Abstract
The aim of this work was to determine the proximate, mineral, amino acid composition, antioxidant activity, anti-nutritional factors, total dietary fiber, total phenolic content and technological properties of C. limetta peels. Moreover, analytical techniques including FT-IR and SEM were also conducted to study the morphological and structural properties of C. limetta peels. Considering the proximate, mineral, and amino acid composition, C. limetta peels was found to be a good source of ash (3.06 ± 0.20%), crude fiber (10.13 ± 0.30%), carbohydrate (64.08 ± 0.55%), protein (7.56 ± 0.25%), potassium (125.9671 mg/100 g), calcium (112.5861 mg/100 g), magnesium (16.43 mg/100 g), asparagine (2111.06 nmol/mg), glutamic acid (1331.96 nmol/g), and aspartic acid (1162.19 nmol/mg). Furthermore, they contain an appreciable amount of total dietary fiber (48.73 ± 0.45%), total phenolic content (14.30 ± 0.03 mg GAE/g), and antioxidant activity (52.65 ± 0.10%). Moreover, the antinutritional factors present in C. limetta peels were observed to be within the threshold limit. The results of technological properties of peels suggested that they can be potentially utilized as good emulsifying, gelling, foaming, and bulking agents in food industries. Therefore, C. limetta peels can be successfully re-utilized as natural food additive with numerous nutritive and bioactive properties in food sector, thereby achieving zero waste generation.
Keywords: Citrus peel, Amino acids, Minerals, Phenolics, Antioxidant, Dietary fibers
Introduction
Citrus species having their origin in the Rutaceae family and are one of the most abundantly grown crops across the world. Since bygone times, citrus fruits are being acknowledged for their exceptional sensorial attributes, promising nutritional and therapeutic properties, thereby making them a vital part of human’s diet. Moreover, it has been valuated that majority of citrus fruits produced are consumed fresh and about 25% are being processed, which is responsible for the production of tons of peels as by-products (Panwar et al. 2021a).
Citrus peels are known to be biologically unstable and organic in nature, therefore legitimate laws and eco-friendly techniques are required for their proper disposal and management. However, according to the research studies, citrus peels are considered as an enticing depository of phenolic compounds, amino acids, minerals, and dietary fiber, that can perform numerous pharmacological functions. Therefore, with an increase in demand for plant-derived products and functional foods, the re-use of citrus peels for the development of food products is gaining popularity. Moreover, variety of sustainable extraction techniques are being used for the recovery of these bioactive compounds from citrus peels and their utilization for the development of value-added marketable products (Panwar et al. 2021b).
The physico-chemical and nutritional composition of citrus peel may diversify according to the different cultivars and species. In addition, study of food composition is required to develop nutritional tables, enhance research studies regarding association of diet and diseases, and evaluation of nutritional status of human population (de Moraes Barros et al. 2012). Considering this, the knowledge regarding composition of citrus peels is extremely vital for its complete utilization in development of any food product and extraction of high value compounds from them. Research studies on some of the citrus species, including C. sinensis, C. limon, and C. reticulata have already been done to investigate their physico-chemical and nutritional properties. However, studies on the physico-chemical properties of C. limetta, also known as mosambi/sweet lime are very scarce. Due to the lack of scientific knowledge regarding its nutritional composition and in order to utilize the natural resource of Citrus limetta peels, the present study was aimed to investigate its proximate composition, mineral profile, amino acid composition, antioxidant, anti-nutritional, and functional properties.
Materials and methods
Plant material
C. limetta peels were collected from local market in Longowal, Punjab, India. Samples were washed using distilled water and dries using tray drier (SICO House, Patiala, India) at 45 °C to obtain a constant weight, grounded using an electric grinder and stored in airtight bags for further use.
Chemicals
Ammonium hydroxide, hydrochloric acid, methanol, gallic acid, and sodium carbonate was purchased from Merck, Germany. Folin- Ciocalteu reagent was purchased from Loba Chemie, India.
Analysis of proximate composition
C. limetta peel powder was analyzed in triplicates for its moisture, ash, fat, crude fiber, pH, and protein content according to the standard methods of Association of Official Analytical Chemists, 2010 (AOAC). The water activity (aw) of peel powder was directly measured using Rotronic HygroLab water activity meter. Furthermore, the total carbohydrate content was determined by calculating from the results of ash, moisture, protein, fat, and, crude fiber.
Analysis of color
The measurement of C. limetta peel color was measured using hunter colorimeter (Model D25 optical sensor, Hunter Associates Laboratory Inc., USA).
Analysis of mineral composition
The mineral composition of C. limetta peels was analyzed using microwave digestion (Anton Paar Microwave Go) of samples using the procedure described by Cindric et al. (2012) using an Inductively coupled plasma- Atomic Absorption Spectrophotometer (ICP-AES, SPECTRO Analytical Instruments GmbH, Germany).
Analysis of total amino acids
The evaluation of total amino acid composition in C. limetta peels was obtained via HR-LCMS using a Q-Exactive Plus Biopharma-High Resolution orbitrap Liquid Chromatograph Mass Spectrophotometer (Thermo Fischer Scientific Pte. Ltd., USA).
Analysis of anti-nutritional factors
Oxalate determination
To determine the oxalate content of citrus peels, sample (2 g) was suspended in distilled water (200 mL) and was digested using hydrochloric acid (6 N, 10 mL) for 1h at 100 °C. The mixture was then filtered and supernatant (125 mL) was collected, to which few drops of methyl red indicator to give a pink-colored solution. Thereafter, ammonium hydroxide was slowly added to the solution with constant stirring until the color changes from pink to faint yellow. The solution was again heated to 90 °C in a water bath, calcium chloride (5%, 10 mL) was added to it with continuous stirring and was kept overnight. The obtained suspension was then centrifuged at 3000 rpm and the precipitate was dissolved in 20% H2SO4. Finally, the solution was titrated against potassium permanganate (0.05 N) until the appearance of pink color and the oxalate concentration in peel sample was calculated (Oke 1996).
Total alkaloid content determination
The total alkaloid content in peel samples was determined according to the procedure of Harborne (1973).
Phytic acid determination
Citrus peel sample was suspended in concentrated hydrochloric acid (2%, 25 mL) and kept for 3 h. The solution was filtered and distilled water (10.7 mL) was added to the filtrate. Finally, the solution was titrated with iron chloride solution using 0.3% ammonium thiocyanate as an indicator until a brownish-yellow color appears (Oyeyinka and Afolayan 2019).
Total saponins determination
The total saponin content in peel sample was determined according to the method of Chapagain and Weisman (2005). The absorbance was taken at 430 nm and total saponin was determined using a calibration curve of diosgenin as standard.
Total tannins determination
Total tannins content in peel samples was determined according to the procedure of Khandelwal et al. (2010) and total tannins content was calculated using a standard curve of catechin.
Analysis of functional properties
Water/oil holding capacity
The water/oil holding capacity (WHC/OHC) of peel powder were determined following the procedure of Garau et al. (2007) and the water/oil retention was recorded using the following formula (Eq. 1):
| 1 |
Emulsifying properties
The emulsifying capacity (EC) and emulsifying stability (ES) of peel powder was determined according to the procedure of Dias et al. (2020) with minor modifications and EC/ES was then calculated.
| 2 |
| 3 |
Foaming properties
The methodology of Chaparro Acuna et al. (2012) was followed to determine the foaming capacity (FC) and foaming stability (FS) of citrus peel powder (Eq. 4):
| 4 |
Finally, the FS was measured according to the change in foam volume after 8 h and was calculated as (Eq. 5):
| 5 |
Swelling capacity
Swelling capacity (SC) of peel powder was determined using the procedure of Mora et al. (2013) with minor modifications and swelling capacity of peel powder was expressed as mL of water/g of dry weight of sample.
Analysis of dietary fiber content
The soluble (SDF), insoluble (IDF), and total dietary fibers (TDF) in citrus peel powder was determined following the AOAC enzymatic-gravimetric procedure of Prosky et al. (1988).
Analysis of total phenolic content
The total phenolic content of C. limetta peel powder was determined spectrophotometrically using Folin- Ciocalteu’s reagent as described by Hegazy and Ibrahium, (2012). Total phenolic content was determined using a standard curve of gallic acid and the results are expressed as milligram gallic acid equivalent (mg GAE/g) of dried peel extract.
Analysis of DPPH radical scavenging activity
The DPPH radical scavenging activity of citrus peel powder was determined using 2,2-diphenyl-1-picrylhydrazyl following the method of Brand-Williams et al. (1995).
Scanning electron microscopy
The morphological structure of dried C. limetta peel powder was observed using scanning electron microscopy (SEM, JSM-7610 F Plus, JEOL, Japan) and the images were collected at a magnification of 100x and 3000x.
Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FT-IR) of C. limetta peel powder was done using an FT-IR spectrometer (RX-U, FTIR, USA) by the procedure of KBr disc method in the wavelength range of 500–4000 cm-1.
Statistical analysis
All the experiments were performed in triplicates and the results are expressed as the means ± standard deviation.
Results and discussion
Proximate composition
The average composition of C. limetta peel powder is shown in Table 1. Moisture content is an important parameter that determines the quality, shelf life, and preservation of product. The high levels of moisture in peels makes them highly perishable, is more susceptible to microbial fermentation. However, C. limetta peel powder was observed to have 11.78 ± 0.06% moisture content, which was relatively low and can be favourable in enhancing the shelf life of peel powder. This value was found to be similar to the values for peels of C. maxima (13.20 ± 0.19%) (Ani and Abel 2018).
Table 1.
Proximate composition of C. limetta peels
| Parameters | Content (dry basis) | |
|---|---|---|
| Moisture (%) | 11.78 ± 0.06 | |
| Ash (%) | 3.06 ± 0.20 | |
| Protein (%) | 7.56 ± 0.25 | |
| Crude fiber (%) | 10.13 ± 0.30 | |
| pH (%) | 4.96 ± 0.01 | |
| Fat (%) | 3.36 ± 0.17 | |
| Water activity (%) | 0.49 ± 0.001 | |
| Carbohydrates (%) | 64.08 ± 0.55 | |
| Color | L* | 77.72 ± 0.01 |
| a* | 2.91 ± 0.02 | |
| b* | 31.39 ± 0.02 | |
| Total phenolic content (mg GAE/g dried extract) | 14.30 ± 0.03 | |
| DPPH radical scavenging activity (%) | 52.65 ± 0.10 | |
| Dietary fiber content | Insoluble dietary fiber (%) | 43.66 ± 0.49 |
| Soluble dietary fiber (%) | 5.07 ± 0.04 | |
| Total dietary fiber (%) | 48.73 ± 0.45 | |
The ash content in peels is the measure of inorganic residues that remains after complete oxidation of organic matter present in the sample. It is an important parameter in determining the concentration of total minerals present in sample. In this study, the ash content of peel powder was determined to be 3.06 ± 0.20% and this result was found to be in close accordance to the ash content of C. sinensis peels (3.18 ± 0.06%) as reported by Hu et al. (2020).
Lipids are another important constituent of food product and are considered as a vital contributor of energy. Moreover, lipids play an important role in determination of overall physical attributes of food products. Therefore, a precise analysis of lipids in food products is required for appropriate labelling of nutrition along with ensuring that the food product meets the manufacturing specifications. The lipid content of C. limetta peels was determined to be 3.36 ± 0.17% which was observed to be lower than that reported for C. macrocarpa peels (4.70 ± 1.31%) (Fronteras et al. 2021).
Crude fiber is an important component in food products owing to its high pharmacological benefits in human body. The crude fiber content of C. limetta peels was found to be 10.13 ± 0.30% which was higher than the fiber content of C. sinensis peels (5.10 ± 0.88%) as mentioned by Agbaje et al. (2020), thereby suggesting its significant potential to be incorporated in food products for the development of high fiber products. Similarly, the pH of citrus peels was observed to be acidic in nature (4.96 ± 0.01) which was found to be similar to the results of Dias et al. (2020) for citrus peels. As a matter of fact, the importance of pH determination in food products is well accepted in the scientific community as it affects the quality, stability, and preservation of the product. The low pH of citrus peels makes them resistant to microbial growth, thereby keeping good quality of the final product.
Protein is a vital nutritional component of food product and its sufficient consumption is important for the general health and well-being of human beings. Protein affects the texture, stability, functional properties, and economic value of food product. In this study, the protein content in C. limetta peels was observed to be 7.56 ± 0.25% which was lower than C. sinensis peels (9.73 ± 0.63%) (Romelle et al. 2016) and higher than the protein content of C. reticulata peel (0.63 ± 0.06%) and C. limetta peel (2.00 ± 0.58%) (Rafiq et al. 2019; Younis et al. 2019). Therefore, it could be suggested that the appreciable protein content of C. limetta peels could make it a potential ingredient to improve the functional properties of food products. Furthermore, water activity of C. limetta peels was recorded as 0.47 ± 0.001 which was quite low that ensures the microbial and biological stability of product. Similar results were explained by Tekgul and Baysal (2018) who studied the effect of different drying techniques on the quality properties of C. limon peels.
Carbohydrate determination is critical for food scientists owing to its significant role as structural components which contributes to the textural, nutritional, and functional properties of food products. A significant content of carbohydrate was observed in C. limetta peels (64.08 ± 0.55%). Studies have demonstrated a lower carbohydrate content of 53.27 ± 0.10% and 58.62 ± 0.42% for C. sinensis peels (Romelle et al. 2016; Dias et al. 2020), respectively. The high carbohydrate content in C. limetta peels could make it an excellent substrate for the production of chemicals and fuels and these peels could also be used as a source of energy for the development of breakfast meals and formulations.
Color
Color of fruit peels is one of the important quality attributes that significantly affects the consumer acceptability of the product. The color value of C. limetta peel powder is expressed as L*, a*, and b* values that corresponds to lightness, redness/greenness, and yellowness/blueness, respectively and the results has been illustrated in Table 1. The L* value of C. limetta peels was observed to be 77.72 ± 0.01 which was found to be higher than those of C. reticulata peels dried using tray drier (37.14 ± 0.90), vacuum drier (57.64 ± 0.30), and freeze drier (59.75 ± 0.05) probably due to browning reactions during drying or caramelization of sugar, thereby causing color deterioration (Rafiq et al. 2019). Therefore, high L* value of C. limetta peels indicated lower deterioration of color. Similarly, the a* value of C. limetta peels was found to be 2.91 ± 0.02 which was higher than peels of two different cultivars of C. natsudaidai (1.13 ± 0.09, 0.50 ± 0.09). The lower a* value of C. natsudaidai peels might be associated with degradation of more carotenoids in dried samples. Furthermore, the b* value of C. limetta peels was 31.39 ± 0.02 which was higher than C. sinensis peels (25.74 ± 0.71) (Dias et al. 2020) and this difference in yellowness for citrus peels could be due to the degradation of flavonoids and carotenoids during drying process.
Mineral composition
Minerals are essential compounds required for proper functioning of various physiological, structural, and metabolic processes in human body. Therefore, analysis of minerals in products is a pivotal consideration to evaluate their role in human nutrition and therefore its importance cannot be overaccentuated. As depicted in Table 2, seven minerals were quantified in C. limetta peels, including five macro minerals (sodium, potassium, calcium, magnesium, and phosphorus) and micro minerals (copper and manganese).
Table 2.
Mineral composition of C. limetta peels
| Sample | Cu | Fe | K | Mn | Na | Zn | Ca | Mg | P |
|---|---|---|---|---|---|---|---|---|---|
| µg/100 g | ppm | mg/100 g | µg/100 g | mg/100 g | ppm | mg/100 g | mg/100 g | mg/100 g | |
| C. limetta peel | 1723 | ND* | 125.96 | 7970 | 4.26 | ND*s | 112.58 | 16.43 | 7.81 |
*ND means less than 0.01 ppm
Potassium was observed to be the pre-dominant mineral present in C. limetta peels with a value of 125.96 mg/100g. Similar content of potassium was observed in peels of different citrus species, including C. maxima (127 mg/100 g), C. limon (127 mg/100 g), and white C. paradisi (129 mg/100 g) (Czech et al. 2020). After potassium, calcium was found to be in high concentration of 112.58 mg/100g in C. limetta peels. It should be noted that the calcium content in C. limetta peels were found to be higher than those reported by Czech et al. (2020) in different citrus species ranging from 28.8 to 63.9 mg/100 g and Matsuo et al. (2019) for different C. natsudaidai cultivars ranging from 58.9 to 97.9 mg/100 g. Magnesium is considered as the fourth most common mineral in human body after potassium, calcium, and sodium. The magnesium content in C. limetta peels was observed to be 16.43 mg/100 g and this result was observed to be higher than the magnesium content of C. reticulata peels (13.4 mg/100 g) and C. maxima peel extract (5.39 mg/100 g) (de Moraes Barros et al. 2012); Ani and Abel 2018).
Sodium is an essential mineral required by human body to maintain normal cellular homeostatis and blood pressure. Sodium content in C. limetta peels was observed to be 4.26 mg/100 g which is quite low in concentration. Higher sodium content was observed in C. sinensis peels (19.44 mg/100 g) as reported by Matuso et al. (2019). It should be noted that the concentration of sodium is much less than potassium in citrus fruits, which is a vital information for people suffering from hypertension and heart related issues. Therefore, owing to the low concentration of sodium in C. limetta peels, they could be included in diets of consumers who requires strict dietary restriction of sodium. Phosphorus is an essential micromineral which is known as a structural component of nucleic acids and cell membranes. Phosphorus content in C. limetta peels was observed to be 7.81 mg/100 g. A high concentration of phosphorus was observed in peels of different citrus fruits (C. sinensis: 25.3 mg/100 g, C. maxima: 21.9 mg/100 g, C. reticulata: 19.9 mg/100 g, C. limon: 23.9 mg/100 g, C. aurantifolia: 20.1 mg/100 g, and C. paradisi: 19.0–22.5 mg/100 g) (Czech et al. 2020).
In addition, small amounts of microminerals, including copper and manganese were also found in C. limetta peels. Microminerals are the mineral elements that is required in less quantities by human body and is necessary for optimum functioning of human body. Copper and manganese were found at concentrations of 1723 µg/100g and 7970 µg/100g, respectively. Smaller amounts of copper and manganese were present in C. reticulata peels (1.40 µg/g and 4.60 µg/g, respectively) (Hayat et al. 2020).
Total amino acids
The total amino acid profile of C. limetta peel powder is presented in Table 3. In this study, the amino acid revealed that four essential amino acids namely threonine (564.43 nmol/mg), methionine (317.94 nmol/mg), leucine (649.79 nmol/mg), and lysine (465.47 nmol/mg) were detected in C. limetta peels, whereas histidine, valine, tryptophan, phenylalanine, and isoleucine were not detected. These essential amino acids play a major role in the proper functioning of biological system. Similarly, amongst the non-essential amino acids, C. limetta peels contained aspartic acid (1162.19 nmol/mg), glutamic acid (1331.96 nmol/mg), asparagine (2111.06 nmol/mg), serine (859.96 nmol/mg), glycine (633.80 nmol/mg), arginine (578.50 nmol/mg), alanine (1007.20 nmol/mg), tyrosine (355.03 nmol/mg), and hydroxyproline (776.90 nmol/mg), whereas, glutamine, cystine, and norvaline was not detected. Studies have explained the role of non-essential amino acids in the growth and repair of tissues, proper functioning of immune system, synthesis of hormones, and formation of red blood cells. Similar studies on the evaluation of amino acid profile for peels of different citrus species have been done by several researchers and it should be noted that the differences in amino acid profiles of citrus species might be related the differences in cultivars, ecological and genetic parameters, stage of fruit maturation, seasonal collection of fruit, and sample preparation process (Matsuo et al. 2019).
Table 3.
Amino acid profile of C. limetta peels
| Sr.no | Amino acids | nmol/mg of protein |
|---|---|---|
| 1 | Aspartic acid | 1162.19 |
| 2 | Glutamic Acid | 1331.96 |
| 3 | Asparagine | 2111.06 |
| 4 | Serine | 859.96 |
| 5 | Glutamine | ND |
| 6 | Histidine | ND |
| 7 | Glycine | 633.80 |
| 8 | Threonine | 564.43 |
| 9 | Arginine | 578.50 |
| 10 | Alanine | 1007.20 |
| 11 | Tyrosine | 355.03 |
| 12 | Cystine | ND |
| 13 | Valine | ND |
| 14 | Methionine | 317.94 |
| 15 | Norvaline | ND |
| 16 | Tryptophan | ND |
| 17 | phenylalanine | ND |
| 18 | Isoleucine | ND |
| 19 | leucine | 649.79 |
| 20 | Lysine | 465.47 |
| 21 | Hydroxproline | 776.90 |
*ND means not determined
Anti-nutritional factors
Anti-nutritional factors are defined as the substances that are either naturally present in plants or are synthetically generated in them by their regular metabolism processes. Moreover, the presence of anti-nutrients in high concentration has the ability to bind nutrients, thereby affecting their digestion and bioavailability. However, it has been reported that an appropriate ratio of anti-nutrients to nutrients can lower the adverse effect on digestibility as well as shows potential health benefits. The levels of different anti-nutritional factors were evaluated in this study and the results were presented in Table 4.
Table 4.
Quantitative content of anti-nutrients in C. limetta peels
| Anti-nutritional factors | Amount |
|---|---|
| Oxalate (mg/100 g) | 1.29 ± 0.04 |
| Alkaloids (%) | 4.16 ± 0.18 |
| Phytic acid (mg/100 g) | 2.17 ± 0.15 |
| Saponins (mg DE/g dry sample) | 4.71 ± 1.16 |
| Tannins (mg CE/g dry sample) | 2.31 ± 0.02 |
DE = diosgenin equivalent; CE = catechin equivalent
Oxalate is a plant-derived toxic compound without any known pharmacological function in human body. The increased consumption of oxalate can form insoluble complex with essential nutrients, thus reducing their bioavailability and causes severe irritation to the gastrointestinal lining and formation of kidney stones. Therefore, consumers have been recommended to limit their dietary oxalate intake to > 200 mg/day. The oxalate content in C. limetta peels was observed to be 1.29 ± 0.04 mg/100 g, which was within the threshold limit of its consumption. This value of oxalate content was observed to be similar to the oxalate content of raw banana peel (1.93 ± 0.02 mg/100g) (Abou-Arab et al. 2018).
Alkaloids are known to be a group of secondary metabolites that are naturally present in plant species which protects them against bacterial, and fungal attacks. According to the reports, alkaloids might exert some toxicity when consumed by humans, however a level of approximately 1–2 mg/kg body weight is considered as safe for human ingestion. The alkaloid concentration of C. limetta peels was determined to be 4.16 ± 0.18 %. This value of alkaloid content in C. limetta peels was observed to be lower than those reported by Romelle et al. (2016) for C. sinensis peels (5.44 ± 0.72%).
Phytic acid is one of the major naturally occurring anti-nutrients present in plants and acts as a chelator of nutrients which binds with the nutrients to form insoluble phytate salts, thereby reducing their bioavailability. Moreover, reports have shown the deficiency of zinc and iron amongst humans consuming diets rich in phytic acid. On the contrary, phytate has been attributed as a natural antioxidant and their low levels (0.1–0.9%) in diet can reduce blood cholesterol response and depression. Therefore, an optimal ratio of phytate is required to be maintained in order to ensure the bioavailability of micronutrients in diet as well as to avoid any adverse effects on health. It should be noted that a normal human body can tolerate the maximum concentration of 250–500 mg/100g of phytic acid (Ekop et al. 2008). The concentration of phytates in C. limetta peels was estimated to be 2.17 ± 0.15 mg/100g, which was far below the permissible range of phytate consumption.
Saponins are the amphiphilic glycosidic metabolites that are present naturally in various plant species and have bitter, toxic, and astringent nature and anti-pathogenic properties, In humans, consumption of saponin enriched products can cause growth impairment, throat irritation, ulcers, and reduced nutrient availability. In this study, the saponin content in C. limetta peels was observed to be 4.71 ± 1.16 mg DE/g dry sample. A higher saponin concentration of 213.03 ± 21.12 mg EE/g dry sample was observed for carrot peel oven dried at 50 °C (Nguyen and Le 2018)
Tannins are a specific group of high molecular weight phenolic compounds that are widely present in almost each part of plant species. Tannins have an ability to interfere with enzyme activities and bind with proteins resulting in reduced protein digestibility. However, tannin consumption within permissible limits have been associated with numerous health benefits. Therefore, an optimum level of consumption must be maintained by consumers in order to avoid any adverse health effects. It has been recorded that the daily intake of tannins in India is approximately 1500–2500 mg and levels below 1.5–2.5 g is considered as safe for consumption without any side effects. The tannin content in C. limetta peels was 2.31 ± 0.02 mg CE/g dry sample. In a study, tannin content of 2.13 mg/100g was investigated for potato peels by Kpanja et al. (2019).
Functional properties
The measured values of WHC/OHC and SC has been listed in Table 5. The WHC/OHC are two fundamental functional properties affecting the technical and psychological properties of food products. Therefore, the WHC of C. limetta peels was evaluated and results showed that 1g of peel powder was able to hold 6.92 ± 0.11 g water. This value of WHC was observed to be higher than that of C. hystrix peel (5.45 ± 0.09 mL water/g) and C. limon peels (5.61 ± 0.06 g water/g) (Abirami et al. 2014; Tekgul and Baysal 2018). Similarly, OHC of C. limetta peels was 2.85 ± 0.09 g oil/g sample, which was observed to be higher than that of C. hystrix peel (1.72 ± 0.09 mL oil/g), C. maxima (red) peel (1.33 ± 0.09 mL oil/g), and C. maxima (white) peel (1.26 ± 0.25 mL oil/g) (Abirami et al. 2014). Moreover, similar value of OHC was found by Younis et al. (2019) for debittered C. limetta peels (2.40 ± 0.05 mL oil/g sample). Therefore, owing to these appreciable values of WHC/OHC, citrus peels can be potentially used as a functional ingredient in food products to improve their textural, sensorial, and rheological properties, avoid syneresis, and formulation of products with high percentage of fat content.
Table 5.
Functional properties of C. limetta peels
| Property | Value |
|---|---|
| Water holding capacity (g water/g peel) | 6.92 ± 0.11 |
| Oil holding capacity (g oil/g peel) | 2.85 ± 0.09 |
| Emulsifying capacity (%) | 39.49 ± 0.81 |
| Emulsifying stability (%) | 32.72 ± 0.63 |
| Foaming capacity (%) | 19.22 ± 0.34 |
| Foaming stability (%) | 18.07 ± 0.33 |
| Swelling capacity (%) | 9.83 ± 0.12 |
As for the emulsifying properties, the EC and ES of C. limetta peels were observed to be 39.49 ± 0.81% and 32.72 ± 0.63%, respectively. EC is the ability of a molecule to facilitate the dispersion or solubilization of two immiscible liquids and ES is known as the capability to maintain an emulsion and their resistance to rupture. Previous studies have explained that the presence of an appreciable quantity of protein, galactose, and arabinose linked to the polysaccharide side chains can contribute to remarkable emulsifying properties (Chen et al. 2016).
Foams are a two-phase system where the gas bubbles are in dispersed phase and are trapped in a continuous phase of liquid. The FC and FS are two important functional parameters that are required to improve the texture and sensorial properties of food products. The FC and FS of C. limetta peels was observed to be 19.22 ± 0.34% and 18.07 ± 0.33%, respectively. It should be noted that the foaming properties are mainly related to the protein and phenolic content of peel samples as they can accumulate in the water-air interface that reduces the surface tension (Ezzati et al. 2020).
The SC of C. limetta peels was 9.83 ± 0.12% which was similar to the result reported by Younis et al. (2019) for mosambi peels (9.57 ± 0.59%). SC of peels is an important hydration parameter and is defined as their capacity to absorb water. It might be possible that the swelling capacity of peels mainly depends on their variety and processing type and is directly related to the number of cellulose units present in them. The determination of functional properties of peels is an important attribute which is required to explain the interaction amongst its structure, component, nature, and physicochemical properties. Therefore, the results of functional properties of peels indicated that C. limetta peels can be potentially utilized as an additive to achieve the desirable textural, sensorial, and physiochemical properties of food products.
Dietary fiber content
Dietary fiber is a group of carbohydrate polysaccharides which is known to be a part of plant cell wall and is resistant to digestion in human intestine. Numerous epidemiological studies have shown the physiological benefits of its adequate consumption. The results obtained in this study indicated that C. limetta peel consisted of 48.73 ± 0.45% TDF, in which the content of IDF was 43.66 ± 0.49% and SDF was 5.07 ± 0.04% (Table 1). These results were higher than that of different C. maxima and C. paradisi cultivars where the range of IDF, SDF, and TDF ranged from 6.1–16.2 to 1.8–5.66 g/100g, and 7.9–22 g/100g, respectively (Deng et al. 2021).
Total phenolic content
Polyphenols of citrus peels are the secondary plant metabolites, that plays an important role in the normal growth of fruits and have potential role in improving human health as well as in prevention of numerous chronic diseases. According to the results of this study, the total phenolic content in C. limetta peels was observed to be 14.30 ± 0.03 mg GAE/g dried extract (Table 1). This result was observed to be higher than those obtained by Medina-Torres et al. (2019), who obtained a total phenolic content of 11.19 ± 0.1 mg GAE/g from C. latifolia waste.
DPPH radical scavenging activity
Free radical scavenging activity was done to analyse the antioxidant potential of C. limetta peels which is due to the presence of high phenolic compounds having poly-hydroxyl groups in it. DPPH radical scavenging activity of C. limetta peel extract was determined to be 52.65 ± 0.10% (Table 1) which was in close proximity to the antioxidant activity of C. reticulata peel extract (53.76 ± 1.42%) (Shehata et al. 2021).
Scanning electron microscopy
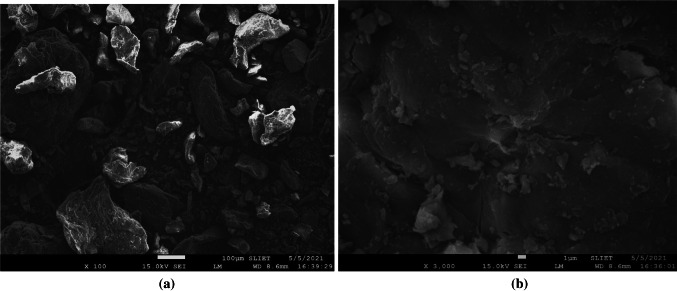
The surface morphology of C. limetta peel was studied using scanning electron microscope (SEM) and the micrographs are depicted in Fig 1a and b. The micrographs revealed that the C. limetta peels have irregular surface having rough, loose and porous morphology. Moreover, it can also be seen that the surface of C. limetta peels have large and irregular particles which suggested the presence of starch granules having high cellulose content. Besides, the thick layer on the peel surface indicated the presence of high fiber content that covers substances such as lignin, pectin, and hemicellulose (Boluda-Aguilar and Lopez-Gomez 2013).
Fig. 1.
SEM micrographs of C. limetta peel powder at (a) 100 × and (b) 3000 × magnification
Fourier transform infrared spectroscopy
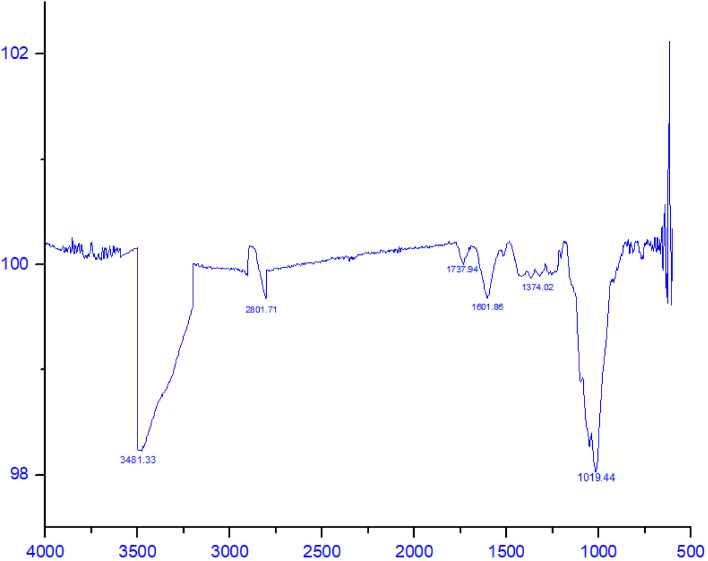
The FTIR spectroscopy of C. limetta peels was done to identify the functional groups present in them and the spectra has been shown in Fig. 2. As evident from the spectra, broad peaks at 3481.33 cm–1 corresponds to the inter/intra-molecular O–H stretching vibrations which indicates the presence of pectin in peel powder. The peak at 2801.71 cm–1 is attributed to the asymmetrical stretching of C–H bonds is associated with the presence of lignin, cellulose, and hemicellulose. Furthermore, the peak at 1737.94 cm–1 and 1601 cm–1 is mainly associated with the C=O stretching and corresponds to the vibrations of uronic ester and acetyl groups of hemicellulose or ester linkages of carboxylic groups of lignin and presence of phenols. The band at 1374.02 cm–1 was due to the bending of C–H bonds of cellulose units. Finally, the peak observed at 1019.44 cm–1 is assigned to the vibrational stretching of C–C–O and C=O bonds that indicates the presence of cellulose and lignin (Indulekha et al. 2017). Therefore, these functional groups suggested that C. limetta peels are a potential source of pectin, fibers, phenols, lignin, cellulose, and, hemicellulose.
Fig. 2.
Fourier Transform Infrared (FT-IR) spectra of C. limetta peel powder
4. Conclusions
The comprehensive characterization of C. limetta peels has reinforced the prospective valorization of this underutilized by-product that has either been used as animal feed or discarded without prior treatment. The experimental data revealed that the citrus peels were an enriched source of carbohydrates, minerals (especially, potassium, calcium, and magnesium), dietary fibers, and amino acids, and depending on the individual dietary requirements, these peels can be included in the diets to ameliorate human health. Moreover, the methanolic extracts of C. limetta peels showed promising phenolic content and antioxidant activities. Besides, remarkable functional properties were exhibited by C. limetta peels which suggested its possible utilization as natural thickening, emulsifying, foaming, and texturizing agent in food industry. In addition, the SEM and FT-IR analysis of citrus peels revealed the presence of diverse compounds and bioactive compounds. Therefore, it could be concluded that C. limetta peels might have a significant potential to be utilized as cost effective additive in food and pharmaceutical sector for the development of functional foods and dietary supplements. Besides, the re-utilization of citrus peels could help in reducing the cost and environment burdens associated with their management and disposal. Further research and clinical studies are recommended at molecular level in order to confirm the pharmacological importance of citrus peels.
Acknowledgements
Significant contribution was made by Divyani Panwar and Parmjit S. Panesar to the present study. Experimental design and concept were designed by Divyani Panwar and Parmjit S. Panesar and performed by Divyani Panwar. The work was supervised and guided by Harish K. Chopra. Divyani Panwar would also like to acknowledge the fellowship support (ICMR Ref. Letter No. 3/1/2/181/2020- (Nut)) from Indian Council of Medical Research (ICMR), New Delhi, India and infrastructural support from Sant Longowal Institute of Engineering and Technology, Longowal, India.
Abbreviations
- aw
Water activity
- C.
Citrus
- SEM
Scanning electron microscopy
- FTIR
Fourier transform infrared spectroscopy
- WHC
Water holding capacity
- OHC
Oil holding capacity
- SC
Swelling capacity
- EC
Emulsifying capacity
- ES
Emulsifying stability
- FC
Foaming capacity
- FS
Foaming stability
- IDF
Insoluble dietary fiber
- SDF
Soluble dietary fiber
- TDF
Total dietary fiber
Author contributions
DP: Conceptualization, Investigation, Formal Analysis, Data curation, Writing- Original draft preparation, Writing- Reviewing and Editing. PSP: Conceptualization, Resources, Supervision, Funding Acquisition, Writing- Reviewing and Editing. HKC: Resources and Supervision.
Funding
Divyani Panwar received the fellowship support (ICMR Ref. Letter No. 3/1/2/181/2020- (Nut)) from Indian Council of Medical Research (ICMR), New Delhi, India.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest regarding the publication of this paper.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abirami A, Nagarani G, Siddhuraju P. Measurement of functional properties and health promoting aspects-glucose retardation index of peel, pulp and peel fiber from Citrus hystrix and Citrus maxima. Bioact Carbohydr Dietary Fibre. 2014;4(1):16–26. [Google Scholar]
- Abou-Arab AA, Abu-Salem FM. Nutritional and anti-Nutritional composition of banana peels as influenced by microwave drying methods. Int J Nutrit Food Eng. 2018;11(12):845–852. [Google Scholar]
- Agbaje RB, Ibrahim TA, Raimi OT. Physico-chemical properties and sensory qualities of juices extracted from five selected fruits and their peels. Int J Eng Appl Sci Technol. 2020;4(11):2455–2143. [Google Scholar]
- Ani PN, Abel HC. Nutrient, phytochemical, and antinutrient composition of Citrus maxima fruit juice and peel extract. Food Sci Nutr. 2018;6(3):653–658. doi: 10.1002/fsn3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boluda-Aguilar M, López-Gómez A. Production of bioethanol by fermentation of lemon (Citrus limon L.) peel wastes pretreated with steam explosion. Ind Crops Prod. 2013;41:188–197. [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset CLWT. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28(1):25–30. [Google Scholar]
- Chapagain B, Wiesman Z. Larvicidal effects of aqueous extracts of Balanites aegyptiaca (desert date) against the larvae of Culex pipiens mosquitoes. Afr J Biotech. 2005;4(11):1351–1354. [Google Scholar]
- Chaparro Acuña SP, Gil González JH, Aristizábal Torres ID. Physicochemical characteristics and functional properties of vitabosa (Mucuna deeringiana) and soybean (Glycine max) Food Sci Technol. 2012;32(1):98–105. [Google Scholar]
- Chen HM, Fu X, Luo ZG. Effect of molecular structure on emulsifying properties of sugar beet pulp pectin. Food Hydrocoll. 2016;54:99–106. [Google Scholar]
- Cindrić IJ, Zeiner M, Krpetić M, Stingeder G. ICP-AES determination of minor and major elements in Cornelian cherry (Cornus mas L.) after microwave assisted digestion. Microchem J. 2012;105:72–76. doi: 10.1016/j.foodchem.2012.07.051. [DOI] [PubMed] [Google Scholar]
- Czech A, Zarycka E, Yanovych D, Zasadna Z, Grzegorczyk I, Kłys S. Mineral content of the pulp and peel of various citrus fruit cultivars. Biol Trace Elem Res. 2020;193(2):555–563. doi: 10.1007/s12011-019-01727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moraes Barros HR, de Castro Ferreira TAP, Genovese MI. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 2012;134(4):1892–1898. doi: 10.1016/j.foodchem.2012.03.090. [DOI] [PubMed] [Google Scholar]
- Deng M, Lin Y, Dong L, Jia X, Shen Y, Liu L, Zhang R. Physicochemical and functional properties of dietary fiber from pummelo (Citrus grandis L Osbeck) and grapefruit (Citrus paradisi Mcfad) cultivars. Food Biosci. 2021;40:100890. [Google Scholar]
- Dias PGI, Sajiwanie JWA, Rathnayaka RMUSK. Chemical composition, physicochemical and technological properties of selected fruit peels as a potential food source. Int J Fruit Sci. 2020;20(2):S240–S251. [Google Scholar]
- Ekop AS, Obot IB, Ikpatt EN. Anti-nutritional factors and potassium bromate content in bread and flour samples in Uyo Metropolis. Nigeria E J Chem. 2008;5(4):736–741. [Google Scholar]
- Ezzati S, Ayaseh A, Ghanbarzadeh B, Heshmati MK. Pectin from sunflower by-product: optimization of ultrasound-assisted extraction, characterization, and functional analysis. Int J Biol Macromol. 2020;165:776–786. doi: 10.1016/j.ijbiomac.2020.09.205. [DOI] [PubMed] [Google Scholar]
- Fronteras JP, Dacera DD, Bello DD, Santos KJLD. Utilization of pesticide-free calamansi (Citrus microcarpa) and mango (Mangifera indica) peels for the production of acetic acid with potential industrial application. Bioresour Technol Rep. 2021;15:100806. [Google Scholar]
- Garau MC, Simal S, Rossello C, Femenia A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chemistry. 2007;104(3):1014–1024. [Google Scholar]
- Hayat K, Zhang X, Abbas S, Hussain S, Hussain A, Tahir MU. Effect of microwave treatment on the nutritional profile of the citrus mandarin cultivars peels. J Food Process Preserv. 2020;44(10):e14791. [Google Scholar]
- Hegazy AE, Ibrahium MI. Antioxidant activities of orange peel extracts. World Appl Sci J. 2012;18(5):684–688. [Google Scholar]
- Herborne JB. Phytochemical methods. Guide Modern Tech Plant Anal. 1973;2:5–11. [Google Scholar]
- Hu X, Zhang X, Ngo HH, Guo W, Wen H, Li C, Ma C. Comparison study on the ammonium adsorption of the biochars derived from different kinds of fruit peel. Sci Total Environ. 2020;707:135544. doi: 10.1016/j.scitotenv.2019.135544. [DOI] [PubMed] [Google Scholar]
- Indulekha J, Siddarth G, Kalaichelvi P, Arunagiri A. Characterization of citrus peels for bioethanol production. In: Mohan BR, Srinikethan G, Meikap B, editors. Materials, energy and environment engineering. Singapore: Springer; 2017. pp. 3–12. [Google Scholar]
- Khandelwal S, Udipi SA, Ghugre P. Polyphenols and tannins in Indian pulses: effect of soaking, germination and pressure cooking. Food Res Int. 2010;43(2):526–530. [Google Scholar]
- Kpanja EJ, Duru S, Omage JJ, Sekoni AA, Gonjoh PT. Proximate composition, anti–nutritional factors and the effect of irish potato (Solanum tuberosum L.) peels on the performance and carcass characteristics of broiler chickens. Niger J Animal Sci. 2019;21(2):214–222. [Google Scholar]
- Matsuo Y, Miura LA, Araki T, Yoshie-Stark Y. Proximate composition and profiles of free amino acids, fatty acids, minerals and aroma compounds in Citrus natsudaidai peel. Food Chem. 2019;279:356–363. doi: 10.1016/j.foodchem.2018.11.146. [DOI] [PubMed] [Google Scholar]
- Medina-Torres N, Espinosa-Andrews H, Trombotto S, Ayora-Talavera T, Patrón-Vázquez J, González-Flores T, Pacheco N. Ultrasound-assisted extraction optimization of phenolic compounds from Citrus latifolia waste for chitosan bioactive nanoparticles development. Molecules. 2019;24(19):3541. doi: 10.3390/molecules24193541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora YN, Contreras JC, Aguilar CN, Meléndez P, De la Garza I, Rodríguez R. Chemical composition and functional properties from different sources of dietary fiber. Am J Food Nutrit. 2013;1(3):27–33. [Google Scholar]
- Nguyen VT, Le MD. Influence of various drying conditions on phytochemical compounds and antioxidant activity of carrot peel. Beverages. 2018;4(4):80. [Google Scholar]
- Oke OL. Chemical studies of some Nigerian vegetables. Exp Agric. 1965;1(2):125–129. [Google Scholar]
- Oyeyinka BO, Afolayan AJ. Comparative evaluation of the nutritive, mineral, and antinutritive composition of Musa sinensis L.(Banana) and Musa paradisiaca L.(Plantain) fruit compartments. Plants. 2019;8(12):598. doi: 10.3390/plants8120598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar D, Panesar PS, Chopra HK. Recent trends on the valorization strategies for the management of citrus by-products. Food Rev Intl. 2021;37(1):91–120. [Google Scholar]
- Panwar D, Saini A, Panesar PS, Chopra HK. Unraveling the scientific perspectives of citrus by-products utilization: Progress towards circular economy. Trends Food Sci Technol. 2021;111:549–562. [Google Scholar]
- Prosky L, Asp NG, Schweizer TF, Devries JW, Furda I. Determination of insoluble, soluble, and total dietary fiber in foods and food products: interlaboratory study. J Assoc off Anal Chem. 1988;71(5):1017–1023. [PubMed] [Google Scholar]
- Rafiq S, Singh B, Gat Y. Effect of different drying techniques on chemical composition, color and antioxidant properties of kinnow (Citrus reticulata) peel. J Food Sci Technol. 2019;56(5):2458–2466. doi: 10.1007/s13197-019-03722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romelle FD, Rani A, Manohar RS. Chemical composition of some selected fruit peels. Europ J Food Sci Technol. 2016;4(4):12–21. [Google Scholar]
- Shehata MG, Awad TS, Asker D, El Sohaimy SA, Abd El-Aziz NM, Youssef MM. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr Res Food Sci. 2021;4:326–335. doi: 10.1016/j.crfs.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekgül Y, Baysal T. Comparative evaluation of quality properties and volatile profiles of lemon peels subjected to different drying techniques. J Food Process Eng. 2018;41(8):e12902. [Google Scholar]
- Younis K, Ahmad S, Osama K, Malik MA. Optimization of de-bittering process of mosambi (Citrus limetta) peel: Artificial neural network, Gaussian process regression and support vector machine modeling approach. J Food Process Eng. 2019;42(6):e13185. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.