Abstract
Background
The purpose of this study was to compare the efficacy of electroacupuncture (EA), diclofenac and their combination in symptomatic treatment of osteoarthritis (OA) of the knee.
Methods
This study was a randomized, single-blind, placebo controlled trial. The 193 out-patients with OA of the knee were randomized into four groups: placebo, diclofenac, EA and combined (diclofenac plus EA). Paracetamol tablets were prescribed as a rescue analgesic during the study. The patients were evaluated after a run-in period of one week (week 0) and again at the end of the study (week 4). The clinical assessments included the amount of paracetamol taken/week, visual analog scale (VAS), Western Ontario and McMaster Universities (WOMAC) OA Index, Lequesne's functional index, 50 feet-walk time, and the orthopedist's and patient's opinion of change.
Results
One hundred and eighty six patients completed the study. The improvement of symptoms (reduction in mean changes) in most outcome parameters was greatest in the EA group. The proportions of responders and patients with an overall opinion of "much better" were also greatest in the EA group. The improvement in VAS was significantly different between the EA and placebo group as well as the EA and diclofenac group. The improvement in Lequesne's functional index also differed significantly between the EA and placebo group. In addition, there was a significant improvement in WOMAC pain index between the combined and placebo group.
Conclusion
EA is significantly more effective than placebo and diclofenac in the symptomatic treatment of OA of the knee in some circumstances. However, the combination of EA and diclofenac treatment was no more effective than EA treatment alone.
Background
Osteoarthritis (OA) is the most common joint disease. Articular cartilage in OA has shown to lose its mechanical resistance, elasticity and smoothness, and is consequently worn out by the movements of the joint. This leads to reactive bone remodeling, forming osteophytes, microfractures, subchondral eburnation and pseudocysts, and exposure of the articular end of the bone [1]. The consequent roughness of the articular cartilage surfaces elicits secondary inflammatory reactions of the synovial membrane and bone. Unlike rheumatoid arthritis and other inflammatory joint diseases, the inflammatory component of OA is relatively mild [2,3]. Clinical manifestations of OA of the knee are joint pain, stiffness in the morning or after rest, pain at night, limited joint motion and/or joint deformity. Joint pain in OA may originate from not only synovitis, but also stretching of the joint capsule or ligaments, periosteal irritation due to osteophyte formation, trabecular microfractures, intraosseous hypertension, or muscle spasm [4-7].
There are many treatment modalities for OA of the knee including nonpharmacologic (e.g. patient education, weight control, physical and occupational therapy, and aerobic exercise programs) and pharmacologic therapy (e.g. intraarticular steroid injections, paracetamol, topical analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs) and opioid analgesics) [8]. Although NSAIDs are the most widely prescribed drugs to reduce joint pain and stiffness, the inflammatory component of OA is usually minimal, therefore, the need for the anti-inflammatory effect of NSAIDs in this condition is controversial [2,9,10]. Moreover, inhibition of prostaglandin biosynthesis is directly related to many common and occasionally severe side effects including gastrointestinal bleeding, hypertension, congestive heart failure, hyperkalemia, and renal insufficiency [11-14]. These disadvantages call for an evaluation of the risks and benefits of the therapy in comparison with a less toxic one for OA.
Since the efficacy of NSAIDs in symptomatic treatment of OA of the knee depends on the analgesic rather than anti-inflammatory effect, paracetamol (analgesic drug) has been recently recommended as the first-line oral drug in the management of OA of the knee [8]. However, the long term use of paracetamol probably leads to hepatic and renal impairment [15,16]. Thus, less toxic pain managing procedures, e.g. electroacupuncture (EA), may be considered as an alternative treatment of this disease. Even though EA is safe and effective in combating pain [17,18], its role in OA of the knee is still controversial and comparative studies between EA and NSAIDs in this disease are rare [19]. Therefore, the aim of this study was to compare the efficacy of EA, diclofenac and their combination in short-term, symptomatic treatment of OA of the knee.
Materials and methods
Research design
This study was a randomized, single-blind, placebo controlled trial. The treatment procedures consisted of placebo tablet plus placebo EA (placebo group), diclofenac tablet plus placebo EA (diclofenac group), placebo tablet plus true EA (EA group) and diclofenac tablet plus true EA (combined group). This study was approved by the Medical Ethics Committee of the Faculty of Medicine, Chiang Mai University and was in compliance with the Helsinki Declaration.
Subjects
Two hundred out-patients of either sex, aged over 40 years, and who had been suffering from unilateral or bilateral OA of the knee according to the criteria of the American College of Rheumatology [8] for more than 3 months duration, were recruited. Lequesne's functional index, which was evaluated at the screening visit, had to be at least 6 points. Subjects had to be able to walk and give both verbal and written information regarding the study. Signed informed consent was obtained prior to entry. Exclusion criteria included an underlying inflammatory arthropathy, expectation of surgery in the future, recent injury in the area affected by OA of the knee, intraarticular corticosteroid injections or EA within the last 3 months, hypersensitivity to NSAIDs or paracetamol, abnormal liver or kidney function tests, evidence of leukopenia and coagulopathies screened by clinical laboratory, concomitantly receiving anticoagulants, history of peptic ulceration, anemia, uncontrolled hypertension, congestive heart failure, hyperkalemia, pregnancy, lactation and malignant tumors.
Treatment procedures
1. Drug administration
During a run-in period of one week, the patients refrained from using any NSAIDs or analgesics except for a "rescue analgesic" (2 tablets of 500 mg paracetamol orally as needed, up to 4 times daily). The patients who had persistent pain and a Lequesne's functional index of at least 6 points at the end of the run-in period were randomized into the four groups mentioned above. Diclofenac sodium, 25 mg film-coated tablets were a gift from Novartis (Thailand) Limited. Placebo and diclofenac were prepared in identical appearance. Either the placebo or diclofenac was prescribed at 1 tablet, 3 times a day immediately after each meal for 4 weeks. In addition, 2 tablets of 500 mg paracetamol were still prescribed as a "rescue analgesic" during this study.
2. True and placebo EA
The true EA treatment was standardized throughout the study. It was performed by the physician acupuncturist who received acupuncture training in the People's Republic of China. Four fine stainless steel needles were inserted into acupuncture points around the affected knee [20] as presented in Table 1 and Figure 1. All needles were used in order to conduct an electrical current through the points, and were inserted superficially (not more than 0.5 inch approximately in depth). Thus, an elicitation of needle sensation (so-called De Qi) during the insertion of the needles was not intended. The first pair of electrodes was connected to the Dubi and nearest adjacent point (medial Xiyan) and the second pair to the trigger point and Ququan. The electrical stimulation was applied slowly and simultaneously to each pair of needles until it reached the maximum toleration level of the patient. Biphasic pulses were used for the electrical stimulation at a frequency of 2 Hz, and it was administered for 20 minutes in each treatment. The patients were treated 3 times a week (Monday, Wednesday, and Friday) for 4 weeks (12 times).
Table 1.
The selected acupuncture points used in this study.
| Acupuncture points | Location | Needling manipulation |
| Dubi (ST-35)1 | In the depression of the lateral part of the patella ligament, when the knee is bent. | Slightly towards the medial side*. |
| Medial Xiyan (Extra)1 | In the depression of the medial part of the patella ligament, when the knee is bent. | Slightly towards the lateral side**. |
| Trigger point2 | At the level of the joint line, midpoint between the medial Xiyan and Ququan. | Straight insertion*. |
| Ququan (Liv-8)2 | At the medial end of the knee crease, in front of the semi-membranous muscle behind the lower end of the femur. | Straight insertion**. |
1First pair of elctrodes 2Second pair of electrodes *Stimulated with positive polarity at visit #1, 3, 5, 7,9,11 and negative polarity at visit #2, 4, 6, 8, 10, 12. **Stimulated with negative polarity at visit #1, 3, 5, 7,9,11 and positive polarity at visit #2, 4, 6, 8, 10, 12.
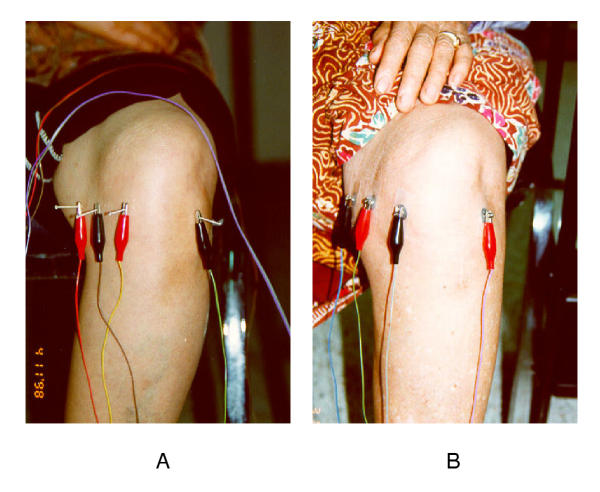
Figure 1.
The selected acupuncture points around the knee, demonstrated in sitting position, during treatment with true (A) and placebo EA (B).
The placebo EA was performed by attaching patch electrodes to the selected acupuncture points. Each electrode was connected to the sound producing dummy mode of the same apparatus, as in the true EA treatment. The duration and frequency of treatment were the same as those in the true EA treatment. Both true and placebo EA were performed by the same physician. Thus, the physician acupuncturist was the only person in the research team who knew which patients received the true or placebo EA.
Compliance with treatment was assessed by counting the number of unused tablets (diclofenac or placebo) and the number of times acupuncture treatment was received. During the study period, all additional therapies (e.g. oral or topical NSAIDs, intraarticular corticosteroid injection, other analgesics, chondro-protective agents, surgical procedures on the knee joint etc.) were not allowed. However, all other treatment for concomitant disorders that did not interfere with the study could be continued, but it had to be documented.
Assessments
Clinical assessments were evaluated for base-line data at the end of the run-in period (week 0) and again at the end of the study (week 4). These assessments included the amount of paracetamol tablets taken/week, 50 feet-walk time, a patient's global pain as 100 mm visual analog scale (VAS) over the previous 3 days, the Western Ontario and McMaster Universities OA Index (WOMAC: score ranging from 0–96) [21], and Lequesne's functional index (score ranging from 0–24) [22]. At the end of this study, the orthopedist's and patient's opinion of change (much better, better, same, worse, much worse) were evaluated. The patients were considered to be responders if they met the following criteria. Firstly, the number of paracetamol tablets taken in week 4 was less than that at week 0, or less than 14 tablets/week. Secondly, at least 4 of the following 5 outcome parameters showed the following improvement: VAS, WOMAC, Lequesne's functional index decreased by at least 50%, and the orthopedist's or patient's overall opinion of change was better or much better. Clinical assessments in each patient were evaluated by the same physician who was blinded to the treatment. Complete physical examination and non-directive questioning for adverse events were also performed weekly for 4 weeks in order to acquire a safety assessment.
Statistical analysis
One-way analysis of variance (ANOVA) was used to determine whether the four treatment groups differed in mean values of change from a base-line in paracetamol consumption, 50 feet-walk time, VAS, WOMAC and Lequesne's functional index. If there was any statistical significance between any of the four groups, the Scheffe method was used to demonstrate statistical significance between each of two groups. Differences among the treatment groups in the overall opinion of change, and number of patients considered to be responders, were evaluated by chi-square or Fisher's exact test.
Results
Of the 200 subjects considered eligible for the study, 5 dropped out and 2 were withdrawn during the run-in period due to hypersensitivity to paracetamol and spontaneous pain relief. The remaining 193 subjects constituted the study population and were randomized into four parallel groups, which were not significantly different in base-line characteristics (Table 2) and radiographic findings of affected knees (Table 3). Only 1 patient in each placebo, EA and combined group as well as 2 patients in the diclofenac group received acupuncture treatment prior to this study.
Table 2.
Base-line characteristics of patients evaluated at the end of the run-in period.
| Treatment groups | |||||
| Characteristic | Placebo | Diclofenac | EA | Combined | p-value ** |
| n (M:F) | 47 (12:35) | 49 (11:38) | 48 (10:38) | 49 (10:39) | 0.93c |
| Age (yr)* | 62.70 (7.22) | 62.14 (7.53) | 65.10 (3.40) | 61.84 (8.95) | 0.16a |
| Body weight (kg)* | 60.65 (10.24) | 57.65 (10.64) | 59.89 (9.74) | 59.92 (9.66) | 0.49a |
| Height (cm)* | 153.94 (6.45) | 151.94 (10.71) | 152.19 (5.89) | 153.32 (6.73) | 0.54a |
| Duration of OA (yr)* | 4.98 (3.32) | 3.94 (2.83) | 6.09 (4.96) | 4.53 (3.86) | 0.05a |
| Localization of OA | 0.40c | ||||
| Right/Left knee | 2/3 | 2/6 | 1/4 | 3/5 | |
| Both knees | 42 | 41 | 43 | 41 | |
| Number of paracetamol tablets taken per week* | 22.06 (13.75) | 18.94 (14.68) | 21.40 (14.97) | 19.04 (14.87) | 0.63a |
| 50 ft-walk time (sec)* | 22.04 (4.81) | 22.36 (6.00) | 24.54 (8.14) | 22.77 (5.13) | 0.21a |
| VAS* | 63.49 (22.36) | 64.79 (23.41) | 66.87 (22.34) | 57.63 (21.21) | 0.21a |
| WOMAC* | |||||
| Pain index | 10.19 (4.20) | 11.02 (4.15) | 10.25 (3.86) | 10.50 (4.18) | 0.75a |
| Stiffness index | 4.51 (1.71) | 4.08 (1.95) | 4.35 (2.10) | 4.27 (1.57) | 0.72a |
| Disability index | 37.04 (12.00) | 35.65 (12.89) | 38.00 (13.18) | 37.94 (13.02) | 0.79a |
| Total score | 51.75 (15.96) | 50.76 (17.98) | 52.60 (18.13) | 52.71 (17.65) | 0.94a |
| Lequesne's functional index* | 13.78 (3.78) | 13.85 (3.22) | 14.14 (2.98) | 13.73 (2.92) | 0.93a |
*Data represent mean (SD) **Statistic analysis: a = one-way ANOVA, c = chi-square test.
Table 3.
The radiographic findings at entry into the study*.
| Treatment groups | |||||
| Radiographic findings | Placebo (89 knees) | Diclofen ac (90 knees) | EA (91 knees) | Combined (90 knees) | p-value |
| Kellgren and Lawrence X-ray grade [32] | |||||
| Grade 1 | 4 | 1 | 3 | 5 | 0.40f |
| Grade 2 | 4 | 6 | 5 | 7 | 0.84f |
| Grade 3 | 19 | 15 | 25 | 23 | 0.31c |
| Grade 4 | 62 | 68 | 58 | 55 | 0.16c |
| Knee compartment with most severe change of OA | |||||
| Medial tibiofemoral | 63 | 77 | 69 | 64 | 0.73c |
| Lateral tibiofemoral | 12 | 5 | 13 | 11 | 0.24c |
| Patellofemoral | 14 | 8 | 9 | 15 | 0.28c |
Data represent number of patients. Statistic analysis: c = chi-square test, f = Fisher's exact test.
Of the 193 study patients, 186 (96.37%) completed the study. The remaining 7 patients were withdrawn from the trial due to flare of pain with joint swelling (2 in the placebo and 1 in the EA group), severe GI side effects (3 in the combined group), and flare of pain from an accidental fall not related to treatment (1 in the EA group). Since there were few patients withdrawn from the trial, the results were, therefore, not substantially affected, whether the analysis was performed by an intention to treat analysis or an analysis on available completers. Thus, the following data showed the findings in only 186 available completers.
During 4 weeks of treatment, change in body weight compared to the base-line values did not significantly differ among the four groups (one-way ANOVA calculated weekly, data not shown). The rates of compliance with medications (placebo or diclofenac) and acupuncture (placebo or EA) in each group were more than 90%, and comparable among the four groups.
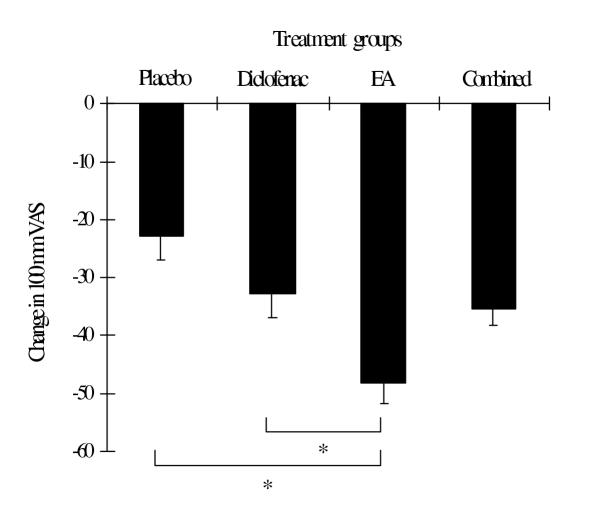
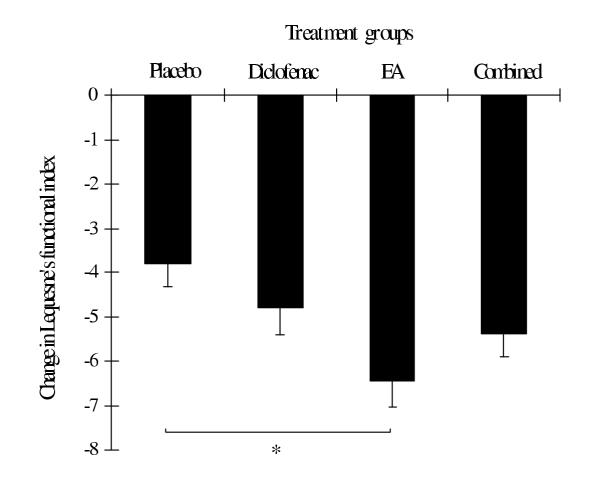
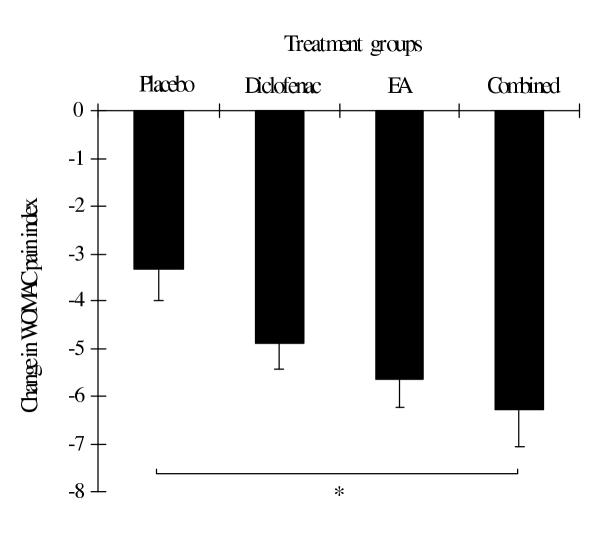
At the end of the study, the improvement of symptoms, which were determined by the reduction in mean changes in most outcome parameters (except WOMAC pain index and WOMAC total score), was greatest in the EA group (Table 4). The mean changes in VAS were significantly different between the EA and placebo group as well as the EA and diclofenac group (Table 4 and Figure 2). The mean changes in the Lequesne's functional index also differed significantly between the EA and placebo group (Table 4, Figure 3). In addition, there were significant differences in mean changes in the subscale of standardized WOMAC (pain index) between the combined and placebo group (Table 4 and Figure 4). The proportion of patients with the orthopedist's and patient's overall opinion of "much better" was greatest in the EA group, followed by the diclofenac, combined and placebo group, respectively. In addition, the proportion of responders was also greatest in the EA group, followed by the combined, diclofenac and placebo group, respectively. However, statistical differences among the four groups were found only in the orthopedist's overall opinion and a number of responders (Table 5). There were no differences between the four groups in the remaining outcome parameters.
Table 4.
Change in outcome parameters after 4 weeks of treatment*.
| Treatment groups | |||||
| Parameter | Placebo (n = 45) | Diclofenac (n = 49) | EA (n = 46) | Combined (n = 46) | p-valuea |
| Number of paracetamol taken (tablets taken per week) | -5.16 (2.33) | -4.43 (1.90) | -7.89 (2.09) | -5.13 (2.06) | NS |
| 50 feet-walk time (sec) | -2.70 (0.52) | -3.52 (0.46) | -4.41 (0.70) | -4.13 (0.54) | NS |
| VAS | -22.86 (4.02) | -32.99 (3.94) | -48.24 (3.59) | -35.59 (2.74) | <0.05† |
| WOMAC | |||||
| Pain index | -3.31 (0.68) | -4.90 (0.53) | -5.65 (0.59) | -6.28 (0.77) | <0.05†† |
| Stiffness index | -1.47 (0.31) | -1.55 (0.27) | -2.24 (0.31) | -2.02 (0.28) | NS |
| Disability index | -12.33 (1.88) | -14.39 (1.77) | -19.17 (2.05) | -18.98 (1.92) | NS |
| Total score | -17.11 (2.73) | -20.84 (2.43) | -27.07 (2.78) | -27.28 (2.79) | NS |
| Lequesne's functional index | -3.82 (0.51) | -4.80 (0.61) | -6.44 (0.59) | -5.39 (0.52) | <0.05††† |
*Compared values of week 4 versus those of week 0 (run-in peroid), data represent mean (SEM). Statistic analysis: a = one-way ANOVA and Scheffe method. NS= no statistical significance. † = EA vs placebo and diclofenac group. †† = combined vs placebo group. ††† = EA vs placebo group.
Figure 2.
Change in 100 mm VAS in each treatment group evaluated at the end of week 4. Values are mean ± SEM. * = p < 0.05 between groups (one-way ANOVA and Scheffe method).
Figure 3.
Change in Lequesne's functional index in each treatment group evaluated at the end of week 4. Values are mean ± SEM. * = p < 0.05 between groups (one-way ANOVA and Scheffe method).
Figure 4.
Change in WOMAC pain index in each treatment group evaluated at the end of week 4. Values are mean ± SEM. * = p < 0.05 between groups (one-way ANOVA and Scheffe method).
Table 5.
Overall opinions of change and number of responders evaluated at week 4.
| Treatment groups | |||||
| Parameter | Placebo (n = 45) | Diclofenac (n = 49) | EA (n = 46) | Combined (n = 46) | p-value |
| Orthopedist's overall opinion* | 0.01† | ||||
| Much better | 6 | 18 | 21 | 16 | |
| Better | 22 | 21 | 20 | 23 | |
| Same | 16 | 10 | 5 | 7 | |
| Worse | 1 | 0 | 0 | 0 | |
| Patient's overall opinion* | 0.09† | ||||
| Much better | 19 | 25 | 31 | 22 | |
| Better | 16 | 17 | 11 | 23 | |
| Same | 9 | 7 | 4 | 1 | |
| Worse | 1 | 0 | 0 | 0 | |
| Number of responders | 13 | 18 | 27 | 24 | 0.02†† |
*Data represent number of patients, † = chi-square test evaluated on the proportions of patients with the opinion of "much better", †† = chi-square test.
The percentage of patients who experienced adverse effects (e.g. gastrointestinal and central nervous system symptoms, rash, edema, and hypertension) during this study did not differ between the four groups (data not shown), whereas, local contusions around the knee were common in the EA and combined group (approximately 45%). However, the contusions usually disappeared within 5–7 days.
When the responders in each group were followed up for 2 months, the proportion of remaining responders was not significantly different between the four groups (Table 6).
Table 6.
Number of responders considered at the end of the study (week 4) and at 1 and 2 month(s) after treatment*.
| 1 month after treatment | 2 months after treatment | ||
| Treatment groups | responders at week 4 | remaining responders/ evaluated responders† | remaining responders/ evaluated responders† |
| Placebo | 13 | 11/12 | 9/12 |
| Diclofenac | 18 | 10/16 | 7/15 |
| EA | 27 | 21/25 | 19/24 |
| Combined | 24 | 14/24 | 14/24 |
| p value** | 0.12 | 0.19 | |
* Only the responders at the end of the study were followed up for 2 months. †Some patients were unable to be evaluated due to loss of follow up or use of NSAIDs for other purposes during the follow-up period. ** Fisher test between the four groups.
Discussion
Although there are several lines of evidence from many controlled and uncontrolled studies for the short-term and long-term effectiveness of acupuncture in relieving clinical pain [23-28], the scientific data concerning the efficacy of acupuncture in OA are rare [19]. In addition, there are several systemic flaws [29] among these studies due to inadequate statistical power, inadequate sessions of acupuncture treatment, failure to control concomitant therapies, or no sham/placebo acupuncture controlled group. In this study, we minimized the methodological limitations of previous studies by using the randomized, single-blind, placebo controlled design with a larger sample size of OA patients coupled with standard outcome assessments. By using the percentage of the responders as the main efficacy criterion, the comparison between true and sham acupuncture needs at least 61 patients per group, whereas, only 35 patients per group are needed to compare between true and placebo acupuncture performed by not puncturing the skin [23,30]. In order to increase the ability of differentiating true from placebo effects and minimize the sample size, we selected the procedure of attaching the acupuncture points with the patch electrodes as placebo EA, and at least 45 completers per group were treated in this trial. In this study, a double-blind design was considered inappropriate, since we used patch electrodes as placebo EA and our patients might have recognized the difference between true and placebo EA. A single blind was, therefore, a reasonable alternative.
The acupuncture points used were selected because we intended to determine only the effects of local points around the affected knee. This applied especially to the medial aspect, which related to the knee compartment that was frequently involved in OA. Using these local points coupled with the needling technique (developed by Chawal Kanchanakul, acupuncturist of Dhammanamai Foundation, Chiang Mai, Thailand) demonstrated a rather simple, convenient, less painful, and more acceptable method for Thai patients, and it was effective in our pilot study. The points selected here were therefore different from other trials [24-26,28], which also included the distal points at medial and lateral aspects of the leg. Low-frequency (2 Hz) EA was selected because it produces an analgesia of long duration, which outlasts the 20-min stimulation session by 30 min to many hours. In addition, its effects are cumulative after several sessions of treatment given either daily or less frequently (2–3 times a week) [30]. For these reasons, the low-frequency EA in this study was therefore given 3 times a week for 4 weeks, as commonly recommended in EA practice. However, to balance the acupuncture point stimulation by positive and negative polarities, each point was stimulated 6 times with positive and negative polarities in an alternate sequence during 12 sessions of treatment. In addition, each pair of electrodes was connected to each pair of adjacent points in order to obtain equal electrical sensation in each point during stimulation.
In this study, the clinical responses observed in the placebo group might result from, 1) the placebo effect or natural fluctuations in the symptoms of OA that are unrelated to the analgesic effect of paracetamol, because some patients demonstrated reductions in these scores without or with minimal analgesic need, or 2) the direct effects of paracetamol as a rescue analgesic. The latter reason made the placebo group not absolutely inert because paracetamol is also the first-line drug in the treatment of OA of the knee. However, the use of a rescue analgesic could not be avoided due to ethical reasons.
At week 4, the improvement of symptom scores (except change in WOMAC pain index and WOMAC total score) and the number of responders/patients with the opinion of "much better" were greatest in the EA group. These data indicate the great potential of EA in the symptomatic treatment of OA of the knee. This study also demonstrated that EA was significantly more effective than placebo with respect to changes in VAS and Lequesne's functional index, and significantly more effective than diclofenac with respect to the changes in VAS. This superiority of EA indicates its genuine efficacy [17,23,30], which was more effective than placebo (or diclofenac) in this study. A previous trial revealed 34% and 14% reductions in the mean values of WOMAC total score and Lequesne's index, respectively after 4 weeks of acupuncture treatment [28], whereas, our study demonstrated a 50% and 45% reduction, respectively. These discrepancies might be due to differences in acupuncture point selection, number of points and the electrical stimulation technique used, or electrical stimulation parameters.
The clinical responses in the combined group were slightly superior to the diclofenac group, but not to the EA group (except for the tendency of being superior with respect to a change in the subscale of the standardized WOMAC: pain index). These responses indicate that EA may exert an adequate analgesic effect, a combination with diclofenac may not be of further benefit. Although a combination was not more efficacious than EA alone, it was significantly more so compared to placebo in terms of improvement in the WOMAC pain index, while EA was not. However, the nonsignificant change in clinical scores between the diclofenac and placebo group indirectly suggests that diclofenac may be as effective as paracetamol (as needed) in symptomatic treatment of OA of the knee.
The nonsignificant change in paracetamol consumption among the four groups during treatment might result from the unnecessary use of paracetamol in a great majority of active treatment groups, despite a significant improvement in some parameters of the EA and combined group, and a tendency to improve parameters in the diclofenac group. Another possibility might be the large variation in paracetamol consumption in each group that contributed to a false negative result. The nonsignificant change in the 50 feet-walk time among the four groups suggests that this parameter may not be sensitive enough to demonstrate existing differences. Walk time determined during stair climbing should thus be a better alternative [31]. The failure to demonstrate the differences in other parameters (i.e. change in WOMAC stiffness, disability, and total scores) between the active treatment and placebo group may be due mainly to the rescue analgesic and partly to inadequacy of the sample size.
This study demonstrated that EA treatment was safe and free from serious adverse effects. However, the indifference in adverse events between the diclofenac and placebo group might be due to the exclusion of patients who were at high risk to the adverse effects of NSAIDs during the screening visit or short-term trial. In this study, the rescue analgesic paracetamol contributed to several confounding effects. Therefore, the randomized placebo controlled trial without using rescue analgesic should be investigated further to confirm the effectiveness of this range of active treatment, especially in EA and its combination with NSAIDs.
In summary, EA was significantly more effective than placebo regarding reductions in 100 mm VAS and Lequesne's functional index, but was significantly more effective than diclofenac in only the reduction of 100 mm VAS. The combination of EA and diclofenac treatment was more effective than placebo with respect to the reduction in the subscale of standardized WOMAC (pain index), but not more effective than EA treatment alone. Local contusions were minor adverse effects commonly found in the EA and combined group. The positive effects far outweigh the serious adverse ones of EA, which make this procedure an attractive alternative treatment for patients with OA of the knee.
Competing Interests
None declared.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This work was supported by the Faculty of Medicine, Chiang Mai University, Thailand.
Contributor Information
Chaichan Sangdee, Email: csangdee@mail.med.cmu.ac.th.
Supanimit Teekachunhatean, Email: steekach@mail.med.cmu.ac.th.
Kanit Sananpanich, Email: ksananpa@mail.med.cmu.ac.th.
Nantawit Sugandhavesa, Email: nsugandh@mail.med.cmu.ac.th.
Siripong Chiewchantanakit, Email: sichiewc@mail.med.cmu.ac.th.
Suwalee Pojchamarnwiputh, Email: spojcham@mail.med.cmu.ac.th.
Subhachai Jayasvasti, Email: sjayasva@mail.med.cmu.ac.th.
References
- Setnikar I. Antireactive properties of chondroprotective drugs. Int J Tissue React. 1992;14:253–61. [PubMed] [Google Scholar]
- Bradley JD, Brandt KD, Katz BP, Kalasinski LA, Ryan SI. Comparison of an antiinflammatory dose of ibuprofen, an analgesic dose of ibuprofen, and acetaminophen in the treatment of patients with osteoarthritis of the knee. N Engl J Med. 1991;325:87–91. doi: 10.1056/NEJM199107113250203. [DOI] [PubMed] [Google Scholar]
- Peyronm J. Inflammation in osteoarthritis (OA): Review of its role in the clinical practice, disease progress, subsets and pathology. Semin Arthritis Rheum. 1981;11:115–9. [Google Scholar]
- Bullough P. Synovial and osseous inflammation in osteoarthritis. Semin Arthritis Rheum. 1981;11:146. [Google Scholar]
- Altman RD, Hochberg MC. Degenerative joint disease. Clin Rheum Dis. 1983;9:681–9. [PubMed] [Google Scholar]
- Miller MR, Kasahara M. Observations of the innervation of human long bone. Anat Rec. 1963;145:13–7. [Google Scholar]
- Lemperg RK, Arnoldi CC. The significance of intraosseous pressure in normal and diseased states with special reference to the intraosseous engorgement-pain syndrome. Clin Orthop. 1978;136:143–56. [PubMed] [Google Scholar]
- Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, Moskowitz RW, Schnitzer TJ. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum. 1995;38:1541–6. doi: 10.1002/art.1780381104. [DOI] [PubMed] [Google Scholar]
- Brooks PM, Potter SR, Buchanan WW. NSAIDs and osteoarthritis-help or hindrance? J Rheumatol. 1982;9:3–5. [PubMed] [Google Scholar]
- Bollet AJ. Analgesic and anti-inflammatory drugs in the therapy of osteoarthritis. Semin Arthritis Rheum. 1981;11:130–2. [Google Scholar]
- Coles LS, Fries JF, Kraines RG, Roth SH. From experiment to experience: side effects of nonsteroidal anti-inflammatory drugs. Am J Med. 1983;74:820–8. doi: 10.1016/0002-9343(83)91073-2. [DOI] [PubMed] [Google Scholar]
- Griffin MR, Piper JM, Daugherty JR, Snowden M, Ray WA. Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med. 1991;114:257–63. doi: 10.7326/0003-4819-114-4-257. [DOI] [PubMed] [Google Scholar]
- Soll AH, Weinstein WM, Kurata J, McCarthy D. Nonsteroidal anti-inflammatory drugs and peptic ulcer disease. Ann Intern Med. 1991;114:307–19. doi: 10.7326/0003-4819-114-4-307. [DOI] [PubMed] [Google Scholar]
- Clive DM, Stoff JS. Renal syndromes associated with nonsteroidal antiinflammatory drugs. N EngI J Med. 1984;310:563–72. doi: 10.1056/NEJM198403013100905. [DOI] [PubMed] [Google Scholar]
- Bailey BO. Acetaminophen hepatotoxicity and overdose. Am Fam Phys. 1980;22:83–73. [PubMed] [Google Scholar]
- Buckalew VM., Jr Habitual use of acetaminophen as a risk factor for chronic renal failure: a comparison with phenacein. Am J Kidney Dis. 1996;28:S7–13. doi: 10.1016/s0272-6386(96)90562-4. [DOI] [PubMed] [Google Scholar]
- He LF. Involvement of endogenous opioid peptides in acupuncture analgesia. Pain. 1987;31:99–121. doi: 10.1016/0304-3959(87)90011-X. [DOI] [PubMed] [Google Scholar]
- Gordon JS. Alternative medicine and the family physician. Am Fam Physician. 1996;54:2205–12. [PubMed] [Google Scholar]
- Perrot S, Menkes CJ. Nonpharmacological approaches to pain in osteoarthritis. Available options. Drugs. 1996;52:21–6. doi: 10.2165/00003495-199600523-00005. [DOI] [PubMed] [Google Scholar]
- Cheng XN. Chinese Acupuncture and Moxibusion. Beijing: Foreign Languages Press. 1987.
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- Lequesne MG, Mery C, Samson M, Gerard P. Indexes of severity for osteoarthritis of the hip and knee validation-value in comparison with other assessment tests. Scand J Rheunatology. 1987;65:85–9. doi: 10.3109/03009748709102182. [DOI] [PubMed] [Google Scholar]
- Richardson PH, Vincent CA. Acupuncture for the treatment of pain: a review of evaluative research. Pain. 1986;24:15–40. doi: 10.1016/0304-3959(86)90023-0. [DOI] [PubMed] [Google Scholar]
- Christensen BV, luhl IU, Vilbek H, Bulow HH, Dreijer NC, Rasmussen HF. Acupuncture treatment of severe knee osteoarthrosis. A long-term study. Acta Anaesthesiol Scand. 1992;36:519–25. doi: 10.1111/j.1399-6576.1992.tb03511.x. [DOI] [PubMed] [Google Scholar]
- Takeda W, Wessel J. Acupuncture for the treatment of pain of osteoarthritic knees. Arthritis Care Res. 1994;7:118–22. doi: 10.1002/art.1790070304. [DOI] [PubMed] [Google Scholar]
- Gaw AC, Chang LW, Shaw LC. Efficacy of acupuncture on osteoarthritic pain. A controlled, double-blind study. N EngI J Med. 1975;293:375–8. doi: 10.1056/NEJM197508212930803. [DOI] [PubMed] [Google Scholar]
- Birch S, Hammerschlag R. Acupuncture efficacy: a compendium of controlled trials. National Academy of Acupuncture and Oriental Medicine. 1996.
- Berman BM, Singh BB, Lao L, Langenberg P, Li H, Hadhazy V, Bareta J, Hochberg M. A randomized trial of acupuncture as an adjunctive therapy in osteoarthritis of the knee. Rheumatol. 1999;38:346–54. doi: 10.1093/rheumatology/38.4.346. [DOI] [PubMed] [Google Scholar]
- Berman BM. Overview of clinical trials on acupuncture for pain. Presentation at NIH Consensus Development Conference on Acupuncture. Program and Abstracts National Institutes of Health, Bethesda, Maryland. 1997. pp. 61–2.
- Stux G, Pomeranz B. Basics of Acupuncture. Springer-Verlag Berlin Heidelberg. 1998.
- Bellamy N. Outcome measurement in osteoarthritis clinical trials. J Rheumatol. 1995;22:49–51. [PubMed] [Google Scholar]
- Kellgren JH, Lawrence JS. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]