AMR), and World Health Organization (WHO) treatment guidelines recommend that local resistance data should determine the choice of antibiotic therapy.6 Where local resistance data are not available, dual therapy is generally recommended over single-agent therapy, using a single dose of either injectable ceftriaxone plus oral azithromycin or oral cefixime plus oral azithromycin.6 Dual therapies were originally introduced for treatment of coinfection with Chlamydia trachomatis,7 reported in 46% of gonorrhoea cases,8 and also based on clinical experience with other bacteria that have developed AMR rapidly.7 Using two antimicrobials with different mechanisms of action may improve treatment efficacy and potentially slow the emergence and spread of resistance to cephalosporins. However, some countries such as the United States of America (USA)9 and the United Kingdom (UK)10 recommend ceftriaxone monotherapy in guidelines published in 2020 and 2018, respectively, as resistance to azithromycin has increased.
N. gonorrhoeae has rapidly developed resistance to successive antimicrobial treatments, first to the sulphonamides introduced in the 1930s and subsequently to penicillins, tetracyclines and fluoroquinolones.7 Recently, N. gonorrhoeae isolates with resistance to extended-spectrum cephalosporins such as cefixime have emerged in the USA and globally, leading to concerns over the possibility of untreatable gonorrhoea in the future.7,11 The first extensively drug-resistant strain of N. gonorrhoeae, showing high-level resistance to ceftriaxone and resistance to previously used antimicrobials, was isolated in Japan,12 followed by the identification of new extensively drug-resistant strains in France13 and Spain.14 Increased spread of some ceftriaxone-resistant N. gonorrhoeae strains or closely genetically related strains has been reported in Australia (2013–2017),15,16 Japan (2014–2015),17,18 Canada,19 Denmark (2017)20 and France (2018),21 predominantly associated with travel to Asia. In China, ceftriaxone resistance was reported in 30% of gonococcal isolates in a study conducted in Hangzhou in 2019.22 The first verified treatment failure for ceftriaxone and azithromycin dual therapy to cure pharyngeal gonorrhoea was reported in the UK in 2016,23 and in 2018 the world's first case of gonorrhoea with combined resistance to ceftriaxone and high-level resistance to azithromycin was reported in England and Australia.24,25
AMR in N. gonorrhoeae is monitored by the WHO Global Gonococcal Antimicrobial Surveillance Programme (GASP), a collaborative global network of reference laboratories,11 and in Latin America and the Caribbean (LAC) by the Latin American and Caribbean Network for Antimicrobial Resistance Surveillance (known by its Spanish acronym ReLAVRA). ReLAVRA was formally established in 1996 by the Pan-American Health Organization (PAHO)/WHO regional office and partnering member states. It is a network responsible for the ongoing collection of reliable, comparable and reproducible AMR data to inform AMR prevention and control policies and interventions in the LAC region.26 It currently comprises 19 countries (Argentina, Bolivia, Brazil, Chile, Colombia, Costa Rica, Cuba, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Dominican Republic, Uruguay and Venezuela), each represented by a national reference laboratory that receives data from sentinel sites. Data from ReLAVRA reported high levels of resistance to tetracycline, penicillin and ciprofloxacin in 2005–2015, and ceftriaxone-resistant N. gonorrhoeae has been reported in four countries in the Americas region (Argentina, Brazil, Canada and the USA) as of October 2017.27 The percentage of isolates with decreased susceptibility to ceftriaxone (defined as a minimum inhibitory concentration [MIC] value of 0.06 to 0.125 mg/L) increased from 2.3% in 2011 to 4.3% in 2013 (all also showed decreased susceptibility to cefixime), and data from Gonococcal Antimicrobial Susceptibility Surveillance Programme–Argentina (GASSP-AR) showed a continuous increase of isolates with decreased susceptibility to extended-spectrum cephalosporins to 7.9% in 2015.28
In Argentina, 5.5% of 237 N. gonorrhoeae samples obtained in 2013 and 2015 showed decreased susceptibility to cefixime and ceftriaxone.29 However, AMR surveillance data for gonorrhoea are absent or very limited in parts of LAC,11 and a low percentage of countries in the Americas region systematically monitor AMR to support treatment decisions.
There is a need for detailed information on the dynamic pattern of AMR in N. gonorrhoeae in LAC to support healthcare decision-makers in planning treatment strategies in these countries. The objective of this systematic review was to describe reported data on AMR in N. gonorrhoeae in LAC countries.
Methods
The analysis presented here was part of a broader systematic review that also included data on the epidemiological and economic burden of gonorrhoeal disease in LAC. The epidemiological and economic findings will be published elsewhere. The findings on AMR are presented in this article. The protocol is registered in PROSPERO CRD UK (registration number: CRD42021253342).
This systematic literature review followed the methods of the Cochrane Systematic Reviews Manual30 and Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses (PRISMA).31,32 For those reviews of observational trials, this review followed Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.33
Inclusion and exclusion criteria
Studies were eligible for inclusion in the review if they were conducted in people regardless of age or sex in any LAC country, published between 1 January 2011 and 13 February 2021, included at least 100 participants (with or without gonorrhoea) or at least 20 cases for a case series (no limit by number of cases/isolates for studies that evaluated AMR), and were of one of the following types: case series; case-control studies; cohort studies; control groups of randomized controlled trials (RCTs) or quasi-RCTs; controlled and uncontrolled before–after studies; cross-sectional studies; epidemiological surveillance reports; interrupted time series (ITS) and controlled ITS (STIC).
Studies had to report data on one of the following outcomes: frequency of antimicrobial resistance to N. gonorrhoeae; and/or patterns of antibiotic resistance. There was no language restriction. Systematic reviews and meta-analyses were considered only as a source of primary studies. When data or subsets of data were reported in more than one publication, we selected the one with the largest sample size.
Search strategy
The following electronic databases were searched for eligible articles: Centre for Reviews and Dissemination (CRD) York; Cochrane Library (CENTRAL); Cumulative Index of Nursing and Allied Health Literature (CINAHL); EconLIT; Embase; Latin American and Caribbean Literature in Health Sciences (LILACS); and PubMed. Detailed search terms for each database are presented in Table S1 (available as Supplementary data at JAC Online). The reference lists of any papers included were hand-searched for additional information. We also searched databases of doctoral theses, websites of major regional medical societies and associations, and proceedings of regional and international congresses, including the Asociación Colombiana de Infectología, Asociación Mexicana de Infectología y Microbiología Clínica, Asociación Panamericana de Infectología, International Society for Sexually Transmitted Diseases Research, Sociedad Argentina de Infectología, Sociedad Brasilera de Infectología, Sociedad Chilena de Infectología, STI & HIV World Congress, Brazilian Society of Sexually Transmissible Diseases, and the International Union against Sexually Transmitted Infections. In addition, grey literature sources were searched, such as websites of the local departments of health in included countries, PAHO, the Virtual Health Library34 and hospital reports. The WHO-GASP and ReLAVRA databases were also searched for information up to 2019.
Article selection and data extraction
Publications were screened by two of the investigators using title and abstract according to the eligibility criteria. Discrepancies were solved by agreement of the entire team. Potentially eligible articles were retrieved in full text for further analysis. All screening phases of the study used COVIDENCE,35 a web-based platform designed to process systematic reviews.
From eligible articles, the research team extracted data on: publication and study characteristics (type of publication, year published, authors, geographical location, study design including domains for risk of bias assessment); study population characteristics (age, sex, sample size, latent immunocompromising conditions, risk evaluation for N. gonorrhoeae, inclusion and exclusion criteria); and outcomes (frequency of AMR). The original authors were contacted if necessary to obtain any missing information or clarification.
Risk of bias assessment
Included studies were assessed for risk of bias by two investigators, with discrepancies resolved by consensus with the whole team. For observational studies and the control arm of trials we used a checklist developed by the USA’s National Heart, Lung, and Blood Institute36 that classifies studies as high risk of bias (Poor), moderate risk of bias (Fair) and low risk of bias (Good). For the evaluation of cohort studies and cross-sectional studies the tool comprises 14 items, and for case series there are 9 items. For RCTs and quasi-RCTs the Cochrane tool37 was used, containing the following domains: sequence generation; assignment concealment; blinding of participants and staff; blinding of outcome evaluators; incomplete results data; selective reporting on results; and other potential threats to validity. For before–after studies, ITS and STIC we used the relevant items from the Cochrane Effective Practice and Organisation of Care Group criteria.38 For before–after studies these were: baseline measurement; characteristics of studies that used a second site as a control (only for controlled studies); blinded assessment of primary outcomes; reliable measurement of primary outcomes; follow-up of professionals; and patient follow-up. For ITS these were: intervention independent of other changes; prior specification of the form of the intervention effect; likelihood that the intervention would affect data collection; blinding to the allocation of interventions by outcome evaluators; incomplete results data; selective notification of results; and other sources of bias. For STIC, the domains were the same as ITS plus three additional domains: imbalance of outcome measures at baseline; comparability of the intervention and control group at the beginning of the study; and protection against contamination. Each criterion was scored as low risk, high risk or uncertain risk. For those rated as uncertain we attempted to obtain more information from the study authors.
Quality assessment of AMR studies
The quality of AMR studies was evaluated according to WHO recommendations.39,40 Studies were scored on whether they specified the location at which the isolates were collected and the collection period, described the method of identifying the isolates and the population from which the isolates were obtained, included at least 100 tested isolates, utilized or implied utilization of control strains recommended by WHO to identify MIC, and described the method for determining the antimicrobial susceptibility of isolates or the MIC values for susceptibility, reduced susceptibility and resistance isolates.
Statistical analysis and reporting
For AMR outcomes from published studies and the GASP and ReLAVRA databases a descriptive overview is presented. The risk of bias results are tabulated.
Results
Literature search and study selection
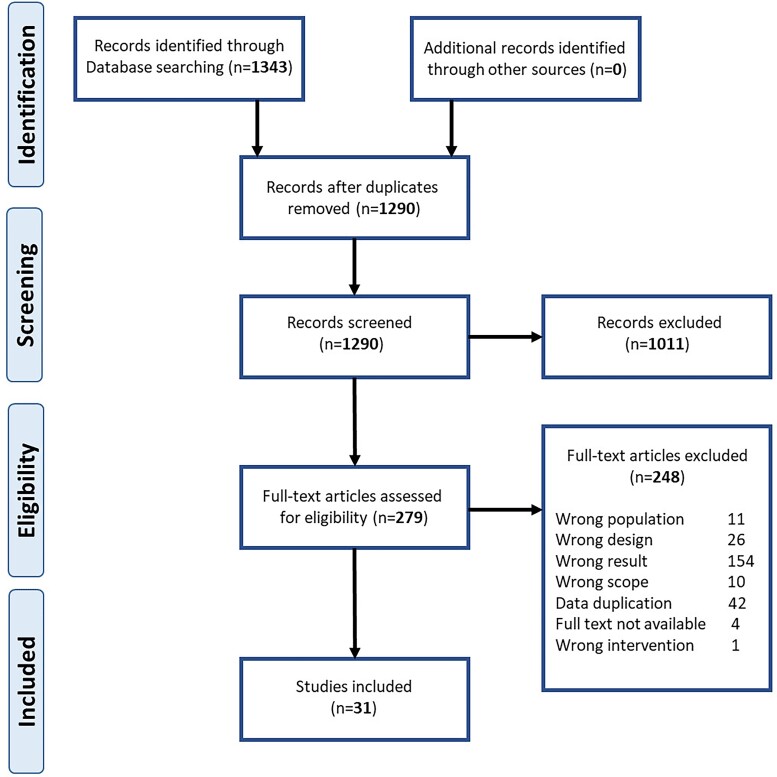
After removal of duplicates, 1290 references were identified from the search and screened by title and abstract, after which 279 were retrieved for full-text review. Of these, 31 references, published between 2011 and 2020, provided AMR data and are included in this article (Figure 1). 29,41–70 Twenty-three of the publications were in English and the remaining eight were in Spanish. As the authors are fluent in both English and Spanish there was no need for translation.
Figure 1.
PRISMA flow chart. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Characteristics of included studies
Table 1 summarizes the characteristics of the included studies. There were 16 full articles and 15 conference abstracts; 30 were cross-sectional studies and 1 was an epidemiological surveillance study. The cross-sectional studies were observational studies conducted by an investigator, whereas the epidemiological surveillance study related to a specific surveillance protocol established to investigate disease burden at a particular place and time. The reported duration of the studies ranged from 12 months46,52,54 to 263 months (21.9 years).49 Eight studies (25.8%) reported the origin of the samples, all from healthcare service outpatients.
Table 1.
Characteristics of included studies
| First author and year of publication | Type of publication | Country | Type of source | Type of sampling | Study start date: dd/mm/yyyy | Study completion date: dd/mm/yyyy | Study length (months) | Study design |
|---|---|---|---|---|---|---|---|---|
| Acevedo 201341 | Conference abstract | Uruguay | NR | Convenience (health system) | 01/01/2010 | 31/12/2011 | 24 | Cross-sectional |
| Barros dos Santos 201942 | Full text | Brazil | NR | Convenience (health system) | 01/03/2014 | 31/10/2017 | 44 | Cross-sectional |
| Bautista 201843 | Conference abstract | Colombia | NR | Convenience (health system) | 01/01/2012 | 31/12/2017 | 72 | Cross-sectional |
| Bazzo 201844 | Full text | Brazil | NR | Convenience (health system) | 01/10/2015 | 31/12/2016 | 24 | Cross-sectional |
| Casco 201145 | Full text | Argentina | Ambulatory | Convenience (health system) | 01/01/2005 | 30/12/2009 | 60 | Cross-sectional |
| Costa 201346 | Full text | Brazil | Ambulatory | Convenience (health system) | 01/03/2011 | 28/02/2012 | 12 | Cross-sectional |
| Costa-Lourenço 201847 | Full text | Brazil | NR | Convenience (health system) | 01/01/2006 | 31/12/2015 | 120 | Cross-sectional |
| de los Méndez 201248 | Conference abstract | Argentina | NR | Convenience (health system) | 01/01/2000 | 31/12/2010 | 132 | Epidemiological surveillance study |
| Dillon 201349 | Full text | LAC | NR | Convenience (health system) | 01/01/1990 | 31/12/2011 | 263 | Cross-sectional |
| dos Santos 201750 | Conference abstract | Brazil | NR | Convenience (health system) | 01/01/2003 | 31/12/2016 | 168 | Cross-sectional |
| Flores Fernández 201251 | Full text | Venezuela | NR | Convenience (health system) | 01/02/2008 | 28/02/2009 | 13 | Cross-sectional |
| Galarza 201452 | Conference abstract | Argentina | NR | Convenience (health system) | 01/01/2012 | 31/12/2012 | 12 | Cross-sectional |
| Gianecini 201953 | Full text | Argentina | Ambulatory | Convenience (health system) | 01/01/2011 | 31/12/2016 | 72 | Cross-sectional |
| Gianecini 201729 | Conference abstract | Argentina | NR | Convenience (health system) | 01/01/2013 | 31/12/2015 | 36 | Cross-sectional |
| Gianecini 201354 | Conference abstract | Argentina | NR | Convenience (health system) | 01/01/2011 | 31/12/2011 | 12 | Cross-sectional |
| Golfetto 201955 | Conference abstract | Brazil | NR | Convenience (health system) | 01/01/2008 | 31/12/2016 | 108 | Cross-sectional |
| Jorge Berrocal 201856 | Full text | Peru | NR | Convenience (health system) | 01/10/2016 | 30/11/2017 | 14 | Cross-sectional |
| Martins 201957 | Full text | Brazil | Ambulatory | Convenience (health system) | 01/01/2003 | 31/12/2015 | 156 | Cross-sectional |
| Medeiros 201358 | Full text | Brazil | Ambulatory | Convenience (health system) | 01/09/2008 | 31/05/2012 | 45 | Cross-sectional |
| Montano 201659 | Conference abstract | Peru | NR | Convenience (health system) | 01/02/2013 | 31/03/2016 | 38 | Cross-sectional |
| Rahman 201760 | Conference abstract | Peru | NR | Convenience (health system) | 01/01/2012 | 31/12/2016 | 60 | Cross-sectional |
| Rivillas-García 202061 | Full text | Colombia | NR | Convenience (health system) | 01/01/2009 | 31/12/2018 | 120 | Cross-sectional |
| Sánchez Palencia 201762 | Full text | Peru | NR | Convenience (health system) | 01/01/2012 | 31/12/2013 | 24 | Cross-sectional |
| Schijman 201863 | Conference abstract | Argentina | NR | Convenience (health system) | 01/01/2012 | 31/12/2017 | 72 | Cross-sectional |
| Thakur 201764 | Full text | LAC | NR | Convenience (health system) | 01/01/2010 | 31/12/2011 | 24 | Cross-sectional |
| Uehara 201165 | Full text | Brazil | NR | Convenience (health system) | 01/01/2006 | 31/12/2010 | 60 | Cross-sectional |
| Vacchino 201366 | Conference abstract | Argentina | NR | Convenience (health system) | 01/01/1993 | NR | NR | Cross-sectional |
| Vacchino 201767 | Conference abstract | Argentina | Ambulatory | Convenience (health system) | 01/01/2014 | 31/12/2015 | 24 | Cross-sectional |
| Le Van 201968 | Conference abstract | Peru | NR | Convenience (health system) | 01/01/2012 | 31/12/2015 | 48 | Cross-sectional |
| Vargas 201969 | Conference abstract | Peru | Ambulatory | Convenience (health system) | 01/01/2013 | 31/12/2016 | 48 | Cross-sectional |
| Zotta 201470 | Full text | Argentina | Ambulatory | Convenience (health system) | 01/01/2005 | 31/12/2010 | 72 | Cross-sectional |
LAC, Latin America and the Caribbean; NR, not reported.
The studies provided data on AMR in the following countries: Argentina (n = 10)29,45,48,52–54,63,66,67,70 during 1993 and between 2000 and 2017; Brazil (n = 9)42,44,46,47,50,55,57,58,65 in 2003 to 2016; Colombia (n = 2)43,61 in 2009 to 2018; Peru (n = 6)56,59,60,62,68,69 in 2012 to 2017; Uruguay (n = 1)41 in 2010 and 2011; and Venezuela (n = 1)51 during 2008. There were also two studies reporting on AMR in various LAC countries from the WHO-GASP-LAC, one of which49 reported resistance between 1990 and 2011 in 23 LAC countries, and the other reported data from 2010 and 2011 in seven LAC countries.64 Five of the studies in Argentina belonged to GASSP-AR,29,52–54,67 two to the Gonococcal Antimicrobial Susceptibility Surveillance Program (ProsVAG),48,70 and one in Brazil to the Brazilian GASP Network.44,71
From 1990 to 2018, 38,417 positive samples of N. gonorrhoeae (out of a total of 40, 653 samples reported for AMR analysis) were processed, with the number of samples evaluated ranging from 2051 to 21,592,49 including urethral, endocervical, urine, rectal, pharyngeal and ocular secretion samples. One study did not report the total number of samples processed.60 Twenty-seven studies (87.1%) analysed AMR for more than one antibiotic, of which one reported only resistance to ciprofloxacin.60 The remaining four studies analysed only ciprofloxacin.62,65,68,69 The antibiotics included in studies evaluating several antibiotics were ciprofloxacin (n = 26), penicillin (n = 25), tetracycline (n = 24), azithromycin (n = 21), ceftriaxone (n = 19), cefixime (n = 8), spectinomycin (n = 7), ceftriaxone/cefixime combined (n = 5), gentamicin (n = 3), erythromycin (n = 1), chloramphenicol (n = 1), cefoxitin (n = 1) and ofloxacin (n = 1).
The methods used to evaluate AMR were agar dilution in 46.7% (n = 14), combined methods in 23.3% (n = 7), agar diffusion (Etest) in 13.3% (n = 4), molecular techniques (PCR) for the detection of genes associated with resistance in 6.7% (n = 2), disk diffusion (DD) in 10% (n = 3) and in one study the method used was not reported.61
The MIC was evaluated in 23 studies (74.1%), and 15 studies (48.4%) reported at least one MIC value. Ten studies reported the MICs of all the antibiotics evaluated, four the MIC of only one, and one study the MICs of two of the antibiotics evaluated. For studies that reported at least one MIC value, 80% (n = 12) used the agar dilution method for evaluation and three studies used combined methods. For the criteria used to evaluate antibiotic susceptibility, 48.4% (n = 15) used the MIC breakpoints established by the Clinical and Laboratory Standards Institute (CLSI), 9.7% (n = 3) used the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria and 16.1% (n = 5) used more than one set of criteria, combining the CLSI MIC breakpoints for all antibiotics except azithromycin, due to the lack of consensus and different established breakpoints (of the five studies, two assessed azithromycin susceptibility with EUCAST criteria42,44, one study used the Centers for Disease Control (CDC) criteria,46 one study used data from literature58 and one study did not report the criteria used45). Eight studies (25.8%) did not report the criteria used, although two used techniques to evaluate genes associated with resistance, specifically mutations in the gyrA gene associated with ciprofloxacin resistance.62,69
Risk of bias assessment
Table S2 summarizes the risk of bias assessment; 22 studies (71%) were assessed as being at moderate (fair) risk; 5 (16%) were assessed as ‘good’ and 4 (13%) as ‘poor’. It should be noted that many domains did not apply to the objectives of the included studies, such as different levels of exposure or blinding of evaluators.
Quality assessment of AMR studies
The results of the quality assessment of AMR studies are summarized in Table S3. The quality was scored as high in 52% (n = 16) of the studies and moderate in the remaining 48% (n = 15). The most frequently missed items were not describing the population from which samples were obtained (64.5%, n = 20) and not specifying whether a reference/control strain was included when assessing antimicrobial susceptibility (48%, n = 15). Other than the studies that reported data from reference laboratories belonging to surveillance networks, participation in external quality assessment was not reported in the studies.
Results of published AMR studies
Azithromycin
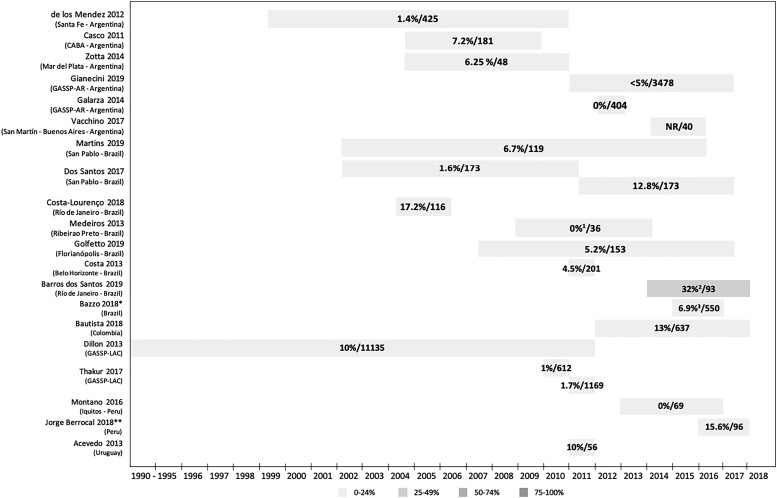
MIC values and resistance percentages were reported as described in each study. Figure 2 shows data on the percentage of strains resistant to azithromycin, and Table 2 shows the data on reported MIC values of azithromycin. Twelve studies (60%) reported resistance to azithromycin in at least 5% of strains evaluated (Figure 2), with the highest percentage in Brazil (32%, 2014–2017).42 The findings of this paper differ from data reported by WHO-GASP and ReLAVRA (see below), where the highest resistance rate reported for Brazil was 6.9% in 2016, and a rate of 61% was reported for Peru. This may reflect the small number of strains evaluated (<100), which could mean the results are not representative. Furthermore, the study conducted in Brazil evaluated strains from Rio de Janeiro, which was not part of the Brazilian GASP Network, raising the possibility that resistance to azithromycin in this city could be higher than in the rest of the country. However, despite discrepancies in the detail, the overall pattern of data is consistent with emerging resistance to azithromycin that is increasing over time.
Figure 2.
Percentage of strains resistant to azithromycin/number of strains evaluated in published studies. *Manaus, Salvador, Brasilia, Belo Horizonte, São Paulo, Florianopolis, Porto Alegre (Brazilian GASP Network). **Lima, Callao, Ancash, Ayacucho, Madre de Dios, Loreto, Ucayali. 119.4% of strains with decreased sensitivity are reported, but MIC data are not provided. 2Percentage of resistant strains according to EUCAST criteria (according to CLSI criteria, 25%). 3Percentage of resistant strains according to EUCAST criteria (according to CLSI criteria, 1.9%). CABA, City of Buenos Aires; CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing; GASSP-AR, Gonococcal Antimicrobial Susceptibility Surveillance Programme—Argentina; GASP-LAC, Gonococcal Antimicrobial Surveillance Programme—Latin America and the Caribbean; MIC, minimum inhibitory concentration; NR, not reported.
Table 2.
Azithromycin MIC values reported in published studiesa
| First author and year of publication | Total isolates R (%)/total isolates evaluated | MIC50 (mg/L) | MIC90 (mg/L) | Range (mg/L) | Method used for MIC determination | Resistance breakpoints used (MIC breakpoint for R) |
|---|---|---|---|---|---|---|
| Casco 2011 (Argentina)45 | 13 (7.2)/181 | 0.125 | 0.5 | 0.001 to 16 | agar dilution | NR (R ≥ 1 mg/L) |
| Zotta 2014 (Argentina)70 | 3 (6.25)/48 | — | — | 0.125 to 1 | agar dilution | CLSI (NR) |
| Galarza 2014 (Argentina)52 | 0 (0)/404 | 0.25 | 0.5 | — | agar dilution | CLSI (NR) |
| Vacchino 2017 (Argentina)67 | NR/40 | 0.25 | 0.5 | — | agar dilution | CLSI (NR) |
| Martins 2019 (Brazil)57 | 8 (6.7)/119 | 0.25 | 0.5 | ≤0.015 to >1 | agar dilution | EUCAST (R > 0.5 mg/L) |
| Costa-Lourenço 2018 (Brazil)47 | 20 (17.2)/116 | 0.25 | 2 | 0.032 to 16 | agar dilution | CLSI (NR) |
| Costa 2013 (Brazil)46 | 9 (4.5)/201 | 0.125 | 0.38 | 0.016 to 12 | DD/Etest | CDC criteria (R ≥ 1 mg/L) |
| Bazzo 2018 (Brazil)44 | 38 (6.9)/550 (EUCAST) 7 (1.3)/550 (CLSI) |
0.06 | 0.5 | 0.03 to 8 | agar dilution | EUCAST/CLSI (R ≥ 1 mg/L) |
All MIC data expressed in mg/L; however, some of the original studies used units of µg/mL. CDC, Centers for Disease Control; CLSI, Clinical and Laboratory Standards Institute; DD, disk diffusion; EUCAST, European Committee on Antimicrobial Susceptibility Testing; MIC, minimum inhibitory concentration; NR, not reported; R, resistant. MIC50: MIC of the antibiotic that inhibits the growth of 50% of the strains. MIC90: MIC of the antibiotic that inhibits the growth of 90% of the strains.
Extended-spectrum cephalosporins
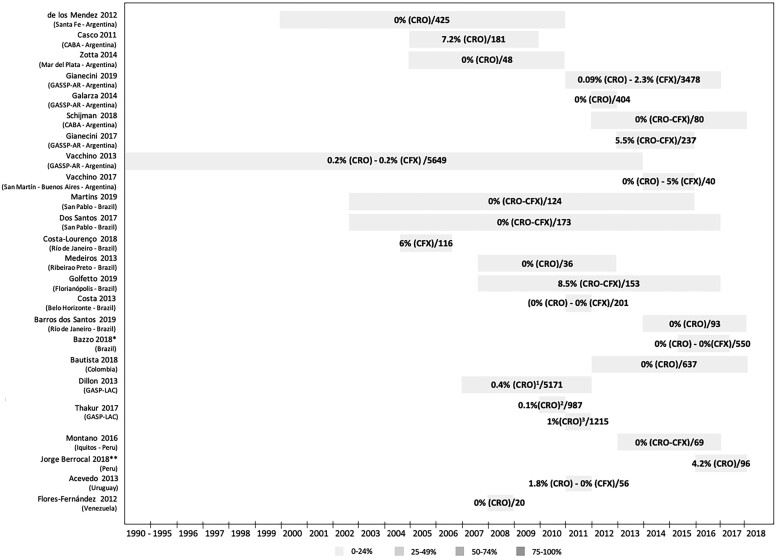
Figure 3 shows data on the percentage of strains resistant to extended-spectrum cephalosporins, and Table 3 shows reported MIC values of extended-spectrum cephalosporins. Consistent with WHO-GASP and ReLAVRA data, resistance to extended-spectrum cephalosporins remains infrequent in the LAC region. The percentage of strains with decreased susceptibility/resistance for ceftriaxone was between 0.09% (Argentina, 2011–2016)53 and 7.2% (Argentina, 2005–2009)45, for cefixime it was between 0.2% (Argentina, 1993–not reported)66 and 6% (Brazil, 2006–2015),47 and for both combined it was 5.5% (Argentina, 2013–2015)29 to 8.5% (Brazil, 2008–2016)55 (Figure 3).
Figure 3.
Percentage of strains with decreased sensitivity or resistant to extended-spectrum cephalosporins/number of strains evaluated in published studies. *Manaus, Salvador, Brasilia, Belo Horizonte, São Paulo, Florianopolis, Porto Alegre (Brazilian GASP Network). **Lima, Callao, Ancash, Ayacucho, Madre de Dios, Loreto, Ucayali. 1Twenty strains reported in Argentina, Brazil, Chile, Cuba and Uruguay. 2One strain reported in Cuba. 3Twelve strains reported in Argentina (eight), Chile (three) and Uruguay (one). CABA, City of Buenos Aires; CFX, cefixime; CRO, ceftriaxone; GASSP-AR, Gonococcal Antimicrobial Susceptibility Surveillance Programme—Argentina; GASP-LAC, Gonococcal Antimicrobial Surveillance Programme—Latin America and the Caribbean; NR, Not reported.
Table 3.
Extended-spectrum cephalosporin MIC values reported in published studiesa
| First author and year of publication | Total isolates DS-R (%)/total isolates evaluated | MIC50 (mg/L) | MIC90 (mg/L) | Range (mg/L) | Method used for MIC determination | Resistance breakpoints used (MIC breakpoint for S) |
|---|---|---|---|---|---|---|
| Ceftriaxone | ||||||
| Casco 2011 (Argentina)45 | 13 (7.2)/181 | 0.004 | 0.032 | 0.001–0.25 | agar dilution | CLSI (NR) |
| Zotta 2014 (Argentina)70 | 0 (0)/48 | — | — | 0.002–0.16 | agar dilution | CLSI (NR) |
| Gianecini 2019 (Argentina)53 | 3 (0.08)/3478 | 0.06 | 0.125 | 0.06–0.5 | agar dilution | EUCAST (NR) |
| Galarza 2014 (Argentina)52 | 0 (0)/404 | 0.008 | 0.032 | — | agar dilution | CLSI (NR) |
| Vacchino 2017 (Argentina)67 | 0 (0)/40 | 0.008 | 0.016 | — | agar dilution | CLSI (NR) |
| Martins 2019 (Brazil)57 | 0 (0)/124 | 0.002 | 0.015 | ≤0.001–0.06 | agar dilution | EUCAST (S ≤ 0.125 mg/L) |
| Costa 2013 (Brazil)46 | 0 (0)/201 | 0.002–0.032 | — | — | DD/Etest | CLSI (S ≤ 0.25 mg/L) |
| Bazzo 2018 (Brazil)44 | 0 (0)/550 | 0.008 | 0.016 | 0.0005–0.125 | agar dilution/Etest | CLSI (S ≤ 0.25 mg/L) |
| Thakur 2017 (LAC)64 | 1 (0.1)/987 (2010) | — | — | — | agar dilution/DD/Etest | CLSI (NR) |
| 12 (0.98)/1215 (2011) | — | — | 0.125–0.25 | |||
| Cefixime | ||||||
| Gianecini 2019 (Argentina)53 | 79 (2.3)/3478 | 0.125 | 0.25 | 0.125–0.5 | agar dilution | EUCAST (NR) |
| Vacchino 2013 (Argentina)66 | 10 (0.17)/5649 | — | — | 0.125–0.5 | agar dilution | CLSI (NR) |
| Vacchino 2017 (Argentina)67 | 2 (5)/40 | 0.016 | 0.03 | — | agar dilution | CLSI (NR) |
| Costa-Lourenço 2018 (Brazil)47 | 7 (6)/116 | 0.016 | 0.125 | 0.001–0.25 | agar dilution | CLSI (NR) |
| Costa 2013 (Brazil)46 | 0 (0)/201 | 0.016–0.125 | — | — | DD/Etest | CLSI (S ≤ 0.25 mg/L) |
| Bazzo 2018 (Brazil)44 | 0 (0)/550 | 0.008 | 0.06 | 0.0005–0.250 | agar dilution/Etest | CLSI (S ≤ 0.25 mg/L) |
All MIC data expressed in mg/L; however, some of the original studies used units of µg/mL. CLSI, Clinical and Laboratory Standards Institute; DD, disk diffusion; DS-R, decreased susceptibility-resistant; EUCAST, European Committee on Antimicrobial Susceptibility Testing; MIC, minimum inhibitory concentration; NR, not reported; S, susceptibility. MIC50: MIC of the antibiotic that inhibits the growth of 50% of the strains. MIC90: MIC of the antibiotic that inhibits the growth of 90% of the strains.
Ciprofloxacin, penicillin and tetracycline
Information on resistance to ciprofloxacin, penicillin and tetracycline is shown in Tables 4–6 and Figures S1–S3. Resistance was high with an indication of an increasing trend over time, although there was no uniform pattern. Resistance to penicillin ranged from 17.6% (Argentina, 2005–2009)45 to 98% (Brazil, 2014–2017),42 to tetracycline from 20.7% (Argentina, 2012)52 to 90% (Venezuela, 2008),51 and to ciprofloxacin from 5.9% (Argentina, 2000–2010)48 to 89% (Peru, 2012–2016).60 The ciprofloxacin findings are consistent with data from WHO-GASP and ReLAVRA (see below).
Table 4.
Ciprofloxacin MIC values reported by published studiesa
| First author and year of publication | Total isolates R (%)/total isolates evaluated | MIC50 (mg/L) | MIC90 (mg/L) | Range (mg/L) | Method used for MIC determination | Resistance breakpoints used (MIC breakpoint for R) |
|---|---|---|---|---|---|---|
| Casco 2011 (Argentina)45 | 47 (25.8)/181 | 0.016 | 16 | 0.001 to 32 | agar dilution | CLSI (NR) |
| Zotta 2014 (Argentina)70 | 14 (29.5)/48 | — | — | 0.004 to 16 | agar dilution | CLSI (NR) |
| Galarza 2014 (Argentina)52 | 198 (49)/404 | 0.016 | 16 | — | agar dilution | CLSI (NR) |
| Vacchino 2017 (Argentina)67 | NR/40 | 1 | 4 | — | agar dilution | CLSI (NR) |
| Martins 2019 (Brazil)57 | 20 (15.3)/124 | 0.002 | >2 | ≤0.00025 to >2 | agar dilution | EUCAST (R > 0.06 mg/L) |
| Costa-Lourenço 2018 (Brazil)47 | 75 (64.7)/116 | 2 | 16 | 0.002 to 32 | agar dilution | CLSI (NR) |
| Costa 2013 (Brazil)46 | 43 (21.4)/201 | 0.002 | 4 | 0.002 to >32 | DD/Etest | CLSI (R ≥ 1 mg/L) |
| Bazzo 2018 (Brazil)44 | 306 (55.6)/550 | 0.016 | 8 | 0.001 to 32 | agar dilution | CLSI (R ≥ 1 mg/L) |
All MIC data expressed in mg/L; however, some of the original studies used units of µg/mL. CLSI, Clinical and Laboratory Standards Institute; DD, disk diffusion; EUCAST, European Committee on Antimicrobial Susceptibility Testing; MIC, minimum inhibitory concentration; NR, not reported; R, resistant. MIC50: MIC of the antibiotic that inhibits the growth of 50% of the strains. MIC90: MIC of the antibiotic that inhibits the growth of 90% of the strains.
Table 5.
Penicillin MIC values reported by published studiesa
| First author and year of publication | Total isolates R (%)/total isolates evaluated | MIC50 (mg/L) | MIC90 (mg/L) | Range (mg/L) | Method used for MIC determination | Resistance breakpoints used (MIC breakpoint for R) | Resistance phenotypes (%)b |
|---|---|---|---|---|---|---|---|
| Casco 2011 (Argentina)45 | 32 (17.6)/181 | 0.5 | 4 | 0.001 to >256 | agar dilution | CLSI (NR) | PPNG (12.6), CMPR (23.7) |
| Zotta 2014 (Argentina)70 | 10 (20.9)/48 | — | — | 0.125 to 128 | agar dilution | CLSI (NR) | PPNG (8.4), CMPR (2.1), CMRNG (10.4) |
| Galarza 2014 (Argentina)52 | 148 (37)/404 | 1 | 8 | — | agar dilution | CLSI (NR) | NR |
| Vacchino 2017 (Argentina)67 | NR/40 | 0.5 | 4 | — | agar dilution | CLSI (NR) | NR |
| Martins 2019 (Brazil)57 | 73 (59)/124 | 4 | >4 | ≤0.015 to >4 | agar dilution | EUCAST (R > 1 mg/L) | NR |
| Costa-Lourenço 2018 (Brazil)47 | 68 (59)/116 | 2 | 16 | 0.064 to 32 | agar dilution | CLSI (NR) | NR |
| Costa 2013 (Brazil)46 | 45 (22.4)/201 | 0.25 | 6 | 0.008 to 256 | DD/Etest | CLSI (NR) | PPNG (19.5), CMPR (4), CMRNG (2) |
| Bazzo 2018 (Brazil)44 | 204 (37)/550 | 0.5 | 8 | 0.016 to 128 | agar dilution | CLSI (R ≥ 2 mg/L) | NR |
All MIC data expressed in mg/L; however, some of the original studies used units of µg/mL. CLSI, Clinical and Laboratory Standards Institute; CMPR, chromosomally mediated penicillin resistance (non-PPNG, tetracycline MIC <2.0 mg/L and penicillin MIC ≥2.0 mg/L); CMRNG, chromosomally resistant N. gonorrhoeae (non-PPNG, non-TRNG [tetracycline-resistant N. gonorrhoeae], penicillin MIC ≥2.0 mg/L and tetracycline MIC 2.0–8.0 mg/L); DD, disk diffusion; EUCAST, European Committee on Antimicrobial Susceptibility Testing; MIC, minimum inhibitory concentration; NR, not reported; PPNG, penicillinase-producing N. gonorrhoeae (β-lactamase positive); R, resistant. MIC50: MIC of the antibiotic that inhibits the growth of 50% of the strains. MIC90: MIC of the antibiotic that inhibits the growth of 90% of the strains.
The percentages refer to the total isolates evaluated.
Table 6.
Tetracycline MIC values reported by published studiesa
| First author and year of publication | Total isolates R (%)/total isolates evaluated | MIC50 (mg/L) | MIC90 (mg/L) | Range (mg/L) | Method used for MIC determination | Resistance breakpoints used (MIC breakpoint for R) | Resistance phenotypes (%)b |
|---|---|---|---|---|---|---|---|
| Casco 2011 (Argentina)45 | 41 (23)/181 | 1 | 4 | 0.032 to 64 | agar dilution | CLSI (NR) | NR |
| Zotta 2014 (Argentina)70 | 13 (27.1)/48 | — | — | 0.125 to 16 | agar dilution | CLSI (NR) | TRNG (4.2), CMTR (12.6), CMRNG (10.4) |
| Galarza 2014 (Argentina)52 | 120 (30)/404 | 1 | 16 | — | agar dilution | CLSI (NR) | NR |
| Vacchino 2017 (Argentina)67 | NR/40 | 1 | 32 | — | agar dilution | CLSI (NR) | NR |
| Costa-Lourenço 2018 (Brazil)47 | 76 (65.5)/116 | 4 | 32 | 0.032 to 32 | agar dilution | CLSI (NR) | NR |
| Costa 2013 (Brazil)46 | 65 (32)/201 | 0.5 | 16 | 0.032 to 32 | DD/Etest | CLSI (≥2 mg/L) | TRNG (16.5), CMTR (15.4), CMRNG (2) |
| Bazzo 2018 (Brazil)44 | 339 (62)/550 | 2 | 32 | 0.125 to >32 | agar dilution | CLSI (R ≥ 2 mg/L) | NR |
All MIC data expressed in mg/L; however, some of the original studies used units of µg/mL. CLSI, Clinical and Laboratory Standards Institute; CMRNG: chromosomally resistant N. gonorrhoeae (non-PPNG, non-TRNG, penicillin MIC ≥2.0 mg/L and tetracycline MIC 2.0–8.0 mg/L); CMTR, chromosomally mediated tetracycline resistance (non-PPNG [penicillinase-producing N. gonorrhoeae], non-TRNG, penicillin MIC <2.0 mg/L and tetracycline MIC ≥2.0–8.0 mg/L); DD, disk diffusion; MIC, minimum inhibitory concentration; NR, not reported; R, resistant; TRNG, tetracycline-resistant N. gonorrhoeae (tetracycline MIC ≥16 mg/L and β-lactamase negative). MIC50: MIC of the antibiotic that inhibits the growth of 50% of the strains. MIC90: MIC of the antibiotic that inhibits the growth of 90% of the strains.
The percentages refer to the total isolates evaluated.
Spectinomycin and gentamicin
Resistance to spectinomycin was reported in 0% of strains in six studies, and in 1% of strains in the remaining study (Peru, 2016–2017)56 (Figure S4, Table 7). Of the three studies evaluating AMR to gentamicin only two reported data, on a total of 35554 and 23729 strains. Reported resistance was 0% in both studies (MIC50 and MIC90: 8 mg/L, range: 4–16 mg/L, criteria used for antimicrobial susceptibility data from literature54; MIC50 and MIC90: 8 mg/L, range: 2–16 mg/L, criteria used for antimicrobial susceptibility not reported29; there is no resistance breakpoint for gentamicin).
Table 7.
Spectinomycin MIC values reported by published studiesa
| First author and year of publication | Total isolates R (%)/total isolates evaluated | MIC50 (mg/L) | MIC90 (mg/L) | Range (mg/L) | Method used for MIC determination | Resistance breakpoints used (MIC breakpoint for R) |
|---|---|---|---|---|---|---|
| Casco 2011 (Argentina)45 | 0 (0)/181 | 16 | 64 | 0.5–64 | agar dilution | CLSI (NR) |
| Zotta 2014 (Argentina)70 | 0 (0)/48 | — | — | 16–32 | agar dilution | CLSI (NR) |
| Costa 2013 (Brazil)46 | 12 (6)/201 | 4–24 | — | — | DD/Etest | CLSI (NR) |
All MIC data expressed in mg/L; however, some of the original studies used units of µg/mL. CLSI, Clinical and Laboratory Standards Institute; DD, disk diffusion; MIC, minimum inhibitory concentration; NR, not reported; R, resistant. MIC50: MIC of the antibiotic that inhibits the growth of 50% of the strains. MIC90: MIC of the antibiotic that inhibits the growth of 90% of the strains.
Other antibiotics
Resistance to the remaining antibiotics evaluated was 32.6% (181 strains evaluated) to erythromycin45 (MIC50: 0.5 mg/L; MIC90: 4 mg/L; range: 0.032–256 mg/L; criteria used for antimicrobial susceptibility not reported; MIC breakpoint for resistance ≥2 mg/L), 11.9% (201 strains evaluated) to chloramphenicol46 (MIC50: 0.38 mg/L; MIC90: 2 mg/L; range: 0.125–12 mg/L; criteria used for antimicrobial susceptibility, Dick Van et al. method; MIC breakpoint for resistance ≥2 mg/L), 36% (36 strains evaluated) to ofloxacin58 (MIC data not reported; criteria used for antimicrobial susceptibility, CLSI; MIC breakpoint for resistance ≥2 mg/L), and 0% (20 strains evaluated) to cefoxitin51 (MIC data not reported; criteria used for antimicrobial susceptibility, CLSI; MIC breakpoint for resistance not reported).
WHO-GASP surveillance data
AMR information was obtained from the WHO-GASP surveillance programme for ciprofloxacin, azithromycin, ceftriaxone and cefixime between 2011 and 2018.71 Fourteen countries provided data on ciprofloxacin resistance, with the number of years varying from 1 (Bolivia, Uruguay) to 14 (Argentina, Colombia) (Table S4). The number of strains evaluated ranged from 1 (Brazil, 2015) to 2091 (Chile, 2018). The countries reporting the highest resistance rates evaluated fewer than 100 strains each year, and not all countries consistently provided data. However, there were high rates of ciprofloxacin resistance, with a trend to increase over time. The highest rates overall were reported in Peru (78.6%–100%) and Ecuador (80%–100%) (Table S4).
Eight countries reported AMR data on azithromycin, for 2011–2013 and 2015–2018, with all countries missing data in at least one year (Table S5). The number of strains evaluated ranged from 5 (Ecuador 2018) to 2091 (Chile 2018). The countries reporting the highest proportion of resistant strains were Colombia (45.4% in 2017), Cuba (46.9% in 2017) and Peru (61% in 2015). Although resistance to azithromycin is not as high as for ciprofloxacin, it reached ≥5% (the level recommended by the WHO to discontinue the use of an antibiotic as first-line empirical gonorrhoea treatment) in several countries and years.
Data on decreased susceptibility/resistance were reported from 14 countries for ceftriaxone (Table S6) and 12 countries for cefixime (Table S7), with missing years in all countries except Argentina. Strains with decreased susceptibility/resistance to ceftriaxone were reported in three countries—Argentina (0.15% in 2014), Peru (2.38% in 2016) and Bolivia (80% in 2014)—and decreased susceptibility/resistance to cefixime was reported in one country, Argentina (0.15% in 2015).
ReLAVRA surveillance data
From ReLAVRA, we obtained data on resistance to ciprofloxacin, azithromycin and ceftriaxone between 2011 and 2019.72 Data on resistance to ciprofloxacin and ceftriaxone were reported from nine countries and data on azithromycin resistance by six. The total number of strains evaluated and the percentage of resistance were the same in both ReLAVRA and WHO-GASP, and therefore we assumed that these countries send information to both surveillance networks. The only difference we found was that in ReLAVRA the antibiotic susceptibility assessment method has been reported since 2017. Argentina, Chile, Cuba and Peru evaluated the MIC; El Salvador, the Dominican Republic and Paraguay used the DD method; and Colombia used both.
For ciprofloxacin in 2016–2018, most of the strains evaluated had MIC values ≥1 mg/L, which categorizes them as resistant. The highest percentage of strains with MIC of 2 mg/L occurred in 2016 (24.73%) and 2018 (21.13%) and the MIC was 4 mg/L in 28.92% of strains in 2017. For azithromycin in 2016–2018, most of the strains evaluated had a MIC within the susceptibility range (≤1 mg/L). Less than 1% of the strains evaluated had a high-level resistance to azithromycin (≥256 mg/L): 0.10% in 2016, 0.17% in 2017% and 0.55% in 2018. Ceftriaxone resistance reported to ReLAVRA was less than 1%. The only country that reported non-susceptible isolates was Argentina in 2014, with 0.2% of non-susceptible strains out of a total of 679 strains evaluated. In 2016–2018, most of the strains evaluated had a MIC within the susceptibility range (<0.25 mg/L).
Discussion
Our literature search did not identify any systematic review evaluating AMR to N. gonorrhoeae in LAC published in the last 10 years. The search identified 31 published studies reporting data from six countries across the LAC region, indicating a shortage of information for the region. Furthermore, although the number of countries reporting data to the WHO-GASP network has increased over time, only 19% of countries in the Americas region participated in 2016,4 highlighting that the surveillance of AMR to N. gonorrhoeae in the region remains suboptimal.
Our results from LAC are generally consistent with published information from other regions of the world. This review identified high resistance to penicillin, tetracycline and ciprofloxacin (resistance to penicillin ranged from 17.6%45 to 98%,42 to tetracycline from 20.7%52 to 90%,51 and to ciprofloxacin from 5.9%48 to 89%60). High resistance to these drugs was also reported in Africa (resistance to penicillin 75%, to tetracycline 91.7% and to ciprofloxacin 37.5%)73 and the Asia-Pacific region (resistance to ciprofloxacin >5% was reported by 88.7% of studies).74 In 2020, Australia reported resistance to penicillin of 26.6%, to tetracycline 30% and to ciprofloxacin 36.4%,75 and in the USA reported resistance to ciprofloxacin in 2019 was 35.4%, the highest recorded.76 Europe reported high resistance to ciprofloxacin of 50.3% in 2018.77
Our results indicate increasing resistance to azithromycin in LAC (60% of studies reported resistance in at least 5% of strains evaluated, with the highest percentage [32%] in Brazil42), and this was also consistent with results from other regions. In Africa, the reported resistance was 4.2%,73 and in the Asia-Pacific region the percentage of resistance was 0.1%–5%, with 23.5% of reports showing resistance >5%, and an increase in reports with resistance to azithromycin >5% from 14.3% in 2011 to 38.9% in 2016.74 Australia reported a decrease in azithromycin resistance from 9.3% in 2017 to 3.9% in 2020, finding a single strain with high-level resistance (MIC ≥256 mg/L),75 although the USA reported 5.1% of isolates with an elevated MIC to azithromycin.76 In Europe, the resistance found was 7.6% (MIC >1 mg/L according to EUCAST) in 2018, and five strains had a MIC ≥256 mg/L.77
Resistance to the extended-spectrum cephalosporins ceftriaxone and cefixime was infrequent in our review, and this is also consistent with findings from other regions. No reported resistance to ceftriaxone was found in Africa.73 Reports with >5% of isolates with decreased susceptibility (MIC ≥0.125 mg/L) increased from 14.3% to 35.3% in Asia-Pacific between 2011 and 2016,74 and remained stable between 2016 and 2018 in Australia at 0.04%–0.06%, increasing to 0.11% in 2019 (five strains with MIC ≥0.25 mg/L) and 0.9% in 2020 (one strain was resistant).75 In the USA, 0.1% of the isolated strains had a high MIC to ceftriaxone in 2019,76 and in Europe three resistant strains were reported (two with MIC = 0.25 mg/L, one with MIC = 0.5 mg/L), compared with zero resistant strains in 2016 and 2017.77 We found less information on resistance to cefixime. Between 1982 and 2012, 118 isolates of N. gonorrhoeae were found with MIC >0.25 mg/L, most in the USA (n = 60) and Japan (n = 42), with a susceptibility rate ranging from 92.2% to 100% (none from LAC countries).39 In Europe, resistance has remained stable at around 2% since 2014, with three strains with MIC >0.5 mg/L in 2017 and five in 2018.77
We found few published data on resistance to gentamicin and spectinomycin. Australia did not report resistance to gentamicin (the first year it included this antibiotic) or spectinomycin in 2020.75 Also, in Canada no resistance to spectinomycin was found in 2014.78 In Africa, however, the reported resistance to gentamicin was 28.6%.73 The reasons for this apparent regional difference in gentamicin resistance are not clear. Further data about patterns of resistance to these two antibiotics would be valuable, because they are potential alternative treatment options for gonorrhoea, although with some limitations (for example, spectinomycin is not available in many countries and is not useful for the treatment of pharyngeal infections).
The first-line empirical treatment for gonorrhoea currently recommended by WHO and in most countries is the combination of ceftriaxone plus azithromycin. However, in 2018 and 2020, respectively, the UK10 and CDC in the USA9 updated their guidelines to recommend monotherapy with ceftriaxone, with the addition of doxycycline in the USA guidelines if concomitant chlamydial infection cannot be excluded. This change is due to growing concern about the potential impact of dual azithromycin therapy on commensal organisms and other concurrent pathogenic microorganisms, along with low resistance to ceftriaxone and increasing resistance to azithromycin. In addition, azithromycin has a prolonged half-life in plasma, up to 14 days, so the use of this antibiotic in the case of an undiagnosed N. gonorrhoeae infection could result in prolonged exposure to subinhibitory concentrations, potentially promoting the emergence of resistance.79
This review has some limitations. We were not able to assess AMR in relation to the demographic characteristics of the population or the type of gonococcal infection, because few studies provided that information. One study correlated the resistance data with the sexual orientation of the population included, finding a higher proportion of strains resistant to ciprofloxacin in MSM compared with heterosexual men.45 It is possible that some AMR data found have been reported by several studies, especially those providing data from WHO-GASP, GASSP-AR and the Brazilian GASP Network. We attempted to exclude, as far as possible, articles with duplicate publication of data. Antibiotic susceptibility outcomes were described as in the original studies, and methodological variations or differences in study quality may have affected our findings. We did not include data on resistance phenotypes, which could have improved assessment of resistance.
In conclusion, the limited evidence identified by this systematic review on AMR in N. gonorrhoeae in LAC indicates that resistance to azithromycin is increasing, consistent with findings elsewhere in the world. This, combined with its long half-life and potential effects on promoting AMR development, raises questions over its usefulness as part of the first-line empirical treatment for gonorrhoeal infections. Treatment guidelines in the UK and USA have already recommended removing azithromycin from first-line therapy. Resistance to extended-spectrum cephalosporins remains infrequent in LAC, supporting their position as the last empirical treatment option available for gonorrhoea. The small number of LAC countries with published studies, and the limited number contributing data to surveillance networks such as WHO-GASP and ReLAVRA, indicates that surveillance for AMR in N. gonorrhoeae is suboptimal in the LAC region. To better understand the development of AMR and to slow the emergence of resistance, it will be important to strengthen antimicrobial susceptibility surveillance networks for N. gonorrhoeae in the LAC region. This can be addressed by putting in place STI surveillance systems that can monitor AMR, thereby increasing the number of countries reporting data on AMR in N. gonorrhoeae, and capacity-building to establish regional networks of laboratories to perform gonococcal culture with quality-assured and comparable AMR surveillance nationally and internationally. Programmes could be established for the rational use of antibiotics to increase awareness of correct use of antibiotics among healthcare providers and consumers, with effective drug regulations and prescription policies. Further possibilities include considering the potential for preventive messages and interventions such as education regarding symptomatic and asymptomatic STIs, promotion of safe sexual behaviours including increased condom use, enhanced sexual partner notification and treatment, and expansion of targeted interventions including screening in some settings for vulnerable populations such as sex workers, MSM and adolescents. Effective early detection and diagnosis of STIs could improve testing and control of gonorrhoea, including testing for cure and systematic monitoring of treatment failures, ideally linked to general HIV/STI clinics, and recommended appropriate treatment regimens, for which public health guidelines and policies are essential. Finally, research would be valuable into identification of alternative effective treatment regimens, especially novel treatment options and vaccines.
Supplementary Material
Acknowledgements
The authors would like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Carole Nadin (Fleetwith Ltd, on behalf of GSK) provided writing support.
Contributor Information
María Macarena Sandoval, Instituto de Efectividad Clínica y Sanitaria (IECS-CONICET), Buenos Aires, Argentina.
Ariel Bardach, Instituto de Efectividad Clínica y Sanitaria (IECS-CONICET), Buenos Aires, Argentina; Centro de Investigaciones Epidemiológicas y Salud Pública (CIESP-IECS), CONICET, Buenos Aires, Argentina.
Carlos Rojas-Roque, Instituto de Efectividad Clínica y Sanitaria (IECS-CONICET), Buenos Aires, Argentina.
Tomás Alconada, Instituto de Efectividad Clínica y Sanitaria (IECS-CONICET), Buenos Aires, Argentina.
Jorge A Gomez, GSK, Buenos Aires, Argentina.
Thatiana Pinto, GSK, Wavre, Belgium.
Carolina Palermo, Instituto de Efectividad Clínica y Sanitaria (IECS-CONICET), Buenos Aires, Argentina.
Agustin Ciapponi, Instituto de Efectividad Clínica y Sanitaria (IECS-CONICET), Buenos Aires, Argentina; Centro de Investigaciones Epidemiológicas y Salud Pública (CIESP-IECS), CONICET, Buenos Aires, Argentina.
Funding
This work was funded by GlaxoSmithKline Biologicals SA, which was involved in all stages of the study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of this manuscript.
Transparency declarations
J.A.G. and T.P. are employed by GSK. J.A.G. holds shares in GSK. A.B., T.A., C.P., C.R.-R., M.M.S. and A.C. received funding from GSK to complete the work disclosed in this article. The authors declare no other financial and non-financial relationships and activities. This study was supported by GlaxoSmithKline Biologicals SA in the form of grants awarded and all costs associated with the development and publication of this article. All data involved in this study are included in the main manuscript and its supplementary information files.
Supplementary data
Figures S1 to S4 and Tables S1 to S7 are available as Supplementary data at JAC Online.
References
- 1. Rowley J, Vander Hoorn S, Korenromp Eet al. . Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 2019; 97: 548–62P. 10.2471/BLT.18.228486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Unemo M, Seifert HS, Hook EWet al. . Gonorrhoea. Nat Rev Dis Primers 2019; 5: 79. 10.1038/s41572-019-0128-6 [DOI] [PubMed] [Google Scholar]
- 3. Chemaitelly H, Majed A, Abu-Hijleh Fet al. . Global epidemiology of Neisseria gonorrhoeae in infertile populations: systematic review, meta-analysis and metaregression. Sex Transm Infect 2021; 97: 157–69. 10.1136/sextrans-2020-054515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Report on global sexually transmitted infection surveillance, 2018. https://www.who.int/publications-detail-redirect/9789241565691
- 5. World Health Organization . Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. 2012. https://www.who.int/publications/i/item/9789241503501
- 6. World Health Organization (WHO) . WHO guidelines for the treatment of Neisseria gonorrhoeae. 2016. https://www.who.int/publications/i/item/9789241549691 [PubMed]
- 7. Barbee LA. Preparing for an era of untreatable gonorrhea. Curr Opin Infect Dis 2014; 27: 282–7. 10.1097/QCO.0000000000000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Datta SD, Sternberg M, Johnson REet al. . Gonorrhea and chlamydia in the United States among persons 14 to 39 years of age, 1999 to 2002. Ann Intern Med 2007; 147: 89–96. 10.7326/0003-4819-147-2-200707170-00007 [DOI] [PubMed] [Google Scholar]
- 9. St Cyr S, Barbee L, Workowski KAet al. . Update to CDC´s treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep2020; 69: 1911–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fifer H, Saunders J, Soni Set al. . 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae. Int J STD AIDS 2020; 31: 4–15. 10.1177/0956462419886775 [DOI] [PubMed] [Google Scholar]
- 11. Wi T, Lahra MM, Ndowa Fet al. . Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 2017; 14: e1002344. 10.1371/journal.pmed.1002344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohnishi M, Golparian D, Shimuta Ket al. . Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 2011; 55: 3538–45. 10.1128/AAC.00325-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Unemo M, Golparian D, Nicholas Ret al. . High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 2012; 56: 1273–80. 10.1128/AAC.05760-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Camara J, Serra J, Ayats Jet al. . Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother 2012; 67: 1858–60. 10.1093/jac/dks162 [DOI] [PubMed] [Google Scholar]
- 15. Lahra MM, Martin I, Demczuk Wet al. . Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis 2018; 24: 735–40. 10.3201/eid2404.171873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lahra MM, Ryder N, Whiley DM. A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N Engl J Med 2014; 371: 1850–1. 10.1056/NEJMc1408109 [DOI] [PubMed] [Google Scholar]
- 17. Deguchi T, Yasuda M, Hatazaki Ket al. . New clinical strain of Neisseria gonorrhoeae with decreased susceptibility to ceftriaxone in Japan. Emerg Infect Dis 2016; 22: 142–4. 10.3201/eid2201.150868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakayama S, Shimuta K, Furubayashi Ket al. . New ceftriaxone- and multidrug- resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother 2016; 60: 4339–41. 10.1128/AAC.00504-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lefebvre B, Martin I, Demczuk Wet al. . Ceftriaxone-resistant Neisseria gonorrhoeae, Canada 2017. Emerg Infect Dis 2018; 24: 381–3. 10.3201/eid2402.171756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Terkelsen D, Tolstrup J, Johnsen CHet al. . Multidrug-resistant Neisseria gonorrhoeae infection with ceftriaxone resistance and intermediate resistance to azithromycin, Denmark, 2017. Euro Surveill 2017; 22: 1273. 10.2807/1560-7917.ES.2017.22.42.17-00659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poncin T, Fouere S, Braille Aet al. . Multidrug-resistant Neisseria gonorrhoeae failing treatment with ceftriaxone and doxycycline in France, November 2017. Euro Surveill 2018; 23: 1800264. 10.2807/1560-7917.ES.2018.23.21.1800264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yan J, Chen Y, Yang Fet al. . High percentage of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone among isolates from a single hospital in Hangzhou, China. J Antimicrob Chemother 2021; 76: 936–9. 10.1093/jac/dkaa526 [DOI] [PubMed] [Google Scholar]
- 23. Fifer H, Natarajan U, Jones Let al. . Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med 2016; 374: 2504–6. 10.1056/NEJMc1512757 [DOI] [PubMed] [Google Scholar]
- 24. Eyre DW, Sanderson ND, Lord Eet al. . Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill 2018; 23: 1800323. 10.2807/1560-7917.ES.2018.23.27.1800323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whiley DM, Jennison A, Pearson Jet al. . Genetic characterization of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect Dis 2018; 18: 717–18. 10.1016/S1473-3099(18)30340-2 [DOI] [PubMed] [Google Scholar]
- 26. Pan-American Health Organization (PAHO). ReLAVRA homepage. https://www.paho.org/en/relavra
- 27. Pan-American Health Organization . Epidemiological alert. Extended-spectrum cephalosporin resistance in Neisseria gonorrhoeae. 2018. https://iris.paho.org/bitstream/handle/10665.2/50516/EpiUpdatee2February2018_eng.pdf
- 28. Gianecini R, Romero MLM, Oviedo Cet al. . Emergence and spread of Neisseria gonorrhoeae isolates with decreased susceptibility to extended-spectrum cephalosporins in Argentina, 2009 to 2013. Sex Transm Dis 2017; 44: 351–5. 10.1097/OLQ.0000000000000603 [DOI] [PubMed] [Google Scholar]
- 29. Gianecini R, Oviedo C, Galarza P. Evaluation of gentamicin susceptibility and resistance phenotypes of Neisseria gonorrhoeae isolates in Argentina. Sex Transm Infect 2017; 93: A124–A5. [Google Scholar]
- 30. Higgins J, Thomas J, Chandler Jet al. . Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated August 2019). Cochrane Collaboration. www.training.cochrane.org/handbook
- 31. Liberati A, Altman DG, Tetzlaff Jet al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moher D, Liberati A, Tetzlaff Jet al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stroup DF, Berlin JA, Morton SCet al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 34. Pan-American Health Organization (PAHO), Institutional Repository for Information Sharing (IRIS) . Virtual Health Library. https://iris.paho.org/handle/10665.2/19171
- 35. Covidence systematic review software. [computer program]. COVIDENCE. https://www.covidence.org/
- 36. National Institutes of Health (NIH) . Study quality assessment tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 37. Sterne JAC, Savovic J, Page MJet al. . Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 38. Cochrane Effective Practice and Organisation of Care Group. Suggested risk of bias criteria for EPOC reviews. https://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/suggested_risk_of_bias_criteria_for_epoc_reviews.pdf
- 39. Yu RX, Yin Y, Wang GQet al. . Worldwide susceptibility rates of Neisseria gonorrhoeae isolates to cefixime and cefpodoxime: a systematic review and meta-analysis. PLoS One 2014; 9: e87849. 10.1371/journal.pone.0087849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fletcher-Lartey S, Dronavalli M, Alexander Ket al. . Trends in antimicrobial resistance patterns in Neisseria gonorrhoeae in Australia and New Zealand: a meta-analysis and systematic review. Antibiotics 2019; 8: 191. 10.3390/antibiotics8040191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Acevedo A, Ingold A, Parnizzari Fet al. . N. gonorrhoeae antimicrobial resistance in Uruguay: period 2010–2011. Sex Transm Infect 2013; 89 Suppl 1: P2.088. 10.1136/sextrans-2013-051184.0352 [DOI] [Google Scholar]
- 42. Barros Dos Santos KT, Skaf LB, Justo-da-Silva LHet al. . Evidence for clonally associated increasing rates of azithromycin resistant Neisseria gonorrhoeae in Rio de Janeiro, Brazil. Biomed Res Int 2019; 2019: 3180580. 10.1155/2019/3180580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bautista A, Sanabria O, Duarte C. Vigilancia por laboratorio de resistencia antimicrobiana de aislamientos colombianos de Neisseria gonorrhoeae, 2012–2017. XI Encuentro Nacional de Investigadores en Enfermedades Infecciosas, Congress, Pereira Colombia, Aug 2-4, 2018; 19.
- 44. Bazzo ML, Golfetto L, Gaspar PCet al. . First nationwide antimicrobial susceptibility surveillance for Neisseria gonorrhoeae in Brazil, 2015–16. J Antimicrob Chemother 2018; 73: 1854–61. 10.1093/jac/dky090 [DOI] [PubMed] [Google Scholar]
- 45. Casco RH, García SD, Perazzi BEet al. . Neisseria gonorrhoeae. Resistencia a los antibióticos. Dermatol argent 2011; 17: 396–401. [Google Scholar]
- 46. Costa LMB, Pedroso ERP, Vieira Neto Vet al. . Antimicrobial susceptibility of Neisseria gonorrhoeae isolates from patients attending a public referral center for sexually transmitted diseases in Belo Horizonte, State of Minas Gerais, Brazil. Rev Soc Bras Med Trop 2013; 46: 304–9. 10.1590/0037-8682-0009-2013 [DOI] [PubMed] [Google Scholar]
- 47. Costa-Lourenço A, Abrams AJ, Dos Santos KTBet al. . Phylogeny and antimicrobial resistance in Neisseria gonorrhoeae isolates from Rio de Janeiro, Brazil. Infect Genet Evol 2018; 58: 157–63. 10.1016/j.meegid.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. De Los Mendez EA, Morano S, Nagel Aet al. . Surveillance of antimicrobial resistance in Neisseria gonorrhoeae from a hospital of Santa Fe City, Argentina; 2000–2010. Int J Infect Dis 2012; 16: e333. 10.1016/j.ijid.2012.05.390 [DOI] [Google Scholar]
- 49. Dillon JAR, Trecker MA, Thakur SDet al. . Two decades of the gonococcal antimicrobial surveillance program in South America and the Caribbean: challenges and opportunities. Sex Transm Infect 2013; 89: iv36–41. 10.1136/sextrans-2012-050905 [DOI] [PubMed] [Google Scholar]
- 50. Dos Santos TM, Golfetto L, Schorner MAet al. . Evolution of Neisseria gonorrhoeae resistance to antimicrobials in a historical series of isolates from São Paulo/Brazil. Sex Transm Infect 2017; 93: A175. doi; 10.1136/sextrans-2017-053264.454 [DOI] [Google Scholar]
- 51. Flores Fernández EM, Márquez Planché YC, Albarado Ysasis LS. Antimicrobial susceptibility and betalactamase production in Neisseria gonorrhoeae, Cumana, Sucre State, Venezuela. Rev Soc Venez Microbiol 2012; 32: 18–21. [Google Scholar]
- 52. Galarza P, Gianecini A, Oviedo C. Locally generated data 2012 from the Argentinean gonoccocal antimicrobial surveillance program. Sex Transm Dis 2014; 41: S87. [Google Scholar]
- 53. Gianecini RA, Golparian D, Zittermann Set al. . Genome-based epidemiology and antimicrobial resistance determinants of Neisseria gonorrhoeae isolates with decreased susceptibility and resistance to extended-spectrum cephalosporins in Argentina in 2011–16. J Antimicrob Chemother 2019; 74: 1551–9. 10.1093/jac/dkz054 [DOI] [PubMed] [Google Scholar]
- 54. Gianecini R, Pagano I, Oviedo Cet al. . Evaluation of gentamicin susceptibility of Neisseria gonorrhoeae isolates in Argentina. Sex Transm Infect 2013; 89: A364. 10.1136/sextrans-2013-051184.1138 [DOI] [Google Scholar]
- 55. Golfetto L, Schörner M, Santos Tet al. . Molecular epidemiology associated with resistance in Neisseria gonorrhoeae isolates from South Brazil during 2008–2016. Sex Transm Infect 2019; 95: A288–A9. [Google Scholar]
- 56. Jorge-Berrocal A, Mayta-Barrios M, Fiestas-Solórzano V. Resistencia antimicrobiana de Neisseria gonorrhoeae en Perú. Rev Peru Ned Exp Salud Publica 2018; 35: 155–6. 10.17843/rpmesp.2018.351.3552 [DOI] [PubMed] [Google Scholar]
- 57. Martins RA, Cassu-Corsi D, Nodari CSet al. . Temporal evolution of antimicrobial resistance among Neisseria gonorrhoeae clinical isolates in the most populated South American metropolitan region. Mem Inst Oswaldo Cruz 2019; 114: e190079. 10.1590/0074-02760190079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Medeiros MIC, Silva JO, Carneiro AMMet al. . Antimicrobial resistance in Neisseria gonorrhoeae isolates from Ribeirão Preto, São Paulo, Brazil. DST-j Bras Doenças Sex Transm 2013; 25: 31–5. 10.5533/DST-2177-8264-201325107 [DOI] [Google Scholar]
- 59. Montano S, Burga R, Rocha Cet al. . Resistance of Neisseria gonorrhoeae in remote Peruvian jungle settings. Am J Trop Med Hyg 2016; 95: 332. [Google Scholar]
- 60. Rahman N, Kluz N, Puplampu-Attram Net al. . Antibiotic resistance and molecular typing of Neisseria gonorrhoeae isolated from the three overseas sites through the global emerging infections surveillance and response system (GEIS). Sex Transm Infect 2017; 93: A154. 10.1136/sextrans-2017-053264.399 [DOI] [Google Scholar]
- 61. Rivillas-García JC, Sanchez SM, Rivera-Montero D. Desigualdades sociales relacionadas con la resistencia a antimicrobianos de N. gonorrhoeae en Colombia. Rev Panam Salud Publica 2020; 44: e49. 10.26633/RPSP.2020.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sánchez Palencia L, Acosta Cáceres J. Mutaciones en la región determinante de resistencia a quinolonas (QRDR) del gen gyrA de Neisseria gonorrhoeae presente en muestras clínicas de hombres que tienen sexo con hombres. Rev Peru Biol 2017; 24: 283. 10.15381/rpb.v24i3.13905 [DOI] [Google Scholar]
- 63. Schijman M, Montoto M, Butori Bet al. . Resistencia antibiótica en aislamientos de Neisseria gonorrhoeae. XIX Congreso Sociedad Argentina de Infectología 2018, Buenos Aires, Argentina, Oct2018; 96. [Google Scholar]
- 64. Thakur SD, Araya P, Borthagaray Get al. . Resistance to ceftriaxone and azithromycin in Neisseria gonorrhoeae isolates from 7 countries of South America and the Caribbean: 2010–2011. Sex Transm Dis 2017; 44: 157–60. 10.1097/OLQ.0000000000000587 [DOI] [PubMed] [Google Scholar]
- 65. Uehara AA, Amorin EL, Ferreira Mde Fet al. . Molecular characterization of quinolone-resistant Neisseria gonorrhoeae isolates from Brazil. J Clin Microbiol 2011; 49: 4208–12. 10.1128/JCM.01175-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vacchino M, Gianecini R, Oviedo Cet al. . Emergence of Neisseria gonorrhoeae isolates with in vitro decreased susceptibility to ceftriaxone in Argentina. Sex Transm Infect 2013; 89: A77. 10.1136/sextrans-2013-051184.0234 [DOI] [PubMed] [Google Scholar]
- 67. Vacchino M, Tilli M, Gianecini Ret al. . Antimicrobial susceptibility profile of Neisseria gonorrhoeae detected in a public hospital in Buenos Aires, Argentina. Sex Transm Infect 2017; 93: A176–A7. [Google Scholar]
- 68. Le Van A, Rahman N, Dozier Net al. . Peruvian gonococcal strains reveal novel ngmast types and false-positive B-lactamase isolates with blatem gene mutations. Sex Transm Infect 2019; 95: A284–A5. [Google Scholar]
- 69. Vargas S, Qquellon L, Durand Det al. . Extra-genital ciprofloaxin-resistant Neisseria gonorrhoeae infections among sexual-health clinic users in Lima, Peru. Sex Transm Infect 2019; 95: A291. [Google Scholar]
- 70. Zotta MC, Layayen S, Galeano Get al. . Infección por Neisseria gonorrhoeae y fenotipos de resistencia antimicrobiana, Mar del Plata, 2005–2010. Acta Bioquím Clín Latinoam 2014; 48: 475–83. [Google Scholar]
- 71. World Health Organization (WHO), Department of Reproductive Health and Research . Gonococcal Antimicrobial Surveillance Programme (GASP). https://www.who.int/initiatives/gonococcal-antimicrobial-surveillance-programme
- 72. OPS—Red Latinoamericana y del Caribe de Vigilancia de la Resistencia a los Antimicrobianos (ReLAVRA) . Resistencia a los antimicrobianos. https://www3.paho.org/data/index.php/es/temas/resistencia-antimicrobiana.html.
- 73. Tadesse BT, Ashley EA, Ongarello Set al. . Antimicrobial resistance in Africa: a systematic review. BMC Infect Dis 2017; 17: 616. 10.1186/s12879-017-2713-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. George CRR, Enriquez RP, Gatus BJet al. . Systematic review and survey of Neisseria gonorrhoeae ceftriaxone and azithromycin susceptibility data in the Asia Pacific, 2011 to 2016. PLoS One 2019; 14: e0213312. 10.1371/journal.pone.0213312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lahra M, Hogan T, Shoushtari Met al. Australian gonococcal surveillance programme annual report, 2020. Department of Health and Aged Care. https://www1.health.gov.au/internet/main/publishing.nsf/Content/cda-pubs-annlrpt-gonoanrep.htm [DOI] [PubMed]
- 76. Centers for Disease Control and Prevention (CDC) . Sexually transmitted disease surveillance 2019. https://stacks.cdc.gov/view/cdc/105136
- 77. European Centre for Disease Prevention and Control . Gonococcal antimicrobial susceptibility surveillance in Europe—results summary, 2018. ECDC. https://www.ecdc.europa.eu/en/publications-data/gonococcal-antimicrobial-susceptibility-surveillance-europe-2018 [Google Scholar]
- 78. Public Health Agency of Canada. Report on sexually transmitted infections in Canada, 2018. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/report-sexually-transmitted-infections-canada-2018.html
- 79. Kong FYS, Horner P, Unemo Met al. . Pharmacokinetic considerations regarding the treatment of bacterial sexually transmitted infections with azithromycin: a review. J Antimicrob Chemother 2019; 74: 1157–66. 10.1093/jac/dky548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.