Abstract
Autism spectrum disorders caused by both genetic and environmental factors are strongly male-biased neuropsychiatric conditions. However, the mechanism underlying the sex bias of autism spectrum disorders remains elusive. Here, we use a mouse model in which the autism-linked gene Cttnbp2 is mutated to explore the potential mechanism underlying the autism sex bias.
Autism-like features of Cttnbp2 mutant mice were assessed via behavioural assays. C-FOS staining identified sex-biased brain regions critical to social interaction, with their roles and connectivity then validated by chemogenetic manipulation. Proteomic and bioinformatic analyses established sex-biased molecular deficits at synapses, prompting our hypothesis that male-biased nutrient demand magnifies Cttnbp2 deficiency. Accordingly, intakes of branched-chain amino acids (BCAA) and zinc were experimentally altered to assess their effect on autism-like behaviours.
Both deletion and autism-linked mutation of Cttnbp2 result in male-biased social deficits. Seven brain regions, including the infralimbic area of the medial prefrontal cortex (ILA), exhibit reduced neural activity in male mutant mice but not in females upon social stimulation. ILA activation by chemogenetic manipulation is sufficient to activate four of those brain regions susceptible to Cttnbp2 deficiency and consequently to ameliorate social deficits in male mice, implying an ILA-regulated neural circuit is critical to male-biased social deficits. Proteomics analysis reveals male-specific downregulated proteins (including SHANK2 and PSD-95, two synaptic zinc-binding proteins) and female-specific upregulated proteins (including RRAGC) linked to neuropsychiatric disorders, which are likely relevant to male-biased deficits and a female protective effect observed in Cttnbp2 mutant mice. Notably, RRAGC is an upstream regulator of mTOR that senses BCAA, suggesting that mTOR exerts a beneficial effect on females. Indeed, increased BCAA intake activates the mTOR pathway and rescues neuronal responses and social behaviours of male Cttnbp2 mutant mice. Moreover, mutant males exhibit greatly increased zinc demand to display normal social behaviours.
Mice carrying an autism-linked Cttnbp2 mutation exhibit male-biased social deficits linked to specific brain regions, differential synaptic proteomes and higher demand for BCAA and zinc. We postulate that lower demand for zinc and BCAA are relevant to the female protective effect. Our study reveals a mechanism underlying sex-biased social defects and also suggests a potential therapeutic approach for autism spectrum disorders.
Keywords: autism spectrum disorders, branched-chain amino acids, infralimbic cortex, nutrient supplementation, zinc
Yen et al. show that mutation of the autism-linked gene Cttnbp2 impairs social behaviours in male but not female mice. Differences in the synaptic proteomes of male and female mutant mice suggest involvement of zinc and of the mTOR pathway in this sex difference; zinc supplementation improves social behaviours in male Cttnbp2 mutant mice.
Introduction
Autism spectrum disorders, a group of early-onset neurodevelopmental disorders, are ∼4–5-fold more prevalent in males than females (https://www.cdc.gov/ncbddd/autism/data.html). Several hypotheses have been proposed to explain the male bias of autism spectrum disorders, including female protective effects, male risk factors, extreme male brain theory, enhanced plasticity hypotheses, sex chromosome theory, sex hormone theory and a neuroendocrine hypothesis.1–8 A multiple-hit hypothesis (encompassing genetic, environmental and sex interactions) has also been proposed.1 Given that autism spectrum disorders are highly heterogeneous, each hypothesis may account for certain conditions and these hypotheses may not be mutually exclusive.
Autism spectrum disorders are characterized by impaired social interaction, communication deficits, repetitive sensory-motor behaviours and cognitive inflexibility (https://www.psychiatry.org/patients-families/autism/what-is-autism-spectrum-disorder). Recently, large-scale whole-exome sequencing of patients with autism spectrum disorders identified hundreds of disease-linked genes.4,9–12 Genes involved in transcriptional regulation and synaptic formation/signalling have been shown to be critical to autism spectrum disorders.4,9–12 These autism-linked genes may be subclassified into three groups, i.e. male-enriched, female-enriched and shared genes based on sex-specific incidence rates. For male-enriched genes, their mutations have been identified far more predominantly in male but not female patients. Moreover, there are more male-enriched genes than for either of the other two groups.13,14 Thus, sex likely differentially influences the outcome of genetic variations, but further validation is needed.
In this report, we investigate the mechanism underlying sex bias in autism spectrum disorders using the cortactin binding protein 2 (Cttnbp2) mouse genetic models. CTTNBP2, a brain-specific cytoskeleton-associated scaffold protein, is highly enriched at dendritic spines where it regulates dendritic spine formation and maintenance in mature neurons.15–17 Controlled by alternative splicing, at least three isoforms of Cttnbp2 transcripts have been identified in mice.15 However, immunoblotting analyses have indicated that only the short form of CTTNBP2, a protein of 630 amino acid residues, is the dominant isoform in the brain.15,18 Thus, variations within the coding region of the short isoform are expected to be more relevant to autism spectrum disorders.19 Indeed, six variations in the short form of CTTNBP2, including A113T, M121I, G343R, P354A, R536* (stop codon) and D580Y, have been reported for patients with autism spectrum disorders but not case controls.12 Moreover, all of the patients carrying those CTTNBP2 variants are male, indicating that CTTNBP2-dependent autism phenotypes are enriched among males.12,13
Among those six CTTNBP2 variants, the impact of the M121I, R536* and D580Y mutations have already been investigated in mice. Similar to Cttnbp2 deletion, the corresponding autism-linked mutations in the mouse Cttnbp2 gene, i.e. M120I, R533* and D570Y, all result in reduced dendritic spine density.18,19 Mouse genetic models carrying a null allele or the autism-linked mutations of Cttnbp2 also exhibit autism-like behaviours, including social deficits, supporting that those CTTNBP2 mutations are disease-causative.18,19
The short form of CTTNBP2 consists of an N-terminal coiled-coil region and a C-terminal intrinsically disordered region.20 The intrinsically disordered region is required for condensate formation via phase separation.20 It also associates with microtubule and F-actin to regulate dendritic arborization and dendritic spine formation, respectively.15–17 The N-terminal coiled-coil region is involved in homo- and hetero-oligomerization and zinc binding.20 Its interaction with zinc induces higher-order CTTNBP2 multimerization and promotes synaptic retention of CTTNBP2.20 In addition, since the intrinsically disordered region is very flexible, it can fold back to interact with the N-terminal coiled-coil region.19,20 Moreover, Cttnbp2 deletion reduces total zinc levels in mouse brains.18 Together, these findings suggest that CTTNBP2 binds zinc, regulates zinc levels in neurons, and that its function is also controlled by zinc.
Although only male but not female autism patients have been found to harbour CTTNBP2 mutations,13 both male and female case control individuals carry variations in the CTTNBP2 gene.12 Thus, one of the possibilities to explain male enrichment of CTTNBP2 mutations in patients is that females are resilient to those mutations. To explore this speculation, we investigated if Cttnbp2 deletion and autism-linked mutation solely elicit social deficits in male but not female mice. Given that the M120I mutation in mice impairs almost all examined functions of CTTNBP2—including the N- and C-terminal interaction, cortactin binding, biological condensate formation, dendritic spine retention, dendritic spine formation, and social interactions18–20—the Cttnbp2 M120I mutant line represents an interesting model for studying autism. Since monoallelic CTTNBP2 variation has been documented among patients, accordingly we explored the mechanism underlying sex bias in autism-like phenotypes using Cttnbp2+/M120I mice.12
Materials and methods
Antibodies, reagents and plasmids
The following antibodies were used in this study: C-FOS (Cell Signaling, #2250, Clone#9F6, rabbit, 1:500); GFP (Abcam, ab13970, chicken, 1:1000); RAGC (Cell Signaling, #5466, rabbit, 1:500); TIPRL (Abcam, #ab70795, rabbit, 1:500); SHANK2 (Cell Signaling, #12218, rabbit, 1:500); SYNPO (Acris, #BM5086P, rabbit, 0.1 μg/ml); PSD95 (Cell Signaling, #2507, rabbit, 1:1000); HSP90 serum (gift from Dr Chung Wang, IMB, Taiwan, rabbit, 1:2000)21; p-mTOR (Cell Signaling, #2448, rabbit, 1:500); mTOR (Cell Signaling, #2972S, rabbit, 1:500); S6K (Cell Signaling, #2708S, rabbit, 1:500); p-S6K70 T389 (Cell Signaling, #9205S, rabbit, 1:500); 4EBP (Cell Signaling, #9644S, rabbit, 1:500). The following reagents were obtained commercially: ProHance (Gadolinium contrast agent, Bracco Diagnostics, #111181); Vectastain Elite ABC kit (Vector Labs, #PK-6102); C21 (Tocris, #6422); OCT (Tissue-TeK, 4583); ZnSO4 (Sigma-Aldrich, #Z0251); 30 ppm zinc diet (Research Diets, D19410B). AAV vectors used in this study were: pAAV-CamKII-hM3D(Gq)-mCherry (Addgene, #50476); pAAV-CamKII-hM4D(Gi)-mCherry (Addgene, #50477)22; pAAV-CAG-tdTomato (Addgene, #59462).
Animals
Cttnbp2 −/− and Cttnbp2+/M120I mice have been described previously.18,19 All mouse lines were maintained by backcrossing to C57BL/6JNarl purchased from the National Laboratory Animal Center, Taiwan. Mice were housed in the animal facility of the Institute of Molecular Biology, Academia Sinica, under controlled temperature (20–23°C) and humidity (48–55%) and a 12 h light/12 h dark cycle (light: 8.00 a.m. to 8.00 p.m.) with free access to water and food (LabDiet #5k54). After weaning at post-natal Day (P) 21–22, mice were moved from the breeding room to the experimental area and housed individually. Temperature and humidity in the test room were also controlled and the light intensity was 240 lx. All experiments were performed during the daytime, from 10.00 a.m. to 5.00 p.m. All animal experiments were performed with the approval of the Academia Sinica Institutional Animal Care and Utilization Committee (Protocol No. 11-12-294) and in strict accordance with its guidelines and those of the Council of Agriculture Guidebook for the Care and Use of Laboratory Animals.
Animal behavioural assays
Four behavioural tests were conducted as described previously, i.e. open field test (OF), light/dark box (LDB), three-chamber test (3C) and reciprocal social interaction (RSI),18,23,24 starting at P28 and ending at P42 (Fig. 1A), to evaluate if Cttnbp2 mutant mice exhibit sex-biased behavioural deficits. An elevated plus maze (EPM) test was also included in experiments related to zinc supplementation. Detailed descriptions of behavioural experiments are presented in the Supplementary material.
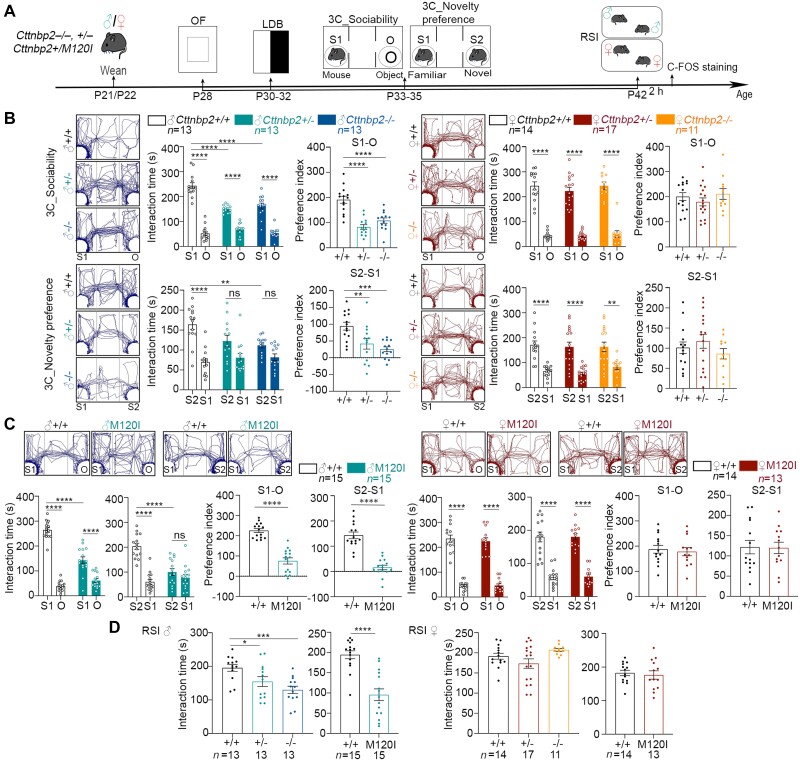
Figure 1.
Cttnbp2 deficiency results in male-biased social deficits in mice. (A) Schematic of the experimental timeline from P28 to P42, together with the behavioural procedures conducted on both sexes of Cttnbp2 mutant mice. O = cage with an object; S1 = cage with unfamiliar mouse 1; S2 = cage with unfamiliar mouse 2. Two hours after RSI at P42, brains were collected for C-FOS staining. (B and C) The results of the 3C social task. Mouse trajectory in the chamber, time spent in each cage, and preference index (i.e. time difference between the indicated cages) are shown for each set of animals. Left = male; right = female. (B) Male, but not female, Cttnbp2+/− and Cttnbp2−/− mice display deficits in sociability and social novelty in the 3C test. (C) Cttnbp2+/M120I mice (denoted M120I mice) also display male-biased deficits. (D) The results of the reciprocal social interaction test. Left = male; right = female. Data are presented as means ± SEM, together with data-points for individual mice. The numbers (n) of examined mice for each group are indicated. For all results of the 3C test, the interaction time was analysed using two-way ANOVA with Tukey’s multiple comparisons post hoc test. The preference indices were analysed using one-way ANOVA in B and two-tailed unpaired t-test in C and D. In E, one-way ANOVA and two-tailed unpaired t-test were used for three- and two-group comparisons, respectively. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns = non-significant. All statistical analysis and results, including the actual P-values, are summarized in Supplementary Table 7.
C-FOS staining
C-FOS immunostaining of brain sections was conducted as described previously.25 In brief, one of four consecutive 50-μm thick brain sections was selected for C-FOS staining using DAB staining (Vectastain Elite ABC kit, Vector). The region of interest (ROI) was identified according to the Allen Brain Atlas (http://help.brain-map.org/display/api/Atlas+Drawings+and+Ontologies, Atlas ID one, mouse P56 coronal) and manually adjusted. The numbers of C-FOS-positive (C-FOS+) cells in each ROI were then determined using ImageJ according to the following sequential steps: (i) the images were converted into 8-bit; (ii) noise signals were processed using ‘subtract background’ (setting: 10 pixels, light background) and ‘threshold’ (B&W mode); (iii) greyscale ‘Binary watershed’ was then applied to segment particles; and (iv) C-FOS+ cells in the ROI were determined using ‘Analyze particles’ with settings ‘size >10 pixels (1 μm/pixel)’ and ‘circularity 0.01–1.00’. Density of C-FOS+ cells in each ROI was then determined.
Chemogenetic modulation
Surgical injection and chemogenetic manipulation were performed as described previously.26,27 To evaluate the role of the ILA in controlling social behaviours of male and female mice, we bilaterally injected AAV-CamKII-hM3Dq-mCherry (4.32 × 1012 vg/ml) and AAV-CamKII-hM4Di-mCherry (>2 × 1012 vg/ml) into the ILA of male and female mice, respectively, at P27/28. AAV-CAG-tdTomato (8.06 × 1012 vg/ml) was used as a negative control in both male and female mice. One hour before the behavioural assay, C21 (1 mg/kg of body weight) was injected intraperitoneally to either activate or inhibit neuronal activity of the ILA.
Liquid chromatography-tandem mass spectrometry analysis
Brains corresponding to section number 39–41 of the Allen Brain Atlas were dissected to collect the ILA. The tissue samples of three mice were pooled as one replicate and four biological repeats were used for each group. Synaptosomal fractionation, liquid chromatography-tandem mass spectrometry (LC-MS-MS) and bioinformatic analyses of the ILA were then performed as described previously18,28 and detailed in the Supplementary material.
Immunoblotting
Five to seven biological replicates (10–15 μg per lane) of ILA or total cortical lysates were subjected to three to four independent immunoblotting assays to validate the results of our proteomic analysis or to evaluate the effect of branched-chain amino acid (BCAA) treatment. The membranes were blocked with 5% non-fat milk, washed with PBS-T (0.2% Tween-20) buffer, and then incubated with primary antibodies overnight at 4°C. After washing, the membrane was incubated with HRP-conjugated secondary antibodies for 2 h at room temperature. HSP90 was used as a loading control for normalization of the protein levels. The results were visualized using a WesternBright ECL Spray or Immobilon Western Chemiluminescent HRP Substrate and recorded using an ImageQuant LAS 4000 system with ImageQuant LAS 4000 Biomolecular Imager software (GE Healthcare). Signal quantification was performed using ImageJ Fiji version 2.1.0/1.53c.
Zinc diet treatment
Mice were randomly assigned to two groups provisioned with different levels of zinc in their diets (Fig. 6A): (i) 30 ppm zinc, i.e. Research Diets D19410B representing a normal zinc diet; or (ii) 150 ppm zinc, i.e. Research Diets D19410B plus 75 ppm ZnSO4 in drinking water representing a higher zinc diet. Behavioural assays and body weight measurements were performed during P30–P42.
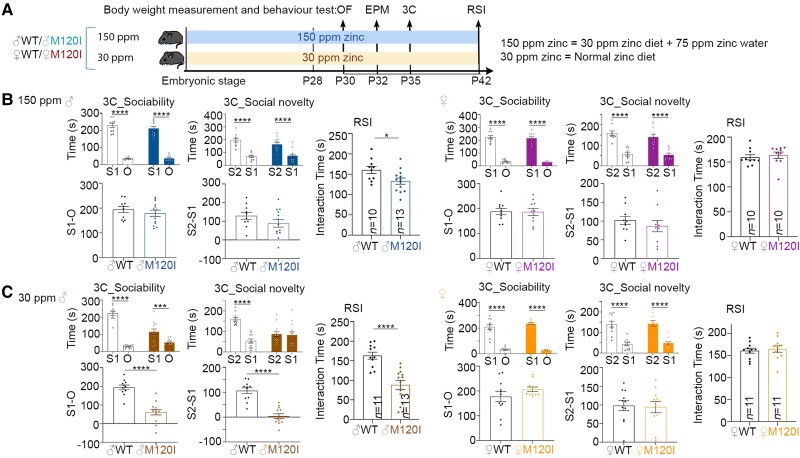
Figure 6.
The social behaviours of female M120I mice are insensitive to the levels of zinc intake. (A) Schematic of the experimental timeline and behavioural tests. Mice were fed on 150 or 30 ppm zinc diets since the embryonic stage, as indicated. (B and C) The results of the 3C and RSI tests are shown as indicated. Left = male; right = female. (B) The results for mice fed on the 150 ppm zinc diet. (C) The results for mice fed on the 30 ppm zinc diet. The same sets of mice were used for the OF, EPM, 3C and RSI tests. The results for OF and EPM are summarized in Supplementary Fig. 9. The sample sizes (n, i.e. numbers of mice) are indicated only for the results of RSI. Data are presented as means ± SEM, and the results of individual mice are shown. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. For the results of the 3C test, the interaction times were analysed using two-way ANOVA with Tukey’s multiple comparisons post hoc test. The preference index and RSI were analysed using a two-tailed unpaired t-test. All statistical analysis and results, including the actual P-values, are summarized in Supplementary Table 7.
Quantification and statistical analysis
All quantitative data in this report are presented as means plus standard error of the mean (SEM). Individual data-points are also presented. GraphPad9 Prism (Graphpad software, La Jolla, CA) was used to perform statistical analysis and to generate the graphs. No statistical method was applied to evaluate the sample size, but our sample sizes are similar to previous publications.25,29 Data collection and analysis were conducted in a random and blind manner. Statistical analyses were performed using a two-tailed unpaired Student’s t-test, two-tailed paired t-test, one-way ANOVA followed by Dunnett’s post hoc test, or two-way ANOVA with Tuckey’s post hoc test. For all comparisons, P < 0.05 was considered significant. All statistical details and results are available in Supplementary Tables 1–5 and 7.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.
Results
Male-biased social deficits in Cttnbp2-deficient mice
We arranged a series of behavioural paradigms from P28 to P42 (Fig. 1A) to investigate if Cttnbp2 deletion and autism-linked mutation result in sex-biased social deficits. In addition to Cttnbp2 knockout mice, Cttnbp2+/M120I mice serve as a mouse model of monoallelic M121I mutation of the human CTTNBP2 gene identified in autism patients.12,19,20 To confirm the observed phenotypes are not caused by the off-target effect of gene editing, we generated two independent Cttnbp2+/M120I mouse lines (#8 and #12, hereafter denoted M120I mice) and backcrossed the mutant mice to wild-type (WT) C57BL/6 for at least six generations before behavioural analysis. The results of line #8 have been published previously,19,20 revealing that the M120I mutation impairs social behaviours of adult male mice. Here, we show that adult male mice of line #12 also exhibit social deficits (Supplementary Fig. 1A), further strengthening the evidence that Cttnbp2 M120I mutation results in social deficits. Since both lines exhibit the same social deficits, we only subjected line #12 to the experiments presented in this report.
We subjected both male and female mice to OF, LDB, 3C and RSI tests (Fig. 1A). For all Cttnbp2+/−, Cttnbp2−/− and M120I mice, both male and female mutant mice behaved normally compared to their WT littermates in an OF (Supplementary Fig. 2B and C). In a LDB, male mice behaved comparably regardless of genotype, but female Cttnbp2+/− and Cttnbp2−/− mice spent more time in the light zone (Supplementary Fig. 2B and C). However, this anxiolytic effect was not detected for female M120I mice (Supplementary Fig. 2C). Thus, anxiolysis in females is not a shared phenotype attributable to Cttnbp2 deficiency, so it was not pursued further in the current report.
We then used a 3C test to evaluate the sociability and novelty preference of male and female mice. Juvenile male Cttnbp2+/−, Cttnbp2−/− and M120I mice all exhibited a clear reduction in interactions with Stranger one (S1) for sociability and in interactions with Stranger two (S2) for novelty preference (Fig. 1B, left). In contrast to male mutant mice, none of the female mutant mice showed any deficits in sociability or novelty preference (Fig. 1B, right).
To further confirm the sex-biased social deficits, we subjected our mutant mice to an RSI assay, which revealed that all male Cttnbp2+/−, Cttnbp2−/− and M120I mice exhibited RSI deficits (Fig. 1C). Female Cttnbp2 mutant mice behaved comparably to their WT littermates (Fig. 1C). Thus, Cttnbp2 mutation elicits male-biased social defects at the juvenile stage.
In addition to the juvenile stage, we further investigated the behaviours of adult female M120I mice. Similarly, adult M120I females behaved normally in an open field (Supplementary Fig. 1B). Importantly, the reciprocal interaction of mutant adult females with female strangers was also comparable to their WT female littermates, regardless of their estrus cycle (Supplementary Fig. 1B). Thus, Cttnbp2 autism-linked mutation results in male-biased social deficits in both juveniles and adults.
Identification of brain regions displaying sex-dependent differences
Next, we used our M120I mice as a model to investigate how autism-linked Cttnbp2 mutation results in male-biased social deficits. First, we used structural MRI to assess neuroanatomical differences between male and female mutant mice and their same-sex WT littermates. We ran linear models to compare genotypes across all groups, and separately in males and females. Additionally, we examined the interaction between genotype and sex. However, we did not detect any significant differences in comparisons of 182 different brain regions (Supplementary Table 1), nor in our voxel-wise comparisons. Thus, there are no obvious neuroanatomical differences among these four groups.
Nevertheless, we speculated that neuronal activity may vary among the mouse groups. Accordingly, we examined C-FOS expression 2 h after RSI (Fig. 1A) across 31 brain regions (Supplementary Tables 2 and 3) involved in social interaction or associated with sexual dimorphism.30,31 First, we compared the mean values of C-FOS+ cell density to determine the following ratios: M120I male/WT male (to indicate a genetic effect on males); M120I female/WT female (to represent a genetic effect on females); WT female/WT male (to show an effect of sex on WT mice); and M120I female/M120I male (to reveal an effect of sex on the Cttnbp2 M120I mutation). Based on the mean values, we observed that differences for the M120I female/WT female and WT female/WT male groups were mainly less than 50% (Fig. 2A, region between 0.66 and 1.5 indicated by two dashed lines). Differences of >50% were mainly attributable to the groups of M120I male/WT male (Fig. 2A, blue circles) and M120I female/M120I male (Fig. 2A, orange triangles). This global comparison of neuronal activity indicates that Cttnbp2 deficiency likely alters neuronal responses in some brain regions in a sex-biased manner.
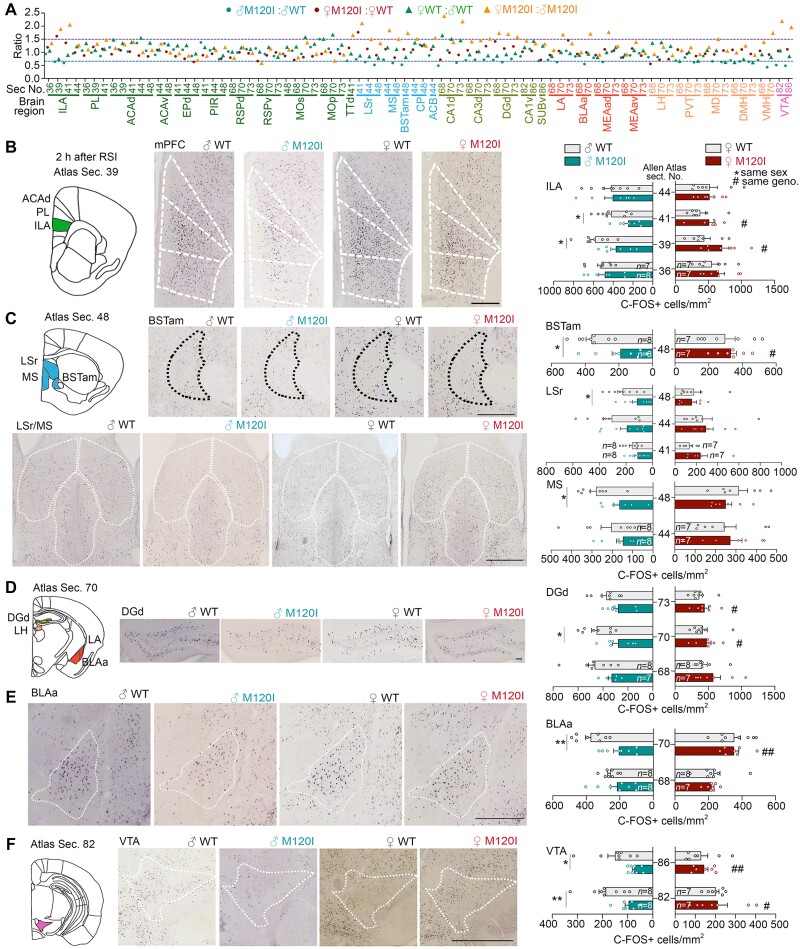
Figure 2.
Cttnbp2 M120I mutation results in reduced neuronal activation of specific brain regions in male mice but not females. Two hours after reciprocal social stimulation at P42 (Fig. 1A), C-FOS staining was performed to identify differentially activated brain regions of male and female M120I mice compared to the same-sex WT littermates. In general, six to eight mouse brains were used for each group. A total of 31 brain regions were screened. The full names of all 31 brain regions are listed in Supplementary Table 2. (A) The ratios of C-FOS+ cell densities for four indicated comparisons are summarized. The screened brain regions and the corresponding section numbers based on the Allen Brain Atlas are indicated. The P-values for all four comparisons and the actual means of C-FOS+ cell density are summarized in Supplementary Tables 2 and 3. (B–F) C-FOS staining in seven brain regions, including the ILA, BSTam, lateral septal nucleus (LSr), medial septal nucleus (MS), DGd, BLAa and VTA, of male Cttnbp2 M120I mice in response to social stimulation. Schematic diagrams and representative corresponding brain sections are shown on the left, with quantitative analyses shown on the right. (B) The mPFC, including the ILA, prelimbic area and ACAd. (C) BSTam, LSr and MS. (D) DGd region. (E) BLAa region. (F) VTA region. Data are presented as means ± SEM and individual data-points are shown. Number of mice (n) for each group is indicated. *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.05, ##P < 0.01. Two-tailed unpaired t-test was used for all analyses in this figure. Asterisk indicates the comparison between male WT and M120I mice; number sign represents the comparison between male and female mutant mice. Scale bars = (B) 600 μm; (C) top, 300 μm; bottom, 600 μm; (D) 100 μm; (E and F) 500 μm. The results of the other brain regions are summarized in Supplementary Figs 3 and 4.
We also performed statistical analysis for each brain region. Compared to male WT mice, male M120I mice exhibited reduced C-FOS+ cell densities in seven brain regions, including the ILA, the anteromedial area of the bed nuclei of the stria terminalis (BSTam), rostral part of the lateral septal nucleus, medial septal nucleus, dentate gyrus of the dorsal hippocampus (DGd), anterior part of basolateral amygdala (BLAa) and ventral tegmental area (VTA) (Fig. 2B–F). No such differences were detected for the comparison between female M120I and WT mice (Fig. 2B–F). When male and female M120I mice were further compared, the numbers of activated neurons in the ILA, BSTam, DGa, BLAa and VTA were also higher in female mutant mice (Fig. 2B–F, indicated with a number sign, or double number sign). Thus, the activities of brain regions were noticeably reduced by Cttnbp2 M120I mutation in male mice.
The same analysis on female M120I mice revealed that only the lateral habenula and lateral amygdala were affected (Supplementary Fig. 3), with C-FOS+ cell density being reduced by M120I mutation in the lateral habenula but increased in the lateral amygdala (Supplementary Fig. 3 and Supplementary Tables 2 and 3). The other brain regions did not display obvious differences (Supplementary Fig. 4 and Supplementary Tables 2 and 3). Thus, the impact of M120I mutation on females is much milder.
Hence, our analysis of C-FOS expression indicates that female and male M120I mouse brains respond differently to social stimulation, with female mice proving more resilient to Cttnbp2 deficiency in terms of neuronal activation upon social stimulation.
The critical role of the ILA-related circuits in sex-biased deficits
Among the seven male-biased regions identified above, we were particularly interested in the ILA because it links many other brain regions to control social interaction, emotional responses and other behaviours.32–34 Accordingly, we evaluated the role of the ILA in controlling social behaviours by means of chemogenetic manipulation (Fig. 3A and Supplementary Fig. 5). AAV-CaMKII-hM3Dq-mCherry (or AAV-CAG-tdTomato as a control) was stereotactically injected into the ILA of male M120I mice at P27-28 (Fig. 3A and Supplementary Fig. 5). We confirmed AAV injection at the ILA in all quantified mice after completing experiments (Supplementary Fig. 5).
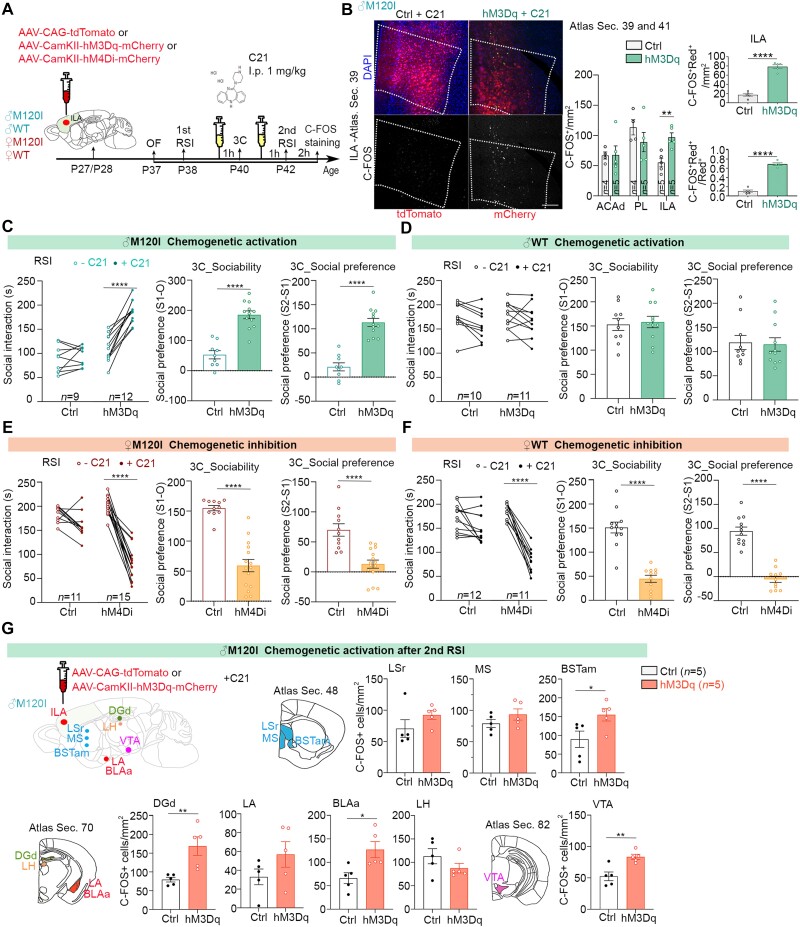
Figure 3.
The ILA is critical to social behaviours. (A) Schematic of the experimental timeline for chemogenetic manipulation and behavioural tests to evaluate the role of the ILA in sex-biased social deficits. Various AAVs, as indicated, were individually injected bilaterally into the ILA. At P40 and P42, mice were intraperitoneally injected with C21 (1 mg/kg) 1 h before undergoing behavioural tests. C-FOS staining was performed 2 h after the second RSI at P42 to monitor neuronal activation. Injection sites were also identified (Supplementary Fig. 5). (B) Chemogenetic activation using hM3Dq and C21 increases C-FOS+ cell numbers in the ILA, but not the prelimbic area or ACAd. Representative images are shown on the left. The density of total C-FOS+ cells in ACAd, PL and ILA are shown in the middle. The density of C-FOS and AAV double-positive cells and the ratios of C-FOS and AAV double-positive cells to AAV-infected cells are shown on the right. Since all AAV constructs express red fluorescent proteins, representing either tdTomato or mCherry, ‘Red+’ is used to indicate AAV-infected cells in the panels. Scale bar = 200 μm. (C–F) The results of behavioural tests upon chemogenetic activation or inhibition, including the first and second RSI tests, and sociability and social preference in the 3C test. The same sets of mice were used here and in Supplementary Fig. 6. (C) Chemogenetic activation of male M120I mice. (D) Chemogenetic activation of male WT mice. (E) Chemogenetic inhibition of female M120I mice. (F) Chemogenetic inhibition of female WT mice. (G) Chemogenetic activation of the male ILA also activates other brain regions, including the BSTam, DGd, BLAa and VTA. Data are presented as means ± SEM and the results for individual mice are shown. Numbers of mice (n) are only indicated on the right panels for each set of data. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Two-tailed unpaired t-tests were used, except that a two-tailed paired t-test was used to compare the first and second RSI tests. All statistical analysis and results, including the sample size and actual P-values, are summarized in Supplementary Table 7.
After recovering from the surgery, the mice were subjected to behavioural assays in the order of OF-RSI-3C-RSI (Fig. 3A). C21, an agonist of designer receptor exclusively activated by designer drugs (DREADD), was intraperitoneally injected into mice one hour before the 3C and the second RSI assays (Fig. 3A). Two hours after the second RSI test at P42, we performed C-FOS staining to confirm our chemogenetic activation enhanced neuronal activation in the ILA (Fig. 3B, middle). In contrast to the ILA, neither the prelimbic area of the medial prefrontal cortex, a region adjacent to the ILA, nor the dorsal part of the anterior cingulate area (ACAd) presented differences in C-FOS+ cell numbers (Fig. 3B, middle). In the ILA, the majority of hM3Dq-expressing cells were also C-FOS+ (Fig. 3B, right), supporting that the responses of ILA neurons were regulated by hM3Dq and C21. Together, these results support the specificity of our experimental manipulation.
Mouse performance in the OF was comparable between hM3Dq- and tdTomato-expressing male M120I mice (Supplementary Fig. 5A). Comparing data from the first and second RSI assays, we found that C21 treatment enhanced the social interaction time of hM3Dq-expressing male M120I mice, but not that of the tdTomato-expressing mutants (Fig. 3C). In the 3C test, C21 treatment increased both sociability and novelty preference of hM3Dq-expressing male M120I mice (Fig. 3C). Unlike for male M120I mice, the same chemogenetic approach did not enhance the behaviours of male WT mice expressing hM3Dq at the ILA (Fig. 3D), likely due to a ceiling effect. Together, these results support that enhanced ILA neuronal activity ameliorates the social deficits of male M120I mice.
Next, we investigated if the ILA is also critical for controlling social interaction among female mice. AAV-CaMKII-hM4Di-mCherry was infected into the ILA to inactivate neuronal activity upon C21 treatment (Fig. 3A). Similar to our observations for male mice, surgery and AAV expression did not alter the behaviours of female WT or M120I mutant mice in the OF (Supplementary Fig. 6C and D). Importantly, ILA inactivation by chemogenetic manipulation noticeably impaired the social behaviours of both WT and M120I female mice in RSI and 3C tests (Fig. 3E and F). Thus, the ILA is a critical region controlling the social behaviours of both male and female mice.
We then investigated if ILA activation is sufficient to activate other male-biased brain regions in M120I mice. Chemogenetic activation was again performed to activate the ILA in M120I male mice. After C21 treatment, we found that the numbers of C-FOS+ cells also increased in four other male-biased regions, including BSTam, DGd, BLAa and VTA (Fig. 3G). The effect was specific, because the lateral septal nucleus, medial septal nucleus, lateral amygdala and lateral habenula were not significantly affected (Fig. 3G), indicating that the ILA influences the BSTam, DGd, BLAa and VTA to control social behaviours. Each of these five regions also had more C-FOS+ cells in mutant females compared to mutant males upon social stimulation (Fig. 2), further strengthening their role in controlling sex-biased social deficits.
Altered ILA synaptic proteomes of M120I mutant mice
Since Cttnbp2 deletion was previously shown to alter synaptic protein targeting,18 we investigated if the M120I mutation also influences the composition of synaptic proteins in the ILA and if any alteration is relevant to the male-biased social deficits of M120I mice. We collected synaptosomal fractions of the ILA from both sexes of M120I mutant mice and WT littermates and subjected them to label-free LC-MS-MS to identify differentially expressed proteins (DEPs). Principal component analysis of entire proteomes indicated that male and female mouse brains were distinguishable (Supplementary Fig. 7A). We then selected proteins with a reliable peptide signal and an adjusted P-value <0.05 for DEP identification (Fig. 4A–F, Supplementary Fig. 6 and Supplementary Tables 4 and 5).
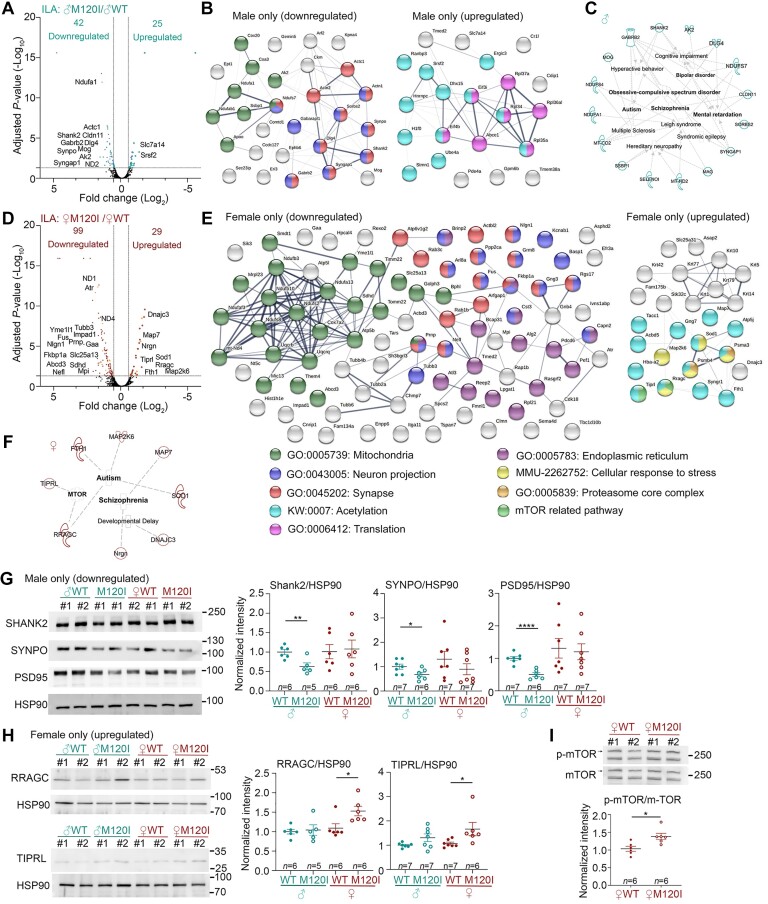
Figure 4.
Molecular features of the ILA synaptic proteome. Crude synaptosomal fractions of the ILA were isolated and analysed. Four biological replicates for each group and a total of four groups of mice (male and female WT and Cttnbp2 M120I mice) were subjected to LC-MS-MS and bioinformatic analysis. (A) Volcano plot of DEPs of male M120I (♂M120I) versus male WT (♂WT). (B) STRING protein network of DEPs of male M120I versus male WT. (C) Disease association of DEPs of male M120I versus male WT using Ingenuity Pathway Analysis (IPA). (D) Volcano plot of DEPs of female M102I (♀M120I) versus female WT (♀WT). (E) STRING protein network of DEPs of female M120I versus female WT. (F) Disease association of DEPs of female M120I versus female WT based on a literature search. For STRING, significant GOs are listed. For instance, proteins related to mitochondria, neuron projection and synapse are highly enriched in the DEP of male only (downregulated) and female only (downregulated). The group of female only (downregulated) further contains several proteins related to the endoplasmic reticulum. Protein acetylation is a common modification of the DEP in the groups of male only (upregulated) and female only (upregulated). Proteins related to translation regulation are specifically present in the group of male only (upregulated). In the group of female only (upregulated), three unique groups related to mTOR regulation, stress response and proteasome core complex are present. (G) Immunoblotting validation of male-specific downregulated DEPs, including SHANK2, SYNPO and PSD-95. (H) Immunoblotting validation of female-specific upregulated DEPs, including RRAGC and TIPRL. Protein levels were normalized to HSP90. (I) Phosphorylation levels of mTOR are higher in female M120I mice than female WT littermates. For immunoblotting in G–I, two lanes for each group in the blot represent two biological replicates labelled as #1 and #2. Each replicate contained a pool of ILA synaptosomal samples isolated from three mice. The sample size (n) indicates the number of biological replicates. The quantification data are based on three to four independent experiments and have been normalized against the levels of HSP90. Uncropped images are available in Supplementary Fig. 10. Data are presented as means ± SEM, and individual data-points are also shown. *P < 0.05, **P < 0.01, ****P < 0.0001. Two-tailed unpaired t-tests were used to analyse the differences between WT and mutant mice of the same sex. All statistical analysis and results, including the actual P-values, are summarized in Supplementary Table 7.
The protein networks and gene ontology (GO) of these DEPs in the ILA were then analysed using STRING (https://string-db.org), Ingenuity Pathway Analysis (IPA, Qiagen), and Syngo (https://www.syngoportal.org). For male-only downregulated DEPs, the most significant GO terms were synapse-, neuron projection- and mitochondrion-related proteins (Fig. 4B, left), which may account for the ILA synaptic deficits displayed by male M120I mice. Indeed, our IPA indicated an association of male-only DEPs with neurological and neuropsychiatric disorders, such as autism, schizophrenia, bipolar disorder, obsessive-compulsive disorders, hyperactivity, and intellectual disability (Fig. 4C and Supplementary Fig. 7C), supporting roles for these DEPs in synaptic function.
Among male-only upregulated DEPs, proteins contributing to translation were apparent (Fig. 4B, right). Since the reduced dendritic spine density attributable to the M120I mutation likely restricts synaptic activity-dependent protein synthesis, these upregulated DEPs likely represent a scenario where neurons were trying to compensate for the synaptic deficits by controlling protein synthesis.
It was unexpected to find more female-only DEPs in the ILA compared to males (Fig. 4D and E and Supplementary Table 5). The primary GO terms were related to mitochondria, neuron projection, synapse and endoplasmic reticulum (Fig. 4E), but the DEPs differed from those of males. Moreover, the IPA did not identify neuropsychiatric disorders relevant to female-only downregulated DEPs, which is consistent with the normal social behaviours displayed by female M120I mice.
The results of our Syngo analysis were similar to those of STRING. We identified several synaptic proteins at both post- and pre-synaptic sites in male up and downregulated DEPs and female downregulated DEPs. However, only two synaptic proteins were identified among female upregulated DEPs (Supplementary Fig. 7D and E). We further explored female-only upregulated DEPs to understand how females are resistant to Cttnbp2 deficiency. STRING analysis identified several proteins related to stress responses and the proteasome, but there were no obvious GO terms associated with synaptic function (Fig. 4E, right). Accordingly, we searched the available literature manually to annotate female upregulated DEPs, which uncovered eight female upregulated DEPs reportedly linked to autism, schizophrenia, microcephaly or developmental delay (Fig. 4F and Supplementary Table 6). Among them, we noticed that two of the DEPs influence mTORC1 activity, i.e. RRAGC (Ras-related GTP-binding protein C) and TIPRL (TOR signalling pathway regulator). RRAGC regulates the relocalization of mTORC1 to lysosomes in response to the availability of amino acids, especially BCAAs, including leucine, isoleucine and valine, and consequently controls protein synthesis.35–37 TIPRL associates with PP2A, playing a positive role in regulating mTOR activity.38 Thus, enhancing RRAGC and TIPRL expression in female M120I mice likely increases mTOR pathway activity to promote protein synthesis.
We adopted an immunoblotting approach to validate our proteomic results. Among male-only downregulated DEPs, we detected reduced synaptic protein levels of SHANK2, PSD95 and SYNPO in the ILA of male M120I mice relative to WT, but that was not the case for female counterparts (Fig. 4G). In the female-only upregulated DEPs group, protein levels of both RRAGC and TIPRL were indeed increased in female M120I mutant mice relative to WT, but again male mice did not present the same outcome (Fig. 4H). Moreover, consistent with the increased levels of RRAGC and TIPRL, we determined that mTOR phosphorylation levels were higher in female M120I mice compared to WT females (Fig. 4I). Together, these immunoblotting results strengthen the reliability of our proteomic analysis and also highlight the involvement of the mTOR pathway in responses to Cttnbp2 deficiency.
Amelioration of the social deficits of male M120I mice via BCAA supplementation
Based on the results of our synaptic proteome analysis and our previous study,18,20 we deduced two potential ways of treating the male-biased social deficits displayed by M120I mice. Our first approach was to enhance protein synthesis. Synaptic stimulation increases protein synthesis, which feeds back to strengthen synaptic responses and connectivity.39–41 Increased mTOR-regulated protein synthesis by means of leucine supplementation was also shown to ameliorate dendritic spine deficits, enhance neuronal activation and improve social behaviours of two other autism mouse models, i.e. Nf1 and Vcp mutant mice.28,42 Based on this evidence, we speculated that increased mTOR activity through enhanced expression of RRAGC and TIPRL in female M120I mice may have provided a beneficial effect in terms of female behaviours. Accordingly, artificially manipulating mTOR activity by means of BCAA supplementation may also exert a beneficial effect on M120I males.
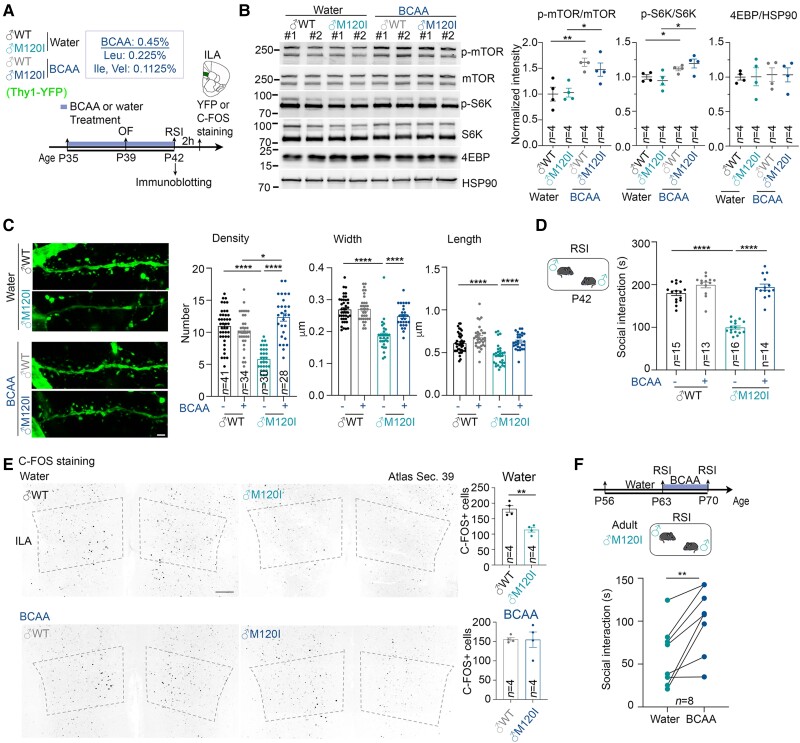
To test that hypothesis, we supplemented the drinking water of juvenile mice from P35 to P42 with BCAA (Leu:Ile:Val = 2:1:1) (Fig. 5A). Then, we monitored mTOR pathway activity, dendritic spine density, social behaviours, and neuronal activation (as represented by C-FOS expression). As anticipated, BCAA supplementation enhanced the levels of phospho-mTOR and phospho-S6K in the ILA of male M120I mice (Fig. 5B). BCAA supplementation also ameliorated the dendritic spine defects in the ILA of male M120I mice (Fig. 5C). However, it did not alter the body weight of WT or mutant mice (Supplementary Fig. 8A). In terms of our behavioural tests, BCAA supplementation did not influence mouse behaviours in an open field, but it did enhance the social interactions of male M120I mice to a level comparable to WT males (Fig. 5D and Supplementary Fig. 8B). Moreover, the numbers of C-FOS+ cells in the ILA were also comparable between WT and mutant mice upon BCAA supplementation (Fig. 5E). These results support our hypothesis that increasing protein synthesis can improve the social deficits of juvenile M120I mice.
Figure 5.
BCAA supplementation increases the social interactions of male Cttnbp2 M120I mice. (A) Schematic of the experimental timeline for BCAA treatment and behavioural tests. Male mice expressing YFP under the control of the Thy1 promoter started drinking BCAA-supplemented water at P35. Mice that drank regular drinking water were included as controls. OF and RSI tests were performed at P39 and P42, respectively. Some mouse brains were collected directly after the RSI test for immunoblotting. Some mouse brains were collected 2 h after the RSI test for C-FOS or Thy1-YFP staining. (B) BCAA treatment increases mTOR activity of the mouse cerebral cortex. Left: The results of immunoblotting using different antibodies as indicated. Right: Quantification of immunoblotting. Two lanes for each group in the blot represent synaptosomal fractions prepared from different mice, #1 and #2. Four mice per group were analysed in two separate experiments. Numbers of mice examined (n) are also indicated. Uncropped images are available in Supplementary Fig. 10. (C) The density, width and length of dendritic spines of ILA neurons were analysed based on YFP signal. Left: Representative images of the first branches of apical dendrites of ILA neurons. Right: Quantitative results. Mouse numbers: four for both water- and BCAA-treated male WT (♂WT); three for both water- and BCAA-treated male M120I (♂M120I). Numbers (n) of examined neurons are indicated in the panel. (D) RSI test results at P42. Numbers of mice (n) are indicated. (E) C-FOS staining results. Left: Representative images of C-FOS staining at the ILA region. Right: C-FOS+ cell density in the ILA. Four mice for each genotype were analysed. (F) Adult M120I mice also respond to BCAA supplementation. Eight adult M120I mice were analysed. Data are presented as means ± SEM, and the results of individual mice are shown. *P < 0.05, **P < 0.01, ****P < 0.0001. (B) Two-tailed unpaired t-test was used to examine the effect of genotype. (C) Two-way ANOVA with Tukey’s multiple comparisons post hoc test. (D) Two-way ANOVA with Tukey’s multiple comparisons post hoc test. (E) Two-tailed unpaired t-test. (F) Two-tailed paired t-test. All statistical analysis and results, including the actual P-values, are summarized in Supplementary Table 7.
We also investigated the effect of BCAA supplementation on adult M120I male mice by performing two RSI tests at 9 and 10 weeks, with BCAA supplemented for 7 days between both tests (Fig. 5F). As per our finding for juvenile mice, BCAA supplementation increased the social interactions of adult male M120I mice (Fig. 5F). Thus, BCAA supplementation ameliorates social defects in both adult and juvenile male mice.
Differential zinc demand contributes to the sex-biased response
The second approach to tackling the male-biased social deficits displayed by M120I mutant mice was zinc supplementation. Our recent study showed that Cttnbp2 knockout results in impaired synaptic targeting of several zinc-binding proteins, including SHANK1, SHANK2 and SHANK3.18 Zinc supplementation can restore the synaptic distribution of SHANK proteins in adult male Cttnbp2−/− mice and improve their social interactions.18 Notably, SHANK2 and PSD-95, two critical synaptic zinc-binding proteins, were identified among the group of male-only downregulated DEPs. Therefore, we investigated the potential beneficial effect of zinc supplementation on juvenile male M120I mice.
For all of the experiments described in the previous sections, the mice were fed on a diet containing 84 ppm zinc. To test the effect of zinc, juvenile M120I mice were fed on diets with 150 ppm zinc (Fig. 6A).43 These mice behaved normally in an open field and in the elevated plus maze (Supplementary Fig. 9). Importantly, male M120I mice behaved comparably to their male WT littermates in the 3C test (Fig. 6B). In the RSI test, although male zinc-supplemented M120I mice still spent a shorter time interacting with strangers compared with WT littermates (Fig. 6B), the difference was smaller than for the groups fed on an 84 ppm zinc diet (Fig. 1D versus 6B). For female M120I mice, zinc supplementation did not appear to elicit any change in behaviour (Fig. 6B). Thus, increased zinc intake ameliorates the social interaction deficits of juvenile male M120I mice, albeit incompletely.
Next, we investigated if reducing zinc intake could induce social deficits in female M120I mice. Given that 30 ppm dietary zinc is sufficient for the entire life-cycle of WT mice,44 we investigated if reducing dietary zinc from 84 to 30 ppm was sufficient to induce social defects in female M120I mice and found that, unlike their male littermates, female M120I mice fed on 30 ppm zinc diets still behaved normally in both 3C and RSI tests (Fig. 6C).
Overall then, our results indicate that the social behaviours of male M120I mice are susceptible to levels of zinc intake. In contrast, female mice are resilient to the change in zinc levels.
Discussion
In this report, we suggest that a deficiency of Cttnbp2 (an autism-associated gene) influences the activity of ILA-related circuits in male mice but not in female mice, consequently impairing social behaviours in a male-biased manner. The differential synaptic proteomes of male and female mutant mice indicate the potential involvement of zinc and mTOR pathways in the sex-biased responses of Cttnbp2 mutant mice. Indeed, zinc and BCAA supplementations both improved the social behaviours of male Cttnbp2 mutant mice. In contrast, females proved resilient to Cttnbp2 deficiency given that their demands for BCAA and zinc are lower than those of male mice.
Note that, to maintain health, males generally require more zinc and protein than females (https://ods.od.nih.gov/HealthInformation/nutrientrecommendations.aspx). Adult men are recommended to take 11–16 mg zinc daily, whereas women require 7–10 mg zinc daily.45–47 Thus, men require at least 30–50% more zinc than women. In terms of protein, men and women need 56 g and 46 g of protein every day, respectively, so protein demand is ∼20% higher in men than women.48,49 A previous study reported that mutations of BCKDK (branched-chain keto acid dehydrogenase kinase) promote BCAA catabolism and reduce BCAA levels in sera and peripheral tissues, such as muscles, consequently reducing BCAA levels in the brain and contributing to autism and epilepsy.50 Thus, it seems likely that higher demand for zinc and protein in the peripheral tissues of males sensitize them to the deficits caused by Cttnbp2 deficiency. It will be intriguing to further explore the crosstalk between genetic variations and nutrients in mouse disease models and human patients.
In the human brain, sex-biased differences have been reported in brain volume and the percentages of grey matter and white matter.51 However, similar to the findings of a previous study using a mouse model,52 we did not detect a brain size difference between male and female mice. Instead, we found that Cttnbp2 M120I mutation results in differential activation across male and female mouse brains. The M120I mutation has an obvious inhibitory effect on neuronal activation in ILA-related circuits (encompassing the BLA, DG, BST and VTA) of male but not female mice. These areas are likely critical to the resilience of female mice to Cttnbp2 mutation, although the mechanism remains unclear.
The female protective effect and multiple-hit hypothesis are two well-known hypotheses postulated to explain the male bias in autism spectrum disorders.1,3,53,54 Our study reveals that genetic variation, sex and nutrients act in concert to influence the outcome of autism spectrum disorders, thus fitting the multiple-hit hypothesis. In addition, our results are consistent with the hypothesis of a female protective effect, which suggests a higher threshold for autism spectrum disorders in females.53–55 The identification of more genetic variations among female patients indeed supports this hypothesis.53–55 However, there is only a single genetic variation, i.e. Cttnbp2 M120I, in the model system we assessed here. Thus, multiple genetic variations are irrelevant to our system. Instead, our results suggest that sex-related nutrient demands elicit autism-linked phenotypes in the presence of Cttnbp2 mutation. Our study indicates that females hosting Cttnbp2 mutations are more protected than males because females have lower demands for BCAA and zinc. Accordingly, differing nutrient demands may be another factor modulating thresholds for the manifestation of autism spectrum disorders.
In addition to our current study, previous studies have also indicated roles for zinc and BCAA in autism spectrum disorders. Zinc directly regulates many autism-linked genes.56–60 For instance, zinc directly binds NMDAR, or enhances SRC kinase activity to phosphorylate NMDAR, thereby regulating NMDAR conductivity.59,61 Zinc also binds SHANK2 and SHANK3 to regulate their synaptic distribution and functions in the brain.43,62–65 Thus, zinc plays multiple roles in controlling synaptic activity. Consequently, zinc supplementation improves behavioural deficits of several autism mouse models, such as Shank3, Shank2 and Tbr1 mutant mice.43,64,66 Moreover, our previous study on Cttnbp2 knockout mice demonstrated that CTTNBP2 controls synaptic targeting of 18 zinc-binding or zinc-regulated proteins.18 Zinc supplementation ameliorates the synaptic targeting deficits of CTTNBP2-regulated proteins and improves the social behaviours of Cttnbp2 knockout mice.42 Here, we have further shown that M120I mutation of Cttnbp2 also results in reduced levels of several synaptic proteins. Zinc supplementation likely improves the defects of M120I mice by bolstering direct targets of CTTNBP2 or enhancing the general activity of neurons via other molecules and/or pathways.
BCAA metabolism has also been linked to autism spectrum disorders.50,67,68 In addition to the BCKDK gene mentioned above, solute carrier transporter 7a5 (SLC7A5), a BCAA transporter localized at the blood–brain barrier, was also found to be relevant to autism spectrum disorders.67 Maintenance of BCAA levels in the brain is critical to neuronal function because deletion of SLC7A5 reduces the levels of BCAA in the brain and results in autism-linked phenotypes. Moreover, a cohort study on 516 autistic children and 164 age-matched children further indicated a reduced level of serum BCAA in the children with autism.68 These studies strongly support a role for BCAA in autism spectrum disorders.
Our own studies have further revealed the function of BCAA in synaptic formation and responses. Previously, we have demonstrated that Cttnbp2 M120I mutation reduces the density and size of dendritic spines in mouse brains.19 Consistent with the reduced dendritic spine density, M120I mutation also decreased the frequency of miniature excitatory postsynaptic currents.19 Here, we found that Cttnbp2 M120I mice derive a benefit from BCAA supplementation. Dendritic spine density, neuronal activation and social behaviours of Cttnbp2 M120I mice are all improved by BCAA supplementation in the diet. In addition to Cttnbp2 M120I mice, our previous study showed that supplementation of leucine, the most potent BCAA for activating the mTOR pathway, induces protein synthesis, increases dendritic spine density, and improves the social behaviours and memory of Nf1+/− and Vcp+/R95G mice.28,42,69 The molecular functions of the proteins encoded by Nf1, Vcp and Cttnbp2 vary considerably. Neurofibromin, the protein product of Nf1, controls dendritic spine density via PKA activation,70 and it interacts with VCP to control endoplasmic reticulum formation and protein synthesis.28,69,71 VCP functions as an AAA ATPase to control many cellular processes, with endoplasmic reticulum formation and consequent protein synthesis being the most critical downstream targets of VCP to regulate dendritic spine formation.28,42,69 Since neurofibromin and VCP act in the same pathway, it is reasonable that neurofibromin and VCP deficits can be ameliorated by the same treatment, i.e. leucine supplementation. In contrast, as a cytoskeleton-associated protein, there is no evidence supporting a direct role for CTTNBP2 in controlling protein synthesis. However, fewer and/or smaller dendritic spines may limit the efficiency of protein synthesis controlled by synaptic activation. An increase in BCAA intake activates the mTOR pathway to induce protein synthesis, independently of synaptic stimulation.39 Accordingly, we expect that many other autism-linked mutations that result in fewer and/or smaller dendritic spines may share the same property of their impacts being counteracted by BCAA supplementation. Thus, it will be important to apply BCAA supplementation to other autism-linked mouse models to test our hypothesis.
Supplementary Material
Acknowledgements
We thank the Transgenic Core, Imaging Core and Animal Facility of the Institute of Molecular Biology, Academia Sinica, for excellent technical assistance. The MS data analysed using an Orbitrap Fusion Lumos mass spectrometer were acquired at the Academia Sinica Common Mass Spectrometry Facilities for Proteomics and Protein Modification Analysis located at the Institute of Biological Chemistry, Academia Sinica, supported by Academia Sinica Core Facility and Innovative Instrument Project (AS-CFII-108-107). We thank Dr Shu-Yu Lin and Mr Ming-Chieh Tsai at the Institute of Biological Chemistry, Academia Sinica, for their excellent technical support regarding MS. For in-gel digestion and sample preparation for proteomic analysis, we thank Cheng-His Lin Hsieh from the Mass Spectrometry Facility of the Genomics Core at the Institute of Molecular Biology for technical assistance. Dr John O’Brien conducted English editing and members of Y.-P.H.’s laboratory relabelled samples for blind experiments.
Contributor Information
Tzu-Li Yen, Molecular and Cell Biology, Taiwan International Graduate Program, Institute of Molecular Biology, Academia Sinica and Graduate Institute of Life Sciences, National Defense Medical Center, Taipei 11529, Taiwan, ROC; Institute of Molecular Biology, Academia Sinica, Taipei 11529, Taiwan, ROC.
Tzyy-Nan Huang, Institute of Molecular Biology, Academia Sinica, Taipei 11529, Taiwan, ROC.
Ming-Hui Lin, Institute of Molecular Biology, Academia Sinica, Taipei 11529, Taiwan, ROC.
Tsan-Ting Hsu, Institute of Molecular Biology, Academia Sinica, Taipei 11529, Taiwan, ROC.
Ming-Hsuan Lu, Institute of Molecular Biology, Academia Sinica, Taipei 11529, Taiwan, ROC.
Pu-Yun Shih, Institute of Molecular Biology, Academia Sinica, Taipei 11529, Taiwan, ROC.
Jacob Ellegood, Mouse Imaging Centre, Hospital for Sick Children, Toronto, Ontario M5T 3H7, Canada; Department of Medical Biophysics, The University of Toronto, Toronto, Ontario M5G 1L7, Canada.
Jason Lerch, Mouse Imaging Centre, Hospital for Sick Children, Toronto, Ontario M5T 3H7, Canada; Department of Medical Biophysics, The University of Toronto, Toronto, Ontario M5G 1L7, Canada; Wellcome Centre for Integrative Neuroimaging, The University of Oxford, Oxford OX3 9DU, UK.
Yi-Ping Hsueh, Molecular and Cell Biology, Taiwan International Graduate Program, Institute of Molecular Biology, Academia Sinica and Graduate Institute of Life Sciences, National Defense Medical Center, Taipei 11529, Taiwan, ROC; Institute of Molecular Biology, Academia Sinica, Taipei 11529, Taiwan, ROC.
Funding
This work was supported by grants from Academia Sinica (AS-TP-110-L10 and AS-IA-111-L01 to Y.-P.H.).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Ferri SL, Abel T, Brodkin ES. Sex differences in autism spectrum disorder: A review. Curr Psychiatry Rep. 2018;20:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gockley J, Willsey AJ, Dong S, Dougherty JD, Constantino JN, Sanders SJ. The female protective effect in autism spectrum disorder is not mediated by a single genetic locus. Mol Autism. 2015;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mottron L, Duret P, Mueller S, et al. Sex differences in brain plasticity: A new hypothesis for sex ratio bias in autism. Mol Autism. 2015;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacquemont S, Coe BP, Hersch M, et al. A higher mutational burden in females supports a “female protective model” in neurodevelopmental disorders. Am J Hum Genet. 2014;94:415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Werling DM. The role of sex-differential biology in risk for autism spectrum disorder. Biol Sex Differ. 2016;7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fester L, Rune GM. Sexual neurosteroids and synaptic plasticity in the hippocampus. Brain Res. 2015;1621:162–169. [DOI] [PubMed] [Google Scholar]
- 8. Schaafsma SM, Pfaff DW. Etiologies underlying sex differences in Autism Spectrum Disorders. Front Neuroendocrinol. 2014;35:255–271. [DOI] [PubMed] [Google Scholar]
- 9. Sanders SJ, Murtha MT, Gupta AR, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iossifov I, O'Roak BJ, Sanders SJ, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robinson EB, St Pourcain B, Anttila V, et al. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet. 2016;48:552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Rubeis S, He X, Goldberg AP, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanders SJ, He X, Willsey AJ, et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron. 2015;87:1215–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y, Li N, Li C, et al. Genetic evidence of gender difference in autism spectrum disorder supports the female-protective effect. Transl Psychiatry. 2020;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen YK, Hsueh YP. Cortactin-binding protein 2 modulates the mobility of cortactin and regulates dendritic spine formation and maintenance. J Neurosci. 2012;32:1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen YK, Chen CY, Hu HT, Hsueh YP. CTTNBP2, But not CTTNBP2NL, regulates dendritic spinogenesis and synaptic distribution of the striatin-PP2A complex. Mol Biol Cell. 2012;23:4383–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shih P-Y, Lee S-P, Chen Y-K, Hsueh Y-P. Cortactin-binding protein 2 increases microtubule stability and regulates dendritic arborization. J Cell Sci. 2014;127(Pt 16):3521–3534. [DOI] [PubMed] [Google Scholar]
- 18. Shih PY, Hsieh BY, Lin MH, et al. CTTNBP2 controls synaptic expression of zinc-related autism-associated proteins and regulates synapse formation and autism-like behaviors. Cell Rep. 2020;31:107700. [DOI] [PubMed] [Google Scholar]
- 19. Shih PY, Hsieh BY, Tsai CY, Lo CA, Chen BE, Hsueh YP. Autism-linked mutations of CTTNBP2 reduce social interaction and impair dendritic spine formation via diverse mechanisms. Acta Neuropathol Commun. 2020;8:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shih PY, Fang YL, Shankar S, et al. Phase separation and zinc-induced transition modulate synaptic distribution and association of autism-linked CTTNBP2 and SHANK3. Nat Commun. 2022;13:2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liou ST, Wang C. Small glutamine-rich tetratricopeptide repeat-containing protein is composed of three structural units with distinct functions. Arch Biochem Biophys. 2005;435:253–263. [DOI] [PubMed] [Google Scholar]
- 22. Nawaratne V, Leach K, Suratman N, et al. New insights into the function of M4 muscarinic acetylcholine receptors gained using a novel allosteric modulator and a DREADD (designer receptor exclusively activated by a designer drug). Mol Pharmacol. 2008;74:1119–1131. [DOI] [PubMed] [Google Scholar]
- 23. Hung YF, Hsueh YP. TLR7 And IL-6 differentially regulate the effects of rotarod exercise on the transcriptomic profile and neurogenesis to influence anxiety and memory. iScience. 2021;24:102384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hung YF, Chen CY, Li WC, Wang TF, Hsueh YP. Tlr7 deletion alters expression profiles of genes related to neural function and regulates mouse behaviors and contextual memory. Brain Behav Immun. 2018;72:101–113. [DOI] [PubMed] [Google Scholar]
- 25. Huang TN, Chuang HC, Chou WH, et al. Tbr1 haploinsufficiency impairs amygdalar axonal projections and results in cognitive abnormality. Nat Neurosci. 2014;17:240–247. [DOI] [PubMed] [Google Scholar]
- 26. Huang TN, Hsu TT, Lin MH, et al. Interhemispheric connectivity potentiates the basolateral amygdalae and regulates social interaction and memory. Cell Rep. 2019;29:34–48.e4. [DOI] [PubMed] [Google Scholar]
- 27. Hsu T-T, Huang T-N, Hsueh Y-P. Anterior commissure regulates neuronal activity of amygdalae and influences locomotor activity, social interaction and fear memory in mice. Front Mol Neurosci. 2020;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shih YT, Huang TN, Hu HT, Yen TL, Hsueh YP. Vcp overexpression and leucine supplementation increase protein synthesis and improve fear memory and social interaction of Nf1 mutant mice. Cell Rep. 2020;31:107835. [DOI] [PubMed] [Google Scholar]
- 29. Huang TN, Yen TL, Qiu LR, Chuang HC, Lerch JP, Hsueh YP. Haploinsufficiency of autism causative gene Tbr1 impairs olfactory discrimination and neuronal activation of the olfactory system in mice. Mol Autism. 2019;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ko J. Neuroanatomical substrates of rodent social behavior: The medial prefrontal cortex and its projection patterns. Front Neural Circuits. 2017;11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen P, Hong W. Neural circuit mechanisms of social behavior. Neuron. 2018;98:16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mukherjee A, Caroni P. Infralimbic cortex is required for learning alternatives to prelimbic promoted associations through reciprocal connectivity. Nat Commun. 2018;9:2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hiser J, Koenigs M. The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol Psychiatry. 2018;83:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang WC, Zucca A, Levy J, Page DT. Social behavior is modulated by valence-encoding mPFC-amygdala sub-circuitry. Cell Rep. 2020;32:107899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care. 2008;11:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsun ZY, Bar-Peled L, Chantranupong L, et al. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol Cell. 2013;52:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakashima A, Tanimura-Ito K, Oshiro N, et al. A positive role of mammalian Tip41-like protein, TIPRL, in the amino-acid dependent mTORC1-signaling pathway through interaction with PP2A. FEBS Lett. 2013;587:2924–2929. [DOI] [PubMed] [Google Scholar]
- 39. Lu MH, Hsueh YP. Protein synthesis as a modifiable target for autism-related dendritic spine pathophysiologies. FEBS J. 2022;289:2282–2300. [DOI] [PubMed] [Google Scholar]
- 40. Lo LH, Lai KO. Dysregulation of protein synthesis and dendritic spine morphogenesis in ASD: Studies in human pluripotent stem cells. Mol Autism. 2020;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakahata Y, Yasuda R. Plasticity of spine structure: Local signaling, translation and cytoskeletal reorganization. Front Synaptic Neurosci. 2018;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang TN, Shih YT, Lin SC, Hsueh YP. Social behaviors and contextual memory of Vcp mutant mice are sensitive to nutrition and can be ameliorated by amino acid supplementation. iScience. 2021;24:101949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fourie C, Vyas Y, Lee K, Jung Y, Garner CC, Montgomery JM. Dietary zinc supplementation prevents autism related behaviors and striatal synaptic dysfunction in Shank3 exon 13-16 mutant mice. Front Cell Neurosci. 2018;12:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. National Research Council (US) Subcommittee on Laboratory Animal Nutrition . Nutrient requirements of the mouse. In: Nutrient requirements of laboratory animals. 4th rev ed. National Academies Press (US); 1995. https://www.ncbi.nlm.nih.gov/books/NBK231918/ [PubMed] [Google Scholar]
- 45. National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances . Recommended dietary allowances. National Academies Press; 1989. [PubMed] [Google Scholar]
- 46. Institute of Medicine (US) Panel on Micronutrients . Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academies Press (US); 2001. [PubMed] [Google Scholar]
- 47. Haase H, Ellinger S, Linseisen J, Neuhäuser-Berthold M, Richter M. Revised D-A-CH-reference values for the intake of zinc. J Trace Elem Med Biol. 2020;61:126536. [DOI] [PubMed] [Google Scholar]
- 48. Ryan-Harshman M, Aldoori W. New dietary reference intakes for macronutrients and fibre. Can Fam Physician. 2006;52:177–179. [PMC free article] [PubMed] [Google Scholar]
- 49. Gardner CD, Hartle JC, Garrett RD, Offringa LC, Wasserman AS. Maximizing the intersection of human health and the health of the environment with regard to the amount and type of protein produced and consumed in the United States. Nutr Rev. 2019;77:197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Novarino G, El-Fishawy P, Kayserili H, et al. Mutations in BCKD-kinase lead to a potentially treatable form of autism with epilepsy. Science. 2012;338:394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jung H, Park H, Choi Y, et al. Sexually dimorphic behavior, neuronal activity, and gene expression in Chd8-mutant mice. Nat Neurosci. 2018;21:1218–1228. [DOI] [PubMed] [Google Scholar]
- 53. Robinson EB, Lichtenstein P, Anckarsater H, Happe F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci USA. 2013;110:5258–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dougherty JD, Marrus N, Maloney SE, et al. Can the “female protective effect” liability threshold model explain sex differences in autism spectrum disorder? Neuron. 2022;110:3243–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Levy D, Ronemus M, Yamrom B, et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–897. [DOI] [PubMed] [Google Scholar]
- 56. Curtin P, Austin C, Curtin A, et al. Dynamical features in fetal and postnatal zinc-copper metabolic cycles predict the emergence of autism spectrum disorder. Sci Adv. 2018;4:eaat1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yasuda H, Yoshida K, Yasuda Y, Tsutsui T. Infantile zinc deficiency: Association with autism spectrum disorders. Sci Rep. 2011;1:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bolte S, Girdler S, Marschik PB. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci. 2019;76:1275–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee EJ, Lee H, Huang TN, et al. Trans-synaptic zinc mobilization improves social interaction in two mouse models of autism through NMDAR activation. Nat Commun. 2015;6:7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pfaender S, Sauer AK, Hagmeyer S, et al. Zinc deficiency and low enterocyte zinc transporter expression in human patients with autism related mutations in SHANK3. Sci Rep. 2017;7:45190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jalali-Yazdi F, Chowdhury S, Yoshioka C, Gouaux E. Mechanisms for Zinc and Proton Inhibition of the GluN1/GluN2A NMDA receptor. Cell. 2018;175:1520–1532.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Grabrucker AM. A role for synaptic zinc in ProSAP/Shank PSD scaffold malformation in autism spectrum disorders. Dev Neurobiol. 2014;74:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Grabrucker AM, Knight MJ, Proepper C, et al. Concerted action of zinc and ProSAP/Shank in synaptogenesis and synapse maturation. EMBO J. 2011;30:569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grabrucker S, Jannetti L, Eckert M, et al. Zinc deficiency dysregulates the synaptic ProSAP/Shank scaffold and might contribute to autism spectrum disorders. Brain. 2014;137(Pt 1):137–152. [DOI] [PubMed] [Google Scholar]
- 65. Hagmeyer S, Sauer AK, Grabrucker AM. Prospects of zinc supplementation in autism spectrum disorders and shankopathies such as phelan McDermid syndrome. Front Synaptic Neurosci. 2018;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee K, Jung Y, Vyas Y, et al. Dietary zinc supplementation rescues fear-based learning and synaptic function in the Tbr1(+/-) mouse model of autism spectrum disorders. Mol Autism. 2022;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tarlungeanu DC, Deliu E, Dotter CP, et al. Impaired amino acid transport at the blood brain barrier is a cause of autism spectrum disorder. Cell. 2016;167:1481–1494.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Smith AM, King JJ, West PR, et al. Amino acid dysregulation metabotypes: Potential biomarkers for diagnosis and individualized treatment for subtypes of autism spectrum disorder. Biol Psychiatry. 2019;85:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shih YT, Hsueh YP. VCP And ATL1 regulate endoplasmic reticulum and protein synthesis for dendritic spine formation. Nat Commun. 2016;7:11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lin YL, Lei YT, Hong CJ, Hsueh YP. Syndecan-2 induces filopodia and dendritic spine formation via the neurofibromin-PKA-Ena/VASP pathway. J Cell Biol. 2007;177:829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang HF, Shih YT, Chen CY, Chao HW, Lee MJ, Hsueh YP. Valosin-containing protein and neurofibromin interact to regulate dendritic spine density. J Clin Invest. 2011;121:4820–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.